Abstract
Signaling via the major excitatory amino acid glutamate has been implicated in the regulation of various aspects of the biology of oligodendrocytes, the myelinating cells of the CNS. In this respect, cells of the oligodendrocyte lineage have been described to express a variety of glutamate-responsive transmembrane proteins including sodium-dependent glutamate transporters. The latter have been well-characterized to mediate glutamate clearance from the extracellular space. However, there is increasing evidence that they also mediate glutamate-induced intracellular signaling events. Our data presented here show that activation of oligodendrocyte expressed sodium-dependent glutamate transporters, in particular GLT-1 and GLAST, promotes the morphological aspects of oligodendrocyte maturation. This effect was found to be associated with a transient increase in intracellular calcium levels and a transient phosphorylation event at the serine (S)371 site of the calcium sensor calcium/calmodulin-dependent kinase IIβ (CaMKIIβ). The potential regulatory S371 site is located within CaMKIIβ’s previously defined actin binding/stabilizing domain, and phosphorylation events within this domain were identified in our studies as a requirement for sodium-dependent glutamate transporter-mediated promotion of oligodendrocyte maturation. Furthermore, our data provide good evidence for a role of these phosphorylation events in mediating detachment of CaMKIIβ from filamentous (F)-actin, thereby allowing a remodeling of the oligodendrocyte’s actin cytoskeleton. Taken together with our recent findings, which demonstrated a crucial role of CaMKIIβ in regulating CNS myelination in vivo, our data strongly suggest that a sodium-dependent glutamate transporter-CaMKIIβ-actin cytoskeleton axis plays an important role in the regulation of oligodendrocyte maturation and CNS myelination.
Keywords: myelin, calcium signaling, differentiation, central nervous system, actin cytoskeleton
INTRODUCTION
Glutamate, the major excitatory amino acid, mediates a wide variety of cellular responses in the developing and adult central nervous system (CNS) not only by affecting signal transduction in neurons but also by regulating glia cells, including the myelinating cells of the CNS, oligodendrocytes (Kolodziejczyk et al. 2010). Interestingly, the primary mode of glutamate release affecting differentiating cells of the oligodendrocyte lineage during development and under physiological conditions appears to be vesicle exocytosis along unmyelinated axons (Kukley et al. 2007; Ziskin et al. 2007), even though release from electrically active axons by reversal of glutamate uptake has also been proposed (Kriegler and Chiu 1993). The prominent release of glutamate from unmyelinated axons raises the possibility that glutamate, in addition to mediating signaling events at synaptic junctions between axons and NG2-positive progenitor cells (De Biase et al. 2011; Etxeberria et al. 2010; Kukley et al. 2010), may be able to trigger cellular responses in differentiating oligodendrocytes that are involved in the regulation of oligodendrocyte maturation and CNS myelination.
Cells of the oligodendrocyte lineage have been described to express members of all of the three major glutamate-responsive transmembrane protein families (Kolodziejczyk et al. 2010; Matute 2011). Out of these, metabotropic glutamate receptors have been found downregulated as oligodendrocytes differentiate (Deng et al. 2004; Luyt et al. 2006). Ionotropic glutamate receptors have primarily been associated with excitotoxicity (Matute 2011) but they have also been implicated in the regulation of myelination (Lundgaard et al. 2013) and the preservation of neuronal integrity (Fruhbeis et al. 2013). Nevertheless, conditional deletion of the key receptor subunit of the NMDA subtype of iontropic glutamate receptors in cells of the oligodendrocyte lineage was not found to lead to an apparent phenotype (De Biase et al. 2011; Guo et al. 2012). Thus, sodium-dependent glutamate transporters emerge as good candidates for mediating glutamate-evoked responses related to the maturation of differentiating oligodendrocytes. Of the known five mammalian sodium-dependent glutamate transporters, also known as excitatory amino acid transporters (EAAT) or members of the solute carrier family 1 (SLC1), three have been found expressed by cells of the oligodendrocyte lineage, namely GLAST (EAAT1, SLC1A3), GLT-1 (EAAT2, SLC1A2) and EAAC1 (EAAT3, SLC1A1) (Arranz et al. 2008; DeSilva et al. 2009; Domercq and Matute 1999; Kukley et al. 2010; Regan et al. 2007). While these transporters have been well-characterized to remove glutamate from the extracellular environment (Beart and O’Shea 2007; Danbolt 2001), increasing evidence suggests that they play functional roles beyond extracellular glutamate clearance (Flores-Mendez et al. 2013; Lopez-Colome et al. 2012; Martinez-Lozada et al. 2011).
Interestingly, signaling initiated by the activation of sodium-dependent glutamate transporters has been proposed to activate calcium/calmodulin-dependent kinase type II (CaMKII) (Flores-Mendez et al. 2013; Martinez-Lozada et al. 2011), and one of the four CaMKII genes, namely CaMKIIβ, has recently been implicated in the regulation of oligodendrocyte maturation and CNS myelination (Waggener et al. 2013). More specifically, CaMKIIβ was implicated in promoting the morphological aspects of oligodendrocyte maturation, which are to a large extent regulated by changes in the cellular cytoskeleton (Bauer et al. 2009), primarily via its actin binding/stabilizing domain rather than its well-known kinase catalytic domain. Thus, and based on the above observations, we investigated here a possible role of a sodium-dependent glutamate transporter-CaMKIIβ-actin cytoskeleton axis in the regulation of the morphological aspects of oligodendrocyte maturation.
MATERIALS AND METHODS
Animals
Sprague–Dawley female rats with early postnatal litters were obtained from Harlan Laboratories, Inc. (Indianapolis, IN). All animal studies were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University.
Antibodies
Supernatants from cultured hybridoma cells (clone A2B5; ATCC, Manassas, VA) were used for immunopanning. Anti-GLAST, anti-GLT-1, and anti-EAAC1 (Abcam, Cambridge, MA) antibodies were used for Western blot analysis as well as immunocytochemistry. Anti-CaMKII, anti-pCaMKII T286/7 (Cell Signaling Technology, Inc. Danvers, MA), anti-pCaMKIIβ S371 (generated and characterized by us; Kim et al. in preparation), anti-GAPDH (EMD Millipore, Billerica, MA), and horseradish peroxidase (HRP)-labeled secondary antibodies (Vector Laboratories, Burlingame, CA) were used for Western blot analysis. Supernatants from cultured hybridoma cells (clone O4; gift from S.E. Pfeiffer), anti-MBP antibodies (EMD Millipore, Billerica, MA) and Alexa 488- or Alexa 564-conjugated secondary antibodies (Life Technologies, Grand Island, NY) were used for immunocytochemistry.
Cell Culture
Primary rat oligodendrocyte progenitors were isolated from postnatal day 3 (P3) rat brains by A2B5 immunopanning (Barres et al. 1992; Lafrenaye and Fuss 2011). Oligodendrocyte progenitors were either used directly in plasmid nucleofection experiments or plated onto fibronectin (10 μg/mL)-coated tissue culture dishes or glass coverslips. Plated oligodendrocyte progenitors were cultured in serum-free differentiation medium (DMEM containing 40 ng/mL tri-iodo-thyronine (T3; Sigma, St Louis, MO) and 1× N2 supplement (Life Technologies Corp., Grand Island, NY); DMEM/T3/N2). In siRNA-mediated gene silencing experiments, differentiating oligodendrocytes were transfected with siRNAs 24h after plating. Otherwise, plated oligodendrocyte progenitors were allowed to differentiate for 48h. Under these conditions, the majority of cells represented post-migratory, premyelinating oligodendrocytes as they expressed the O4 antigen (Sommer and Schachner 1982; Warrington et al. 1993; data not shown). Such populations of differentiating oligodendrocytes were either directly analyzed or treated as indicated with the following compounds: L-glutamate (Glu), D-aspartate (Asp), the competitive, non-transportable inhibitor of sodium-dependent glutamate transport DL-threo-β-benzyloxyaspartic acid (TBOA), the GLT-1-selective non-transportable inhibitor of glutamate/aspartate uptake dihydrokainic acid (DHK) (all from R&D Systems, Inc. Minneapolis, MN), the selective GLAST inhibitor UCPH-101 (Abcam, Cambridge, MA), the membrane permeable pharmacological inhibitor of CaMKII activity KN-93 or its inactive derivative KN-92 (EMD Millipore, Billerica, MA). In the case of dual treatments, inhibitors were added 30 min prior to the application of L-glutamate or D-aspartate. Cells were analyzed 6h after addition of L-glutamate or D-aspartate unless stated otherwise. Typically, at least three independent experiments were performed, whereby an independent experiment refers to an experiment in which cells were isolated from a separate P3 rat litter at an independent time-point (day) and treated separately from all other independent experiments. In each experiment triplicate coverslips (cultures) were prepared for all conditions/treatments.
Cells of the immortalized mouse oligodendroglial cell line CIMO were cultured in DMEM/5% FCS/1 μg/mL interferon-γ (EMD Millipore, Billerica, MA) at 33 °C (Bronstein et al. 1998) and then used in plasmid nucleofection experiments.
Quantitative (q)RT-PCR Analysis
For the determination of relative mRNA expression levels, qRT-PCR was performed on a CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA) using the iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) and the following gene-specific primer pairs: Glast: forward (5′-AGCCTGGGGTGTCTTCCACCA-3′), reverse (5′-ACCACAGCCTTGCACTTCAGTGTCT-3′); Glt-1: forward (5′-TGGCGGCTCCCATCCACCCT-3′), reverse (5′-GGCGGCCGCTGGCTTTAGCA-3′); Eaac1: forward (5′-GCCCACGAGCTCGGGATGCG-3′), reverse (5′-CACGATGCCCAGTACCACGGC-3′). Ppia (reference gene): forward: (5′-GGAGACGAACCTGTAGGACG-3′) and reverse: (5′-GATGCTCTTTCCTCCTGTGC-3′) Pgk1 (reference gene): forward: (5′-ATGCAAAGACTGGCCAAGCTAC-3′) and reverse: (5′-AGCCACAGCCTCAGCATATTTC-3′)
PCR conditions were as follows: 95°C for 3 min followed by 40 cycles of 95°C for 15s, 58°C for 30s, and 95°C for 10s. For comparing the expression levels of the different genes, R0 values were determined as described by Peirson et al. (2003). To determine relative expression levels the ΔΔCT method was used (Livak and Schmittgen 2001).
