SUMMARY
Recent cumulative data shows that various transcription factors are recruited to the chromatin in an iron responsive manner to affect diverse cellular functions in the pathogenic fungus Candida albicans. Here we identified groups of iron-responsive genes in C. albicans by chromatin remodeling analysis at gene promoters, using micrococcal nuclease (MNase) digestion followed by deep sequencing. Chromatin in the promoter regions of iron uptake and utilization genes showed repressed and active configuration, respectively, under iron replete conditions. GO Term enrichment analysis of genes with differentially remodeled chromatin, in respective promoter locales, suggested that many genes involved in adhesion are also iron-responsive. C. albicans was observed to be more self-adherent (2-fold increase) and formed higher biofilm mass (77% increase) in the presence of iron. Furthermore, we identified various known and novel adhesion-related genes with iron-dependent active chromatin profiles that are indicative of potential up-regulation under iron replete conditions. Transcription factor Cph1 that is activated upon Cek1 phosphorylation also showed an active chromatin profile under iron replete conditions and cells showed iron-responsive Cek1 MAPK phosphorylation in the presence of iron. Thus, iron affects diverse biological functions by modulating chromatin profiles of large gene sets and by signaling through Cek1 MAPK in C. albicans.
Keywords: Iron, MAP Kinase, Signaling, Chromatin remodeling, Nucleosome, Candida albicans
INTRODUCTION
Assimilation and utilization of iron are highly regulated biological processes because iron is toxic when allowed to accumulate in excess of cellular needs (Hentze et al., 2004). Acquisition of iron from the human host is a challenge for microbial pathogens since the majority of iron is sequestered by iron binding proteins or is associated within iron-containing proteins or the heme moiety of hemoglobin (Sutak et al., 2008). Therefore, successful microbial colonizers have evolved tightly regulated systems that allow them to acquire iron while ensuring that their intracellular levels do not become toxic. Microbial iron homeostasis is accomplished through up-regulation of genes involved in iron uptake and down-regulation of genes involved in iron utilization under iron deplete conditions; while iron replete environments repress genes involved in iron uptake and allow expression of genes involved in processes that require iron.
Regulation of iron metabolism assumes greater importance for the opportunistic pathogenic fungus Candida albicans since it must adapt to iron-limited (iron deplete) blood or iron-rich (iron replete) gastrointestinal (GI) environment (McCance and Widdowson, 1938, Miret et al., 2003). Mechanisms related to iron-mediated regulation of genes directly involved in iron homoeostasis in C. albicans are well known. C. albicans utilizes multiple transcriptional regulatory elements for adaptation to high and low iron environments, involving an intricate interplay between the GATA-type Sfu1 transcriptional repressor, the Cys6Zn2 transcriptional activator Sef1, the CCAAT-binding complex (CBC) proteins Hap2/3/5, and Hap43 (Chen et al., 2011) that can act both as a transcriptional activator and repressor (Singh et al., 2011).
Iron also affects the expression of genes unrelated to iron metabolism (Lan et al., 2004, Chen et al., 2011, Hameed et al., 2011) including genes involved in yeast to hyphae transition, genes encoding cell wall proteins, and lipid homeostasis genes. Furthermore, a microarray study using a hap43Δ/Δ mutant identified numerous Hap43-dependent gene clusters beyond iron homeostasis (Singh et al., 2011), while diverse cellular processes such as adhesion, ribosome biogenesis, and low nitrogen induced filamentation were found to be regulated by the CBC complex independent of Hap43 (Hsu et al., 2013). It is, however, tempting to believe that many of these processes are also iron-responsive since CBC components are regulated by Hap43 in an iron dependent manner (Singh et al., 2011). Interestingly, in another pathogenic fungus Cryptococcus neoformans, the GATA-type transcription factor Cir1 is responsible for iron acquisition as well as for maintenance of other virulence traits such as capsule formation, melanin production, and growth at 37° C (Jung et al., 2006).
