Abstract
Nerve conduction within the mammalian central nervous system is made efficient by oligodendrocyte-derived myelin. Historically, thyroid hormones have a well described role in regulating oligodendrocyte differentiation and myelination during development, however, it remains unclear which thyroid hormone receptors are required to drive these effects. This is a question with clinical relevance since non-specific thyroid receptor stimulation can produce deleterious side-effects. Here we report that GC-1, a thyromimetic with selective thyroid receptor β action and a potentially limited side-effect profile, promotes in vitro oligodendrogenesis from both rodent and human oligodendrocyte progenitor cells. In addition, we used in vivo genetic fate tracing of oligodendrocyte progenitor cells via PDGFαR-CreER;Rosa26-eYFP double-transgenic mice to examine the effect of GC-1 on cellular fate and find that treatment with GC-1 during developmental myelination promotes oligodendrogenesis within the corpus callosum, occipital cortex and optic nerve. GC-1 was also observed to enhance the expression of the myelin proteins MBP, CNP and MAG within the same regions. These results indicate that a β receptor selective thyromimetic can enhance oligodendrocyte differentiation in vitro and during developmental myelination in vivo and warrants further study as a therapeutic agent for demyelinating models.
Keywords: myelination, thyroid hormone, oligodendrocytes, GC-1
INTRODUCTION
Thyroid hormones (THs) play a critical role in developmental oligodendrogenesis and myelination. Acting through nuclear hormone receptors, THs have been shown to promote the expression of a number of oligodendrocyte specific genes including myelin basic protein (MBP), myelin-associated glycoprotein (MAG), proteolipid protein (PLP) and cyclic nucleotide 3’-phosphodiesterase (CNP) (Farsetti et al., 1992; Farsetti et al., 1991; Rodriguez-Pena et al., 1993; Tosic et al., 1992). In vivo, numerous studies of hypo- and hyperthyroid animals as well as genetically modified rodents have provided clear evidence that THs regulate oligodendrocyte differentiation and maturation (Bernal, 2002; Bernal, 2007; Darras, 2008; Dugas et al., 2012; O'Shea and Williams, 2002; Zoeller and Rovet, 2004). Despite unequivocal evidence of the important role played by THs in myelination, we do not yet clearly understand which thyroid hormone receptors (THRs) are required to mediate these effects (Baas et al., 1997; Bernal, 2002; Bernal, 2007; Darras, 2008; O'Shea and Williams, 2002; Zoeller and Rovet, 2004). Moreover, long-term non-specific THR activation can cause serious side effects that limit the translational potential of THs for the treatment of myelin disorders occurring both developmentally (for example cerebral palsy) and in the adult central nervous system (CNS) (multiple sclerosis, monophasic optic neuritis and transverse myelitis). We investigated the potential for a β THR selective thyromimetic, GC-1, to stimulate oligodendrocyte differentiation in rodent and human cultures in vitro and in vivo during development in the mouse.
There are two circulating THs: thyroxine (T4) and tri-iodothyronine (T3). T4 is the principal product of the thyroid gland and the most abundant circulating TH. However, most classic TH actions are believed to be mediated by T3 (Yen, 2001). T4 is converted to T3 by deiodinases within target organs.
TH actions are mediated through nuclear hormone receptors that are encoded by two different genes. The THRα gene encodes two isoforms (α1 and α2) while an additional four isoforms are encoded by the β gene (β1–4) (Tagami et al., 2010; Williams, 2000; Yen, 2001). In general, THRαs regulate cardiac rate and contractility (Gloss et al., 2001; Johansson et al., 1998) while THRβs control inner-ear and retina development (Rusch et al., 2001), cholesterol homeostasis (Gullberg et al., 2002), lipoprotein metabolism (Tancevski et al., 2009), and TH levels (Weiss et al., 1997).
Potential side effects mediated by excessive, non-selective THR stimulation are akin to hyperthyroidism and include tachycardia, arrhythmia, decreased mineral bone density and muscle wasting (Baxter et al., 2001; Johansson et al., 1998; Mansen et al.). Selective modulation of the β receptor has been shown to circumvent many of these undesirable effects, thus widening the safety margin between therapeutic and harmful effects (Borngraeber et al., 2003; Freitas et al., 2003; Grover et al., 2004; Miyabara et al., 2005; Scanlan, 2010; Trost et al., 2000). However, it is not known whether THRβ1 activation is sufficient to promote oligodendrogenesis.
In vitro studies indicate that oligodendrocyte progenitor cells (OPCs) express both THRα and THRβ1 and expression of the latter increases with oligodendrocyte maturation (Barres et al., 1994; Gao et al., 1998). Overexpression of THRβ1 in mouse OPCs was found to accelerate TH-induced differentiation (Billon et al., 2001). Furthermore, THRβ1 has been shown to be a better transactivator of the MBP TRE versus THRα1 (Jeannin et al., 1998). Taken together, these studies suggest an important role for THRβ1 in OL maturation.
Here we report that GC-1, a thyromimetic with selective THRβ action (Chiellini et al., 1998; Manzano et al., 2003; Trost et al., 2000), promotes oligodendrogenesis from both rodent and human OPCs in vitro. Using in vivo genetic fate tracing of OPCs, we found that GC-1 enhances oligodendrogenesis during development and increases production of the myelin proteins MBP, CNP and MAG. These results indicate that thyromimetics selective for the β receptor can enhance OL differentiation and promote myelination while limiting the potential for serious side effects elicited by non-specific THR activation.
MATERIALS AND METHODS
Animals
PLP-enhanced green fluorescent protein (eGFP) mice were a kind gift from Dr. Wendy Macklin (Mallon et al., 2002). NG2-DsRed BAC transgenic mice and PDGFαR-CreER mice were generated as described previously (Kang et al., 2010; Ziskin et al., 2007). Rosa26-eYFP mice were purchased from the Jackson Laboratory. C57BL/6 mice were purchased from National Cancer Institute, Frederick. Timed pregnant Sprague Dawley rats were purchased from Charles River. The care and treatment of animals in all procedures strictly followed the NIH Guide for the Care and Use of Laboratory Animals and the Johns Hopkins University IACUC. Mice and rats were housed at standard temperature (21°C) and in a light controlled environment with ad libitum access to the food and water.
In Vitro Studies
Isolation and Culture of Murine DsRed+ OPCs
NG2-DsRed and PLP-eGFP mice were crossbred to create double transgenics. Postnatal day (P) 2–6 mice were sacrificed and the cerebrum rapidly dissected in ice-cold Hank’s Balanced Salt Solution (HBSS). Following removal of the meninges, tissue was diced with a razor blade prior to cellular dissociation using the MACS® Neural Dissociation Kit (Miltenyi Biotec Inc.) according to the manufacturer’s instructions. DsRed expressing cells were fluorescence-activated cell sorted (FACS) using a MoFlo MLS high speed cell sorter (Beckman coulter) with Summit version 4.3 software. Cell suspension preparation and FACS was completed within 2–3 hours.
Sorted cells were plated onto either poly-L-lysine (PLL; 10 µg/mL, Sigma) coated glass coverslips (for immunocytochemistry) or 96 well, black-walled plates (Greiner Bio-One; for reporter assay) and cultured in Sato media (modified from (Bottenstein and Sato, 1979) Dulbecco’s Modified Eagle Medium containing B27, glutamine, penicillin/streptomycin, sodium pyruvate, (all from Life Technologies), 100 µg/mL apo-transferrin, 100 µg/mL BSA, 60 ng/mL progesterone, 16 µg/mL putrescine, 40 ng/mL sodium selenite, 50 µg/mL insulin, 5 µg/mL n-acetyl cysteine, 10 ng/mL biotin, 50 ng/mL hydrocortisone (all from Sigma) and trace elements B (Cellgro)) supplemented with 10 ng/ml recombinant human platelet derived growth factor-AA (PDGF-AA, PeproTech). After four days in vitro, cells were either maintained in PDGF (proliferation media) or switched to Sato media containing T3 (Sigma) or GC-1 (generous gift from Dr. Thomas Scanlan (Chiellini et al., 1998)) for a further four days. Media changes were made every other day. For proliferation studies, 10 µM 5-bromo-2'-deoxyuridine (BrdU, Sigma) was added to cultures at the time they were switched to differentiation media and 24 hrs prior to fixation.
Immunocytochemistry and Microscopy
Cells were fixed in 4% paraformaldehyde, rinsed and then permeabilized in phosphate buffered saline (PBS) containing 5% normal goat serum (NGS) and 0.4% Triton-X100 for 1 h at room temperature (RT). For BrdU immunostaining, cells were preincubated in 2N HCl at RT for 20 min, followed by neutralization with 0.1 M sodium borate buffer (pH 8.5) prior to immunolabeling. Cells were then incubated overnight at 4 °C in PBS containing 3% NGS, 0.1% Triton X-100 and primary antibody as follows; guinea pig anti-NG2 (1:300); rabbit anti-platelet derived growth factor α receptor (PDGFαR) (1:600, both courtesy of Dr. Bill Stallcup); rabbit anti-Olig2 (1:1000, Millipore); mouse anti-MBP (smi99, 1:1000, Covance); rat anti-BrdU (1:100, Accurate); mouse anti-CNP (1:750, Millipore) and chicken anti-GFP (1:1000, AbCam). The next day, cells were rinsed and incubated for 1 h at RT with species-specific secondary antibodies directly conjugated to Alexa fluorophores (1:1000, Life Technologies). Coverslips were mounted in ProLong® Gold anti-fade reagent with 4',6-diamidino-2-phenylindole (DAPI) and visualized on a Zeiss Axio Observer Z1 epifluorescence microscope. Images were captured and processed using AxioVision 4.8 software. Cell counts were made at 200× from three fields of view per condition in three separate experiments.
Quantification of Murine in vitro Oligodendrogenesis
Quantification of eGFP and MBP immunoreactivity was performed using In-cell Western analysis (Odyssey Infrared Imaging System; Li-Cor Biosciences) according to the manufacturer’s guidelines. Cells cultured in 96-well plates were fixed and prepared for immunocytochemistry with either anti-GFP or anti-MBP as indicated above. Species-specific secondary antibodies conjugated to 800CW infrared dye (1:5000, LiCor Biosciences) were incubated in PBS containing 3% NGS and 0.1% Tween-20 along with the nuclear stain Syto60 (1:10000, Life Technologies) for 1 h at RT. After washing, plates were scanned simultaneously at 700 and 800 nm using the Odyssey Infrared Imaging System.
Isolation and Culture of Rat OPCs
P6 Sprague Dawley rat pups were sacrificed and cortices rapidly dissected in ice-cold HBSS. Tissue was mechanically diced prior to cellular dissociation using the MACS® Neural Dissociation Kit (Miltenyi Biotec Inc.) as described above. OPCs were selected by immunomagnetic bead selection according to the manufacturer’s instructions using mouse monoclonal anti-A2B5 IgM antibodies conjugated to microbeads (Miltenyi Biotec Inc.). The A2B5+ cell fraction was resuspended in Sato media and cultured as described for the murine OPCs above. Rat OPCs were proliferated in 10 ng/ml PDGF for four days prior to differentiation for an additional four to eight days.
