SUMMARY
Objective
In this study, we use time-locked video and electroencephalograph (EEG) recordings to characterize acute seizures and EEG abnormalities in an animal model that replicates many salient features of human neonatal hypoxic-ischemic encephalopathy (HIE) including the brain injury pattern and long-term neurologic outcome.
Methods
Hypoxia-ischemia (HI) was induced in 7-day-old rats by ligating the right carotid artery and exposing the pups to hypoxia for 2 hours (Rice-Vannucci method). To identify seizures and abnormal EEG activity, pups were monitored by video-EEG during hypoxia and at various time points after HI. Occurrence of electroclinical seizures, purely electrographic seizures and other abnormal discharges in the EEG were quantified manually. A power spectrum analysis was done to evaluate the effects of HI on EEG spectra in the 1 to 50 Hz frequency band.
Results
During hypoxia, all pups exhibit short duration, but frequent electroclinical seizures. Almost all pups continue to have seizures in the immediate period following termination of hypoxia. In over half of the HI rats seizures persisted for 24 hours, for some of them, the seizures continued for more than 48 hours. Seizures were not observed in any rats at 72 hours after HI-induction. A significant reduction in background EEG voltage in the cortex ipsilateral to the ligated carotid artery occurred in rats subjected to HI. In addition, purely electrographic seizures, spikes, sharp waves and brief runs of epileptiform discharges (BRED) were also observed in these rats.
Significance
HI-induction in P7 rats using the Rice-Vannucci method resulted in the development of seizures and EEG abnormalities similar to that seen in human neonates with HIE. Therefore, we conclude that this is a valid model to test the efficacy of novel interventions to treat neonatal seizures.
Keywords: Epilepsy, EEG, video, electroclinical, hypoxia, ischemia
INTRODUCTION
Seizures occur more often in the neonatal period than at any other time of human life. The most common cause of neonatal seizures is hypoxic-ischemic encephalopathy (HIE),1, 2 a serious condition with a suggested incidence of 1 to 8 cases per 1000 live births.3 Hypoxia-ischemia (HI) in neonates also results in injury to various brain regions4 and survivors of such injury can experience a multitude of neurological problems such as cerebral palsy, learning deficits and epilepsy.2, 5, 6 Clinical as well as basic science research studies suggest that seizures may exacerbate HI-induced brain injury and contribute to poor neurological outcome7–13 (but also see14, 15). Current antiepileptic drugs were developed using adult animal models and are not fully effective in treating neonatal seizures. Due to developmental differences, an immature brain may respond differently to an injury and a treatment than a mature brain. Therefore, to find the most effective treatment for a neonatal disease, it is imperative to test the efficacy of novel drugs in an animal model that not only accurately replicates the etiology and symptoms of a disease, but also the age of onset of the disease.
The Rice-Vannucci model of HI-induction in the postnatal day 7 (P7) rat16 is widely used to study neonatal HIE. This model exhibits many of the salient features of human neonatal HIE such as the extent of brain injury,17–19 development of epilepsy in some, but not all animals in later life,20 and the learning and memory deficits.21 However, a complete characterization of acute seizures in this model is still lacking. In the current study, we use a synchronized video and electroencephalograph (EEG) recording technique to study characteristics of HI-induced neonatal seizures that occur during and shortly following the time of insult. For certain disease conditions such as epilepsy, continuous video-EEG recording has become the gold standard to confirm the presence of convulsive seizures and also to detect non-convulsive or subclinical seizures. Video-EEG is particularly useful in the assessment of neonates in whom a large percentage of seizures are subclinical and for whom differentiation of normal movements from ictal motor activity can be difficult if not impossible based on behavioral observation alone. Further, in neonates, there is often dissociation between clinical (behavioral) seizures and EEG phenomena (known as electroclinical uncoupling), resulting in resolution of behavioral manifestations of seizures despite on-going electrographic seizures. Because of this phenomenon, it has been observed that some drugs effectively stop behavioral seizures in neonates without stopping electrographic seizures. Therefore, the use of video-EEG is being increasingly employed in clinical settings to identify and manage neonatal seizures. However, because of technical challenges, EEG is rarely obtained in animal models of neonatal diseases. In the current study, we describe the characteristics of acute electroclinical seizures and the background EEG in the Rice-Vannucci P7 rat model of neonatal HIE. This information will be helpful in assessing how closely this model replicates the human neonatal HIE condition as well as the model’s validity for testing the efficacy of drugs used in the treatment of neonatal seizures.
METHODS
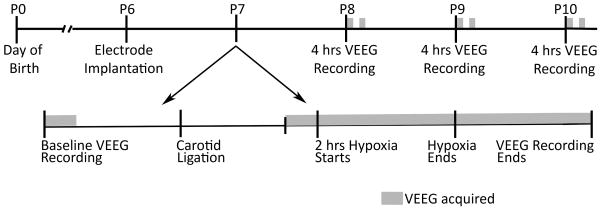
All animal procedures were performed according to the protocol approved by the Institutional Animal Care and Use Committee of the University of Colorado Anschutz Medical Campus (UC-AMC). Also, all efforts were made to reduce animal suffering and the number of animals used. Timed pregnant Sprague-Dawley rats were obtained from Charles River Laboratories (Wilmington, MA). The pregnant rats were at the 14th day of gestation (E14) on arrival at the laboratory animal facility of the UC-AMC, and delivered the pups at E22 or E23. The litter size varied from 11 to 13 pups and the weights ranged between 12.5 to 15 grams at postnatal day 6 (P6). Only male pups were used in the current study. The experiments were performed in a sequence depicted in figure 1.
Figure 1.
Schematic representation of the time course of the experimental procedures performed to characterize HI-induced seizure activities and abnormalities in the EEG. VEEG = video-EEG, Hrs = hours, P = postnatal day.