Possible explanations have emerged for the role of iron in affecting these diverse phenotypes in C. albicans. For example, germination in C. albicans was negatively correlated with the presence of iron (Hameed et al., 2008) that may be explained by the involvement of the global transcriptional co-repressor Tup1 in both hyphae formation (Braun and Johnson, 1997) and iron assimilation (Knight et al., 2002). Sfu1 and Hap43 have also been suggested to interact with Tup1 (Pelletier et al., 2007, Hsu et al., 2011) and in S. cerevisiae, Tup1 regulates transcription through chromatin-mediated mechanisms (Buck and Lieb, 2006, Rizzo et al., 2011). Additionally, chromatin remodeling through the SWI/SNF pathway has previously been linked to hyphae formation in C. albicans (Mao et al., 2006). This cumulative data suggests that multiple transcription factors are recruited in response to iron, potentially allowing for changes in chromatin organization to affect diverse cellular processes.
Mitogen activated protein kinase (MAPK) pathways, allowing nuclear localization of specific transcription factors, represent another mechanism for condition-specific recruitment to the chromatin. Iron is also capable of signaling through the MAPK Hog1 stress pathway to affect cell surface flocculation in C. albicans (Kaba et al., 2013). However, it is not known whether iron can induce signaling via the Cek1 MAPK pathway, which has a well-known role in modulating the cell surface of C. albicans by maintaining the structural integrity of cell wall mannans (Li et al., 2009) and by controlling β-glucan exposure (Galan-Diez et al., 2010) and the glycosylation status of cell wall proteins (Cantero and Ernst, 2011).
In light of the involvement of a complex interplay of transcriptional regulators as well as MAPK signaling in response to environmental iron levels, we investigated the role of iron in affecting diverse processes in C. albicans using micrococcal nuclease (MNase) digestion followed by deep sequencing. In contrast to previous studies of iron response that used microarrays, our approach was to examine iron-responsive global events in chromatin organization in terms of changes to a more open chromatin configuration (active chromatin preceding activation of gene expression) or a more closed chromatin (repressed chromatin that is a hallmark of gene repression), as determined by mapping the position and occupancy of nucleosomes with MNase digestion. When combined with next-generation sequencing, MNase-seq can determine all locations across the genome where nucleosome organization has changed (Rizzo et al., 2012). Using MNase-seq in iron deplete and replete conditions, we identified chromatin remodeling events across the genome to show that iron influences the potential expression of large sets of genes involved in adhesion and hyphae formation at the chromatin level. We further show here that iron also signals into Cek1 MAPK pathway and affects cell adhesion and biofilm formation. This study presents the first iron chromatome of an AIDS-related fungal pathogen.
RESULTS
Iron modulates specific changes in the chromatin landscape to affect diverse biological processes
To analyze comprehensive iron-mediated chromatin changes that potentially precede changes in gene expression, we performed MNase-seq on iron replete and deplete C. albicans cells. Iron free YNB media was supplemented with the iron chelator BPS (50µM) to further limit endogenous iron levels and served as our deplete iron media (DIM; representative of iron levels in iron-limited niches in the body); while DIM supplemented with 100 µM iron was used as replete iron media (RIM; representative of iron levels in average laboratory medium YPD and iron-rich niches in the body). To specifically test the role of iron alone on C. albicans growth, we used glucose as carbon source and maintained the incubation temperature at 30°C, in order to exclude conditions that induce hyphae such as N-AcetylGlucosamine (NAG) as carbon source or a growth temperature of 37°C.
The resulting MNase-protection scores reflect the relative level of DNA protection from MNase digestion and at most sites represent the occupancy/density of nucleosomes at each genomic location. To identify gene promoters where chromatin organization changed in the presence of iron, we compared iron deplete and replete MNase-protection profiles by Pearson correlation (CORR) and by average change in MNase-protection (average MNase-protection Fe deplete - average MNase-protection Fe replete = ΔOCC). Thus promoters with a low CORR represent locations where the nucleosome organization has changed dramatically and promoters with large ΔOCC represent locations where nucleosomes have been lost between conditions. If a promoter has a large positive ΔOCC, then the chromatin is becoming more ‘active’ as observed when gene expression increases. On the other hand, if a promoter has a large negative ΔOCC, then the chromatin is becoming more ‘repressed’, as seen when gene expression decreases.
