Abstract
Premenstrual dysphoric disorder (PMDD) is the prototypical sex‐specific disorder in which symptom onset and offset require a particular hormonal milieu and for which there is moderate heritability. The present study investigated brain emotion processing in PMDD and healthy controls, as well as functional polymorphisms in two candidate genes for PMDD, the serotonin transporter (5‐HTT) and brain derived neurotrophic factor (BDNF). The 5‐HTT linked polymorphic region (5‐HTTLPR) and BDNF Val66Met polymorphisms were genotyped in 31 patients with PMDD and 31 healthy controls. A subset of 16 patients and 15 controls participated in two functional magnetic resonance imaging‐sessions performing an emotion processing task; once in the mid‐follicular, and once in the late luteal phase which corresponds with maximum severity of mood symptoms. Genotypes were not directly associated with PMDD. A main effect of group was found in the whole brain analysis, with patients having lower activation of the pre‐genual anterior cingulate and ventro‐medial prefrontal cortex, independent of menstrual cycle phase. Post‐hoc functional ROI analyses in the fronto‐cingulate cluster showed no effect of 5‐HTTLPR genotype but a genotype‐by‐group‐by‐phase interaction effect of BDNF Val66Met. Women with PMDD who were carriers of the Met‐allele had lower fronto‐cingulate cortex activation in the luteal phase compared to Met‐allele carrying controls. The results provide suggestive evidence of impaired emotion‐induced fronto‐cingulate cortex activation in PMDD patients. Although limited by a small sample, the potential influence of BDNF Val66Met in PMDD is in line with preclinical findings. Hum Brain Mapp 35:4450–4458, 2014. © 2014 The Authors. Human Brain Mapping Published by Wiley Periodicals, Inc.
Keywords: anterior cingulate cortex, BDNF Val66Met, emotion, fMRI, premenstrual dysphoric disorder, 5‐HTTLPR
INTRODUCTION
Premenstrual dysphoric disorder (PMDD) is categorized as a mood disorder [APA, 2013] with onset of functionally impairing or distressing mood and physical symptoms in the luteal phase of the menstrual cycle, a decline in symptom severity after onset of menstruation, and an absence of symptoms in the postmenstrual week [Yonkers et al., 2008]. Hallmark mood symptoms include mood lability, irritability, anxiety, tension, and depression [Epperson et al., 2012]. The disorder affects roughly 5% of women of reproductive age [Wittchen et al., 2002], and has a moderate heritability [Kendler et al., 1998]. However, no abnormality in peripheral levels of ovarian hormones has been observed [Yonkers et al., 2008], and no genetic marker has consistently been associated with PMDD. The neurobiological underpinnings of PMDD thus remain largely unknown.
Ovarian hormones have profound organizational effects during brain development, and important and diverse activational effects in adulthood [Cahill, 2006; Gillies and McArthur, 2010; Savic, 2010]. Neuroimaging findings in PMDD suggest menstrual cycle phase‐by‐diagnosis interaction effects, indeed highlighting the relevance of hormone fluctuations in this disorder [Epperson, 2013]. Moreover, a proton magnetic resonance spectroscopy (1H‐MRS) study implicated a gamma‐aminobutyric acid (GABA) interaction with ovarian hormones and neurosteroids in the pathophysiology of PMDD [Epperson et al., 2002], and a PET study suggested a role for serotonin in PMDD [Jovanovic et al., 2006].
Emotions drive cognitive and behavioral processes, and are biologically basic and inherited states [Lindquist et al., 2012]. The functional neuroanatomy of emotion processing and response comprises mainly the amygdala, medial PFC, and insular cortex [Etkin et al., 2011; Shin and Liberzon, 2010]. The medial PFC and ACC have been linked to sadness, anxiety and depressive states [Etkin et al., 2011; Lindquist et al., 2012], typical symptomatic characteristics of PMDD. The ACC, in particular the pregenual region (pgACC) and the ventro‐medial PFC (vmPFC), are involved in the top‐down regulation of anxiety, through inhibition of amygdala reactivity to stressful stimuli; and deficient pregenual‐prefrontal functioning and amygdala hyper‐responsiveness are typical characteristics of anxiety disorders (Rauch et al, 2006). Interestingly, patients with PMDD have increased amygdala activation in response to emotional stimuli [Gingnell et al., 2012; Protopopescu et al., 2008], suggesting an impaired anxiety regulation. In addition, patients with PMDD, who have high scores of anxiety, display increased emotion‐induced amygdala reactivity in the luteal phase [Gingnell et al., 2012].
Association studies of PMDD have indicated markers in genes related to the monoamine and sex gonadal hormone systems [Adams and McCrone, 2012; Dhingra et al., 2007; Gingnell et al., 2010; Huo et al., 2007]. Selective serotonin reuptake inhibitors (SSRIs) are the first‐line treatment for PMDD, and patients preferentially respond to serotonergic rather than noradrenergic antidepressants [Freeman et al., 1999]. Complementary to the serotonin (5‐HT) system is the brain derived neurotrophic factor (BDNF). A constellation of molecular, pharmacological and genetic findings support an interaction between 5‐HT and BDNF with implication for mood disorders [Martinowich and Lu, 2008]. A mouse and a human model provided translational evidence of impaired extinction of fear among carriers of the Met allele, a phenotype that is associated with anxious and depressive behavior [Soliman et al., 2010]. More recently one study of mice, making use of a genetic knock‐in animal model, provided evidence of an association between the BDNF Met66 allele and anxiety‐related behavior during the estrous phase, when both estradiol and progesterone decline [Bath et al., 2012]. All together these findings make these two functional polymorphisms, the 5‐HT linked polymorphic region (5‐HTTLPR) and the single nucleotide polymorphism (SNP) adenine/guanine (A/G), Valine66Methionine (Val66Met), BDNF (rs6265), candidate genetic markers for PMDD. It is thus plausible that the 5‐HTTLPR and BDNF Val66Met are potential modulators of pregenual‐prefrontal region reactivity to emotional stimuli in PMDD.
