Abstract
Visual cortical areas in the mammalian brain are linked through a system of interareal feedforward and feedback connections, which presumably underlie different visual functions. We characterized the refinement of feedback projections to primary visual cortex (V1) from multiple sources in juvenile ferrets ranging in age from four to ten weeks postnatal. We studied whether the refinement of different aspects of feedback circuitry from multiple visual cortical areas proceeds at a similar rate in all areas. We injected the neuronal tracer cholera toxin B (CTb) into V1, and mapped the areal and laminar distribution of retrogradely labeled cells in extrastriate cortex. Around the time of eye opening at four weeks postnatal, the retinotopic arrangement of feedback appears essentially adultlike; however, Suprasylvian cortex supplies the greatest proportion of feedback, whereas area 18 supplies the greatest proportion in the adult. The density of feedback cells and the ratio of supragranular/infragranular feedback contribution declined in this period at a similar rate in all cortical areas. We also find significant feedback to V1 from layer IV of all extrastriate areas. The regularity of cell spacing, the proportion of feedback arising from layer IV, and the tangential extent of feedback in each area all remained essentially unchanged during this period, except for the infragranular feedback source in area 18 which expanded. Thus, while much of the basic pattern of cortical feedback to V1 is present before eye opening, there is major synchronous reorganization after eye opening, suggesting a crucial role for visual experience in this remodeling process.
Keywords: Extrastriate, Visual development, Feedback projections, Neuroanatomy, V1, RRID: AB_10013220, RRID: nif-0000-10294, RRID: nlx_153890
Introduction
The mammalian brain is immature at birth, with major developmental events such as the establishment of basic neural circuitry and its subsequent refinement ensuing during the postnatal period. A major developmental strategy commonly used by many developing inter- and intra-areal corticocortical pathways is the initial overproduction of projections, followed by the selective removal of a subset to yield the mature adult pattern (Changeux and Danchin, 1976; Rakic et al., 1986; Innocenti and Price, 2005). Intracortical connections within primary visual cortex (V1) of several species (cat, ferret, human, monkey) undergo substantial postnatal refinement (Huttenlocher, 1979; Price, 1986; Callaway and Katz, 1990; Burkhalter et al., 1993; Coogan and Van Essen, 1996; Durack and Katz, 1996; Ruthazer and Stryker, 1996). Similarly, corticocortical connections between different mammalian visual cortical areas have been shown to refine and reorganize as well (Dehay et al., 1988; Price and Blakemore, 1989; Price and Zumbroich, 1989; Barone et al., 1995; Batardiere et al., 1998, 2002). The anatomical refinement of cortical circuits presumably underlies the maturation of neuronal physiological properties, and is thus one indicator of functional maturity. We are particularly interested in characterizing the postnatal refinement of interareal connections in the ferret visual cortex; specifically we wish to know whether feedback projections to V1 from multiple extrastriate areas refine with a similar postnatal timecourse. Differential development of distinct cortical circuits could underlie the known differences in the rate at which various perceptual abilities mature (Harwerth et al., 1986; Lewis and Maurer, 2005; Braddick and Atkinson, 2011).
There are currently two prevailing theories that describe the developmental sequence of anatomical and functional maturation. The hierarchical theory holds that the maturation of a given functional property or anatomical circuit proceeds in a sequential fashion with more basic functional properties or anatomical circuits maturing before more complex ones. The earliest accounts supporting the concept of hierarchical maturation of cortical areas come from classic studies by Flechsig (1901) on the human brain. He showed that during the first years of postnatal life, pathways to the primary motor, somatosensory, visual, and auditory cortical areas are the first to be myelinated. Consistent with this, Condé et al. (1996) showed that parvalbumin expression in a subset of GABAergic neurons in the monkey brain appears during development in a sequential fashion across the visual cortical hierarchy - first in V1, and later in more rostral areas. More recent in vivo imaging studies in humans have also confirmed Flechsig’s findings, showing similar regional differences in the rate of cortical maturation (Girard et al., 1991; Paus et al., 2001). Zhang et al. (2005) provided physiological evidence supporting the hierarchical theory. The authors reported that the receptive field surround of monkey V1 neurons is mature by four weeks of age, whereas in V2 the receptive field surround size and surround suppression were not adultlike until after eight weeks of age. In contrast, the concurrent theory posits that functional or anatomical aspects of multiple brain regions mature in concert. This theory derives from anatomical data in monkey suggesting that synaptogenesis and synapse elimination occur concurrently in multiple diverse regions of cerebral cortex (Rakic et al., 1986; Bourgeois et al., 1994). What remains unclear is which aspects of cortical function and circuitry mature synchronously, and which in a sequential or hierarchical fashion.
Several aspects of corticocortical connectivity refine postnatally, such as the laminar and tangential distribution of cells furnishing interareal projections. In cats and monkeys, there is a developmental refinement of the topography of interareal projections, a decline in the infragranular contribution to feedforward projections (also observed in the rat by Coogan and Burkhalter, 1988), and a decline in the supragranular contribution to feedback projections (Price and Blakemore, 1985; Kato et al., 1991; Price et al., 1994; Barone et al., 1995; Batardiere et al., 1998, 2002). The selective reduction in supragranular feedback cells leads to a unique laminar distribution characteristic of each area in the adult. Feedforward and feedback pathways may not refine similarly. Batardiere et al. (2002) suggested a difference in the development of different types of cortical connections of primate area V4; the laminar distribution of the feedforward pathway from area V2 to V4 is adultlike prior to birth, while feedback projections from V4 to V2 undergo extensive remodeling. Burkhalter (1993) demonstrated a similar developmental sequence of events in human infants; the laminar termination pattern of feedforward connections matures before that of feedback connections. Likewise, Berezovski et al. (2011) examined the development of feedforward and feedback connections in mouse visual cortex between V1, anterolateral area (AL), and lateromedial area (LM). Feedforward connections were present at the earliest time point examined (postnatal day 2), while feedback connections were not detectable until postnatal day 11. Thus, changes during postnatal development in certain aspects of the interareal connectivity of primary visual cortex have been reported.
What is lacking is simultaneous quantitative descriptions of the organization of the feedback pool to V1 from multiple visual areas at different postnatal ages; this will clarify whether there is synchronous versus sequential development of cortical areas and the links among them. Here we describe the developmental refinement of feedback projections arising from multiple visual areas in ferret visual cortex from four weeks to ten weeks postnatal. We have quantified different anatomical aspects related to the topography of feedback projections. Specifically, we are interested in determining whether different anatomical features of feedback circuitry refine simultaneously in all visual cortical areas. We find that the overall retinotopic pattern of feedback connections from extrastriate cortex is qualitatively adultlike as early as 4 weeks of age (in the period just before eye opening), though there are differences from the adult in areal, tangential, and laminar distribution; these all refine in the weeks after the eyes open. Significantly, the refinement of different anatomical aspects of cortical feedback maturation appears to follow a similar developmental timecourse in all visual cortical areas. Our results suggest that while the overall pattern of feedback projections from extrastriate cortex to primary visual cortex is present before eye opening, the fine scale refinement of the spatial layout of feedback connections in multiple visual areas occurs largely synchronously, and requires early postnatal visual experience.
Materials and Methods
Anatomical tracer injections
We studied 16 female sable ferrets (Mustela putorius furo) at six postnatal ages: 4 weeks (n=3), 5 weeks (n=3), 6 weeks (n=2), 7 weeks (n=2), 8 weeks (n=3), and 10 weeks (n=3). Animals were obtained from Marshall Farms (North Rose, NY); kits were housed with the jill under a 12 h light/dark cycle. All procedures conformed to National Institutes of Health guidelines. Prior to surgery, ferrets were sedated with an intramuscular injection of ketamine (25 mg/kg) and xylazine (2 mg/kg). The animal’s head was fixed in a stereotaxic apparatus, and secured with ear bars. The animals were respired using a pump, which delivered a mixture of 1%–2% isoflurane in O2. A small mask was placed on the nose and snout to administer isoflurane throughout the surgery. The EKG, pulse, tissue oxygenation, and rectal temperature were continuously monitored throughout the rest of the surgery, and maintained at appropriate levels. During a sterile surgery, Lidocaine HCl was injected into the scalp prior to incisions. The scalp was retracted, and a craniotomy and durotomy were performed on either the left or right hemisphere. Cholera toxin B subunit (CTb: List Biological Laboratories, Campbell CA, Cat# 104) was reconstituted in 0.1M potassium phosphate buffer (1%, pH 6.0), and either pressure injected or delivered with current into primary visual cortex. Iontophoretic injections using glass micropipettes (aperture 10–15 μm) were made by passing current at 2 μA for 10 minutes with a 7-second on-off cycle at two cortical depths to ensure that the extent of the injection site spanned both the upper and lower layers of the cortex. This method of injection typically yields an injection core with a diameter of 800–1000 μm. Pressure injections were delivered with a Picospritzer (Parker Hannifin, Fairfield, NJ), using glass micropipettes (aperture 30–40 μm) at two cortical depths with 2 X 10 msec pulses at each location. Both injection methods yielded comparable injection core volumes.
Following the injections, craniotomies were filled with sterile Gelfoam. Lidocaine was injected into the wound margins before the scalp was sutured, and an intramuscular injection of a broad spectrum antibiotic (ampicillin: 25 mg/kg) and analgesic (buprenorphine: 0.05 mg/kg) was administered for 2 days postoperatively. After a survival period of five to seven days, the animals were sedated with ketamine (25 mg/kg) + xylazine (2 mg/kg), given an intraperitoneal injection of sodium selenite (15 mg/kg) for subsequent labeling of synaptic zinc, then euthanized with an intraperitoneal overdose of pentobarbital (100 mg/kg).
Tissue fixation and histological processing
Animals were transcardially perfused with a 0.9% NaCl solution followed by a 4% paraformaldehyde in 0.1M phosphate buffer (PB) solution, then a 4% paraformaldehyde plus 10% sucrose solution. The brains were removed from the skull, then the posterior cortex was blocked and placed in a postfix solution of 4% buffered paraformaldehyde plus 30% sucrose for 2–3 hours. The brains were then placed into a 0.1 M PB solution with 30% sucrose for 2 days until they were sunk.
Frozen tangential or sagittal sections were cut at 40 microns using a sliding microtome. The sections were separated into four numbered series. The first and the third series were processed to reveal the CTb label using a modified version of the standard CTb protocol (Angelucci et al., 1996; Cantone et al., 2005). All procedures were done on free-floating sections, and all solutions were made with 0.1M phosphate buffered saline (PBS) (pH 7.4). Sections were rinsed in PBS, incubated in a 1% H2O2 solution to eliminate endogenous peroxidase, and rinsed again in PBS. This was followed by a short incubation in 0.1M glycine solution, rinsed in PBS, then incubated overnight at 4° C in a blocking solution containing 4% normal rabbit serum (NRS), 2.5% bovine serum albumin (BSA), and 1% Triton-X solution or 4% fish gelatin to reduce non-specific staining. The sections were rinsed in PBS, then incubated for 48 hours in a solution containing a 1:5,000 dilution of anti-choleragenoid (primary antibody, List Biological Laboratories, Campbell CA, Cat# 703, RRID: AB_10013220), 2% NRS, 2.5% BSA, and 1% Triton-X. The sections were then rinsed in PBS, and incubated in a 1:200 dilution of biotinylated rabbit anti-goat IgG (secondary antibody, Vector Laboratories, Burlingame CA, Cat# BA5000), 2% NRS, 2.5% BSA, and 2% Triton. After several rinses in PBS and a brief incubation in blocking solution, the tissue sections were incubated in a solution containing Standard Elite ABC Kit (Vector Laboratories, Burlingame CA, Cat# PK-6100). Finally the tracer was developed with diaminobenzidine, and sections were mounted on subbed slides, dehydrated and cleared in xylene, and coverslipped in Permount.