Electrode implantation
To record electrical activity of the brain, P6 rats (n = 16) were implanted bilaterally in the parietal cortex with silver electrodes (0.008″ outer diameter; A-M Systems, Carlsborg, WA). The holes for implantation were made 2.5 mm behind the bregma and 3 mm lateral from midline sutures. The length of the electrodes from the bottom of the pedestal was 3 mm. This length assured that the recording electrode was sufficiently inside the brain, but not too deep (to decrease cortical damage), to obtain good quality signal (supplemental figure 1). Two electrode placement schemes were employed, differing only in the placement of the reference electrode. In the first, a common reference electrode was placed near the lambda over the left hemisphere; in the second, the active electrode in each hemisphere was referenced to a separate electrode positioned near the lambda in the same hemisphere as itself. Except for the seizure characterization data, which were obtained from both configurations and are presented in the table 1, all data were acquired using the second configuration. The electrode assembly was fixed to the skull with tissue adhesive and dental acrylic cement. The entire implantation procedure was performed under isoflurane anesthesia (2–4% for induction, initiated at 2%; 1–1.5% for maintenance). After the surgery, the rats were treated with an analgesic (0.1 mg/kg buprenorphine) once every 12 hours for 48 hours.
Table 1.
Characteristics of seizure and EEG activity observed in neonatal rats with HIE
| No. pups ECS | No. pups EGS | No. pups BRED | Latency to ECS (minutes) | No. ECS/hour | Total duration of ECS/hour (minutes) | No. EGS/hour | Total duration of EGS/hour (minutes) | No. BRED/hour | Total duration of BRED/hour (minutes) | |
|---|---|---|---|---|---|---|---|---|---|---|
| During HI | 12/12 | 2/12 | 10/12 | 7.3 ± 13 | 10.6 ± 5 | 4.8 ± 2.4 | 1.3 ± 1 | 1.4 ± 0.5 | 11.2 ± 7.5 | 1.1 ± 0.7 |
| Immediately after HI | 11/12 | 3/12 | 11/12 | - | 6.4 ± 8.1 | 2.2 ± 8.1 | 1.3 ± 1.4 | 0.7 ± 0.7 | 9.6 ± 8.5 | 1 ± 1 |
| 24 hours after HI | 8/12 | 7/12 | 9/12 | - | 4.4 ± 4.2 | 1.6 ± 1.8 | 0.6 ± 0.4 | 0.6 ± 1.1 | 6.5 ± 4.3 | 0.6 ± 0.4 |
| 48 hours after HI | 3/12 | 0/12 | 7/12 | - | 0.8 ± 0.6 | 0.3 ± 0.2 | 0 | 0 | 5.8 ± 5.4 | 0.5 ± 0.4 |
| 72 hours after HI | 0/12 | 0/12 | 6/12 | - | 0 | 0 | 0 | 0 | 0.7 ± 0.3 | 0.06 ± 0.02 |
Data shown are mean ± standard deviation, n = 12, ECS = Electroclinical seizure, EGS = Electrographic seizure, BRED = Brief Runs of Epileptiform Discharge
Hypoxia-ischemia induction
HI was induced in P7 pups (n = 12) according to a published protocol.16,20 The pups were anesthetized with isoflurane and, after infusion of Marcaine (0.5%) at the incision site, a small longitudinal cut was made along the midline of ventral cervical skin. The right common carotid artery was then identified and double ligated with 4-0 polyglycolic acid suture. The incision was closed with 4-0 nylon Dermalon sutures or with skin glue. The entire operation lasted for 10 to 12 minutes. Following carotid ligation, the pups remained with the dam in a warm cage for 60 to 105 minutes. The pups were then separated and monitored for 30 minutes by video-EEG, following which they were exposed to hypoxia for 2 hours in an airtight chamber that was filled with 8% oxygen and 92% nitrogen gas mixture. The oxygen content of the chamber was monitored using an oxygen sensor (Dräger Pac 7000, Pittsburg, PA) and was maintained between 8 and 8.3%. The temperature and humidity of the chamber were also tightly controlled and maintained at 36.5° Celsius and 60 to 70% respectively as variations in these parameters can affect both mortality rate and extent of the HI-induced injury.
Video-EEG recording
EEG signals synchronized with digital video were recorded using the Stellate Harmonie system (Natus Medical, San Carlos, CA). The EEG data were collected with a sampling rate of 1000 Hz and stored on a hard disk for off-line analysis. P6 pups (n = 12) were implanted with electrodes and, at P7, underwent 30 minutes of baseline video-EEG recording prior to carotid ligation. Following ligation and prior to hypoxia, a second 30-minute video-EEG record was acquired. The pups were continuously monitored by video-EEG during hypoxia. The video-EEG recording was continued for 2 more hours after completion of hypoxia. To find out if the pups experienced acute spontaneous seizures following HI-induction, they were monitored by video-EEG for 4 hours a day (two 2 hour sessions separated by a 2 hour break, during which the pups were housed with the dam) 24, 48 and 72 hours after HI-induction. A separate group of rats whose carotid artery was not ligated and who were not exposed to hypoxia (control rats, n = 4) was monitored using video-EEG under the same conditions and for the same duration as HI rats.
Seizure characterization
Video-EEG records were analyzed by the first author of the current paper (DS) and all the events (electroclinical and purely electrographic seizures) identified by DS were checked for accuracy by a board certified clinical epileptologist (AW) and a researcher with experience in animal EEG analyses (YR). Electroclinical seizures were defined by an EEG pattern that differed from background in either amplitude, frequency or both, evolved over time and contained spikes or sharps lasting for 10 seconds or more and were associated with a change in the rat’s behavior. Electrographic seizures were defined as seizures observed in the EEG that were not associated with a behavioral correlate on video. Brief runs of epileptiform discharges (BRED) were defined as EEG patterns similar to an electrographic seizure with duration greater than 2 but less than 10 seconds. These might or might not have an associated change in the rat’s behavior.