Quantitative PCR
Rat cells were lysed directly in 6 well plates using 600 µL of buffer RLT (Qiagen) and immediately homogenized in QIAshredder columns (Qiagen). Homogenized lysates were stored at −80 °C until the conclusion of each trial and then processed simultaneously with the RNeasy Plus Mini Kit (Qiagen) according to supplementary protocol RY26 (Qiagen). Genomic DNA was removed using gDNA elimination columns included with the kit. Samples of total RNA (1600 ng) were reverse transcribed in a 40 µL reaction volume with the NCode VILO cDNA synthesis kit (Life Technologies). The cDNA samples were diluted to 10 ng/µL in water and 5 – 50 ng of diluted cDNA was used for qPCR. Reactions were performed in triplicate using a 20 µL volume, 400 nM primer, SensiMix SYBR master mix (Bioline), and CFX384 Touch or iCycler iQ Real-Time PCR Detection Systems (Bio-Rad). Cycling conditions were as follows: 10 minute initial denature, followed by 40 cycles of 95 °C for 10 seconds, 55 °C for 15 seconds, and 72 °C for 15 seconds. The following primers were used; THRα1: 5' CCTCCACATGAAAGTCGAGTG 3' and 5' CCCAGCTTTGTCCCTTCTC 3'; THRβ1: 5' AAACATCCTCACACCTCATCC 3' and 5' ATCGTTCACATCGTCTGGATC 3'. Primer specificity was validated by melt curve analysis. Fold-changes were calculated with qbase+ data analysis software (Biogazelle) with expression scaled to Day 0 controls. All data were normalized to four reference genes including beta actin (Actb), eukaryotic translation elongation factor 1 alpha (Eef1a1), hypoxanthine phosphoribosyltransferase 1 (HPRT1), and large ribosomal protein P0 (Rplp0). The mean coefficient of variation (CV) and stability (M) values for reference genes were 0.157 / 0.363 (Actb), 0.147 / 0.351 (Eef1a1), 0.205 / 0.435 (Hprt1), and 0.134 / 0.332 (Rplp0).
Western Blot Analysis of Rat OPCs
Rat cells were lysed in radio immunoprecipitation assay (RIPA) buffer (Boston BioProducts) and protein abundance quantified using the Bradford assay (Bio-Rad). Equal amounts of protein were denatured in Laemmli’s SDS sample buffer containing β-mercaptoethanol (Boston BioProducts) prior to separation on 12% polyacrylamide gels (Critereon, TGX, Bio-Red) by electrophoresis. For detection of MAG, protein samples were prepared in a non-denaturing Laemli's sample buffer (Boston BioProducts). Samples were then transferred to PVDF membrane. Membranes were blocked in 0.1% Tween-20 TBS containing 5% non-fat milk for 1 hr prior to incubation with primary antibodies. Primary antibodies were diluted in the same solution as follows; anti-MBP (clone smi99; Covance) 1:1000; anti-TRβ1 (clone J52; Santa Cruz) 1:300; and anti-β-actin (clone 20–33; Sigma) 1:5000. Anti-mouse or anti-rabbit secondary antibodies conjugated to IRDye® 680 or 800 were incubated for 1 hr prior to scanning the membrane on a LI-COR Odyssey® imager. Images were analyzed using the Odyssey® infrared imaging system application software version 3.0. Protein expression was normalized to β-actin.
Isolation and Culture of Human OPCs
OPCs were isolated from grey matter tissue surgically resected from patients with intractable epilepsy. Tissue procurement and use was conducted in accordance with the Johns Hopkins Institutional Review Board (study number NA_00040083). Patients were aged 2–19 yr. Brain resections were taken from nonpathological tissue surrounding the epileptic focus. Using a dissecting microscope, meninges were removed and grey matter dissected in chilled HBSS. In preliminary experiments, we achieved a lower OPC yield from white matter tissue (most likely due to the additional processing required for myelin removal). Tissue was diced with a razor blade prior to cellular dissociation using the MACS® Neural Dissociation Kit (Miltenyi Biotec Inc.). Cells were selected based on expression of a known human OPC antigen, A2B5 (Miron et al., 2007; Sim et al., 2008; Wilson et al., 2003). A2B5+ cells were collected using magnetic cell sorting (MACS) according to the manufacturer’s instructions (Miltenyi Biotec Inc.) and plated onto PLL (10 µg/mL, Sigma) coated glass coverslips. Sato media (as described above) supplemented with 10 ng/mL PDGF was used to proliferate the OPCs for the first seven days in culture. After this time, PDGF was either withdrawn or replaced with 20 nM T3 or GC1. Seven days later, cells were fixed and immunostained as described above.
In Vivo Studies
GC-1 Treatment
P7 C57BL/6 mice were treated daily with 3 µg GC-1 or an equal volume of vehicle by intraperitoneal injection daily until the time of sacrifice one week later.
Western Blot Analysis
For THRβ1 studies, P0, P7, P14 and 8-week-old C57BL/6 mice were sacrificed, perfused transcardially with chilled PBS and the brains dissected. For myelin protein studies following GC-1 treatment, P14 mice were perfused with chilled PBS and the optic nerves and brains collected. The corpus callosum and occipital cortex were dissected with the aid of a dissecting microscope. Nuclear and cytoplasmic extracts were prepared from tissue using NU-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific) according to manufacturer’s instructions. Immunoblotting was conducted as described above. THRβ1 levels were assessed in nuclear protein fractions using anti-THRβ1 (clone J52; Santa Cruz) 1:300. The identity of the THRβ1-specific band was verified by comparison to a positive control (Fig. S2). Myelin proteins were assessed using cytoplasmic protein fractions and using the same immunoblotting conditions as described for the in vitro experiments above.
In vivo Fate Tracing of OPCs
4-hydroxytamoxifen (4-HT, Sigma H7904) was prepared as described previously (Badea et al., 2003). PDGFαR-CreER;Rosa26-eYFP mice were injected with a total of 0.3 mg over three days from P7-P10. Starting on P7, mice were administered 3 µg GC-1 or an equal volume of vehicle by intraperitoneal injection daily until the time of sacrifice one week later. Mice were deeply anesthetized with sodium pentobarbital (100 mg/kg b.w.) and perfused trancardially with chilled PBS followed by 4% paraformaldehyde. Brains and optic nerves were dissected, post-fixed overnight and cryoprotected in 30% sucrose for approximately 48 hrs prior to freezing. Tissue was sectioned on a cryostat (20 µm) and mounted onto glass slides (Superfrost Plus; Fisher).
For adenomatous polyposis coli (APC) immunostaining, tissue was pretreated in LAB solution (Polysciences) for 10 mins before blocking. Sections were blocked and permeabilized in PBS containing 5% NGS and 0.4% Triton-X for 1 h at RT and then incubated overnight at 4 °C in PBS containing 3% NGS, 0.1% Triton X-100 and primary antibody as follows; mouse anti-APC (clone CC-1, 1:50, Calbiochem); rabbit anti-PDGFαR (as above) and chicken anti-GFP (as above). Species-specific secondary antibodies directly conjugated to Alexa fluorophores (1:1000) were used to visualize immunostaining. Sections were incubated in secondary antibodies for 1 hr at RT prior to mounting in anti-fade reagent with DAPI.
Mounted slides were imaged using a Zeiss Axio Observer Z1 epifluorescence microscope and Axiovision software with the appropriate excitation and emission filters. A total of 3 – 5 sections were examined per mouse, and 3 – 5 mice were analyzed per group. Areas were chosen randomly within the indicated regions of brain or optic nerve.
Statistical Analyses
Data are expressed as mean ± standard error of the mean. Unpaired Student's t-tests (two-tailed) were used to distinguish significant differences in reporter OPC immunoreactivity in vitro and lineage fate in vivo. All other in vitro studies were analyzed using One-way ANOVA followed by Dunnett’s Multiple Comparison Test. These statistical tests were conducted using GraphPad Prism 5.03 (GraphPad Prism Software). P values of < 0.05 were considered significant.
RESULTS
Generation of a novel in vitro system to monitor oligodendrogenesis
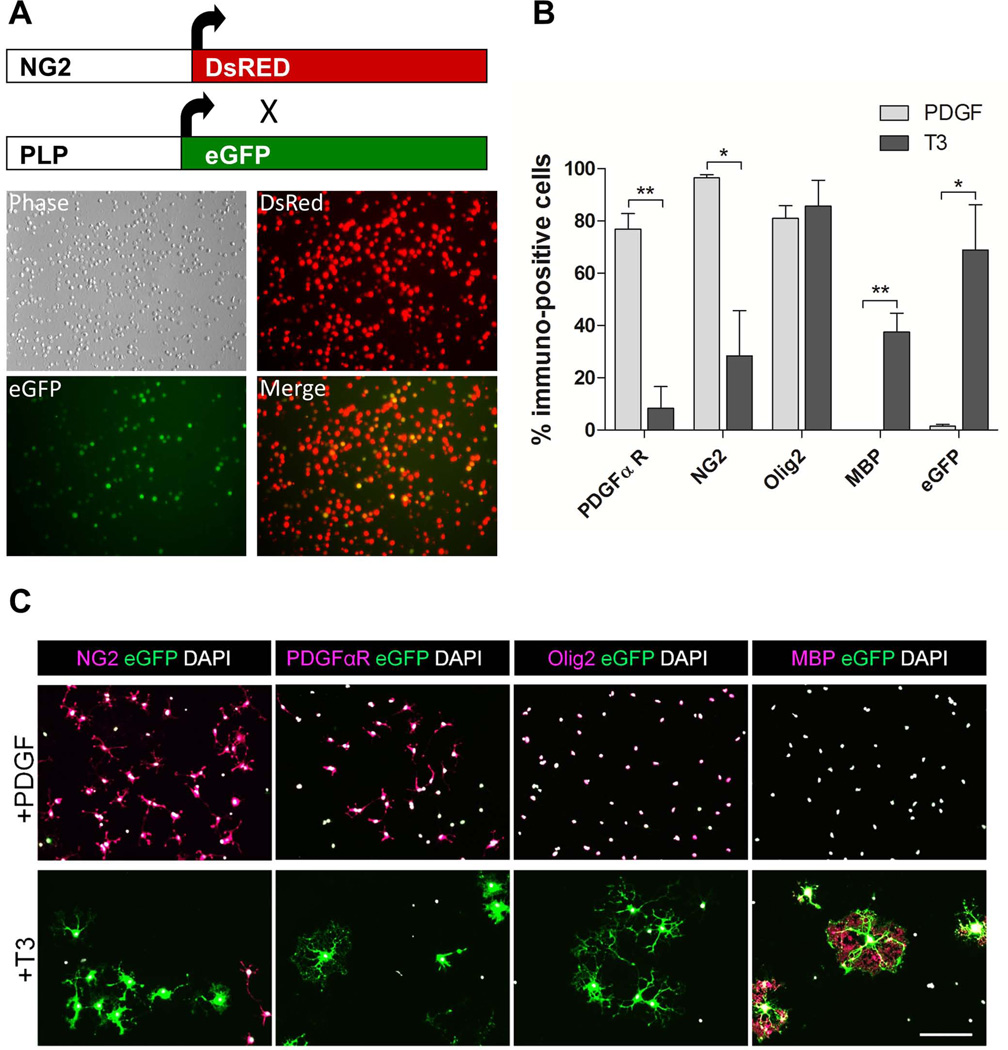
We wished to develop an in vitro technique that would allow us to monitor oligodendrogenesis in a sensitive and quantitative manner. To generate mice that would allow isolation of OPCs based on their expression of NG2 and then track their capacity to differentiate into mature OLs, we crossed PLP-eGFP transgenic mice (Mallon et al., 2002) to NG2-DsRed BAC transgenics (Ziskin et al., 2007) and used FACS to select all DsRed+ cells from the brains of mice aged between P2 and P6 (Fig. 1A). We did not exclude cells that were DsRed+/eGFP+ since activity from the DM20 promoter of the PLP-eGFP mouse can induce limited eGFP expression in progenitor cells (Mallon et al., 2002). Immunocytochemical characterization of DsRed+-selected cells eight days post-isolation revealed a high percentage of PDGFαR (77%) and NG2 (94%) immunoreactive cells when maintained in media supplemented with the mitogen, PDGF (Fig. 1B, C). Under these conditions, there was no detectable expression of MBP and very few (1.5%) eGFP+ cells. Although there was no change in the expression of Olig2 (a pan OL lineage marker) following treatment with T3 (81% vs. 86%), there was a significant decrease in expression of PDGFαR and NG2, a marked increase in MBP expression and an increase in eGFP+ cells (Fig. 1B, C). These results show that TH-stimulated differentiation of isolated DsRed+ OPCs is faithfully reported by increased expression of eGFP in OLs.