Sections from the remaining series were processed for cytochrome oxidase (CO) (Wong-Riley, 1979), Nissl substance, or synaptic zinc following the protocol described in Khalil and Levitt (2013). These were compared with adjacent CTb stained sections to assign cells to particular areas and layers.
Antibody characterization
The antibodies used in this study are listed in Table 1. The anti-cholera toxin B subunit is a polyclonal antibody raised in goat (primary antibody, List Biological Laboratories, Campbell CA, Cat# 703, RRID: AB_10013220). Reactivity to cholera toxin B subunit was confirmed by an immunodiffusion assay. The biotinylated rabbit anti-goat IgG is a polyclonal antibody raised in rabbit (secondary antibody, Vector Laboratories, Burlingame CA, Cat# BA5000). This antibody is purified by affinity chromatography using a goat IgG column, and cross-reactivities that are likely to interfere with specific labeling are removed by solid phase adsorption techniques. Cross-reactivity to various immunoglobulins is analyzed by solid phase immunoassay. Furthermore, the specificity and sensitivity of this antibody are also tested on a panel of tissues.
Table 1.
Antibodies used in this study
| Antibody | Source | Manufacturer | Dilution |
|---|---|---|---|
| Anti-Cholera Toxin B Subunit | Goat polyclonal | List Biological Laboratories, Campbell CA (Cat# 703) | 1:5,000 |
| Biotinylated anti-goat IgG | Rabbit polyclonal | Vector Laboratories, Burlingame CA (Cat# BA5000) | 1:200 |
Reconstruction of label
We relied on the following criteria to ensure that our injections were restricted to area 17 and did not intrude onto area 18 or white matter. The laminar location of the injection core was visually inspected using adjacent sections stained for Nissl, CO, or synaptic zinc to ensure that none of our cases intruded on white matter. Injection core was defined as the uniform, densely labeled region of CTb. We observed a typical pattern of label in all the layers (A, A1, and C) of the lateral geniculate nucleus (LGN) following our area 17 injections. Consistent with previous reports (Baker et al., 1998), the density of labeled cells was greatest in the two A layers, but a small number of cells were also present in the C layers. In contrast, if the injection intruded on area 18, there would be many more cells in the C-layers of the LGN. Furthermore, the lack of extensive label in ventral cortex (which results from area 18 injections: unpublished results) was interpreted as further evidence that the location of our injections was indeed restricted to area 17. All the cases analyzed in this study had injection cores that were restricted to area 17 without intruding onto area 18 or white matter.
Section outlines from every fourth semi-tangential section containing CTb label were traced, and retrogradely labeled cells found within each visual area were plotted in the Neurolucida tracing and reconstruction program (MicroBrightField, Williston VT, RRID: nif-0000-10294). Fiducial landmarks such as blood vessels were marked, and comparison of CTb tracings with adjacent CO, synaptic zinc, or Nissl stained sections was used for precise local alignment. CTb-labeled cells were then assigned to a cortical area and layer. Accurate laminar assignment of cells was further verified by measuring the distance from the pial surface to the bottom of particular layers and subsequently compared with similar measurements in adjacent CO, synaptic zinc, or Nissl stained sections. Three-dimensional reconstructions of section outlines containing CTb label were generated by carefully stacking and aligning tracings of serial sections containing retrogradely labeled cells. Vascular landmarks and other anatomical features were used to accomplish precise alignment by pairing two consecutive tracings. Correct determination of laminar and areal boundaries was critical to our analysis. We have previously shown that visual cortical areas in juvenile ferrets may reliably be distinguished in sections stained for synaptic zinc (Khalil and Levitt, 2013); observed areal boundaries in zinc-stained sections were well correlated with adjacent CO sections and prior descriptions (Innocenti et al., 2002). Histochemical methods did not always reveal sharp areal boundaries, but rather showed transition zones. Therefore, cells found in transition zones were assigned to border zones 18/19 and 19/21. The proportion of cells that were assigned to border zones was less than 2% of the total pool of feedback cells and were therefore not included in subsequent figures.
We note that oftentimes, clusters of feedback cells were offset from one another, which further facilitated the assignment of cells to areas. Sections containing CTb-labeled cells or stained for CO, zinc, or Nissl were examined and photographed with bright field illumination using a Nikon Eclipse Ti inverted scope with a low power (4×) lens. Contrast and brightness of photomicrographs were enhanced in image processing software (Adobe Photoshop CS5, v.12) for display purposes, but were otherwise unaltered. All figures were assembled in Adobe Photoshop (CS5, v.12), and all line graphs and histograms were generated in MATLAB (The Mathworks, Natick, MA, RRID: nlx_153890).
Cell counts and cell densities
Because of variation in the absolute number of labeled cells in extrastriate cortex due to differences in injection core size and extent of laminar intrusion, we determined the proportion of labeled feedback cells in each area as a fraction of the total pool of labeled cells to more accurately reflect developmental changes in the distribution across areas. The relative proportion of labeled cells located in each visual area was determined by dividing the number of cells in a given visual area by the total number of labeled cells in extrastriate cortex. Similarly, the proportion of labeled cells located in the different layers of each area was determined by dividing the number of labeled cells in the supragranular or infragranular layers of a given area by the total number of labeled cells found within that area. We then computed supragranular to infragranular ratios (supra/infra) to determine the relative contribution of the upper vs. lower layers, and used this ratio as a measure to assess developmental changes in the contribution of each set of layers. We also determined the proportion of feedback to area 17 arising from layer IV of each extrastriate area. We determined the peak density of feedback cells in each area, defined in the region in each area with the highest density within a cluster of cells in any section of the reconstructed stack (this was often found in at least 5–6 sections in the stack). A circumscribed area that contained the highest peak density was subsequently delineated with a 200 μm diameter circle. We chose a small area to include the highest peak density in the middle of the cluster, and to exclude cells in the periphery of the cluster. The volume of each sample was then calculated by multiplying the area of the circle by the section thickness (40 μm). Peak density values were then calculated using the sample volumes obtained.
To further assess the spatial distribution of feedback cells, we assessed the distribution of nearest neighbor distances (NND) of labeled cells in a given visual area. The nearest neighbor distance represents the distance of a single cell to that of the closest adjacent cell in a 2D plane within that visual area. We determined nearest neighbor distances within a 300 μm diameter circle centered over a cluster of feedback cells. This region includes cells in the densest region of the cluster as well as those in the periphery. We separately computed nearest neighbor distances in the supra- and infragranular layers in each area, and constructed frequency histograms of these values. To assess developmental changes in the spatial distribution of nearest neighbor distances, we computed the median and third quartile values (Q3) in each area at each developmental age. The Q3 is the value below which 75% of the NND values lie.
Stereological methods were used to determine neuronal density, using Nissl-stained sections from areas 17, 18, 19, 21, and SSy at 4 and 6 weeks of age, as well as in adults. Using Neurolucida, columns encompassing a region of interest were drawn perpendicular to the pial surface, extending to the white matter. Sample boxes were then randomly chosen in each column, superimposed on each of the five layers, except for layer 1, which is cell sparse. Sample boxes were 45 μm × 45 μm. Three sections were used from each animal, and two to three samples were obtained from each section. Neurons were counted using a light microscope at 100× oil immersion. Neurons with a reliably distinct nucleolus were counted through the depth of each box, with an exclusion zone of 4 μm at the top and bottom of the section. To minimize overcounting, nucleoli were counted if they fell entirely within the box, or touched the top and right sides; nucleoli touching the bottom and left sides of the counting box were excluded. Glial cells containing multiple granules (lacking clear single nucleoli) were not counted. Section shrinkage in the z-axis varied from 50 to 75% and the resulting sample volumes were adjusted accordingly.
To more explicitly reveal an underlying regularity in the spatial distribution of nearest neighbor distances we also computed a “conformity ratio” (CR), which is the mean of the NND divided by the standard deviation (Cook, 1996); the more regular the arrangement of neurons, the higher the CR. This ratio is immune to boundary effects as well as undersampling effects in the region of interest. To test if any of our NND distributions were significantly different from a random arrangement of neurons (at the p<0.01 significance level), we compared our values to Cook’s calculations of critical values of conformity ratios (Figure 2, Cook 1996).
All statistical analyses were performed in MATLAB (The Mathworks, Natick, MA). Initially, Barlett’s test was used to confirm that each data set had a normal distribution; we then assessed statistical differences between slopes (the slope of each line corresponding to the change with time of a particular measure in an area) by applying the ANCOVA (analysis of covariance) with a significance level of p<0.05. When a value of p<0.05 was obtained, post hoc pair-wise comparisons between slopes were then computed using a Bonferroni correction. Where appropriate, a non-parametric permutation test was used to address whether there was a significant correlation between other measured variables in each area and developmental age. The permutation test estimates the probability of obtaining our data by chance. Statistical testing consisted of randomly permuting the raw data points within a single visual area between different age groups 10,000 times (without replacement). On each iteration, a random reassignment is accomplished and a correlation is computed. After many iterations, we compared the distribution of reshuffled correlations with our observed correlation. Our observed correlation was deemed significant if it resulted in a p<0.05. We used a more stringent criterion value of p<0.01 to test for a significant change in the conformity ratios. A permutation test was separately conducted for each visual area. To asses statistical significance among age groups, areas, and layers in our neuron density counts we used the Kruskal-Wallis test with a significance level of p < 0.05. Subsequent post hoc comparisons were computed using a Bonferroni correction when significance was observed.
Results
All injection cases are summarized in Table 2 and 3. The injection core was defined as the uniformly dense region of CTb label, with a mean diameter of 1020 μm at all ages studied. We confirmed that all injection sites were confined to area 17, and spanned all six layers without intruding on the white matter. This was verified by comparing tracings of the injection core with adjacent sections stained for CO, synaptic zinc, and Nissl substance; this allowed us to identify the areal borders between areas 17 and 18 in juvenile visual cortex (Innocenti et al., 2002; Khalil and Levitt, 2013). Correct identification of visual cortical areas in the juvenile was crucial to our analysis as one of our primary goals was to track simultaneously the postnatal refinement of feedback projections from multiple visual areas to primary visual cortex.
Table 2.
