Abstract
Herpes virus type 1 (HSV-1) is one of the most widespread human pathogens and accounts for more than 90% of cases of herpes simplex encephalitis (HSE) causing severe and permanent neurologic sequelae among surviving patients. We hypothesize such CNS deficits are due to HSV-1 infection of neural progenitor cells (NPCs). In vivo, HSV-1 infection was found to diminish NPC numbers in the subventricular zone. Upon culture of NPCs in conditions that stimulate their differentiation, we found HSV-1 infection of NPCs resulted in the loss of neuronal precursors with no significant change in the percentage of astrocytes or oligodendrocytes. We propose this is due a direct effect of HSV-1 on neuronal survival without alteration of the differentiation process. The neuronal loss was prevented by the addition of microglia or conditioned media from NPC/microglia co-cultures. Using neutralizing antibodies and recombinant cytokines, we identified interleukin-6 (IL-6) as responsible for the protective effect by microglia, likely through its downstream Signal Transducer and Activator of Transcription 3 (STAT3) cascade.
Keywords: Virus, stem cells, encephalitis, protection, cytokines
Introduction
Herpes virus type 1 (HSV-1) is a common human neurotropic virus that accounts for more than 90% of the cases of herpes simplex encephalitis (HSE). This devastating disease causes severe impairment of neurological function that typically engages the frontal and temporal lobes causing characteristic clinical features including personality changes, cognitive impairment, aphasia, seizures and focal weakness (Kennedy and Steiner, 2013; Whitley and Gnann, 2002). HSV-1 has been associated with 70% mortality in untreated HSE patients and 30% mortality and a high incidence of severe and permanent neurological deficits among treated cases (Steiner, 2011). To date, the link between HSV-1 infection and lifelong severe neurological impairment experienced by HSE survivors is not understood.
Neural stem cells (NSCs) residing in the subventricular zone (SVZ) and the subgranular zone of the hippocampal dentate gyrus in the mammalian brain remain active into adulthood, providing neurons, astrocytes and oligodendrocytes in response to brain insults by producing new progenitor cells that migrate to regions affected by neurodegenerative pathology (Arvidsson et al., 2002; Eriksson et al., 1998; Ming and Song, 2011). In response to inflammation as a result of trauma, ischemia, or autoimmune disease, NPCs proliferate, migrate, and differentiate driven by signals immediately downstream of the NPCs as well as the injured tissue (Kazanis et al., 2007; Nait-Oumesmar et al., 2007; Yu et al., 2008). Such signals include neurotransmitters and growth factors expressed by injured cells that ultimately affect neurogenesis as a result of proliferation of neuroblasts (Banasr et al., 2004; Maurer, 2010).
In a mouse model of HSE, we recently discovered that upon reaching the brain stem, HSV-1 specifically targets and replicates in the ependymal cells lining the lateral ventricles prior to expansion into the parenchyma of the brain (Conrady et al., 2013). The proximity of the lateral ventricles to the SVZ provides a basis by which the virus could gain direct access to NPCs. As such, HSV-1 could influence the proliferation or differentiation of NPCs either directly or indirectly through the induction of inflammatory factors emanating from resident microglia or astrocytes infected with the virus. To this end, adult mouse NPCs were evaluated for susceptibility to HSV-1 infection and differentiation patterns following infection. We found NPCs were highly susceptible to HSV-1 infection with a specific loss of neuroblasts upon differentiation. The addition of microglia prevented this loss in an IL-6-dependent manner likely through the downstream STAT3 signaling pathway.
Materials and Methods
Animals
All animal procedures were approved by the University of Oklahoma Health Sciences Center and Dean McGee Eye Institute Institutional Animal and Care and Use Committee. C57BL/6 mice were obtained from The Jackson Laboratory. Transgenic mice to visualize NPCs in the adult brain (nestin-GFP transgenic mice) (Mignone et al., 2004) were obtained from Dr. Enikolopov (Cold Spring Harbor laboratory, Cold Spring Harbor, New York) and mated as homozygous pairs in the DMEI vivarium. Mice were 6-8 weeks old at the time of performing experiments.
Virus and in vivo infection
HSV-1 strain McKrae was propagated on HSV-1 susceptible green monkey kidney (Vero) cells and maintained at a stock concentration of 108 PFU/ml. Anesthetized mice were infected by scarification of the corneal surface followed by the application of 3.0 μl of PBS containing virus (105 PFU/eye) as previously described (Conrady et al., 2012). Alternatively, anesthetized mice were directly inoculated with the virus in the right lateral ventricle (105 PFU). Specifically, hair and skin overlaying the skullcap were resected and a pen-size hole drilled 0.5 mm anterior and 0.6 mm lateral of the bregma. A stereotactic injector (Stoelting Co, Wood Dale, IL) was used to inoculate the virus or PBS as procedure control at a depth of 2.5 mm from the skullcap. Skin was then sutured closed, and mice were treated with antibiotic-supplemented (trimethoprim and sulfamethazole) water and remained in the vivarium until sample collection.
Flow cytometry
For detection of NPCs in the SVZ of HSV-1 infected or uninfected mouse brains, anesthetized nestin-GFP transgenic mice were ocularly infected with 105 PFU HSV-1 or left uninfected. At 8d post infection (pi), the mice were exsanguinated and their brains were removed. The olfactory bulb and cerebellum were removed in order to reveal the hippocampus with the latter peeled from the cortex and removed to expose the wall of the lateral ventricle as described previously (Mirzadeh et al., 2010). Once the cortex and thalamus were removed, a single-cell suspension from the resulting lateral wall of the ventricle including the ependyma and SVZ regions was prepared using a neural tissue dissociation kit (MACS Miltenyi Biotec, #130-096-733), MACS columns, and magnetic MACS separators. Single-cell suspensions were resuspended in 1% bovine serum albumin (BSA) in PBS and analyzed using a flow cytometer (MACS Quant Analyzer, Miltenyi Biotec Inc, Auburn, CA) to detect GFP+ cells.
NPC and NPC-microglia culture system
A mouse GFP-expressing NPC cell line (M. Young, Harvard University) was used. To determine susceptibility of non-differentiated NPCs to HSV-1 infection, 100,000 NPCs/well were seeded on coverslips on 12-well plastic plates containing growth media: DMEM/F12 with glutamax (Invitrogen, #15168) supplemented with 20 ng/ml recombinant human epithelial growth factor (EGF) (Invitrogen, #13247-051), and antibiotic/antimycotic reagents. Upon a 3h incubation period, the cells were infected at a range of 0.0001-0.1multiplicity of infection (MOI) for 1h. The media was then removed and replaced with 1.0 ml of fresh media. NPC cultures were subsequently analyzed by immunocytochemistry at times pi.
For NPC differentiation studies, 30,000 NPCs/well were seeded on sterile coverslips on plates previously covered with Growth Factor Reduced BD Matrigel matrix (BD Biosciences, #354230; final concentration 0.3 mg/ml) in media that lacks EGF supplementation (Carbajal et al., 2010). Upon a 3h incubation period, the cells were infected with HSV-1 at a MOI of 0.0001 for 1h. The media was then replaced with 1.0 ml of fresh media. The cultures were then co-cultured in the presence or absence of microglia (MG) for different periods of time. For NPC/MG co-cultures, 200,000 freshly isolated microglia cells were added on top of NPCs seeded onto Matrigel matrix as described above. Specifically, single-cell suspensions from uninfected C57/BL6 mouse brains were prepared using a neural tissue dissociation kit (MACS Miltenyi Biotec, #130-092-628).The CD11b+ cells were isolated using CD11b microbeads (MACS Miltenyi Biotec, #130-093-634), MACS columns, and magnetic MACS separators according to the manufacturer’s protocol to achieve enrichment of 89-96 % CD11b+. As a control, some NPC cultures received an equivalent number of HSV-1 glycoprotein B (gB)-specific CD8+ T cells instead of microglia, which were isolated from spleens of HSV gBT-I.1 TCR transgenic mice (Mueller et al., 2002) using CD8a (Ly-2) microbeads (MACS Miltenyi Biotec, #130-049-401). In all cell culture experiments, the media was replaced with fresh media 2d pi.
Viral plaque assay
At indicated time points, the media from cell cultures was collected, and analyzed for viral content as previously described (Austin et al., 2006).
Conditioned media treatments
Following 2d of incubation, the media from uninfected NPC, MG cultures, and NPC/MG co-cultures was collected in 15 ml conical tubes, centrifuged (285 × g for 5min), and the supernatant passed through a 0.1 μm diameter pore filter. NPC cultures plated on Matrigel matrix were infected with HSV-1 for 1h, and the media replaced by 1.0 ml of fresh media, NPC-conditioned media (CM), MG-CM, or NPC/MG-CM. For immunocytochemical analysis, the CM was replaced 2d pi, and the cultures fixed at 4d pi. For detection of phosphorylated STAT3, NPCs were cultured an additional 12 h pi after which cells were collected for protein extraction and analysis.
Suspension protein array
Culture media from infected and uninfected cell cultures was collected 2 days following the initiation of culture. The media was clarified by centrifugation (10,000 × g for 1.5 min), and aliquots of the supernatants were evaluated for analyte content including IL-6, interleukin (IL)-1α, IL-1β, IL-10, leukemia inhibitory factor (LIF), tumor necrosis factor α (TNF-α), CXC chemokine ligand 10 (CXCL10), and interferon- γ (IFN-γ) (Milliplex, Millipore) by a Bioplex suspension array system (BioRad) according to the manufacturer’s instructions. The amount of each analyte is expressed in pg/ml. For assessment of IL-6 in nestin-GFP mice, the cerebrospinal fluid (CSF) was accessed through the cisterna magna as previously described (Conrady et al., 2013) and collected from uninfected and infected Nestin-GFP mice and analyzed at 8d pi.
In vitro neutralization of IL-6 and TNF-α
NPC and NPC/MG co-cultures were established on Matrigel matrix and infected with HSV-1 as described above. Following the addition of MG to the NPC cultures, anti-IL-6 antibody, anti-TNF-α antibody, or rat IgG1 isotype control (R&D systems, #MAB406, #MAB4101, and #MAB005 respectively) was added to the cultures at a final concentration of 6.0 μg/ml. At 2d pi, the media was replaced by fresh media containing neutralizing antibodies or isotype control IgG1. Sample media from 2d old cultures was used for measurement of cytokines, and cells were fixed at 4d and processed for immunocytochemical analysis.