Power Spectrum Analysis
The power spectra were determined using a Fast Fourier Transform (FFT) algorithm written using Visual Basic (Microsoft, Redmond, WA) subroutines. A rectangular window was used with epoch sizes of 8192 points (sampling rate = 1000 Hz) and frequency bins of 1 Hz spanning 1 to 50 Hz. To compare groups of epochs characterizing different animal states (e.g., baseline, ictal during hypoxia, interictal during hypoxia, interictal post-HI background), we identified at least 10 EEG epochs (contiguous or not) that were acquired during that state and were devoid of movement artifacts. The average of the power for the different epochs in each frequency bin was calculated for each of the two groups (animal states) to be compared. To investigate the change in the shape of the power distribution, the actual power was normalized. To statistically compare groups, the power within the frequency bins was integrated into delta (1–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–25 Hz), and gamma (25–50 Hz) bands. Comparisons were performed using methods described below.
Statistical Analysis
GraphPad Prism 5 statistical software (GraphPad Software Inc., San Diego, CA) was used for statistical analysis. Friedman test with Dunn’s test for multiple comparisons was used to compare integrated power spectral values of EEG obtained up to 72 hours after carotid ligation with the corresponding pre-ligation baseline value for each rat, and to analyze age-dependent changes in EEG power in the control rats. An unpaired t-test was used to identify statistical differences in integrated power spectral values of EEG at P10 between control and HI rats. A one-way analysis of variance (ANOVA) with Tukey’s post-hoc test was used to evaluate the effect of HI on spike or sharp wave activity. A p value of less than 0.05 was considered statistically significant.
RESULTS
Characteristics of hypoxia-ischemia induced seizures
Seizures were not observed in the control rats during any video-EEG recording sessions. In the HI group of rats, seizures were not detected during baseline EEG recording or in the period between carotid ligation and exposure to hypoxia. However, all pups quickly developed behavioral seizures with a clear electrographic correlate (electroclinical seizure) upon exposure to the hypoxic environment following carotid artery ligation (n = 12, table 1). The behavioral seizures consisted of clonic seizures, tonic posturing of the trunk, tonic-clonic seizures, facial twitching and stiffening of the tail (video 1, supplemental material). The clonic and tonic seizures could involve all extremities or originate unilaterally. Those which did originate unilaterally did frequently generalize. The EEG activity associated with the electroclinical seizures showed an evolution of amplitude, frequency or both and contained spikes and sharps (figure 2A). The rats experienced frequent, typically short duration seizures during hypoxia (table 1). Two out of twelve pups (16%) also developed purely electrographic seizures during hypoxia at an average frequency of 1 seizure per hour (table 1).
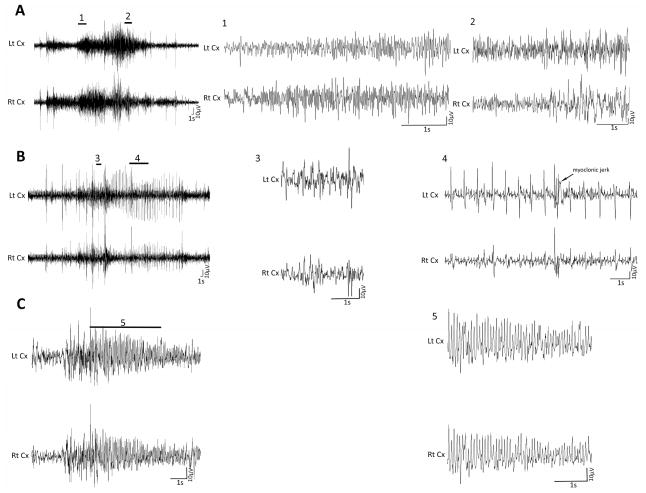
Figure 2.
Representative EEG tracings during a clinical seizure in P7 rats. (A) EEG in the left panel shows ictal activities during hypoxia. Magnified excerpts of a part of clinical seizure associated with these activities, marked by a bar above the EEG tracing in the left panel, are provided in the middle and right panel of the figure. The EEG seizure activity was associated with tonic (1) and clonic (2) seizure. (B) Electroclinical seizure during the reperfusion period, i.e., immediate period following hypoxia termination. The EEG ictal activity was associated with a brief body tonus (3) and multiple body jerks (4). (C) Electroclinical seizures were observed even 24 hours after HI. The representative EEG ictal discharge shown here was associated with tonic hind limb extension and stiffening of tail (5). Lt = left, Rt = right, Cx = cortex, S = seconds, V = volts.
In the period immediately following hypoxia i.e., during the reperfusion period, 11 out of 12 rats (91%) continued to have electroclinical seizures. These seizures, similar to seizures of the hypoxic period, were brief and frequent (table 1). The behavioral seizures, which correlated with a change in EEG activity, consisted of clonic, tonic and tonic-clonic seizures (figure 2B). Some of the rats (3/12) also developed purely electrographic seizures (table 1). Twenty-four hours after the initial insult, 66% of the rats (8/12) continued to exhibit electroclinical seizures (table 1). For these rats, both the seizure frequency and the total time seizing were lower than during the hypoxic and the reperfusion period (table 1). The behavioral seizures consisted of body jerks associated with tonic and clonic seizures (figure 2C). Many of these rats (7/12) also manifested purely electrographic seizures (table 1). Only 25% of HI rats (3/12) continued to have electroclinical seizures 48 hours following HI-induction (table 1). Neither electroclinical nor purely electrographic seizures were observed in any rats 72 hours after HI-induction (n = 12, table 1).
To examine whether there was any change in the shape of the EEG power spectrum within the 1 to 50 Hz frequency band during the electroclinical seizures, the sum of the raw EEG powers in each frequency bin was normalized to one. The normalized power was then compared between ictal and inter-ictal epochs of the hypoxia period. The results revealed that during the electroclinical seizures, the relative distribution of power shifts from slower to faster frequencies. Specifically, increases in power at about 9 and 25 Hz were observed during the ictal activity in both cortices (supplemental figure 2).