Characteristics of injection cases
| Age | Case | Core diameter (μm) | Core volume (mm3) | Mean Core volume ( mm3) | Max DV extent (μm) | Max ML extent (μm) | Laminar intrusion | Total # labeled cells | Normalized # labeled cells | Mean # labeled cells |
|---|---|---|---|---|---|---|---|---|---|---|
| 4 wks | 219 | 800 | 0.32 | 900 | 480 | Mostly SG/very little IG | 1180 | |||
| 220 | 900 | 0.42 | 1.156 | 1000 | 800 | More SG/little less IG | 2412 | 2271 | 2644 | |
| 247 | 1450 | 2.73 | 2300 | 1280 | Equal uptake SG & IG | 4340 | ||||
| 5 wks | 231 | 1100 | 1.54 | 1500 | 1280 | Equal uptake SG & IG | 4912 | |||
| 241 | 900 | 1.03 | 1.193 | 1000 | 960 | Mostly SG/very little IG | 3642 | 3225 | 3848 | |
| 249 | 1000 | 1.01 | 1500 | 960 | Mostly SG/some IG | 2990 | ||||
| 6 wks | 255 | 1100 | 1.26 | 1.92 | 1500 | 800 | Equal uptake SG & IG | 3683 | 6193 | 11890 |
| 256 | 1500 | 2.58 | 2400 | 1280 | Equal uptake SG & IG | 8207 | ||||
| 7 wks | 215 | 950 | 0.97 | 1.04 | 1600 | 800 | Equal uptake SG & IG | 4850 | 3668 | 3815 |
| 248 | 900 | 1.11 | 1300 | 800 | Mostly SG/some IG | 2779 | ||||
| 8 wks | 200 | 850 | 0.46 | 800 | 640 | Mostly SG/some IG | 1221 | |||
| 229 | 800 | 0.46 | 0.446 | 900 | 800 | Mostly SG/very little IG | 2200 | 4626 | 2066 | |
| 235 | 700 | 0.42 | 1000 | 640 | Mostly SG/some IG’ | 2778 | ||||
| 10 wks | 250 | 1600 | 2.51 | 2300 | 1440 | Equal uptake SG & IG | 15351 | |||
| 251 | 600 | 0.21 | 1.34 | 700 | 480 | Mostly SG/very little IG | 1677 | 4808 | 6443 | |
| 258 | 1000 | 1.30 | 1300 | 640 | Mostly SG/some IG | 2300 |
Table 3.
Areal and laminar proportion of feedback cells in all injection cases
| Age | Case | Area 18 # cells %Supra/Infra | Area 19 # cells %Supra/Infra | Area 21 # cells %Supra/Infra | Area Ssy # cells %Supra/Infra | Area 18/19 border | Area 19/21 border |
|---|---|---|---|---|---|---|---|
| 4 wks | 219 | 228 / 0.62 / 0.33 | 136 / 0.46 / 0.54 | 273 / 0.24 / 0.75 | 535 / 0.42 / 0.33 | ||
| 220 | 850 / 0.65 / 0.16 | 553 / 0.56 / 0.44 | 219 / 0.73 / 0.27 | 790 / 0.56 / 0.26 | |||
| 247 | 1285 / 0.64 / 0.23 | 888 / 0.41 / 0.22 | 866 / 0.64 / 0.21 | 1208 / 0.53 /0.29 | 31 | 25 | |
| 5 wks | 231 | 810 / 0.31 / 0.43 | 1178 / 0.36 / 0.42 | 943 / 0.35 / 0.60 | 1690 / 0.54 / 0.35 | ||
| 241 | 1736 / 0.30 / 0.44 | 900 / 0.39 / 0.41 | 180 / 0.34 / 0.66 | 808 / 0.50 / 0.25 | |||
| 249 | 944 / 0.31 / 0.49 | 138 / 0.38 / 0.60 | 594 / 0.60 / 0.40 | 1186 / 0.42 / 0.33 | 19 | ||
| 6 wks | 255 | 2109 / 0.24 / 0.53 | 727 / 0.29 / 0.55 | 365 / 0.36 / 0.53 | 455 / 0.40 / 0.37 | 18 | |
| 256 | 2544 / 0.36 / 0.47 | 1688 / 0.28 / 0.64 | 892 / 0.14 / 0.86 | 2359 / 0.35 / 0.50 | 53 | ||
| 7 wks | 215 | 1631 / 0.21/ 0.61 | 1137 / 0.28 / 0.63 | 969 / 0.41 / 0.59 | 947/ 0.36 / 0.44 | ||
| 248 | 1590 / 0.21 / 0.53 | 313 / 0.47 / 0.35 | 150 / 0.08 / 0.98 | 699 / 0.29 / 0.34 | |||
| 8 wks | 200 | 743 / 0.17 / 0.53 | 235 / 0.26 / 0.65 | 92 / 0.29 / 0.65 | 151 / 0.26 / 0.44 | ||
| 229 | 1166 / 0.39 / 0.46 | 489 / 0.27 / 0.49 | 154 / 0.32 / 0.58 | 391 / 0.39 / 0.47 | |||
| 235 | 1092 / 0.25 / 0.52 | 710 / 0.37 / 0.47 | 314 / 0.31 / 0.61 | 639 / 0.26 / 0.48 | |||
| 10 wks | 250 | 6296 / 0.22 / 0.52 | 2935 / 0.27 / 0.34 | 1818 / 0.16 / 0.77 | 2805 / 0.29 / 0.45 | 130 | 234 |
| 251 | 1078 / 0.24 / 0.43 | 267 / 0.24 / 0.55 | 130 / 0.26 / 0.82 | 202 / 0.32 / 0.49 | |||
| 258 | 1315 / 0.26/ 0.49 | 419 / 0.15 / 0.80 | 285 / 0.33 / 0.66 | 281 / 0.38 / 0.48 |
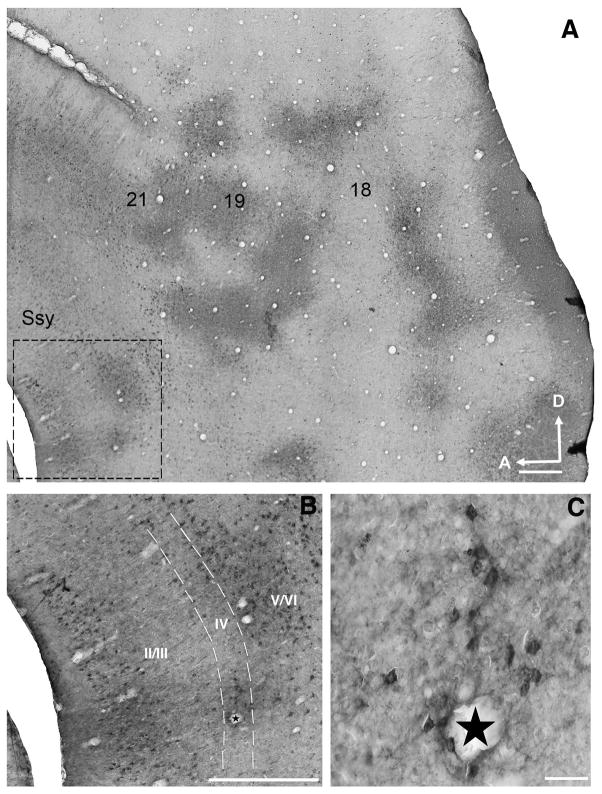
The pattern of feedback labeling in extrastriate cortex of a juvenile ferret (5 weeks old) is depicted in a representative photomicrograph of a semi-tangential section in Fig. 1A. The numbers indicate visual areas that provide substantial feedback to area 17. We find that in all areas examined, feedback cells and feedforward terminal clusters are typically found at corresponding locations, reflecting the reciprocity of connections between primary visual cortex and these extrastriate visual areas. Genuine cell label was distinguished from nonspecific labeling by its greater intensity and discontinuous cellular distribution. The ringlike pattern of feedback label found in Fig. 1A is qualitatively similar to the pattern of label we observe in the visual cortex of the adult. Fig. 1B is a higher magnification image of the dashed rectangle in panel A within the Suprasylvian region (Ssy); feedback cells can be found throughout all laminae. Although the majority of feedback cells were found in layers II/III and V/VI, labeled cells are also found in layer IV (see Fig. 3C below). Fig. 1C is a higher magnification image of layer IV of Ssy with labeled feedback cells.
Figure 1. Representative feedback label in extrastriate cortex in a 5 week old ferret.
Photomicrographs of a semi-tangential brain section showing the typical pattern of feedback labeling in extrastriate cortex after a CTb injection in area 17. A: Label resulting from an area 17 injection site (not shown) with overlapping clusters of orthogradely labeled terminals and retrogradely labeled cells in areas 18, 19, 21, and Ssy. Dashed square indicates region shown at higher magnification in B. B: Laminar distribution of feedback cells in Suprasylvian cortex (Ssy). Dashed lines indicate boundaries of layer IV. Most labeled cells are found in the infragranular layers, but supragranular and layer IV cells are labeled as well. C: High power photomicrograph of feedback cells in area 18. Arrows point to feedback cells. Stars in panels B and C indicate corresponding blood vessel. Ssy: Suprasylvian cortex, A: anterior, D: dorsal. Scale in A= 500 μm, scale in B= 200 μm, scale in C= 50 μm.
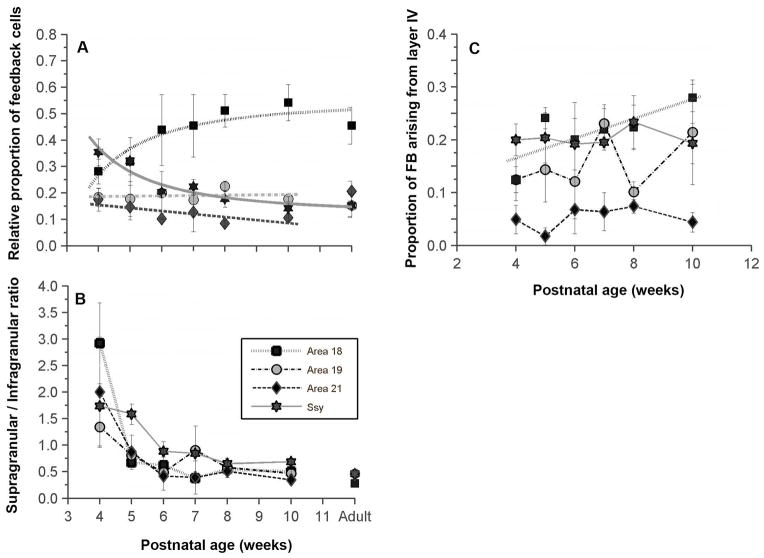
Figure 3. Developmental changes in the areal and laminar distribution of feedback cells in the visual cortex of juvenile ferrets.
A: Proportion of feedback projections to area 17 arising from areas 18, 19, 21, and Ssy as a function of age. Before eye opening, Ssy makes the largest feedback contribution to area 17; by 6 weeks postnatal, area 18 feedback input is largest. B: Ratio of the proportion of feedback to area 17 arising from supragranular to that of infragranular layers in each area. The supragranular contribution to feedback declines synchronously in all extrastriate areas from 4 to 6 weeks. C: The proportion of feedback arising from layer IV in each area changes little in this period, except for feedback from area 18 whose layer IV contribution increases. Adult mean values are plotted on the right for comparison in panels A and B. Error bars represent ±SEM
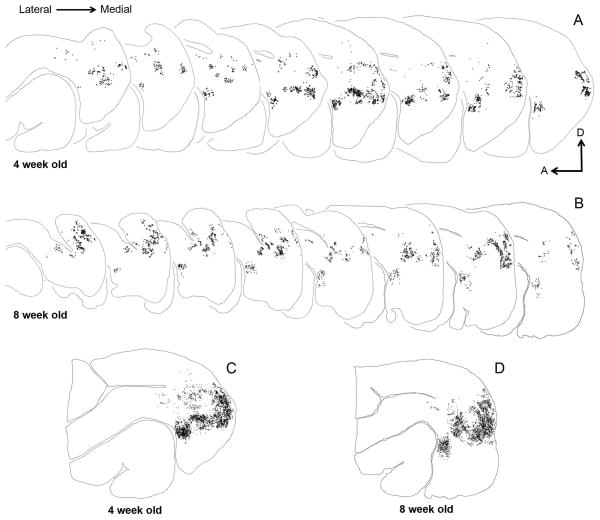
To examine the overall pattern of feedback label in extrastriate cortex, and to assess developmental changes in the pattern, we generated serial reconstructions of semi-tangential sections in a young and an older juvenile (Fig. 2). We accomplished this by outlining the contour of every fourth section, and plotting every labeled feedback cell in extrastriate cortex. Each dot represents a single feedback cell. In both reconstructions (Fig. 2A, B), superficial sections are to the left with successive sections being more medial. Fiducial landmarks and blood vessels were used for precise local alignment of all the serial sections in Fig. 2A and 2B to yield a collapsed image of the overall pattern in Fig. 2C and 2D. One clear and striking similarity between the 4 week old (Fig. 2C) and 8 week old (Fig. 2D) reconstructions is the stereotypical ring-like pattern of feedback label that is qualitatively similar to the pattern observed in adult ferret visual cortex (Cantone et al., 2005). Therefore, it appears that by 4 weeks of age (shortly before eye opening) the pattern of feedback label is broadly comparable to the feedback pattern of adult labeling for an injection at a similar retinotopic location. We observed this ring-like pattern at all postnatal ages studied.
