Abstract
Despite extensive studies of the mucosal immune system in the female reproductive tract (FRT) and its regulation by sex hormones, relatively little attention has been paid to the tissue environment in the FRT that regulates immune cell function. Consisting of secretions from epithelial cells, stromal fibroblasts and immune cells in tissues from the upper (Fallopian tubes, uterus and endocervix) and lower (ectocervix and vagina) tracts, each tissue compartment is unique and precisely regulates immune cells to optimize conditions for successful pregnancy and protection against sexually transmitted diseases including HIV. Our goal in this Review is to focus on the mucosal (tissue) environment in the upper and lower FRT. Specifically, this review will identify the contributions of epithelial cells and fibroblasts to the tissue environment and examine the impact of this environment on HIV-target cells. Much remains to be learned about the complex interactions with the tissue environment at different sites in the FRT and the ways in which they are regulated by sex hormones and chemical contraceptives. Awareness of the involvement of the tissue environment in determining immune cell function and HIV acquisition is crucial for the understanding the mechanisms that lead to HIV prevention, acquisition and the development of new therapeutic modalities of immune protection.
Keywords: Female reproductive tract, sex hormones, epithelial cells, fibroblasts, innate and adaptive immune systems, estradiol, progesterone
Introduction
Three decades after the discovery of Human Immunodeficiency Virus (HIV), with no effective vaccine available and limited success with microbicides, the HIV pandemic continues to spread with more than 30 million people living with HIV and almost 2.7 million new infections in 20101. Sexual contact remains the predominant mechanism of transmission worldwide, with young women most vulnerable to HIV infection with rates 2-fold higher than that in young men 2. There is increasing evidence that the first contact between HIV and immune cells in the female reproductive tract (FRT) is vital in determining the outcome of HIV infection. However, despite our growing understanding of the mucosal immune system in the FRT, much remains to be learned about the underlying mechanisms that regulate immune cell susceptibility to infection at different sites in the FRT.
The mucosal immune system in the FRT contains a spectrum of immune cells including NK cells, neutrophils, macrophages and T cells that are different from their blood counterparts al 3,4,52, 53. In addition to their unique phenotypic characteristics, these cells are functionally unique and responsive to changes in hormone balance during the menstrual cycle 5. Recent studies indicate that immune cells vary with the mucosal site analyzed. For example, when macrophages isolated from the gastro-intestinal (GI) and compared to those from the vagina, vaginal macrophages were more susceptible to HIV infection than those from the GI tract 6. While much remains to be learned about the unique characteristics of FRT mucosal immune cells, of equal importance is defining the role of the local tissue environment in regulating immune cell phenotype and function.
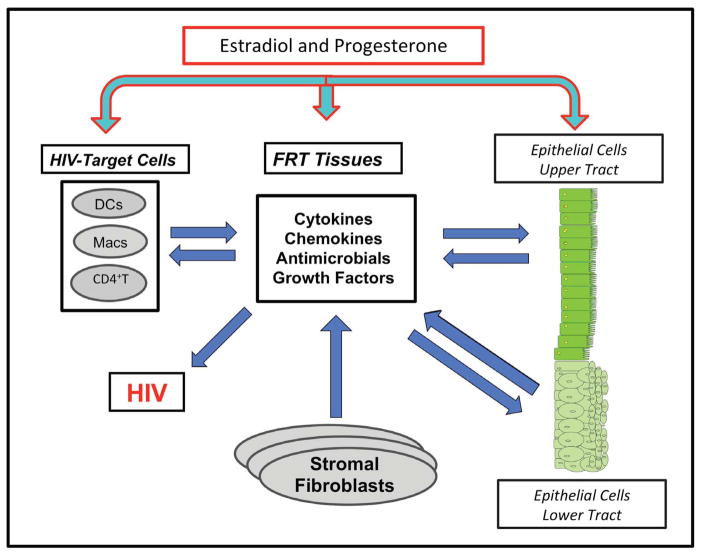
Epithelial cells, fibroblasts and immune cells that reside in FRT tissues contribute to the pool of cytokines, chemokines and growth factors present in the tissue environment. As seen in Figure 1, HIV-target cells (CD4+T cells, macrophages and dendritic cells) in FRT tissues are essential for reproductive success. Once immune cells or their precursors enter the FRT, their phenotypes are determined by direct interactions with resident cells in the tissue and the combined effect of their secretions. Additionally, the presence or absence of the sex hormones estradiol (E2) and progesterone (P4) influence the makeup of the tissue environment and both directly and indirectly modulate immune cell function 7,8. Adding to the complexity of this system is that hormone responsiveness of different cell types varies with anatomical location in the FRT.
Figure 1.
Representation of tissue environment immune protection against HIV in the female reproductive tract. Sex hormones regulate cellular interaction and communication between epithelial cells, fibroblasts and HIV-target cells to provide defense against HIV infection.
Our goal in this Review is to examine our understanding of FRT immunobiology by focusing on the mucosal (tissue) environment in the upper and lower FRT. Specifically, as outlined in Figure 1, we will examine the contributions of epithelial cells and fibroblasts to the tissue environment and examine the impact of this environment on HIV-target cells (CD4+T cells, macrophages and dendritic cells) against HIV. Awareness of the role of the tissue environment to immune cell function and HIV acquisition is crucial for understanding the barriers that prevent HIV infection and the development of new modalities of immune protection.
Changes in immune responses in the FRT during the menstrual cycle: Window of Vulnerability
It is not widely appreciated that HIV has multiple portals of entry through both the lower and upper FRT. Once deposited in the vagina, bacteria, HIV, sperm and radio-opaque dyes move rapidly into the cervix, uterus, Fallopian tubes and ovaries 9,10 (Hope, T: Personal Communication), potentially leading to viral exposure over a large and phenotypically diverse mucosal surface. The immune system in the FRT is unique in that it is hormonally controlled to support fertilization, and implantation 5, 11. We found that E2 and P4 act directly and indirectly through cytokines, chemokines and growth factors to enhance and suppress essential components of the humoral, cell-mediated and innate immune systems in the upper and lower FRT 12. For example, in the uterus during the proliferative phase, lymphoid aggregates (LA) consist of discrete CD4+ or CD8+ T cell clusters (300 cells/LA). During the secretory phase, LA increased to 3000–4000 cells/LA. Moreover, uterine CTL activity, but not vaginal, is suppressed during the secretory phase, most likely to optimize conditions for implantation 13. These findings led us to propose the hypothesis of a “window of vulnerability” (starting at ovulation and lasting 7–10 days during the secretory phase of the cycle) in which immune events to optimize fertilization and implantation place women at greatest risk for infection by sexually transmitted infections (STI)/HIV 12.