Supplementation of NPC cultures with recombinant IL-6
NPC cultures were established on Matrigel matrix as described above. Two days pi, the cultures received mouse recombinant IL-6 (Millipore, #IL017) (0.1, 1.0, 10.0, and 50.0 ng/ml final concentration) or PBS vehicle. Four days pi, the cultures were fixed and processed for immunocytochemical analysis.
Immunochemistry
For immunohistochemistry (IHC), at indicated time points pi, Nestin-GFP transgenic mice were anesthetized and transcardially perfused with 4% paraformaldehyde (PFA). The brains were subsequently removed, fixed for additional 4h in 4% PFA at room temperature, sagitally sliced into halves, embedded in 2% agarose, and vibrotome-sectioned (Vibratome 3000 Sectioning System, The Vibratome Company, St. Louis, MO). Fifty μm-thick sections were blocked and permeated for 2h in PBS containing 3% BSA and 0.2% Triton, and processed for fluorescence as previously described (Encinas and Enikolopov, 2008).
For immunocytochemistry (ICC), at indicated time points, cell cultures were fixed with 4% PFA for 15 min, followed by a permeation step with 0.5% Triton in PBS for 10 min (note: permeation was avoided when staining cells for galactocerebroside [GALC] antigen). After 3 washes with 1 × PBS, samples were incubated with 10% normal donkey serum for 1h, followed by overnight incubation with primary antibodies. Following the overnight incubation, the samples were washed 3x with PBS, incubated with fluorescently-labeled secondary antibody for 1h (1:150), stained with nuclear dye (DAPI), washed 3x with PBS, and mounted in 50% glycerol for confocal imaging. The primary antibodies used were: anti-doublecortin (DCX) (Millipore, #AB2253, 1:2,000), anti-glial acidic fibrillar protein (GFAP) (DAKO, #Z0334, 1:500), anti-GALC (Millipore, #MAB342, 1:200), anti-HSV-1 (DAKO, #, B0114, 1:200), anti-CD11b (Leinco Technologies, #C227, 1:100), anti-HVEM receptor (Santa Cruz Biotechnology, # sc-365971, 1:200), and anti-phospho Histone H-3 (Millipore, #06-570, 1:100). All fluorescently labeled secondary antibodies were purchased from Jackson Laboratories. TUNEL assay was performed according to the manufacturer’s protocol for the Click-iT TUNEL Alexa Fluor 594 Imaging Assay kit (Invitrogen, #C10246). 2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI) and propidium iodide (PI) dyes were purchased from Sigma Aldrich (#D9542 and #P4170, respectively). Imaging was performed on an Olympus FluoView confocal laser scanning microscope (Olympus, Center Valley, PA, v5.0). Imaging and microscope settings were identical for all samples within experiments. For in vitro cell quantification, the MetaMorph offline software (v 7.7.0.0) was used to manually count cells of interest on acquired confocal images. Cells were counted in 5-7 fields of view per sample with the numbers summarized to obtain a total count for each sample of each cell type. The summary was then used to generate the percent of neuronal or glial phenotypes per sample and averaged to express the number of GFP+cells/field of view within samples.
Western blots
NPCs were seeded on Matrigel matrix-covered 6 well-plates at a density of 300,000 cells per well and infected with HSV-1 (MOI=0.0001) as described above. At indicated times, the cells were washed with PBS, scraped with a rubber scraper, pooled (3 wells/treatment), and lysed with RIPA lysis buffer (Santa Cruz Biotechnology, #sc-24948). In some experiments, a kit for extraction of nuclear and cytoplasmic protein (Thermo Scientific, #78833) was used to enrich for the nuclear protein fraction. For protein extraction from brain, tissue was harvested and dissected as described above and lysed with RIPA lysis buffer using a tissue sonicator. Protein content was measured using the BCA protein assay (Thermo Scientific, #23225). Equal amounts of protein were resolved on SDS 4-20% gradient polyacrylamide gels (Invitrogen, #EC60285). The proteins were transferred onto nitrocellulose membranes. Proteins were detected using the following primary antibodies: anti-Beta III tubulin (abcam, #ab18207, 1:2,000), anti-GFAP (DAKO, #Z0334, 1:1,000), anti-Beta actin (abcam, #ab6276, 1:10,000), anti- pCREB to phosphorylated CREB (Cell Signaling Technology, #9198, 1:2,000), anti-CREB (Cell Signaling Technology, #9197, 1:2,000), anti-NeuroD (Cell Signaling Technology, #4373, 1:1,000), anti-SOX2 (abcam, #ab97959, 1:2,000), anti-GAPDH (abcam, #ab8245, 1:2,000), anti-TATA binding protein TBP (abcam, #63766, 1:2,000), anti-pSTAT3 to phosphorylated STAT3 (Cell Signaling Technology, #9145, 1:2,000), and anti-STAT3 (Cell Signaling Technology, #4904, 1:2,000). For re-probing blots, membranes were stripped (Thermo Scientific, #46430) prior to blocking and incubation with a different primary antibody. For secondary chemiluminiscent detection, blots were incubated with corresponding HRP-conjugated secondary antibodies (Amersham Biosciences). Imaging of the blots and analysis of bands intensities were performed by conventional image analysis using an Image Station 4000R and Kodak software (IS4000 R; v.4.5.1).
Statistical analysis
Statistical analysis was performed by using Graph pad Prism 5.0 software. The data are expressed as the mean ±SEM for each group. The paired or unpaired t-test was performed to assess the significant differences (p<0.05) between groups. For multiple comparisons, one-way analysis of variance was performed followed by the Bonferroni’s post hoc test.
Results
Neural progenitor cells are susceptible to HSV-1 infection and upon differentiation the neuronal precursor population is significantly reduced
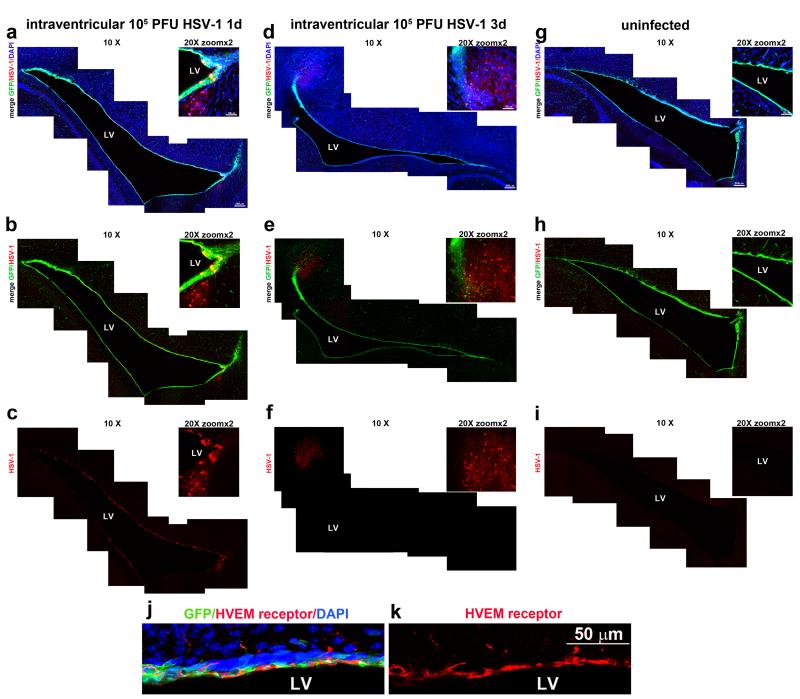
Ocular infection with HSV-1 has been found to spread to the ependyma from the brainstem prior to infection of the brain parenchyma (Conrady et al., 2013). As such, it is predicted NPCs residing in the neurogenic niche within the SVZ in close proximity to the ependyma could be susceptible to infection. To demonstrate the susceptibility of NPCs to virus, HSV-1 was administered into the lateral ventricles of nestin-GFP transgenic mice and assessed for localization relative to GFP-expressing NPCs. The results revealed that by day 1 pi GFP+ expressing cells in the SVZ surrounding the lateral ventricle (LV) stained positive for HSV-1 antigen as did GFP− cells adjacent to the SVZ (Figure 1 A, B, C). By day 3 pi, HSV-1 antigen expression spread radially and was primarily localized outside the SVZ in parenchymal areas of the brain (Figure 1 D, E, F). There was no evidence for co-localization of GFP-expressing cells and HSV-1 antigen around the borders of the LV at this time point. However, on occasion there was a loss in GFP expression in some areas of the LV lining in close proximity to HSV-1 antigen expression. Some of the GFP− cells expressing HSV-1 antigen exhibited neuronal morphology. Multiple entry receptors have been described to mediate infection by HSV-1, such as herpes virus entry mediator (HVEM), nectin-1 and -2, and paired Ig-like receptor alpha (PILRα) (Kopp et al., 2009). HVEM receptor was found constitutively expressed lining the walls of the lateral ventricle of the brain, co-localizing with GFP+ cells (Figure 1 j-k) as well as in other cell types throughout the brain (data not shown). Taken together, the data suggest that upon inoculation into the LV, NPCs are the first target of HSV-1 through at least one of its entry receptors, HVEM, and as time progresses, the infection spreads to the parenchyma of the brain.
Figure 1. Susceptibility of NPCs present in the ependymal and subventricular cell layers of the lateral ventricle to HSV-1 infection.
Nestin-GFP transgenic mice were inoculated with 105 PFU HSV-1 or PBS as control in the right lateral ventricle of the brain. At 1 (panels a-c) or 3 (panels d-f) days post infection, the mice were perfused with 4% PFA, their brains removed and sectioned (50μm), and subsequently processed. Confocal images of representative brain slices were used to reconstruct the whole margins of the lateral ventricle (LV). GFP-expressing NPCs are depicted in green, HSV-1-expressing cells are shown in red and DAPI-stained nuclei in blue. Brain slices from uninfected mice (panels g-i) served as controls for HSV-1 antigen expression. Note, co-localization of GFP and HSV-1 antigen at day 1 pi (panel b) within the ependyma and SVZ. By day 3 pi, the HSV-1 antigen expression is primarily expressed in the SVZ proximal to the ependyma. The HSV-1 entry receptor, HVEM, was found constitutively expressed within the ependymal and SVZ of naïve uninfected mice, shown in red, co-localizing with GFP+ expressing cells (green) (panels j-k). LV: lateral ventricle. This figure is representative of n=3 mice/time point or uninfected controls.