Figure 2. Serial reconstructions of retrogradely labeled cells in the visual cortex of juvenile ferrets.
The semi-tangential sections are arranged serially (lateral=left). Black dots represent retrogradely labeled cells. A, B: Feedback label in every fourth section in a 4 week old (A) and an 8 week old (B) ferret. C, D: Superimposed and aligned images of the serial sections from the reconstructions shown in A and B to reveal the complete pattern of label. At 4 weeks postnatal, the overall label pattern resembles that seen in the adult. The sparse labeling anterior to area 21 and dorsal to the suprasylvian area (Ssy) is located in areas PPr and PPc. A: anterior, D: dorsal.
We first asked whether the absolute number of extrastriate cells providing feedback to area 17 decreases with age. Table 2 shows that mean injection volumes were essentially the same at all ages studied (with the exception of 8 week olds, in which injection volumes were somewhat smaller than at other ages). We determined the total number of feedback cells labeled in extrastriate cortex as a function of injection core total volume (reasoning that injection core total volume was a better metric than maximal tangential extent, as the spread of CTb tracer is usually anisotropic). The total number of feedback cells shown in Table 2 reflects cells that were counted in every 4th section. We found a highly significant linear correlation between the total number of labeled cells in extrastriate cortex and injection core total volume (r2= 0.82, p =0.0006), with no differences among areas in this relationship. Since absolute number of labeled cells in extrastriate cortex scaled linearly with injection core volume, we determined a normalized number of labeled cells at each age by using the regression line to determine the number of cells in each case if the injection volume had been 1 mm3. Normalized cell counts for each age group are recorded in Table 2. With the exception of a few cases (250, 256) that had larger injection cores and thus a greater number of labeled cells, there was no obvious change with age in the number of cells in extrastriate cortex providing feedback to area 17. Therefore, it appears that at all of the postnatal ages studied here, similarly sized injection cores in area 17 yielded comparable number of labeled feedback cells in extrastriate cortex. We see no compelling evidence for a decrease with age in absolute numbers of feedback cells.
Given that the total number of feedback cells resulting from an area 17 injection appears not to change significantly with age, we wondered what spatial features of the feedback pool change throughout development. In the adult ferret, Cantone et al. (2005) have shown that area 18 provides the greatest proportion of the total number of cells providing feedback to area 17 (mean 45.5%). Areas 19, 21, and Ssy each provide equivalent smaller feedback contributions. We were therefore interested in revealing whether the proportion of feedback arising from each visual area changes throughout postnatal development. Secondarily, we wanted to know whether the refinement of feedback connections in each visual area proceeds at a similar rate or if each visual area has a unique developmental trajectory. Since absolute number of labeled feedback cells could vary with injection core size and laminar intrusion, the proportion of feedback from each area is a normalized measure that more appropriately reflects changes in the total pool of feedback to area 17.
The change in the relative proportion of feedback connections arising from each visual area as a function of age is plotted in Fig. 3A. Adult values are plotted on the right for comparison. Unlike the adult, in which area 18 (black squares) provides the greatest proportion of feedback, at 4 weeks of age we find that area Ssy provides the greatest feedback input (about 36% of the total), somewhat greater than that from area 18. Within 2 weeks, by the age of 6 weeks (soon after eye opening), the adultlike distribution of feedback is attained: area 18 provides the greatest amount of feedback (mean 44%), while areas 19, 21, and Ssy provide roughly the same amount of feedback (areal means range from 10%–20%).
The proportion of feedback arising from area 18 increased throughout development and was best fit with a hyperbolic function (r2= 0.8308), while the proportion of feedback arising from Ssy decreased throughout development and was similarly fit with a hyperbolic function (r2= 0.9114). Although the period of greatest increase in the proportion of feedback arising from area 18 is between 4–6 weeks of age, 95% of the peak value is achieved by 8 weeks of age. Similarly, the period of major decline in the proportion of feedback arising from Ssy is between 4–6 weeks of age, and 95% of the decline to minimum value is achieved by 10 weeks of age. There was no significant change with age in the feedback contribution made by areas 19 and 21 (r2= 0.021 and r2= 0.51, respectively). After 6 weeks, there is little change in the proportion of feedback arising from different cortical areas. A permutation test was separately conducted for each area to determine whether there was a significant correlation between the proportion of feedback in each area and developmental age.
The permutation test estimates the probability of obtaining our data by chance. Statistical testing consisted of randomly permuting the raw data points within a single visual area between different age groups 10,000 times (without replacement). This repeated random reassignment between age groups was used to test the null hypothesis that there is no relationship between proportion of feedback and developmental age. The permutation test yielded a p<0.0032 for area 18, p<0.5077 for area 19, p<0.05832 for area 21, and p<0.0002 for Ssy. Thus, there is a significant change with age in the proportion of feedback to area 17 arising from areas 18 and Ssy, but there is not a significant change in the proportion of feedback arising from areas 19 and 21. Feedback cells were also labeled in the lateral temporal (LT), posterior parietal (PP), and auditory areas in the younger animals. The proportion of feedback deriving from PP was about 3%, 6% in LT, and less than 1% in auditory cortex. These small contributions did not appear to change with age. We also assessed the developmental change in the laminar distribution of feedback cells in each visual area. Fig. 3B shows the ratio of the proportion of feedback arising in each area from the supragranular layers to that from the infragranular layers. Higher supra/infra ratios reflect a greater proportion of feedback arising from the supragranular layers. At 4 weeks of age, the majority of labeled cells in all areas were found in the supragranular layers; area 18 seems to have the greatest proportion of feedback arising from the supragranular layers, while area 19 appears to have a more balanced contribution from the supragranular and infragranular layers (ratio 1.3).
A permutation test was separately conducted for each area to determine whether there was a significant correlation between the ratio of supra- to infragranular neurons in each area and developmental age. The permutation test yielded a p<0.0032 for area 18, p<0.0077 for area 19, p<0.01832 for area 21, and p<0.0002 for Ssy. Thus, with age, there is a significant change in the supragranular contribution resulting in lower supra/infra ratios. By 6 weeks of age, similar to the areal rearrangements described above, the laminar pattern of feedback appears similar to that in the adult; the infragranular contribution to feedback was dominant in all extrastriate areas.
We consistently found labeled feedback cells in layer IV of all the visual areas that provide input to area 17. We therefore quantified the relative proportion of feedback cells in layer IV of areas 18, 19, 21, and Ssy to determine if the contribution from layer IV changes with age (Fig. 3C). Areas 18, 19, and Ssy had a substantial proportion of their feedback arise from layer IV (means 10–20%). At all ages, Layer IV of area 21 seems to provide a minor feedback contribution (roughly 5%). We performed a permutation test to reveal if there was a significant change with age in the proportion of feedback arising from layer IV of each visual area. We found that the proportion of feedback arising from layer IV of areas 19, 21, and Ssy does not change significantly with age (area 19: p=0.297; area 21: p=0.298; Ssy: p=0. 369). However, we found that the increase in the proportion of feedback arising from layer IV of area 18 is statistically significant (p=0.01).
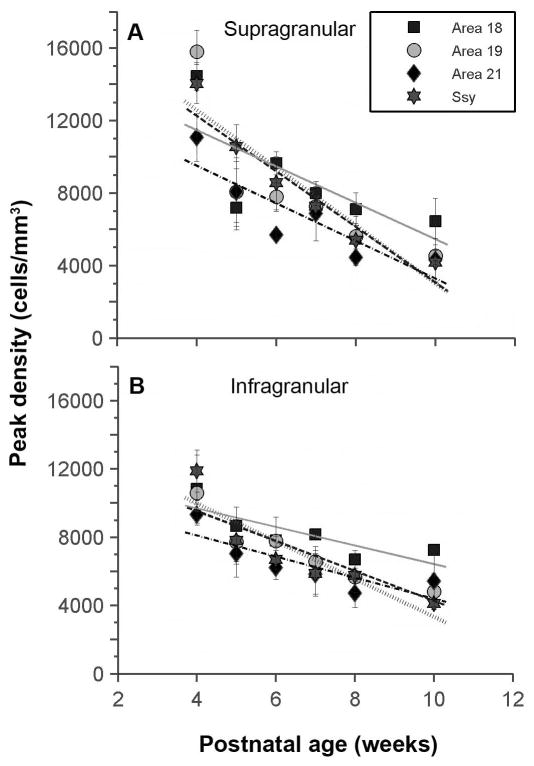
We next compared the peak densities of labeled cells in these areas at different developmental stages. We defined the peak density within a cortical area as the region of highest density in any one of the reconstructed sections, delineated with a 200 μm diameter circle. The peak density of feedback cells in the supragranular layers of all areas at 4 weeks of age is initially very high (mean=13,840 cells/mm3) (Fig. 4A). The peak density of feedback cells in the supragranular layers of all areas examined decreased monotonically with age. By 10 weeks of age, the peak densities declined dramatically (mean=4,860 cells/mm3). Comparison of the regression lines for each area’s data revealed that the slopes were not significantly different from each other (ANCOVA, p=0.6004); this indicates that feedback cell density declined at a similar rate in all areas. We similarly plotted the peak density of feedback cells in the infragranular layers of all areas as a function of age (Fig. 4B). The peak density at 4 weeks of age averages 10,600 cells/mm3 across all areas. Like the supragranular layers, the peak density of cells in infragranular layer also declines linearly, albeit at a slower rate. We likewise found that the slopes were not significantly different from each other (ANCOVA, p=0.2730), indicating indistinguishable rates of feedback loss.
Figure 4. Developmental decreases in the peak densities of labeled cells within the cortical areas providing feedback to area 17.
Peak density of feedback cells in the supragranular (A) and infragranular (B) layers of areas 18, 19, 21, and Ssy declines monotonically with age. Regression lines are plotted for each visual area. Error bars represent ±SEM.