Evidence in support of a “window” comes from two studies. Using repeated, low-dose simian-human immunodeficiency virus (SHIVSF162P3) vaginal exposures during normal menstrual cycles 14, 18 macaques (95%) first displayed viremia in the follicular phase, compared with 1 macaque (5%) in the luteal phase. Taking into account a viral eclipse phase of 7–14 days before viremia could be detected, Vishwanathan et al. estimated a “window” of most frequent virus transmission between days 24 and 31 of the menstrual cycle (late secretory phase). More recently, Saba et al used human cervical explants to demonstrate that productive HIV-1 infection of human cervical tissue ex vivo is associated with the secretory and not the proliferative phase of the menstrual cycle 15. Taken together, these findings suggest that endocrine changes in part mediated through the tissue environment in parts of the FRT modulate immune protection in a way that increases the likelihood of HIV infection.
Contribution of epithelial cells to the FRT environment
Epithelial cells protect against infection by providing a physical barrier and secreting chemokines, cytokines and antimicrobials that contribute to mucosal defense against pathogens, including HIV 5, 7, 16–18. Layers of stratified squamous epithelial cells line the vagina and ectocervix, while tight junctions between the columnar epithelial cells maintain the integrity of the mucosal monolayer in the endocervix, endometrium, and Fallopian tubes (see Figure 1). The transition between squamous and columnar epithelial cells occurs at the cervical transformation zone – the junction of the ecto- and endocervix 5. The tight junction barrier permits epithelial cells of the upper tract to functionally polarize in order to respond to different stimuli from the apical (lumen) and basolateral (tissue) compartments, as well as to release IgA from the tissues into the lumen via the polymeric immunoglobulin receptor (pIgR) to prevent infection 17, 19. In a series of elegant studies, Nazli, Kaushic and colleagues showed that when upper tract genital epithelial cells were exposed to HIV, the mucosal barrier was compromised and correlated with secretion of pro-inflammatory cytokines by the epithelial cells 20–22.
FRT epithelial cell secretions
Although the effects of FRT epithelial cell secretions, both constitutive and induced, are well documented, less attention has been paid to discriminate between secretions that are released basolaterally into the tissues compared to those that are secreted apically into the lumen. Basolateral secretions have direct effects on the HIV target cells as well as on the underlying fibroblasts, and these in turn contribute to the FRT immune environment via their own secretions. We have shown that significant quantities of IL-8, IL-6, G-CSF, MCP-1, GM-CSF, TNFα, MIP-1β are constitutively secreted by purified uterine, endocervical and Fallopian tube epithelial cells into the basolateral compartment in culture 23. We have also shown that uterine epithelial cells preferentially release transforming growth factor-beta (TGFβ) into the basolateral chamber (approximately 70% > apical) and tumor necrosis factor-alpha (TNFα) into the apical compartment (approximately 30% > basolateral) 24. When epithelial cells on cell culture inserts were transferred to plates containing stromal cells, co-culture for 24–48hr decreased TNFα release into both the apical and basolateral chambers (approximately 30%-50%). Similar results were found when conditioned stromal medium (CSM) was placed in the basolateral chamber. These studies indicate that uterine stromal cells produce a soluble factor(s) that regulates the secretion of TNFαby uterine epithelial cells.
The secretion profile of FRT epithelial cells is partially dependent on the anatomical site within the FRT. For example, MIP3α (CCL20) has a potential secretion gradient among epithelial cells of Fallopian tubes>uterus>endocervix>ectocervix/vagina 25 (Patel et al. unpublished). Secretions by FRT epithelial cells have significant effects in determining whether or not an HIV infection will occur. For example, many of these epithelial cell factors, including IL-8, CCL20 and RANTES, are chemokines/cytokines that will attract/activate immune cells to/at the site of HIV infection. The number of activated HIV target cells (CD4+T cells, macrophages and dendritic cells) present at the point of HIV exposure may increase chances for infection. Alternatively, some of the same factors have anti-HIV activity and prevent HIV infection 7. This apparent paradox with relation to HIV infection is dependent on many factors, including the quantity of the epithelial cell factors secreted, the site within the FRT, the presence of co-infecting pathogens, the presence of proteolytic enzymes, the sex hormone balance, the activation status of cells, the interaction with other factors secreted by other cells as well as epithelial cells, and the types of immune cells present.
Epithelial cell secretions are also important in modulating the phenotype of local immune cells within the tissue. Studies from our laboratory addressed the influence of the FRT mucosal environment on dendritic cells. Using conditioned media (CM) from uterine epithelial cells to treat monocyte-derived DC, Ochiel et al 26 demonstrated that DC phenotype and function changes in response to secreted soluble factors in CM. As seen in Figure 2, uterine epithelial cell CM down-regulated DC-SIGN expression, but increased surface expression of CXCR4 on iDC. TGFβ blockade abolished the effect of CM on DC-SIGN but not CXCR4 suggesting that other molecules in CM are involved in regulating iDC phenotype. CM also inhibited iDC cell-mediated Trans infection of HIV-1 to TZM-bl target cells 27. This is the first evidence that EC basolaterally secreted TGFβ, a known estrogen-mediated soluble factor 28, lowers HIV infection of a target cell. In other studies, Smith et al. showed that TGFβ is partially responsible for the non-permissiveness of intestinal MØs to HIV infection 29. After CM treatment, DC responded with a tolerogenic pattern to stimulation with lipopolysaccharide (LPS) and poly (I:C), which included down-regulation of co-stimulatory molecules, production of IL-10, increased IDO and reduced induction of allogeneic proliferation 26. In agreement with this tolerogeneic environment, a great proportion of macrophages (CD68+) in the endometrium express CD163, a molecule that identifies alternatively activated macrophages, rather than CD14 30.
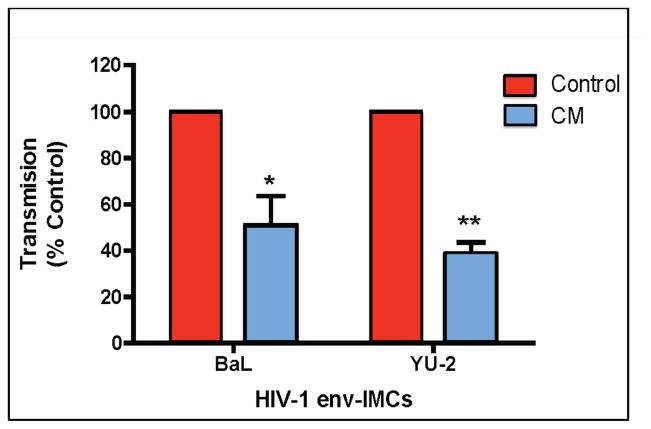
Figure 2.
Basolateral conditioned media (CM) from primary polarized epithelial cells inhibit trans infection by immature dendritic cells (iDC). The effect of primary uterine epithelial cell CM on trans infection by DC of HIV-1 infectious molecular clones encoding envelope ectodomains (env-IMC) of reference HIV-1 (BaL or YU-2) is shown. Media or CM from the basolateral compartments of primary uterine epithelial cells grown in cell inserts were incubated with immature DC differentiated from human monocyte derived-dendritic cells. Immature DC were pulsed with HIV-1 derived from infectious mononuclear clones expressing env genes for BaL or YU-2 in an isogenic reporter background. HIV-1 trans infection assay was performed using TZM-bl reporter cells as targets. Virus-pulsed DC were co-cultured with the TZM-bl reporter cell line and virus infection was assayed by measuring β-galactosidase expression with a Luminometer. The data are presented as % transmission +/− SEM (Calculated from 5 separate experiments) of HIV-1 by CM DC relative to Control DC. * p<0.05, ** p<0.001. Similar results were obtained with 4 transmitted/founder HIV-1. From 27.