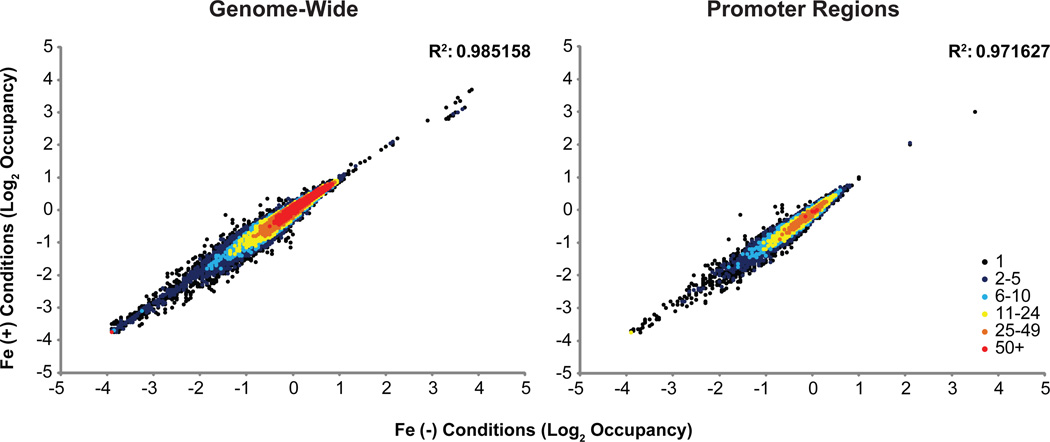
We examined the reproducibility of our MNase-seq experiments by comparison of MNase protection datasets between experimental replicates and found that 95% of 1000 bp windows were extremely highly correlated (>0.8 R) between the replicate experiments (Figure S1); this is a result of additional MNase-seq standardization steps in our protocol (Rizzo et al., 2012). Two replicates of iron replete and deplete conditions were then averaged and MNase protection profiles were plotted across the entire genome for 500 bp segments to compare between conditions (Figure 1). Across the entire genome, iron replete and deplete conditions were extremely correlated (R2 = 0.99; Figure 1, left) and promoters had an R2 = 0.97 (Figure 1, right).
Figure 1.
Density heat scatterplot of log2 nucleosome occupancy. Average log2 occupancy of non-overlapping 500 bp windows at 10 bp resolution genome-wide (left) and at promoters regions defined at 500 bp upstream of ATG (right) were mapped in + versus - Fe conditions. Replete and deplete conditions correlate extremely well across the genome as well as for promoter regions.
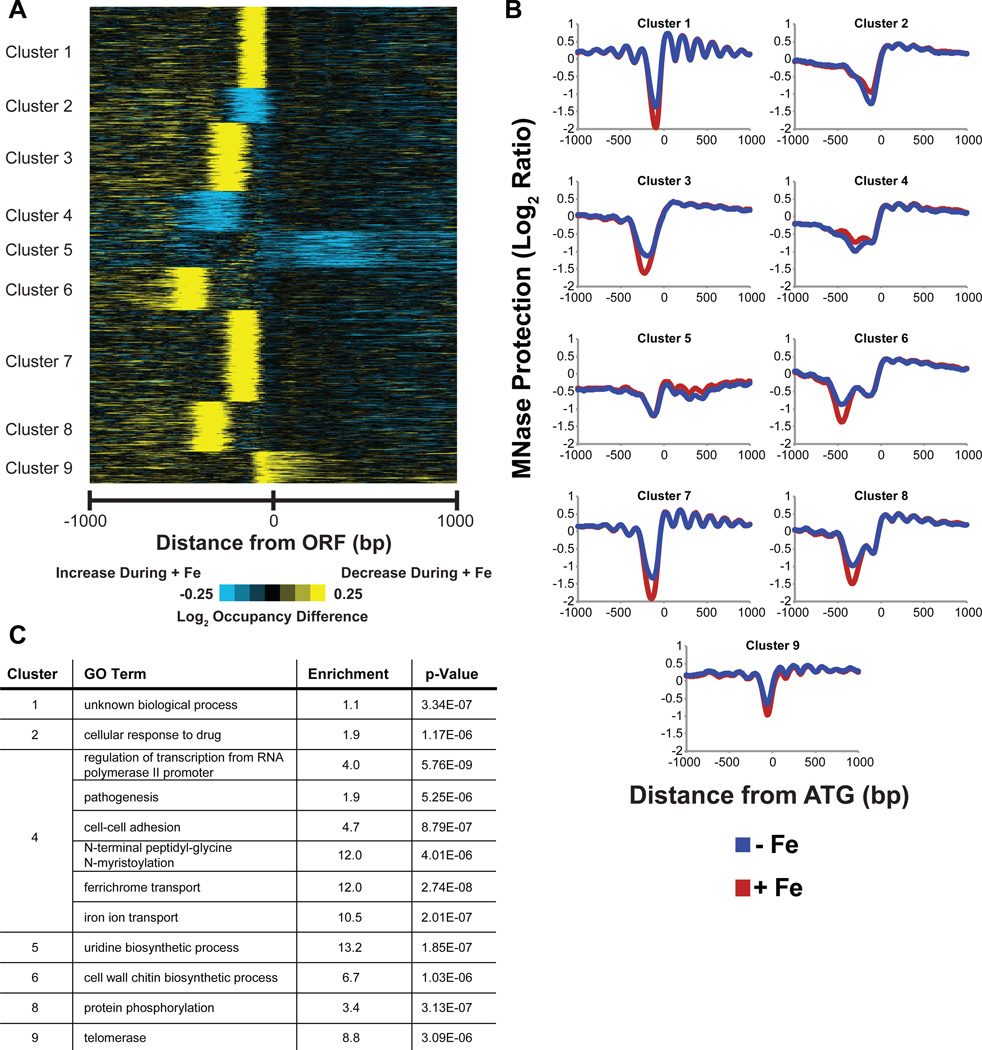
To identify iron-responsive gene clusters, we examined MNase protection over 1000 bp centered at ATG and clustered changes in MNase protection between replete and deplete conditions (Figure 2A; list of Orfs present in each cluster is provided in Table S1A). These cluster differences were also plotted in composite format to view the average change in occupancy in response to iron (Figure 2B), illustrating graphically the extent of effect of iron on