Declining density of feedback cells could result either because a fixed proportion of cells in the neuropil provide feedback but overall neuronal density declines with age, or because neuron density remains unchanged but fewer of those cells furnish feedback axons to area 17. To distinguish these possibilities, we measured neuronal density in Nissl-stained material from each extrastriate area. We find that in area 18, neuronal density in adults declined to about 49%–65% of the value in 4 week olds (layer II: 280,000 to 140,000 cells/mm3; layer III: 210,000 to 100,000 cells/mm3; layer V: 110,000 to 75,000 cells/mm3; layer VI: 200,000 to 120,000 cells/mm3. Similarly, in area Ssy, neuronal density in adults declined to 50–73% of the value in 4 week olds (layer II: 240,000 to 120,000 cells/mm3; layer III: 160,000 to 115,000 cells/mm3; layer V: 120,000 to 77,000 cells/mm3; layer VI: 160,000 to 110,000 cells/mm3. We found no clear evidence for declines in cell density in areas 19 and 21. For comparison, by 10 weeks postnatal peak density of feedback cells declined more: in areas 18 and Ssy to about 43% and 29% (respectively) of the 4 week old value in the supragranular layers, and to about 64% and 33% in the infragranular layers; in areas 19 and 21, the declines in feedback cell density were to 25–50% of the 4 week value (Fig. 4). Cantone et al. (2005) reported even lower peak density of feedback cells in these areas in the adult. Thus, the developmental decline in density of feedback cells may in part reflect decreasing neuronal density. However, this cannot be entirely attributed to declines in overall neuronal density (presumably reflecting not neuronal loss but rather brain growth due to increased volume occupied by axons, dendrites, and glial cells).
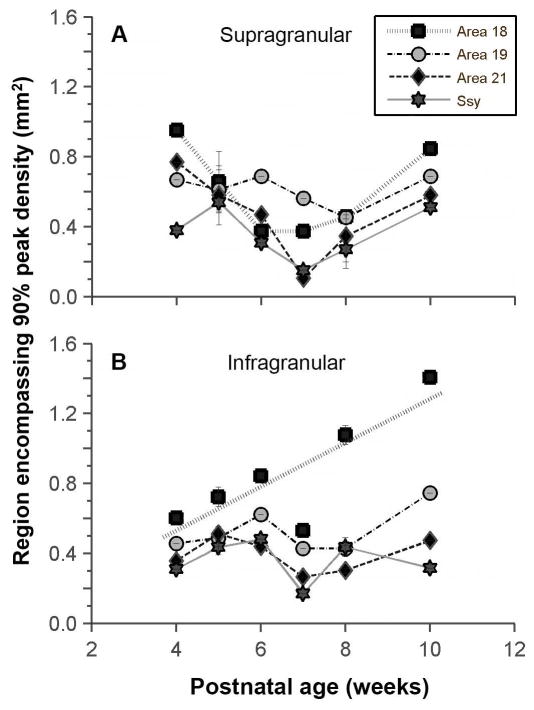
The decline in peak density could reflect an increase with age in the tangential areal extent of the region providing feedback. We determined whether the size of the region in each area encompassing 90% of the peak density of feedback cells changes with age. Fig. 5A shows that there is no significant change with age in the areal extent of this region in the supragranular layers of all areas examined (permutation test, area 18 p=0.661; area 19=0.713; area 21 p=0.785; Ssy p=0.829). Fig. 5B similarly shows that the areal extent of the region that encompasses 90% of the peak density of feedback cells in the infragranular layers remains unchanged throughout development in areas 19, 21, and Ssy (area 19 p=0.156; area 21 p=0.486; Ssy p=0.793). However, in area 18 the areal extent of the region that encompasses 90% of the peak density in the infragranular layers does appear to increase with age (permutation test, p=0.004; r2=0.62).
Figure 5. Developmental changes in the tangential extent of feedback label.
Area of region that encompasses 90% of the peak density of feedback cells in the supragranular (A) and infragranular (B) layers of areas 18, 19, 21, and Ssy as a function of age. With the exception of infragranular area 18, the region in each cortical area furnishing area17 feedback remains essentially unchanged in extent. Regression line is plotted for area 18. Error bars represent ±SEM.
We next assessed developmental changes in the spatial distribution of the feedback population by quantifying nearest neighbor distances (NND) between cells in each visual area providing feedback to area 17. For every cell within a region of interest, the distance to its nearest neighbor was computed and a frequency histogram for all the nearest neighbor distances was subsequently constructed. This measure also indicates the density of feedback projections from each visual area (high NND values reflecting lower cell density), but also reveals more about the spatial layout of the feedback pool. Specifically, it can reveal if the distribution reflects spatial randomness, clustering, uniform tiling, or a more dispersed distribution.
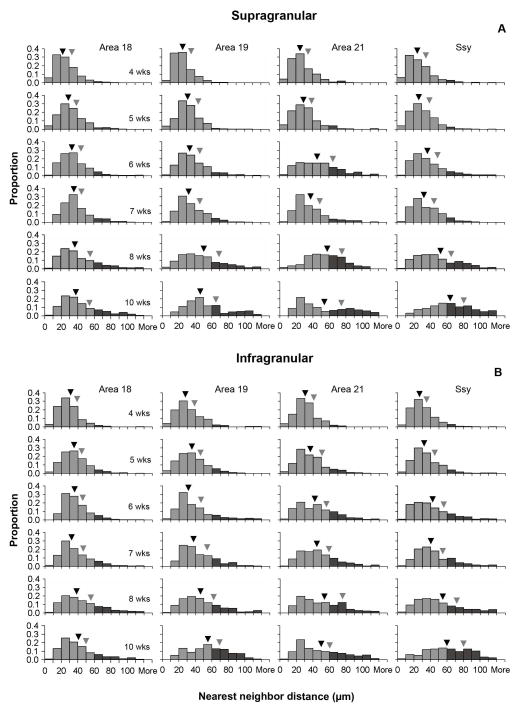
Figures 6A and 6B illustrate the distribution of nearest neighbor distances between feedback cells in the supra- and infragranular layers of each area at all postnatal ages studied. The black and gray arrowheads indicate the median and third quartile values, respectively. The shape of the distribution is positively skewed for all areas at all postnatal ages. However, at younger ages (4 and 5 weeks), the distribution of NNDs is more peaked, with lower median NND values in all areas. With age, the median NND values as well as the Q3 values shift towards longer distances. The shape of the distribution also changes with age; the tail of the distribution becomes more prominent, indicating that with age the proportion of longer NND distances increases (indicated by bins with dark shading, representing values exceeding 60 μm).
Figure 6. Developmental changes in the distribution of nearest neighbor distances (NNDs) between feedback cells in juvenile visual cortex.
Frequency distribution histograms of NNDs in the supragranular (A) and infragranular (B) layers of areas 18, 19, 21, and Ssy. Dark shaded bins represent NND values exceeding 60 μm. Black arrowheads indicate median NND values, and gray arrowheads indicate the 3rd quartile NND values. In all areas, with age there is an increase in median NND, and an increase in the proportion of NNDs exceeding 60 μm.
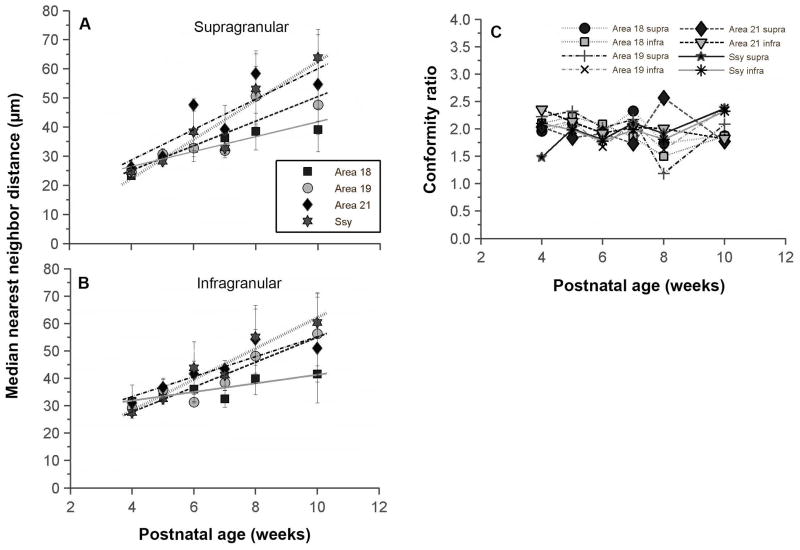
We plotted the median nearest neighbor distance value in the supragranular layers of each visual area as a function of age (Fig. 7A). At 4 weeks of age, the median NND value in all areas is quite low and similar in all areas (mean= 25 μm). To 10 weeks postnatal, the median NND value appears to increase in all areas to a value between 40–60 μm, and the median NND values seem to diverge among areas. We compared regression lines among areas, and find that there are no significant differences among the slopes (ANCOVA, p=0.1205). We also plotted the change with age in median nearest neighbor distance in the infragranular layers of each visual area (Fig. 7B). At 4 weeks of age, the median NND value in all areas is quite low and similar in all areas (mean= 30 μm). We find that there is a significant slope difference between area 18 and Ssy (ANCOVA, posthoc test; p=0.009); the increase in the median NND value in the infragranular layers of area 18 seems to occur at a slower rate than in Ssy. Therefore, it appears that the median NND value in the supragranular and infragranular layers of all areas share a broadly similar developmental trajectory. However, the increase in the median NND values in the infragranular layers of all areas seems to proceed at a slower rate.
Figure 7. Age dependent increase in the median nearest neighbor distance (NND) between feedback cells in the juvenile ferret visual cortex.
A, B: Median nearest neighbor distance between feedback cells in the supragranular (A) and infragranular (B) layers of areas 18, 19, 21, and Ssy as a function of age. Median NND increases monotonically at the same rate in all areas. Regression lines are plotted for each visual area. Error bars represent ±SEM C: Conformity ratio (mean NND/Standard deviation of NND) in supra- and infragranular layers of each area as a function of age. This measure of regularity of spacing between feedback cells remains unchanged with age, and provides no strong evidence for a nonrandom distribution of cells.
The nearest neighbor analysis is informative in revealing details of how the spacing of neurons changes with age. To more explicitly reveal an underlying regularity in the spatial distribution of nearest neighbor distances we also computed conformity ratios (Cook, 1996) for our NND samples. This measure is the mean of the NND divided by the standard deviation; the more regular the arrangement of neurons, the higher the conformity ratio. In (Fig. 7C) the conformity ratios in the supra- and infragranular layers of areas 18, 19, 21, and Ssy are plotted as a function of age. The conformity ratios appear to fluctuate around the value of 2 during this postnatal period, but appear not to change with age. A permutation test was separately conducted for each area to assess whether there was a significant correlation between the conformity ratio in each area and developmental age. We found no significant change in conformity ratio with age.. We also tested whether conformity ratios for any of the NND distributions were significantly different from a random arrangement. Almost all conformity ratio values were not significantly different from complete randomness (p<0.001: although conformity ratios for at least one area at each postnatal age suggested statistically significant deviation from randomness). Thus, during this postnatal period while peak density declines and nearest neighbor distances increase, the underlying regularity of the feedback pool in each area seems indistinguishable from a random arrangement, and does not change with age.