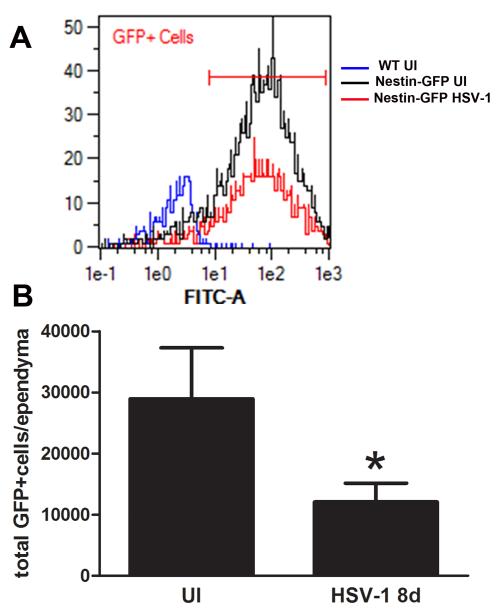
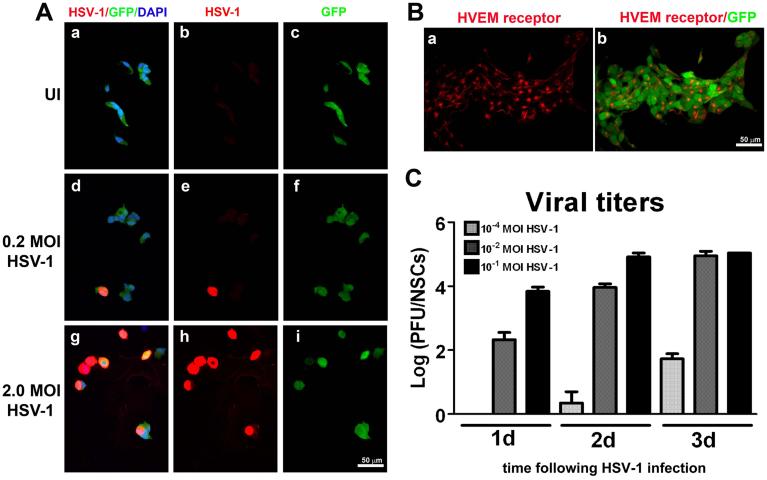
Next, nestin-GFP transgenic mice were infected in the cornea and assessed for the impact of HSV-1 on the pool of NPCs within the SVZ at day 8 pi. Flow cytometry analysis (Figure 2B) of single-cell suspensions of the ependymal and SVZ brain regions showed infected mice had greater than a 50% reduction in the total number of GFP+ cells compared to their uninfected counterparts. These results suggested two possibilities: 1) a deficit in NPCs due to cytopathic effects of viral replication causing cell death, or 2) a deficit in NPCs due to HSV-1 favoring the differentiation process. To more closely focus on the impact of HSV-1 on NPC differentiation, an in vitro approach was taken. GFP-expressing NPCs were initially evaluated for susceptibility to HSV-1. In a dose-dependent fashion, NPCs were highly susceptible to HSV-1 infection (Figure 3A). In proliferation conditions, NPCs were found to constitutively express HVEM (Figure 3B) and therefore, being one of the mediators of susceptibility to infection. In a time-dependent fashion, NPC cultures were maximally infected 72 h pi at an MOI of 0.01(Figure 3C).
Figure 2. HSV-1 results in a loss of NPCs residing in the ependymal and subventricular cell layers of the lateral ventricle.
Nestin-GFP transgenic mice were ocularly inoculated with 105 PFU HSV-1 or left uninfected as control (UI) and at day 8pi, their brains removed, dissected and processed. A) Representative flow cytometry histograms of GFP-expressing cells (GFP+) corresponding to NPCs in the ependyma and SVZ of GFP-nestin transgenic brains. An overlay of representative histograms of total GFP+ cells corresponding to HSV-1 8d (red), UI (black), and WT GFP-negative- control groups (blue). B) Flow cytometry of the total number of GFP+ cells in the ependymal and SVZ regions of infected mice in comparison to uninfected mice. The results are expressed as GFP+cells/ependyma ± SEM and represent 3 independent experiments (8 total replicates for infected group and 5 total replicates for UI group, *p<0.05 comparing the UI to HSV-1infected group by unpaired T-test).
Figure 3. A) Detection of HSV-1 infection in NPCs.
NPC cultures were labeled with antibody against HSV-1 (red) 24 h pi with 0.2 MOI (d-f) or 2.0 MOI (g-i) HSV-1. Uninfected (a-c) (UI) NPC cultures served as controls. The merge represents the signal of HSV-1, GFP (green; NPCs), and DAPI (blue; nuclei). B) Immunodetection of HSV-1 receptor in uninfected NPC culture using an antibody against HVEM receptor (a: red). The merge (b) represents the signal of HVEM and GFP. NPCs constitutively express the HSV-1 entry receptor HVEM. C) Viral titers of HSV-1 infected NPC cultures. At the indicated time point pi, aliquots of supernatants from NPC cultures infected with 10−4 HSV-1 MOI, 10−2 HSV-1 MOI, or 10−1 MOI HSV-1 were collected and assayed for viral titer. The viral titers are expressed as log PFU ± SEM (n=3 at each time point). Each panel represents two independent experiments with n=3 for each time point.
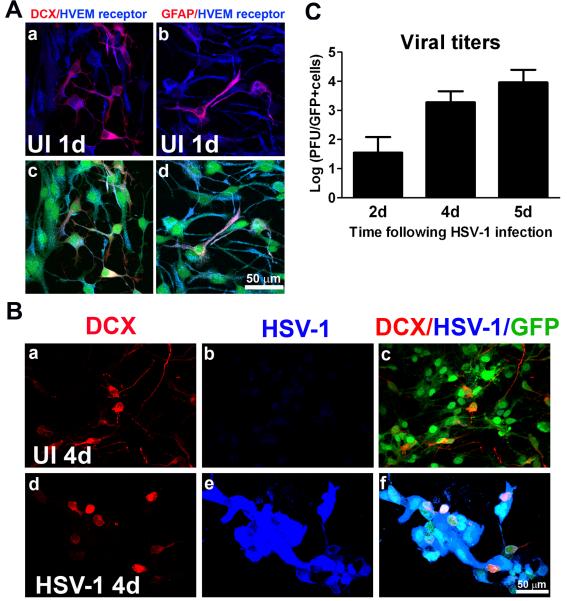
NPCs undergo differentiation toward neural and astroglial phenotypes when cultured on a matrigel matrix without EGF (Carbajal et al., 2010). Within 24 h upon culturing cells under these conditions, NPCs began to undergo differentiation with neuroblast (DCX) and astrocytic (GFAP) phenotypic markers detectable (Figure 4A). These cells constitutively expressed HVEM receptor when subjected to a differentiation environment (Figure 4A). We noted that unlike for NPCs in growth media (Figure 3B), the staining pattern for HVEM receptor was diffuse in the cells plated on matrigel matrix (Figure 4A). Four days pi, GFP+ cells including neuroblasts stained positive for HSV-1 antigen suggesting that the infection was propagated from the undifferentiated NPCs to cells committed to the neuronal lineage (Figure 4B). It is presumed astrocytic and oligodendrocytic lineages were also infected since previous results have indicated neurons, astrocytes and oligodendrocytes can be infected with HSV-1 (Markovitz et al., 1997). In these cultures, viral titers reached their maximum level at day 5 pi corresponding with cell death (Figure 4C). Therefore, the first 4-day window was used to further assess the effects of HSV-1 on NPC differentiation.
Figure 4. Infection of NPCs in culture conditions that induce cell differentiation into neuronal and glial lineages.
NPCs were seeded on Matrigel matrix covered plates in EGF-free media and 3 h later, the cultures were infected with HSV-1 (10−4 MOI) or left uninfected (UI). After 1, 2, 4, or 5d pi, cells were fixed and processed. A) Immunostaining of GFP- expressing cells (GFP+ cells; green) with antibodies against HVEM receptor (blue), the neuronal marker DCX (a and c; red), or with the astrocytic marker GFAP (b and d; red) showed expression of HVEM entry receptor in differentiated and undifferentiated NPCs. B) Detection of HSV-1 infection in GFP+ cells with antibody against HSV-1 (blue) in neuronal (d-f: red) committed cells. Four days pi, DCX- positive and DCX-negative cells expressed HSV-1 antigen. C) HSV-1 infected GFP+ cell cultures were harvested at the indicated time point and assayed for viral content by plaque assay. The viral titers are expressed as Log PFU±SEM (n=3 at each time point).
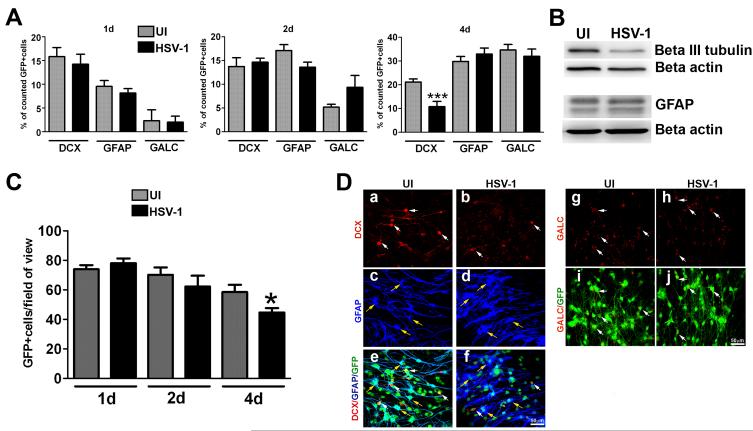
NPCs induced to undergo differentiation in culture and infected with HSV-1 revealed no significant changes in the percentages of neuroblasts, astrocytes, or oligodendrocytes or in the absolute number of cells per field of view detected at early time points pi (Figure 5A). However, by day 4 pi there was a selective, significant reduction in the percentage of neuroblasts in the infected cultures compared to the uninfected counterparts without changes in the percentage of astrocytes or oligodendrocytes (Figure 5A). Cell lysates obtained from NPC cultures at day 4 pi showed a loss in the neuronal-associated protein, beta III tubulin, but not the astrocytic-associated protein, GFAP (Figure 5B). These results were consistent with the reduction in the absolute number of GFP+ cells per field of view (Figure 5C). Representative confocal images in Figure 5D illustrate the selective loss of neuroblasts at day 4 pi showing the reduction of DCX+ cells comparing HSV-1 infected to uninfected cultures (panel a vs b) while no changes were observed in astrocyte (panel c vs d),and oligodendrocyte (panel g vs h) populations. Detection of GFAP- and DCX- expressing cells with GFP+ NSC demonstrated the loss of GFP+ cells in the HSV-1-infected cultures (panels e vs f and i vs j).