Western blot analysis
Cells were homogenized in lysis buffer (150 mM NaCl, 10 mM KCl, 20 mM HEPES (pH 7.0), 1 mM MgCl2, 20% glycerol, 1% Triton X-100) including the complete protease and phosphatase inhibitor cocktail (Thermo Scientific, Rockford, IL), and 12 μg (30 μg when using anti-GLAST antibodies) were used for Western blot analysis. Bound antibodies were detected using HRP-conjugated secondary antibodies in combination with the ECL Prime Western blotting detection reagent (GE Healthcare Life Sciences, Piscataway, NJ). Chemiluminescent signals were detected by exposure of photographic film (Kodak BioMax MR, Eastman Kodak Company, Rochester, NY) and quantified by densitometry using the ImageJ software package (Abramoff et al. 2004).
Immunocytochemistry
For immunocytochemistry using O4 hybridoma supernatants, cells were fixed in 4% paraformaldehyde/PBS, nonspecific binding sites were blocked in 10% FCS/DMEM and cells were incubated with the supernatant (1:1 diluted in 10% FCS/DMEM) overnight. For immunocytochemical detection of GLAST, GLT-1, EAAC1 or MBP, cells were fixed in 4% paraformaldehyde/PBS and then permeabilized using 0.5% Triton X-100/0.4 M sucrose/PBS. Subsequently, cells were incubated for 30 min in 10% FCS/DMEM and then overnight with anti-GLAST, anti-GLT-1, anti-EAAC1 (Abcam, Cambridge, MA) or anti-MBP (SMI99; Covance, Princeton, NJ) antibodies. Primary antibodies were detected using Alexa 488- or Alexa 564-conjugated secondary antibodies (Life Technologies Corp., Grand Island, NY) and nuclei were counterstained using Hoechst 33342 (EMD Millipore, Billerica, MA).
siRNA-mediated Gene Silencing
Differentiating oligodendrocytes were transfected with ON-TARGETplus siRNA SMARTpools directed against rat Glast, Glt-1 or Eaac1 (Thermo Fisher Scientific Inc., Pittsburg, PA) using Lipofectamine 2000 (Life Technologies Corp., Grand Island, NY). As control, an ON-TARGETplus non-targeting siRNA pool (Thermo Fisher Scientific Inc., Pittsburg, PA) was used. Transfection medium containing siRNA-Lipofectamine complexes was replaced with serum-free differentiation medium (DMEM/T3/N2) after 3h and cells were cultured for an additional 72h. Knock-down of gene expression was assessed by qRT-PCR and Western blot analysis.
Process Morphology Analysis
Oligodendrocyte morphology was analyzed and quantified as previously described in detail (Dennis et al. 2008). Briefly, oligodendrocytes were immunostained using O4 hybridoma cell supernatants and images of approximately 30 cells were taken for each treatment group in each experiment (n≥3; i.e. at least 90 cells per condition) using an Olympus BX51 inverted fluorescent microscope (Olympus America Inc., Center Valley, PA). Cells were chosen over the entire field of the coverslip by scanning from the upper left to the lower right. Only cells that displayed features of a healthy cell (based on nuclear stain and membrane appearance) and were without overlap with any neighboring cell were selected for analysis. IP Lab imaging software (BD Biosciences Bioimaging, Rockville, MD) was used to determine the network area (total area within the radius of the O4 immunopositive process network minus the cell body). For the bar graphs representing network area, the mean value for cells cultured under control conditions was calculated and set to 100%. Adjusted, i.e. normalized, values for all cells were then averaged for each experimental condition. For the generation of representative images, confocal laser scanning microscopy was used (Zeiss LSM 510 META NLO; Carl Zeiss Microscopy, LLC, Thornwood, NY). Images represent 2D maximum projections of stacks of 0.5 μm optical sections.
Cell Count Analysis
To determine the number of MBP immunopositive cells, images of four fields per coverslip were taken with a 20× objective using an Olympus BX51 fluorescence microscope equipped with an Olympus DP72 CCD camera (Olympus America Inc., Center Valley, PA). Three coverslips per condition for each of three independent experiments were analyzed, and Hoechst 33342-positive nuclei as well as MBP immunopositive oligodendrocytes were counted using the Cell Counter plugin to the ImageJ software package (Abramoff et al. 2004).
Intracellular Calcium Measurement
Intracellular calcium concentrations were determined in principle as previously described (Grynkiewicz et al. 1985). Briefly, differentiating oligodendrocytes were loaded with the calcium indicator fura-2 AM ester (2.5 μM) and pluronic F-127 (0.01%) (Life Technologies Corp., Grand Island, NY) in differentiation medium for 30 min at 37°C. Cells were washed and incubated in differentiation medium for an additional 30 min at 37°C. Ratiometric calcium measurements were made at 340 and 380 nm excitation and 510-520 nm emission wavelengths with cells cultured in differentiation medium (unless mentioned otherwise) using a Zeiss Observer.Z1 microscope in combination with the Axio VisionRel 4.8 software package (Carl Zeiss Microscopy, LLC, Thornwood, NY). Measurements were taken from at least 9 cells per treatment group and experiment and from 3 independent experiments (i.e. a total of 27 cells per treatment group) before and after the application of the indicated compounds. To calculate intracellular free calcium concentrations (in nM), a calibration curve (Calcium Calibration Buffer Kit, Life Technologies Corp., Grand Island, NY) was used.
Plasmid Nucleofection
A2B5 immunopanned oligodendrocyte progenitors or CIMO cells were nucleofected (Lonza Cologne GmbH, Cologne, Germany) with the following constructs: a plasmid encoding eGFP-CaMKIIβ (Okamoto et al. 2004), a control plasmid encoding eGFP alone, and plasmids encoding eGFP-CaMKIIβallA or eGFP-CaMKIIβallD in which alanine or aspartic acid residues replace all serine/threonine residues located within the variable domain (aa 317-396) (Kim et al. in preparation). All the above plasmids have the same plasmid backbone derived from the eukaryotic expression vector pEGFP-C1 (Clontech Laboratories, Inc., Mountain View, CA). It is also of note that the eGFP tag has been shown to not interfere with CaMKIIβ function (Okamoto et al. 2004; Okamoto et al. 2007).
To visualize F-actin in CIMO cells, cells were fixed in 4% paraformaldehyde/0.5% glutaraldehyde/0.4M sucrose/PBS and then incubated with Acti-stain 555 phalloidin (Cytoskeleton, Inc., Denver, CO). Colocalization was quantified by determining weighted colocalization coefficients (Manders et al. 1993) using the ZEN software package (Carl Zeiss MicroImaging, LLC, Thornwood, NY) and dual-color confocal images of cellular protrusion that were imaged using a 63× objective and 2× digital zoom. At least 20 cells per condition and experiment were analyzed in 3 independent experiments (i.e. a total of at least 60 cells per condition).
To visualize F-actin in differentiating oligodendrocytes, a plasmid (pCAGGS backbone; Niwa et al. 1991) encoding Lifeact (Riedl et al. 2008) fused to mRuby (Kredel et al. 2009) was used. For the generation of representative images, confocal laser scanning microscopy was used (Zeiss LSM 510 META NLO; Carl Zeiss Microscopy, LLC, Thornwood, NY). Images represent 2D maximum projections of stacks of 0.5 μm optical sections.
Statistical Analysis
For statistical analysis the GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA) was used. In the case of two or more groups of data composed of variable values, two-tailed Student’s t-tests or Kruskal–Wallis one-way analyses of variance (ANOVA) combined with post hoc Dunn’s or Student–Newman-Keuls tests were performed. When comparing a single control group with experimental groups of data, ANOVA with post hoc Dunnett tests were used. In the case data were compared to a set control value (1 or 100%) lacking variability, one-sample t-tests were used (Dalgaard 2008; Skokal and Rohlf 1995).
RESULTS
Sodium-dependent glutamate transport, mediated primarily by GLAST and GLT-1, promotes the morphological maturation of differentiating oligodendrocytes
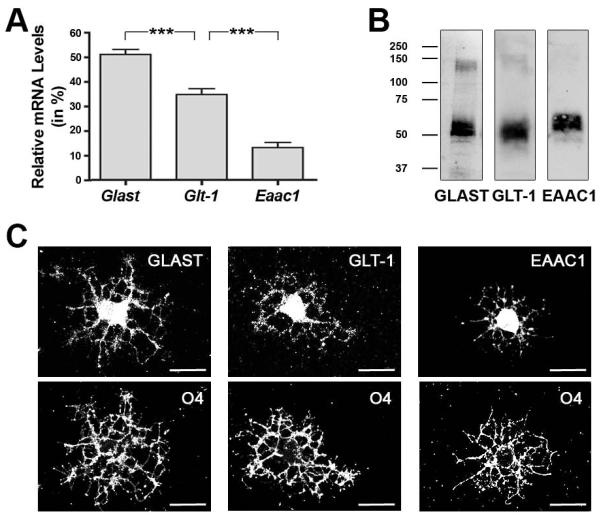
As introduced above, the sodium-dependent glutamate transporter genes Glast, Glt-1 and Eaac1 have been found to be expressed by cells of the oligodendrocyte lineage. To confirm such expression in differentiating oligodendrocytes in our culture system, qRT-PCR analysis was performed and revealed relative mRNA expression levels of Glast > Glt-1 > Eaac1 (Fig. 1A). Further validation was obtained through Western blot analysis and immunocytochemistry (Fig. 1B,C). Notably, in particular GLAST and GLT-1 were readily detectable not only in the cell body but also in cellular processes.
FIGURE 1.
Sodium-dependent glutamate transporters are expressed in differentiating oligodendrocytes. A: Bar graph representing sodium-dependent glutamate transporter mRNA levels as determined by qRT-PCR analysis. Total glutamate transporter mRNA levels (Glas t+Glt-1+Eaac1) were set to 100% and the values for the individual gene-specific mRNA levels were adjusted accordingly. Data represent means ± SEM (n = 3 independent experiments, ***p≤0.001, ANOVA). B: Representative Western blots depicting sodium-dependent glutamate transporter protein expression. Numbers to the left indicate molecular weights in kDa. C: Representative images of differentiating oligodendrocytes double-immunostained using anti-GLAST, -GLT-1, or -EAAC1 antibodies in combination with O4 hybridoma supernatants. Scale bars: 20 μm.