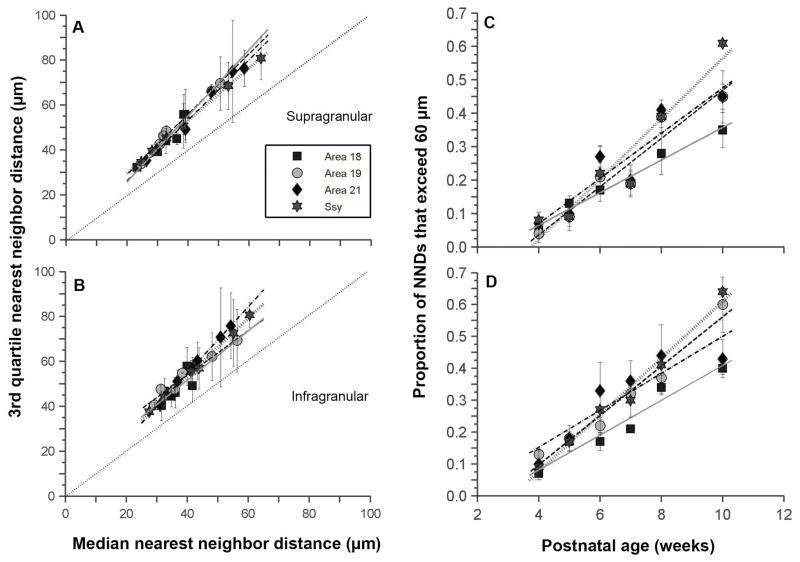
To further examine the refinement of feedback connections, we used the 3rd quartile (Q3) value in conjunction with the median NND value to better characterize the change in the shape of the NND distribution. An increase in the median NND with age reflects a decrease in the density of feedback cells. However, this measure alone does not inform about the underlying distribution. The median NND value could conceivably increase without reflecting a change in the distribution. For example, with age the entire distribution of NND values could simply shift to higher values, and subsequently yield a higher median NND value; in this case the Q3 value would increase by the same amount as the median value. However, if the change in the Q3 value differs from that of the median NND value, this suggests a change in the shape of the NND distribution (and hence the underlying arrangement of feedback cells). We plotted the Q3 value in the supragranular layers of each area as a function of median NND value (Fig. 8A); individual data points represent values at different ages. The dashed line through the origin with a slope of one indicates equal change in Q3 and median values. We find a strong correlation between median NND and the Q3 value in each area (area 18, r2 =0.90: area 19, r2 =0.99; area 21, r2 =0.98; Ssy, r2 =0.99). The slopes do not differ significantly among areas (ANCOVA, p=0.147). However, the slopes do differ significantly from 1 (slope test, p=0.01). Thus, in all areas examined, the rate at which the Q3 value increases is greater than that for median NND. The same holds in the infragranular layers (Fig. 8B). We find a strong correlation between median NND and Q3 value (area 18, r2 =0.55: Area 19, r2 =0.95; area 21, r2 =0.99; Ssy, r2 =0.99). The slopes do not differ significantly among areas (ANCOVA, p=0.064). However, the slopes do differ significantly from 1 (slope test, p=0.01). Taken together, the results suggest that both the median and Q3 NND value in the supragranular and infragranular layers of all areas examined increase monotonically with age, but the Q3 value increases at a greater rate. The greater rate of increase in the Q3 value confirms a change in shape of the NND distribution (emergence of larger values), rather than a simple shift of the entire distribution to larger values.
Figure 8. Developmental changes in the distribution of nearest neighbor distances (NND) between feedback cells in juvenile ferret visual cortex.
A, B: Correlation between the 3rd quartile NND values (Q3) and median NND values in the supragranular (A) and infragranular (B) layers of areas 18, 19, 21, and Ssy. In all areas, the Q3 NND similarly increases with age faster than the median NND, indicating that the distribution of NNDs is not simply uniformly shifting with age to larger values, but is also specifically developing a subset of larger NND values. C, D: The proportion of NNDs in the supragranular (C) and infragranular (D) layers of areas 18, 19, 21, and Ssy that exceed 60 μm as a function of age. These increase with age at the same rate in all areas. Regression lines are plotted for each visual area. Error bars represent ±SEM
We quantified the emergence of these longer nearest neighbor distances among feedback cells in each area. We chose a criterion distance of 60 μm, since at 4 weeks of age almost no NNDs exceeded this value. We determined whether the proportion of NNDs in each area that exceeded 60 μm changed with age. In both the supra- and infragranular layers of all areas examined, the proportion increased linearly (Fig. 8C, 8D). At 4 weeks of age, the proportion of NNDs that exceed 60 μm is on average 6–10%. However, by 10 weeks of age, that proportion rises considerably (areal mean range between 35% to 64%). The rate of increase of this population did not significantly differ among areas in the supragranular layers (ANCOVA, p=0.103). The rate of increase similarly did not differ among areas in the infragranular layers as well (ANCOVA, p=0.0472).
Discussion
We have shown substantial refinement in the spatial organization of feedback projections from multiple visual areas to ferret primary visual cortex during the period after eye opening. There is major reorganization in the areal and laminar proportion of feedback arising from each visual area from 4 to 6 weeks postnatal (in the period after eye-opening). However, peak density declines, and both median and 3rd quartile NND values increase monotonically from 4 to 10 weeks postnatal. Our results also indicate that much of the overall pattern of feedback label in extrastriate cortex is present as early as four weeks postnatal, before the eyes open. Our results are in agreement with previous studies showing that feedback projections undergo a period of prolonged remodeling (Barone et al., 1995; Batardiere et al., 1998, 2002; Burkhalter, 1993). Our results provide evidence for a developmental process operating with the same time constant in all visual areas to refine peak density and nearest neighbor distances among feedback cells, with another process governing the refinement of areal and laminar distribution of feedback projections to area 17. But there may be differences among areas in these refinement processes even if they occur at similar rates; for example, the decline in peak density of feedback cells in area 18 can be largely attributed to a reduction in the overall neuronal density, while the decline in peak density of feedback cells in area 19,21, and Ssy cannot be so explained as neuronal density declines little if at all in those areas. This suggests feedback pathways may differ in their initial exuberance and subsequent pruning of projections.
1) Relationship to feedback circuitry in the adult ferret
In the adult ferret, Cantone et al. (2005) have shown that area 18 provides the greatest proportion of the total number of cells providing feedback to primary visual cortex. Areas 19, 21, and Ssy each provide similar smaller feedback contributions. However, at four weeks of age, we find that area Ssy provides the greatest proportion of feedback to primary visual cortex. Moreover, in the adult ferret, the majority of feedback cells are found in the infragranular layers with fewer cells in the supragranular layers. The inverse relationship exists in the juvenile ferret; at the youngest age examined (4 weeks postnatal) the contribution from the supragranular layers is greater than that from the infragranular layers. Our results are in agreement with previous studies showing that feedback cells are more numerous in the supragranular layers of visual cortical areas early in development (Barone et al., 1995; Batardiere et al., 1998). Peak density of feedback cells in both the supragranular and infragranular layers was roughly six to eight times greater in each area at four weeks of age than in the adult (adult values obtained from Cantone et al., 2005 who did not distinguish supra- and infragranular densities). Peak density of feedback cells in the supragranular and infragranular layers declines monotonically with age, yet adult values are not reached by ten weeks of age. The magnitude of decline in peak density is far greater from 4 to 10 weeks postnatal, with a modest decline occurring between 10 weeks and adulthood. Thus, it appears that there is a prolonged period that extends later than ten weeks in which the peak density continues to decrease.
The decline in peak density of feedback projections we observe is partly due to the decline in overall neuronal density (presumably because of brain growth and not cell death). However, the developmental decline in peak density cannot be entirely attributed to simple declines in overall neuronal density. We also show that the area encompassing 90% of the peak density does not change significantly with age (except for area 18, which increases with age). These data may seem incompatible. However, the region used to calculate the peak density in each area was a 200 μm diameter circle, while the region used to calculate the NND values encompassed more of the feedback cluster (300 μm diameter circle). Furthermore, it is certainly possible that while the region in each cortical area encompassing 90% of the peak density does not change with age, the total extent of the region containing the feedback pool does expand with age. Alternatively, if the region encompassing 90% of the peak density does not change with age, yet peak density declines, it is possible that there is selective pruning of feedback cells in the periphery of the region.
Our nearest neighbor analysis data corroborate our peak density counts; median nearest neighbor distance is substantially lower at four weeks of age and increases monotonically with age in all areas. In all areas examined, from 4 to 10 weeks of age peak density of feedback cells in the supragranular and infragranular layers declined on average by a factor of 2.25, while the median NND increased by a similar factor of 1.90. However, the change in the NND distributions can not be entirely attributed to the decline in peak density. Firstly, a decline in density would lead to a simple rightward shift in the distributions of NNDs (i.e. to larger values), yet we find that the shapes of the distributions change to become more skewed.. Secondly, because of the difference in size of the region used to calculate the peak density and NNDs (200 μm vs. 300 μm), selective pruning of cells in the periphery of cluster (300 μm) could account for the additional changes we observe in the NND distributions (i.e longer NNDs). To our knowledge, this is the first study to quantify the nearest neighbor distances of feedback cells in multiple visual areas throughout development. Cantone et al. (2005) did not quantify the spatial distribution of feedback cells in the adult ferret, therefore we cannot unequivocally claim that the nearest neighbor values at ten weeks of age are comparable to adult values. Interestingly, the authors did claim that feedback label in Ssy of the adult was observed as sparse. This is in agreement with our NND analysis; the median NND value in Ssy in both the supra- and infragranular layers increased to ten weeks of age. Our data also suggest that NNDs do not simply uniformly increase; the emergence with age of a population of longer NNDs suggests the selective retention of a minority population of feedback cells more spatially segregated from the rest of the feedback pool in each area. This could reflect a selective developmental loss of inappropriate feedback connections from extrastriate visual areas to primary visual cortex to attain the adult-like spatial configuration.
2) Comparison with other aspects of anatomical development in the ferret
A number of other anatomical features appear to refine during this same postnatal period shortly after eye opening. We have shown (Khalil and Levitt, 2013) that the major period of zinc circuit refinement (reflecting a subset of feedforward and feedback pathways) occurs shortly after eye opening. This phase of major decline in the synaptic zinc levels in areas 17 and 18 (particularly in layer IV) coincides with the period of reorganization of feedback projections from multiple visual areas to area 17. This is not surprising given that zinc-positive inputs to area 17 are a subset of feedback projections from multiple visual cortical areas. Similarly, the refinement of horizontal projections in layers II/III of ferret visual cortex begins around P22 and continues until distinct adult-like terminal clusters are observed at P45 (Durack and Katz, 1996; Ruthazer and Stryker, 1996). Around the time of eye opening, these long-range horizontal connections are limited in spatial extent, and are less clustered than in the adult. While ocular dominance columns are established early in the ferret (P16: Crowley and Katz, 2000), the critical period for ocular dominance plasticity roughly coincides with the period of major reorganization of feedback circuitry, and continues until the end of the second postnatal month (Issa et al., 1999).
Therefore, it appears that the period of major refinement of feedback circuitry in ferret visual cortex occurs on a similar timescale as the refinement of certain other features of anatomical circuitry. The onset of visual experience seems to be a critical factor for this refinement process of feedback projections as well as other anatomical features of the visual cortex. For example, the density of thalamocortical synapses in layer 4 of ferret area 17 increases rapidly in the month that follows the onset of patterned visual experience (Erisir and Harris, 2003). Furthermore, manipulation of sensory experience has been shown to affect the anatomical refinement of circuits. For instance Callaway and Katz (1991) demonstrated that depriving young cats of patterned visual experience by binocular lid suture prior to eye opening reduced the specificity of terminal clusters of horizontal connections in V1. The refinement of different aspects of feedback projections we document here appears to be differentially affected by visual experience. The slow monotonic rate of refinement in peak density, and median and Q3 NND values (which start before eye opening), contrasts with the sharp decline in areal and laminar proportion of feedback we observe right after eye-opening. We suggest that the critical period for the refinement of the areal and laminar proportion of feedback is from four to six week postnatal. However, since peak density and NND values seem to change prior to eye opening, the critical period for their normal refinement may start before eye opening and extend for a longer period of time.