Characteristics of background or inter-ictal EEG
The baseline EEG of P7 rats consisted of low amplitude activity (~30 μV) and appeared continuous, with intermittent periods of voltage attenuation (no movement during this period) alternating with high voltage activities (no movement except occasional twitches). Some spike and sharp wave like activity was also observed in the baseline EEG record. These activities, which were not associated with any visible movement artifact, were high amplitude (3 times the average amplitude) sharply-contoured waveforms with duration 20–200 msec (supplemental figure 3A). However, because of the invasive nature of the recording technique used in this study, it is not possible to conclude with certainty whether these activities were physiologic or pathologic. A significant increase in the number of spikes and sharp waves occurred following HI-induction (supplemental figure 3 B, C). No such change was observed in control rats at the similar time in the recording session as for the HI rats. This suggests that the increased spike and sharp wave activity observed in HI rats resulted from HI-induced injury. Along with many individual spikes and sharp waves, BREDs (supplemental figure 4), which first appeared during the hypoxia period, were also observed in the inter-ictal EEG record. During hypoxia, the EEG of the majority of rats (10/12) contained BREDs, which continued to appear during the reperfusion period (table 1). In some of the rats, unlike electroclinical and electrographic seizures, BREDs were observed even 72 hours after HI-induction. However, the frequency and the duration of BREDs continued to decline with increasing time from initiation of hypoxia (table 1).
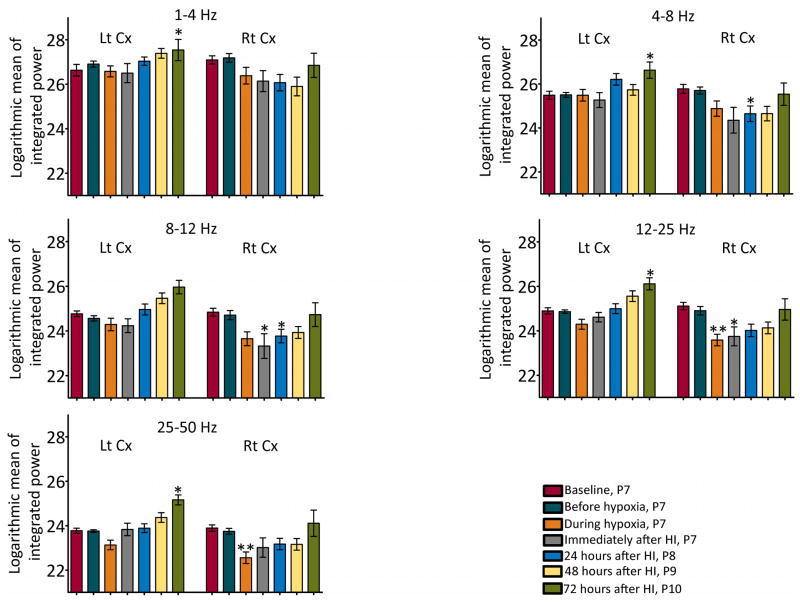
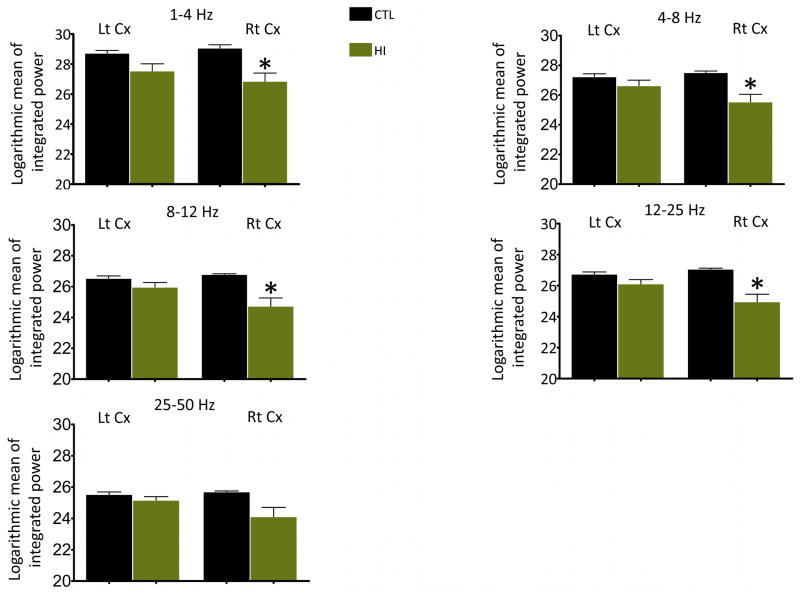
Severe voltage suppression of the background or inter-ictal EEG was evident in both cortices during hypoxia and in the period immediately following hypoxia, but it was more pronounced in the cortex ipsilateral to the ligated carotid artery (right cortex). Quantitative analyses showed a significant reduction in the integrated EEG power in the beta and gamma frequency bands during the inter-ictal period in the right cortex while undergoing hypoxia as compared to the power in the same frequency bands in the baseline EEG (figure 4). In the period immediately following hypoxia, a significant drop in power in the alpha and beta bands was observed in the right cortex (figure 4). The reduction in the power in the alpha band persisted for another 24 hours. Also, a significant decrease in the inter-ictal theta power was observed in the right cortex 24 hours after HI-induction (figure 4). By 72 hours after HI (at P10), the EEG power in all the frequency bands in the right cortex was similar to the power in the baseline EEG acquired at P7 (figure 4). However, the EEG power in 1 to 25 Hz frequency band at 72 hours after HI was significantly lower than the EEG power in the control rats at P10 (figure 5). In the hemisphere contralateral to the ligated carotid artery (left cortex), power in the alpha, beta and gamma frequency bands in the inter-ictal period during hypoxia showed a decreasing trend, but was not significantly different from the baseline power (figure 4). Further, the EEG power at 72 hours after HI was higher than the power in the baseline EEG that was acquired at P7 (figure 4); it was, however, similar to the EEG power of P10 control rats (figure 5). In fact, the developmental changes in the EEG power in the left cortex of HI rats were similar to that of the control rats (supplemental figure 5).