Figure 5. Differentiation patterns of NPCs following infection with HSV-1.
NPCs were seeded on Matrigel matrix covered plates in EGF free media and 3 h later, the cultures were infected with HSV-1 (MOI = 10−4) or left uninfected (UI). At the indicated time pi (day 1-3), cultures were fixed and immunostained for neuron (DCX), astrocyte (GFAP) and oligodendrocyte (GALC) markers (A, C, D). The bar graph in A shows the percentage of positive cells for each marker ±SEM comparing infected to UI cultures (***p<0.005 by paired T test comparison between UI and HSV-1 infected for DCX). B. Representative blots from protein lysates probed with antibodies against β III tubulin (neuronal marker) and GFAP 4 days pi. The graph in C shows the averaged absolute number of cells per field of view ± SEM comparing HSV-1 and UI cultures at each time point. To quantify cell numbers, 5-7 fields of view were counted within a sample for each cell marker in 3 independent experiments (at least 6 total replicates per measurement), *p<0.05 comparing the UI to HSV-1infected group at day 4 pi by paired T test comparison. D. Representative confocal images show a selective decrease in the percentage (and number, data not shown) of DCX-positive cells 4 days pi. White arrows indicate DCX+ or GALC+ cells and yellow arrows indicate GFAP+cells.
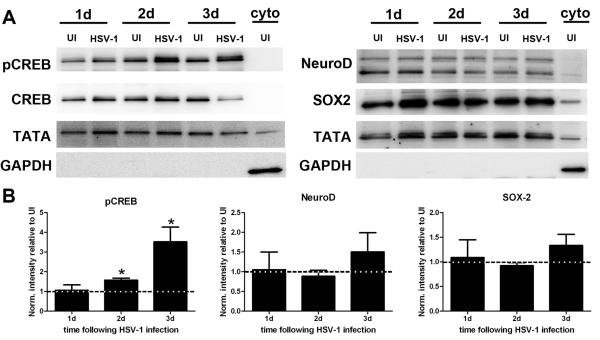
Since there was a selective loss of neuroblasts in differentiated NPC cultures following HSV-1 infection, the expression of transcription factors associated with neurogenesis including NeuroD, necessary for neuronal maturation (Gao et al., 2009), SOX2, required for proliferation of NPCs (Ferri et al., 2004), and CREB, necessary for neuronal survival signaling (Giachino et al., 2005; Jagasia et al., 2009) were evaluated. Time points ranging from 1 to 3 days would be appropriate to study such transcriptional changes since changes in expression would be expected to occur earlier than the loss of neuroblasts observed at 4 days pi in culture (Figure 5A). By western blot analysis of nuclear fractions, the data show a significant increase in the phosphorylation of CREB at day 2 and 3 pi in the infected cultures compared to their uninfected counterparts (Figure 6A and 6B). This finding suggested the specific decrease in the neuronal phenotype following HSV-1 infection was the consequence of the virus affecting neuronal survival rather than the differentiation/maturation process as neither the expression of NeuroD or SOX2 showed any noticeable change between groups at any time point studied (Figure 6A and 6B). TUNEL and PI staining was conducted to assess cell death in the NPC cultures at time points following HSV-1 infection (Supplemental Figure 1). Occasional TUNEL+ DCX+ cells that were not GFAP+ were observed at 3 days pi (Supplemental Figure A, white arrows), suggestive of necrotic/apoptotic events affecting the neuronal population. An increase in PI+ cells was observed following 2 days pi (Supplemental Figure B, white arrows) consistent with HSV-1- triggered cell death and a decrease of total GFP+ cells present in the culture at day 4 pi (Figure 5C).
Figure 6. Expression of neuronal-associated transcription factors in NPC cultures following HSV-1 infection.
A. Representative blots from nuclear protein lysates probed with antibodies against phospho-CREB (pCREB) and total CREB (neuronal survival), NeuroD (maturation of neuroblasts), and SOX2 (NPC/progenitor cell proliferation) 1 (1d), 2 (2d), and 3 (3d) pi. Antibodies against TATA binding protein and GAPDH were used as nuclear and cytoplasm loading controls respectively. B. Densitometry analysis of pCREB, NeuroD, and SOX2 at each time point pi. All band intensity was normalized to TATA and the signal for pCREB was also normalized to the intensity of CREB. The results are expressed as intensity of infected to UI ± SEM (n=at least 3 total replicates from 2 independent experiments for each group *p<0.05 comparing infected to uninfected samples by paired T test comparison).
Microglia prevent the loss of neuroblasts following HSV-1 infection
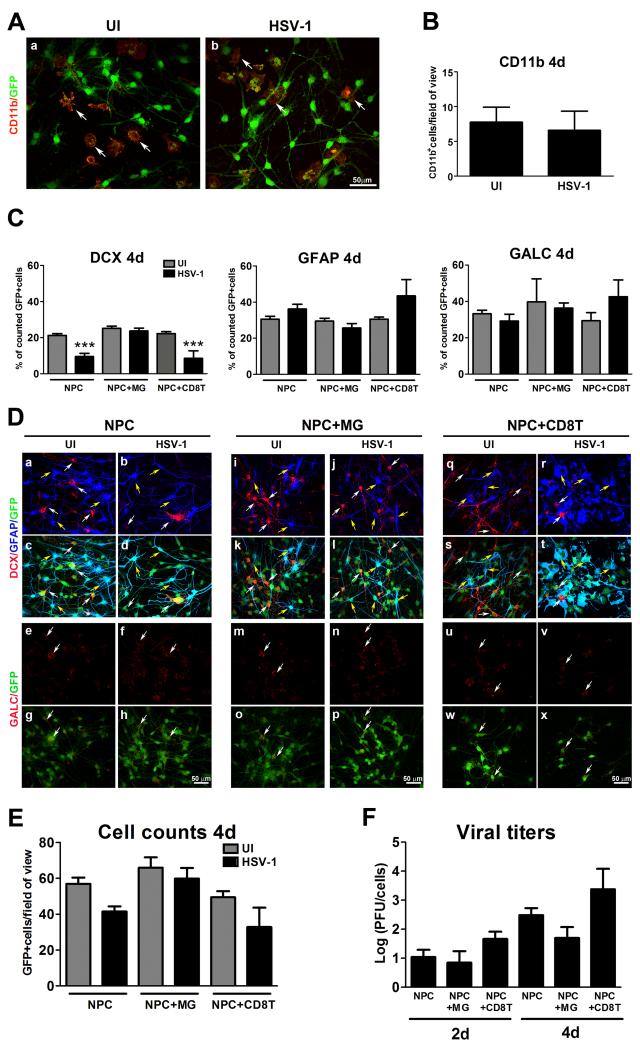
MG are densely populated and proliferative cells found throughout the parenchyma of the brain and in neurogenic niches that interact with NPCs (Mosher et al., 2012). As such, MG were added to NPC cultures and resultant cultures were evaluated for resistance to infection and NPC differentiation. The number of MG added on top of a layer of NPCs in culture did not differ comparing uninfected to infected co-cultures at day 4 pi (Figure 7A, B). Of interest, the addition of MG to the NPC cultures prevented the specific loss of neuroblasts (DCX+ cells) and the reduction in the absolute number of GFP+ cells at day 4 pi (Figure 7C-E). By comparison, there was no noticeable change in the glial phenotypes (astrocytes and oligodendrocytes) comparing cultures with or without MG infected with or without HSV-1 (Figure 7C, 7D). Conversely, the addition of CD8+T cells, known to traffic to the encephalitic brain (Sciammas et al., 1997) did not rescue the loss of neuroblasts as a result of HSV-1 (Figure 7C-E). To investigate whether MG prevented the loss of neuroblasts by reducing viral replication, infectious virus content was measured at 2 and 4 days pi. The results showed that in comparison to NPC-infected cultures alone, the addition of MG did not significantly suppress HSV-1 replication (Figure 7F).
Figure 7. Effect of microglia on NPC differentiation patterns following HSV-1 infection.
NPCs were seeded on Matrigel matrix covered plates in EGF free media and 3 h later, the cultures were infected with HSV-1 (10−4 MOI) for 1h or left uninfected (UI). Following the incubation period, 200,000 enriched microglial cells (NPC+MG) or an equivalent number of HSV-specific CD8+T cells (NPC+CD8T) were added. Four days pi, cultures were fixed and immunostained for microglia (CD11b) (A,B) and for neuroblast (DCX), astrocyte (GFAP) and oligodendrocyte (GALC)-specific markers (C,D). The total number of GFP+ cells were recorded (E) and the supernatant collected to assay for viral content (F). The bar graph in B shows the mean number of CD11b+ cells per field of view+ SEM. The bar graph in C shows the percentage of positive cells for each marker ±SEM compared to the GFP+ cells. The graph in E shows the mean number of cells per field of view ±SEM comparing infected (HSV-1) or uninfected (UI) cultures of NPC alone, co-cultures of NPC+MG, and NPC+CD8T at 4 days pi. D. Representative confocal images of the quantitative findings in C and E. Numerical values included in B, C and E were obtained from counting 5-7 fields of view within a sample for each cell marker in at least 4 independent experiments (6 total replicates per measurement). (***p<0.0001 compared to all uninfected experimental groups and to infected NPC+MG group by ANOVA followed by Bonferroni’s multiple comparison test). F. The viral titers are expressed as log PFU±SEM (n=3 for each experimental group). White arrows in A depict CD11b+ cells (stained in red) and in D DCX+ cells (stained in red). Yellow arrows in D depict GFAP+ cells.