Fig. 1.
OPCs isolated from the cerebrum of NG2-DsRed;PLP-eGFP mice express eGFP following T3-mediated differentiation into OLs. A. OPCs are generated from NG2-DsRed crossed to PLP-eGFP mice. DsRed+ cells 1 hr post-sorting are dimly eGFP positive due to activity from the DM/20 promoter. B. Characterization of PDGFαR, NG2, Olig2, MBP and eGFP expression 8 days after sorting in OPCs either maintained in proliferation media (10 ng/ml PDGF) or switched to differentiation media (PDGF withdrawal and 45 nM T3). Data are presented as mean ± SEM, n = 3, **P < 0.005, *P < 0.05, Student’s t-test PDGF vs. T3. C. Representative images from immunolabeling characterization of OPCs. Scale bar represents 100 µm.
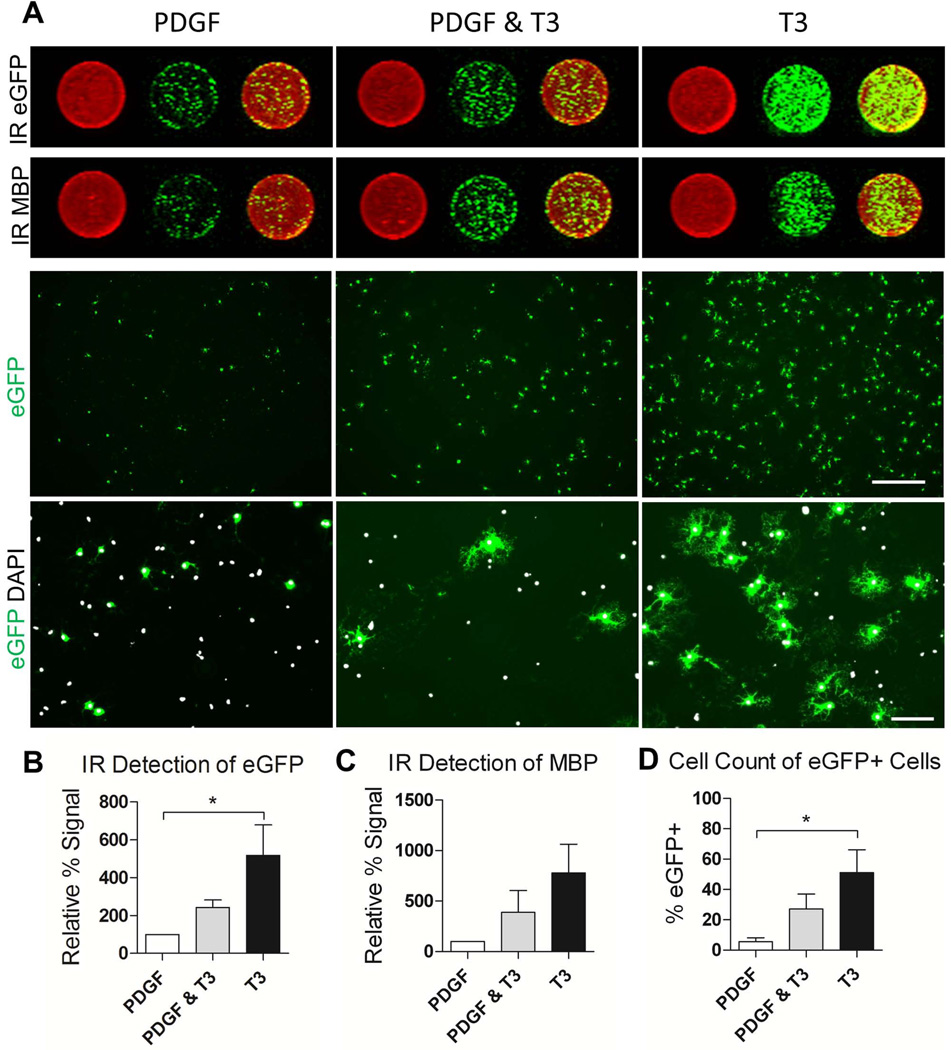
Once it was established that DsRed+/eGFP+ OPCs function as reporter cells for OL differentiation, we developed a quantitative in vitro assay to screen for OL generation. In DsRed+ cells isolated from the brains of NG2-DsRed;PLP-eGFP transgenic mice, OL differentiation results in increased eGFP expression and this increase can be detected and quantified using an in-cell western approach (Fig. 2). Specifically, an antibody against eGFP was used to immunolabel differentiated cells which were then detected using an infrared secondary and the LI-COR Odyssey system. This facilitated a quantitative assessment of oligodendrogenesis in a 96-well format using infrared fluorescence imaging and quantification. To verify the fidelity of this method, we compared it to two additional methods of quantification; manual cell counts of eGFP+ cells and infrared detection of MBP immunolabeled cells. Comparable results were obtained when estimating oligodendrogenesis under three different conditions (+PDGF, T3 +PDGF and T3 –PDGF) using these techniques (Fig. 2).
Fig. 2.
Validation of infrared fluorescence imaging as a method to quantify oligodendrogenesis from DsRed+-selected OPCs. A. Representative images of OL lineage cells cultured for 4 days in media supplemented with 10 ng/ml PDGF followed by an additional 4 days in PDGF, PDGF supplemented with 45 nM T3 or PDGF withdrawal and 45 nM T3. Circular insets show representative infrared scanned wells from a 96-well plate. Anti-eGFP and anti-MBP s are pseudo-colored green, Syto60 is pseudo-colored red. The native eGFP signal from the same condition is shown under low (top panel; scale bar represents 500 µm) and high (bottom panel; scale bar represents 100 µm) magnification. B. Quantification of eGFP signal for proliferation and differentiation conditions using IR scan. The signal from each well was normalized to the nuclear stain, Syto60 to correct for cell number. C. Quantification of MBP signal normalized to Syto60 using IR scan. D. Cells counts of eGFP+ cells normalized to DAPI (a minimum of 315 cells were counted per condition). *P < 0.05, One-Way ANOVA, Dunnett’s Multiple Comparison Test.
GC-1 Promotes Oligodendrogenesis in Murine Cells in vitro
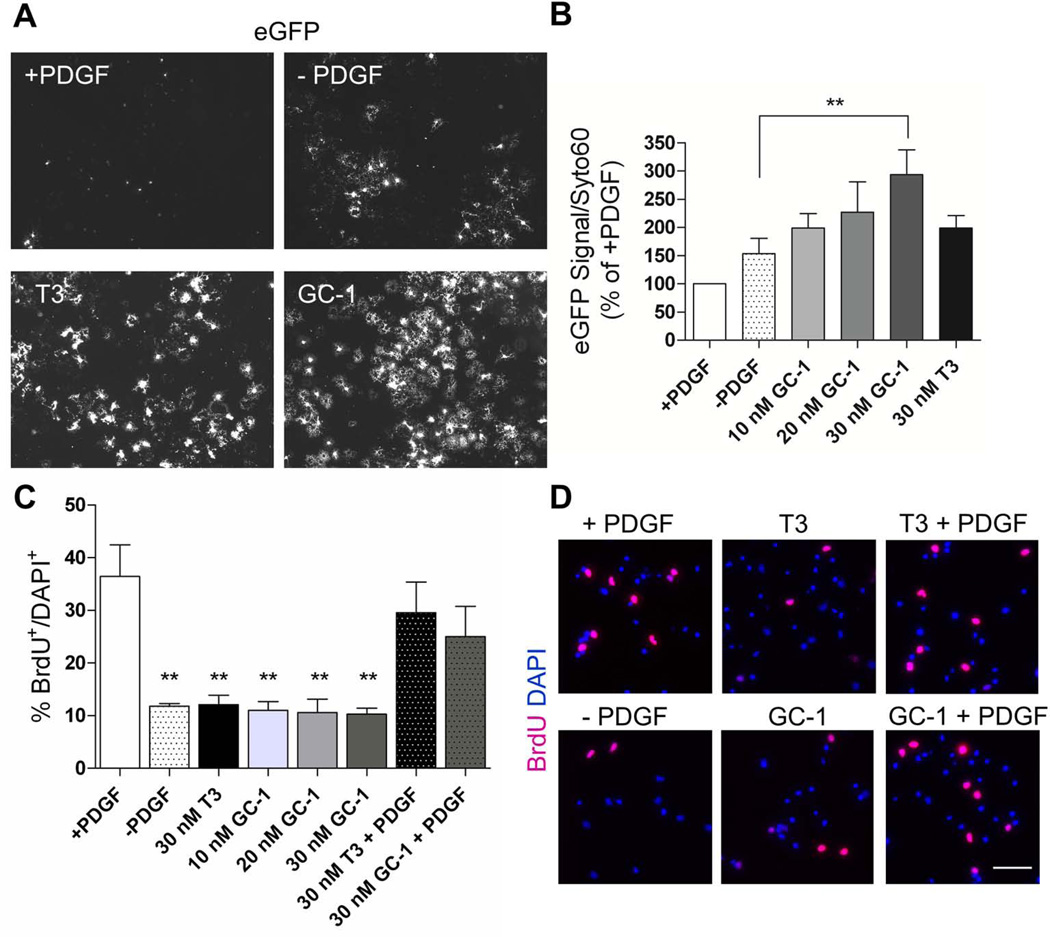
To determine if GC-1, a THRβ selective agonist, promotes oligodendrogenesis, DsRed+ cells were cultured in the presence of PDGF for four days. Withdrawal of PDGF for an additional four days increased eGFP expression, but the effect was not significant (Fig. 3A, B). Exposure to 10 and 20 nM GC-1 and 30 nM T3 induced a two-fold increase in eGFP as compared to PDGF withdrawal alone. At a concentration of 30 nM, GC-1 produced an approximately three-fold increase in eGFP, which was significantly greater than PDGF withdrawal alone, showing that GC-1 can effectively promote murine OL differentiation in vitro.
Fig. 3.
GC-1 promotes oligodendrogenesis without significantly decreasing mitogen-induced proliferation. DsRed+ OPCs were cultured for 4 days in media supplemented with 10 ng/ml PDGF followed by an additional 4 days under the following conditions; PDGF, PDGF withdrawal, PDGF withdrawal & 30 nM T3, PDGF withdrawal & 10, 20 or 30 nM GC-1. A. Native eGFP expression in OPCs under indicated conditions. B. 30 nM GC-1 significantly increases oligodendrogenesis when compared to PDGF withdrawal alone. n ≥ 3, ** P < 0.005, One-Way ANOVA, Dunnett’s Multiple Comparison Test. C. OPC proliferation in a 24 hr period is reduced by withdrawal of PDGF. There is no further decrease in proliferation mediated by addition of either T3 or GC-1. In the presence of PDGF, neither T3 nor GC-1 has a significant effect on OPC proliferation n = 3 **P < 0.005, One-Way ANOVA, Dunnett’s Multiple Comparison Test. D. Representative images of BrdU (magenta) incorporation into the nuclei (DAPI: blue) of OPCs cultured under the indicated conditions. Scale bar represents 50 µm.