While there is a growing literature demonstrating an effect of menstrual cycle phase and/or ovarian hormones in neuroimaging of PMDD, no previous study has considered genetic factors in relation to emotional areas with regulatory functions such as pgACC and vmPFC. Hence, the present study aimed to investigate whether pregenual‐prefrontal activation during emotion processing is lower in patients with PMDD than in healthy controls in the late luteal phases of the menstrual cycle. The study also aimed to elucidate the potential mediating effect of the functional polymorphisms 5‐HTTLPR and BDNF Val66Met on pregenual‐prefrontal activation.
MATERIALS AND METHODS
Participants
The study sample included 31 patients with PMDD and 31 healthy controls with regular menstrual cycles (25–31 days). Included patients met the criteria for a PMDD diagnosis, as defined by DSM‐IV TR, and they were recruited among women seeking help for premenstrual symptoms at the out‐patient ward of the Department of Obstetrics and Gynecology, Uppsala University Hospital or from newspaper advertisement. Diagnosis was based on daily, prospective symptom ratings on the cyclicity diagnoser (CD) scale during two consecutive menstrual cycles [Sundstrom et al., 1999]. The scale consists of nine negative mood symptoms (depression, decreased interest in usual activities, fatigue, irritability, tension, mood swings, lability, difficulties in concentrating, and sleeping disturbances), two positive mood symptoms (cheerfulness and energy), four somatic symptoms (food cravings, swelling, breast tenderness, and menstrual bleeding), and one parameter for measuring impact on daily life. The CD scale is a Likert scale ranging from 0 to 8 with 0 indicating a complete absence of the symptom and eight maximal severity of the symptom. Patients were considered to have PMDD if they had a clinically relevant 100% increase in five symptoms (at least one of them being one of the core symptoms) during seven premenstrual days compared to seven mid‐follicular days, associated with a significant social or occupational impairment [Hammarbäck et al., 1989]. All patients displayed at least 1 week of sparse symptoms (mean scores <2) in the follicular phase. The controls were physically healthy women with no self‐reported premenstrual symptoms. They displayed no luteal phase symptoms (mean scores <2), no significant cyclicity (<50% increase) in affective symptoms between the follicular and luteal phases, and no functional impairment according to the CD scale.
Exclusion criteria for all participants were pregnancy; breast feeding; treatment with hormonal contraceptives; benzodiazepines or other psychotropic drugs (including SSRI) within 3 months prior to inclusion; previous brain surgery; visual impairment (>5 degrees myopic/hyperopic or profound astigmatism); and profound fear of confined spaces. Participants with on‐going depression, anxiety, or other psychiatric disorders were excluded using the Swedish version of the Mini International Neuropsychiatric Interview (M.I.N.I) [Sheehan et al., 1998]. All participants provided written informed consent prior to inclusion, and the procedures were approved by the Regional Ethical Review Board, Uppsala, Sweden.
Neuroimaging Study Design
A subset including 16 patients and 15 controls, all right‐handed, participated in two fMRI sessions, once in the mid‐follicular phase (6–12 days after onset of menstruation) and once in the late luteal phase (postovulatory day 8–13). Data on 14 out of the 16 PMDD patients and all controls have previously been presented in a ROI‐based approach focusing on the amygdale [Gingnell et al., 2012].
Late luteal phase testing was scheduled according to the positive luteinizing hormone (LH) assay (Clearplan, Unipath, Bedford, UK), and was verified with progesterone serum concentrations and onset of the next menstrual bleeding. The luteal phase interval was chosen to correspond with maximum severity of mood symptoms rather than peak progesterone levels. To avoid test order effects across the menstrual cycle, half of the participants were scheduled to start in the follicular phase, while the remaining participants entered the study in the late luteal phase.
Hormonal Analyses
Serum progesterone and estradiol levels were measured before each scanning session and were analyzed by competitive immunometry electrochemistry luminescence detection at the accredited Clinical Chemistry Laboratory, Uppsala University Hospital. The samples were run on a Roche Cobas e601 with Cobas Elecsys progesterone or estradiol reagent kits (Roche Diagnostics, Bromma, Sweden). For progesterone the measurement interval was 0.1–191 nmol l−1 and for estradiol 18.4–15,781 pmol l−1. The progesterone intra‐assay coefficient of variation was 2.2% at 2.4 nmol l−1 and 2.8% at 31.6 nmol l−1, while the estradiol intra‐assay coefficient of variation was 6.8% at 85.5 pmol l−1 and 2.8% at 1640 pmol l−1.