Figure 4.
Integrated power values for various background EEG frequency bands following HI-induction. A significant reduction in the integrated background EEG power in all the frequency bands was observed in the right cortex, the cortex ipsilateral to the ligated carotid artery, following HI-induction. N = 7 rats; *, **statistically different from baseline values; *P<0.05, **P<0.01, Lt = left, Rt = right, Cx = cortex.
Figure 5.
Comparison of background EEG power between HI and control (CTL) rats at P10. A significant difference in the integrated background EEG power in all the frequency bands, except the gamma band, in the right cortex was observed between P10 HI (n =7) and P10 CTL rats (n = 4). *P<0.05, **P<0.01, Lt = left, Rt = right, Cx = cortex.
Discussion
To determine if an intervention, such as the administration of a drug, results in a significant change, it is best to, as accurately as possible, characterize the baseline (control) state so that deviations from that baseline can be identified. This paper is the first to describe the characteristics and evolution of both behavioral and EEG seizures over a 72 hour time frame in a rat model of neonatal HIE, derived using the Rice-Vannucci method. The uniqueness and accuracy of the descriptions were facilitated through the use of video monitoring that was time-locked with EEG. This technique allowed us to accurately correlate each EEG waveform to a rat’s behavior. Due to the size and thickness of the skull of a neonatal rat, and because they cannot be kept separate from the dam for a very long-time, it is technically very challenging to implant multiple electrodes and keep them intact for a prolonged period before significant deterioration of the EEG signal occurs. Even with these difficulties, EEG is still the most reliable method for identifying seizures, especially in neonates in whom it is difficult to differentiate uncoordinated movement from behavioral seizure activity. Further, EEG is necessary to detect subclinical seizures, which are common in neonates with HIE. Thus, using the synchronized video-EEG technique, we demonstrated that induction of HI in P7 rats by the Rice-Vannucci method16, a commonly used animal model to study neonatal HIE, resulted in the development of recurrent short duration electroclinical seizures, purely electrographic seizures as well as abnormalities in background EEG.
To be an accurate model of the human condition, we need to demonstrate that seizures seen in the Rice-Vannucci model are similar to those experienced by human neonates. In human neonates, seizures are often brief and repetitive22 and consist of clonic, tonic, myoclonic and subtle seizure subtypes.23–25 In our study, clonic, tonic and tonic-clonic seizures that were clearly associated with changes in the EEG activity were observed following the introduction of pups to the hypoxic environment. Myoclonic jerks, often preceded by a spike in the EEG, were also observed along with other seizure activity and, most commonly occurred just before an electroclinical seizure began. In this paper, we significantly expand on the current literature. In a study similar to our own, Hayakawa and colleagues26 also observed generalized tonic and tonic-clonic seizures associated with bursts of polyspikes or spike-and-wave complexes during hypoxia in P6 rats whose left carotid artery was ligated (Rice-Vannucci method). A recent study documented that induction of HI in P12 rats (P11, if the day of birth is considered as P0) by Rice-Vannucci method results in the development of behavioral seizure activity such as forelimb or hindlimb paddling which correlated with alterations in EEG activity during hypoxia.27 However, these studies did not determine whether seizures persisted following HI-induction. Electroclinical seizures consisting of myoclonic jerks, tonic posturing, clonic convulsions, cycling movements and generalized tonic-clonic episode have been observed in P7 rats,28,29 P10 rats,28 P12 mice,30 newborn piglets,12 and fetal sheep31 in which hypoxia/ischemia was induced by methods that were different from the Rice-Vannucci method.
Our study demonstrates that following HI-induction, electroclinical seizures occurred spontaneously for 24 hours in the majority and for 48 hours in 25% of HI rat pups. In human patients with neonatal HIE, first seizures (sub-clinical or electroclinical) have been observed 6 to 20 hours after birth, and these seizures often continue to occur for 48 hours or more.32,33 It is important to note that because of practical reasons, human EEG monitoring usually does not begin before many hours have passed after the birth. Similar to electroclinical seizures, in our study, electrographic seizures were first observed during hypoxia and continued to appear for 24 hours after HI-induction. However, unlike electroclinical seizures, which occurred in almost all of the HI rats, electrographic seizures were observed in only a few of the HI rats. In human neonates subclinical or electrographic seizures without clinical correlates are common, especially subsequent to antiepileptic treatment.34
Along with electroclinical and electrographic seizures, multiple abnormalities in the EEG background activities were observed in the HI animals. The changes observed in the EEG background activities have been used as a diagnostic tool to prognosticate long-term outcome.35 The baseline EEG of P7 rats and the EEG of control rats in our study appeared continuous, contained spikes and sharp wavelike discharges as well as alternate periods of suppression and higher amplitudes. Following HI-induction, a visually obvious increase in the number of spikes and sharp waves was observed. In a study of human neonates by Clancy and Legido, the presence of spikes or sharp waves at 3-month follow-up was associated with a 100% rate of postnatal epilepsy development, whereas only 27% patients without spikes or sharp waves developed postnatal epilepsy.36 Another abnormality in the EEG background that is commonly observed in the neonates with HIE and has a very strong prognostic value is voltage attenuation. In our study there was a significant and persistent attenuation of inter-ictal EEG voltage following the introduction of the pups to hypoxia after carotid ligation. In the cortex ipsilateral to ligated carotid artery of the HI rats, the EEG power at P10 was significantly lower than that of P10 control rats, which may suggest a delay in EEG maturation (dysmaturity). In both rats and humans, a severely depressed EEG background that persists for a period of time is considered to be indicative of severe brain damage and a marker for an abnormal long-term neurologic outcome. According to a study carried out by Murray and colleagues, an EEG with background suppression (> 50% reduction from the normal values) in neonates with HIE is associated with abnormal outcomes.37 Their study also suggests that if the amplitude remains attenuated at 48 hours after the birth, the prognosis is very poor.