each gene cluster. Clusters were then tested for enrichment for GO Term processes (Figure 2C), and as expected, iron-related processes were among the most highly enriched processes (cluster 4). However, the results show that various non-iron homeostasis processes are also affected by iron at the chromatin level (Figure 2C). Gene promoter regions where ΔOCC or CORR changed significantly were identified using a False Discovery Rate (FDR) of 0.1, to identify C. albicans genes most affected by iron at the chromatin level (Table S2; containing 844 genes that were most dissimilar between conditions by CORR (R < 0.87) or genes having ΔOCC scores greater than +38.95 and less than −37.1226). Based on this cut-off criterion, genes that are significant in each cluster in Figure 2A are highlighted in yellow in Table S1A and the total number of genes significantly affected by iron in each cluster is enlisted in Table S1B. Functions of these genes regulate a wide range of cellular processes, including iron homeostasis (Table S2).
Figure 2.
Clustering of MNase protection differences at genome-wide promoters. (A) Clustergram generated using k-medians clustering with k = 9 of log2 occupancy differences between + and - Fe conditions at 1 kb window at 10 bp resolution centered at ATG of C. albicans genes reveal distinct groups of iron responsive genes. (B) Composite maps show distinct patters for occupancy change for each cluster. (C) GO Biological term enrichment of the iron-responsive clusters identified from MNase-seq data analysis show iron and various non-iron homeostasis related biological processes as being affected by iron.