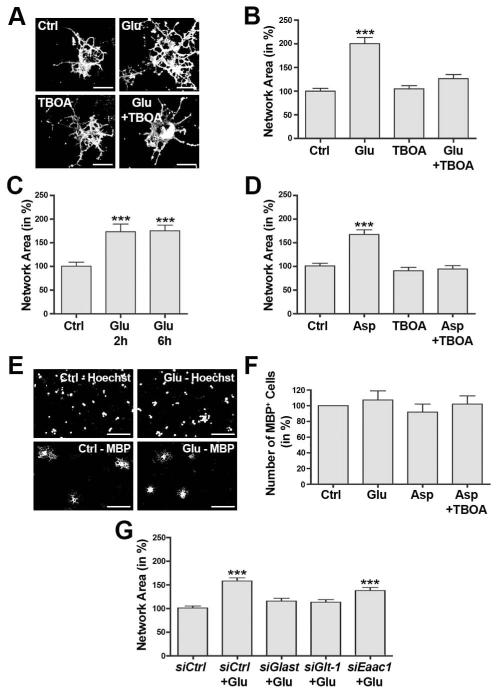
To assess a potential role of sodium-dependent glutamate transporters beyond extracellular glutamate clearance, differentiating oligodendrocytes were treated with 100 μM L-glutamate and morphological aspects of oligodendrocyte maturation were assessed via a quantification of the network area as described in detail previously (Dennis et al. 2008). As shown in Fig. 2A-C, L-glutamate treatment had a maturation-promoting effect that was seen as early as 2h and up to 6h. Importantly, this effect could be blocked by pre-treatment with TBOA (Fig. 2A,B), a competitive and non-transportable inhibitor of sodium-dependent glutamate transport (Shigeri et al. 2001; Shimamoto et al. 1998). In agreement with previous studies, no obvious effects on cell survival were noted in the presence of the L-glutamate and TBOA concentrations used here and within the time frames analyzed (Deng et al. 2006 and data not shown).
FIGURE 2.
Activation of sodium-dependent glutamate transporters promotes the morphological aspects of oligodendrocyte differentiation. A-F: Differentiating oligodendrocytes were treated for 6h (unless noted otherwise) as indicated: control (Ctrl), L-glutamate (Glu, 100 μM), a non-transportable inhibitor of sodium-dependent glutamate transport (TBOA, 100 μM), D-aspartate (Asp, 100 μM). A: Representative images of differentiating oligodendrocytes immunostained using O4 hybridoma supernatants. Scale bars: 20 μm. B-D: Bar graphs representing quantitative analyses of oligodendrocyte network areas (Dennis et al. 2008). Data represent means ± SEM (***p≤0.001 compared to control, ANOVA). E: Representative images of differentiating oligodendrocytes stained with an antibody specific for myelin basic protein (MBP) as well as with Hoechst 33342 (Hoechst) to visualize nuclei. Scale bars: 100 μm. F: Bar graph depicting the number of MBP immunopositive cells normalized to the number of Hoechst-positive nuclei. Data represent means ± SEM. ANOVA revealed no statistically significant difference (p≤0.05). G: Bar graph depicting oligodendrocyte network areas upon siRNA-mediated knock-down of individual sodium-dependent glutamate transporters (as indicated) and subsequent treatment with L-glutamate (Glu, 100 μM). Data represent means ± SEM (***p≤0.001 compared to siCtrl non-treated, ANOVA).
To further substantiate that sodium-dependent glutamate transporters play a predominant role in the observed maturation-promoting effect exerted by L-glutamate, the naturally occurring amino-acid D-aspartate was used as a glutamate-equivalent agonist. Similar to L-glutamate, D-aspartate is efficiently taken up through the sodium-dependent glutamate transporter system (Danbolt and Storm-Mathisen 1986; Davies and Johnston 1976; Kanai and Hediger 1992; Palacin et al. 1998; Pines et al. 1992). In contrast to L-glutamate, however, D-aspartate does not activate non-NMDA receptor ionotropic and metabotropic glutamate receptors (Domercq et al. 2005; Errico et al. 2008; Sugiyama et al. 1989), and it is not metabolized by glutamine synthetase (Bender et al. 1997). Thus, the use of D-aspartate eliminates potential confounding effects that may be due to an activation of AMPA/kainate and/or metabotropic glutamate receptors and/or the release of glutamine via the sodium-dependent neutral amino acid transporter system. As shown in Fig. 2D, D-aspartate elicited an effect on the oligodendrocyte process network that was comparable to the one seen upon treatment with L-glutamate (compare Fig. 2B with 2D).
It has been well-demonstrated that morphological maturation of oligodendrocytes occurs during development concurrently with changes in gene expression (Baumann and Pham-Dinh 2001; Emery 2010; Pfeiffer et al. 1993). Under experimental conditions, however, molecular mechanisms regulating cellular morphology may be uncoupled from those that regulate gene expression (Buttery and ffrench-Constant 1999; Kim et al. 2006; Lafrenaye and Fuss 2011; Osterhout et al. 1999; Waggener et al. 2013). Thus, and to assess a potential role of L-glutamate and the activity of sodium-dependent glutamate transporters on myelin gene expression, potential changes in myelin basic protein (Mbp) expression were assayed using immunocytochemistry. In these experiments, no significant differences in the number of MBP-positive cells were noted (Fig. 2E,F), thus suggesting that the sodium-dependent glutamate transporter-mediated effect on oligodendrocyte maturation is primarily associated with the morphological aspects of this process.
As shown in Fig. 1, differentiating oligodendrocytes express more than one of the known sodium-dependent glutamate transporter genes. To evaluate the role of individual transporter genes, differentiating oligodendrocytes were transfected with siRNA pools specifically silencing Glast, Glt-1 or Eaac1 expression. Knock-down of gene expression was confirmed by Western blot analysis, which revealed a specific reduction in transporter protein levels of at least 70% (Fig. S1). Importantly, down-regulation of a particular transporter gene was not associated with compensatory up-regulation of any other transporter gene (Fig. S1). In addition, no effect on the oligodendrocyte network area was noted upon knock-down of sodium-dependent glutamate transporter expression alone (data not shown). However, knock-down of either Glast or Glt-1 expression was found to eliminate the effect of L-glutamate on the oligodendrocyte network area (Fig. 2G). For Eaac1, the sodium-dependent glutamate transporter with much lower mRNA expression levels when compared with Glast or Glt-1, knock-down of gene expression was seen to only partially attenuate the effect of L-glutamate on the oligodendrocyte network area (siCtrl+Glu: 157±7%, siEaac1+Glu: 136±6%, p=0.007, ANOVA).
Taken together, the above data demonstrate that L-glutamate can promote the morphological aspects of oligodendrocyte maturation via a sodium-dependent glutamate transporter-mediated mechanism. In addition, they suggest that even a slight decline in the oligodendrocyte-derived expression of sodium-dependent glutamate transporters can reduce the effect of L-glutamate on the maturation of differentiating oligodendrocytes.
Activation of sodium-dependent glutamate transporters in differentiating oligodendrocytes leads to a transient increase in intracellular calcium levels
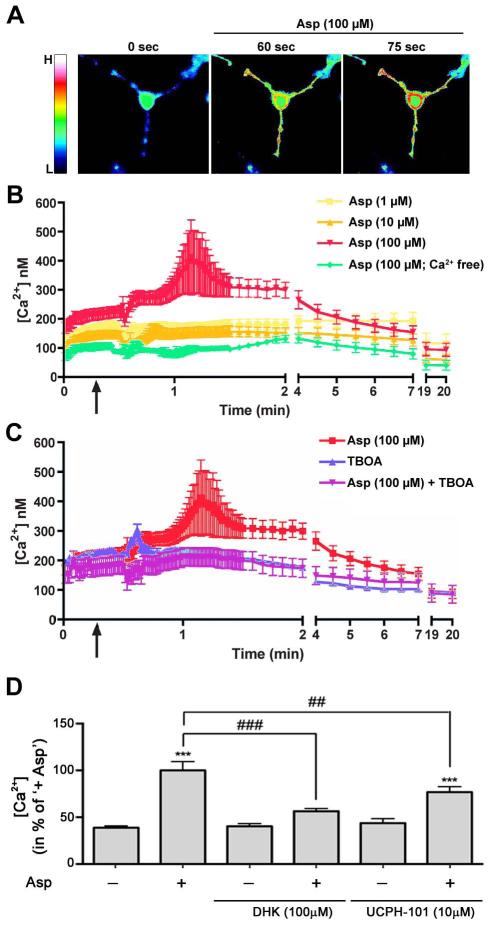
Having established that activation of sodium-dependent glutamate transporters promotes the morphological maturation of differentiating oligodendrocytes, we next wished to explore potential downstream signaling events involved in this process. These signaling-related studies were focused on calcium-mediated events, since it had previously been demonstrated that glutamate transport can activate the reverse mode of the sodium/calcium exchanger and thereby lead to a transient increase in intracellular calcium levels (Lopez-Colome et al. 2012; Martinez-Lozada et al. 2011; Rojas et al. 2007). To assess the effect of an activation of sodium-dependent glutamate transporters on intracellular calcium levels, D-aspartate was used as a glutamate-equivalent agonist, and calcium measurements were taken within the first minutes of the experiment, i.e. at an early stage of oligodendrocyte maturation. It is of note that the cells shown in Fig. 2A were imaged 6h after glutamate application, i.e. subsequent to a period of significant process outgrowth. As shown in Fig. 3, D-aspartate treatment elicited a transient increase in intracellular calcium levels that appeared to occur first within cellular processes (Fig. 3A). This D-aspartate-induced transient increase in free intracellular calcium levels was found to be dose-dependent (Fig. 3B) and absent in the presence of TBOA (Fig. 3C). In addition, no such increase was observed in the absence of extracellular calcium (Fig. 3B).
FIGURE 3.
The activation of sodium-dependent glutamate transporters increases intracellular calcium levels in the processes of early stage differentiating oligodendrocytes. A: Representative pseudo-colored images of fura-2 AM fluorescence ratio measurements upon treatment with D-aspartate (Asp). The bar to the left represents a relative color scale indicating low (L) and high (H) calcium levels. B-C: Time course of changes in free intracellular calcium concentrations [Ca2+] upon different treatments as indicated in the inset shown in the upper right. Start of treatment is indicated by the arrow. The graphs represent means ± SEM. D: Bar graph depicting a quantitative analysis of free intracellular calcium concentrations [Ca2+] at the time-point of highest response to D-aspartate. The mean value for D-aspartate treated cells (+ Asp) was set to 100% and the remaining values were calculated accordingly. Treatments are indicated along the x-axis. Data represent means ± SEM (***p≤0.001 compared to control (untreated), ###p≤0.001 and ##p≤0.01 compared to ‘+ Asp’, ANOVA).