3) Comparison with anatomical development in other species
Other studies in monkey (Barone et al., 1995; Batardiere et al., 2002) have shown that feedback connections undergo extensive remodeling involving selective pruning of a subset to yield the adultlike pattern of connections. Barone et al. (1995) investigated the refinement of the laminar distribution of feedback connections reporting a decrease in the proportion of supragranular neurons that differs among areas. This laminar refinement of feedback connections is largely complete by one to two months after birth. Similarly, the laminar distribution of feedback neurons to area 17 in kittens is uniform across individual extrastriate areas, and the selective reduction in the supragranular neurons yields the laminar distribution characteristic of each area in the adult (Batardiere et al., 1998). Price and Blakemore (1985) have reported that feedforward neurons in area 17 projecting to area 18 in the kitten are distributed in bands of uniform density. These bands refine in the following two weeks to yield discrete dense clusters of feedforward projections, characteristic of the adult pattern.
Developmental studies investigating the sequence of refinement of interareal feedforward and feedback connections in the visual cortex of human infants have concluded that while the laminar distribution of labeled fibers and cell bodies in V1 and V2 indicate that feedforward and feedback connections emerge shortly before birth, the laminar termination pattern of feedforward connections appears relatively mature before feedback connections reach their mature form (Burkhalter, 1993). A similar study in mouse visual cortex was conducted more recently by Berezovski et al (2011). The authors examined the development of feedforward and feedback connections between mouse V1, anterolateral area (AL), and lateromedial area (LM). The findings showed that FF connections were present at the earliest time point examined (postnatal day 2), while FB connections were not detectable until P11 and suggests that feedforward connections refine earlier than feedback connections.
4) Relationship to V1 neuronal properties
Feedback projections from extrastriate visual areas have been shown to influence the response properties of V1 neurons. It is now accepted that different visual response properties of V1 neurons mature and attain adultlike characteristics at different times and rates in postnatal development. Orientation and direction selectivity of V1 neurons appear to refine and become adult like during the same refinement period of feedback projection. There appears to be some degree of orientation selectivity as early as visual responses can be elicited, two weeks before the time of natural eye opening, but that adult-like selectivity is not reached until about a week after eye opening (Chapman and Stryker 1993, Krug et al., 2001). Moreover, orientation maps in V1 are present prior to eye opening and the overall map layout does not change much throughout development (Chapman et al., 1996). The basic structure of orientation maps is therefore innate, but experience is necessary for specific features of these maps, and for maintaining selectivity of cortical neurons. Optical imaging and electrophysiological techniques have revealed the absence of direction selectivity in ferret visual cortex at eye-opening, which develops during the subsequent two weeks from P30 to P45 (Li et al., 2006). Patterned visual input appears to be critical in the development of cortical direction selectivity, as dark rearing ferrets after eye opening precludes the formation of direction maps and V1 neurons lack direction tuning.
Our results are consistent with those of Li et al. (2006) who reported that direction selectivity maps in V1 were adultlike by 6 weeks of age. Similarly, we have shown that the proportion of feedback from Ssy decreases to the adultlike level from 4 to 6 weeks postnatal, and remains fairly stable thereafter. Ssy has been suggested to be the primate homologue of area MT, an area implicated in motion perception. Feedback connections from Ssy presumably contribute to direction selective-properties of V1 cells. Therefore, if at least some aspects of feedback circuits from Ssy to V1 are adult-like by 6 weeks of age (for example the supragranular/infragranular ratio of feedback, as well as the proportion of total feedback to V1 provided by Ssy), it is possible that maturation of these circuits contribute to the maturation of direction-selective responses of V1 cells.
Understanding how the spatial organization of feedback projections refines in ferret visual cortex during development to generate the adult-like pattern is a prerequisite for assessing their physiological relevance. In the monkey, Zhang et al. (2005) have shown that the sizes of receptive field centers of infant V1 and V2 neurons are considerably larger than those of the adult. This might reflect the immaturities in the feedback pool that we describe here. Moreover, Zhang et al. (2005) reported that the development of both the receptive field center and surround of V2 neurons lags behind that of V1 neurons. This could reflect one example of cortical areas developing sequentially.
5) Comparison with behavioral studies
The anatomical refinement of feedback circuits presumably parallels the maturation of neuronal physiological properties and more broadly visual perceptual abilities, and is therefore one indicator of functional maturity. Visual cortical areas subserve different perceptual functions as a result of their interareal anatomical circuits, and feedback circuits are one class of interareal projections. Different visual functions attain maturity at different times and rates in development. The timecourse and duration of refinement varies significantly as more basic visual functions mature earlier and require less time than more complex ones, which require considerable postnatal time to refine. The variability in the rates and duration of the postnatal refinement period appears to reflect the maturational status of distinct cortical circuits that mediate these specific perceptual abilities.
Behavioral studies on human infants have revealed that many basic visual functions are absent at birth, but mature soon thereafter. For instance, by isolating infants’ VEPs (visual evoked potentials) Braddick et al. (1986) found that orientation-selective responses first appeared around 6 weeks of age. Wattam-Bell (1991) reported that responses to direction of motion by isolating VEPs was not possible until 10–12 weeks of age. Furthermore, human infants do not show stereopsis until about 4 – 6 months of age (Birch, 1993; Birch et al., 1983; Brown et al., 2007; Held et al., 1980). Similarly, newborn monkeys are also unable to detect objects embedded in random dot stereograms and stereopsis emerges suddenly around 4 weeks of age (O’Dell et al., 1991).
Complex visual functions that presumably depend on the maturation of extrastriate visual areas have an extended developmental timecourse. For instance, contour integration abilities develop comparatively late. Kovacs et al. (1999) reported that children younger than 3 years of age were unable to identify a coherent contour defined by a circular ring of Gabor patches imbedded in noise, and their ability to perform the task improved into their teenage years. In monkeys, this integrative task is late to develop as well, with an inability to perform contour integration prior to about 16 weeks, and full maturation requiring at least one year (Kiorpes and Bassin, 2003). Kiorpes and Movshon (2004) showed that unlike contour integration tasks, monkeys could perform a motion direction discrimination task at the earliest ages studied (3–5 weeks), but the full maturation continued up to 3 years. Although human infants can detect directional motion cues at about 2 months of age (Braddick et al., 2003), coherent motion sensitivity was found to be adultlike at around 3 years of age (Parrish et al., 2005). Some aspects of motion perception appear immature even up to 7 years or more (Ellemberg et al., 2003; Giaschi and Regan, 1997; Parrish et al., 2005), and perhaps even into adolescence (Bucher et al., 2006). Therefore, the different developmental trajectories of different aspects of feedback connections (dramatic change in areal and laminar proportion from 4–6 weeks, versus the slow monotonic rate of refinement in peak density and nearest neighbor distance among feedback cells) mirrors the multiple developmental trajectories of different visual functions.
Conclusion
We have shown that feedback connections to ferret primary visual cortex from the supra- and infragranular layers of multiple visual areas undergo extensive remodeling in their spatial layout during the period after eye-opening. Remarkably, the presence of aspects of adult-like connections at four weeks of age suggests that the basic pattern of feedback connections to primary visual cortex is present before eye opening. Baldwin et al. (2012) similarly demonstrated that the adultlike pattern of cortical connections between V1 and V2 is present in early postnatal monkeys, with refinement of these connections between 2 to 8 weeks postnatal. We have shown that several features of feedback circuitry to V1 from multiple sources refine at similar rates. Our results confirm and extend the notion that at least some aspects of cortical maturation occur in concert in multiple visual areas. Furthermore, the protracted postnatal period of remodeling of cortical feedback projections provides a window for visual experience to potentially modify these circuits.
Acknowledgments
Supported by grants from the NSF (0619290), National Center for Research Resources (2G12RR03060-26A1) and the National Institute on Minority Health and Health Disparities (8G12MD007603-27) from the National Institutes of Health, and Professional Staff Congress-City University of New York (PSC-CUNY)
We are grateful to Violeta Contreras Ramirez and Syeda Hasan for assistance with cell counting, and to Ahmad Farhat for technical assistance.
Footnotes
Conflict of interest statement.
No identified conflict: RK, JBL
Role of Authors
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: RK, JBL. Acquisition of data: RK, JBL. Analysis and interpretation of data: RK, JBL. Drafting of the manuscript: RK, JBL. Critical revision of the manuscript for important intellectual content: RK, JBL. Statistical analysis: RKf, JBL. Obtained funding: JBL. Study supervision: JBL.