IL-6 is responsible for the protection of the neuronal phenotype in HSV-1 infected NPC/MG co-cultures undergoing differentiation
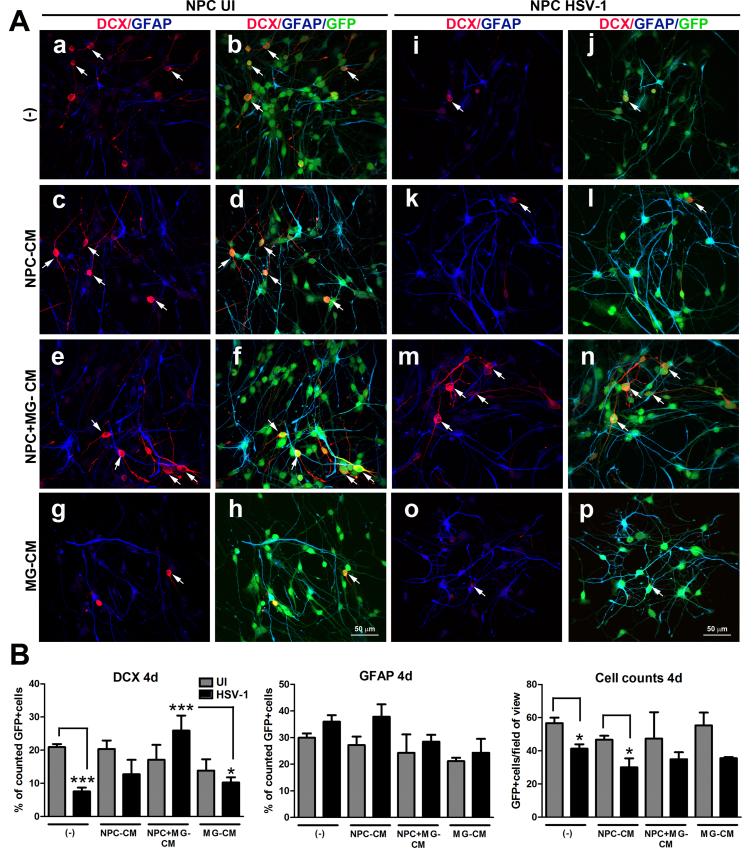
To determine whether the protective effect of MG on neuroblast preservation in differentiating NPC/MG co-cultures infected with HSV-1 is due to the secretion of a soluble factor(s), conditioned media (CM) from uninfected NPC+MG-, NPC only-, and MG only- cultures was added to HSV-1-infected NPC cultures under conditions that drive differentiation. NPC cultures treated with NPC+MG-CM showed significant preservation of the percentage of neuroblasts in comparison to infected cultures supplemented with fresh media (no CM), NPC only-CM, and MG only-CM, while no significant changes were observed in the percentage of GFAP+ cells (Figure 8A,B).
Figure 8. Conditioned media from NPC-microglia co-cultures prevents the specific loss of the DCX+ neuroblasts in NPC differentiated cultures infected with HSV-1.
NPCs were seeded on Matrigel matrix covered plates in EGF free media and 3 h later, the cultures were infected with HSV-1 (MOI=10−4) for 1h or left uninfected (UI). Next, the media was removed from each well, and cultures were supplemented with 1 ml of conditioned media from 2 day old NPC-microglia cultures (NPC+MG-CM) or an equal amount of 2 day old conditioned media from NPC only (NPC-CM) or microglia only (MG-CM) cultures. Other control cultures received fresh media (−). At 4 days pi, cultures were fixed and immunostained for neuroblast (DCX) and astrocyte (GFAP) -specific markers. (A) Representative confocal images for the quantitative findings in (B), where the bar graphs show the percentage of positive cells for each marker ±SEM and the averaged absolute number of cells per field of view ±SEM. The selective decrease in the percentage of DCX+ cells in no CM-, NPC-CM-, and MG-CM- treated cultures was blocked only in the NPC cultures treated with NPC+MG-CM. The data presented in B were obtained from counting 5-7 fields of view within a sample for each cell marker in 2 independent experiments (4 total replicates per measurement). DCX day 4 (4d) graph: a) ***p<0.0001 comparing infected NPC with no CM (−) to uninfected NPC with no CM (−), b) ***p<0.0001 comparing infected NPC treated with NPC+MG− CM group to infected NPC with no CM (−), and c) *p<0.01 comparing NPC HSV-1-treated group with MG-CM to NPC HSV-1-treated group with NPC+MG-CM; in the Cell counts day 4 graph: a) *p<0.01 comparing infected NPC with no CM (−) to uninfected NPC with no CM (−) and b)) *p<0.01 comparing infected NPC treated with NPC-CM to uninfected NPC treated with NPC-CM by ANOVA followed by Bonferroni’s multiple comparison test. White arrows in A depict DCX+ cells (stained in red).
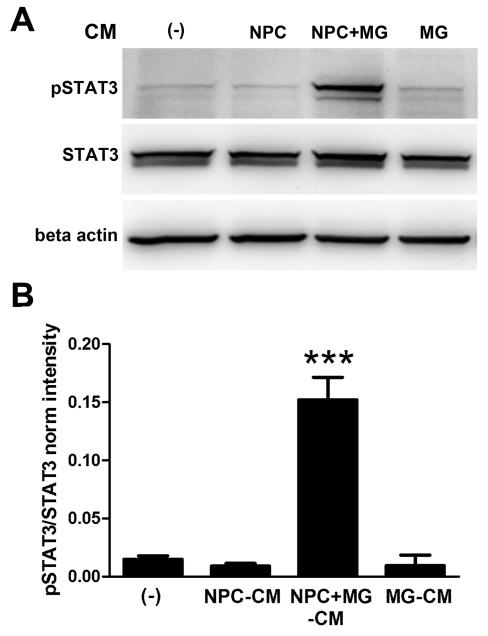
As these results suggested a soluble molecule secreted by uninfected NPC+MG co-cultures provided protection, analysis of CM for candidate molecules was next undertaken. Since microglia have been reported to express receptors and the cognate ligands for inflammatory cytokines, IL-1 (Hanisch, 2002), IL-6 and LIF (Nakanishi et al., 2007), TNF-α (Chao et al., 1992; Koka et al., 1995; Lokensgard et al., 2001), IL-10 (Lodge and Sriram, 1996), and chemokines, including CXCL-10 (Cheeran et al., 2003), the measurement of these and additional soluble factors in the CM from infected and uninfected NPC cultures in the presence or absence of MG was undertaken. Only IL-6 (Figure 9A), CXCL-10(Figure 9B), and TNF-α (Figure 9C) were detected in the CM samples from NPC+MG co-cultures compared to NPC cultures alone, independent of HSV-1 infection. Of these three candidate cytokines, IL-6 has been reported to be neuroprotective in both in vivo models of cerebral ischemia (Loddick et al., 1998) and in vitro systems where it was described to promote neuronal survival, axon outgrowth and neuronal differentiation (Hama et al., 1991). Emphasis on the possible contribution of IL-6 in the protective effect on neuroblast preservation in the NPC+MG co-cultures infected with HSV-1 was reinforced with the elevation in the phosphorylated STAT3 expression in NPC from cultures treated with CM from NSC+MG cultures but not NPC only or MG only CM (Figure 10).
Figure 9. Inflammatory cytokine/chemokine expression in NPC− and NPC+MG cultures following HSV-1 infection.
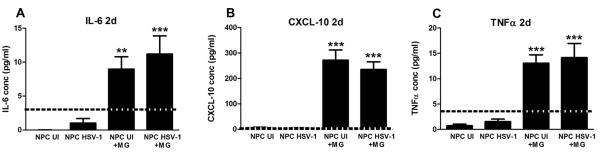
NPCs were seeded on Matrigel matrix covered plates in EGF free media and 3 h later, the cultures were infected with HSV-1 (10−4 MOI) for 1h or left uninfected (UI). Following the incubation period, 200,000 microglial cells (NPC+MG) were added on top of the NPC, and 2 days later, culture media samples were collected and assayed for cytokine/chemokine content by suspension array. Bars represent the concentration of the indicated molecule expressed in pg/ml ± SEM (n=8 total replicates from 3 independent experiments for each group). **p<0.001 and ***p<0.0001 comparing NPC UI or NPC HSV-1 groups to the NPC+ MG infected or UI groups as determined by ANOVA followed by Bonferroni’s multiple comparison test. The dotted line in each graph represents the lower limit within the linear range of the standard provided by the kit.
Figure 10. Activation of STAT3 in NPC cultures treated with conditioned media from NPC+MG co-cultures.
NPCs were seeded on Matrigel matrix covered plates in EGF free media and 3 h later, the media was removed from each well and cultures supplemented with 2 ml of conditioned media from 2 day old NPC-microglia cultures (NPC+MG-CM) or equal amounts of 2 day old conditioned media from NPC only (NPC-CM), or microglia only (MG-CM) cultures. Other control cultures received fresh media (−) for the same period of time. After a 12 h incubation time, cells were detached from the wells and processed for western blot analysis. (A) Representative blots from protein extracts in which only NPC cultures that received NPC+MG-CM had significant phosphorylation of STAT3 (pSTAT3). (B) Summary densitometric analysis of STAT3 activation. STAT3 was used to measure total STAT3 and β-actin as a loading control. Internal normalizations for each band were done to β-actin and the results are expressed as ratio pSTAT3/STAT3 normalized intensity ± SEM (n=3 total replicates from 2 independent experiments for each group ***p<0.0001 comparing the NPC+MG-CM group to all other groups as determined by ANOVA followed by Bonferroni’s multiple comparison test).