To assess the functional contributions of each of the two main sodium-dependent glutamate transporters expressed by differentiating oligodendrocytes, namely GLAST and GLT-1, dihydrokainic acid (DHK), a non-transportable inhibitor of L-glutamate uptake selective for GLT-1 (Arriza et al. 1994), and UCPH-101, a non-substrate inhibitor selective for GLAST (Abrahamsen et al. 2013; Erichsen et al. 2010), were used. As shown in Fig. 3D, the D-aspartate-mediated transient increase in intracellular calcium levels was completely abolished in the presence of DHK but only partially blocked in the presence of UCPH-101. Such a more prominent functional role of GLT-1 compared with GLAST in differentiating oligodendrocytes could be further validated by performing glutamate uptake assays (data not shown).
Taken together, the above data support the idea that in differentiating oligodendrocytes L-glutamate/D-aspartate activates sodium-dependent glutamate transporters and in particular GLT-1, which in turn mediate a transient increase in intracellular calcium levels via entry from the extracellular environment.
The maturation-promoting effect of sodium-dependent glutamate transporters in differentiating oligodendrocytes is mediated by a transient phosphorylation event within CaMKIIβ’s actin binding/stabilizing domain
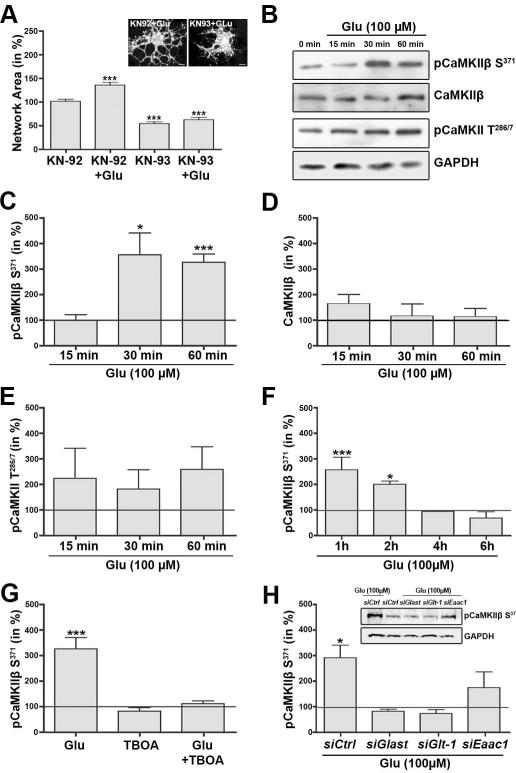
Our recent data provided good evidence for a critical role of the calcium sensor CaMKIIβ and in particular its actin binding/stabilizing domain in regulating oligodendrocyte maturation and CNS myelination (Waggener et al. 2013). Thus, CaMKIIβ and its actin binding/stabilizing domain may be directly involved in the sodium-dependent glutamate transporter-mediated effect described here. Indeed, pre-treatment of differentiating oligodendrocytes with KN-93, a membrane-permeable pharmacological inhibitor of CaMKIIβ’s kinase catalytic as well as actin binding/stabilizing activity (Lin and Redmond 2008; Sumi et al. 1991), was found to block the maturation-promoting effect of L-glutamate on the oligodendrocyte process network (Fig. 4A). It is of note that KN-93 treatment alone and thus a continuous inhibition of CaMKII activity attenuated the morphological maturation of differentiating oligodendrocytes (Fig. 4A). This effect has been previously described and was found to not be associated with a change in cellular viability (Waggener et al. 2013).
FIGURE 4.
Activation of sodium-dependent glutamate transporters leads to a transient phosphorylation event at CaMKIIβ’s S371 site. A: Bar graph depicting a quantitative analysis of the oligodendrocyte network area (Dennis et al. 2008). Cells were pre-treated with the pharmacological CaMKII inhibitor KN-93 or its inactive derivative KN-92 and then incubated in the absence or presence (+Glu) of 100 μM L-glutamate. The mean values for control cells (pre-treated with KN-92 and incubated in the absence of L-glutamate) were set to 100% and experimental values were calculated accordingly. Data represent means ± SEM (***p≤0.001 compared to control, ANOVA). The inset (upper right) depicts representative images of differentiating oligodendrocytes treated with KN-92 plus L-glutamate (left) or KN-93 plus L-glutamate (right) and immunostained using O4 hybridoma supernatants. Scale bars: 5 μm. B, inset in H: Representative Western blots depicting CaMKII phosphorylation (pCaMKIIβ S371, pCaMKII T286/7) or total CaMKIIβ protein levels. GAPDH protein levels are shown representatively for the Western blot for which anti-pCaMKII T286/7 (B) or anti-pCaMKIIβ S371 (inset in H) antibodies were used. C-H: Bar graphs depicting the levels of pCaMKIIβ S371 (C,F- H), total CaMKIIβ (D) or pCaMKII T286/7 (E) at different time-points after addition of L-glutamate (Glu) (C-F), at the time-point of 60 min after addition of L-glutamate and prior pre-treatment with or without TBOA (G) or after transfection with siRNA pools as indicated and L-glutamate treatment for 60 min (H). All CaMKII protein levels were normalized to GAPDH protein levels. The mean normalized values for control (non-treated) cells were set to 100% (horizontal line) and experimental values were calculated accordingly. Data represent means ± SEM of 3 independent experiments (***p≤0.001, *p≤0.05 compared to control, ANOVA).
To further assess the effect of L-glutamate on CaMKII and in particular CaMKIIβ’s actin binding/stabilizing domain in differentiating oligodendrocytes, phosphorylation events at CaMKII’s T286/7 and CaMKIIβ’s S371 site were analyzed. CaMKII’s T286/7 site represents CaMKII’s autophosphorylation site, which regulates autonomous CaMKII kinase catalytic activity and calcium/calmodulin binding affinity (Coultrap and Bayer 2012). Due to sequence conservation, phosphorylation events at this site could only be detected by pan pCaMKII T286/7 antibodies. CaMKIIβ’s S371 site is a phosphorylation site that is located within CaMKIIβ’s unique actin binding/stabilizing domain (Kim et al. 2011; Lin and Redmond 2009; O’Leary et al. 2006; Okamoto et al. 2007; Sanabria et al. 2009), and antibodies specifically recognizing this site have been generated by us. These antibodies were found to not recognize CaMKIIβ S371A, a mutant form of CaMKIIβ that cannot be phosphorylated at its S371 site (data not shown). As shown in Fig. 4B,C, treatment of differentiating oligodendrocytes with L-glutamate led to a significant increase in phosphorylation at CaMKIIβ’s S371 site. This effect was time-dependent and transient, since an increase in phosphorylation was not seen prior to 30 min of treatment and at 4h and beyond (Fig. 4C,F). No such increase in phosphorylation was observed at CaMKII’s autophosphorylation (T286/7) site within 15-60 min of L-glutamate treatment (Fig. 4E), nor was a change in total protein levels of CaMKIIβ noted (Fig. 4D). Importantly, the effect of L-glutamate on the phosphorylation at CaMKIIβ’s S371 site could be blocked by pre-treatment with TBOA (Fig. 4G).
To further assess the contribution of individual sodium-dependent glutamate transporter genes in this L-glutamate-mediated increase in pCaMKIIβ S371 levels, differentiating oligodendrocytes were transfected with siRNA pools specifically targeting Glast, Glt-1 or Eaac1 expression. Similar to what was observed for the effect of gene-specific knock-down of sodium-dependent glutamate transporter expression on the oligodendrocyte network area (Fig. 2F), knock-down of Glast or Glt-1 expression eliminated the effect of L-glutamate on the phosphorylation of CaMKIIβ’s S371 site, while the effect of a knock-down of Eaac1 expression appeared less pronounced (Fig. 4H).
Our data described so far demonstrate that in differentiating oligodendrocytes, L-glutamate can activate sodium-dependent glutamate transporters, which in turn can mediate a transient increase in intracellular calcium levels, a transient phosphorylation event within CaMKIIβ’s actin binding/stabilizing domain (S371 site) and a promotion of the morphological aspects of oligodendrocyte maturation.
Phosphorylation events within CaMKIIβ’s actin binding/stabilizing domain regulate the association of CaMKIIβ with filamentous (F)-actin and the effect of L-glutamate on the oligodendrocyte process network
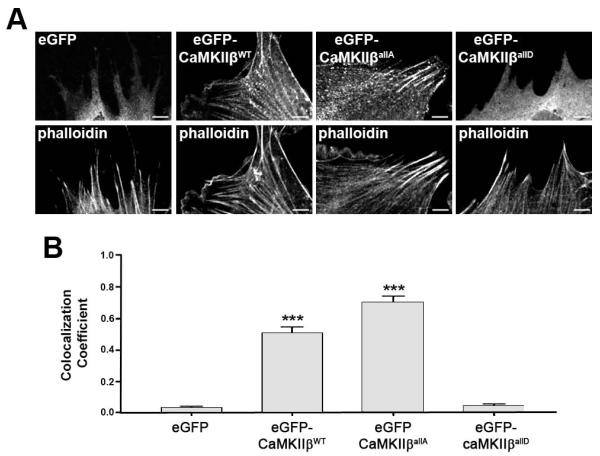
Our recent data, investigating the role of CaMKIIβ in the structural plasticity of dendritic spines, demonstrated that upon increases in intracellular calcium levels and serine (including S371)/threonine phosphorylation events within CaMKIIβ’s actin binding/stabilizing domain CaMKIIβ detaches from F-actin (Kim et al. 2011). These data raised the possibility that sodium-dependent glutamate transporter-mediated phosphorylation events within CaMKIIβ’s actin binding/stabilizing domain may affect the oligodendrocyte process network via a change in CaMKIIβ’s association with F-actin. To assess this idea, eGFP fusion proteins of CaMKIIβ mutant forms were generated in which the serine/threonine residues within the actin binding/stabilizing domain are either non-phosphorylatable (eGFP-CaMKIIβallA) or their phosphorylated state is mimicked (eGFP-CaMKIIβallD). In addition, eGFP-CaMKIIβWT and eGFP-CaMKIIβK43R, a mutant that is impaired in ATP binding and thus inactive with regard to its kinase catalytic but not actin binding activity (Okamoto et al. 2007), were used. To validate the proposed involvement of phosphorylation events within CaMKIIβ’s actin binding/stabilizing domain on CaMKIIβ’s association with F-actin, nucelofection studies were performed in cells of the oligodendroglial cell line CIMO. As shown in Fig. 5, non-phosphorylatable eGFP-CaMKIIβallA as well as eGFP-CaMKIIβWT largely colocalized with F-actin. In contrast, the phospho-mimetic eGFP-CaMKIIβallD showed little colocalization with F-actin and appeared similar to eGFP distributed diffusely and throughout the cytoplasm.