Experimental Paradigm
The emotion processing task used in this study has been previously described [Hariri et al., 2002]. Briefly, participants viewed images of faces displaying angry or fearful emotions (emotion task) or vertical/horizontal ellipses (sensorimotor control task) displayed in blocks of six. All images consisted of three photos or geometrical figures ordered in a triangle, one on top and two below (Supporting Information Fig. 1). For each task, subjects were instructed to compare the top image with the two images below and decide which one displayed the same facial expression, alternatively the same geometrical orientation, as the top image. Subjects answered by pressing a button with the left or right index finger. Each emotion block had an equal mix of target and nontarget emotions as well as sex of the actors. Images were presented for 4 s and were interspaced with a fixation cross (2 s for the sensorimotor control tasks and randomly selected 2, 4, or 6 s for the emotion task).
fMRI Data Acquisition and Analysis
MR imaging was performed according to standard procedures. MR imaging was performed using a 3T whole body scanner (Achieva 3T X Philips scanner, Philips Medical Systems, Best, The Netherlands) equipped with an eight‐channel head coil. An anatomical T1‐weighted reference data set to a voxel size of 0.8 × 1.0 × 2.0 mm3 and 60 slices was acquired at the beginning of each scanning session. During stimulus presentation BOLD imaging was performed using a single shot EPI sequence with parameters TE/TR 35/3,000 ms, flip angle 90°, acquisition matrix 76 × 77, acquired voxel size 3.0 × 3.0 × 3.0 mm3 and 30 slices.
Subjects were lying on their back in the scanner with the head lightly fixated with Velcro strips. During scanning, visual stimuli were presented through goggles mounted on the head coil (VisualSystem, NordicNeuroLab, Bergen, Norway). The stimulus paradigm was implemented using the commercial software package E‐prime (Psychology Software Tools, Sharpsburg, PA). To synchronize the paradigm and the MR‐scanner, trigger pulses from the scanner were fed to the paradigm‐controlling PC through SyncBox (NordicNeuroLab, Bergen, Norway).
All image processing and data analysis was conducted using Statistical Parametric Mapping (SPM8) implemented in MATLAB using default parameters, unless specified otherwise. The pre‐processing steps, which were independent of previous analyses [Gingnell et al., 2012], included slice timing correction (reference = middle slice) [Sladky et al., 2011], movement correction (reference = mean image), as well as spatial normalization to an EPI‐template in stereotactic space as defined by the Montreal Neurological Institute (MNI). Spatial smoothing was applied with a Gaussian kernel of 8 × 8 × 8 mm3 full‐width at half‐maximum. Single subject activation maps were computed with the general linear model in SPM8. Here, one regressor for each condition (emotion and sensorimotor) was convolved with the default hemodynamic response function. The realignment parameters obtained from the motion correction were used as nuisance regressors in the first‐level analysis. For the contrast of interest used in the group analyses the individual difference between emotional vs. sensorimotor condition was computed.
Genetic Analyses
DNA was isolated from blood and used to genotype 5‐HTTLPR and BDNF Val66Met polymorphisms with standard methods. DNA was isolated from blood samples using QIAamp DNA Mini Kit (http://www.qiagen.com/). The 5‐HTTLPR and BDNF Val66Met were genotyped. The 5‐HTTLPR was amplified using the following primer sequences: forward 5′‐AAC ATG CTC ATT TAA GAA GTG GAA C‐3′ and reverse 5′‐XCT AGA GGG ACT GAG CTG GAC AAC‐3′. The reverse primer was labeled with the fluorescent dye 5′‐hex. PCR was performed in a 10 μl reaction mixture containing DNA, 1.0 mM PCR 1xBuffer, 1.5 mM MgCl2, 0.2 mM dNTPs; 7%DMSO; 0.8 μM of two primers and 0.5 U Fast Start Taq DNA polymerase (Roche Diagnostics, Germany). The PCR reactions were performed on a GeneAmp 9700 (Applied Biosystems) at the following profile: starting at 94°C for 4 min, followed by 35 cycles of denaturation at 94°C for 45 s, annealing at 61°C for 1 min and elongation at 72°C for 90 s, with final extension at 72°C for 7 min. The PCR products were analyzed by capillary electrophoresis ABI PRISM@3700 DNA Analyzer (Applied Biosystem, USA) and allele size were determined by manually checking the chromatograms using Gene Marker1.5® AFLP/Genotyping software (SoftGenetics LLC®2004. State College, PA). To estimate the rate of genotyping errors, one‐third of the sample has been analyzed twice and the PCR products were resolved by electrophoresis on a 2% agarose gel, run 1 h at 120 V, and visualized under UV light using SYBR ® Safe DNA Gel Stain (Invitrogen TM). Buffer used as a running buffer was 0.5 × Tris—EDTA‐Buffer (TEB) and sizes were determined by comparison with a 100 bp DNA sequencing ladder. No inconsistencies were identified. PCR was separately performed for BDNF Val66Met in a 5‐μl reaction mixture containing TaqMan®Universal PCR Master Mix (Applied Biosystems) 2.5 μl; 40× Custom TaqMan® SNP Genotyping Assays Mix (Applied Biosystems) 0.125 μl, and DNA. The Allele Discrimination PCR reaction was performed on an ABI PRISM®7900HT Sequence Detection System at the following thermal cycler conditions: initial step of 10 min at 95°C, followed by 40 cycles of 15 s at 92°C and of 60 s at 60°C. Genotypes were analyzed using SDS 2.3 (Applied Biosystem®). To estimate the rate of genotyping errors, the BDNF Val66Met polymorphism was amplified a second time using a fluorescence‐based competitive allele‐specific PCR (KASPar) assay (KBioscience, England) based on public genome sequence (http://www.ensembl.org/). Allele discrimination was done using SNPviewer2®. No inconsistencies were found. Genotypes of 5‐HTTLPR and BDNF Val66Met were in Hardy–Weinberg equilibrium (P = 1; and P = 0.30, respectively).