Anti-HIV factors
HIV can cross the epithelial barrier and contact underlying target cells through several mechanisms including: (i) gaps in the epithelial lining, (ii) transcytosis or (iii) paracellular movement 31. Fortunately, FRT epithelial cells secrete a variety of anti-HIV molecules including mucus, human beta defensins (HBD), MIP3α, MIP1β, secretory leukocyte protease inhibitor (SLPI), lysozyme, tracheal anti-microbial peptide, trappin-2/elafin, cathelicidin, lysozyme and many others. These factors can function as receptor/co-receptor antagonists of HIV, directly inhibit the virus, prevent enhancement of HIV infection by eliminating co-infections, reduce inflammation or by other means. Interestingly, whereas secretions from uterine or Fallopian tube epithelial cells directly inhibit N. gonorrhoeae, C. albicans, and HIV-1, they had no effect on the commensal Lactobacillus crispatus 7. This suggests that the endogenous antimicrobials secreted by FRT epithelial cells will not reduce the protective effect of commensals in preventing HIV infection.
Pro-inflammatory secretions
Epithelial cells can recognize foreign pathogens through the expression pattern recognition receptors (PRR) that detect conserved moieties present in these pathogens such as nucleic acids (dsRNA, ssRNA) or bacterial components (LPS, flagellin). Upon recognition, epithelial cells upregulate their expression and secretion of pro-inflammatory cytokines 5, 7, 32–35. These function as signals that lead to increased trafficking of immune cells to the mucosal surface. Furthermore, an inflammatory environment within the FRT is associated with increased susceptibility to HIV acquisition. Similarly, co-infections and exposure of FRT epithelial cells to PRR and sexually transmitted pathogens stimulates secretion of pro-inflammatory factors that can lead to an immune activation environment of HIV infectivity 36–38. Additionally, semen, the vector by which pathogens are introduced into the FRT induces pro-inflammatory cytokine secretion by FRT epithelial cells that activates HIV-1 LTR in T cells 39, 40.
Role of Sex hormones
We have previously shown that E2 increases secretion of upper tract epithelial cell endogenous antimicrobials such as HBD2 and SLPI with known anti-HIV activity and inhibits the secretion of pro-inflammatory cytokines cytokines such as TNFα after viral or bacterial stimulation 41. The latter effect may be due to the antimicrobials in that E2-induced SLPI is known to inhibit NF-kB function and therefore production of pro-inflammatory factors by epithelial cells. Since pro-inflammatory cytokines, perhaps secreted in response to a bacterial or viral co-infection, are known to enhance HIV infectivity, E2 most likely has multiple effects on epithelial cells from different sites in the FRT that influence HIV acquisition. For example, in contrast to uterine epithelial cells in which E2 stimulates secretion of HBD2 and other immune molecules, E2 decreases vaginal epithelial cell secretion of innate immune proteins including HBD2 42,43.
FRT epithelial cells as targets in HIV infection
Recent results suggest that FRT epithelial cells may serve as a direct conduit of HIV to HIV-target cells, making the epithelial cells themselves de facto HIV-target cells. Asin and collaborators showed that primary uterine epithelial cells and fibroblasts treated with HIV released viral particles that were able to infect CD4+T cells 44. Liu and coworkers demonstrated that gastric epithelial cells act as a reservoir for HIV and also induce an inflammatory state that contributes to infectivity 45. Whether FRT epithelial cells can productively produce virus is controversial but a recent report showed that epithelial cells are infected with HIV and the epithelial cells pass on de novo virus in a contact-dependent mechanism to CD4+T cells 46. Recently, we found that uterine epithelial cells are susceptible to HIV infection with mucosally transmitted molecular clones of HIV-1 (Ochsenbauer, unpublished observation). Overall, studies to date indicate that the microenvironment of the female genital tract is complex and undergoes dynamic changes due to hormones and other factors that could influence HIV infection. Epithelial cells are critical contributors to the tissue environment and that contributions in the form of cytokines, chemokines and growth factors are site specific and under hormonal control.
Contribution of stromal fibroblasts to the FRT environment
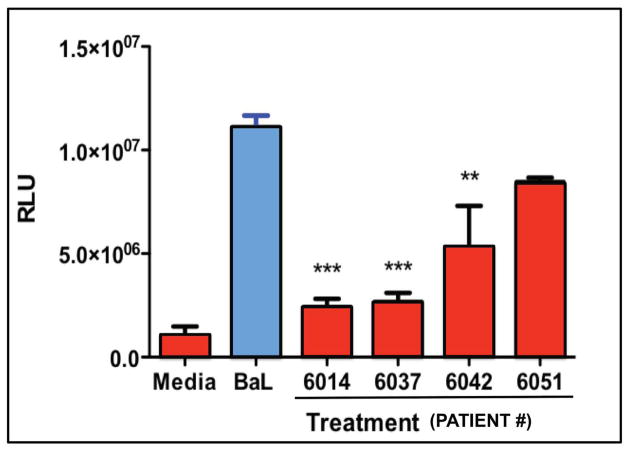
Fibroblasts are located beneath the epithelial layer and comprise the majority cell type in the sub-epithelial tissues. Despite this, relatively little is known about their role in innate immune protection. Previous studies have reported that secretions from a uterine stromal fibroblast cell line influence the phenotype of monocytes and macrophages and vice versa 47, 48. Fibroblasts from the uterus and Fallopian tubes express TLRs and upregulate the secretion of proinflammatory cytokines in response to the bacterial TLR4 ligand, LPS 49. In other studies, we have found that uterine, endocervical and ectocervical fibroblasts secrete both Type I and Type III IFNs in response to poly (I:C), leading to the upregulation of intracellular genes including MxA, OAS2 and APOBEC3G (Patel et al. unpublished observations). Additionally, they also secrete IL-8, IL-6, and MCP-1 in response to poly (I:C). To determine whether fibroblasts secrete antimicrobials, we analyzed conditioned media (CM) following 48hr in culture and found that cells from the upper FRT (Cx, FT and EM), but not the lower tract (ECx, VG), secrete CCL20/MIP3α and HBD2 (not shown), both of which we have found to inhibit HIV infection 25, 27. In preliminary studies, we tested diluted CM (1:4) from EM fibroblasts of 4 women for anti-HIV activity. As seen in Figure 3, fibroblast CM from 3/4 women had potent anti-HIV activity against an R5 (BaL) reference virus.
Figure 3.
Effect of 48hr fibroblast conditioned media (CM) derived from 4 patients on the infection of HIV BaL. TZM-bl cells were incubated with media control, Bal, or Bal and CM from quadruplicate fibroblast cultures of 4 patients. Relative Light Units (RLU) of β-galactosidase expression were determined with a Luminometer. Conditioned Media from three out of four fibroblast cell cultures significantly decreased infection. **, p<0.01, ***, p<0.001.