FIGURE 5.
Phosphorylation events within CaMKIIβ’s actin binding/stabilizing domain regulate the association of CaMKIIβ with filamentous (F)-actin. A: Representative images of CIMO cells nucleofected with plasmids encoding eGFP fusion proteins of CaMKIIβ (WT or mutant form as indicated) and stained for F-actin (phalloidin). Scale bars: 5 μm. B: Bar graph depicting the weighted colocalization coefficients for eGFP fusion proteins and phalloidin. Data represent means ± SEM (***p≤0.001 compared to eGFP, ANOVA).
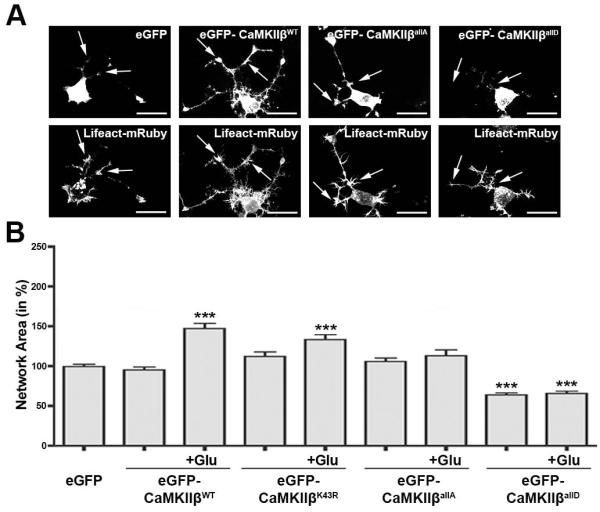
Having established the F-actin binding characteristics of the different forms of CaMKIIβ, we performed co-nucleofection experiments using a plasmid encoding Lifeact fused to mRuby in differentiating oligodendrocytes. Lifeact is a 17-amino-acid peptide, which binds to F-actin without interfering with actin organization and dynamics (Riedl et al. 2008) and thus allows reliable visualization of F-actin-rich structures. Using this approach, CaMKIIβallA and eGFP-CaMKIIβWT were readily detectable within F-actin-enriched regions located within cellular processes of differentiating oligodendrocytes (see arrows in Fig. 6A). In contrast, eGFP-CaMKIIβallD was similar to eGFP found predominantly localized to the cell body (Fig. 6A).
FIGURE 6.
Phosphorylation events within CaMKIIβ’s actin binding/stabilizing domain regulate the glutamate-mediated promotion of the morphological aspects of oligodendrocyte maturation. A: Representative images of differentiating oligodendrocytes co-nucleofected with plasmids encoding an eGFP fusion protein of CaMKIIβ (WT or mutant form as indicated) and Lifeact-mRuby. Arrows point toward F-actin-enriched regions along cellular processes as visualized via Lifeact-mRuby fluorescence. Scale bars: 20 μm. B: Bar graph depicting a quantitative analysis of the oligodendrocyte network area (Dennis et al. 2008). Cells were nucleofected as indicated and then incubated in the absence or presence (+Glu) of 100 μM L-glutamate. The mean values for control cells (nucleofected with an eGFP encoding plasmid and incubated in the absence of L-glutamate) were set to 100% and experimental values were calculated accordingly. Data represent means ± SEM (***p≤0.001 compared to control, ANOVA).
To further assess the role of the phosphorylation state of CaMKIIβ’s actin binding/stabilizing domain and thus its level of association with F-actin in the L-glutamate-mediated promotion of the morphological aspects of oligodendrocytes maturation, the effect of the expression of the different eGFP-CaMKIIβ forms on the oligodendrocyte network area was evaluated. As shown in Fig. 6B, expression of non-phosphorylatable eGFP-CaMKIIβallA blocked the maturation-promoting effect of L-glutamate treatment. No such effect was observed when eGFP-CaMKIIβWT or eGFP-CaMKIIβK43R were expressed, suggesting that the L-glutamate-mediated maturation-promoting effect may not require CaMKIIβ’s kinase catalytic activity. Importantly and consistent with our previous findings (Waggener et al. 2013; Fig. 4A), constitutive expression of the phospho-mimetic eGFP-CaMKIIβallD attenuated the morphological maturation of differentiating oligodendrocytes, likely due to a dominant-negative effect with regard to F-actin binding. In addition, it eliminated the effect of L-glutamate treatment on the oligodendrocyte process network.
Taken together, the above data support the idea that transient phosphorylation within CaMKIIβ’s actin binding/stabilizing domain leading to transient detachment of CaMKIIβ from F-actin, is a required step in the molecular mechanism that promotes the morphological aspects of oligodendrocyte maturation via an activation of sodium-dependent glutamate transporters. Notably, constitutive (vs. transient) phosphorylation within CaMKIIβ’s actin binding/stabilizing domain appears to have an opposing effect, i.e. to attenuate oligodendrocyte maturation.
DISCUSSION
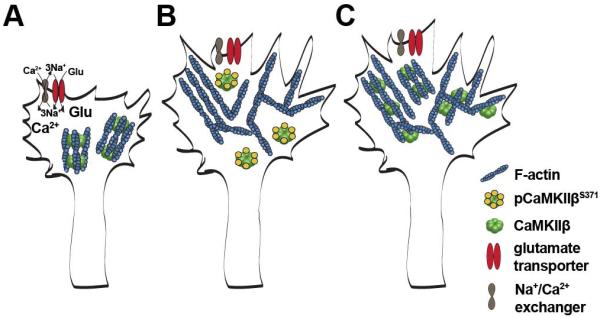
The studies described here investigated the role of sodium-dependent glutamate transporters in the regulation of oligodendrocyte maturation. Based on the data presented and in combination with the ascribed function of CaMKIIβ’s actin binding/stabilizing domain in the regulation of dendritic spine morphology (Okamoto et al. 2009), we propose the following model for the role of sodium-dependent glutamate transporters in the regulation of oligodendrocyte maturation (Fig. 7). Under basal conditions, CaMKIIβ is, in differentiating oligodendrocytes, to a large extent associated with the actin cytoskeleton. Glutamate release from, for example, unmyelinated axonal segments activates oligodendroglial sodium-dependent glutamate transporters, which in turn activate the reverse mode of oligodendroglial sodium/calcium exchangers resulting in a transient increase in intracellular calcium levels (Fig. 7A). This increase in intracellular calcium levels leads to phosphorylation events within CaMKIIβ’s actin binding/stabilizing domain (including CaMKIIβ’s S371 site), inactivation of CaMKIIβ’s actin binding activity and detachment of CaMKIIβ from the actin cytoskeleton. Such transient inactivation of CaMKIIβ’s actin binding activity opens a time window during which actin cytoskeleton remodeling events and actin polymerization are favored (Hoffman et al. 2013; Okamoto et al. 2007; Sanabria et al. 2009) (Fig. 7B). This dynamic phase is followed by a phase of actin cytoskeleton stabilization via re-activation of CaMKIIβ’s actin binding activity through de-phosphorylation (Fig. 7C). It is of note that the actin binding activity of CaMKIIβ appears to be independent of its kinase catalytic activity, a concept that is supported by recent in vitro and in vivo studies (Borgesius et al. 2011; van Woerden et al. 2009). Interestingly, actin binding properties have also been described for other members of the CaMKII gene family. These are, however, mediated by a structure-function domain different from the one identified in CaMKIIβ and analyzed here (Caran et al. 2001; Hoffman et al. 2013). Thus, further studies will be necessary to evaluate a potential contribution of CaMKII isozymes other than CaMKIIβ in the molecular mechanism described here. Importantly, and in contrast to its physiological role as proposed above, inactivation of CaMKIIβ’s actin binding properties in a constitutive (vs. transient) fashion appears to attenuate the morphological maturation of differentiating oligodendrocytes, an effect that is likely mediated by de-stabilization of the actin cytoskeleton over an extended period of time (Waggener et al. 2013). Thus, it is cycles of activation and de-activation of CaMKIIβ’s actin binding activity that allow re-organization of the actin cytoskeleton and a promotion of the morphological aspects of oligodendrocyte maturation. This idea is further supported by recent findings demonstrating that balanced activation and de-activation of the actin filament severing and depolymerizing factor cofilin regulates the function of the myelinating cells of the peripheral nervous system, namely Schwann cells (Sparrow et al. 2012).
FIGURE 7.
Proposed model for the role of a sodium-dependent glutamate transporter-CaMKIIβ-actin cytoskeleton axis in the regulation of oligodendrocyte maturation. A: L-glutamate (Glu) stimulates sodium-dependent glutamate transporter activity, which in turn leads to an increase in intracellular sodium (Na+) levels, activation of the reverse mode of the sodium/calcium exchanger and a transient increase in intracellular calcium (Ca2+) levels. B: The transient increase in intracellular calcium levels leads to phosphorylation events within CaMKIIβ’s actin binding/stabilizing domain (pCaMKIIβS371), detachment of CaMKIIβ from filamentous (F)-actin and the opening of a time window during which cytoskeletal rearrangements and morphological remodeling can occur. C: Upon de-activation (de-phosphorylation), CaMKIIβ binds to F-actin and thereby stabilizes the newly arranged cytoskeleton. Such cycles of activation and de-activation of CaMKIIβ’s actin binding activity allow re-organization of the actin cytoskeleton while at the same time preventing its uncontrolled disintegration. adapted from Okamoto et al. (2009).
In addition to the above, functional significance of oligodendroglial sodium-dependent glutamate transporters for glutamate uptake has been well-demonstrated (Arranz et al. 2008; DeSilva et al. 2009; Domercq et al. 2005; Pitt et al. 2003; Regan et al. 2007). Consistent with our observations made when assessing calcium levels in differentiating oligodendrocytes upon treatment with D-aspartate (Fig. 3), it has been shown that, at least in isolated optic nerve preparations, uptake of D-aspartate occurs particularly within oligodendrocyte processes. This observation led to the idea that glutamate homeostasis may be tightly regulated at the zones where axons and oligodendrocyte processes meet and in a fashion that may be analogous to what has been described for the tripartite synapse (Arranz et al. 2008). At the tripartite synapse, a so called “glutamate/glutamine shuttle” has been described (Martinez-Lozada et al. 2013; Rodriguez and Ortega 2012; Uwechue et al. 2012) in which, upon its uptake, glutamate is converted to glutamine by the enzymatic activity of glutamine synthetase. Glutamine is then released via sodium-dependent neutral amino acid transporters to be re-taken up by neurons and to serve as a precursor for glutamate synthesis. Glutamine synthetase is well-known to be expressed by cells of the oligodendrocyte lineage (D’Amelio et al. 1990; Tansey et al. 1991; Warringa et al. 1988), and microarray studies indicate the expression of, in particular, the system N amino acid transporter 2 (SNAT2 or SLC38A2) in differentiating oligodendrocytes (Cahoy et al. 2008). Nevertheless, more detailed studies will be necessary to clearly demonstrate that such a glutamate/glutamine shuttle exists at axon-oligodendrocyte process interaction zones.
