Abstract
Liver interstitial dendritic cells (DC) have been implicated in immune regulation and tolerance induction. We found that the transmembrane immuno-adaptor DNAX-activating protein of 12kDa (DAP12) negatively regulated conventional liver myeloid (m) DC maturation and their in vivo migratory and T cell allostimulatory ability. Livers were transplanted from C57BL/6(H2b) (B6) wild-type (wt) or DAP12−/− mice into wt C3H (H2k) recipients. Donor mDC (H2-Kb+CD11c+) were quantified in spleens by flow cytometry. Anti-donor T cell reactivity was evaluated by ex vivo CFSE-MLR and delayed-type hypersensitivity responses, while T effector and regulatory T cells (Treg) were determined by flow analysis. A 3–4-fold increase in donor-derived DC was detected in spleens of DAP12−/− liver recipients compared with those given wt grafts. Moreover, pro-inflammatory cytokine gene expression in the graft, IFNγ production by graft-infiltrating CD8+ T cells, and systemic levels of IFNγ were all elevated significantly in DAP12−/− liver recipients. DAP12−/− grafts also exhibited reduced incidences of CD4+Foxp3+ cells and enhanced CD8+ T cell IFNγ secretion in response to donor antigen challenge. Unlike wt grafts, DAP12−/− livers failed to induce tolerance and were rejected acutely. Thus, DAP12 expression in liver grafts regulates donor mDC migration to host lymphoid tissue, alloreactive T cell responses and transplant tolerance.
Keywords: DAP12, liver transplant, dendritic cells, T cells, tolerance
Introduction
Liver grafts between MHC-mismatched mice (1), certain rat strains (2, 3), and outbred pigs (4) are accepted without immunosuppressive therapy. Whereas in mice, liver allografts induce robust, donor-specific tolerance (1), in humans, the liver is generally regarded as the most tolerogenic of transplanted whole organs (5, 6). Mechanisms underlying liver transplant tolerance are not well understood, but production of soluble MHC class I by the graft and regulatory functions of donor-derived hematopoietic cells have been proposed (3, 7, 8). The liver is an immune organ, with a unique consistency of both non-parenchymal and parenchymal cells that have been implicated in tolerance induction (9–11). These include professional and non-professional antigen (Ag)-presenting cells (APC), i.e. dendritic cells (DC) and hepatic macrophages (Kupffer cells) (12, 13), sinusoid-lining endothelial cells (14, 15), hepatic stellate cells (16, 17), and hepatocytes (18). There is strong evidence that these hepatic APC play important roles in immune regulation and tolerance induction (10). DC are uniquely well-equipped APC that promote self tolerance in the steady-state (19) and regulate immunity (20, 21). Liver-resident DC comprise several subsets (10, 12). In addition to conventional CD11b+ CD11c+ myeloid (m)DC, other DC subsets found in the mouse liver include rarer, non-conventional plasmacytoid (p)DC (CD11b-CD11clo B220+ plasmacytoid DC Ag+ [PDCA]-1+) (22). They are characterized by their immaturity and resistance to maturation (23, 24), and display tolerogenic properties (8, 25, 26). Liver DC can attenuate hepatic inflammation and fibrosis (27−29) and regulate liver warm and transplant-induced ischemia-reperfusion injury (30, 31). Moreover, liver DC can subvert T cell responses (12, 25, 26, 32–34) and prolong allograft survival (35).
Molecular mechanisms whereby hepatic APCs regulate/inhibit T cell responses include the expression of B7 homologue 1 (B7-H1) (14, 34, 36, 37), IL-10 (38, 39), FasL (40, 41), the Notch ligand Jagged 1 (18), CD39 (31) and DNAX-activating protein of 12kDa (DAP12) (42). DAP12 is a homodimeric immunoreceptor tyrosine-based activation motif (ITAM)-bearing transmembrane adaptor protein that is highly expressed in lymphoid tissues and the lung and to a much lesser degree in whole liver tissue (43, 44). It is expressed by DC, macrophages and natural killer (NK) cells and integrates signals through multiple receptors, including triggering receptor expressed on myeloid cells (TREM)-1 and -2, NKG2D, Ly49, myeloid DAP12-associating lectin-1 and CD200R (45–48). By associating with distinct receptors, DAP12-can potentiate or inhibit leukocyte activation, with the outcome determined by the avidity between the DAP12-associated receptor and its ligand (49). Macrophages from DAP12−/− mice have increased phagocytic capacity (50) and DAP12/TREM-1/2 activation modulates phagocytosis (51, 52).
Conventional mDC propagated from the bone marrow of DAP12−/− mice exhibit a more mature phenotype than those from wild-type (wt) controls (53), while DAP12-deficient lung CD11c+ APC enhance Ag-specific T cell responses in vivo (54). Recently, using small interfering RNA (siRNA) to silence DAP12, we found (42) that DAP12 promoted the expression of IL-1 receptor (R)-associated kinase (IRAK)-M, a negative regulator of Toll-like receptor (TLR) signaling and the production of IL-10 by liver mDC. Consequently, DAP12 restrained their T cell allostimulatory activity. In this study, we examined the role of DAP12 in determining the in vivo migrational function of liver-derived mDCs, the survival of liver transplants from DAP12−/− mice, and underlying effects on T cell responses to the allograft. Our data suggest that DAP12 expression in liver grafts regulates the migration of donor mDCs to host secondary lymphoid tissue, Th1 cell-mediated alloimmune responses and the induction of transplant tolerance.
Materials and Methods
Mice
Male C57BL/6 (B6;H-2b), BALB/c (H-2d) and C3H (H2k) mice (8- to 12-wk old) were purchased from The Jackson Laboratory, Bar Harbor, ME. DAP12−/− mice (55) generated initially in the 129/SvJ and B6 hybrid background as described (56), were backcrossed onto the B6 background and kindly provided by Dr. Marco Colonna, Washington University School of Medicine, St. Louis, MO. Animals were maintained in the specific pathogen-free Central Animal Facility of the University of Pittsburgh School of Medicine. Experiments were conducted under an Institutional Animal Care and Use Committee-approved protocol and in accordance with criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health. Animals were fed a diet of Purina rodent chow (Ralston Purina, St. Louis, MO) and received tap water ad libitum.
Reagents
Complete culture medium comprised RPMI-1640 (BioWhittaker, Walkersville, MD) supplemented with 10% (v/v) fetal calf serum (Nalgene, Miami, FL), non-essential amino acids, L-glutamine, sodium pyruvate, penicillin-streptomycin, and 2-mercaptoethanol (all from Life Technologies, Gaithersburg, MD). Lipopolysaccharide (LPS) and CpG A; ODN 1585 were purchased from InvivoGen (San Diego, CA). Chinese hamster ovary cell-derived recombinant human fms-like tyrosine kinase 3 ligand (Flt3L) was obtained from Amgen (Seattle, WA).
Isolation of mouse liver and spleen DC
DC were isolated and purified as described in detail (24). Briefly, livers and spleens were harvested from mice given the endogenous DC poietin fms-like tyrosine kinase-3 ligand (Flt3L) (10μg/ day i.p.; 10 days) and digested in collagenase (Sigma). Bulk DC were enriched by density gradient centrifugation using Histodenz (Sigma). For pDC purification (>95%) PDCA-1+ cells were positively selected from the DC-enriched fraction using immunomagnetic beads and a paramagnetic LS column (Miltenyi Biotec) (22). mDC (CD11b+CD11c+PDCA-1−) were isolated from the pDC-depleted, DC-enriched fraction using anti-CD11c immunomagnetic beads (Miltenyi Biotec) as described (22). The purity of mDC consistently exceeded 95%.