To determine the effect of GC-1 on OPC proliferation, we measured BrdU incorporation during a 24-hour time period (Fig. 3C, D). As expected, withdrawal of the mitogen, PDGF, significantly decreased proliferation from 36% to 11% (n = 3, P < 0.005, One-Way ANOVA). However, an additional significant decrease in proliferation was not observed upon exposure to T3 or GC-1 at doses up to 30 nM. In the presence of PDGF and either T3 or GC-1, we noted a trend, but no significant decrease in OPC proliferation when compared to PDGF alone.
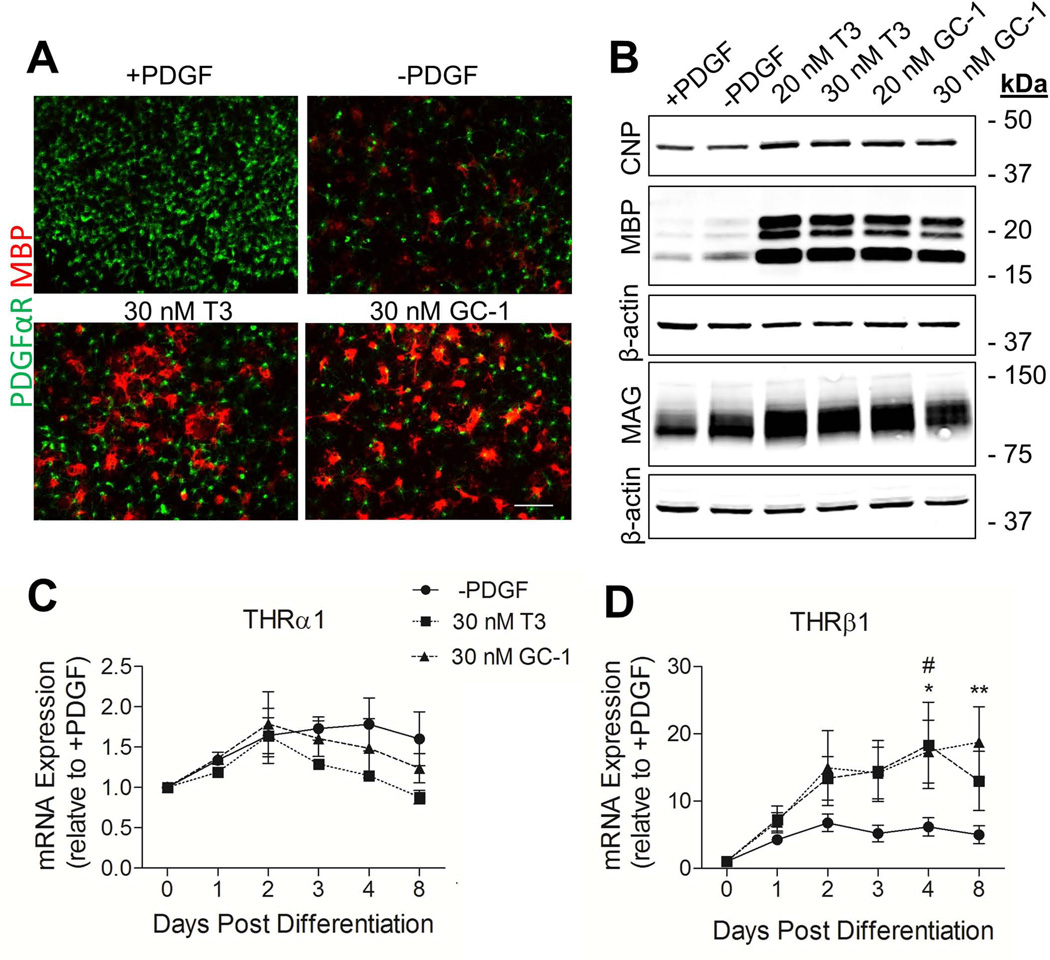
THRβ1 is Upregulated in Response to GC-1-Mediated OL Differentiation
A2B5+ OPCs derived from rat cortices were used to investigate if GC-1-mediated differentiation was accompanied by a change in the expression of THRβ1. As for murine cultures, 30 nM T3 and GC-1 were both observed to induce oligodendrogenesis in rat cells as indicated by shifts in PDGFR and MBP immunostaining (Fig. 4A). Western blot analyses also revealed increased production of the myelin proteins CNP, MBP and MAG in T3 and GC-1 treated cultures (Fig. 4B). Neither T3 nor GC-1 was found to effect cell survival in vitro (Fig. S1). Using quantitative PCR, we determined that, when compared to PDGF withdrawal, no significant changes in THR1 expression were observed post differentiation (Fig. 4C). In contrast, both T3 and GC-1 induced an increase in THRβ1 expression at days 4 and 8 post-differentiation that was significantly above PDGF withdrawal (Fig. 4D). Moreover, the observed increase at day 8 with GC-1 was significantly greater than T3.
Fig. 4.
THRβ1 is upregulated in response to GC-1-mediated OL differentiation. OPCs were isolated from rat cortices based on expression of A2B5. Following 4 days of proliferation in 10 ng/ml PDGF, cells were either maintained in PGDF for an additional 4 days, or switched to differentiating conditions; PDGF withdrawal; PDGF withdrawal and 20/30 nM T3; PDGF withdrawal and 20/30 nM GC-1. A. Double labeling immunoflorescence for PDGFαR (green) and MBP (red) comparing differentiating conditions. Scale bar represents 100 µm. B. Western blot analysis of CNP, MBP and MAG protein showing increased expression following treatment with either T3 or GC-1. Q-PCR analysis of THRα1 (C.) and THRβ1 (D.) under differentiating conditions. Data are presented as mean ± SEM, n = 3, **P < 0.005 GC-1 versus PDGF withdrawal, *P < 0.05 GC-1 versus PDGF withdrawal, #P < 0.05 T3 versus PDGF withdrawal, 2 Way ANOVA.
GC-1 Promotes Oligodendrogenesis from Human Cells in vitro
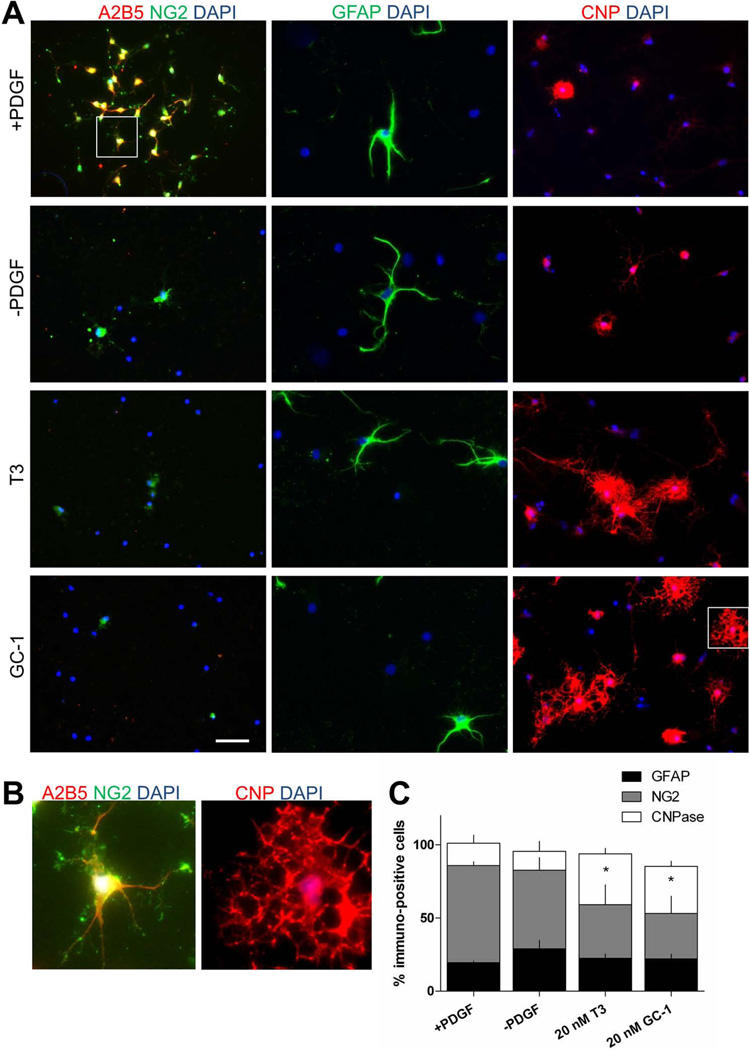
To determine if GC-1 is able to facilitate OL differentiation not only from rodent, but also from human cells, we isolated OPCs from human cerebral tissue. Tissue was derived from surgically resected grey matter tissue taken from patients with intractable epilepsy (see methods). Cells isolated using A2B5 immunoselection and maintained under proliferation conditions (+PDGF) for two weeks, were found to be NG2+ (66%) GFAP+ (19%) and CNP+ (15%) (Fig. 5). As previously reported in the literature, we found that T3 promoted human CNP+ OL differentiation (Othman et al., 2011; Wilson et al., 2003). Treatment with GC-1 (20 nM) induced an increase (from 15 to 35%) in the number of CNP+ human OLs similar to that of T3 treatment and significantly greater than that of PDGF withdrawal alone (15 to 19%, n = 3, P < 0.05, One-Way ANOVA).
Fig. 5.
GC-1 promotes differentiation of human OPCs into CNP positive OLs. A. Immunofluorescence characterization of A2B5-selected human OPCs. OPCs were cultured for 7 days in media supplemented with 10 ng/ml PDGF followed by an additional 7 days under the following conditions; PDGF, PDGF withdrawal, PDGF withdrawal & 20 nM T3, PDGF withdrawal & 20 nM GC-1. B. Magnified view of boxed regions shown in A. C. Exposure to either 20 nM T3 or GC-1 reduced the number of NG2 positive cells and increased the number of CNP positive cells. No significant change in the number of GFAP positive cells was observed. Data are presented as mean ± SEM, n = 3, *P < 0.05, One-Way ANOVA.