Our studies revealed a prominent expression of the sodium-dependent glutamate transporter gene Glast in differentiating oligodendrocytes. This finding is in agreement with previous observations made in tissue culture as well as in vivo in both rodents and humans (DeSilva et al. 2009; Domercq et al. 1999; Pitt et al. 2003; Regan et al. 2007; Vallejo-Illarramendi et al. 2006). In addition, a low level expression of Eaac1 is consistent with the reported presence of few Eaac1-positive cells in the developing optic nerve (Domercq et al. 1999). In contrast, the expression of Glt-1 in cells of the oligodendrocyte lineage appears more complicated, since it has in vivo and in rodents been described to be developmentally regulated and to be predominant at stages at which oligodendrocytes are premyelinating (DeSilva et al. 2009; Desilva et al. 2007; Domercq et al. 1999). However, in human tissue the expression of Glt-1 has been reported to occur in mature oligodendrocytes (Pitt et al. 2003; Werner et al. 2001). More importantly, our data suggest that on a functional level GLT-1 is the more prominent sodium-dependent glutamate transporter in differentiating oligodendrocytes, even though GLAST also contributes to glutamate-mediated uptake and transient increases in intracellular calcium levels. Such a predominant functional sodium-dependent glutamate transporter role of GLT-1 in cells of the oligodendrocyte lineage is in agreement with previous findings made in rodents and humans (Pitt et al. 2003; Regan et al. 2007). However, a predominant role of EAAC1 in glutamate uptake in maturing oligodendrocytes has also been described (DeSilva et al. 2009). Thus, additional studies will be necessary to more precisely define individual sodium-dependent glutamate transporter contributions in cells of the oligodendrocyte lineage. Also noteworthy is the finding that development in mice with single knock-outs for Glast, Glt-1 or Eaac1 occurs without apparent CNS gross phenotypes (Peghini et al. 1997; Tanaka et al. 1997; Watase et al. 1998), while double Glast/Glt-1 knockout mice die in utero and show multiple developmental brain defects (Matsugami et al. 2006). These data indicate that during CNS development the loss of one sodium-dependent glutamate transporter may be compensated for functionally by at least one of the remaining transporters, i.e. by a mechanism that could further complicate a delineation of individual roles for individual sodium-dependent glutamate transporters.
Our model proposed in Fig. 7 is suggestive of pivotal roles for oligodendroglial sodium/calcium exchangers and intracellular calcium levels in the regulation of oligodendrocyte maturation. This idea is supported by recent findings implicating the sodium/calcium exchanger NCX3 in the regulation of oligodendrocyte differentiation (Boscia et al. 2012). In addition, process outgrowth has been found to be regulated by intracellular calcium levels in differentiating oligodendrocytes (Yoo et al. 1999). Interestingly, in our studies (Fig. 3) intracellular calcium levels remained slightly above control levels after the initial rise. Such a calcium response is consistent with previously described calcium responses mediated by signaling through sodium-dependent glutamate transporters in astrocytes (Rojas et al. 2007). In the case of astrocytes the initial rise in intracellular calcium concentration was found to be amplified by calcium release from ryanodine sensitive calcium stores (Rojas et al. 2007), which are also expressed by cells of oligodendrocyte lineage (Simpson et al. 1998).
In the major demyelinating disease in human, Multiple Sclerosis (MS), a block in oligodendrocyte differentiation is considered one of the main causes of inefficient remyelination and repair (Chang et al. 2002; Fancy et al. 2010; Kremer et al. 2011; Kuhlmann et al. 2008). Interestingly, changes in sodium-dependent glutamate transporter protein levels have been reported for white matter areas surrounding MS lesions (Pitt et al. 2003; Vallejo-Illarramendi et al. 2006; Werner et al. 2001) and glutamate levels have been found elevated in MS brains (Srinivasan et al. 2005; Trapp and Stys 2009; Werner et al. 2001). Such changes in glutamate homeostasis have been implicated in mediating excitotoxicity (Matute 2011; Pitt et al. 2003). However, it has also been suggested that mature rodent as well as human oligodendrocytes are largely resistant to such glutamate-mediated injury (Kolodziejczyk et al. 2009; Rosenberg et al. 2003; Wosik et al. 2004). Thus, and in light of our findings, it is tempting to speculate that changes in glutamate homeostasis and sodium-dependent glutamate transporter signaling may primarily contribute to the differentiation block seen in MS rather than mediate oligodendrocyte cell death.
Supplementary Material
FIGURE S1: siRNA-mediated gene silencing results in a significant knock-down of sodium-dependent glutamate transporter expression in differentiating oligodendrocytes. A: Bar graph depicting sodium-dependent glutamate transporter protein levels upon siRNA-mediated gene silencing and normalized to GAPDH protein levels. The mean value for cells treated with the control siRNA pool (siControl) was set to 100% (horizontal gray line) and experimental values were calculated accordingly. Data represent means ± SEM of 3 independent experiments (***p≤0.001,*p≤0.05, ANOVA). B: Representative Western blots depicting protein levels for GLAST, GLT-1, EAAC1 and GAPDH (as control). GAPDH protein levels are shown representatively for the Western blot, for which anti-EAAC1 antibodies were used.
Main Points.
The exact mechanisms regulating oligodendrocyte maturation and CNS myelination are still not fully understood. The data presented here reveal a novel sodium-dependent glutamate transporter-CaMKIIβ-actin cytoskeleton signaling axis that stimulates the morphological aspects of oligodendrocyte maturation and is likely to play an important role in the regulation of CNS myelination.
ACKNOWLEDGEMENTS
This work was supported by a grant from the NIH-NINDS (BF), by NIH grant R01DA17310 (YH), by Conacyt-Mexico (AO) as well as a scholarship from Conacyt-Mexico (ZML), and by a Grant-in-Aid for Scientific Research and Grant-in-Aid for Scientific Research on the Innovative Area “Foundation of Synapse and Neurocircuit Pathology” from the Ministry of Education, Culture, Sports, Science and Technology of Japan (YH). Microscopy was performed at the VCU Department of Anatomy and Neurobiology Microscopy Facility, supported, in part, with funding from NIH-NINDS Center Core Grant 5 P30 NS047463. Special thanks go to Steve Pfeiffer and Jeff Bronstein for providing the O4 hybridoma and CIMO cells, respectively. YH is partly supported by Takeda Pharmaceuticals Co. Ltd. and Fujitsu Laboratories. Other authors have no conflicts of interest to declare.
REFERENCES
- Abrahamsen B, Schneider N, Erichsen MN, Huynh TH, Fahlke C, Bunch L, Jensen AA. Allosteric modulation of an excitatory amino acid transporter: the subtype-selective inhibitor UCPH-101 exerts sustained inhibition of EAAT1 through an intramonomeric site in the trimerization domain. J Neurosci. 2013;33:1068–87. doi: 10.1523/JNEUROSCI.3396-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with Image J. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Arranz AM, Hussein A, Alix JJ, Perez-Cerda F, Allcock N, Matute C, Fern R. Functional glutamate transport in rodent optic nerve axons and glia. Glia. 2008;56:1353–67. doi: 10.1002/glia.20703. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci. 1994;14:5559–69. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Bauer NG, Richter-Landsberg C, ffrench-Constant C. Role of the oligodendroglial cytoskeleton in differentiation and myelination. Glia. 2009;57:1691–705. doi: 10.1002/glia.20885. [DOI] [PubMed] [Google Scholar]
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- Beart PM, O’Shea RD. Transporters for L-glutamate: an update on their molecular pharmacology and pathological involvement. Br J Pharmacol. 2007;150:5–17. doi: 10.1038/sj.bjp.0706949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender AS, Woodbury DM, White HS. The rapid L- and D-aspartate uptake in cultured astrocytes. Neurochem Res. 1997;22:721–6. doi: 10.1023/a:1027358211472. [DOI] [PubMed] [Google Scholar]
- Borgesius NZ, van Woerden GM, Buitendijk GH, Keijzer N, Jaarsma D, Hoogenraad CC, Elgersma Y. betaCaMKII plays a nonenzymatic role in hippocampal synaptic plasticity and learning by targeting alphaCaMKII to synapses. J Neurosci. 2011;31:10141–8. doi: 10.1523/JNEUROSCI.5105-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscia F, D’Avanzo C, Pannaccione A, Secondo A, Casamassa A, Formisano L, Guida N, Annunziato L. Silencing or knocking out the Na(+)/Ca(2+) exchanger-3 (NCX3) impairs oligodendrocyte differentiation. Cell Death Differ. 2012;19:562–72. doi: 10.1038/cdd.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein JM, Hales TG, Tyndale RF, Charles AC. A conditionally immortalized glial cell line that expresses mature myelin proteins and functional GABA(A) receptors. J Neurochem. 1998;70:483–91. doi: 10.1046/j.1471-4159.1998.70020483.x. [DOI] [PubMed] [Google Scholar]
- Buttery PC, ffrench-Constant C. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol Cell Neurosci. 1999;14:199–212. doi: 10.1006/mcne.1999.0781. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–78. doi: 10.1523/JNEUROSCI.4178-07.2008. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caran N, Johnson LD, Jenkins KJ, Tombes RM. Cytosolic targeting domains of gamma and delta calmodulin-dependent protein kinase II. J Biol Chem. 2001;276:42514–9. doi: 10.1074/jbc.M103013200. [DOI] [PubMed] [Google Scholar]
- Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346:165–73. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- Coultrap SJ, Bayer KU. CaMKII regulation in information processing and storage. Trends Neurosci. 2012;35:607–18. doi: 10.1016/j.tins.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amelio F, Eng LF, Gibbs MA. Glutamine synthetase immunoreactivity is present in oligodendroglia of various regions of the central nervous system. Glia. 1990;3:335–41. doi: 10.1002/glia.440030504. [DOI] [PubMed] [Google Scholar]