Literature cited
- Angelucci A, Clasca F, Sur M. Anterograde axonal tracing with the subunit B of cholera toxin: a highly sensitive immunohistochemical protocol for revealing fine axonal morphology in adult and neonatal brains. J Neurosci Methods. 1996;65:101–112. doi: 10.1016/0165-0270(95)00155-7. [DOI] [PubMed] [Google Scholar]
- Baker GE, Thompson ID, Krug K, Smyth D, Tolhurst DJ. Spatial-frequency tuning and geniculocortical projections in the visual cortex (areas 17 and 18) of the pigmented ferret. Eur J Neurosci. 1998;10:2657–2668. doi: 10.1046/j.1460-9568.1998.00276.x. [DOI] [PubMed] [Google Scholar]
- Baldwin MK, Kaskan PM, Zhang B, Chino YM, Kaas JH. Cortical and subcortical connections of V1 and V2 in early postnatal macaque monkeys. J Comp Neurol. 2012;520(3):544–69. doi: 10.1002/cne.22732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks MS, Stephens BR, Hartmann EE. The development of basic mechanisms of pattern vision:Spatial-frequency channels. J Exp Child Psych. 1985;40:501–527. doi: 10.1016/0022-0965(85)90080-3. [DOI] [PubMed] [Google Scholar]
- Barone P, Dehay C, Berland M, Bullier J, Kennedy H. Developmental remodeling of primate visual cortical pathways. Cereb Cortex. 1995;5:22–38. doi: 10.1093/cercor/5.1.22. [DOI] [PubMed] [Google Scholar]
- Batardiere A, Barone P, Dehay C, Kennedy H. Area-specific laminar distribution of cortical feedback neurons projecting to cat area 17: quantitative analysis in the adult and during ontogeny. J Comp Neurol. 1998;396(4):493–510. doi: 10.1002/(sici)1096-9861(19980713)396:4<493::aid-cne6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Batardière A, Barone P, Knoblauch K, Giroud P, Berland M, Dumas AM, Kennedy H. Early specification of the hierarchical organization of visual cortical areas in the macaque monkey. Cereb Cortex. 2002;12(5):453–65. doi: 10.1093/cercor/12.5.453. [DOI] [PubMed] [Google Scholar]
- Berezovskii VK, Nassi JJ, Born RT. Segregation of feedforward and feedback projections in mouse visual cortex. J Comp Neurol. 2011;519(18):3672–83. doi: 10.1002/cne.22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Gwiazda J, Held R. The development of vergence does not account for the development of stereopsis. Perception. 1983;12:331–336. doi: 10.1068/p120331. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Patricia S, Goldman-Rakic, Rakic P. Synaptogenesis in the Prefrontal Cortex of Rhesus Monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Braddick OJ, Wattam-Bell J, Atkinson J. Orientation specific cortical responses develop in early infancy. Nature. 1986;320:617–619. doi: 10.1038/320617a0. [DOI] [PubMed] [Google Scholar]
- Braddick OJ. Orientation- and motion-selective mechanisms in infants. In: Simons K, editor. Early visual development: Normal and abnormal. New York: Oxford University Press; 1993. pp. 163–177. [Google Scholar]
- Braddick OJ, Atkinson J, Wattam-Bell J. Normal and anomalous development of visual motion processing: Motion coherence and ‘dorsal stream vulnerability’. Neuropsychologia. 2003;41:1769–1784. doi: 10.1016/s0028-3932(03)00178-7. [DOI] [PubMed] [Google Scholar]
- Braddick OJ, Birtles D, Wattam-Bell J, Atkinson J. Motion- and orientation-specific cortical responses in infancy. Vision Res. 2005;45:3169–3179. doi: 10.1016/j.visres.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Braddick OJ, Atkinson J. Development of human visual function. Vision Res. 2011;51(13):1588–609. doi: 10.1016/j.visres.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Brown AM, Lindsey DT, Satgunam P, Miracle JA. Critical immaturities limiting infant binocular stereopsis. Invest Opthalmol Vis Sci. 2007;48(3):1424–34. doi: 10.1167/iovs.06-0718. [DOI] [PubMed] [Google Scholar]
- Bucher K, Dietrich T, Marcar VL, Brem S, Halder P, Boujraf S. Maturation of luminance- and motion-defined form perception beyond adolescence: A combined ERP and fMRI study. Neuroimage. 2006;31(4):1625–1636. doi: 10.1016/j.neuroimage.2006.02.032. [DOI] [PubMed] [Google Scholar]
- Burkhalter A, Bernardo KL, Charles V. Development of local circuits in human visual cortex. J Neurosci. 1993;13(5):1916–31. doi: 10.1523/JNEUROSCI.13-05-01916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhalter A. Development of forward and feedback connections between areas V1 and V2 of human visual cortex. Cereb Cortex. 1993;3(5):476–87. doi: 10.1093/cercor/3.5.476. [DOI] [PubMed] [Google Scholar]
- Callaway EM, Katz LC. Emergence and refinement of clustered horizontal connections in cat striate cortex. J Neurosci. 1990;10(4):1134–53. doi: 10.1523/JNEUROSCI.10-04-01134.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM, Katz LC. Effects of binocular deprivation on the development of clustered horizontal connections in cat striate cortex. Proc Natl Acad Sci USA. 1991;88(3):745–9. doi: 10.1073/pnas.88.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantone G, Xiao J, McFarlane N, Levitt JB. Feedback connections to ferret straite cortex: Direct evidence for visuotopic convergence of feedback inputs. J Comp Neurol. 2005;487:312–331. doi: 10.1002/cne.20570. [DOI] [PubMed] [Google Scholar]
- Changeux JP, Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976;264(5588):705–12. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- Chapman B, Stryker MP. Development of orientation selectivity in ferret visual cortex and effects of deprivation. J Neurosci. 1993;13(12):5251–62. doi: 10.1523/JNEUROSCI.13-12-05251.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B, Stryker MP, Bonhoeffer T. Development of orientation preference maps in ferret primary visual cortex. J Neurosci. 1996;16(20):6443–53. doi: 10.1523/JNEUROSCI.16-20-06443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condé F, Lund JS, Lewis DA. The hierarchical development of monkey visual cortical regions as revealed by the maturation of parvalbumin-immunoreactive neurons. Brain Res Dev Brain Res. 1996;96(1–2):261–76. doi: 10.1016/0165-3806(96)00126-5. [DOI] [PubMed] [Google Scholar]
- Coogan TA, Burkhalter A. Sequential development of connections between striate and extrastriate visual cortical areas in the rat. J Comp Neurol. 1988;278(2):242–52. doi: 10.1002/cne.902780207. [DOI] [PubMed] [Google Scholar]
- Coogan TA, Van Essen DC. Development of connections within and between areas V1 and V2 of macaque monkeys. J Comp Neurol. 1996;(3):327–42. doi: 10.1002/(SICI)1096-9861(19960826)372:3<327::AID-CNE1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Cook JE. Spatial properties of retinal mosaics: an empirical evaluation of some existing measures. Vis Neurosci. 1996;13(1):15–30. doi: 10.1017/s0952523800007094. [DOI] [PubMed] [Google Scholar]
- Crowley JC, Katz LC. Early development of ocular dominance columns. Science. 2000;290(5495):1321–4. doi: 10.1126/science.290.5495.1321. [DOI] [PubMed] [Google Scholar]
- Dehay C, Kennedy H, Bullier J. Characterization of transient cortical projections from auditory, somatosensory, and motor cortices to visual areas 17, 18, and 19 in the kitten. J Comp Neurol. 1988;272(1):68–89. doi: 10.1002/cne.902720106. [DOI] [PubMed] [Google Scholar]
- Durack JC, Katz LC. Development of horizontal projections in layer 2/3 of ferret visual cortex. Cereb Cortex. 1996;6:178–183. doi: 10.1093/cercor/6.2.178. [DOI] [PubMed] [Google Scholar]
- Ellemberg D, Lewis TL, Meghji KR, Maurer D, Guillemot JP, Lepore F. Comparison of sensitivity to first- and second-order local motion in 5-year olds and adults. Spatial Vision. 2003;16(5):419–428. doi: 10.1163/156856803322552748. [DOI] [PubMed] [Google Scholar]
- Erisir A, Harris JL. Decline of the critical period of visual plasticity is concurrent with the reduction of NR2B subunit of the synaptic NMDA receptor in layer 4. J Neurosci. 2003;23(12):5208–18. doi: 10.1523/JNEUROSCI.23-12-05208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flechsig P. Developmental (myelogenetic) localisation of the cerebral cortex in the human subject. Lancet. 1901;2:1027–1029. [Google Scholar]
- Giaschi D, Regan D. Development of motion-defined figure-ground segregation in preschool and older children, using a letter-identification task. Optometry Vision Sci. 1997;74:761–767. doi: 10.1097/00006324-199709000-00024. [DOI] [PubMed] [Google Scholar]
- Girard N, Raybaud C, du Lac P. MRI study of brain myelination. J Neuroradiol. 1991;18(4):291–307. [PubMed] [Google Scholar]
- Harwerth RS, Smith EL, 3rd, Duncan GC, Crawford ML, von Noorden GK. Multiple sensitive periods in the development of the primate visual system. Science. 1986;232(4747):235–8. doi: 10.1126/science.3952507. [DOI] [PubMed] [Google Scholar]
- Held R, Birch EE, Gwiazda J. Stereoacuity of human infants. Proc Natl Acad Sci USA. 1980;77:5572–5574. doi: 10.1073/pnas.77.9.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Price DJ. Exuberance in the development of cortical networks. Nat Rev Neurosci. 2005;6(12):955–65. doi: 10.1038/nrn1790. [DOI] [PubMed] [Google Scholar]
- Issa NP, Trachtenberg JT, Chapman B, Zahs KR, Stryker MP. The critical period for ocular dominance plasticity in the ferret’s visual cortex. J Neurosci. 1999;19(16):6965–78. doi: 10.1523/JNEUROSCI.19-16-06965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N, Ferrer JM, Price DJ. Regressive changes among corticocortical neurons projecting from the lateral suprasylvian cortex to area 18 of the kitten’s visual cortex. Neurosci. 1991;43(2–3):291–306. doi: 10.1016/0306-4522(91)90294-x. [DOI] [PubMed] [Google Scholar]
- Khalil R, Levitt JB. Zinc histochemistry reveals circuit refinement and distinguishes visual areas in the developing ferret cerebral cortex. Brain Struct Funct. 2013;218(5):1293–1306. doi: 10.1007/s00429-012-0458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L, Bassin SA. Development of contour integration in macaque monkeys. Vis Neurosci. 2003;20(5):567–75. doi: 10.1017/s0952523803205101. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Movshon JA. Development of sensitivity to visual motion in macaque monkeys. Vis Neurosci. 2004;21:851–859. doi: 10.1017/S0952523804216054. [DOI] [PubMed] [Google Scholar]
- Kovács I, Kozma P, Fehér A, Benedek G. Late maturation of visual spatial integration in humans. Proc Natl Acad Sci USA. 1999;96:12204–12209. doi: 10.1073/pnas.96.21.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug K, Akerman CJ, Thompson ID. Responses of neurons in neonatal cortex and thalamus to patterned visual stimulation through the naturally closed lids. J Neurophysiol. 2001;85(4):1436–1443. doi: 10.1152/jn.2001.85.4.1436. [DOI] [PubMed] [Google Scholar]
- Lewis TL, Maurer D. Multiple sensitive periods in human visual development: evidence from visually deprived children. Dev Psychobiol. 2005;46(3):163–83. doi: 10.1002/dev.20055. [DOI] [PubMed] [Google Scholar]
- Li Y, Fitzpatrick D, White LE. The development of direction selectivity in ferret visual cortex requires early visual experience. Nat Neurosci. 2006;9(5):676–81. doi: 10.1038/nn1684. [DOI] [PubMed] [Google Scholar]
- Manger PR, Kiper D, Masiello I, Murrilo L, Tettoni L, Hunyadi Z, Innocenti GM. The representation of the visual field in three extrastriate areas of the ferret (Mustela putorius) and the relationship of retinotopy and field boundaries to callosal connectivity. Cereb Cortex. 2002;12:423–437. doi: 10.1093/cercor/12.4.423. [DOI] [PubMed] [Google Scholar]
- O’Dell C, Boothe RG. The development of stereoacuity in infant rhesus monkeys. Vision Res. 1997;37(19):2675–2684. doi: 10.1016/s0042-6989(97)00080-1. [DOI] [PubMed] [Google Scholar]
- Parrish EE, Giaschi DE, Boden C, Dougherty R. The maturation of form and motion perception in school age children. Vision Res. 2005;45:827–837. doi: 10.1016/j.visres.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull. 2001;54(3):255–66. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Price DJ. The postnatal development of clustered intrinsic connections in area 18 of the visual cortex in kittens. Brain Res. 1986;389(1–2):31–8. doi: 10.1016/0165-3806(86)90170-7. [DOI] [PubMed] [Google Scholar]
- Price DJ, Blakemore C. Regressive events in the postnatal development of association projections in the visual cortex. Nature. 1985;316(22):721–724. doi: 10.1038/316721a0. [DOI] [PubMed] [Google Scholar]
- Price DJ, Ferrer JM, Blakemore C, Kato N. Postnatal development and plasticity of corticocortical projections from area 17 to area 18 in the cat’s visual cortex. J Neurosci. 1994;14:2747–62. doi: 10.1523/JNEUROSCI.14-05-02747.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DJ, Zumbroich TJ. Postnatal development of corticocortical efferents from area 17 in the cat’s visual cortex. J Neurosci. 1989;9(2):600–13. doi: 10.1523/JNEUROSCI.09-02-00600.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232(4747):232–5. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Ruthazer ES, Stryker MP. The role of activity in the development of long-range horizontal connections in area 17 of the ferret. J Neurosci. 1996;15(22):7253–69. doi: 10.1523/JNEUROSCI.16-22-07253.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattam-Bell J. The development of motion-specific cortical responses in infants. Vision Res. 1991;31:287–297. doi: 10.1016/0042-6989(91)90119-p. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171(1):11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Zhang B, Zheng J, Watanabe I, Maruko I, Bi H, Smith EL, 3rd, Chino Y. Delayed maturation of receptive field center/surround mechanisms in V2. Proc Natl Acad Sci USA. 2005;102(16):5862–7. doi: 10.1073/pnas.0501815102. [DOI] [PMC free article] [PubMed] [Google Scholar]