Psychiatric Symptoms
The validated self‐rated Swedish version of the Montgomery—Åsberg depression rating scale (MADRS‐S) [Montgomery and Asberg, 1979], and the Spielberger State‐Trait Anxiety Inventory—State version (STAI‐S) [Hodgues and Spielberger, 1969] were used to assess depression and anxiety symptoms at each test day, once during the mid‐follicular and once during the late‐luteal phase. The trait version of the STAI (STAI‐T) [Hodgues and Spielberger, 1969] was also administered.
Statistical Analyses
For the functional MRI data, repeated‐measures analysis of variance (ANOVA) was done in SPM8 with menstrual cycle phase (within subject), subject (between subjects) and patient/control (between groups) as independent factors. Statistical inference was drawn at P < 0.05 corrected for multiple comparisons with the family wise error rate (FWE) at cluster level following an uncorrected voxel level of P < 0.001. The alpha‐thresholds for the region‐of‐interest (ROI) analysis, ANOVA test, was set to be P < 0.05 since there was only one ROI (fronto‐cingulate cluster). Chi‐square or Mann–Whitney U‐tests were used to assess differences between groups regarding descriptive statistics; repeated measures ANOVA was used to test for differences in depression and anxiety symptoms by BDNF Val66Met and 5‐HTTLPR genotype between patients with PMDD and healthy controls. A three‐way repeated measure ANOVA of mean activation values of the fronto‐cingulate cluster was performed to test for group‐by‐phase‐by‐genotype interaction effects (IBM ® SPSS Statistics 20).
RESULTS
Demographic characteristics suggest that the healthy controls were well matched to the PMDD patients. Description of demographic variables, reproductive history, and depressive‐ and anxiety symptoms is found in Supporting Information Table I. Genotype and allele frequencies of BDNF Val66Met and 5‐HTTLPR did not differ between PMDD patients and healthy controls (Supporting Information Table II). Estradiol and progesterone levels were in agreement with ovulatory cycles, and did not differ between PMDD patients and healthy controls (Supporting Information Table III). Depression and anxiety symptoms did not differ by BDNF Val66Met and 5‐HTTLPR genotypes in either the PMDD patients or healthy controls (Table 1).
Table 1.
Depression and anxiety symptoms by BDNF Val66Met and 5‐HTTLPR genotype among patients with PMDD and healthy controls 1a
| Patients with PMDD (n = 31) | Healthy controls (n = 31) | |||||||
|---|---|---|---|---|---|---|---|---|
| BDNF Val66Met | BDNF Val66Met | |||||||
| GG (Val/Val) | GA + AA (Met carriers) | GG (Val/Val) | GA + AA (Met carriers) | |||||
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |
| MADRS follicular phase (score) | 24 | 5.75 (5.8) | 7 | 7.6 (6.8) | 22 | 3.5 (3.3) | 8 | 2.9 (1.8) |
| MADRS luteal phase (score) | 23 | 12.0 (8.1) | 7 | 13.7 (10.4) | 23 | 3.0 (3.0) | 8 | 2.5 (2.0) |
| STAI‐S folllicular phase (score) | 24 | 35.3 (8.7) | 7 | 32.3 (5.7) | 21 | 29.9 (4.5) | 8 | 29.3 (5.2) |
| STAI‐S luteal phase (score) | 23 | 41.3 (10.5) | 7 | 43.4 (15.0) | 22 | 29.6 (5.9) | 8 | 29.1 (6.9) |
| STAI‐T (score) | 14 | 39.8 (16.3) | 4 | 36.0 (8.2) | 11 | 31.4 (7.3) | 4 | 25.5 (6.6) |
| Patients with PMDD | Healthy controls | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5‐HTTLPR | 5‐HTTLPR | |||||||||||
| LL | SL | SS | LL | SL | SS | |||||||
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |
| MADRS follicular phase (score) | 11 | 7.5 (7.9) | 12 | 5.0 (5.0) | 8 | 6.0 (4.6) | 6 | 2.5 (2.2) | 19 | 3.3 (3.2) | 5 | 4.6 (2.5) |
| MADRS luteal phase (score) | 10 | 9.2 (6.3) | 12 | 14.3 (8.2) | 8 | 13.5 (11.1) | 7 | 2.3 (2.2) | 19 | 2.6 (2.9) | 5 | 4.6 (2.7) |
| STAI‐S folllicular phase (score) | 11 | 34.8 (7.8) | 12 | 33.1 (7.3) | 8 | 36.8 (10.2) | 5 | 30.0 (4.0) | 19 | 29.4 (4.0) | 5 | 30.4 (7.8) |
| STAI‐S luteal phase (score) | 10 | 37.1 (7.6) | 12 | 44.2 (11.7) | 8 | 44.1 (14.4) | 6 | 28.5 (7.1) | 19 | 29.7 (6.3) | 5 | 29.8 (4.6) |
| STAI‐T (score) | 5 | 38.8 (16.8) | 8 | 35.5 (17.5) | 5 | 44.6 (6.2) | 2 | 27.0 (9.9) | 10 | 28.0 (6.1) | 3 | 37.7 (6.5) |
A: adenine; G: guanine; L: long; Met; methionine; S: short; Val: valine.