Fibroblasts also have site-specific differences when treated with E2. When fibroblast chemokines were measured in secretions, E2 stimulated SDF-1 production but had no effect on either MCP-1 or IL-8 (Patel et al. unpublished observations). Intriguingly, the withdrawal of sex hormones from uterine stromal fibroblasts treated with E2 and P4 for several days in vitro, increases their secretion of proinflammatory cytokines including IL-6, CCL2, CXCL8 and IFNα amongst others 50. Because epithelial-stromal cell interactions are essential in facilitating hormone-induced growth and development in the EM, we examined the effects of E2 on hepatocyte growth factor (HGF) made by FRT fibroblasts and found that secretion is regulated by E2 in the EM, but not in Cx and Ecx 51, 52. Given that HGF regulates epithelial cell (EC) tight junction barrier integrity, these findings indicate that sex hormones mediate communication between fibroblasts and epithelial cells with potential consequences for HIV acquisition. Overall, these data demonstrate that FRT fibroblasts are able to communicate with other cells as well as recognize and respond to pathogens and may represent an unappreciated player in innate immune protection within the FRT.
Effect of the tissue environment on immune cells
The role that the mucosal environment of the FRT plays in modifying immune cell susceptibility to HIV infection remains largely unknown. Many studies have demonstrated that the immune cell populations found in the FRT differ from cells found in peripheral blood and from other mucosal surfaces. These differences are driven by the effect of sex hormones acting directly on the immune cells and by the surrounding tissue environment, which is also responsive to hormonal fluctuations. Differences include cell activation, differentiation and HIV-coreceptor expression, which will greatly influence susceptibility to HIV-infection.
Cell activation and differentiation
The immune cell populations found in the uterus are unique when compared to other mucosal surfaces, most likely due to the need to tolerate foreign fetus antigens. Leukocytes increase in numbers in the endometrium during the luteal phase of the menstrual cycle 53 and continue to increase if pregnancy occurs. Several studies have proven that the composition and function of immune cells in the uterus is different than those found in blood and other tissues. In the endometrium T cells represent the main lymphocyte population 54. They display a memory phenotype with high expression of CD45RO 13. Additionally, there is an inverse CD4/CD8 ratio 13, 55, 56. Using endometrial biopsies, Shanmugasundaram et al 3 demonstrated that CD4+ and CD8+ T cells in the endometrium are mostly of the effector memory phenotype (TEM) and express higher levels of CD38 and HLA-DR than cells in peripheral blood, indicating a more activated state. NK cells are far more abundant in the endometrium than in blood and are characterized by high expression of CD56 and lack of CD16 57, 58, which represents an immunomodulatory subset of NK cells 59. A recent study compared leuckocyte composition from menstrual blood and peripheral blood from the same women and found fundamental differences between them 60, consistent with previous studies using hysterectomies. The total percentage of lymphocytes was reduced compared to PBMC, as described before 54. NK cells and T cells in menstrual blood had increased expression of CD103, indicating that they were not of peripheral origin. Also they found that T cells in menstrual blood were more recently activated, as shown by the increase in CD69 expression 60.
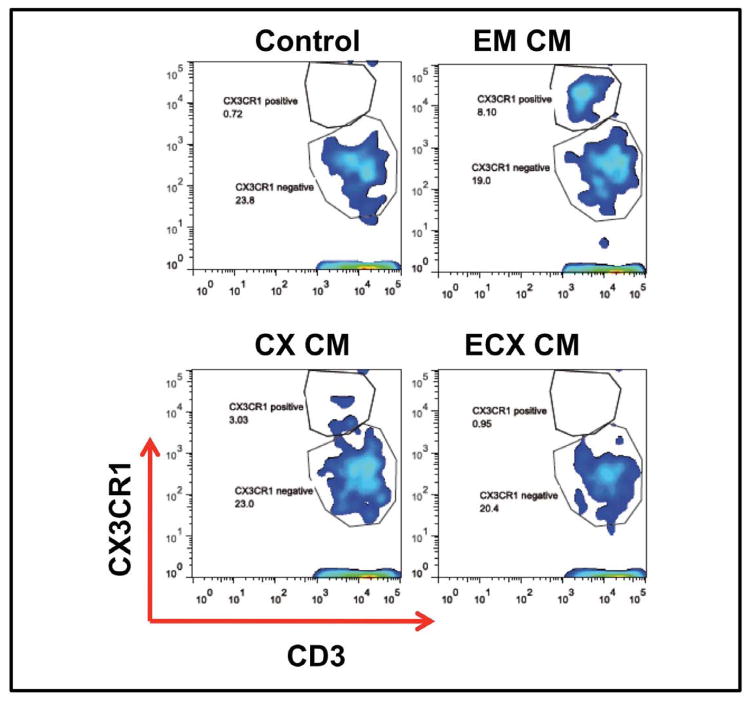
Optimization of the conditions for fertilization and pregnancy take place during the “window of vulnerability” and is dependent on hormonally controlled secretion of chemokines by epithelial cells that recruit and retain immune cells by enhancing or suppressing their expression of chemokine receptors 61–63. Many of these same chemokine receptors are also involved in HIV-infection and act by facilitating viral entry (CXCR4, CCR5) and spread to lymph nodes (CCR7) 64. In recent studies comparing blood and tissue CD4+T cells by RT-PCR analysis, we found that CCR7 was significantly lower in CD4+T cells from tissue relative to that seen with blood. In contrast, CCR6 expression was higher on FRT CD4+T cells than in blood. Within the FRT, CCR6 expression was highest in the Cx followed by ECx and EM. As seen in Figure 4, surface expression of CX3CR1 (Fractalkine receptor), the ligand for CX3CL1 (also called neurotactin or Fractalkine), which attracts and in the bound form retains T cells and monocytes, increased when blood CD4+T cells were treated for 48hr with CM from EM and Cx, but not with CM from ECx epithelial cells.
Figure 4.
Basolateral conditioned media from uterine and endocervical EC up-regulates CX3CR1 expression in CD4+T cells. CD4+T cells were purified from peripheral blood, incubated with basolateral EC conditioned media (CM) from the endometrium, endocervix and ectocervix for 48hr and analyzed for CX3CR1 expression by flow cytometry. CM from endometrium and endocervix, but not ectocervix up-regulated the expression of CX3CR1 compared to control cells incubated with media alone.