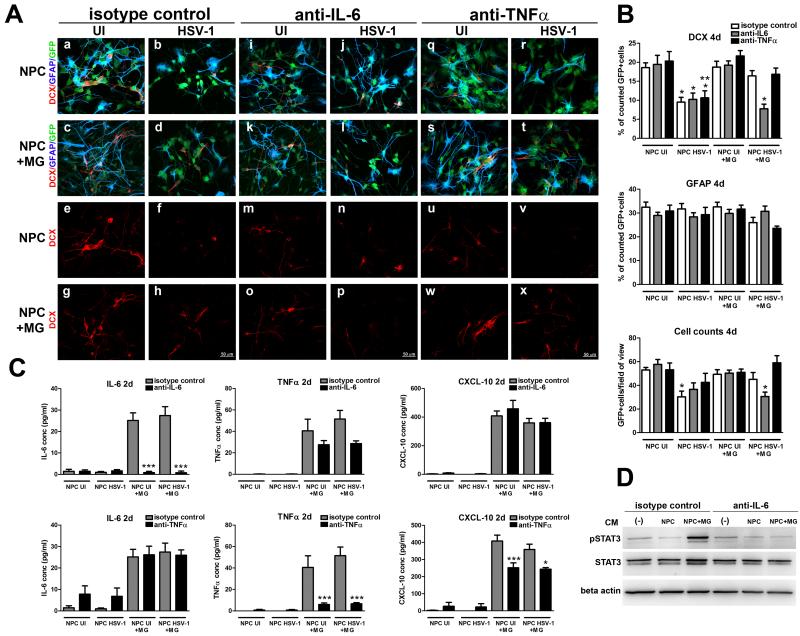
To determine whether IL-6 was the key component of the MG-induced neuroprotection, neutralizing antibody specific to IL-6 was added to infected or uninfected NPC or NPC+ MG cultures in comparison to cultures that received anti-TNF-α or isotypic-control antibody. The percentage of neuroblasts, astrocytes, and total number of GFP+ cells per field of view were assessed at day 4 pi when the significant loss of neuroblasts caused by HSV-1 infection occurred. By confocal image analysis, the addition of anti-IL-6 antibody completely abrogated the preservation of neuroblast phenotype in the infected NPC+MG co-cultures (Figure 11A, 11B). However, there was no added effect when the antibody was included in HSV-1-infected NPC cultures alone suggesting a 50% loss of neuroblasts may be the maximum level that can be achieved under these conditions. By comparison, the addition of anti-TNF-α or rat IgG isotypic-control antibody did not alter the preservation of neuroblasts in the HSV-1-infected NPC+MG co-cultures compared to the infected NPC cultures alone (Figure 11A, 11B). The neutralizing or isotypic control antibodies did not affect NPC differentiation in the absence of HSV-1 infection. Furthermore, the anti-IL-6 antibody completely blocked the expression of IL-6 measured in the infected and uninfected NPC+MG co-cultures without altering the expression of TNF-α or CXCL-10 levels (Figure 11C). The use of anti-TNF-α antibody blocked the expression of TNF-α without altering the expression of IL-6. Unlike anti-IL-6, anti-TNF-α caused a 30% reduction of CXCL-10 in the CM from NSC+MG co-cultures. Consistent with specific neutralization of IL-6, the increased expression of STAT3 phosphorylation of NSC-cultures treated with NPC+MG-CM was lost when the CM from the NPC+MG co-cultures was pre-treated with anti-IL-6 antibody (Figure 11D).
Figure 11. Neutralization of IL-6 blocks microglia-induced preservation of neuroblasts.
A. NPCs were seeded on Matrigel matrix covered plates in EGF free media and 3 h later, the cultures were infected with HSV-1 (MOI=10−4) for 1h or left uninfected (UI). Following the incubation period, 200,000 microglial cells (NPC+MG) were added along with 6 μg/ml neutralizing antibody against IL-6, TNFα, or the isotype control (rat IgG1). At day 4 pi, infected and uninfected NPC and NPC+MG cultures treated with the indicated neutralizing antibody or isotypic IgG1 control were fixed and immunostained for neuroblast (DCX)- and astrocyte (GFAP) -specific markers. Representative images for each treatment regimen are shown. B. The summary of the findings shown in A is displayed here in which the bar graphs show the percentage of positive cells for DCX and GFAP markers ±SEM, and the averaged absolute number of cells per field of view ±SEM. These results were obtained from counting 5-7 fields of view within a sample for each cell marker in 2 independent experiments (4 total replicates per measurement). C. CM samples were collected in A at 2 days pi and analyzed for the concentration of IL-6, TNFα, and CXCL10, expressed as pg/ml ± SEM (n= at least 4 total replicates from 2 independent experiments for each group). D. CM samples were also evaluated for induction of STAT3 phosphorylation of NPC or NPC+ MG co-cultures by western blot analysis (representative blot images from n=2 from 1 experiment). *p< 0.01, **p< 0.001 and ***p< 0.0001 assessed by ANOVA followed by Bonferroni’s multiple comparison test. In B, *under NPC HSV-1+ MG group, comparing anti-IL-6- to isotype control- and to anti-TNFα-treatments; * under NPC HSV-1 group, comparing to equal treatment under the NPC UI group;**under NPC HSV-1 group, comparing to the same treatment under NPC UI+ MG group. In C, comparisons are depicted between anti-IL-6- and isotype control- treatments within NPC UI+MG and NPC HSV-1+MG groups.
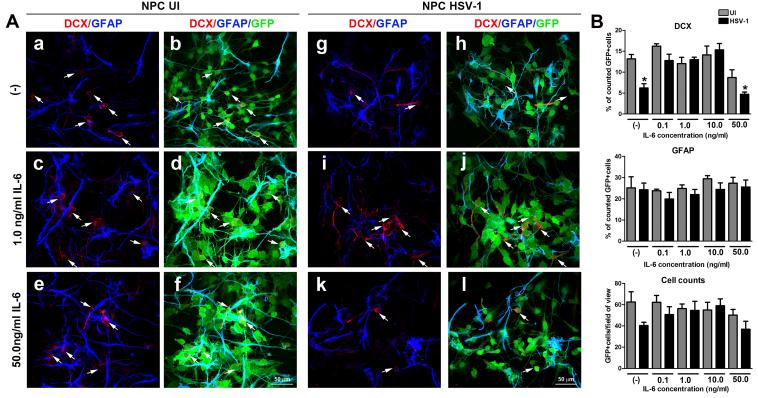
To reinforce the role of IL-6 in the preservation of neuroblasts following differentiation of NPC in HSV-1 infected cultures, the addition of recombinant IL-6 was found to block the loss of neuroblasts in the HSV-1-infected NPC cultures undergoing differentiation without any significant effect of the generation of astrocytes (Figure 12). Of interest, from the range of concentrations tested, the protective effect of IL-6 was found from 0.1 to 10.0 ng/ml. The highest concentration used, 50.0 ng/ml induced a drop in the percentage of neuroblasts following 4d of differentiation. Collectively, the data demonstrate the IL-6/STAT3 axis is a critical pathway in the preservation of the neuronal phenotype following HSV-1 infection under these experimental conditions.
Figure 12. Supplementation with recombinant IL-6 prevents the specific loss of the DCX+ neuroblasts in NPC differentiated cultures infected with HSV-1.
NPCs were seeded on Matrigel matrix covered plates in EGF free media and 3 h later, the cultures were infected with HSV-1 (MOI=10−4) for 1h or left uninfected (UI). Following 2 days of infection, the media was removed from each well and cultures were supplemented with 1 ml of fresh media supplemented with different concentrations of recombinant IL-6 (0.1-50.0 ng/ml final concentration). Other control cultures received fresh media alone (−). At 4 days pi, cultures were fixed and immunostained for neuroblast (DCX) and astrocyte (GFAP) -specific markers. (A) Representative confocal images for the quantitative findings in (B), where the bar graphs show the percentage of positive cells for each marker ±SEM, and the average number of cells per field of view ±SEM. The data presented in B were obtained from counting 5-7 fields of view within a sample for each cell marker in 2 independent experiments (at least 4 total replicates per measurement). In the DCX graph (4d) *p<0.01 comparing NPC HSV1-treated group (no IL-6) to the rest of the groups except those treated with 50.0ng/ml IL-6 and comparing NPC HSV1-treated group (with 50ng/ml IL-6) to the rest of the groups except NPC HSV1-treated group (no IL-6) by ANOVA followed by Bonferroni’s multiple comparison test. White arrows in A depict DCX+ cells (stained in red).
Elevated levels of IL-6 and STAT3 activation are found in the encephalitic brain
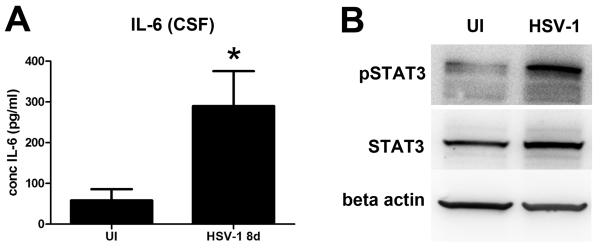
To determine if the IL-6/STAT3 pathway plays a role in the encephalitic brain following HSV-1 infection, nestin-GFP mice were infected with 105 PFU HSV-1/cornea or left uninfected. At 8 days pi when infected mice display acute encephalitis and experience a loss of progenitor cells in the SVZ/ependymal area (Figure 2), CSF was collected from the cisterna magna for analysis of IL-6 concentration. IL-6 levels were significantly higher in the CSF of infected mice (Figure 13 A). Consistently, western blot analysis of STAT3 phosphorylation in protein extract from SVZ/ependymal tissue showed increased activation of STAT3 in the infected condition (Figure 13 B). The observed changes in IL-6 and pSTAT3 might signify the same pathway we identified in vitro is relevant in vivo during acute HSE.
Figure 13. Elevated levels of IL-6 and STAT3 activation are found in the encephalitic brain.
Nestin-GFP transgenic mice were ocularly inoculated with 105 PFU HSV-1 or left uninfected as control (UI) and at day 8pi, extracted CSF and homogenized ependymal and SVZ samples collected. A) CSF samples were assayed for IL-6 content by suspension array. Bars represent IL-6 concentration expressed in pg/ml ± SEM (*p<0.05 comparing the UI to HSV-1infected group by unpaired T-test; n=at least 5 total replicates from 2 independent experiments for each group). B) Representative blots showing increased pSTAT3 in ependyma/ SVZ protein extracts from infected compared to the uninfected group (n=at least 3 for each group).