Iron homeostasis genes show changes at the chromatin level
We next sorted Table S2 for the key word “iron” in gene descriptions provided in the Candida genome database (CGD; http://www.candidagenome.org/) to select candidate genes directly involved in iron homeostasis (Table 1). Almost all known iron homeostasis genes, including ferric reductases and genes involved in iron utilization, previously shown to be iron-regulated by microarray analyses of iron replete versus deplete cells (Lan et al., 2004, Chen et al., 2011), were found within our strict inclusion (Table 1). An almost perfect correlation can be seen in the directionality of gene expression observed in these two previous studies with the chromatin changes identified in this study (Table 1). This validated our choice of cut-off scores used to generate the master list (Table S2) of genes potentially regulated by iron at the chromatin level. Most of the iron homeostasis genes in Table 1 had correspondingly low correlation scores and high absolute ΔOCC scores, typical of chromatin changes linked with highly regulated genes under a given condition (Rizzo et al., 2011).
Table 1.
Iron homeostasis genes repressed or activated at the chromatin level under iron replete conditions.
| ORF | CORR | ΔOCC | NAME | DESCRIPTION | mRNA expression data | |
|---|---|---|---|---|---|---|
| Lan et al 2004 | Chen et al 2011 | |||||
| Repressed ORFs | ||||||
| orf19.5636 | 0.89 | −251.78 | RBT5 | GPI-anchored cell wall protein, role in hemoglobin utilization;regulated by iron | −187.4 | −41.1 |
| orf19.1264 | 0.75 | −131.87 | CFL2 | Putative oxidoreductase, iron utilization | −22.2 | −17.3 |
| orf19.5634 | 0.63 | −120.37 | FRP1 | Ferric reductase; iron-chelation-induced by CCAAT-binding factor | −200.9 | −36.6 |
| orf19.1930 | 0.76 | −99.94 | CFL5 | Ferric reductase; expression greater in low iron | −30.9 | −34.9 |
| orf19.4215 | 0.95 | −68.47 | FET34 | Putative multicopper ferroxidase; expression greater in low iron | −3.3 | −9 |
| orf19.2179 | 0.93 | −65.29 | SIT1 | Ferrichrome siderophore transporter; iron-regulated transcription | −89 | −27 |
| orf19.5779 | 0.98 | −57.75 | RNR1 | Ribonucleotide reductase large subunit; expression greater in low iron | −4.4 | −3.7 |
| orf19.1415 | 0.80 | −45.65 | FRE10 | Major cell-surface ferric reductase under low-iron conditions | NA | −4.4 |
| orf19.7219 | 0.73 | −45.09 | FTR1 | High-affinity iron permease; required for low-iron growth | −1.5 | −17.6 |
| orf19.1932 | 0.82 | −44.12 | CFL4 | C-terminus similar to ferric reductases; expression high in low iron | −25.5 | −3.6 |
| orf19.4328 | 0.64 | −41.24 | CCC2 | Copper-transporting P-type ATPase of Golgi; induced by iron starvation | −2.6 | −3.6 |
| orf19.853 | 0.74 | −35.97 | SAP99 | Putative secreted aspartyl protease; expression greater in low iron | −9.4 | −3.7 |
| orf19.3753 | 0.86 | −17.76 | SEF1 | Zn2-Cys6 transcription factor; regulates iron uptake | NA | −3 |
| orf19.681 | 0.70 | −17.27 | HAP43 | CCAAT-binding factor-dependent transcriptional repressor required for low iron response | NA | −4.6 |
| Activated ORFs | ||||||