Characterization of T cell populations in cervix also shows differences with peripheral blood and other tissues 65, 66. Saba et al. 15 performed extensive characterization T cell phenotype and activation in cervico-vaginal tissues (endocervix and ectocervix). T cells (CD3+) represent the majority of lymphocytes (80%) but, unlike the endometrium, about 50% of them are CD4+ and 50% CD8+ 15 (Rodriguez-Garcia unpublished). Of the CD4+T cells, more than 90% were of the effector memory phenotype (TEM) while CD8+ T cells were 70% TEM. More than 90% of T cells expressed the activation markers CD69 and CD95, while other activation markers such as CD57, CD38, HLA-DR or CD25 were expressed in only 10% or less of the T cells15. A comparative analysis of gene expression and cell phenotype between cervical immune cells and PBMC showed clear differences in cell distribution and immune regulatory pathways between both populations 67. Three pathways relevant for susceptibility to HIV-infection showed to be over-expressed in the cells from the cervix compared to blood: toll-like receptor signaling pathway, complement and coagulation cascades and cytokine-cytokine receptor interaction pathway. Some of the cytokines that were over expressed in cervical cells include MIP-1α, MIP-1β and TNFα, which play a role in immune cell activation and recruitment but also in HIV-coreceptor blockade. TLR 1, 2, 4, 5, 6 and 8 and MyD88 were over-expressed in cervical cells while TLR 7 was under-expressed. Additionally, genes responsible for pro-apoptotic end functions were under expressed, suggesting an anti-apoptotic environment in the cervix 67.
Macrophages represent approximately 10% of the FRT leukocytes 54 and are more frequent in endometrial stroma and myometrial connective tissue 68 than in the endo- or ectocervix. The Influx of macrophages into the endometrium increases prior to menstruation under the influence of E2 and P4 69. In contrast, sex hormones do not regulate vaginal macrophage numbers, which remain constant throughout the menstrual cycle 68.
HIV-Coreceptor expression and HIV-infection
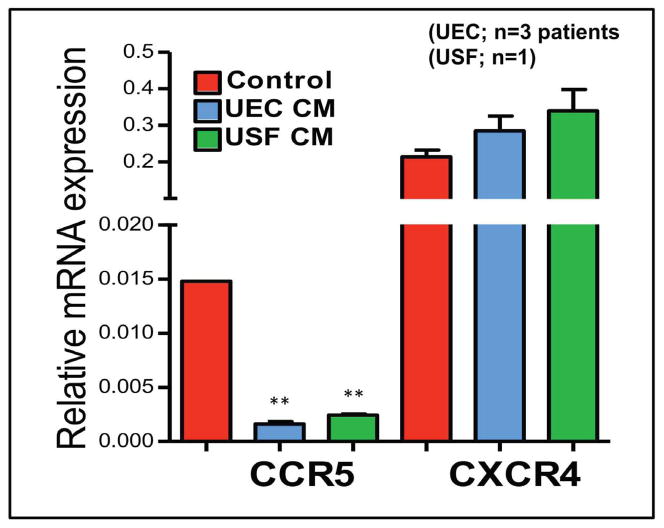
Different studies report increased expression of CCR5 in cells from the FRT 3, 15, 65, 66. Additionally, CCR5 expression may be regulated by the menopausal status, since cells from postmenopausal women expressed more CCR5 than premenopausal women 70 (Rodriguez-Garcia et al, unpublished data). Saba et al. 15 found that about 50% of CD4+T cells expressed HIV-coreceptor CCR5. Interestingly, ex-vivo HIV infection of these cervico-vaginal tissues was only possible with R5 strains, but tissues were very resistant to infection with X4 viruses, even though about 50% of cells also expressed the CXCR4 receptor, suggesting that additional factors beyond HIV-coreceptor expression are involved in susceptibility to HIV-acquisition. More recently (Figure 5), we found that basolateral CM from purified polarized uterine epithelial cell and fibroblast CM down-regulates CCR5 but not CXCR4 mRNA expression in blood CD4+T cells. This observation provides direct evidence that basolateral secretions from epithelial cells and fibroblasts alter susceptibility to HIV infection.
Figure 5.
Basolateral CM from purified polarized uterine epithelial cell and fibroblast CM down-regulate CCR5 but not CXCR4 mRNA expression in CD4+T cells. Purified blood T cells were incubated with CM from primary cultures of uterine epithelial cells and fibroblasts for 24hr. mRNA was isolated and expression of CCR5 and CXCR4 determined by RT-PCR. These findings provide direct evidence that basolateral secretions from FRT epithelial cells and fibroblasts alter susceptibility to HIV infection. **, Significantly p<0.01 lower than control cells.
Productive HIV-infection of cervico-vaginal tissue explants ex-vivo was recently demonstrated to be associated with the stage of the menstrual cycle 15. Only tissues from women in the secretory phase of the menstrual cycle, but not in proliferative or with atrophic endometrium, were able to support productive viral replication. Analysis of the cells supporting HIV-infection demonstrated that both CD4+T cells and macrophages were involved. Tissues supporting productive HIV-replication contained higher percentages of CD209+ (DC-SIGN) macrophages while higher production of CCL5 (RANTES) was found in non-productive infection 15.
A study comparing susceptibility to HIV-infection between macrophages from the vagina and intestinal macrophages showed that vaginal macrophages expressed higher levels of CD14, CD4, CCR5 and CXCR4 and displayed greater susceptibility to HIV infection 71. The reduced susceptibility of intestinal macrophages was due to TGFβ present in the intestinal tissue environment 7, similar to that seen with uterine epithelial conditioned media 27.
Nucleotidase Expression and microbicide availability in the tissue environment of the FRT
Microbicides have demonstrated promise for the prevention of HIV infection. However, recent clinical trials have shown that Preexposure Prophylaxis (PrEP) microbicides such as Tenofovir have been less efficacious in women than in men, possibly due to drug adherence as well as other factors. We discovered that E2 might influence the metabolism of Tenofovir in the FRT by regulating nucleotidase enzymes in fibroblasts and epithelial cells from the upper and lower FRT 72. Nucleotidases have phosphatase activity and are involved in the catabolism of nucleotides through dephosphorylation of the nucleotide terminal phosphate with a preference for nucleotide monophosphates 73 and have been implicated in TFV metabolism. Epithelial cells and fibroblasts were isolated from FRT tissues (Fallopian tubes, endometrium, endocervix and ectocervix) from hysterectomy patients, grown to confluence and treated with E2 prior to RNA isolation. The expression of 6 of 7 nucleotidase (NT) genes, as analyzed by RT-PCR, was measurable in both epithelial cells and fibroblasts from FRT tissues. Of those genes analyzed, E2 increased Cytosolic 5′-nucleotidase IA (NT5C1A) expression at 2 or 4h in endometrial epithelial cells. In parallel studies using a modified Diazyme 5′-Nucleotidase Assay, E2 treatment of epithelial cells and fibroblasts (24 and 48h) increased 5′-nucleotidase biological activity levels. The increase in nucleotidase expression and biological activity induced by E2 suggests that, when E2 levels are elevated during the menstrual cycle, FRT epithelial cells and fibroblasts may contribute to enhanced delivery of Tenofovir to HIV-target cells (macrophages, CD4+T cells) in the FRT. Further studies are needed to define the complex interactions of the endocrine system and its influence on microbicide efficacy.