Discussion
We recently reported that ependymal cells, lining the lateral ventricles in the brain and in close proximity to the SVZ, are susceptible to HSV-1 infection in a mouse model of encephalitis (Conrady et al., 2013). Based on this observation, we hypothesized NPCs may be vulnerable to HSV-1 either by direct cytopathic effect or alternatively, by altering the population of differentiated progeny cells. Consistent with this hypothesis, a recent study reported intranasal infection with HSV-1 led to an initial rise then subsequent decrease in transplanted nestin+ NSCs in the brain of mice (Rotschafer et al., 2013). In fact, we found ocular infection with HSV-1 led to a loss of NPCs within the SVZ by day 8 when nestin-GFP transgenic mice show signs of encephalitis. Since the loss could be due to a direct lytic effect of the virus on the NPC or suppress GFP expression due to the differentiation of the nestin-GFP+ NPCs, we further investigated the relationship of HSV-1 and NPCs using an in vitro culture system. We found HSV-1 infection of NPCs resulted in a specific decrease in the percentage of neuroblasts derived from NPCs cultured under conditions that induced cell differentiation with no noticeable change in the percentages of astrocytes or oligodendrocytes. In addition, a decrease in the total number of cells was observed in the infected cultures. The loss could be due to changes in the programming of infected NPCs reflected by aberrant expression of transcription factors that facilitate neuroblast formation. Western blot analysis revealed that at days 2 and 3 pi, HSV-1 induced phosphorylation of CREB. However, the neuronal and glial differentiation patterns and the expression levels of the transcription factors NeuroD and SOX2 were not different between infected and uninfected groups. Therefore, we interpret these findings to support the idea that HSV-1 affected the survival of neuroblasts rather than the differentiation of NPCs in the culture system.
A missing “ingredient” of our NPC cultures found profusely throughout the brain constantly surveying tissue is the MG (Ekdahl et al., 2009; Kreutzberg, 1996). MG have been postulated as candidates for modulating neurogenesis in both the healthy and injured brain in part, due to their proximity to the neurogenic niche. Furthermore, pro-inflammatory cytokines like IL-1, IL-6 and TNF released by activated MG were suggested to be mediators between MG and the newly formed neurons (Iosif et al., 2006; Monje et al., 2003). In vitro models suggested MG and MG-CM could direct NPC migration and facilitate the differentiation of NPC toward a neuronal phenotype (Aarum et al., 2003; Walton et al., 2006). In contrast, the analysis of in vivo models using HSV-1 have led some to suggest MG activation along with leukocyte infiltration contribute to the neuropathology of HSE (Marques et al., 2008) including a detrimental outcome to NPC maintenance (Rotschafer et al., 2013). Our data support a beneficial role of MG in maintaining the neuronal phenotype of NPC cultures challenged with HSV-1. The protective effect was not associated with a robust reduction in the viral load of MG/NPC and neither seemed to implicate increased cell proliferation to replenish neuronal precursors. In fact, in comparison to uninfected NPC cultures alone, NPC cultures allowed to differentiate for 3 days in the presence of MG exhibited no significant differences in the percentage of cells positive for the mitotic marker phospho-histone H3 (data not shown). Furthermore, in the conditioned media from NPC-MG co-cultures we were unable to detect FGF-2, an established neurogenic factor for cell proliferation and differentiation of NPCs (Tao et al., 1997). Our findings implicate IL-6 as the soluble factor elicited in the NPC-MG co-cultures that protects neuroblasts following HSV-1 infection of NPC/MG co-cultures. Of interest, infection of the co-cultures was not a prerequisite for IL-6 secretion. Such observations might be due to an activated state of microglia upon isolation from their natural environment. Other cytokines surveyed including LIF, a member of the IL-6 family of cytokines, was not detected (data not shown). Presently, the source of IL-6 in the MG/NPC co-cultures is unknown since MG and astrocytes, both of which would be present in the MG/NPC co-cultures, are potential sources (Hanisch and Kettenmann, 2007; Norris and Benveniste, 1993).
IL-6 is a prominent pro-inflammatory molecule generated in response to HSV-1 infection in the CNS (Lewandowski et al., 1994). It is closely aligned with resistance to primary HSV-1 infection (Carr and Campbell, 1999), accelerates nerve regeneration following trauma (Hirota et al., 1996), is neuroprotective following cerebral ischemia (Loddick et al., 1998) or in spinal cord injury (Yang et al., 2012), and has been found to promote neuronal survival, axon outgrowth and neuronal differentiation in in vitro model systems (Hama et al., 1991). In the present study, neutralization of IL-6 but not TNF-α resulted in a loss in the neuroblast/neuronal cells undergoing differentiation in the HSV-1-infected MG/NPC co-cultures and such an outcome was correlative with a loss in pSTAT3 levels. A STAT3-dependent, FGF-2 maintenance of NPC in the mouse neocortex has been reported (Yoshimatsu et al., 2006) consistent with the observation that STAT3 facilitates the terminal differentiation of olfactory bulb neurons from NSC (Yu et al., 2009). Relative to HSV-1 infection in the CNS, the administration of FGF-2 by the intraventricular route was found to stimulate DCX+ cell proliferation and possibly preserve neurogenesis (Rotschafer et al., 2013). FGF-2 is thought to drive STAT3 phosphorylation through Erk1/2 activation (Dong et al., 2012) and therefore, FGF-2 and IL-6 may share a common signaling molecule within the NPC or neuroblast population. Our in vivo data demonstrated an increase activation of STAT3 in the ependyma/SVZ and an elevation of IL-6 in the CSF of HSV-1-infected mice day 8 pi. Taking into account our finding that at 8 days pi there is a significant reduction in the number of NPCs in the ependymal/SVZ, it is tempting to speculate that an increase in IL-6/STAT3 signaling at this time point acts on infiltrating cells absent in our cultures that are detrimental to NPC survival. However, IL-6 may also be necessary to preserve and maintain neuroblasts and NPCs that do not succumb in the inflammatory environment. Further studies are critical to reconcile the beneficial effect of IL-6 found in our in vitro settings with the in vivo role of IL-6 in HSE.
Since MG are present within the neurogenic niches of the brain and are protective for NPC and neuroblasts in the in vitro culture system during acute HSV-1 infection, why do we and others find a loss in the NPC residing in the ependyma and SVZ following HSV-1 infection in vivo? Firstly, in the in vitro MG/NPC co-culture system, the window to evaluate differentiation is confined to the first four days pi. By day 5 pi, the virus has overwhelmed the system with massive cell death in all populations of differentiated cells and non-differentiated NPCs even at the low (0.0001) MOI. In addition, the in vitro system employs 200,000 MG and 30,000 NPCs/well. In vivo, we routinely recover approximately 30,000 NPC and 50,000-100,000 MG from the SVZ/ependymal region/mouse. Taken together, we are exceeding the ratio of MG to NPCs by roughly 2-4 fold. We recognize there are other features in the mouse brain that are not found in the in vitro MG/NPC co-culture including the organized tissue structure, the availability of astrocytes as a rich source of IL-6 (and other soluble factors), and fully differentiated neurons that along with astrocytes produce soluble factors and act as a target for the virus. Additional factors and cell types may also be instrumental in the differentiation of NPC during acute HSV-1 infection in the CNS but such systems have not been clearly identified.
While we have identified IL-6 as a critical cytokine that preserves the neuronal differentiation cascade of NPCs, the dynamics of IL-6 expression relative to HSV-1 and NPCs over the course of acute HSV-1 infection within the SVZ has not been elucidated particularly as it pertains to the replacement of neurons lost during HSE. Moreover, whether intervention to maximize neuronal recovery is adequate in addressing the cognitive deficits often observed in HSE patients is another important question that remains unanswered.
Supplementary Material
Supplemental Figure 1. TUNEL and PI staining of NPC cultures.
NPCs were seeded on Matrigel matrix covered plates in EGF free media and 3 h later, the cultures were infected with HSV-1 (MOI=10−4) for 1h or left uninfected (UI). A) Following 3 days of infection, cells were fixed and processed for TUNEL staining (red staining) in combination with staining with GFAP (green; pointed by white arrows) and DCX (green; pointed by white arrows) specific antibodies. Nuclei were counterstained with DAPI (blue) to assess nuclear location of TUNEL positive staining. Most nuclei detected TUNEL positive did not co-localize with GFAP positive cells and co-localized with DCX positive cells. B) Following 2 days of infection, PI staining of the cell cultures (red). The bar graph in B shows the percentage of positive cells for PI staining ±SEM comparing infected to uninfected cultures (n=2).
Main points.
HSV-1 infection of NPCs reduces the survival of NPC-derived neuroblasts.
Microglia prevents the neuronal loss upon infection of NPCs.
We identified IL-6 as a key factor for the protective effect of microglia.
Acknowledgements
The authors would like to thank Derek Royer and Blake Hopiavuori for technical assistance. We would like to thank Dr. Grigori Enikolopov (Cold Spring Harbor Laboratory, Cold Spring Harbor, New York) for his critical comments in reading the manuscript. This work was supported by grants from the Oklahoma Center for Adult Stem Cell Research through the Oklahoma Tobacco Settlement Endowment Trust, National Institutes of Health (NIH) R01 grant AI053108, and an OUHSC Presbyterian Health Foundation Presidential Professorship award (to DJJC) and NIH R01 NS074987 (TEL). Additional support includes P30 EY021725.