There are some limitations to the current study. Due to the age of the animals, they cannot be kept separate from the dam for a very long duration and therefore, prolonged continuous video-EEG record could not be acquired. As a result, we could have underestimated the frequency of recurring seizures, the percentage of animals that develop acute spontaneous seizures following HI-induction, and the length of the period during which seizures acutely recurred after the initial injury. In our study, because of the size of the skull and the animal, we could efficiently record from only two brain regions (more electrodes will increase the size and the weight of the implant). Hence, we could have missed those seizures that originated from deep brain structures and remained focal. In future studies, continuous monitoring may be achieved through the use of a wireless telemetry system to record EEG. In a recent publication, Zayachkivsky and colleagues describe the features of a novel wireless telemetry system that was well-tolerated by the pups and the EEG was recorded continuously from the pups while they were housed with the dam.29 While this system allows for more continuous monitoring, it is however limited in that there is only one active recording electrode and therefore, EEG signals from multiple areas cannot be obtained.
In this paper, not only have we shown that induction of HI in P7 rats by the Rice-Vannucci method replicates most of the features of acute seizures and EEG abnormalities associated with the human condition, but we have also, through direct EEG, power spectra, and video observation and analysis, sufficiently characterized the baseline model results such that we can compare these with a perturbed system to determine the impact of drugs or other interventions. Since this model also replicates chronic changes observed in human HIE condition, it can be used to study the effects of the treatment of acute seizures on chronic brain injury and long-term neurologic outcome. A shorter latency to acute seizures has been shown to directly correlate with the extent of the acute lesion in the Rice-Vannucci model of neonatal HIE.27 Similarly, long-term studies may help explain the relationship between acute seizures and the chronic effects of neonatal HIE.
Supplementary Material
Supplemental figure 1: Representative brain sections show the location of electrode placement. A schematic diagram of the coronal brain section on the right illustrates the placement of a recording electrode (solid black line), and the cresyl violet stained brain section on the left shows an example of corresponding placement (black arrow). The schematic brain section was reproduced, with the permission from Elsevier, from the 5th edition of The Rat Brain in Stereotaxic Co-ordinates by Paxinos G and Watson C. HP = Hippocampus.
Supplemental Figure 2: Comparison of distribution of EEG power between ictal and inter-ictal periods. The line chart depicts the normalized EEG power of the ictal and inter-ictal epochs recorded from 7 rats during hypoxia in the frequency band 1 to 50 Hz. The EEG power was normalized by dividing the raw power in each frequency bin by the total power, which was obtained by summing up the power in all of the frequency bins. Note an increase of EEG power at ~9 and 25 Hz frequency (black arrows) during electroclinical seizures in both right and left cortex.
Supplemental figure 3: A significant increase in spike and sharp wave number was observed following HI-induction. An example of a spike and sharp waves observed in the baseline EEG (A) and in the EEG acquired during the reperfusion period following HI-induction (B) are given. (C) A thirty-minute EEG record was analyzed manually to determine the number of spikes and sharp waves in the left and right cortex of control (CTL, n = 4) and HI (n = 7) rats. Sharp transients where there was obvious animal movement were excluded from the count. If the spike and/or sharp wave occurred at the same time in both the cortices, it was counted as a single event. The baseline EEG record for HI rats was obtained before carotid ligation. The reperfusion period for HI rats refers to the immediate time following hypoxia exposure, by which time the rat had been monitored using video-EEG for ~3 hours. A comparable recording epoch was analyzed for the CTL group of rats. ***P<0.001
Supplemental figure 4: Representative trace shows an episode of BRED in an HI rat.
Supplemental figure 5: Developmental changes in the EEG power in control rats. An apparent change in the integrated power values for various background EEG frequency bands was observed as the animals matured (n = 4). *, **statistically different from baseline values acquired at P7; *P<0.05, **P<0.01, Lt = left, Rt = right, Cx = cortex.
Representative video-EEG shows an electroclinical seizure episode in a P7 rat during hypoxia.
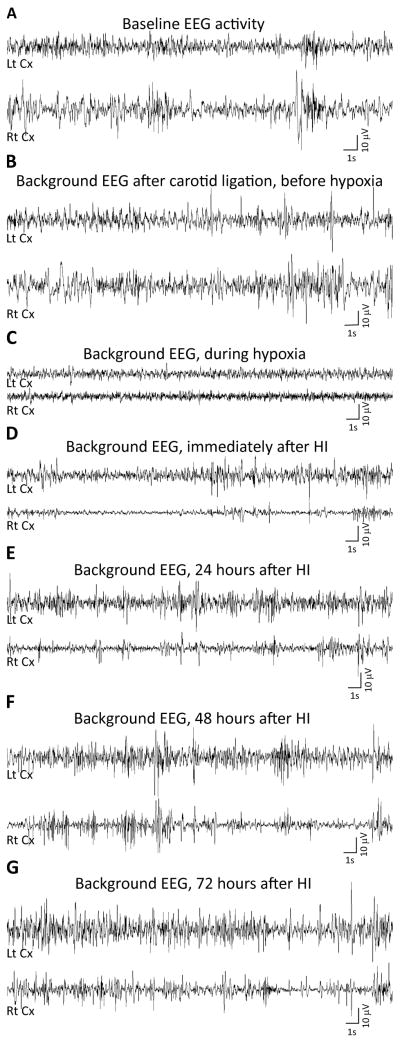
Figure 3.
Representative background EEG tracings from a P7 rat. EEG was acquired before carotid ligation, i.e., during the baseline period (A), after carotid ligation, but before the rat was exposed to the hypoxic environment (B), during hypoxia (C), immediately following HI (D) and, 24 (E), 48 (F) and 72 (G) hours after HI-induction. During all the representative EEG epochs the rat was in a similar behavioral state i.e., it was lying quietly on its side and exhibited slight intermittent limb or tail movements. Lt = left, Rt = right, Cx = cortex, S = seconds, V = volts.