Conclusions
In contrast to immune cells in peripheral blood, entry of immune cells and their precursors into the FRT is characterized by changes in phenotype and immune function. In addition to the influence of sex hormones, a growing body of evidence indicates that the tissue environment in the FRT, which consists of contributions from epithelial cells, stromal fibroblasts and immune cells, is responsible for immunological changes that are unique and contribute to the prevention of HIV infection. Understanding the complexities of the tissue environment and the mechanisms through which sex hormones selectively modulate immune function in the upper and lower FRT are crucial to protection against HIV and other microbial diseases as well as more fully understanding the events responsible for successful fertilization pregnancy and parturition.
Acknowledgments
We thank all study participants. We also thank the Pathologists, Obstetrics and Gynecology surgeons, operating room nurses and support personnel at Dartmouth-Hitchcock Medical Center. This study was supported by NIH grants AI102838 and AI071761 (CRW).
References
- 1.van der Straten A, Van Damme L, Haberer JE, Bangsberg DR. Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS. 2012;26:F13–19. doi: 10.1097/QAD.0b013e3283522272. [DOI] [PubMed] [Google Scholar]
- 2.CAC. HIV/AIDS Surveillance Report. 2003 [Google Scholar]
- 3.Shanmugasundaram U, Critchfield JW, Pannell J, Perry J, Giudice LC, Smith-McCune K, Greenblatt RM, Shacklett BL. Phenotype and Functionality of CD4 and CD8 T Cells in the Upper Reproductive Tract of Healthy Premenopausal Women. Am J Reprod Immunol. 2014;71:95–108. doi: 10.1111/aji.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith JM, Shen Z, Wira CR, Fanger MW, Shen L. Effects of menstrual cycle status and gender on human neutrophil phenotype. Am J Reprod Immunol. 2007;58:111–119. doi: 10.1111/j.1600-0897.2007.00494.x. [DOI] [PubMed] [Google Scholar]
- 5.Wira CR, Fahey JV, Ghosh M, Patel MV, Hickey DK, Ochiel DO. Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. Am J Reprod Immunol. 2010;63:544–565. doi: 10.1111/j.1600-0897.2010.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen R, Richter HE, Smith PD. Early HIV-1 target cells in human vaginal and ectocervical mucosa. Am J Reprod Immunol. 2011;65:261–267. doi: 10.1111/j.1600-0897.2010.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wira CR, Ghosh M, Smith JM, Shen L, Connor RI, Sundstrom P, Frechette GM, Hill ET, Fahey JV. Epithelial cell secretions from the human female reproductive tract inhibit sexually transmitted pathogens and Candida albicans but not Lactobacillus. Mucosal Immunol. 2011;4:335–342. doi: 10.1038/mi.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wira C, Rodríguez-García M, Patel M. Mucosal Immunity in the Human Reproductive Tract: Balancing Reproductive Potential with Protection against Sexually Transmitted Pathogens. In: Stanberry L, editor. Sexually Transmitted Diseases. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Racicot K, Cardenas I, Wunsche V, Aldo P, Guller S, Means RE, Romero R, Mor G. Viral infection of the pregnant cervix predisposes to ascending bacterial infection. J Immunol. 2013;191:934–941. doi: 10.4049/jimmunol.1300661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zervomanolakis I, Ott HW, Hadziomerovic D, Mattle V, Seebea BE, Virgolini I, Heute D, Kissler S, Leyendecker G, Wildt L. Physiology of upward transport in the human female genital tract. Ann NY Acad Sci. 2007;1101:1–20. doi: 10.1196/annals.1389.032. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Garcia M, Patel MV, Wira CR. Innate and adaptive anti-HIV immune responses in the female reproductive tract. Journal of reproductive immunology. 2013;97:74–84. doi: 10.1016/j.jri.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22:1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeaman GR, Guyre PM, Fanger MW, Collins JE, White HD, Rathbun W, Orndorff KA, Gonzalez J, Stern JE, Wira CR. Unique CD8+ T cell-rich lymphoid aggregates in human uterine endometrium. J Leukoc Biol. 1997;61:427–435. [PubMed] [Google Scholar]
- 14.Vishwanathan SA, Guenthner PC, Lin CY, Dobard C, Sharma S, Adams DR, Otten RA, Heneine W, Hendry RM, McNicholl JM, Kersh EN. High Susceptibility to Repeated, Low-Dose, Vaginal SHIV Exposure Late in the Luteal Phase of the Menstrual Cycle of Pigtail Macaques. J Acquir Immune Defic Syndr. 2011 doi: 10.1097/QAI.0b013e318220ebd3. [DOI] [PubMed] [Google Scholar]
- 15.Saba E, Grivel JC, Vanpouille C, Brichacek B, Fitzgerald W, Margolis L, Lisco A. HIV-1 sexual transmission: early events of HIV-1 infection of human cervico-vaginal tissue in an optimized ex vivo model. Mucosal Immunol. 2010;3:280–290. doi: 10.1038/mi.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fahey JV, Schaefer TM, Wira CR. Sex hormone modulation of human uterine epithelial cell immune responses. Integr Comp Biol. 2006;46:1082–1087. doi: 10.1093/icb/icl036. [DOI] [PubMed] [Google Scholar]
- 17.Ochiel DO, Fahey JV, Ghosh M, Haddad SN, Wira CR. Innate Immunity in the Female Reproductive Tract: Role of Sex Hormones in Regulating Uterine Epithelial Cell Protection Against Pathogens. Curr Womens Health Rev. 2008;4:102–117. doi: 10.2174/157340408784246395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wira CR, Fahey JV. The innate immune system: gatekeeper to the female reproductive tract. Immunology. 2004;111:13–15. doi: 10.1111/j.1365-2567.2003.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 20.Kaushic C. HIV-1 infection in the female reproductive tract: role of interactions between HIV-1 and genital epithelial cells. Am J Reprod Immunol. 2011;65:253–260. doi: 10.1111/j.1600-0897.2010.00965.x. [DOI] [PubMed] [Google Scholar]
- 21.Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, Arsenault AL, Kaushic C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6:e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nazli A, Kafka JK, Ferreira VH, Anipindi V, Mueller K, Osborne BJ, Dizzell S, Chauvin S, Mian MF, Ouellet M, Tremblay MJ, Mossman KL, Ashkar AA, Kovacs C, Bowdish DM, Snider DP, Kaul R, Kaushic C. HIV-1 gp120 induces TLR2- and TLR4-mediated innate immune activation in human female genital epithelium. J Immunol. 2013;191:4246–4258. doi: 10.4049/jimmunol.1301482. [DOI] [PubMed] [Google Scholar]
- 23.Fahey JV, Schaefer TM, Channon JY, Wira CR. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Hum Reprod. 2005;20:1439–1446. doi: 10.1093/humrep/deh806. [DOI] [PubMed] [Google Scholar]