- Dalgaard P. Introductory Statistics with R. New York: Springer. Danbolt NC. 2001. Glutamate uptake. Prog Neurobiol. 2008;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Danbolt NC, Storm-Mathisen J. Na+-dependent “binding” of D-aspartate in brain membranes is largely due to uptake into membrane-bounded saccules. J Neurochem. 1986;47:819–24. doi: 10.1111/j.1471-4159.1986.tb00684.x. [DOI] [PubMed] [Google Scholar]
- Davies LP, Johnston GA. Uptake and release of D- and L-aspartate by rat brain slices. J Neurochem. 1976;26:1007–14. doi: 10.1111/j.1471-4159.1976.tb06485.x. [DOI] [PubMed] [Google Scholar]
- De Biase LM, Kang SH, Baxi EG, Fukaya M, Pucak ML, Mishina M, Calabresi PA, Bergles DE. 2011. NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination. J Neurosci. 31:12650–62. doi: 10.1523/JNEUROSCI.2455-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Wang H, Rosenberg PA, Volpe JJ, Jensen FE. Role of metabotropic glutamate receptors in oligodendrocyte excitotoxicity and oxidative stress. Proc Natl Acad Sci U S A. 2004;101:7751–6. doi: 10.1073/pnas.0307850101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Yue Q, Rosenberg PA, Volpe JJ, Jensen FE. Oligodendrocyte excitotoxicity determined by local glutamate accumulation and mitochondrial function. J Neurochem. 2006;98:213–22. doi: 10.1111/j.1471-4159.2006.03861.x. [DOI] [PubMed] [Google Scholar]
- Dennis J, White MA, Forrest AD, Yuelling LM, Nogaroli L, Afshari FS, Fox MA, Fuss B. Phosphodiesterase-Ialpha/autotaxin’s MORFO domain regulates oligodendroglial process network formation and focal adhesion organization. Mol Cell Neurosci. 2008;37:412–24. doi: 10.1016/j.mcn.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSilva TM, Kabakov AY, Goldhoff PE, Volpe JJ, Rosenberg PA. Regulation of glutamate transport in developing rat oligodendrocytes. J Neurosci. 2009;29:7898–908. doi: 10.1523/JNEUROSCI.6129-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desilva TM, Kinney HC, Borenstein NS, Trachtenberg FL, Irwin N, Volpe JJ, Rosenberg PA. The glutamate transporter EAAT2 is transiently expressed in developing human cerebral white matter. J Comp Neurol. 2007;501:879–90. doi: 10.1002/cne.21289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domercq M, Etxebarria E, Perez-Samartin A, Matute C. Excitotoxic oligodendrocyte death and axonal damage induced by glutamate transporter inhibition. Glia. 2005;52:36–46. doi: 10.1002/glia.20221. [DOI] [PubMed] [Google Scholar]
- Domercq M, Matute C. Expression of glutamate transporters in the adult bovine corpus callosum. Brain Res Mol Brain Res. 1999;67:296–302. doi: 10.1016/s0169-328x(99)00072-8. [DOI] [PubMed] [Google Scholar]
- Domercq M, Sanchez-Gomez MV, Areso P, Matute C. Expression of glutamate transporters in rat optic nerve oligodendrocytes. Eur J Neurosci. 1999;11:2226–36. doi: 10.1046/j.1460-9568.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330:779–82. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- Erichsen MN, Huynh TH, Abrahamsen B, Bastlund JF, Bundgaard C, Monrad O, Bekker-Jensen A, Nielsen CW, Frydenvang K, Jensen AA. Structure-activity relationship study of first selective inhibitor of excitatory amino acid transporter subtype 1: 2-Amino-4-(4-methoxyphenyl)-7-(naphthalen-1-yl)-5-oxo-5,6,7,8-tetrahydro-4H-chrom ene-3-carbonitrile (UCPH-101) J Med Chem. 2010;53:7180–91. doi: 10.1021/jm1009154. and others. [DOI] [PubMed] [Google Scholar]
- Errico F, Rossi S, Napolitano F, Catuogno V, Topo E, Fisone G, D’Aniello A, Centonze D, Usiello A. D-aspartate prevents corticostriatal long-term depression and attenuates schizophrenia-like symptoms induced by amphetamine and MK-801. J Neurosci. 2008;28:10404–14. doi: 10.1523/JNEUROSCI.1618-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etxeberria A, Mangin JM, Aguirre A, Gallo V. Adult-born SVZ progenitors receive transient synapses during remyelination in corpus callosum. Nat Neurosci. 2010;13:287–9. doi: 10.1038/nn.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy SP, Kotter MR, Harrington EP, Huang JK, Zhao C, Rowitch DH, Franklin RJ. Overcoming remyelination failure in multiple sclerosis and other myelin disorders. Exp Neurol. 2010;225:18–23. doi: 10.1016/j.expneurol.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Flores-Mendez MA, Martinez-Lozada Z, Monroy HC, Hernandez-Kelly LC, Barrera I, Ortega A. Glutamate-dependent translational control in cultured Bergmann glia cells: eIF2alpha phosphorylation. Neurochem Res. 2013;38:1324–32. doi: 10.1007/s11064-013-1024-1. [DOI] [PubMed] [Google Scholar]
- Fruhbeis C, Frohlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, Kirchhoff F, Mobius W, Goebbels S, Nave KA. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11:e1001604. doi: 10.1371/journal.pbio.1001604. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–50. [PubMed] [Google Scholar]
- Guo F, Maeda Y, Ko EM, Delgado M, Horiuchi M, Soulika A, Miers L, Burns T, Itoh T, Shen H. Disruption of NMDA receptors in oligodendroglial lineage cells does not alter their susceptibility to experimental autoimmune encephalomyelitis or their normal development. J Neurosci. 2012;32:639–45. doi: 10.1523/JNEUROSCI.4073-11.2012. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L, Farley MM, Waxham MN. Calcium-calmodulin-dependent protein kinase II isoforms differentially impact the dynamics and structure of the actin cytoskeleton. Biochemistry. 2013;52:1198–207. doi: 10.1021/bi3016586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360:467–71. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- Kim HJ, DiBernardo AB, Sloane JA, Rasband MN, Solomon D, Kosaras B, Kwak SP, Vartanian TK. WAVE1 is required for oligodendrocyte morphogenesis and normal CNS myelination. J Neurosci. 2006;26:5849–59. doi: 10.1523/JNEUROSCI.4921-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Hayashi M, Narayanan R, Suzuki A, Matsuura K, K O, Hayashi Y. CaMKII gates rapid structural plasticity in hippocampal dendirtic spines. Program No 87207/E3 2011 Neuroscience Meeting Planner Washington, DC: Society for Neuroscience. 2011 2011 Online. [Google Scholar]
- Kolodziejczyk K, Hamilton NB, Wade A, Karadottir R, Attwell D. The effect of N-acetyl-aspartyl-glutamate and N-acetyl-aspartate on white matter oligodendrocytes. Brain. 2009;132:1496–508. doi: 10.1093/brain/awp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziejczyk K, Saab AS, Nave KA, Attwell D. Why do oligodendrocyte lineage cells express glutamate receptors? F1000 Biol Rep. 2010;2:57. doi: 10.3410/B2-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredel S, Oswald F, Nienhaus K, Deuschle K, Rocker C, Wolff M, Heilker R, Nienhaus GU, Wiedenmann J. mRuby, a bright monomeric red fluorescent protein for labeling of subcellular structures. PLoS One. 2009;4:e4391. doi: 10.1371/journal.pone.0004391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer D, Aktas O, Hartung HP, Kury P. The complex world of oligodendroglial differentiation inhibitors. Ann Neurol. 2011;69:602–18. doi: 10.1002/ana.22415. [DOI] [PubMed] [Google Scholar]
- Kriegler S, Chiu SY. Calcium signaling of glial cells along mammalian axons. J Neurosci. 1993;13:4229–45. doi: 10.1523/JNEUROSCI.13-10-04229.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann T, Miron V, Cuo Q, Wegner C, Antel J, Bruck W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131:1749–58. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10:311–20. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- Kukley M, Nishiyama A, Dietrich D. The fate of synaptic input to NG2 glial cells: neurons specifically downregulate transmitter release onto differentiating oligodendroglial cells. J Neurosci. 2010;30:8320–31. doi: 10.1523/JNEUROSCI.0854-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafrenaye AD, Fuss B. Focal adhesion kinase can play unique and opposing roles in regulating the morphology of differentiating oligodendrocytes. J Neurochem. 2011;115:269–82. doi: 10.1111/j.1471-4159.2010.06926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Redmond L. CaMKIIbeta binding to stable F-actin in vivo regulates F-actin filament stability. Proc Natl Acad Sci U S A. 2008;105:15791–6. doi: 10.1073/pnas.0804399105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Redmond L. Neuronal CaMKII acts as a structural kinase. Commun Integr Biol. 2009;2:40–1. doi: 10.4161/cib.2.1.7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lopez-Colome AM, Martinez-Lozada Z, Guillem AM, Lopez E, Ortega A. Glutamate transporter-dependent mTOR phosphorylation in Muller glia cells. ASN Neuro. 2012 doi: 10.1042/AN20120022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgaard I, Luzhynskaya A, Stockley JH, Wang Z, Evans KA, Swire M, Volbracht K, Gautier HO, Franklin RJ, Attwell D. Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol. 2013;11:e1001743. doi: 10.1371/journal.pbio.1001743. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyt K, Varadi A, Durant CF, Molnar E. Oligodendroglial metabotropic glutamate receptors are developmentally regulated and involved in the prevention of apoptosis. J Neurochem. 2006;99:641–56. doi: 10.1111/j.1471-4159.2006.04103.x. [DOI] [PubMed] [Google Scholar]
- Manders EMM, Verbeek FJ, Aten JA. Measurement of co-localization of objects in dualcolor confocal images. J Microsc. 1993;169:375–382. doi: 10.1111/j.1365-2818.1993.tb03313.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Lozada Z, Guillem AM, Flores-Mendez M, Hernandez-Kelly LC, Vela C, Meza E, Zepeda RC, Caba M, Rodriguez A, Ortega A. GLAST/EAAT1-induced glutamine release via SNAT3 in Bergmann glial cells: evidence of a functional and physical coupling. J Neurochem. 2013;125:545–54. doi: 10.1111/jnc.12211. [DOI] [PubMed] [Google Scholar]