Flow cytometry
Liver mDC, hepatic non-parenchymal cells (NPC) and spleen cells were treated with FcγR-blocking rat anti-mouse CD16/32 mAb (2.4G2) to prevent non-specific antibody (Ab) binding. They were then incubated for 30 min with fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, APC-, PE-cyanin (Cy)5-, or PE-Cy7-conjugated monoclonal Abs (mAbs) to detect expression of CD3 (145-2C11, CD4 (GK1.5), CD8 (53-6.7), CD11c (HL3), B220/CD45R (RA3-6B2), I-Ab β-chain (25-9-17) (all eBioscience, San Diego, CA), CD40 (3/23), CD80 (16-10A1), CD86 (GL1), H2-Kb (AF6-88.5) and B7 homologue-1 (B7-H1; CD274) (MIH5) (BD Biosciences, San Diego, CA). For intracellular cytokine staining, cells were fixed with 4% paraformaldehyde and permeabilized using 0.1% saponin, then stained with anti-mouse IFN-γ Ab (XMG1.2) (BioLegend). For Foxp3 staining, cells were fixed and permeabilized using Foxp3 Fix Permkit (eBioscience) and stained with anti-Foxp3 mAb (FJK-16s) (eBioscience). Appropriate Ig isotype controls were obtained from BD PharMingen (San Diego, CA). Flow analysis was performed using an LSR Fortessa flow cytometer (BD Biosciences). Results are expressed as percent positive cells and mean fluorescence intensity (MFI).
T cell purification
Bulk splenocyte suspensions were incubated with a mAb cocktail consisting of anti-CD45R/B220 (RA3-6B2), anti-CD16/CD32 (2.4G2), anti-TER-119, anti-CD11b (M1/70), and anti-Ly6G (RB-8C5) obtained from BD PharMingen and non-T cells eliminated by immunomagnetic negative selection using Dynabeads (InvitroGen, Grand Island, NY) following the manufacturer's instructions.
Mixed leukocyte reaction (MLR)
To assess the T cell allostimulatory activity of liver mDC or pDC, freshly-isolated, unstimulated or stimulated DC were used as stimulators of allogeneic BALB/c T cells (2×105/well) in 72 hr MLR using 96-well, round-bottom plates. For ex vivo T cell re-stimulation, or measurement of anti-donor responses, bulk splenocytes of sensitized or transplanted mice were used as responders, and T cell-depleted (CD3ε Microbeads kit; Miltenyi) B6 splenocytes as stimulators. For the final 18 h of culture, 1 μCi of [3H]-thymidine (Perkin Elmer, Waltham, MA) was added to each well. Radioisotope incorporation was determined using a beta scintillation counter (Perkin Elmer) and results expressed as mean cpm ± 1SD of triplicate wells. Alternatively, T cell proliferation was determined by carboxyfluorescein succinimidyl ester (CFSE)-MLR using responder T cells labeled with CFSE (Invitrogen) as described (42).
Cytokine Assays
Cytokine levels in culture supernatants or serum samples were determined by cytometric bead array flex sets (BD Bioscience) (IL-6, tumor necrosis factor [TNF]α and IFNγ) or by ELISA (IL-12p40 and IFNα) (BioLegend and PBL Biomedical Labs, Piscataway, NJ, respectively), following the suppliers' instructions.
Real-Time Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
Messenger RNA (mRNA) expression was quantified by SYBR Green real-time RT-PCR using an ABI-Prism 7000 sequence detection system (PE Applied Biosystems, Foster City, CA) and primers specific for IFN-γ (F: 5'-CACGGCACAGTCATTGAAAG-3'; R; 5'-TTTTGCCAGTTCCTCCAGAT-3'), TNF-α (F: 5'-CATCTTCTCAAAATTCGAGTGA-3'; R; 5'-TGGGAGTAGACAAGGTACAAC-3'), IL-6 (F: 5'-TCAATTCCAGAAACCGCTATGA-3'; R: 5'-CACCAGCATCAGTCCCAAGA-3'), IL-12 (F: 5'-AACCATCTCCTGGTTTGCCA-3'; R: 5'-CGGGAGTCCAGTCCACCTC-3'), granzyme B (F: 5'-CGATCAAGGATCAGCAGCC-3'; R; 5'-CTGGGTCTTCTCCTGTTCT-3'), perforin (F: 5'-GAAGACCTATCAGGACCAGTACAACTT -3'; R: 5'-CAAGGTGGAGTGGAGGTTTTG -3'), Foxp3 (F: 5'-CACCTATGCCACCCTTATCC-3'; R: 5'-CGAACATGCGAGTAAACCAA-3') or β-actin (F: 5'-AGAGGGAAATCGTGCGTGAC-3'; R: 5'-CAATAGTGATGACCTGGCCGT-3'). The expression of each gene was normalized to the expression of β-actin mRNA using the comparative cycle threshold method (57).
Delayed-type hypersensitivity
BALB/c mice were immunized by s.c. injection at the base of the tail with 10.106 purified wt B6 or DAP12−/− liver mDC. Seven days later, the mice were challenged s.c. in the hind footpad with 10.106 B6 splenocytes. PBS alone was injected into the contralateral hind footpad as a control. Footpad thickness was measured as described (34) at time 0, and at 24 and 48 hr after challenge, using Quick Mini Series 700 digital calipers (Mitutoyo, Kawasaki, Japan).
Liver transplantation and histopathology
Liver harvesting and orthotopic liver transplantation without hepatic artery reconstruction, were performed as described initially by Qian et al (1, 58), with minor modifications. Liver grafts (wt B6 or DAP12−/−) were perfused with University of Wisconsin solution via the portal vein, then transplanted orthotopically into normal C3H recipients by anastomosis of the suprahepatic vena cava with a running 10–0 suture and by anastomosis of the portal vein and inferior vena cava using the cuff technique. The bile duct was connected via ligation over the stent. Liver enzyme (ALT) levels were quantified in serum as described (59). Allograft survival was determined by host survival and rejection assessed histologically. H&E-stained tissue sections were graded in a `blinded' fashion by a transplant pathologist (AJD) using the Banff schema for acute liver rejection (60).
Immunohistochemistry
Immunofluorescence staining for Foxp3+ cells in cryostat sections of liver tissue was performed as described (61).
DC trafficking
CFSE-labeled, purified liver mDC (10.106) were injected s.c. into one hind footpad of normal, allogeneic recipients and CFSE+CD11c+ cells determined in the draining popliteal lymph node (LN), 24 hr later by flow cytometry. Following liver transplantation, donor-derived DC (IAb+CD11c+) were quantified similarly in host spleen cell suspensions.
Statistical analyses
The significances of differences between means were ascertained using the unpaired Student `t' test, two-way ANOVA or log-rank test using Prism version 5.00 (Graphpad Software, San Diego, CA). p<0.05 was considered significant.
Results
DAP12−/− conventional liver mDC exhibit a more mature phenotype, enhanced production of pro-inflammatory cytokines and greater T cell allostimulatory ability
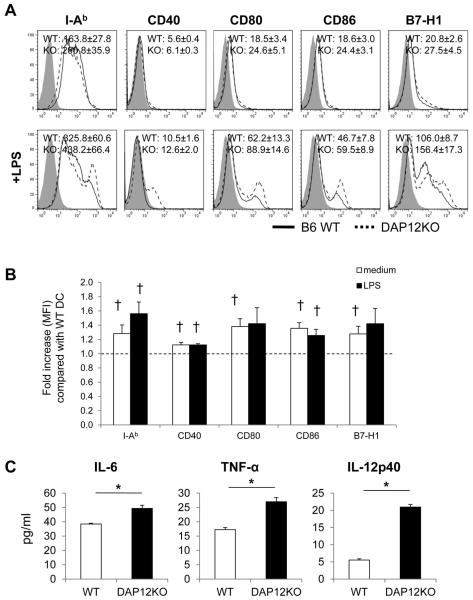
We first examined the cell surface phenotype of conventional liver mDC freshly-isolated from wt B6 and DAP12−/− animals. Previous studies (24, 32, 38, 62, 63) have described in detail, the comparative immaturity and maturation-resistance of normal mouse liver DC compared with those in other parenchymal organs and secondary lymphoid tissues. Using flow cytometry, we observed that, typically for mouse steady-state liver mDC, moderate levels of MHC class II (IAb), but very low levels of costimulatory and co-regulatory molecules (CD40, CD80, CD86, B7-H1) were expressed on unstimulated cells. These levels were increased significantly on both wt and DAP12KO−/− liver mDC after TLR4 ligand (LPS) stimulation (Figure 1A). The expression levels of MHC II, co-stimulatory and co-regulatory molecules were enhanced by DAP12 deficiency, both in the steady-state and following DC activation (Figure 1A & 1B). In addition, secretion of pro-inflammatory cytokines (IL-6, TNFα and especially IL-12p40, that was increased 4-fold) by DAP12−/− liver mDC was enhanced significantly following LPS stimulation compared to wt controls (Figure 1C).