| orf19.6126 | 0.84 | 73.01 | KGD2 | Putative dihydrolipoamide S-succinyltransferase; expression greater in high iron | +8.6 | +2.1 |
| orf19.238 | 0.57 | 59.36 | CCP1 | Similar to cytochrome-c peroxidase N terminus; transcription induced by low iron | +57.7 | +9.0 |
| orf19.2593 | 0.84 | 55.35 | BIO2 | Putative biotin synthase; transcriptionally upregulated in high iron | +107.6 | +2.9 |
| orf19.1195 | 0.98 | 55.09 | − | Putative metalloendopeptidase, role in iron homeostasis | NA | NA |
| orf19.637 | 0.48 | 54.02 | SDH2 | Succinate dehydrogenase, Fe-S subunit; expression greater in high iron | +9 | +8.1 |
| orf19.2803 | 0.90 | 53.93 | HEM13 | Coproporphyrinogen III oxidase; iron-regulated expression | +6.6 | NA |
| orf19.5098 | 0.96 | 52.25 | NTG1 | Protein similar to S. cerevisiae Ntg1p and Ntg2p DNA repair glycosylases; expression greater in high iron | +6 | +4 |
| orf19.1179 | 0.99 | 51.06 | − | Transcriptionally regulated by iron; expression greater in high iron | +4.8 | +5.3 |
| orf19.1926 | 0.88 | 48.79 | SEF2 | Putative zinc cluster protein; expression is repressed by Sfu1p under high-iron conditions | NA | +2.2 |
| orf19.1744 | 0.92 | 45.85 | HEM4 | Putative uroporphyrinogen III synthase; expression greater in high iron | +9.5 | +6.6 |
| orf19.6724 | 0.98 | 42.64 | FUM12 | Putative fumarate hydratase; expression greater in high iron | +14.4 | NA |
| orf19.6538 | 0.98 | 41.90 | VMA11 | Predicted ortholog of S. cerevisiae Tfp3p; required for hemoglobin-iron utilization | NA | NA |
| orf19.3182 | 0.94 | 40.69 | GIS2 | Putative transcription factor; expression is increased in high iron | +5.6 | +4.6 |
| orf19.2765 | 0.91 | 40.68 | PGA62 | Adhesin-like cell wall protein; expression greater in high iron | NA | NA |
| orf19.6229 | 0.53 | 35.60 | CAT1 | Catalase; regulated by iron | +51.1 | +4.2 |
| orf19.1354 | 0.83 | 30.16 | UCF1 | Hap43p–repressed gene; transcription induced in high iron | +5.7 | NA |
| orf19.2013 | 0.72 | 25.48 | KAR2 | Similar to Hsp70p family; expression greater in high iron | +2.2 | NA |
| orf19.6548 | 0.86 | 22.66 | ISU1 | Protein with similarity to NifU; possible role in Fe-S cluster biogenesis; expression greater in low iron | −3.5 | NA |
| orf19.336 | 0.75 | 21.86 | YAH1 | Similar to oxidoreductases; expression greater in high iron | +3.4 | +3.3 |
| orf19.6385 | 0.77 | 16.06 | ACO1 | Aconitase; expression greater in high iron | +48.5 | +5.4 |
| orf19.4647 | 0.86 | 13.52 | HAP3 | Similar to CCAAT-binding transcription factor that regulates respiration; Cap2-dependent upregulation in low iron | −77.4 | −17.5 |
| orf19.2435 | 0.86 | 9.75 | MSI3 | Antigenic HSP70 family protein; expression greater in high iron | +3.2 | NA |
| orf19.1715 | 0.82 | 3.33 | IRO1 | Putative transcription factor; role in iron utilization | −2.5 | NA |
Orfs are sorted by absolute ΔOCC scores. Positive ΔOCC scores reflecting potential activation at the chromatin level under iron replete conditions are highlighted in bold text. mRNA expression data from two previous studies for genes identified in this study is shown in the last 2 columns, respectively.