- Martinez-Lozada Z, Hernandez-Kelly LC, Aguilera J, Lopez-Bayghen E, Ortega A. Signaling through EAAT-1/GLAST in cultured Bergmann glia cells. Neurochem Int. 2011;59:871–9. doi: 10.1016/j.neuint.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Matsugami TR, Tanemura K, Mieda M, Nakatomi R, Yamada K, Kondo T, Ogawa M, Obata K, Watanabe M, Hashikawa T. Proc Natl Acad Sci U S A. 2006;103:12161–6. doi: 10.1073/pnas.0509144103. and others. From the Cover: Indispensability of the glutamate transporters GLAST and GLT1 to brain development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C. Glutamate and ATP signalling in white matter pathology. J Anat. 2011;219:53–64. doi: 10.1111/j.1469-7580.2010.01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–9. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- O’Leary H, Lasda E, Bayer KU. CaMKIIbeta association with the actin cytoskeleton is regulated by alternative splicing. Mol Biol Cell. 2006;17:4656–65. doi: 10.1091/mbc.E06-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Bosch M, Hayashi Y. The roles of CaMKII and F-actin in the structural plasticity of dendritic spines: a potential molecular identity of a synaptic tag? Physiology (Bethesda) 2009;24:357–66. doi: 10.1152/physiol.00029.2009. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7:1104–12. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Narayanan R, Lee SH, Murata K, Hayashi Y. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc Natl Acad Sci U S A. 2007;104:6418–23. doi: 10.1073/pnas.0701656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhout DJ, Wolven A, Wolf RM, Resh MD, Chao MV. Morphological differentiation of oligodendrocytes requires activation of Fyn tyrosine kinase. J Cell Biol. 1999;145:1209–18. doi: 10.1083/jcb.145.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacin M, Estevez R, Bertran J, Zorzano A. Molecular biology of mammalian plasma membrane amino acid transporters. Physiol Rev. 1998;78:969–1054. doi: 10.1152/physrev.1998.78.4.969. [DOI] [PubMed] [Google Scholar]
- Peghini P, Janzen J, Stoffel W. Glutamate transporter EAAC-1-deficient mice develop dicarboxylic aminoaciduria and behavioral abnormalities but no neurodegeneration. EMBO J. 1997;16:3822–32. doi: 10.1093/emboj/16.13.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirson SN, Butler JN, Foster RG. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res. 2003;31:e73. doi: 10.1093/nar/gng073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3:191–97. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- Pines G, Danbolt NC, Bjoras M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm-Mathisen J, Seeberg E, Kanner BI. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992;360:464–7. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- Pitt D, Nagelmeier IE, Wilson HC, Raine CS. Glutamate uptake by oligodendrocytes: Implications for excitotoxicity in multiple sclerosis. Neurology. 2003;61:1113–20. doi: 10.1212/01.wnl.0000090564.88719.37. [DOI] [PubMed] [Google Scholar]
- Regan MR, Huang YH, Kim YS, Dykes-Hoberg MI, Jin L, Watkins AM, Bergles DE, Rothstein JD. Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. J Neurosci. 2007;27:6607–19. doi: 10.1523/JNEUROSCI.0790-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak TA, Werb Z. Lifeact: a versatile marker to visualize F-actin. Nat Methods. 2008;5:605–7. doi: 10.1038/nmeth.1220. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Ortega A. The Glia Connection of the Glutamate/Glutamine Shuttle. Am J Neurosci. 2012;3:32–8. [Google Scholar]
- Rojas H, Colina C, Ramos M, Benaim G, Jaffe EH, Caputo C, DiPolo R. Na+ entry via glutamate transporter activates the reverse Na+/Ca2+ exchange and triggers Ca(i)2+-induced Ca2+ release in rat cerebellar Type-1 astrocytes. J Neurochem. 2007;100:1188–202. doi: 10.1111/j.1471-4159.2006.04303.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg PA, Dai W, Gan XD, Ali S, Fu J, Back SA, Sanchez RM, Segal MM, Follett PL, Jensen FE. Mature myelin basic protein-expressing oligodendrocytes are insensitive to kainate toxicity. J Neurosci Res. 2003;71:237–45. doi: 10.1002/jnr.10472. and others. [DOI] [PubMed] [Google Scholar]
- Sanabria H, Swulius MT, Kolodziej SJ, Liu J, Waxham MN. {beta}CaMKII regulates actin assembly and structure. J Biol Chem. 2009;284:9770–80. doi: 10.1074/jbc.M809518200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeri Y, Shimamoto K, Yasuda-Kamatani Y, Seal RP, Yumoto N, Nakajima T, Amara SG. Effects of threo-beta-hydroxyaspartate derivatives on excitatory amino acid transporters (EAAT4 and EAAT5) J Neurochem. 2001;79:297–302. doi: 10.1046/j.1471-4159.2001.00588.x. [DOI] [PubMed] [Google Scholar]
- Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- Simpson PB, Holtzclaw LA, Langley DB, Russell JT. Characterization of ryanodine receptors in oligodendrocytes, type 2 astrocytes, and O-2A progenitors. J Neurosci Res. 1998;52:468–82. doi: 10.1002/(SICI)1097-4547(19980515)52:4<468::AID-JNR11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Skokal RR, Rohlf FJ. Biometry: the principle and practice in biological research. W. H. Freeman and Company; New York: 1995. [Google Scholar]
- Sommer I, Schachner M. Cell that are O4 antigen-positive and O1 antigen-negative differentiate into O1 antigen-positive oligodendrocytes. Neurosci Lett. 1982;29:183–8. doi: 10.1016/0304-3940(82)90351-2. [DOI] [PubMed] [Google Scholar]
- Sparrow N, Manetti ME, Bott M, Fabianac T, Petrilli A, Bates ML, Bunge MB, Lambert S, Fernandez-Valle C. The actin-severing protein cofilin is downstream of neuregulin signaling and is essential for Schwann cell myelination. J Neurosci. 2012;32:5284–97. doi: 10.1523/JNEUROSCI.6207-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Sailasuta N, Hurd R, Nelson S, Pelletier D. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain. 2005;128:1016–25. doi: 10.1093/brain/awh467. [DOI] [PubMed] [Google Scholar]
- Sugiyama H, Ito I, Watanabe M. Glutamate receptor subtypes may be classified into two major categories: a study on Xenopus oocytes injected with rat brain mRNA. Neuron. 1989;3:129–32. doi: 10.1016/0896-6273(89)90121-9. [DOI] [PubMed] [Google Scholar]
- Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, Hidaka H. The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem Biophys Res Commun. 1991;181:968–75. doi: 10.1016/0006-291x(91)92031-e. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–702. doi: 10.1126/science.276.5319.1699. and others. [DOI] [PubMed] [Google Scholar]
- Tansey FA, Farooq M, Cammer W. Glutamine synthetase in oligodendrocytes and astrocytes: new biochemical and immunocytochemical evidence. J Neurochem. 1991;56:266–72. doi: 10.1111/j.1471-4159.1991.tb02591.x. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Stys PK. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 2009;8:280–91. doi: 10.1016/S1474-4422(09)70043-2. [DOI] [PubMed] [Google Scholar]
- Uwechue NM, Marx MC, Chevy Q, Billups B. Activation of glutamate transport evokes rapid glutamine release from perisynaptic astrocytes. J Physiol. 2012;590:2317–31. doi: 10.1113/jphysiol.2011.226605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo-Illarramendi A, Domercq M, Perez-Cerda F, Ravid R, Matute C. Increased expression and function of glutamate transporters in multiple sclerosis. Neurobiol Dis. 2006;21:154–64. doi: 10.1016/j.nbd.2005.06.017. [DOI] [PubMed] [Google Scholar]
- van Woerden GM, Hoebeek FE, Gao Z, Nagaraja RY, Hoogenraad CC, Kushner SA, Hansel C, DeZeeuw CI, Elgersma Y. betaCaMKII controls the direction of plasticity at parallel fiber-Purkinje cell synapses. Nat Neurosci. 2009;12:823–5. doi: 10.1038/nn.2329. [DOI] [PubMed] [Google Scholar]
- Waggener CT, Dupree JL, Elgersma Y, Fuss B. CaMKIIbeta Regulates Oligodendrocyte Maturation and CNS Myelination. J Neurosci. 2013;33:10453–8. doi: 10.1523/JNEUROSCI.5875-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warringa RA, van Berlo MF, Klein W, Lopes-Cardozo M. Cellular location of glutamine synthetase and lactate dehydrogenase in oligodendrocyte-enriched cultures from rat brain. J Neurochem. 1988;50:1461–8. doi: 10.1111/j.1471-4159.1988.tb03031.x. [DOI] [PubMed] [Google Scholar]
- Warrington AE, Barbarese E, Pfeiffer SE. Differential myelinogenic capacity of specific developmental stages of the oligodendrocyte lineage upon transplantation into hypomyelinating hosts. J Neurosci Res. 1993;34:1–13. doi: 10.1002/jnr.490340102. [DOI] [PubMed] [Google Scholar]
- Watase K, Hashimoto K, Kano M, Yamada K, Watanabe M, Inoue Y, Okuyama S, Sakagawa T, Ogawa S, Kawashima N. Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice. Eur J Neurosci. 1998;10:976–88. doi: 10.1046/j.1460-9568.1998.00108.x. and others. [DOI] [PubMed] [Google Scholar]
- Werner P, Pitt D, Raine CS. Multiple sclerosis: altered glutamate homeostasis in lesions correlates with oligodendrocyte and axonal damage. Ann Neurol. 2001;50:169–80. doi: 10.1002/ana.1077. [DOI] [PubMed] [Google Scholar]
- Wosik K, Ruffini F, Almazan G, Olivier A, Nalbantoglu J, Antel JP. Resistance of human adult oligodendrocytes to AMPA/kainate receptor-mediated glutamate injury. Brain. 2004;127:2636–48. doi: 10.1093/brain/awh302. [DOI] [PubMed] [Google Scholar]
- Yoo AS, Krieger C, Kim SU. Process extension and intracellular Ca2+ in cultured murine oligodendrocytes. Brain Res. 1999;827:19–27. doi: 10.1016/s0006-8993(99)01282-2. [DOI] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–30. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1: siRNA-mediated gene silencing results in a significant knock-down of sodium-dependent glutamate transporter expression in differentiating oligodendrocytes. A: Bar graph depicting sodium-dependent glutamate transporter protein levels upon siRNA-mediated gene silencing and normalized to GAPDH protein levels. The mean value for cells treated with the control siRNA pool (siControl) was set to 100% (horizontal gray line) and experimental values were calculated accordingly. Data represent means ± SEM of 3 independent experiments (***p≤0.001,*p≤0.05, ANOVA). B: Representative Western blots depicting protein levels for GLAST, GLT-1, EAAC1 and GAPDH (as control). GAPDH protein levels are shown representatively for the Western blot, for which anti-EAAC1 antibodies were used.