No difference in reaction time and accuracy were found between groups or cycle phases. Functional MRI analyses showed significant main effects for group and menstrual cycle phase but no significant group‐by‐phase interaction. More precisely, patients had decreased activation during the emotional discrimination task in the pregenual anterior cingulate cortex (pgACC) and the ventro‐medial prefrontal cortex (vmPFC), which was independent of menstrual phase (Figure 1, Supporting Information Table IV). Further regions included the occipital and temporal lobes. On the other hand, patients exhibited increased activations in parietal and frontal regions as well as the supplementary motor area (Supporting Information Table IV). In addition, a main effect of menstrual phase was observed with the follicular phase being associated with higher activation in the motor cortex and the middle frontal gyrus compared to the luteal phase. No increased activations in the luteal phase were found (Supporting Information Table IV). In the present analysis on the whole‐brain level no significant difference was detected in the amygdale, which was present when using a hypothesis‐driven ROI approach [Gingnell et al., 2012], thus indicating that differences between PMDD patients and controls are more pronounced in the fronto‐cingulate cortex than in the amygdale.
Figure 1.
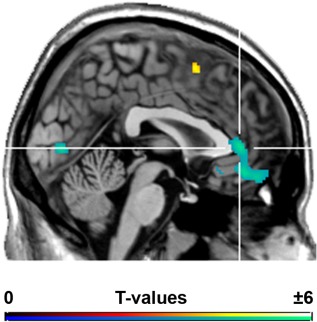
Brain regions showing significantly different activation between PMDD patients and healthy controls during an emotion processing task independent of menstrual cycle phase. Decreased activations in PMDD subjects were found in the fronto‐cingulate cortex (peak t = −5.28 in the pregenual ACC). P < 0.05 FWE‐corrected cluster level following P < 0.001 uncorrected voxel level. Crosshair is at x / y / z = −2/40/4 mm MNI‐space. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Based on the a priori hypothesis from the literature and the present fMRI results concerning impaired functionality of the pregenual‐prefrontal region in PMDD patients, genetic influences were only tested in the fronto‐cingulate cluster. Post‐hoc functional ROI analyses in the fronto‐cingulate cluster of patients vs. controls showed no effect of 5‐HTTLPR genotype, but a genotype‐by‐group‐by‐phase interaction effect of BDNF Val66Met (df = 1, 27; f = 12.38; P = 0.002). The effect was present in the luteal phase, where PMDD patients carrying the BDNF Met allele (N = 4) had lower fronto‐cingulate cortex reactivity compared to the Met allele carrying controls (N = 4), Figure 2. Adjustment for estradiol and/or progesterone levels did not change the results.
Figure 2.
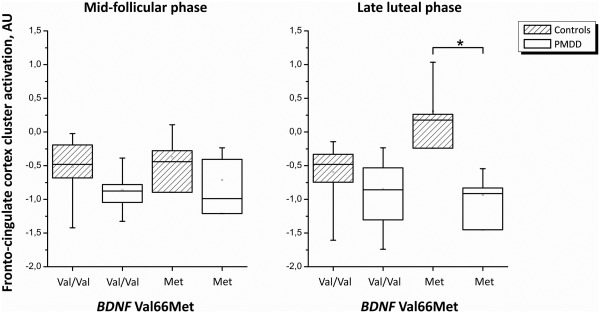
Mean fronto‐cingulate cortex ROI activation in patients with PMDD and healthy comparison subjects during early follicular (left) and late luteal (right) phase by BDNF Val66Met genotype.
DISCUSSION
Emotion Processing in PMDD
Women with PMDD had significantly lower, but phase‐independent, emotion‐induced fronto‐cingulate cortex activation in comparison with healthy controls. These findings are in line with a meta‐analysis in patients with generalized anxiety and/or major depressive disorders where impaired ventral ACC, and amygdala, activation was demonstrated [Etkin and Schatzberg, 2011]. On the basis of the present data, previous ROI‐based studies in PMDD patients [Gingnell et al., 2012; Protopopescu et al., 2008], and patients with stress and anxiety disorders [Rauch et al., 2006; Shin and Liberzon, 2010], it is plausible that impaired functionality of the pregenual‐prefrontal region, and the amygdala, are characteristics of PMDD. However, longitudinal prospective studies are required to shed light on whether this is a risk factor or an acquired feature of the disorder.
Supportive evidence from genetic studies also points to the importance of the cingulate‐amygdala circuitry in affective disorders [Pezawas et al., 2005; Soliman et al., 2010]. The functional polymorphisms 5‐HTTLPR and BDNF Val66Met were, hence, evaluated in relation to the lowered emotion‐induced fronto‐cingulate cortex reactivity, and a phase‐dependent genotype‐by‐group interaction effect of BDNF Val66Met was found. Women with PMDD who were carriers of the Met allele had lower fronto‐cingulate cortex activation in the luteal phase compared to Met‐allele carrying controls.