- 24.Grant KS, Wira CR. Effect of mouse uterine stromal cells on epithelial cell transepithelial resistance (TER) and TNFalpha and TGFbeta release in culture. Biology of reproduction. 2003;69:1091–1098. doi: 10.1095/biolreprod.103.015495. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh M, Shen Z, Schaefer TM, Fahey JV, Gupta P, Wira CR. CCL20/MIP3alpha is a novel anti-HIV-1 molecule of the human female reproductive tract. Am J Reprod Immunol. 2009;62:60–71. doi: 10.1111/j.1600-0897.2009.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochiel DO, Ghosh M, Fahey JV, Guyre PM, Wira CR. Human uterine epithelial cell secretions regulate dendritic cell differentiation and responses to TLR ligands. J Leukoc Biol. 2010;88:435–444. doi: 10.1189/jlb.1009700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochiel DO, Ochsenbauer C, Kappes JC, Ghosh M, Fahey JV, Wira CR. Uterine epithelial cell regulation of DC-SIGN expression inhibits transmitted/founder HIV-1 trans infection by immature dendritic cells. PloS one. 2010;5:e14306. doi: 10.1371/journal.pone.0014306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chegini N, Zhao Y, Williams RS, Flanders KC. Human uterine tissue throughout the menstrual cycle expresses transforming growth factor-beta 1 (TGF beta 1), TGF beta 2, TGF beta 3, and TGF beta type II receptor messenger ribonucleic acid and protein and contains [125I]TGF beta 1-binding sites. Endocrinology. 1994;135:439–449. doi: 10.1210/endo.135.1.8013382. [DOI] [PubMed] [Google Scholar]
- 29.Smith PD, Smythies LE, Shen R, Greenwell-Wild T, Gliozzi M, Wahl SM. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 2011;4:31–42. doi: 10.1038/mi.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen AL, Collins J, Shipman EP, Wira CR, Guyre PM, Pioli PA. A subset of human uterine endometrial macrophages is alternatively activated. Am J Reprod Immunol. 2012;68:374–386. doi: 10.1111/j.1600-0897.2012.01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carias AM, McCoombe S, McRaven M, Anderson M, Galloway N, Vandergrift N, Fought AJ, Lurain J, Duplantis M, Veazey RS, Hope TJ. Defining the interaction of HIV-1 with the mucosal barriers of the female reproductive tract. J Virol. 2013;87:11388–11400. doi: 10.1128/JVI.01377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh M, Schaefer TM, Fahey JV, Wright JA, Wira CR. Antiviral responses of human Fallopian tube epithelial cells to toll-like receptor 3 agonist poly(I:C) Fertil Steril. 2008;89:1497–1506. doi: 10.1016/j.fertnstert.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hickey DK, Patel MV, Fahey JV, Wira CR. Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: stratification and integration of immune protection against the transmission of sexually transmitted infections. J Reprod Immunol. 2011;88:185–194. doi: 10.1016/j.jri.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel MV, Ghosh M, Fahey JV, Wira CR. Uterine epithelial cells specifically induce interferon-stimulated genes in response to polyinosinic-polycytidylic acid independently of estradiol. PLoS One. 2012;7:e35654. doi: 10.1371/journal.pone.0035654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaefer TM, Desouza K, Fahey JV, Beagley KW, Wira CR. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology. 2004;112:428–436. doi: 10.1111/j.1365-2567.2004.01898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peipert JF. Clinical practice. Genital chlamydial infections. N Engl J Med. 2003;349:2424–2430. doi: 10.1056/NEJMcp030542. [DOI] [PubMed] [Google Scholar]
- 37.Plummer FA, Simonsen JN, Cameron DW, Ndinya-Achola JO, Kreiss JK, Gakinya MN, Waiyaki P, Cheang M, Piot P, Ronald AR, et al. Cofactors in male-female sexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1991;163:233–239. doi: 10.1093/infdis/163.2.233. [DOI] [PubMed] [Google Scholar]
- 38.Schust DJ, Ibana JA, Buckner LR, Ficarra M, Sugimoto J, Amedee AM, Quayle AJ. Potential mechanisms for increased HIV-1 transmission across the endocervical epithelium during C. trachomatis infection. Curr HIV Res. 2012;10:218–227. doi: 10.2174/157016212800618093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferreira VH, Nazli A, Khan G, Mian MF, Ashkar AA, Gray-Owen S, Kaul R, Kaushic C. Endometrial epithelial cell responses to coinfecting viral and bacterial pathogens in the genital tract can activate the HIV-1 LTR in an NF{kappa}B-and AP-1-dependent manner. J Infect Dis. 2011;204:299–308. doi: 10.1093/infdis/jir260. [DOI] [PubMed] [Google Scholar]
- 40.Kafka JK, Sheth PM, Nazli A, Osborne BJ, Kovacs C, Kaul R, Kaushic C. Endometrial epithelial cell response to semen from HIV-infected men during different stages of infection is distinct and can drive HIV-1-long terminal repeat. AIDS. 2012;26:27–36. doi: 10.1097/QAD.0b013e32834e57b2. [DOI] [PubMed] [Google Scholar]
- 41.Fahey JV, Wright JA, Shen L, Smith JM, Ghosh M, Rossoll RM, Wira CR. Estradiol selectively regulates innate immune function by polarized human uterine epithelial cells in culture. Mucosal Immunol. 2008;1:317–325. doi: 10.1038/mi.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel MV, Fahey JV, Rossoll RM, Wira CR. Innate immunity in the vagina (part I): estradiol inhibits HBD2 and elafin secretion by human vaginal epithelial cells. Am J Reprod Immunol. 2013;69:463–474. doi: 10.1111/aji.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han JH, Kim MS, Lee MY, Kim TH, Lee M-K, Kim HR, Myung SC. Modulation of human β-defensin-2 expression by 17β-estradiol and progesterone in vaginal epithelial cells. Cytokine. 2010;49:209–214. doi: 10.1016/j.cyto.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Asin SN, Fanger MW, Wildt-Perinic D, Ware PL, Wira CR, Howell AL. Transmission of HIV-1 by primary human uterine epithelial cells and stromal fibroblasts. J Infect Dis. 2004;190:236–245. doi: 10.1086/421910. [DOI] [PubMed] [Google Scholar]
- 45.Liu R, Huang L, Li J, Zhou X, Zhang H, Zhang T, Lei Y, Wang K, Xie N, Zheng Y, Wang F, Nice EC, Rong L, Huang C, Wei Y. HIV Infection in gastric epithelial cells. J Infect Dis. 2013;208:1221–1230. doi: 10.1093/infdis/jit314. [DOI] [PubMed] [Google Scholar]
- 46.Micsenyi AM, Zony C, Alvarez RA, Durham ND, Chen BK, Klotman ME. Postintegration HIV-1 Infection of Cervical Epithelial Cells Mediates Contact-Dependent Productive Infection of T Cells. J Infect Dis. 2013;208:1756–1767. doi: 10.1093/infdis/jit362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eyster KM, Hansen KA, Winterton E, Klinkova O, Drappeau D, Mark-Kappeler CJ. Reciprocal Communication Between Endometrial Stromal Cells and Macrophages. Reproductive Sciences. 2010;17:809–822. doi: 10.1177/1933719110371854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klinkova O, Hansen KA, Winterton E, Mark CJ, Eyster KM. Two-way communication between endometrial stromal cells and monocytes. Reproductive Sciences. 2010;17:125–136. doi: 10.1177/1933719109348922. [DOI] [PubMed] [Google Scholar]