Figure 1. DAP12−/− mouse (C57BL/6; B6) liver myeloid (m) dendritic cells (DC) express elevated levels of cell surface MHC class II (IAb), co-stimulatory and co-regulatory molecules and secrete increased levels of pro-inflammatory cytokines.
(A) flow cytometric analyses of mAb-stained cells wild-type (WT) or DAP12−/− liver mDC cultured overnight in the absence or presence of LPS. Grey profiles indicate isotype controls. Representative data are shown, together with the mean fluorescence intensity (MFI) +/−1SD for each molecule. (B) Fold increase in MFI for each molecule expressed by DAP12−/− compared with WT liver mDC. (C) Concentrations of IL-6, TNFα and IL-12p40 in culture supernatants of unstimulated or LPS-stimulated WT and DAP12−/− liver mDC. Data shown are means +/−1SD obtained from n=4 independent experiments.
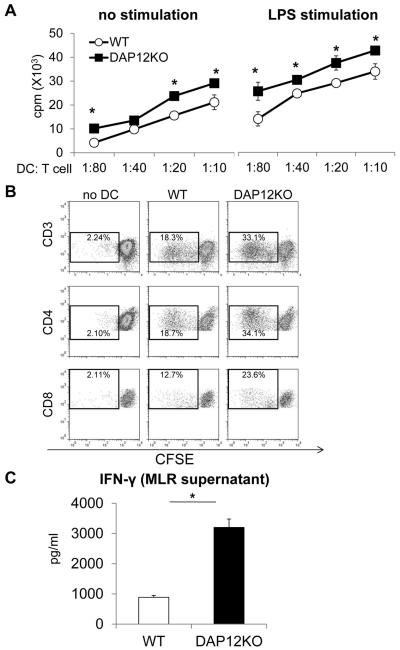
Consistent with their low levels of cell surface co-stimulatory molecules, unstimulated wt liver mDC elicited low levels of allogeneic T cell proliferation in MLR. As anticipated, they displayed greater allostimulatory activity before and after LPS stimulation (Figure 2A & 2B). In addition, DAP12−/− liver mDC enhanced significantly (3-fold) the production of IFNγ by the responder T cell population compared with wt DC (Figure 2C). Taken together, these data confirm and extend our earlier findings using siRNA (42) to silence DAP12 which showed that this transmembrane adaptor protein plays a significant role in negative regulation of mouse liver mDC maturation and T cell stimulatory function.
Figure 2. Enhanced in vitro T cell allostimulatory activity of DAP12−/− compared with WT liver mDC.
Liver mDC were cultured with normal bulk allogeneic BALB/c T cells for 72 hr as described in the Materials and Methods. (A) Extent of T cell proliferation induced by unstimulated or LPS-stimulated DC at various DC:T cell ratios determined by thymidine incorporation. *, p< 0.05. (B) Extent of CD4 and CD8 T cell proliferation induced by WT or DAP12−/− liver mDC at a DC:T cell ratio of 1:10 determined by CFSE-MLR. (C) levels of IFNγ detected in MLR supernatants following T cell stimulation by WT or DAP12−/− liver mDC. *, p<0.01. Data are from n=4 independent experiments.
We also examined the phenotype and function of DAP12−/− liver pDC. As shown in Supplemental Figure 1, although there was no marked change in their surface expression of MHC class II and co-regulatory molecules, DAP12−/− liver pDC secreted increased amounts of IFNα in response to CpG stimulation, and exhibited increased T cell allostimulatory activity compared with wt liver pDC.
DAP12−/− liver mDC exhibit enhanced ability to migrate to host secondary lymphoid tissue and to prime alloreactive T cells in vivo
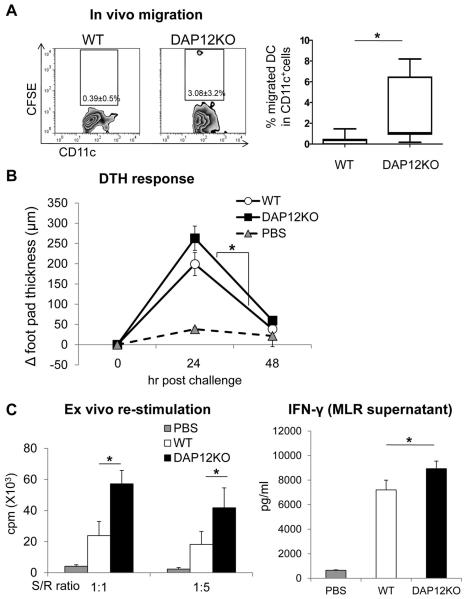
We next examined the impact of DAP12 deficiency on the in vivo migratory and T cell allostimulatory abilities of liver mDC. Previously, migration of allogeneic liver DC from the periphery to host secondary lymphoid tissue has been documented, both in normal wt mice following cell infusion (23, 64) and in recipients of liver allografts that develop donor-specific tolerance (1). Following local (sc) injection of 10.106 CFSE-labeled B6 wt liver mDC into one hind footpad of BALB/c mice, very few cells (< 0.5%) trafficked to the draining popliteal LN within 24hr (Figure 3A). By contrast, significantly greater numbers of injected DAP12−/− liver DC were detected in the draining lymphoid tissue under the same experimental conditions.
Figure 3. Enhanced in vivo migratory ability of DAP12−/− liver mDC to secondary lymphoid tissue of allogeneic recipients and their increased capacity to induce delayed-type hypersensitivity (DTH) responses.
(A) B6 CFSE-labeled WT or DAP12−/− liver mDC (10.106) were injected subcutaneously (sc) into one hind footpad of normal BALB/c recipients. CFSE CD11c+ (donor) DC were enumerated 24hr later in popliteal lymph nodes by flow cytometry. Representative data are shown on the left and means +/−1SD obtained from n=7 separate experiments are shown on the right.*, p < 0.05. (B) Groups of 6 BALB/c mice were sensitized by sc injection at the base of the tail of 10.106 DC and DTH responses elicited 7 days later. Increases in footpad thickness over the ensuing 48 hr are shown. *, p<0.05 (C) Ex vivo proliferative responses and IFNγ production by splenic T cells from mice immunized with WT or DAP12−/− liver mDC. *, p<0.05.
When DAP12−/− compared with wt B6 liver mDC were injected s.c. (base of tail) into fully-allogeneic BALB/c recipients that were challenged 7 days later by local (footpad) injection of donor-strain (B6) splenocytes, significantly increased T cell-mediated DTH responses were observed (Figure 3B), indicating enhanced ability of DAP12−/− liver mDC to prime allogeneic T cells in vivo. Moreover, ex vivo re-stimulation of host T cells with donor APCs revealed markedly enhanced anti-donor T cell proliferative responses (Figure 3C) and significantly increased IFNγ levels in MLR supernatants (Figure 3D) of mice immunized with DAP12−/− liver DC. Thus, DAP12 deficiency augments the ability of liver mDC to migrate to allogeneic host secondary lymphoid tissue, prime allogeneic T cells and elicit anti-donor inflammatory responses in vivo.