A decade ago, an in vitro study and a knock‐in mice model of BDNF Val66Met provided convergent evidence that the Met allele leads to impaired activity‐dependent secretion, distribution to neuronal dendrites, and intracellular trafficking [Chen et al., 2006; Egan et al., 2003], and furthermore suggested that the Met allele is associated with anxiety‐like behavior and lack of responsiveness to SSRI [Chen et al., 2006]. Later studies on BDNF in human anxiety and depression have confirmed these findings [Martinowich et al., 2007]. Recently, Soliman et al. reported diminished vmPFC and elevated amygdala activity in BDNF Met carriers during fear extinction, a feature implicated in anxiety [Soliman et al., 2010]. Additionally, neuroticism, which is a personality trait linked to PMDD [Gingnell et al., 2010], has been associated with the BDNF Met allele, with low serum BDNF levels [Lang et al., 2004], and with the 5‐HTTLPR S allele among women with PMDD [Gingnell et al., 2010].
Moreover, a recent study on healthy women reported higher rostral ACC and amygdala activation during emotion processing in carriers of the BDNF Met allele and of the 5‐HTTLPR short allele, separately and in an epistatic mode, compared to other genotypes [Outhred et al., 2012]. Our data partially confirm these findings, with healthy Met allele carrying subjects displaying enhanced fronto‐cingulate cortex activation only during the luteal phase. However, no effect was found for the 5‐HTTLPR, and interaction effects could not be tested in the present study due to the limited sample size. Thus the present results call for further investigation in larger case‐control samples.
In the present study women have been assessed during the late luteal phase of the menstrual cycle, which corresponds to the peak of symptoms and increased, but declining, progesterone levels [Yonkers et al., 2008]. Thus, as the effect of the fronto‐cingulate cortex—BDNF Val66Met interaction is present in this time period, and disappears during the follicular phase, it is likely to be related to changes in progesterone levels. In fact, the effects of progesterone, which reaches its peak during the luteal phase to rapidly decrease at the end of the menstrual cycle and acts via the action of its metabolite allopregnanolone on GABA receptor A [Backstrom et al., 2013], might be mediated by the direct or chloride pump‐mediated influence of BDNF on the GABAergic system. Additionally to the inhibition of the cation‐chloride transporter KCC2 by BDNF, estrogen enhances the cation‐chloride transporter NKCC1, thus leading to increased intracellular chloride and loss of GABA receptor A inhibitory effects [Galanopoulou, 2008; Nakamura et al., 2004; Price et al., 2005]. However, somewhat complex bidirectional effects between BDNF and estrogen and progesterone impede further speculation and call for further molecular studies [McNamara and Scharfman, 2012].
The BDNF Val66Met has been implicated in the sex differences of affective disorders [Epperson and Bale, 2012], and as a biological link between sex hormones and BDNF [Pluchino et al., 2013]. A putative estrogen response element in the BDNF gene has been suggested [Sohrabji et al., 1995], but the mechanisms behind the interaction between sex hormones and BDNF remain largely unknown [Sohrabji and Lewis, 2006]. Insights on the link between sex hormones and BDNF Val66Met, and its relevance to sex differences in affective disorders, predominantly come from rodent studies [Epperson and Bale, 2012]. In fact, a region‐dependent influence of estrogen and progesterone on BDNF in the brain has been found in female rats [Gibbs, 1999], and the BDNF Val66Met and estrous cycle stage interactive effect on hippocampal memory function and molecular markers of dendritic spine formation has been demonstrated in knock‐in mice [Spencer et al., 2010]. More recently one study of mice, making use of a knock‐in animal model, provided evidence of an association between the Met allele and anxiety‐related behavior during the estrous stage, when both estradiol and progesterone levels decline [Bath et al., 2012]. Interestingly, this phase corresponds to the late luteal phase in humans, and the timing of anxiety symptoms in mice matches with the peak of PMDD symptoms. All together, preclinical and clinical findings suggest the BDNF Met allele as a strong candidate for affective disorders with sex differences or those that are related to sex hormone changes, such as PMDD [Epperson and Bale, 2012].
The present study furthers the understanding of a sex‐specific and sex hormone‐triggered affective disorder [Yonkers et al., 2008] but the psychoneuroendocrine mechanism behind the present results needs to be investigated. If peripheral BDNF levels are related to BDNF Val66Met, and whether these two are related to central BDNF levels in different brain regions remains to be determined [Gibbs, 1999; Lang et al., 2009; Ozan et al., 2010]. Furthermore, no effect of the 5‐HTTLPR was found in the present study. However, as 5‐HT and BDNF are two highly interlinked systems [Martinowich and Lu, 2008], a role of 5‐HT in PMDD cannot be excluded. For instance the BDNF Met allele lower the sensitivity to 5‐HT signaling [Martinowich and Lu, 2008], which may influence antidepressant efficacy in PMDD women. More, both systems carry out development and plasticity functions in the brain, and BDNF Val66Met and 5‐HTTLPR are likely to exert their influence at a neurotrophic level during development. Thus the present findings call also for further studies on the relation between sex hormones and neurotransmitters. Finally, no other study so far has investigated the BDNF Val66Met in PMDD, thus well‐powered genetic association studies are warranted.
CONCLUSIONS
Altogether these findings lead to speculation that PMDD is characterized by impaired fronto‐cingulate cortex activation in response to emotions, and that the BDNF Val66Met polymorphism contributes to this association in the luteal phase. This is the first study investigating the interaction between emotional fronto‐cingulate activation in PMDD and the functional polymorphisms 5‐HTTLPR and BDNF Val66Met, thus contributing to the genetic dissection of women's mental health. Finally the finding on the BDNF Val66Met provides a pattern of association in line with preclinical findings [Bath et al., 2012], and a translational attempt to investigate the neuropsychoendocrinology of PMDD. However the small sample size calls for independent replication of the genetic imaging findings.