THRβ1, the Cellular Target of GC-1, is Expressed During Myelination in the Mouse Brain
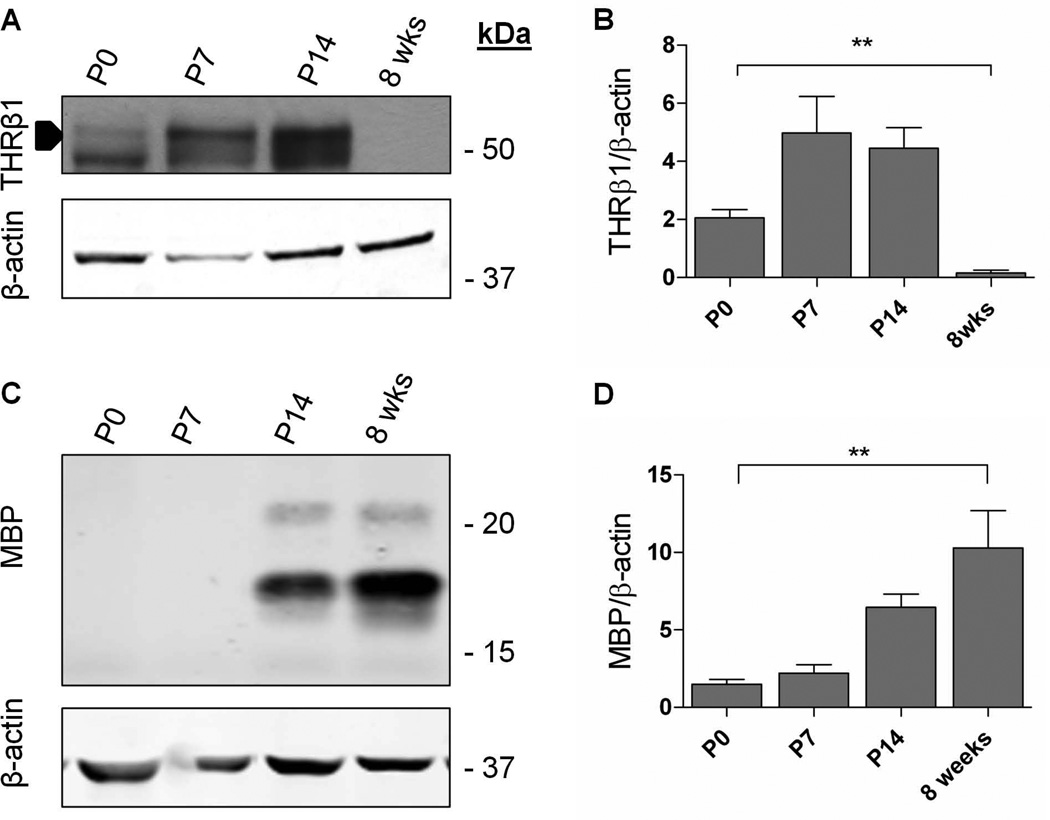
Since GC-1 is a selective agonist for THRβ1, we evaluated expression of this receptor during development in the mouse brain. We found that THRβ1 expression peaked at P7-P14, just prior to and during accumulation of the myelin protein MBP (Fig. 6). Notably, by eight weeks, when myelination is mostly complete (Baumann and Pham-Dinh, 2001; Foran and Peterson, 1992), TRβ1 levels were diminished (~ 8% of P7 levels, Fig. 5). These data indicate that THRβ1 is developmentally regulated in a manner that corresponds with onset of MBP production.
Fig. 6.
THRβ1 expression is regulated during myelination. A. Representative immunoblot showing expression of THRβ1 during development in the mouse brain. B. Quantification of A. n = 3. **P < 0.005, One-way ANOVA. C. Immunoblot showing increased expression of MBP with age. D. Quantification of C. n = 3, **P < 0.005, One-way ANOVA.
GC-1 Enhances Oligodendrogenesis During Myelination
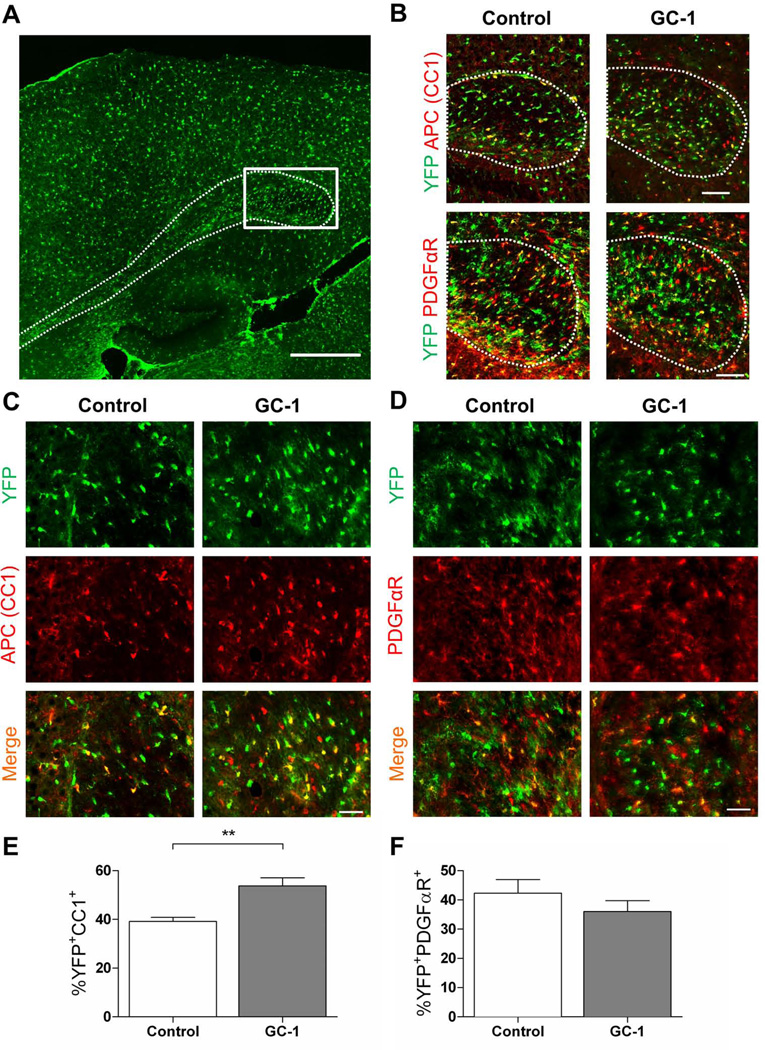
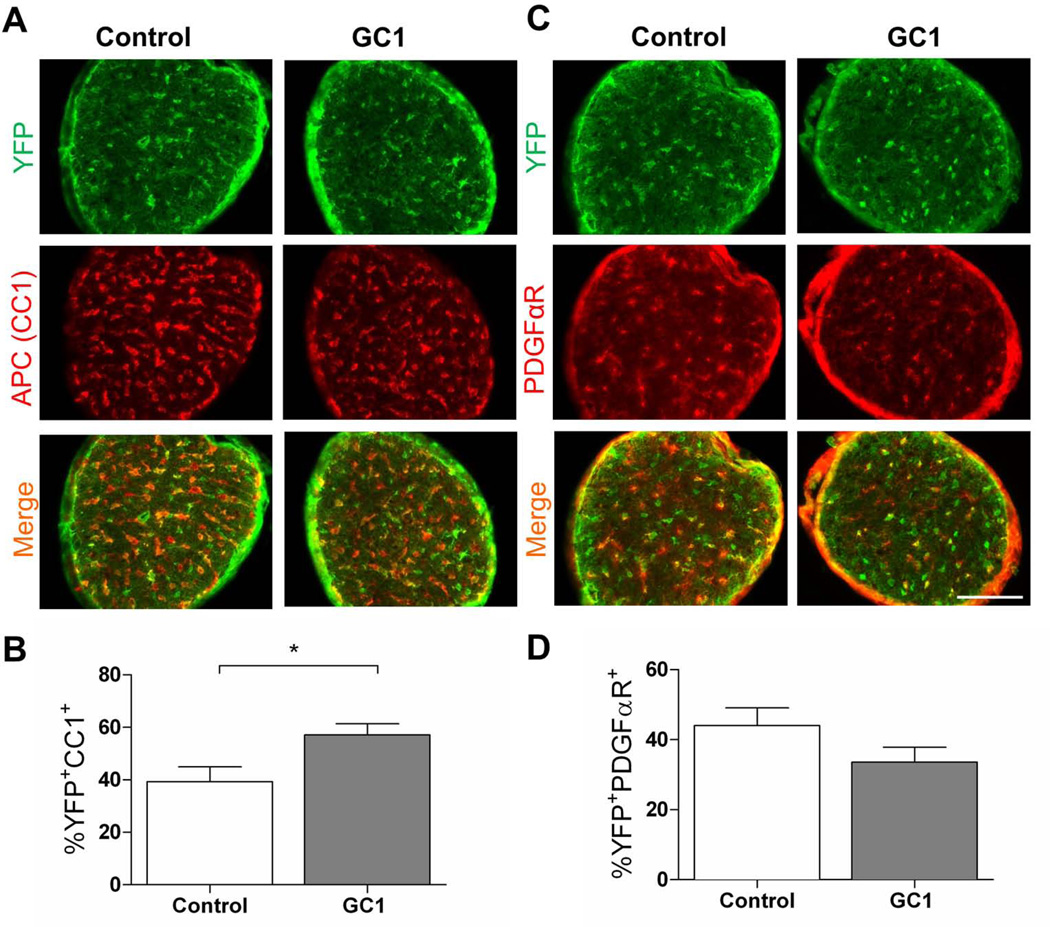
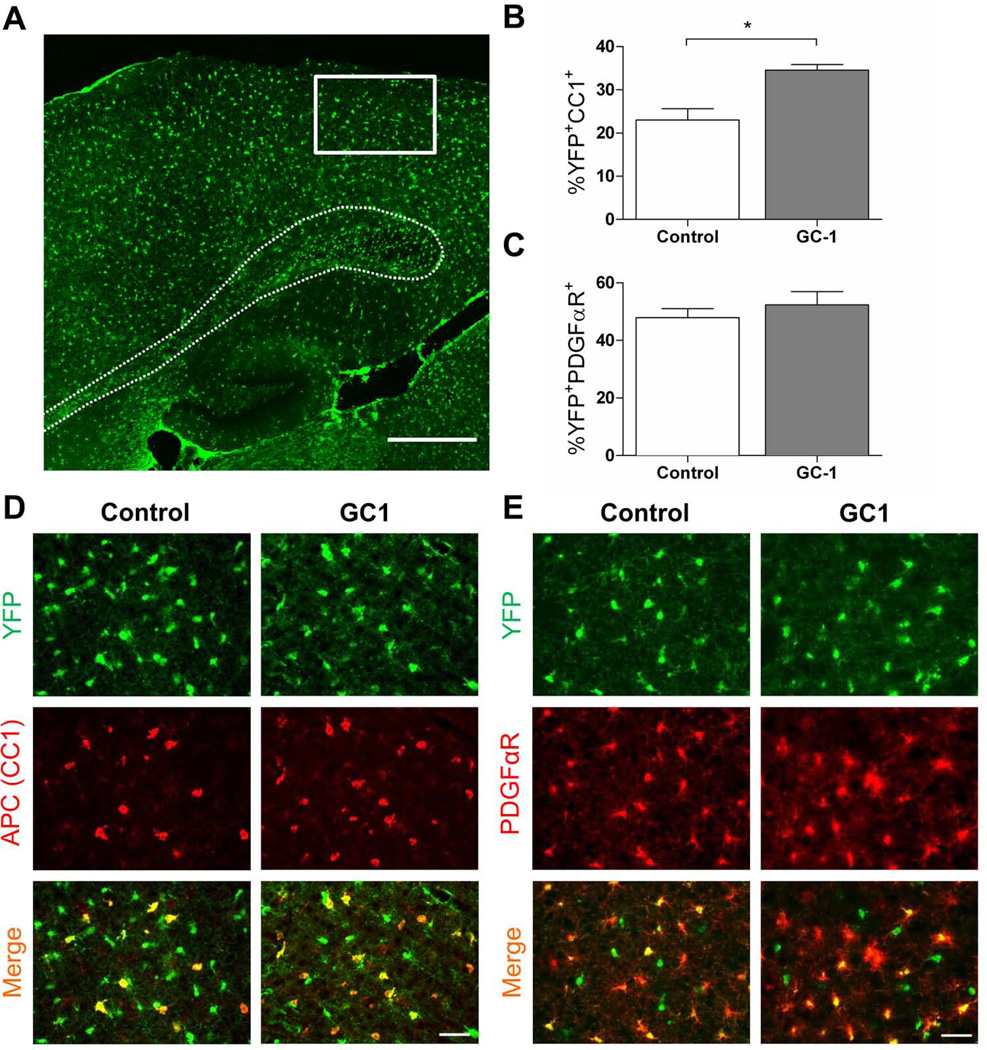
To determine if GC-1 enhances production of OLs in vivo during a critical myelination period (P7-P14), we made use of PDGFαR-CreER transgenic mice (Kang et al., 2010) to trace the fate of OPCs following GC-1 treatment. After crossing to Rosa26-eYFP reporter mice, we induced recombination of the reporter cassette and expression of eYFP within PDGFαR+ cells. Starting at P7, we treated mice with 3 µg GC-1 or an equal volume of vehicle for seven days until P14 when the mice were sacrificed. We examined the effect of GC-1 treatment in three regions of the CNS; the splenium of the corpus callosum, the occipital cortex and the optic nerve. Although OLs appear and myelinate different structures within the CNS at regulated times during development (Foran and Peterson, 1992), we observed that GC-1 was able to significantly increase oligodendrogenesis in all three regions examined (Figs 7–9). In control mice, we observed that ~39% of eYFP+ cells in the splenium of the corpus callosum exhibited immunoreactivity to APC, a marker of mature OLs (Fig. 7C, E). This number increased to ~54% with GC-1 treatment. There was also a trend for decreased numbers of PDGFαR immunoreactive cells following GC-1 treatment (Fig. 7D, F). Similar results were obtained in the occipital cortex (~23% eYFP+APC+ cells in controls versus ~35% following GC-1 treatment, Fig. 8) and optic nerve (~40% eYFP+APC+ cells in controls versus ~57% following GC-1 treatment, Fig. 9) indicating that GC-1 promotes oligodendrogenesis in vivo. We did not note a significant change in the diameter of optic nerve fibers between the two groups (the average diameter of optic nerves in the control groups was 260 ± 46 µm and in the GC-1 treated groups was 270 ± 20 µm).