DAP12 deficiency in donor livers increases graft inflammation and abrogates transplant tolerance
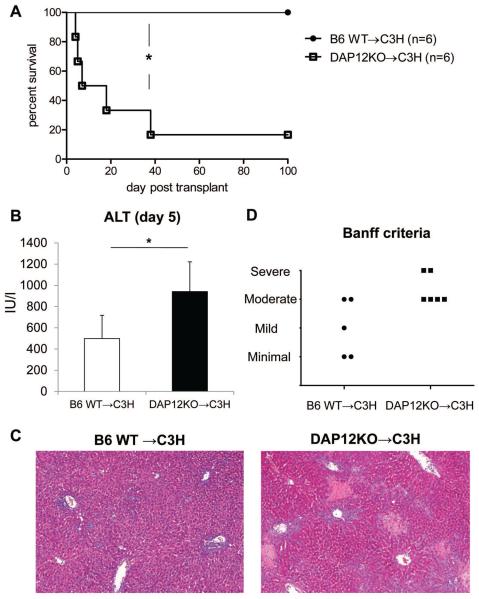
To examine the role of DAP12 deficiency in orthotopic liver transplantation, wt or DAP12−/− B6 (H2b) livers were transplanted into normal C3H (H2k) recipients without immunosuppressive therapy. Whereas in keeping with previous reports (1, 65, 66), wt liver allografts survived indefinitely (MST: > 100 days; n= 6) all DAP12−/− grafts were rejected acutely (MST: 13 days; n=6; p< 0.005) (Figure 4A). To evaluate rejection, we euthanized graft recipients and evaluated liver injury by serum ALT and histological examination using Banff criteria, on day 5 after transplantation. Serum ALT levels were elevated significantly in recipients of DAP12−/− livers compared with those given wt grafts (Figure 4B). Consistent with serum ALT levels, more severe rejection was observed in DAP12−/− allografts compared with wt grafts (Figure 4C & 4D).
Figure 4. DAP12−/− liver allografts are rejected acutely.
(A) Whereas normal WT B6 livers transplanted into C3H recipients were accepted indefinitely (> 100 days), those from DAP12−/− donors were rejected acutely. Actuarial graft survival curve (n= 6 transplants per group). (B) serum ALT levels 5 days post transplant, *, p<0.01. (C) Histopathological appearance of the allografts showing enhanced inflammation and necrosis in DAP12−/− grafts and (D) Banff criteria assessment of rejection.
DAP12 deficiency enhances donor liver mDC migration to host lymphoid tissue
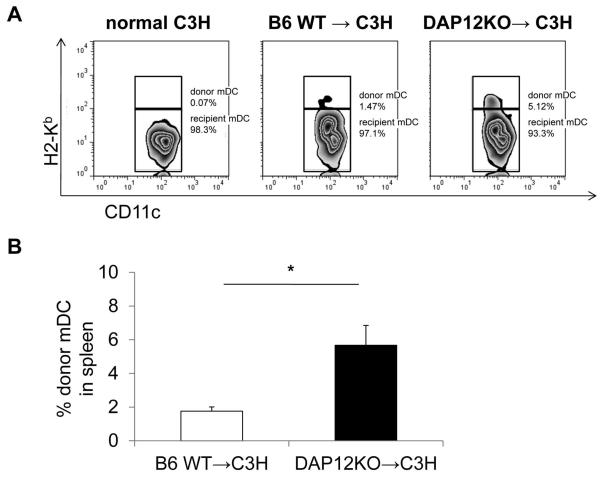
Liver transplantation in mice is associated with the migration of immature donor interstitial DC and their precursors to host lymphoid tissues (32, 64), an event that has been implicated in the induction of liver transplant tolerance (32, 67). There was no influence of DAP12 deficiency on the yield of mDC from BM precursors in vitro (Supplemental Figure 2). To examine the migration of donor liver mDC to spleens of allogeneic recipients, wt or DAP12−/− B6 (H2b) livers were transplanted into normal C3H (H2k) recipients without immunosuppressive therapy. As shown in Figure 5A, 24 hr after wt liver transplantation, a small proportion (<2%; 1.76 ± 0.25%) of spleen CD11c+ cells were of donor origin, whereas a significantly higher (approx 3-fold) incidence (5.67 ± 1.17%; p<0.01) of donor DC was observed in the recipients of DAP12−/− livers (Figure 5B).
Figure 5. Increased numbers of donor mDC migrate from DAP12−/− compared with WT liver allografts.
Livers were transplanted orthotopically from B6 WT or DAP12−/− donors to WT C3H recipients without immunosuppression and the incidences of donor-derived (H2Kb+) CD11c+ DC determined in host spleens 24 hr later by flow cytometry. Representative data (n=4 transplants) are shown in the upper panel (A) and means +/−1SD in the lower panel (B). *, p<0.01.
Rejection of liver allografts from DAP12−/− donors correlates with enhanced anti-donor effector T cell responses and reduced Treg
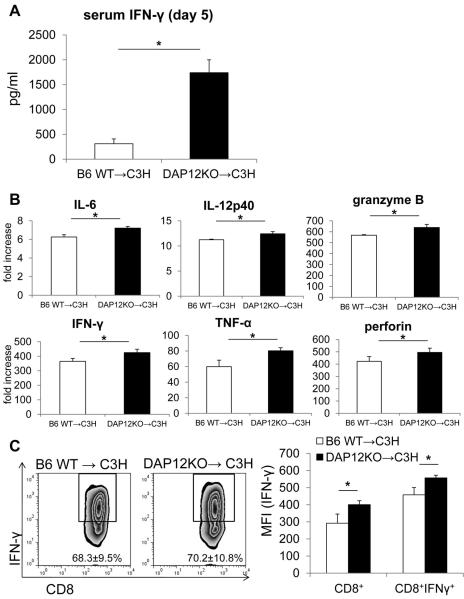
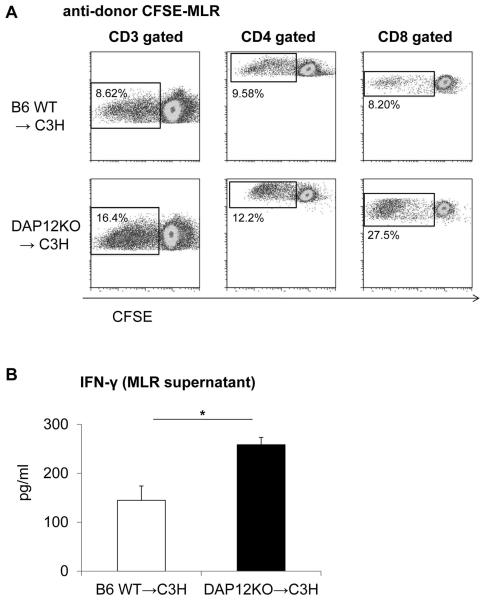
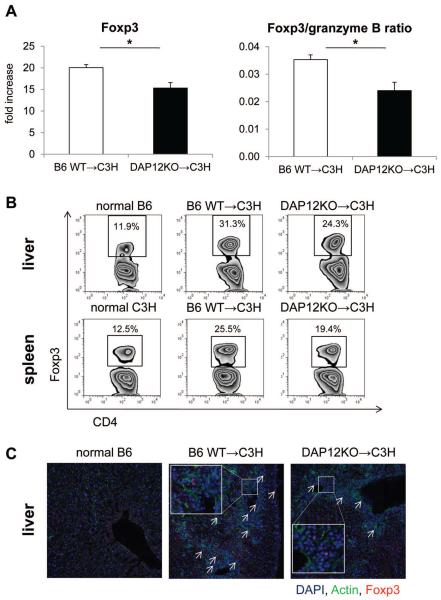
To investigate the mechanistic basis of loss of tolerance/allograft rejection in recipients of DAP12−/− livers, we measured levels of IFNγ and proinflammatory cytokines in serum and allograft tissue. Serum and intragraft levels of IFNγ and expression of proinflammatory cytokines within the graft were significantly higher in DAP12−/− liver recipients (Figure 6A & 6B). Although the incidence of CD8+IFNγ+ T cells in the wt and DAP12−/− grafts were similar, the intensity of IFNγ expression in CD8+/CD8+IFNγ+ T cells was significantly higher in DAP12−/− grafts (Figure 6C). To measure anti-donor immune responses, we harvested spleen cells from recipient mice on day 5 post transplant. Recipient splenocytes were re-stimulated with T cell depleted donor (normal B6) splenocytes for 5 days to evaluate anti-donor T cell proliferation. As shown in Figure 7A, compared with animals given wt livers, those given DAP12−/− grafts displayed significantly enhanced anti-donor CD4+ and CD8+ T cell proliferative responses in CFSE-MLR. Moreover, DAP12−/− liver recipient splenocytes produced significantly higher amounts of IFNγ in response to donor alloAg stimulation in ex vivo MLR (Figure 7B). There is evidence that “spontaneous” mouse liver transplant tolerance is dependent on host CD4+Foxp3+Treg (66). Examination of the incidence of CD4+Foxp3+ Treg, that rose progressively post-transplant (day 5) in both liver grafts and host spleens, revealed a reduction (determined by flow cytometry and immunofluorescence microscopy) in recipients of DAP12−/− compared with wt grafts. This was consistent with the significant reduction in Foxp3 expression in whole liver tissue (Figure 8). Overall, these data indicate that loss of tolerance in recipients of DAP12-deficient liver grafts is associated with enhanced anti-donor proliferative and effector T cell responses and a reduction in intragraft and systemic Treg.