ACKNOWLEDGMENTS
The authors sincerely thank all the women who participated in this study.
Supporting information
Supporting Information
Conflict of Interest: Sundström‐Poromaa I., serves occasionally on advisory boards or act as invited speaker at scientific meetings for MSD, Novo Nordisk, Bayer Health Care, and Lundbeck A/S. Epperson C.N., receives research grant support from Pfizer, Shire and Novartis. Lanzenberger R, received travel grants and conference speaker honoraria from AstraZeneca, Lundbeck A/S and Roche Austria. Comasco E., Hahn A., Ganger S., Gingnell M., Bannbers E., Oreland L., and Wikström J. Report no financial relationships with commercial interests.
REFERENCES
- Adams M, McCrone S (2012): SRD5A1 genotype frequency differences in women with mild versus severe premenstrual symptoms. Issues Ment Health Nurs 33:101–108. [DOI] [PubMed] [Google Scholar]
- APA (2013): Diagnostic and Statistical Manual of Mental Disorders, 5th ed. DSM‐5. Washington DC: American Psychiatric Association. [Google Scholar]
- Backstrom T, Bixo M, Johansson M, Nyberg S, Ossewaarde L, Ragagnin G, Savic I, Stromberg J, Timby E, van Broekhoven F, van Wingen G (2013): Allopregnanolone and mood disorders. Prog Neurobiol 2014;113:88–94. [DOI] [PubMed] [Google Scholar]
- Bath KG, Chuang J, Spencer‐Segal JL, Amso D, Altemus M, McEwen BS, Lee FS (2012): Variant brain‐derived neurotrophic factor (Valine66Methionine) polymorphism contributes to developmental and estrous stage‐specific expression of anxiety‐like behavior in female mice. Biol Psychiatry 72:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L (2006): Why sex matters for neuroscience. Nat Rev Neurosci 7:477–484. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS (2006): Genetic variant BDNF (Val66Met) polymorphism alters anxiety‐related behavior. Science 314:140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra V, Magnay JL, O'Brien PM, Chapman G, Fryer AA, Ismail KM (2007): Serotonin receptor 1A C(‐1019)G polymorphism associated with premenstrual dysphoric disorder. Obstet Gynecol 110:788–792. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR (2003): The BDNF val66met polymorphism affects activity‐dependent secretion of BDNF and human memory and hippocampal function. Cell 112: 257–269. [DOI] [PubMed] [Google Scholar]
- Epperson CN (2013): Premenstrual dysphoric disorder and the brain. Am J Psychiatry 170:248–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Bale TL (2012): BDNF Val66Met polymorphism and brain‐derived neurotrophic factor levels across the female life span: Implications for the sex bias in affective disorders. Biol Psychiatry 72:434–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Haga K, Mason GF, Sellers E, Gueorguieva R, Zhang W, Weiss E, Rothman D, Krystal JH (2002): Cortical y‐aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: A 1H‐MRS study. Arch Gen Psychiatry 59:851–858. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Steiner M, Hartlage SA, Eriksson E, Schmidt PJ, Jones I, Yonkers KA (2012): Premenstrual dysphoric disorder: Evidence for a new category for DSM‐5. Am J Psychiatry 169:465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Schatzberg AF (2011): Common abnormalities and disorder‐specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am J Psychiatry 168:968–978. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R (2011): Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 15:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EW, Rickels K, Sondheimer SJ, Polansky M (1999): Differential response to antidepressants in women with premenstrual syndrome/premenstrual dysphoric disorder: A randomized controlled trial. Arch Gen Psychiatry 56:932–939. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS (2008): Sexually dimorphic expression of KCC2 and GABA function. Epilepsy Res 80:99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB (1999): Treatment with estrogen and progesterone affects relative levels of brain‐derived neurotrophic factor mRNA and protein in different regions of the adult rat brain. Brain Res 844:20–27. [DOI] [PubMed] [Google Scholar]
- Gillies GE, McArthur S (2010): Estrogen actions in the brain and the basis for differential action in men and women: A case for sex‐specific medicines. Pharmacol Rev 62:155–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingnell M, Comasco E, Oreland L, Fredrikson M, Sundstrom‐Poromaa I (2010): Neuroticism‐related personality traits are related to symptom severity in patients with premenstrual dysphoric disorder and to the serotonin transporter gene‐linked polymorphism 5‐HTTPLPR. Arch Womens Ment Health 13:417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingnell M, Morell A, Bannbers E, Wikstrom J, Sundstrom Poromaa I (2012): Menstrual cycle effects on amygdala reactivity to emotional stimulation in premenstrual dysphoric disorder. Horm Behav 62:400–406. [DOI] [PubMed] [Google Scholar]
- Hammarbäck S, Bäckström T, MacGibbon‐Taylor B (1989): Diagnosis of premenstrual tension syndrome: Description and evaluation of a procedure for diagnosis and differential diagnosis. J Psychosom Obstet Gynaecol 10:25–42. [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR (2002): Serotonin transporter genetic variation and the response of the human amygdala. Science 297:400–403. [DOI] [PubMed] [Google Scholar]
- Hodgues WF, Spielberger CD (1969): An indicant of trait or state anxiety? J Consult Clin Psychol 33:430–434. [DOI] [PubMed] [Google Scholar]