Fig. 7.
GC-1 enhances oligodendrogenesis in the corpus callosum during myelination. P7 PDGFαR-Cre;Rosa-YFP mice were treated with 0.3 mg 4-HT to induce expression of YFP specifically within PDGFαR+ OPCs. Mice received 3 µg of GC-1 or an equal volume of vehicle (control) from P7 until the time of sacrifice at P14. A. Sagittal view of corpus callosum (outlined by dashed line) showing region used to examine OPC fate (boxed area). Scale bar represents 500 µm. B. Representative images of the region of interest for each treatment condition. APC was used to identify mature OLs and PDGFαR was used to identify OPCs. Scale bar represents 100 µm. C. Double immunofluorescence labeling of APC (red) and YFP (green) tissue from control and GC-1 treated tissue mice. D. Representative images from control and GC-1 treated tissue immunostained for PDGFαR+ (red) and YFP (green). Scale bar represents 50 µm. E. GC-1 treatment increases the number of YFP+APC+ cells derived from PDGFαR+ OPCs. A total of 862 YFP cells in the control and 1187 cells in the GC-1 group were counted. F. GC-1 does not significantly alter the number of OPCs that remain PDGFαR+. A total of 632 YFP cells in the control and 778 cells in the GC-1 group were counted. Data are presented as mean ± SEM, n ≥ 4/group, **P < 0.005 calculated using a two-tailed Student’s t-test.
Fig. 9.
GC-1 enhances oligodendrogenesis in the optic nerve during myelination. P7 PDGFαR-Cre;Rosa-YFP mice were treated with 0.3 mg 4-HT to induce expression of YFP specifically within PDGFαR+ OPCs. Mice received 3 µg of GC-1 or an equal volume of vehicle (control) from P7 until the time of sacrifice at P14. APC was used to identify mature OLs and PDGFαR was used to identify OPCs. A. Representative images of optic nerve from control and GC-1 treated mice immunolabeled for APC (red) and YFP (green). B. GC-1 treatment increases the number of YFP+APC+ cells derived from PDGFαR+ OPCs. A total of 1055 YFP cells in the control and 994 cells in the GC-1 group were counted. C. Representative images of optic nerve from control and GC-1 treated mice immunolabeled for PDGFαR (red) and YFP (green). Scale bar represents 100 µm D. GC-1 does not significantly alter the number of OPCs that remain PDGFαR+. A total of 888 YFP cells in the vehicle and 637 cells in the GC-1 group were counted. Data are presented as mean ± SEM, n ≥ 4/group, *P < 0.05 calculated using a two-tailed Student’s t-test.
Fig. 8.
GC-1 enhances oligodendrogenesis in the occipital cortex during myelination. P7 PDGFαR-Cre;Rosa-YFP mice were treated with 0.3 mg 4-HT to induce expression of YFP specifically within PDGFαR+ OPCs. Mice received 3 µg of GC-1 or an equal volume of vehicle (control) from P7 until the time of sacrifice at P14. A. Sagittal view of brain section showing region used to examine OPC fate. Scale bar represents 500 µm. B. GC-1 treatment increases the number of YFP+APC+ cells derived from PDGFαR+ OPCs. A total of 523 YFP cells in the control and 432 cells in the GC-1 group were counted. C. GC-1 does not significantly alter the number of OPCs that remain PDGFαR+. A total of 547 YFP cells in the control and 630 cells in the GC-1 group were counted. D. Representative images of occipital cortex from control and GC-1 treated mice immunolabeled for APC (red) and YFP (green). E. Representative images of occipital cortex from control and GC-1 treated mice immunolabeled for PDGFαR (red) and YFP (green). Scale bar represents 50 µm. Data are presented as mean ± SEM, n ≥ 4/group, *P < 0.05 calculated using a two-tailed Student’s t-test.
GC-1 Increases Expression of the Myelin Proteins MBP, CNP and MAG
Having observed an increase in numbers of newly generated OLs following GC-1 treatment from P7 to P14, we next wished to investigate if this effect was associated with an increase in myelin protein expression. By Western blot, we determined that GC-1 significantly increased the expression of MBP, CNP and MAG within the same three regions (corpus callosum, occipital cortex and optic nerve) where we observed enhanced OL numbers (Fig. 10).
Fig. 10.
Increased expression of myelin proteins MBP, CNP and MAG in GC-1 treated mice. Starting at P7, mice received 3 µg of GC-1 or an equal volume of vehicle (control) from P7 until the time of sacrifice at P14. A. Immunoblots showing MBP, CNP and MAG protein levels in the corpus callosum, occipital cortex and optic nerve following GC-1 treatment. B. Quantification of immunoblots including those shown in A. Values for each myelin protein, MBP, CNP and MAG are shown normalized to β-actin. Data are presented as mean ± SEM, n ≥ 4/group, ****P < 0.0001, ***P < 0.001, **P < 0.005, *P < 0.05 calculated using a two-tailed Student’s t-test.
DISCUSSION
THs have a number of potentially therapeutic effects, including oligodendrocyte differentiation and myelination (Barres et al., 1994; Billon et al., 2001; Calza et al., 2002; Dugas et al., 2012; Franco et al., 2008) that could be relevant to the treatment of demyelinating disorders. However, they also have the potential for serious side effects elicited by thyroid receptor signaling in non-CNS tissues. GC-1 is a thyromimetic selective for THRβ (Baxter et al., 2001) that has been shown to mediate specific TH functions with significantly reduced risk for undesirable side effects, including cardiac stimulation mediated through THRα1 (Scanlan, 2010; Tancevski et al., 2009). While GC-1’s effects on plasma cholesterol have been well described and have led to its consideration as a treatment for dyslipidemia (Tancevski et al., 2011), it is not yet understood whether selective stimulation of THRβ1 is sufficient to drive oligodendrogenesis and myelination.
Here we show that GC-1 increases OL differentiation in both rodent and human derived OPC cultures. In vivo genetic fate tracing revealed that GC-1 also augments oligodendogenesis during development and this is associated with increased expression of myelin proteins.
Role of TRβ1 in Oligodendrocyte Maturation and Myelination
Data presented herein indicate that THRβ-selective agonism has the ability to effectively enhance both in vitro and in vivo oligodendrocyte differentiation. GC-1 has approximately the same affinity for THRβ1 as T3 (~70 pM versus ~80 pM respectively), but is selective for THRβ1 over THRα1 (Chiellini et al., 1998). Its selectivity rather than specificity for THRβ1 means that we cannot rule out a contributing role of THRα in OPC differentiation. However, in our murine reporter assay (Fig. 3), 30 nM GC-1 produced a significant increase in oligodendrogenesis that was not achieved with an equimolar dose of T3. This result suggests that selective THRβ1 stimulation enhances differentiation.
OL differentiation can occur through a number of distinct pathways including mitogen-withdrawal and exposure to T3 (Barres et al., 1994; Barres et al., 1993; Dugas et al., 2010). When OPCs are exposed to T3 in the presence of PDGF, they continue to proliferate and only differentiate following multiple rounds of cell division (Barres et al., 1994; Durand and Raff, 2000; Temple and Raff, 1986). We found that like T3, GC-1 does not significantly affect OPC proliferation in the presence of PDGF (Fig. 3). This is important from a therapeutic standpoint as inappropriate OL differentiation could deplete OPC numbers and hinder myelination. Similarly, we found that neither T3 nor GC-1 inhibited proliferation in vitro beyond the reduction produced by PDGF withdrawal itself.
THRβ1 expression within the oligodendrocyte lineage has been somewhat controversial. While some groups (Baas et al., 1994a; Baas et al., 1994b; Billon et al., 2002; Billon et al., 2001; Bury et al., 2002) report expression in differentiated OLs but not OPCs, others have found THRβ1 to be present in both cell types (Barres et al., 1994). These discrepant findings may be explained by changes in the expression of THRβ1 within OPCs that are dependent on the “age” of the cell. Barres et al. (Barres et al., 1994) found that OPCs derived from the P14 rat optic nerve were more sensitive to T3 than P1-derived OPCs that have presumably undergone fewer rounds of proliferation. This possibility is further supported by the observation THRβ1 is found in greater abundance in OPCs isolated from P7 versus embryonic day 18 optic nerves and can progressively accumulate in vitro as the younger cells proliferate (Gao et al., 1998). Together these studies support a role for THRβ1 in the “cellular clock” mechanism that determines an OPC’s propensity to differentiate. In agreement with these data, we find that THRβ1 mRNA expression increases with time in vitro and that this effect is enhanced by exposure to either T3 or GC-1 (Fig. 4D). T3 is known to regulate expression of THRβ1, but not THRα1 (Baas et al., 1998; Liu et al., 2000). Here we find that, like T3, exposure to GC-1 also increases the expression of THRβ1 mRNA, but has no significant effect on THRα1 (Fig. 4C).
Thyroid Hormone and Developmental Myelination
THs are known to play an important role in developmental myelination (Bernal, 2002; Besnard et al., 1994; Sarlieve et al., 2004) and treatment of neonatal rats with thyroxine (T4) has been shown to accelerate the appearance of OLs in the optic nerve (Barres et al., 1994). Since GC-1 is known to cross the blood-brain barrier in rodents (Trost et al., 2000) and THRβ1 is expressed during development (Fig. 6), we investigated whether treatment of neonate mice with GC-1 would augment OL differentiation. Using PDGFαR-CreER mice to trace the lineage of OPCs during a weeklong period from P7 to P14, we found that GC-1 enhanced the generation of OLs in the corpus callosum, the occipital cortex and the optic nerve. In agreement with Kang et al. (Kang et al., 2010), we observed that PDGFαR+ cells remain committed to the oligodendrocyte lineage in the developing brain and did not deviate to either an astrocytic of neuronal fate (Fig. S3). Treatment with GC-1 did not change these outcomes.