Figure 6. Systemic levels of IFNγ and intra-graft expression of pro-inflammatory cytokines are increased following transplantation of DAP12−/− compared with WT liver allografts.
(A) Serum IFNγ levels 5 days post-transplant in recipients of either B6 WT or DAP12−/− liver grafts. (B) Intragraft pro-inflammatory cytokine, granzyme B and perforin levels on day 5 post-transplant. (C) Expression of IFNγ by graft CD8+ T cells. Results (means +/−1SD) were obtained from groups of 3 transplanted animals. *, p<0.05
Figure 7. Anti-donor T cell responses are enhanced in recipients of DAP12−/− liver allografts.
(A) CD4+ and CD8+ T cell proliferative responses to donor alloAgs determined by CFSE-MLR, 5 days post-transplant. (B) IFNγ production by host splenocytes. Results are means +/−1SD from groups of 3–5 transplanted animals. *, p<0.05.
Figure 8. Foxp3 gene expression in allograft tissue and Foxp3+ T cells in livers and spleens are reduced in recipients of DAP12−/− livers.
(A) Foxp3 expression in allografts determined by RT-PCR. (B) Incidences of Foxp3+CD4+ T cells in recipients of WT or DAP12−/− liver grafts determined by flow cytometry. Data are representative of results obtained from 3 animals in each group. *, p<0.05. (C) Immunofluorescence staining of Foxp3+ (arrowed) cells in normal and allografted livers (5 days post-transplant). Insets show higher power views of Foxp3+ cells.
Discussion
The surgically-demanding mouse orthotopic liver transplant model (1) has provided valuable insights into factors that underlie the development of experimental transplant tolerance to donor alloAgs in the absence of immunosuppressive therapy (65, 66, 68, 69). These observations include findings that implicate donor-derived hematopoietic cells, particularly DC (32, 70) and host Treg (66) in the promotion of tolerance. Few studies however, have identified molecular pathways by which these innate or adaptive immune regulatory cells may contribute to liver transplant tolerance.
DAP12, the molecule central to this study, is a highly-conserved, ITAM-bearing signaling immuno-adaptor protein expressed mainly by APCs, such as DC and macrophages, in association with several receptors (47, 49, 50, 71). These receptors include triggering receptor expressed by myeloid cells 1 and 2 (TREM-1, -2), that belongs to a family of activating and inhibitory isoforms encoded by a gene cluster linked to the MHC locus (52). DAP12-associated receptors recognize both host-encoded ligands and those encoded by microbial pathogens, and it seems likely that DAP12 has been preserved to provide important functions for the innate and adaptive immune systems (47). Indeed, DAP12 appears to be critical for the function of mononuclear phagocytes (72).
In the present study, we found that, compared with normal mouse liver mDC, that are weak stimulators of allogeneic T cells and resistant to maturation (23, 24, 38, 73), DAP12−/− liver mDC secreted elevated levels of pro-inflammatory cytokines (IL-6; TNFα) following TLR ligation. This is consistent with evidence that macrophages from DAP12-deficient mice are hyper-responsive, exhibit enhanced phagocytic capacity (50) and produce elevated levels of TNFα, IL-12, and IL-6 when stimulated with TLR ligands (54, 74). In addition, we (42) and others (75) have shown that lack of DAP12 reduces APC IL-10 production and that blockade of IL-10 prevents the ability of DAP12-competent APCs to suppress Th1 cell activation (75). We also observed in this study that DAP12−/− liver mDC expressed enhanced levels of cell surface co-stimulatory molecules and IL-12/IL-23p40 secretion, together with increased allogeneic T cell stimulatory ability, both in vitro and in vivo. These findings are consistent with and extend our previous observations using siRNA to inhibit DAP12 expression in liver mDC (42). They are also in keeping with the enhanced migration we observed in the current study of freshly-isolated DAP12−/− liver mDC or of mDC from DAP12−/− liver grafts to secondary lymphoid tissue of allogeneic hosts. The more mature phenotype of DAP12-incompetent cells is also consistent with the ability of these DAP12−/− cells to induce increased cell-mediated immunity (DTH responses) to donor alloAgs.
The acute rejection, in the absence of DAP12 on donor cells, of MHC mis-matched liver allografts (that are normally accepted without immunosuppressive therapy), is consistent with the enhanced migration that we observed of donor mDC able to induce augmented host anti-donor Th1 cell responses and both local and systemic IFNγ production. Our findings are also consistent with evidence of enhanced immunity in DAP12-deficient mice that control Listeria monocytogenes, Mycobacterium bovis and Mycobacterium tuberculosis infection better than wild-type mice (54, 74). In a recent study, Jeyanathan et al (75) found that lack of DAP12 reduced APC IL-10 production, and increased their Th1 cell-activating ability, resulting in enhanced protection of mice against M. tuberculosis infection. Thus, DAP12 has been identified as an important, novel immune regulatory molecule, that acts via APCs to control the level of antimicrobial type-1 T cell activation and immunopathology (54). We cannot, however, ascribe the loss of liver transplant tolerance solely to absence of DAP12 on donor-derived mDC. Other innate immune cells in DAP12−/− liver grafts could also contribute to/play an important role in the loss of tolerance. These could include liver macrophages (Kupffer cells), NK cells and other DC subsets. Thus, DAP12 has been implicated in regulation of mouse pDC function (48, 76) and we show that DAP12−/− liver pDC have enhanced T cell allostimulatory activity. Loss of DAP12 signaling in donor liver pDCs could, therefore, conceivably contribute to loss of allograft tolerance. Direct demonstration that the absence of DAP12 solely in donor liver mDC is responsible for the switch from liver transplant tolerance to acute rejection would require transplantation of chimeric liver allografts in which only the mDC in the donor hematopoietic cell population were either DAP12−/− or wt control.
The present findings suggest a regulatory of DAP12 in liver DC maturation that may be mediated via inhibitory co-receptors. DAP12 associates with several activating and inhibitory receptors on innate immune cells. However, the role of these DAP12-associated receptors in regulation of immunity and in transplantation has yet to be elucidated. It has been reported recently that TREM-1 inhibition leads to reduced differentiation and proliferation of IFNγ-producing Th1 cells and prolongation of heart allograft survival (77). By contrast, blockade of TREM-2 exacerbates experimental autoimmune encephalitis (78). Thus, both TREM-1 and TREM-2 appear to play important roles in the control of T cell-mediated inflammatory responses. Further studies are required to ascertain the functional inter-relationships between the function of these co-receptors and the expression of DAP12.