Acknowledgments
This work is supported by NIH/NICHD R01 HD065534 grant (YHR), CURE Prevention of Acquired Epilepsy award (YHR) and NIH/NINDS K08 NS053610-05 grant (AMW). We thank the University of Colorado Anschutz Medical Campus Rodent In Vivo Neurophysiology Core for providing facilities to acquire and review video-EEG data. We also thank Dr. Michael Hall and the Neuroscience Core Machine Shop for help with the construction of hypoxia chamber. We thank Dr. Zhaoxing Pan, Research Institute, Children’s Hospital Colorado, for help with statistical analyses, and Dr. Marco Gonzalez and Philip Lam for their help with histology. The authors also wish to thank staff members of the University of Colorado Anschutz Medical Campus Biorepository Core Facility for their assistance with brain imaging (the facility is supported by NIH/NCATS Colorado CTSI Grant Number UL1 TR001082).
Footnotes
DISCLOSURE OF CONFLICTS OF INTEREST
None of the authors have any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Ronen GM, Buckley D, Penney S, et al. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology. 2007;69:1816–1822. doi: 10.1212/01.wnl.0000279335.85797.2c. [DOI] [PubMed] [Google Scholar]
- 2.Legido A, Clancy RR, Berman PH. Neurologic outcome after electroencephalographically proven neonatal seizures. Pediatrics. 1991;88:583–596. [PubMed] [Google Scholar]
- 3.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early human development. 2010;86:329–338. doi: 10.1016/j.earlhumdev.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Huang BY, Castillo M. Hypoxic-ischemic brain injury: imaging findings from birth to adulthood. Radiographics. 2008;28:417–439. doi: 10.1148/rg.282075066. [DOI] [PubMed] [Google Scholar]
- 5.de Vries LS, Jongmans MJ. Long-term outcome after neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2010;95:F220–224. doi: 10.1136/adc.2008.148205. [DOI] [PubMed] [Google Scholar]
- 6.Bergamasco B, Benna P, Ferrero P, et al. Neonatal hypoxia and epileptic risk: a clinical prospective study. Epilepsia. 1984;25:131–136. doi: 10.1111/j.1528-1157.1984.tb04168.x. [DOI] [PubMed] [Google Scholar]
- 7.Glass HC, Glidden D, Jeremy RJ, et al. Clinical Neonatal Seizures are Independently Associated with Outcome in Infants at Risk for Hypoxic-Ischemic Brain Injury. J Pediatr. 2009;155:318–23. doi: 10.1016/j.jpeds.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller SP, Weiss J, Barnwell A, et al. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology. 2002;58:542–548. doi: 10.1212/wnl.58.4.542. [DOI] [PubMed] [Google Scholar]
- 9.Wirrell EC, Armstrong EA, Osman LD, et al. Prolonged seizures exacerbate perinatal hypoxic-ischemic brain damage. Pediatr Res. 2001;50:445–454. doi: 10.1203/00006450-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Dzhala V, Ben-Ari Y, Khazipov R. Seizures accelerate anoxia-induced neuronal death in the neonatal rat hippocampus. Ann Neurol. 2000;48:632–640. [PubMed] [Google Scholar]
- 11.McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology. 2000;55:506–513. doi: 10.1212/wnl.55.4.506. [DOI] [PubMed] [Google Scholar]
- 12.Bjorkman ST, Miller SM, Rose SE, et al. Seizures are associated with brain injury severity in a neonatal model of hypoxia-ischemia. Neuroscience. 2010;166:157–167. doi: 10.1016/j.neuroscience.2009.11.067. [DOI] [PubMed] [Google Scholar]
- 13.van Rooij LG, Toet MC, van Huffelen AC, et al. Effect of treatment of subclinical neonatal seizures detected with aEEG: randomized, controlled trial. Pediatrics. 2010;125:e358–366. doi: 10.1542/peds.2009-0136. [DOI] [PubMed] [Google Scholar]
- 14.Kwon JM, Guillet R, Shankaran S, et al. Clinical seizures in neonatal hypoxic-ischemic encephalopathy have no independent impact on neurodevelopmental outcome: secondary analyses of data from the neonatal research network hypothermia trial. J Child Neurol. 2011;26:322–328. doi: 10.1177/0883073810380915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Towfighi J, Housman C, Mauger D, et al. Effect of seizures on cerebral hypoxic-ischemic lesions in immature rats. Brain Res Dev Brain Res. 1999;113:83–95. doi: 10.1016/s0165-3806(99)00004-8. [DOI] [PubMed] [Google Scholar]
- 16.Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 17.Kadam SD, Dudek FE. Neuropathogical features of a rat model for perinatal hypoxic-ischemic encephalopathy with associated epilepsy. J Comp Neurol. 2007;505:716–737. doi: 10.1002/cne.21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Northington FJ, Ferriero DM, Graham EM, et al. Early neurodegeneration after hypoxia-ischemia in neonatal rat is necrosis while delayed neuronal death is apoptosis. Neurobiol Dis. 2001;8:207–219. doi: 10.1006/nbdi.2000.0371. [DOI] [PubMed] [Google Scholar]
- 19.Yang J, Khong PL, Wang Y, et al. Manganese-enhanced MRI detection of neurodegeneration in neonatal hypoxic-ischemic cerebral injury. Magn Reson Med. 2008;59:1329–1339. doi: 10.1002/mrm.21484. [DOI] [PubMed] [Google Scholar]
- 20.Kadam SD, White AM, Staley KJ, et al. Continuous electroencephalographic monitoring with radio-telemetry in a rat model of perinatal hypoxia-ischemia reveals progressive post-stroke epilepsy. J Neurosci. 2010;30:404–415. doi: 10.1523/JNEUROSCI.4093-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almli CR, Levy TJ, Han BH, et al. BDNF protects against spatial memory deficits following neonatal hypoxia-ischemia. Exp Neurol. 2000;166:99–114. doi: 10.1006/exnr.2000.7492. [DOI] [PubMed] [Google Scholar]
- 22.Clancy RR, Legido A. The exact ictal and interictal duration of electroencephalographic neonatal seizures. Epilepsia. 1987;28:537–541. doi: 10.1111/j.1528-1157.1987.tb03685.x. [DOI] [PubMed] [Google Scholar]