While all three regions examined demonstrated a significant increase in oligodendrogenesis, the magnitude of the effect could be influenced by the intrinsic rate of myelination in each region. For example, in the optic nerve, myelination is known to proceed in a retinal-to-chiasmal direction following the appearance of MBP positive cells on P7 (Foran and Peterson, 1992). In contrast, MBP positive cells are not observed in the corpus callosum until P9. Therefore, while P7–14 is a critical period for CNS myelination in general, the exact timing and rate of the process varies for each region. Thus, the magnitude of the effect mediated by GC-1 may also vary. It may be more difficult to drive myelination in a region that is not yet fully populated by OPCs or by axons receptive to myelination.
Since GC-1 promoted oligodendrogenesis during development, we next asked whether this change was associated with an increase in myelin protein expression during the same time period. We found that the abundance of the myelin proteins MBP, CNP and MAG were all significantly increased at P14 following treatment with GC-1 for one week prior. Although we have not investigated whether this effect is sustained, it does indicate the capacity for GC-1 to enhance myelination.
In summary, GC-1 treatment of both rodent and human derived OPCs was found to enhance oligodendrogenesis in vitro. Examining the lineage progression of PDGFαR+ OPCs during development, we also observed a significant increase in OL generation in vivo. GC-1 may hold therapeutic potential for demyelinating disorders, but carry a lower risk of side effects compared to treatment with non-selective thyroid hormones.
Supplementary Material
Fig. S1. GC-1 and T3 do not significantly affect cell survival during differentiation. Rat A2B5+ OPCs were cultured in 10 ng/ml PDGF for 4 days prior to differentiation for an additional 4 days under the conditions indicated above. On days 2 and 4 post-differentiation, cell supernatants were taken and lactate dehydrogenase (LDH) levels assayed. LDH levels are expressed relative to maximum LDH release (supernatant from cells exposed to 0.8% triton-X for 45 mins.). When compared to PDGF withdrawal, no significant changes between treatment conditions were observed at 2 days post-differentiation. At 4 days, cells maintained in PDGF released significantly more LDH compared to withdrawal. Neither T3 nor GC-1 affected LDH release at 4 days post-differentiation versus PDGF withdrawal. **P < 0.005, One-Way ANOVA, Dunnett’s Multiple Comparison Test.
Fig. S2. Detection of THRβ1 by immunoblot. A. To confirm the identity of THRβ1 by immunolot, HEK 293T cells were transfected with pCMV_THRβ1, pCMV_THRβ4 or empty vector (CTRL). A single band at ~52 kDa (the predicted molecular weight of THRβ1) was identified. B. Long exposure of immunoblot in Fig. 6A. comparing position of THRβ1 positive control relative to protein from experimental conditions.
Fig. S3. GC-1 does not alter lineage commitment of PDGFαR+-derived cells. P7 PDGFαR-Cre;Rosa-YFP mice were treated with 0.3 mg 4-HT to induce expression of YFP specifically within PDGFαR+ OPCs. Mice received 3 µg of GC-1 or an equal volume of vehicle (control) from P7 until the time of sacrifice at P14. A. Double immunolabeling for YFP (green) and the astrocyte marker GFAP (red) within the corpus callosum. Images on the right (scale bar represents 50 µm) are magnified from the left (scale bar represents 500 µm). B. Double immunolabeling for YFP (green) and the neuronal marker NeuN (red) within the corpus callosum. Images on the right (scale bar represents 50 µm) are magnified from the left (scale bar represents 500 µm). C. Table documenting occurrence of GFAP+/YFP+ and NeuN+/YFP+ cells under both treatment conditions.
Main Points.
Thyroid hormones regulate oligodendrocyte differentiation during development, but it remains unclear which receptors are required to drive this effect. We report that GC-1, a thyromimetic with selective receptor β action and a limited side-effect profile, promotes oligodendrogenesis.
ACKNOWLEDGEMENTS
The authors thank Dr. Thomas Scanlan (Oregon Health Sciences University, Portland, OR) for the kind gift of GC-1, Dr. William Stallcup (Burnham Institute, San Diego, CA) for antibodies to NG2 and PDGFαR, Dr. Ronald Evans (Salk Institute, La Jolla, CA) and Dr. Tetsuya Tagami (Kyoto Medical Center, Kyoto, Japan). Hao Zhang and Ada Tam provided excellent technical assistance with FACS. This work was supported by a grant from National Institutes of Health: R21 NS081418-01 (PAC), NMSS Collaborative Center Grant, and The Silverman Foundation. Emily G. Baxi received a Post Doctoral Fellowship Grant from the Maryland Stem Cell Research Fund (2010-MSCRFF-0061-00).
REFERENCES
- Baas D, Bourbeau D, Carre JL, Sarlieve LL, Dussault JH, Puymirat J. Expression of alpha and beta thyroid receptors during oligodendrocyte differentiation. Neuroreport. 1994a;5:1805–1808. doi: 10.1097/00001756-199409080-00030. [DOI] [PubMed] [Google Scholar]
- Baas D, Bourbeau D, Sarlieve LL, Ittel ME, Dussault JH, Puymirat J. Oligodendrocyte maturation and progenitor cell proliferation are independently regulated by thyroid hormone. Glia. 1997;19:324–332. doi: 10.1002/(sici)1098-1136(199704)19:4<324::aid-glia5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Baas D, Fressinaud C, Ittel ME, Reeber A, Dalencon D, Puymirat J, Sarlieve LL. Expression of thyroid hormone receptor isoforms in rat oligodendrocyte cultures. Effect of 3,5,3'-triiodo-L-thyronine. Neurosci Lett. 1994b;176:47–51. doi: 10.1016/0304-3940(94)90868-0. [DOI] [PubMed] [Google Scholar]
- Baas D, Puymirat J, Sarlieve LL. Posttranscriptional regulation of oligodendroglial thyroid hormone (T3) receptor beta 1 by T3. Int J Dev Neurosci. 1998;16:461–467. doi: 10.1016/s0736-5748(98)00053-7. [DOI] [PubMed] [Google Scholar]
- Badea TC, Wang Y, Nathans J. A noninvasive genetic/pharmacologic strategy for visualizing cell morphology and clonal relationships in the mouse. J Neurosci. 2003;23:2314–2322. doi: 10.1523/JNEUROSCI.23-06-02314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Lazar MA, Raff MC. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;120:1097–1108. doi: 10.1242/dev.120.5.1097. [DOI] [PubMed] [Google Scholar]
- Barres BA, Schmid R, Sendnter M, Raff MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118:283–295. doi: 10.1242/dev.118.1.283. [DOI] [PubMed] [Google Scholar]
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- Baxter JD, Dillmann WH, West BL, Huber R, Furlow JD, Fletterick RJ, Webb P, Apriletti JW, Scanlan TS. Selective modulation of thyroid hormone receptor action. J Steroid Biochem Mol Biol. 2001;76:31–42. doi: 10.1016/s0960-0760(01)00052-8. [DOI] [PubMed] [Google Scholar]
- Bernal J. Action of thyroid hormone in brain. J Endocrinol Invest. 2002;25:268–288. doi: 10.1007/BF03344003. [DOI] [PubMed] [Google Scholar]
- Bernal J. Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab. 2007;3:249–259. doi: 10.1038/ncpendmet0424. [DOI] [PubMed] [Google Scholar]
- Besnard F, Luo M, Miehe M, Dussault JH, Puymirat J, Sarlieve LL. Transient expression of 3,5,3'-triiodothyronine nuclear receptors in rat oligodendrocytes: in vivo and in vitro immunocytochemical studies. J Neurosci Res. 1994;37:313–323. doi: 10.1002/jnr.490370304. [DOI] [PubMed] [Google Scholar]
- Billon N, Jolicoeur C, Tokumoto Y, Vennstrom B, Raff M. Normal timing of oligodendrocyte development depends on thyroid hormone receptor alpha 1 (TRalpha1) EMBO J. 2002;21:6452–6460. doi: 10.1093/emboj/cdf662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon N, Tokumoto Y, Forrest D, Raff M. Role of thyroid hormone receptors in timing oligodendrocyte differentiation. Dev Biol. 2001;235:110–120. doi: 10.1006/dbio.2001.0293. [DOI] [PubMed] [Google Scholar]
- Borngraeber S, Budny MJ, Chiellini G, Cunha-Lima ST, Togashi M, Webb P, Baxter JD, Scanlan TS, Fletterick RJ. Ligand selectivity by seeking hydrophobicity in thyroid hormone receptor. Proc Natl Acad Sci U S A. 2003;100:15358–15363. doi: 10.1073/pnas.2136689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bury F, Carre JL, Vega S, Ghandour MS, Rodriguez-Pena A, Langley K, Sarlieve LL. Coexpression of thyroid hormone receptor isoforms in mouse oligodendrocytes. J Neurosci Res. 2002;67:106–113. doi: 10.1002/jnr.10111. [DOI] [PubMed] [Google Scholar]
- Calza L, Fernandez M, Giuliani A, Aloe L, Giardino L. Thyroid hormone activates oligodendrocyte precursors and increases a myelin-forming protein and NGF content in the spinal cord during experimental allergic encephalomyelitis. Proc Natl Acad Sci U S A. 2002;99:3258–3263. doi: 10.1073/pnas.052704499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiellini G, Apriletti JW, Yoshihara HA, Baxter JD, Ribeiro RC, Scanlan TS. A high-affinity subtype-selective agonist ligand for the thyroid hormone receptor. Chem Biol. 1998;5:299–306. doi: 10.1016/s1074-5521(98)90168-5. [DOI] [PubMed] [Google Scholar]
- Darras VM. Endocrine disrupting polyhalogenated organic pollutants interfere with thyroid hormone signalling in the developing brain. Cerebellum. 2008;7:26–37. doi: 10.1007/s12311-008-0004-5. [DOI] [PubMed] [Google Scholar]
- Dugas JC, Cuellar TL, Scholze A, Ason B, Ibrahim A, Emery B, Zamanian JL, Foo LC, McManus MT, Barres BA. Dicer1 and miR-219 Are required for normal oligodendrocyte differentiation and myelination. Neuron. 2010;65:597–611. doi: 10.1016/j.neuron.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas JC, Ibrahim A, Barres BA. The T3-induced gene KLF9 regulates oligodendrocyte differentiation and myelin regeneration. Mol Cell Neurosci. 2012;50:45–57. doi: 10.1016/j.mcn.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand B, Raff M. A cell-intrinsic timer that operates during oligodendrocyte development. Bioessays. 2000;22:64–71. doi: 10.1002/(SICI)1521-1878(200001)22:1<64::AID-BIES11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Farsetti A, Desvergne B, Hallenbeck P, Robbins J, Nikodem VM. Characterization of myelin basic protein thyroid hormone response element and its function in the context of native and heterologous promoter. J Biol Chem. 1992;267:15784–15788. [PubMed] [Google Scholar]
- Farsetti A, Mitsuhashi T, Desvergne B, Robbins J, Nikodem VM. Molecular basis of thyroid hormone regulation of myelin basic protein gene expression in rodent brain. J Biol Chem. 1991;266:23226–23232. [PubMed] [Google Scholar]