Studies by Hall et al (79), using a mouse model of type-1 diabetes, have suggested that signaling through a DAP12-associated receptor on APC could facilitate activation of Treg in pancreatic lymph nodes and thereby contribute to the maintenance of peripheral tolerance to pancreatic β cell-derived Ags. In the present study, we could demonstrate modest reductions in the incidence of Treg in DAP12−/− liver allografts and host spleens post-transplant. Thus, it appears that DAP12 expression may not play a major role in the control of Treg responses during the induction of mouse liver transplant tolerance. Nevertheless, there is evidence that recipient Foxp3+CD25+CD4+ Treg may be necessary for `spontaneous' acceptance of mouse liver allografts via mechanisms that involve cytotoxic lymphocyte Ag-4 (CTLA4) and IL-4 signaling and apoptosis of graft-infiltrating T cells (66). Acute rejection of mouse liver allografts (that is dependent on interventional strategies to precipitate rejection) has been ascribed to Th1/Th17 polarization and anti-donor CD8+ CTL activities (58, 65, 80, 81). In the present study, rejection of allografts lacking DAP12 was associated with enhanced anti-donor effector CD8+ T cell responses, consistent with previous reports implicating these cells in the rejection process.
Recently, based on the use of blocking mAb or KO mice, it has been suggested that upregulated expression of the co-regulatory molecule B7-H1 on non-parenchymal cells of the allograft tissue may contribute to mouse liver transplant tolerance by promoting the apoptosis of graft-infiltrating T cells (65). Conceivably, DAP12 and B7-H1 may act in concert/sequentially to down-modulate the early induction (DC-induced) and effector phases (T cell-mediated) respectively, of the anti-donor T cell response. Overall, our data suggest that the novel immune regulatory molecule DAP12 plays a key role in the induction of mouse liver transplant tolerance and that “spontaneous” liver allograft acceptance is dependent on donor cell expression of DAP12, that can negatively regulate the allostimulatory function of donor-derived liver mDC.
Supplementary Material
Figure S1: DAP12−/− liver pDC secrete more IFN-α and induce greater allogeneic T cell proliferation. (A) Flow cytometric analyses of the surface phenotype of B6 WT or DAP12−/− liver pDC cultured overnight in the absence or presence of CpG. Grey profiles indicate isotype controls. (B) IFN-α secretion by B6 WT or DAP12−/− liver pDC with/without overnight CpG stimulation,- determined by ELISA. CM: culture medium. *, p<0.01. (C) Extent of CD3, CD4 and CD8 allogeneic (BALB/c) T cell proliferation induced by B6 WT or DAP12−/− liver pDC at a DC:T cell ratio of 1:5 in 5-day culture determined by CFSE-MLR. Data are representative of 2 independent experiments.
Figure S2: Yield of mDC from BM of DAP12−/− mice is unaffected. DC were generated from B6 WT or DAP12−/− BM using r mouse GM-CSF and IL-4. DC numbers were counted at days 3, 5, 7 and 10. Data are from n=5 independent experiments.
Acknowledgements
The work was supported by National Institutes of Health grant P01AI81678 (AWT). OY was supported by an American Society of Transplantation Basic Science Fellowship and by a non-concurrent NIH T32 AI74490 post-doctoral fellowship (AWT). BMM was supported by NIH T32 AI89443 (P.A. Morel). We thank Dr. A. Jake Demetris for expert assessment of graft histology, Dr. Tina L. Sumpter for valuable discussion, Mark Ross for performing immunofluorescence staining and Miriam Freeman for excellent administrative support.
Abbreviations
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- DAP12
DNAX-activating protein of 12kDa
- DC(s)
dendritic cell(s)
- DTH
delayed-type hypersensitivity
- Treg
regulatory T cells(s)
Footnotes
Disclosure The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supplemental Information Additional Supporting Information is available in the online version of this article.
References
- 1.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19(4):916–924. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamada N. The immunology of experimental liver transplantation in the rat. Immunology. 1985;55(3):369–389. [PMC free article] [PubMed] [Google Scholar]
- 3.Sun J, McCaughan GW, Gallagher ND, Sheil AG, Bishop GA. Deletion of spontaneous rat liver allograft acceptance by donor irradiation. Transplantation. 1995;60(3):233–236. doi: 10.1097/00007890-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223(205):472–476. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 5.Lerut J, Sanchez-Fueyo A. An appraisal of tolerance in liver transplantation. Am J Transplant. 2006;6(8):1774–1780. doi: 10.1111/j.1600-6143.2006.01396.x. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Fueyo A, Strom TB. Immunologic basis of graft rejection and tolerance following transplantation of liver or other solid organs. Gastroenterology. 2011;140(1):51–64. doi: 10.1053/j.gastro.2010.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benseler V, McCaughan GW, Schlitt HJ, Bishop GA, Bowen DG, Bertolino P. The liver: a special case in transplantation tolerance. Semin Liver Dis. 2007;27(2):194–213. doi: 10.1055/s-2007-979471. [DOI] [PubMed] [Google Scholar]
- 8.Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101–118. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 9.Crispe IN. Liver antigen-presenting cells. J Hepatol. 2011;54(2):357–365. doi: 10.1016/j.jhep.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10(11):753–766. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 11.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 12.Sumpter TL, Abe M, Tokita D, Thomson AW. Dendritic cells, the liver, and transplantation. Hepatology. 2007;46(6):2021–2031. doi: 10.1002/hep.21974. [DOI] [PubMed] [Google Scholar]
- 13.You Q, Cheng L, Kedl RM, Ju C. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology. 2008;48(3):978–990. doi: 10.1002/hep.22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schurich A, Berg M, Stabenow D, Bottcher J, Kern M, Schild HJ, et al. Dynamic regulation of CD8 T cell tolerance induction by liver sinusoidal endothelial cells. J Immunol. 2010;184(8):4107–4114. doi: 10.4049/jimmunol.0902580. [DOI] [PubMed] [Google Scholar]
- 15.Limmer A, Ohl J, Wingender G, Berg M, Jungerkes F, Schumak B, et al. Cross-presentation of oral antigens by liver sinusoidal endothelial cells leads to CD8 T cell tolerance. Eur J Immunol. 2005;35(10):2970–2981. doi: 10.1002/eji.200526034. [DOI] [PubMed] [Google Scholar]
- 16.Dangi A, Sumpter TL, Kimura S, Stolz DB, Murase N, Raimondi G, et al. Selective expansion of allogeneic regulatory T cells by hepatic stellate cells: role of endotoxin and implications for allograft tolerance. J Immunol. 2012;188(8):3667–3677. doi: 10.4049/jimmunol.1102460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumpter TL, Dangi A, Matta BM, Huang C, Stolz DB, Vodovotz Y, et al. Hepatic stellate cells undermine the allostimulatory function of liver myeloid dendritic cells via STAT3-dependent induction of IDO. J Immunol. 2012;189(8):3848–3858. doi: 10.4049/jimmunol.1200819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burghardt S, Erhardt A, Claass B, Huber S, Adler G, Jacobs T, et al. Hepatocytes contribute to immune regulation in the liver by activation of the notch signaling pathway in T cells. J Immunol. 2013;191(11):5574–5582. doi: 10.4049/jimmunol.1300826. [DOI] [PubMed] [Google Scholar]