- 49.Itoh H, Nasu K, Nishida M, Matsumoto H, Yuge A, Narahara H. Human oviductal stromal fibroblasts, but not oviductal epithelial cells, express Toll-like receptor 4: the site-specific mucosal immunity of the human fallopian tube against bacterial infection. Am J Reprod Immunol. 2006;56:91–101. doi: 10.1111/j.1600-0897.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 50.Evans J, Salamonsen LA. Decidualized Human Endometrial Stromal Cells Are Sensors of Hormone Withdrawal in the Menstrual Inflammatory Cascade. Biology of reproduction. 2013 doi: 10.1095/biolreprod.1113.108175. [DOI] [PubMed] [Google Scholar]
- 51.Coleman KD, Ghosh M, Crist SG, Wright JA, Rossoll RM, Wira CR, Fahey JV. Modulation of hepatocyte growth factor secretion in human female reproductive tract stromal fibroblasts by poly (I:C) and estradiol. Am J Reprod Immunol. 2012;67:44–53. doi: 10.1111/j.1600-0897.2011.01063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coleman KD, Wright JA, Ghosh M, Wira CR, Fahey JV. Estradiol modulation of hepatocyte growth factor by stromal fibroblasts in the female reproductive tract. Fertility and sterility. 2009;92:1107–1109. doi: 10.1016/j.fertnstert.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 54.Givan AL, White HD, Stern JE, Colby E, Gosselin EJ, Guyre PM, Wira CR. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol. 1997;38:350–359. doi: 10.1111/j.1600-0897.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 55.Flynn L, Byrne B, Carton J, Kelehan P, O’Herlihy C, O’Farrelly C. Menstrual cycle dependent fluctuations in NK and T-lymphocyte subsets from non-pregnant human endometrium. Am J Reprod Immunol. 2000;43:209–217. doi: 10.1111/j.8755-8920.2000.430405.x. [DOI] [PubMed] [Google Scholar]
- 56.Sabbaj S, Hel Z, Richter HE, Mestecky J, Goepfert PA. Menstrual blood as a potential source of endometrial derived CD3+ T cells. PLoS One. 2011;6:e28894. doi: 10.1371/journal.pone.0028894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lukassen HG, Joosten I, van Cranenbroek B, van Lierop MJ, Bulten J, Braat DD, van der Meer A. Hormonal stimulation for IVF treatment positively affects the CD56bright/CD56dim NK cell ratio of the endometrium during the window of implantation. Mol Hum Reprod. 2004;10:513–520. doi: 10.1093/molehr/gah067. [DOI] [PubMed] [Google Scholar]
- 58.Mselle TF, Meadows SK, Eriksson M, Smith JM, Shen L, Wira CR, Sentman CL. Unique characteristics of NK cells throughout the human female reproductive tract. Clin Immunol. 2007;124:69–76. doi: 10.1016/j.clim.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 59.Hanna J, Mandelboim O. When killers become helpers. Trends Immunol. 2007;28:201–206. doi: 10.1016/j.it.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 60.van der Molen RG, Schutten JH, van Cranenbroek B, Ter Meer M, Donckers J, Scholten RR, van der Heijden OW, Spaanderman ME, Joosten I. Menstrual blood closely resembles the uterine immune micro-environment and is clearly distinct from peripheral blood. Hum Reprod. 2013:det398. doi: 10.1093/humrep/det1398. pii]3. [DOI] [PubMed] [Google Scholar]
- 61.Guerin LR, Moldenhauer LM, Prins JR, Bromfield JJ, Hayball JD, Robertson SA. Seminal fluid regulates accumulation of FOXP3+ regulatory T Cells in the preimplantation mouse uterus through expanding the FOXP3+ cell pool and CCL19-mediated recruitment. Biol Reprod. 2011;85:397–408. doi: 10.1095/biolreprod.110.088591. [DOI] [PubMed] [Google Scholar]
- 62.Hannan NJ, Evans J, Salamonsen LA. Alternate roles for immune regulators: establishing endometrial receptivity for implantation. Expert Rev Clin Immunol. 2011;7:789–802. doi: 10.1586/eci.11.65. [DOI] [PubMed] [Google Scholar]
- 63.Ozturk S, Demir R. Particular functions of estrogen and progesterone in establishment of uterine receptivity and embryo implantation. Histol Histopathol. 2010;25:1215–1228. doi: 10.14670/HH-25.1215. [DOI] [PubMed] [Google Scholar]
- 64.Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol. 2006;6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McKinnon LR, Kaul R. Quality and quantity: mucosal CD4+ T cells and HIV susceptibility. Curr Opin HIV AIDS. 2012;7:195–202. doi: 10.1097/COH.0b013e3283504941. [DOI] [PubMed] [Google Scholar]
- 66.McKinnon LR, Nyanga B, Chege D, Izulla P, Kimani M, Huibner S, Gelmon L, Block KE, Cicala C, Anzala AO, Arthos J, Kimani J, Kaul R. Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. J Immunol. 2011;187:6032–6042. doi: 10.4049/jimmunol.1101836. [DOI] [PubMed] [Google Scholar]
- 67.Horton RE, Kaefer N, Songok E, Guijon FB, Kettaf N, Boucher G, Sekaly RP, Ball TB, Plummer FA. A comparative analysis of gene expression patterns and cell phenotypes between cervical and peripheral blood mononuclear cells. PLoS One. 2009;4:e8293. doi: 10.1371/journal.pone.0008293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005;206:306–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 69.Starkey PM, Clover LM, Rees MC. Variation during the menstrual cycle of immune cell populations in human endometrium. Eur J Obstet Gynecol Reprod Biol. 1991;39:203–207. doi: 10.1016/0028-2243(91)90058-s. [DOI] [PubMed] [Google Scholar]
- 70.Meditz AL, Moreau KL, MaWhinney S, Gozansky WS, Melander K, Kohrt WM, Wierman ME, Connick E. CCR5 expression is elevated on endocervical CD4+ T cells in healthy postmenopausal women. J Acquir Immune Defic Syndr. 2012;59:221–228. doi: 10.1097/QAI.0b013e31823fd215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shen R, Richter HE, Clements RH, Novak L, Huff K, Bimczok D, Sankaran-Walters S, Dandekar S, Clapham PR, Smythies LE, Smith PD. Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type 1 infection. J Virol. 2009;83:3258–3267. doi: 10.1128/JVI.01796-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen Z, Fahey JV, Bodwell JE, Rodriguez-Garcia M, Rossoll RM, Crist SG, Patel M, Wira CR. Estradiol regulation of nucleotidases in female reproductive tract epithelial cells and fibroblasts. PloS one. 2013;8(7):e69854. doi: 10.1371/journal.pone.0069854. doi:10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hunsucker SA, Mitchell BS, Spychala J. The 5′-nucleotidases as regulators of nucleotide and drug metabolism. Pharmacol Ther. 2005;107:1–30. doi: 10.1016/j.pharmthera.2005.01.003. [DOI] [PubMed] [Google Scholar]