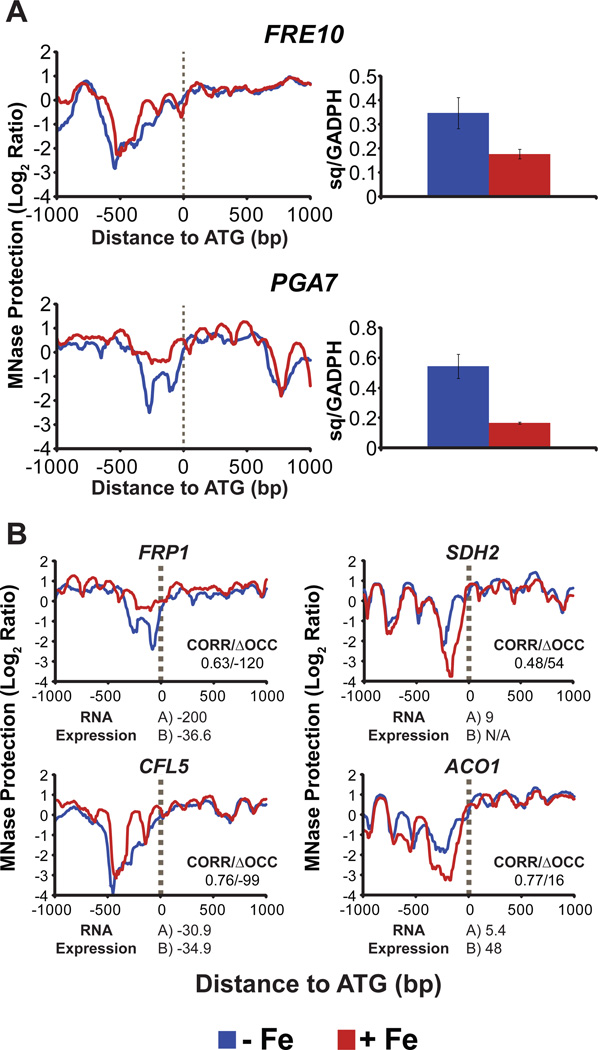
To illustrate that transcriptional changes in iron-regulated genes are dependent on changes in chromatin organization, we determined mRNA expression levels using RT-qPCR and compared it with MNase-Seq data (CORR/ΔOCC scores and ArchTex generated nucleosome profiles) for two highly regulated genes involved in iron uptake: the ferric reductase gene FRE10 (CORR/ΔOCC scores = 0.8/-45) and the gene involved in iron utilization from heme, PGA7 (CORR/ΔOCC scores = 0.6/-156). A highly occupied and well-positioned nucleosome appeared in the promoter region (upstream of ATG) for both FRE10 and PGA7, under iron replete conditions (red lines), relative to deplete cells, indicative of a repressed chromatin in the presence of iron (Figure 3A, left). qPCR data revealed a comparative reduction in mRNA expression levels (almost 2 and 3 fold respectively, for FRE10 and PGA7) in iron replete cells (red bars; Figure 3A, right). We also compared mRNA expression from previously published microarray studies (Lan et al., 2004, Chen et al., 2011) with our MNase-seq data (nucleosome maps and CORR/ΔOCC scores) for several other genes known to be up- or down-regulated in the presence of iron. Some of those, selected on the importance of their function in iron homeostasis, are shown in Figure 3B. Our MNase results showed an increase (FRP1 and CFL5) or decrease (SDH2 and ACO1) in nucleosome occupancy and positioning for respective genes in the presence of iron (red lines); this is in agreement with the decreased (FRP1 and CFL5) or increased (SDH2 and ACO1) mRNA expression under iron replete conditions (Figure 3B), as determined by the previous microarray studies. Also, negative ΔOCC scores were observed for genes down-regulated in the presence of iron (FRP1 and CFL5), while genes up-regulated under iron replete conditions (SDH2 and ACO1) had positive ΔOCC scores (Figure 3B). Our findings of differential gene expression for genes identified by MNase-seq data (CORR/ΔOCC scores and nucleosome maps) provide evidence that chromatin remodeling is an essential prerequisite for condition-specific changes in gene expression.
Figure 3.
Comparison of nucleosome occupancy with gene expression for iron homeostasis genes. (A) Increased promoter occupancy and nucleosome positioning (left) in + Fe conditions (red lines) predicted decreased gene expression for known iron-regulated genes under replete conditions, as confirmed by mRNA levels of respective genes (right), normalized to GADPH, determined by RT-qPCR. Sq is “starting quantity”. (B) Nucleosome maps along with chromatin promoter correlation and ΔOCCupancy scores between iron deplete and replete conditions (shown as CORR/ΔOCC scores, above × axis) are compared with RNA expression data (below x axis) from two published microarray studies, A (Lan et al., 2004) and B (Chen et al., 2011), for each gene. Decreased gene expression (negative numbers for mRNA expression) for FRP1 and CFL5 under iron replete conditions is consistent with increased nucleosome occupancy and greater nucleosome positioning in the promoter regions (red lines) in the presence of iron. In contrast, nucleosome occupancy in promoter regions drops in replete conditions (red lines) for genes up-regulated in + Fe conditions, SDH2 and ACO1 (positive numbers for mRNA expression).