- Huo L, Straub RE, Roca C, Schmidt PJ, Shi K, Vakkalanka R, Weinberger DR, Rubinow DR (2007): Risk for premenstrual dysphoric disorder is associated with genetic variation in ESR1, the estrogen receptor alpha gene. Biol Psychiatry 62:925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic H, Cerin A, Karlsson P, Lundberg J, Halldin C, Nordstrom AL (2006): A PET study of 5‐HT1A receptors at different phases of the menstrual cycle in women with premenstrual dysphoria. Psychiatry Res 148:185–193. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Corey LA, Neale MC (1998): Longitudinal population‐based twin study of retrospectively reported premenstrual symptoms and lifetime major depression. Am J Psychiatry 155:1234–1240. [DOI] [PubMed] [Google Scholar]
- Lang UE, Hellweg R, Gallinat J (2004): BDNF serum concentrations in healthy volunteers are associated with depression‐related personality traits. Neuropsychopharmacology 29:795–798. [DOI] [PubMed] [Google Scholar]
- Lang UE, Hellweg R, Sander T, Gallinat J (2009): The Met allele of the BDNF Val66Met polymorphism is associated with increased BDNF serum concentrations. Mol Psychiatry 14:120–122. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss‐Moreau E, Barrett LF (2012): The brain basis of emotion: A meta‐analytic review. Behav Brain Sci 35:121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinowich K, Lu B (2008): Interaction between BDNF and serotonin: Role in mood disorders. Neuropsychopharmacology 33:73–83. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B (2007): New insights into BDNF function in depression and anxiety. Nat Neurosci 10:1089–1093. [DOI] [PubMed] [Google Scholar]
- McNamara JO, Scharfman HE (2012): Temporal lobe epilepsy and the BDNF receptor, TrkB In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado‐Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies [Internet]. 4th edition Bethesda (MD): National Center for Biotechnology Information (US). [PubMed] [Google Scholar]
- Montgomery SA, Asberg M (1979): A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389. [DOI] [PubMed] [Google Scholar]
- Nakamura NH, Rosell DR, Akama KT, McEwen BS (2004): Estrogen and ovariectomy regulate mRNA and protein of glutamic acid decarboxylases and cation‐chloride cotransporters in the adult rat hippocampus. Neuroendocrinology 80:308–323. [DOI] [PubMed] [Google Scholar]
- Outhred T, Das P, Dobson‐Stone C, Griffiths K, Felmingham KL, Bryant RA, Malhi G, Kemp AH (2012): The functional epistasis of 5‐HTTLPR and BDNF Val66Met on emotion processing: A preliminary study. Brain Behav 2:778–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozan E, Okur H, Eker C, Eker OD, Gonul AS, Akarsu N (2010): The effect of depression, BDNF gene val66met polymorphism and gender on serum BDNF levels. Brain Res Bull 81:61–65. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer‐Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR (2005): 5‐HTTLPR polymorphism impacts human cingulate‐amygdala interactions: A genetic susceptibility mechanism for depression. Nat Neurosci 8:828–834. [DOI] [PubMed] [Google Scholar]
- Pluchino N, Russo M, Santoro AN, Litta P, Cela V, Genazzani AR (2013): Steroid hormones and BDNF. Neuroscience 239:271–279. [DOI] [PubMed] [Google Scholar]
- Price TJ, Cervero F, de Koninck Y (2005): Role of cation‐chloride‐cotransporters (CCC) in pain and hyperalgesia. Curr Top Med Chem 5:547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopescu X, Tuescher O, Pan H, Epstein J, Root J, Chang L, Altemus M, Polanecsky M, McEwen B, Stern E, Silbersweig D (2008): Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord 108:87–94. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA (2006): Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research—Past, present, and future. Biol Psychiatry 60:376–382. [DOI] [PubMed] [Google Scholar]
- Savic I (2010): Sex Differences in the Human Brain, Their Underpinnings and Implications, Vol. 186. Elsevier. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998): The mini‐international neuropsychiatric interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry 59 (Suppl 20): 22–33;quiz 34–57. [PubMed] [Google Scholar]
- Shin LM, Liberzon I (2010): The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35:169–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladky R, Friston KJ, Trostl J, Cunnington R, Moser E, Windischberger C (2011): Slice‐timing effects and their correction in functional MRI. Neuroimage 58:588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Lewis DK (2006): Estrogen‐BDNF interactions: Implications for neurodegenerative diseases. Front Neuroendocrinol 27:404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran‐Allerand CD (1995): Identification of a putative estrogen response element in the gene encoding brain‐derived neurotrophic factor. Proc Natl Acad Sci USA 92:11110–11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Jing D, Tottenham N, Amso D, Somerville LH, Voss HU, Glover G, Ballon DJ, Liston C, Teslovich T, Van Kempen T, Lee FS, Casey BJ (2010): A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science 327:863–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Milner TA, Lee FS, McEwen BS (2010): BDNF variant Val66Met interacts with estrous cycle in the control of hippocampal function. Proc Natl Acad Sci USA 107:4395–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom I, Nyberg S, Bixo M, Hammarback S, Backstrom T (1999): Treatment of premenstrual syndrome with gonadotropin‐releasing hormone agonist in a low dose regimen. Acta Obstet Gynecol Scand 78:891–899. [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Becker E, Lieb R, Krause P (2002): Prevalence, incidence and stability of premenstrual dysphoric disorder in the community. Psychol Med 32:119–132. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, O'Brien PM, Eriksson E (2008): Premenstrual syndrome. Lancet 371:1200–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