Bibliography
- Aarum J, Sandberg K, Haeberlein SL, Persson MA. Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci U S A. 2003;100:15983–15988. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Austin BA, Halford W, Silverman RH, Williams BR, Carr DJ. OAS and PKR are not required for the antiviral effect of Ad:IFN-gamma against acute HSV-1 in primary trigeminal ganglia cultures. J Interferon Cytokine Res. 2006;26:220–225. doi: 10.1089/jir.2006.26.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Hery M, Printemps R, Daszuta A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004;29:450–460. doi: 10.1038/sj.npp.1300320. [DOI] [PubMed] [Google Scholar]
- Carbajal KS, Schaumburg C, Strieter R, Kane J, Lane TE. Migration of engrafted neural stem cells is mediated by CXCL12 signaling through CXCR4 in a viral model of multiple sclerosis. Proc Natl Acad Sci U S A. 2010;107:11068–11073. doi: 10.1073/pnas.1006375107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DJ, Campbell IL. Transgenic expression of interleukin-6 in the central nervous system confers protection against acute herpes simplex virus type-1 infection. J Neurovirol. 1999;5:449–457. doi: 10.3109/13550289909045373. [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu S, Close K, Choi CS, Molitor TW, Novick WJ, Peterson PK. Cytokine release from microglia: differential inhibition by pentoxifylline and dexamethasone. J Infect Dis. 1992;166:847–853. doi: 10.1093/infdis/166.4.847. [DOI] [PubMed] [Google Scholar]
- Cheeran MC, Hu S, Sheng WS, Peterson PK, Lokensgard JR. CXCL10 production from cytomegalovirus-stimulated microglia is regulated by both human and viral interleukin-10. J Virol. 2003;77:4502–4515. doi: 10.1128/JVI.77.8.4502-4515.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrady CD, Zheng M, Fitzgerald KA, Liu C, Carr DJ. Resistance to HSV-1 infection in the epithelium resides with the novel innate sensor, IFI-16. Mucosal Immunol. 2012;5:173–183. doi: 10.1038/mi.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrady CD, Zheng M, van RN, Drevets DA, Royer D, Alleman A, Carr DJ. Microglia and a functional type I IFN pathway are required to counter HSV-1-driven brain lateral ventricle enlargement and encephalitis. J Immunol. 2013;190:2807–2817. doi: 10.4049/jimmunol.1203265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Li Y, Cao J, Liu F, Pier E, Chen J, Xu Z, Chen C, Wang RA, Cui R. FGF2 regulates melanocytes viability through the STAT3-transactivated PAX3 transcription. Cell Death Differ. 2012;19:616–622. doi: 10.1038/cdd.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Enikolopov G. Identifying and quantitating neural stem and progenitor cells in the adult brain. Methods Cell Biol. 2008;85:243–272. doi: 10.1016/S0091-679X(08)85011-X. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ferri AL, Cavallaro M, Braida D, Di CA, Canta A, Vezzani A, Ottolenghi S, Pandolfi PP, Sala M, DeBiasi S, Nicolis SK. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004;131:3805–3819. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, Goebbels S, Eisch AJ, Hsieh J. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci. 2009;12:1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachino C, De MS, Giampietro C, Parlato R, Perroteau I, Schutz G, Fasolo A, Peretto P. cAMP response element-binding protein regulates differentiation and survival of newborn neurons in the olfactory bulb. J Neurosci. 2005;25:10105–10118. doi: 10.1523/JNEUROSCI.3512-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama T, Kushima Y, Miyamoto M, Kubota M, Takei N, Hatanaka H. Interleukin-6 improves the survival of mesencephalic catecholaminergic and septal cholinergic neurons from postnatal, two-week-old rats in cultures. Neuroscience. 1991;40:445–452. doi: 10.1016/0306-4522(91)90132-8. [DOI] [PubMed] [Google Scholar]
- Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hirota H, Kiyama H, Kishimoto T, Taga T. Accelerated Nerve Regeneration in Mice by upregulated expression of interleukin (IL) 6 and IL-6 receptor after trauma. J Exp Med. 1996;183:2627–2634. doi: 10.1084/jem.183.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26:9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagasia R, Steib K, Englberger E, Herold S, Faus-Kessler T, Saxe M, Gage FH, Song H, Lie DC. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci. 2009;29:7966–7977. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanis I, Belhadi A, Faissner A, Ffrench-Constant C. The adult mouse subependymal zone regenerates efficiently in the absence of tenascin-C. J Neurosci. 2007;27:13991–13996. doi: 10.1523/JNEUROSCI.3279-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PG, Steiner I. Recent issues in herpes simplex encephalitis. J Neurovirol. 2013 doi: 10.1007/s13365-013-0178-6. [DOI] [PubMed] [Google Scholar]
- Koka P, He K, Zack JA, Kitchen S, Peacock W, Fried I, Tran T, Yashar SS, Merrill JE. Human immunodeficiency virus 1 envelope proteins induce interleukin 1, tumor necrosis factor alpha, and nitric oxide in glial cultures derived from fetal, neonatal, and adult human brain. J Exp Med. 1995;182:941–951. doi: 10.1084/jem.182.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp SJ, Banisadr G, Glajch K, Maurer UE, Grunewald K, Miller RJ, Osten P, Spear PG. Infection of neurons and encephalitis after intracranial inoculation of herpes simplex virus requires the entry receptor nectin-1. Proc Natl Acad Sci U S A. 2009;106:17916–17920. doi: 10.1073/pnas.0908892106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Lewandowski G, Hobbs MV, Bloom FE. Alteration of intracerebral cytokine production in mice infected with herpes simplex virus types 1 and 2. J Neuroimmunol. 1994;55:23–34. doi: 10.1016/0165-5728(94)90143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loddick SA, Turnbull AV, Rothwell NJ. Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1998;18:176–179. doi: 10.1097/00004647-199802000-00008. [DOI] [PubMed] [Google Scholar]
- Lodge PA, Sriram S. Regulation of microglial activation by TGF-beta, IL-10, and CSF-1. J Leukoc Biol. 1996;60:502–508. doi: 10.1002/jlb.60.4.502. [DOI] [PubMed] [Google Scholar]
- Lokensgard JR, Hu S, Sheng W, vanOijen M, Cox D, Cheeran MC, Peterson PK. Robust expression of TNF-alpha, IL-1beta, RANTES, and IP-10 by human microglial cells during nonproductive infection with herpes simplex virus. J Neurovirol. 2001;7:208–219. doi: 10.1080/13550280152403254. [DOI] [PubMed] [Google Scholar]
- Markovitz NS, Baunoch D, Roizman B. The range and distribution of murine central nervous system cells infected with the gamma(1)34.5-mutant of herpes simplex virus 1. J Virol. 1997;71:5560–5569. doi: 10.1128/jvi.71.7.5560-5569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques CP, Cheeran MC, Palmquist JM, Hu S, Urban SL, Lokensgard JR. Prolonged microglial cell activation and lymphocyte infiltration following experimental herpes encephalitis. J Immunol. 2008;181:6417–6426. doi: 10.4049/jimmunol.181.9.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer MH. Proteomics of brain extracellular fluid (ECF) and cerebrospinal fluid (CSF) Mass Spectrom Rev. 2010;29:17–28. doi: 10.1002/mas.20213. [DOI] [PubMed] [Google Scholar]
- Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469:311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z, Doetsch F, Sawamoto K, Wichterle H, Alvarez-Buylla A. The subventricular zone en-face: wholemount staining and ependymal flow. J Vis Exp. 2010 doi: 10.3791/1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Mosher KI, Andres RH, Fukuhara T, Bieri G, Hasegawa-Moriyama M, He Y, Guzman R, Wyss-Coray T. Neural progenitor cells regulate microglia functions and activity. Nat Neurosci. 2012;15:1485–1487. doi: 10.1038/nn.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Heath W, McLain JD, Carbone FR, Jones CM. Characterization of two TCR transgenic mouse lines specific for herpes simplex virus. Immunol Cell Biol. 2002;80:156–163. doi: 10.1046/j.1440-1711.2002.01071.x. [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B, Picard-Riera N, Kerninon C, Decker L, Seilhean D, Hoglinger GU, Hirsch EC, Reynolds R, Baron-Van EA. Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. Proc Natl Acad Sci U S A. 2007;104:4694–4699. doi: 10.1073/pnas.0606835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi M, Niidome T, Matsuda S, Akaike A, Kihara T, Sugimoto H. Microglia-derived interleukin-6 and leukaemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur J Neurosci. 2007;25:649–658. doi: 10.1111/j.1460-9568.2007.05309.x. [DOI] [PubMed] [Google Scholar]
- Norris JG, Benveniste EN. Interleukin-6 production by astrocytes: induction by the neurotransmitter norepinephrine. J Neuroimmunol. 1993;45:137–145. doi: 10.1016/0165-5728(93)90174-w. [DOI] [PubMed] [Google Scholar]
- Rotschafer JH, Hu S, Little M, Erickson M, Low WC, Cheeran MC. Modulation of neural stem/progenitor cell proliferation during experimental Herpes Simplex encephalitis is mediated by differential FGF-2 expression in the adult brain. Neurobiol Dis. 2013;58:144–155. doi: 10.1016/j.nbd.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciammas R, Kodukula P, Tang Q, Hendricks RL, Bluestone JA. T cell receptor-gamma/delta cells protect mice from herpes simplex virus type 1-induced lethal encephalitis. J Exp Med. 1997;185:1969–1975. doi: 10.1084/jem.185.11.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner I. Herpes simplex virus encephalitis: new infection or reactivation? Curr Opin Neurol. 2011;24:268–274. doi: 10.1097/WCO.0b013e328346be6f. [DOI] [PubMed] [Google Scholar]
- Tao Y, Black IB, DiCicco-Bloom E. In vivo neurogenesis is inhibited by neutralizing antibodies to basic fibroblast growth factor. J Neurobiol. 1997;33:289–296. [PubMed] [Google Scholar]
- Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, Scheffler B, Steindler DA. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–825. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- Whitley RJ, Gnann JW. Viral encephalitis: familiar infections and emerging pathogens. Lancet. 2002;359:507–513. doi: 10.1016/S0140-6736(02)07681-X. [DOI] [PubMed] [Google Scholar]
- Yang P, Wen H, Ou S, Cui J, Fan D. IL-6 promotes regeneration and functional recovery after cortical spinal tract injury by reactivating intrinsic growth program of neurons and enhancing synapse formation. Exp Neurol. 2012;236:19–27. doi: 10.1016/j.expneurol.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Yoshimatsu T, Kawaguchi D, Oishi K, Takeda K, Akira S, Masuyama N, Gotoh Y. Non-cell-autonomous action of STAT3 in maintenance of neural precursor cells in the mouse neocortex. Development. 2006;133:2553–2563. doi: 10.1242/dev.02419. [DOI] [PubMed] [Google Scholar]
- Yu TS, Zhang G, Liebl DJ, Kernie SG. Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. J Neurosci. 2008;28:12901–12912. doi: 10.1523/JNEUROSCI.4629-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Ren W, Ren B. Expression of signal transducers and activator of transcription 3 (STAT3) determines differentiation of olfactory bulb cells. Mol Cell Biochem. 2009;320:101–108. doi: 10.1007/s11010-008-9911-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. TUNEL and PI staining of NPC cultures.
NPCs were seeded on Matrigel matrix covered plates in EGF free media and 3 h later, the cultures were infected with HSV-1 (MOI=10−4) for 1h or left uninfected (UI). A) Following 3 days of infection, cells were fixed and processed for TUNEL staining (red staining) in combination with staining with GFAP (green; pointed by white arrows) and DCX (green; pointed by white arrows) specific antibodies. Nuclei were counterstained with DAPI (blue) to assess nuclear location of TUNEL positive staining. Most nuclei detected TUNEL positive did not co-localize with GFAP positive cells and co-localized with DCX positive cells. B) Following 2 days of infection, PI staining of the cell cultures (red). The bar graph in B shows the percentage of positive cells for PI staining ±SEM comparing infected to uninfected cultures (n=2).