- 23.Volpe J. Neonatal Seizures. In: Volpe J, editor. Neurology of the newborn. Philadelphia: Elsevier; 2008. pp. 203–220. [Google Scholar]
- 24.Mizrahi EM, Kellaway P. Characterization and classification of neonatal seizures. Neurology. 1987;37:1837–1844. doi: 10.1212/wnl.37.12.1837. [DOI] [PubMed] [Google Scholar]
- 25.Mizrahi E, Kellaway P. Diagnosis and management of neonatal seizures. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- 26.Hayakawa T, Higuchi Y, Nigami H, et al. Zonisamide reduces hypoxic-ischemic brain damage in neonatal rats irrespective of its anticonvulsive effect. Eur J Pharmacol. 1994;257:131–136. doi: 10.1016/0014-2999(94)90704-8. [DOI] [PubMed] [Google Scholar]
- 27.Cuaycong M, Engel M, Weinstein SL, et al. A novel approach to the study of hypoxia-ischemia-induced clinical and subclinical seizures in the neonatal rat. Dev Neurosci. 2011;33:241–250. doi: 10.1159/000331646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen FE, Applegate CD, Holtzman D, et al. Epileptogenic effect of hypoxia in the immature rodent brain. Ann Neurol. 1991;29:629–637. doi: 10.1002/ana.410290610. [DOI] [PubMed] [Google Scholar]
- 29.Zayachkivsky A, Lehmkuhle MJ, Fisher JH, et al. Recording EEG in immature rats with a novel miniature telemetry system. J Neurophysiol. 2013;109:900–911. doi: 10.1152/jn.00593.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Comi AM, Trescher WH, Abi-Raad R, et al. Impact of age and strain on ischemic brain injury and seizures after carotid ligation in immature mice. Int J Dev Neurosci. 2009;27:271–277. doi: 10.1016/j.ijdevneu.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams CE, Gunn AJ, Synek B, et al. Delayed seizures occurring with hypoxic-ischemic encephalopathy in the fetal sheep. Pediatr Res. 1990;27:561–565. doi: 10.1203/00006450-199006000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Ahn MO, Korst LM, Phelan JP, et al. Does the onset of neonatal seizures correlate with the timing of fetal neurologic injury? Clin Pediatr. 1998;37:673–676. doi: 10.1177/000992289803701105. [DOI] [PubMed] [Google Scholar]
- 33.Filan P, Boylan GB, Chorley G, et al. The relationship between the onset of electrographic seizure activity after birth and the time of cerebral injury in utero. BJOG. 2005;112:504–507. doi: 10.1111/j.1471-0528.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 34.Clancy RR, Legido A, Lewis D. Occult neonatal seizures. Epilepsia. 1988;29:256–261. doi: 10.1111/j.1528-1157.1988.tb03715.x. [DOI] [PubMed] [Google Scholar]
- 35.Holmes GL, Lombroso CT. Prognostic value of background patterns in the neonatal EEG. J Clin Neurophysiol. 1993;10:323–352. doi: 10.1097/00004691-199307000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Clancy RR, Legido A. Postnatal epilepsy after EEG-confirmed neonatal seizures. Epilepsia. 1991;32:69–76. doi: 10.1111/j.1528-1157.1991.tb05614.x. [DOI] [PubMed] [Google Scholar]
- 37.Murray DM, Boylan GB, Ryan CA, et al. Early EEG findings in hypoxic-ischemic encephalopathy predict outcomes at 2 years. Pediatrics. 2009;124:e459–467. doi: 10.1542/peds.2008-2190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1: Representative brain sections show the location of electrode placement. A schematic diagram of the coronal brain section on the right illustrates the placement of a recording electrode (solid black line), and the cresyl violet stained brain section on the left shows an example of corresponding placement (black arrow). The schematic brain section was reproduced, with the permission from Elsevier, from the 5th edition of The Rat Brain in Stereotaxic Co-ordinates by Paxinos G and Watson C. HP = Hippocampus.
Supplemental Figure 2: Comparison of distribution of EEG power between ictal and inter-ictal periods. The line chart depicts the normalized EEG power of the ictal and inter-ictal epochs recorded from 7 rats during hypoxia in the frequency band 1 to 50 Hz. The EEG power was normalized by dividing the raw power in each frequency bin by the total power, which was obtained by summing up the power in all of the frequency bins. Note an increase of EEG power at ~9 and 25 Hz frequency (black arrows) during electroclinical seizures in both right and left cortex.
Supplemental figure 3: A significant increase in spike and sharp wave number was observed following HI-induction. An example of a spike and sharp waves observed in the baseline EEG (A) and in the EEG acquired during the reperfusion period following HI-induction (B) are given. (C) A thirty-minute EEG record was analyzed manually to determine the number of spikes and sharp waves in the left and right cortex of control (CTL, n = 4) and HI (n = 7) rats. Sharp transients where there was obvious animal movement were excluded from the count. If the spike and/or sharp wave occurred at the same time in both the cortices, it was counted as a single event. The baseline EEG record for HI rats was obtained before carotid ligation. The reperfusion period for HI rats refers to the immediate time following hypoxia exposure, by which time the rat had been monitored using video-EEG for ~3 hours. A comparable recording epoch was analyzed for the CTL group of rats. ***P<0.001
Supplemental figure 4: Representative trace shows an episode of BRED in an HI rat.
Supplemental figure 5: Developmental changes in the EEG power in control rats. An apparent change in the integrated power values for various background EEG frequency bands was observed as the animals matured (n = 4). *, **statistically different from baseline values acquired at P7; *P<0.05, **P<0.01, Lt = left, Rt = right, Cx = cortex.
Representative video-EEG shows an electroclinical seizure episode in a P7 rat during hypoxia.