- 19.Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, et al. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206(3):549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 21.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7(8):610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 22.Castellaneta A, Sumpter TL, Chen L, Tokita D, Thomson AW. NOD2 ligation subverts IFN-alpha production by liver plasmacytoid dendritic cells and inhibits their T cell allostimulatory activity via B7-H1 up-regulation. J Immunol. 2009;183(11):6922–6932. doi: 10.4049/jimmunol.0900582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu L, Woo J, Rao AS, Li Y, Watkins SC, Qian S, et al. Propagation of dendritic cell progenitors from normal mouse liver using granulocyte/macrophage colony-stimulating factor and their maturational development in the presence of type-1 collagen. J Exp Med. 1994;179(6):1823–1834. doi: 10.1084/jem.179.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Creus A, Abe M, Lau AH, Hackstein H, Raimondi G, Thomson AW. Low TLR4 expression by liver dendritic cells correlates with reduced capacity to activate allogeneic T cells in response to endotoxin. J Immunol. 2005;174(4):2037–2045. doi: 10.4049/jimmunol.174.4.2037. [DOI] [PubMed] [Google Scholar]
- 25.Xia S, Guo Z, Xu X, Yi H, Wang Q, Cao X. Hepatic microenvironment programs hematopoietic progenitor differentiation into regulatory dendritic cells, maintaining liver tolerance. Blood. 2008;112(8):3175–3185. doi: 10.1182/blood-2008-05-159921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29(3):464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang M, Ueki S, Kimura S, Yoshida O, Castellaneta A, Ozaki KS, et al. Roles of dendritic cells in murine hepatic warm and liver transplantation-induced cold ischemia/reperfusion injury. Hepatology. 2013;57(4):1585–1596. doi: 10.1002/hep.26129. [DOI] [PubMed] [Google Scholar]
- 28.Henning JR, Graffeo CS, Rehman A, Fallon NC, Zambirinis CP, Ochi A, et al. Dendritic cells limit fibroinflammatory injury in nonalcoholic steatohepatitis in mice. Hepatology. 2013;58(2):589–602. doi: 10.1002/hep.26267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiao J, Sastre D, Fiel MI, Lee UE, Ghiassi-Nejad Z, Ginhoux F, et al. Dendritic cell regulation of carbon tetrachloride-induced murine liver fibrosis regression. Hepatology. 2012;55(1):244–255. doi: 10.1002/hep.24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bamboat ZM, Ocuin LM, Balachandran VP, Obaid H, Plitas G, Dematteo RP. Conventional DCs reduce liver ischemia/reperfusion injury in mice via IL-10 secretion. J Clin Invest. 2010;120:559–569. doi: 10.1172/JCI40008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshida O, Kimura S, Jackson EK, Robson SC, Geller DA, Murase N, et al. CD39 expression by hepatic myeloid dendritic cells attenuates inflammation in liver transplant ischemia-reperfusion injury in mice. Hepatology. 2013;58(6):2163–2175. doi: 10.1002/hep.26593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu L, Rudert WA, Qian S, McCaslin D, Fu F, Rao AS, et al. Growth of donor-derived dendritic cells from the bone marrow of murine liver allograft recipients in response to granulocyte/macrophage colony stimulating factor. J Exp Med. 1995;182:379–387. doi: 10.1084/jem.182.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khanna A, Morelli AE, Zhong C, Takayama T, Lu L, Thomson AW. Effects of liver-derived dendritic cell progenitors on Th1- and Th2-like cytokine responses in vitro and in vivo. J Immunol. 2000;164(3):1346–1354. doi: 10.4049/jimmunol.164.3.1346. [DOI] [PubMed] [Google Scholar]
- 34.Matta BM, Raimondi G, Rosborough BR, Sumpter TL, Thomson AW. IL-27 production and STAT3-dependent upregulation of B7-H1 mediate immune regulatory functions of liver plasmacytoid dendritic cells. J Immunol. 2012;188(11):5227–5237. doi: 10.4049/jimmunol.1103382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rastellini C, Lu L, Ricordi C, Starzl TE, Rao AS, Thomson AW. Granulocyte/macrophage colony-stimulating factor-stimulated hepatic dendritic cell progenitors prolong pancreatic islet allograft survival. Transplantation. 1995;60(11):1366–1370. [PMC free article] [PubMed] [Google Scholar]
- 36.Diehl L, Schurich A, Grochtmann R, Hegenbarth S, Chen L, Knolle PA. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology. 2008;47(1):296–305. doi: 10.1002/hep.21965. [DOI] [PubMed] [Google Scholar]
- 37.Banshodani M, Onoe T, Shishida M, Tahara H, Hashimoto S, Igarashi Y, et al. Adoptive transfer of allogeneic liver sinusoidal endothelial cells specifically inhibits T-cell responses to cognate stimuli. Cell Transplant. 2013;22(9):1695–1708. doi: 10.3727/096368912X657738. [DOI] [PubMed] [Google Scholar]
- 38.Bamboat ZM, Stableford JA, Plitas G, Burt BM, Nguyen HM, Welles AP, et al. Human liver dendritic cells promote T cell hyporesponsiveness. J Immunol. 2009;182(4):1901–1911. doi: 10.4049/jimmunol.0803404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lian ZR, Xu YF, Wang XB, Gong JP, Liu ZJ. Suppression of histone deacetylase 11 promotes expression of IL-10 in Kupffer cells and induces tolerance following orthotopic liver transplantation in rats. J Surg Res. 2012;174(2):359–368. doi: 10.1016/j.jss.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Liu Z, Liang S, Luan X, Long F, Chen J, et al. Role of Kupffer cells in the induction of tolerance of orthotopic liver transplantation in rats. Liver Transpl. 2008;14(6):823–836. doi: 10.1002/lt.21450. [DOI] [PubMed] [Google Scholar]
- 41.Tokita D, Shishida M, Ohdan H, Onoe T, Hara H, Tanaka Y, et al. Liver sinusoidal endothelial cells that endocytose allogeneic cells suppress T cells with indirect allospecificity. J Immunol. 2006;177(6):3615–3624. doi: 10.4049/jimmunol.177.6.3615. [DOI] [PubMed] [Google Scholar]
- 42.Sumpter TL, Packiam V, Turnquist HR, Castellaneta A, Yoshida O, Thomson AW. DAP12 promotes IRAK-M expression and IL-10 production by liver myeloid dendritic cells and restrains their T cell allostimulatory ability. J Immunol. 2011;186(4):1970–1980. doi: 10.4049/jimmunol.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aoki N, Kimura S, Takiyama Y, Atsuta Y, Abe A, Sato K, et al. The role of the DAP12 signal in mouse myeloid differentiation. J Immunol. 2000;165(7):3790–3796. doi: 10.4049/jimmunol.165.7.3790. [DOI] [PubMed] [Google Scholar]
- 44.Yim D, Jie HB, Lanier LL, Kim YB. Molecular cloning, gene structure, and expression pattern of pig immunoreceptor DAP12. Immunogenetics. 2000;51(6):436–442. doi: 10.1007/s002510050642. [DOI] [PubMed] [Google Scholar]
- 45.Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391(6668):703–707. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 46.Tomasello E, Vivier E. KARAP/DAP12/TYROBP: three names and a multiplicity of biological functions. Eur J Immunol. 2005;35(6):1670–1677. doi: 10.1002/eji.200425932. [DOI] [PubMed] [Google Scholar]
- 47.Lanier LL. DAP10- and DAP12-associated receptors in innate immunity. Immunol Rev. 2009;227(1):150–160. doi: 10.1111/j.1600-065X.2008.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sjolin H, Robbins SH, Bessou G, Hidmark A, Tomasello E, Johansson M, et al. DAP12 signaling regulates plasmacytoid dendritic cell homeostasis and down-modulates their function during viral infection. J Immunol. 2006;177(5):2908–2916. doi: 10.4049/jimmunol.177.5.2908. [DOI] [PubMed] [Google Scholar]
- 49.Turnbull IR, Colonna M. Activating and inhibitory functions of DAP12. Nat Rev Immunol. 2007;7(2):155–161. doi: 10.1038/nri2014. [DOI] [PubMed] [Google Scholar]
- 50.Hamerman JA, Ni M, Killebrew JR, Chu CL, Lowell CA. The expanding roles of ITAM adapters FcRgamma and DAP12 in myeloid cells. Immunol Rev. 2009;232(1):42–58. doi: 10.1111/j.1600-065X.2009.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tessarz AS, Cerwenka A. The TREM-1/DAP12 pathway. Immunol Lett. 2008;116(2):111–116. doi: 10.1016/j.imlet.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 52.Gorycka A, Jurkowska M. Structure, expression pattern and biological activity of molecular complex TREM-2/DAP12. Hum Immunol. 2013;74(6):730–737. doi: 10.1016/j.humimm.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 53.Chu CL, Yu YL, Shen KY, Lowell CA, Lanier LL, Hamerman JA. Increased TLR responses in dendritic cells lacking the ITAM-containing adapters DAP12 and FcRgamma. Eur J Immunol. 2008;38(1):166–173. doi: 10.1002/eji.200737600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Divangahi M, Yang T, Kugathasan K, McCormick S, Takenaka S, Gaschler G, et al. Critical negative regulation of type 1 T cell immunity and immunopathology by signaling adaptor DAP12 during intracellular infection. J Immunol. 2007;179(6):4015–4026. doi: 10.4049/jimmunol.179.6.4015. [DOI] [PubMed] [Google Scholar]