- Foran DR, Peterson AC. Myelin acquisition in the central nervous system of the mouse revealed by an MBP-Lac Z transgene. J Neurosci. 1992;12:4890–4897. doi: 10.1523/JNEUROSCI.12-12-04890.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco PG, Silvestroff L, Soto EF, Pasquini JM. Thyroid hormones promote differentiation of oligodendrocyte progenitor cells and improve remyelination after cuprizone-induced demyelination. Exp Neurol. 2008;212:458–467. doi: 10.1016/j.expneurol.2008.04.039. [DOI] [PubMed] [Google Scholar]
- Freitas FR, Moriscot AS, Jorgetti V, Soares AG, Passarelli M, Scanlan TS, Brent GA, Bianco AC, Gouveia CH. Spared bone mass in rats treated with thyroid hormone receptor TR beta-selective compound GC-1. Am J Physiol Endocrinol Metab. 2003;285:E1135–E1141. doi: 10.1152/ajpendo.00506.2002. [DOI] [PubMed] [Google Scholar]
- Gao FB, Apperly J, Raff M. Cell-intrinsic timers and thyroid hormone regulate the probability of cell-cycle withdrawal and differentiation of oligodendrocyte precursor cells. Dev Biol. 1998;197:54–66. doi: 10.1006/dbio.1998.8877. [DOI] [PubMed] [Google Scholar]
- Gloss B, Trost S, Bluhm W, Swanson E, Clark R, Winkfein R, Janzen K, Giles W, Chassande O, Samarut J, et al. Cardiac ion channel expression and contractile function in mice with deletion of thyroid hormone receptor alpha or beta. Endocrinology. 2001;142:544–550. doi: 10.1210/endo.142.2.7935. [DOI] [PubMed] [Google Scholar]
- Grover GJ, Egan DM, Sleph PG, Beehler BC, Chiellini G, Nguyen NH, Baxter JD, Scanlan TS. Effects of the thyroid hormone receptor agonist GC-1 on metabolic rate and cholesterol in rats and primates: selective actions relative to 3,5,3'-triiodo-L-thyronine. Endocrinology. 2004;145:1656–1661. doi: 10.1210/en.2003-0973. [DOI] [PubMed] [Google Scholar]
- Gullberg H, Rudling M, Salto C, Forrest D, Angelin B, Vennstrom B. Requirement for thyroid hormone receptor beta in T3 regulation of cholesterol metabolism in mice. Mol Endocrinol. 2002;16:1767–1777. doi: 10.1210/me.2002-0009. [DOI] [PubMed] [Google Scholar]
- Jeannin E, Robyr D, Desvergne B. Transcriptional regulatory patterns of the myelin basic protein and malic enzyme genes by the thyroid hormone receptors alpha1 and beta1. J Biol Chem. 1998;273:24239–24248. doi: 10.1074/jbc.273.37.24239. [DOI] [PubMed] [Google Scholar]
- Johansson C, Vennstrom B, Thoren P. Evidence that decreased heart rate in thyroid hormone receptor-alpha1-deficient mice is an intrinsic defect. Am J Physiol. 1998;275:R640–R646. doi: 10.1152/ajpregu.1998.275.2.R640. [DOI] [PubMed] [Google Scholar]
- Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YW, Lo LJ, Chan WK. Temporal expression and T3 induction of thyroid hormone receptors alpha1 and beta1 during early embryonic and larval development in zebrafish, Danio rerio. Mol Cell Endocrinol. 2000;159:187–195. doi: 10.1016/s0303-7207(99)00193-8. [DOI] [PubMed] [Google Scholar]
- Mallon BS, Shick HE, Kidd GJ, Macklin WB. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J Neurosci. 2002;22:876–885. doi: 10.1523/JNEUROSCI.22-03-00876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansen A, Tiselius C, Sand P, Fauconnier J, Westerblad H, Rydqvist B, Vennstrom B. Thyroid hormone receptor alpha can control action potential duration in mouse ventricular myocytes through the KCNE1 ion channel subunit. Acta Physiol (Oxf) 198:133–142. doi: 10.1111/j.1748-1716.2009.02052.x. [DOI] [PubMed] [Google Scholar]
- Manzano J, Morte B, Scanlan TS, Bernal J. Differential effects of triiodothyronine and the thyroid hormone receptor beta-specific agonist GC-1 on thyroid hormone target genes in the b ain. Endocrinology. 2003;144:5480–5487. doi: 10.1210/en.2003-0633. [DOI] [PubMed] [Google Scholar]
- Miron VE, Rajasekharan S, Jarjour AA, Zamvil SS, Kennedy TE, Antel JP. Simvastatin regulates oligodendroglial process dynamics and survival. Glia. 2007;55:130–143. doi: 10.1002/glia.20441. [DOI] [PubMed] [Google Scholar]
- Miyabara EH, Aoki MS, Soares AG, Saltao RM, Vilicev CM, Passarelli M, Scanlan TS, Gouveia CH, Moriscot AS. Thyroid hormone receptor-beta-selective agonist GC-24 spares skeletal muscle type I to II fiber shift. Cell Tissue Res. 2005;321:233–241. doi: 10.1007/s00441-005-1119-3. [DOI] [PubMed] [Google Scholar]
- O'Shea PJ, Williams GR. Insight into the physiological actions of thyroid hormone receptors from genetically modified mice. J Endocrinol. 2002;175:553–570. doi: 10.1677/joe.0.1750553. [DOI] [PubMed] [Google Scholar]
- Othman A, Frim DM, Polak P, Vujicic S, Arnason BG, Boullerne AI. Olig1 is expressed in human oligodendrocytes during maturation and regeneration. Glia. 2011;59:914–926. doi: 10.1002/glia.21163. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A, Ibarrola N, Iniguez MA, Munoz A, Bernal J. Neonatal hypothyroidism affects the timely expression of myelin-associated glycoprotein in the rat brain. J Clin Invest. 1993;91:812–818. doi: 10.1172/JCI116301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch A, Ng L, Goodyear R, Oliver D, Lisoukov I, Vennstrom B, Richardson G, Kelley MW, Forrest D. Retardation of cochlear maturation and impaired hair cell function caused by deletion of all known thyroid hormone receptors. J Neurosci. 2001;21:9792–9800. doi: 10.1523/JNEUROSCI.21-24-09792.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarlieve LL, Rodriguez-Pena A, Langley K. Expression of thyroid hormone receptor isoforms in the oligodendrocyte lineage. Neurochem Res. 2004;29:903–922. doi: 10.1023/b:nere.0000021235.83952.9a. [DOI] [PubMed] [Google Scholar]
- Scanlan TS. Sobetirome: a case history of bench-to-clinic drug discovery and development. Heart Fail Rev. 2010;15:177–182. doi: 10.1007/s10741-008-9122-x. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Lang JK, Ali TA, Roy NS, Vates GE, Pilcher WH, Goldman SA. Statin treatment of adult human glial progenitors induces PPAR gamma-mediated oligodendrocytic differentiation. Glia. 2008;56:954–962. doi: 10.1002/glia.20669. [DOI] [PubMed] [Google Scholar]
- Tagami T, Yamamoto H, Moriyama K, Sawai K, Usui T, Shimatsu A, Naruse M. Identification of a novel human thyroid hormone receptor beta isoform as a transcriptional modulator. Biochem Biophys Res Commun. 2010;396:983–988. doi: 10.1016/j.bbrc.2010.05.038. [DOI] [PubMed] [Google Scholar]
- Tancevski I, Demetz E, Eller P. Sobetirome: a selective thyromimetic for the treatment of dyslipidemia. Recent Pat Cardiovasc Drug Discov. 2011;6:16–19. doi: 10.2174/157489011794578473. [DOI] [PubMed] [Google Scholar]
- Tancevski I, Eller P, Patsch JR, Ritsch A. The resurgence of thyromimetics as lipid-modifying agents. Curr Opin Investig Drugs. 2009;10:912–918. [PMC free article] [PubMed] [Google Scholar]
- Temple S, Raff MC. Clonal analysis of oligodendrocyte development in culture: evidence for a developmental clock that counts cell divisions. Cell. 1986;44:773–779. doi: 10.1016/0092-8674(86)90843-3. [DOI] [PubMed] [Google Scholar]
- Tosic M, Torch S, Comte V, Dolivo M, Honegger P, Matthieu JM. Triiodothyronine has diverse and multiple stimulating effects on expression of the major myelin protein genes. J Neurochem. 1992;59:1770–1777. doi: 10.1111/j.1471-4159.1992.tb11009.x. [DOI] [PubMed] [Google Scholar]
- Trost SU, Swanson E, Gloss B, Wang-Iverson DB, Zhang H, Volodarsky T, Grover GJ, Baxter JD, Chiellini G, Scanlan TS, et al. The thyroid hormone receptor-beta-selective agonist GC-1 differentially affects plasma lipids and cardiac activity. Endocrinology. 2000;141:3057–3064. doi: 10.1210/endo.141.9.7681. [DOI] [PubMed] [Google Scholar]
- Weiss RE, Forrest D, Pohlenz J, Cua K, Curran T, Refetoff S. Thyrotropin regulation by thyroid hormone in thyroid hormone receptor beta-deficient mice. Endocrinology. 1997;138:3624–3629. doi: 10.1210/endo.138.9.5412. [DOI] [PubMed] [Google Scholar]
- Williams GR. Cloning and characterization of two novel thyroid hormone receptor beta isoforms. Mol Cell Biol. 2000;20:8329–8342. doi: 10.1128/mcb.20.22.8329-8342.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HC, Onischke C, Raine CS. Human oligodendrocyte precursor cells in vitro: phenotypic analysis and differential response to growth factors. Glia. 2003;44:153–165. doi: 10.1002/glia.10280. [DOI] [PubMed] [Google Scholar]
- Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J Neuroendocrinol. 2004;16:809–818. doi: 10.1111/j.1365-2826.2004.01243.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. GC-1 and T3 do not significantly affect cell survival during differentiation. Rat A2B5+ OPCs were cultured in 10 ng/ml PDGF for 4 days prior to differentiation for an additional 4 days under the conditions indicated above. On days 2 and 4 post-differentiation, cell supernatants were taken and lactate dehydrogenase (LDH) levels assayed. LDH levels are expressed relative to maximum LDH release (supernatant from cells exposed to 0.8% triton-X for 45 mins.). When compared to PDGF withdrawal, no significant changes between treatment conditions were observed at 2 days post-differentiation. At 4 days, cells maintained in PDGF released significantly more LDH compared to withdrawal. Neither T3 nor GC-1 affected LDH release at 4 days post-differentiation versus PDGF withdrawal. **P < 0.005, One-Way ANOVA, Dunnett’s Multiple Comparison Test.
Fig. S2. Detection of THRβ1 by immunoblot. A. To confirm the identity of THRβ1 by immunolot, HEK 293T cells were transfected with pCMV_THRβ1, pCMV_THRβ4 or empty vector (CTRL). A single band at ~52 kDa (the predicted molecular weight of THRβ1) was identified. B. Long exposure of immunoblot in Fig. 6A. comparing position of THRβ1 positive control relative to protein from experimental conditions.
Fig. S3. GC-1 does not alter lineage commitment of PDGFαR+-derived cells. P7 PDGFαR-Cre;Rosa-YFP mice were treated with 0.3 mg 4-HT to induce expression of YFP specifically within PDGFαR+ OPCs. Mice received 3 µg of GC-1 or an equal volume of vehicle (control) from P7 until the time of sacrifice at P14. A. Double immunolabeling for YFP (green) and the astrocyte marker GFAP (red) within the corpus callosum. Images on the right (scale bar represents 50 µm) are magnified from the left (scale bar represents 500 µm). B. Double immunolabeling for YFP (green) and the neuronal marker NeuN (red) within the corpus callosum. Images on the right (scale bar represents 50 µm) are magnified from the left (scale bar represents 500 µm). C. Table documenting occurrence of GFAP+/YFP+ and NeuN+/YFP+ cells under both treatment conditions.