- 55.Kaifu T, Nakahara J, Inui M, Mishima K, Momiyama T, Kaji M, et al. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J Clin Invest. 2003;111(3):323–332. doi: 10.1172/JCI16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76(3):519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 57.Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem. 2000;285(2):194–204. doi: 10.1006/abio.2000.4753. [DOI] [PubMed] [Google Scholar]
- 58.Qian S, Lu L, Fu F, Li W, Pan F, Steptoe RJ, et al. Donor pretreatment with Flt-3 ligand augments antidonor cytotoxic T lymphocyte, natural killer, and lymphokine-activated killer cell activities within liver allografts and alters the pattern of intragraft apoptotic activity. Transplantation. 1998;65(12):1590–1598. doi: 10.1097/00007890-199806270-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ueki S, Castellaneta A, Yoshida O, Ozaki K, Zhang M, Kimura S, et al. Hepatic B7 homolog 1 expression is essential for controlling cold ischemia/reperfusion injury after mouse liver transplantation. Hepatology. 2011;54(1):216–228. doi: 10.1002/hep.24360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Demetris A, Batts KP, Dhillon AP, Ferrell L, Fung JJ, Geller DA, et al. Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25(3):658–663. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 61.Turnquist H, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol. 2007;178(11):7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 62.Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172(2):1009–1017. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- 63.Lunz JG, 3rd, Specht SM, Murase N, Isse K, Demetris AJ. Gut-derived commensal bacterial products inhibit liver dendritic cell maturation by stimulating hepatic interleukin-6/signal transducer and activator of transcription 3 activity. Hepatology. 2007;46(6):1946–1959. doi: 10.1002/hep.21906. [DOI] [PubMed] [Google Scholar]
- 64.Thomson AW, Lu L, Subbotin VM, Li Y, Qian S, Rao AS, et al. In vitro propagation and homing of liver-derived dendritic cell progenitors to lymphoid tissues of allogeneic recipients. Implications for the establishment and maintenance of donor cell chimerism following liver transplantation. Transplantation. 1995;59(4):544–551. doi: 10.1097/00007890-199502270-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morita M, Fujino M, Jiang G, Kitazawa Y, Xie L, Azuma M, et al. PD-1/B7-H1 interaction contribute to the spontaneous acceptance of mouse liver allograft. Am J Transplant. 2010;10(1):40–46. doi: 10.1111/j.1600-6143.2009.02859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li W, Kuhr CS, Zheng XX, Carper K, Thomson AW, Reyes JD, et al. New insights into mechanisms of spontaneous liver transplant tolerance: the role of Foxp3-expressing CD25+CD4+ regulatory T cells. Am J Transplant. 2008;8(8):1639–1651. doi: 10.1111/j.1600-6143.2008.02300.x. [DOI] [PubMed] [Google Scholar]
- 67.Thomson AW, Lu L. Are dendritic cells the key to liver transplant tolerance? Immunol Today. 1999;20(1):27–32. doi: 10.1016/s0167-5699(98)01378-4. [DOI] [PubMed] [Google Scholar]
- 68.Qian S, Lu L, Fu F, Li Y, Li W, Starzl TE, et al. Apoptosis within spontaneously accepted mouse liver allografts: evidence for deletion of cytotoxic T cells and implications for tolerance induction. J Immunol. 1997;158(10):4654–4661. [PMC free article] [PubMed] [Google Scholar]
- 69.Li W, Lu L, Wang Z, Wang L, Fung JJ, Thomson AW, et al. IL-12 antagonism enhances apoptotic death of T cells within hepatic allografts from Flt3 ligand-treated donors and promotes graft acceptance. J Immunol. 2001;166(9):5619–5628. doi: 10.4049/jimmunol.166.9.5619. [DOI] [PubMed] [Google Scholar]
- 70.Starzl TE, Murase N, Thomson A, Demetris AJ. Liver transplants contribute to their own success [comment] Nat Med. 1996;2(2):163–165. doi: 10.1038/nm0296-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Underhill DM, Goodridge HS. The many faces of ITAMs. Trends Immunol. 2007;28(2):66–73. doi: 10.1016/j.it.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 72.Otero K, Turnbull IR, Poliani PL, Vermi W, Cerutti E, Aoshi T, et al. Macrophage colony-stimulating factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and beta-catenin. Nat Immunol. 2009;10(7):734–743. doi: 10.1038/ni.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khanna KV, Yu XF, Ford DH, Ratner L, Hildreth JK, Markham RB. Differences among HIV-1 variants in their ability to elicit secretion of TNF-alpha. J Immunol. 2000;164(3):1408–1415. doi: 10.4049/jimmunol.164.3.1408. [DOI] [PubMed] [Google Scholar]
- 74.Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat Immunol. 2005;6(6):579–586. doi: 10.1038/ni1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jeyanathan M, Damjanovic D, Shaler CR, Lai R, Wortzman M, Yin C, et al. Differentially imprinted innate immunity by mucosal boost vaccination determines antituberculosis immune protective outcomes, independent of T-cell immunity. Mucosal immunology. 2013;6(3):612–625. doi: 10.1038/mi.2012.103. [DOI] [PubMed] [Google Scholar]
- 76.Blasius AL, Cella M, Maldonado J, Takai T, Colonna M. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood. 2006;107(6):2474–2476. doi: 10.1182/blood-2005-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schiechl G, Brunner SM, Kesselring R, Martin M, Ruemmele P, Mack M, et al. Inhibition of innate co-receptor TREM-1 signaling reduces CD4(+) T cell activation and prolongs cardiac allograft survival. Am J Transplant. 2013;13(5):1168–1180. doi: 10.1111/ajt.12186. [DOI] [PubMed] [Google Scholar]
- 78.Piccio L, Buonsanti C, Mariani M, Cella M, Gilfillan S, Cross AH, et al. Blockade of TREM-2 exacerbates experimental autoimmune encephalomyelitis. Eur J Immunol. 2007;37(5):1290–1301. doi: 10.1002/eji.200636837. [DOI] [PubMed] [Google Scholar]
- 79.Hall HT, Sjolin H, Brauner H, Tomasello E, Dalod M, Vivier E, et al. Increased diabetes development and decreased function of CD4+CD25+ Treg in the absence of a functional DAP12 adaptor protein. Eur J Immunol. 2008;38(11):3191–3199. doi: 10.1002/eji.200838259. [DOI] [PubMed] [Google Scholar]
- 80.Li W, Zheng XX, Kuhr CS, Perkins JD. CTLA4 engagement is required for induction of murine liver transplant spontaneous tolerance. Am J Transplant. 2005;5(5):978–986. doi: 10.1111/j.1600-6143.2005.00823.x. [DOI] [PubMed] [Google Scholar]
- 81.Ye Y, Yan S, Jiang G, Zhou L, Xie H, Xie X, et al. Galectin-1 prolongs survival of mouse liver allografts from Flt3L-pretreated donors. Am J Transplant. 2013;13(3):569–579. doi: 10.1111/ajt.12088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: DAP12−/− liver pDC secrete more IFN-α and induce greater allogeneic T cell proliferation. (A) Flow cytometric analyses of the surface phenotype of B6 WT or DAP12−/− liver pDC cultured overnight in the absence or presence of CpG. Grey profiles indicate isotype controls. (B) IFN-α secretion by B6 WT or DAP12−/− liver pDC with/without overnight CpG stimulation,- determined by ELISA. CM: culture medium. *, p<0.01. (C) Extent of CD3, CD4 and CD8 allogeneic (BALB/c) T cell proliferation induced by B6 WT or DAP12−/− liver pDC at a DC:T cell ratio of 1:5 in 5-day culture determined by CFSE-MLR. Data are representative of 2 independent experiments.
Figure S2: Yield of mDC from BM of DAP12−/− mice is unaffected. DC were generated from B6 WT or DAP12−/− BM using r mouse GM-CSF and IL-4. DC numbers were counted at days 3, 5, 7 and 10. Data are from n=5 independent experiments.