Iron-mediated modulation of genes at the chromatin level causes enhanced cell adhesion
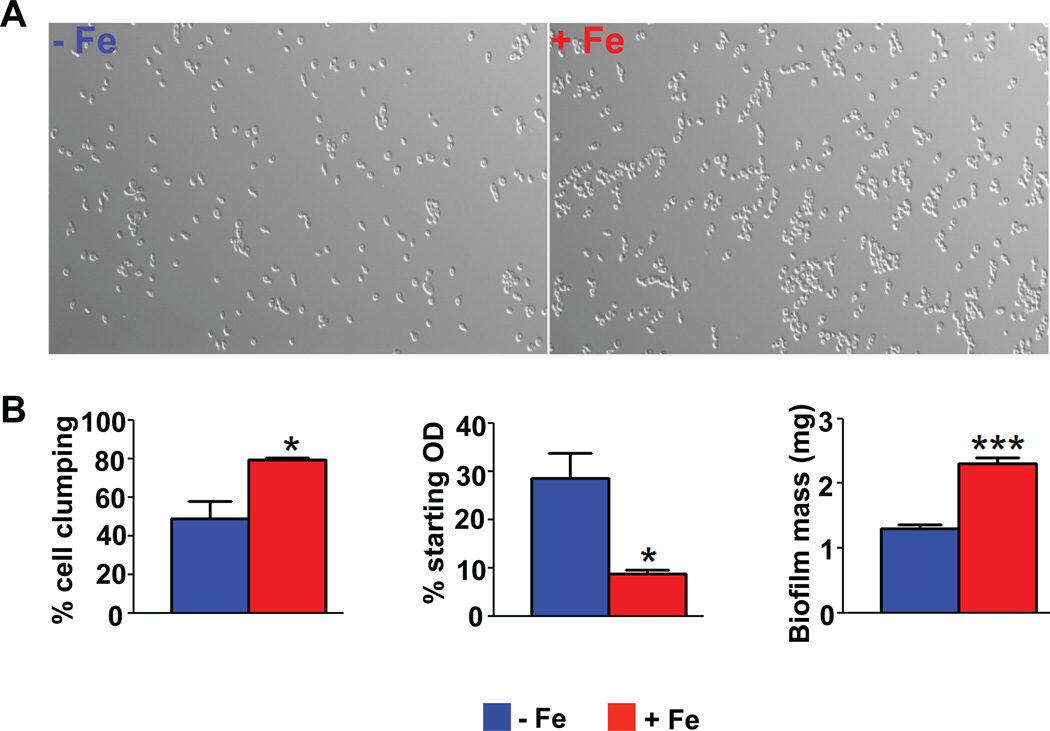
Interestingly, we observed noticeable cell clumping and adhesion to culture tubes for cells grown under iron replete conditions, as compared to deplete cells. Consistent with this phenotype, we observed that both GO Term and GO Slim Term (emphasizing specific gene functions of clusters) enrichment analysis showed cell adhesion processes to be affected at the chromatin level in an iron-responsive manner (cluster 4, GO Term analysis; Figure 2C and cluster 4 as well as cluster 8, Go Slim Term analysis; data not shown). Therefore we examined iron replete and deplete cells under the microscope and observed significantly enhanced cell-to-cell adhesion for iron replete C. albicans cells when compared with iron deplete cells (Figure 4A). Upon quantification of our microscopic results, we observed that cell clumping was almost 2-fold higher for Candida cells grown in iron replete conditions (79.5%) when compared with cells grown in the absence of the metal (48.7%) (Figure 4B, left). Since cells that clump together sediment at a faster rate, sedimentation was quantified by measuring the decrease in optical density (OD) of undisturbed cells over 3 h (Figure 4B, middle). Iron replete cells showed three times the reduction in starting OD when compared with iron deplete cells (Figure 4B, middle), indicative of higher cell sedimentation rates in the presence of iron. We therefore further hypothesized that cells that are iron replete may form better biofilms since adhesion abilities of cells contribute positively towards biofilm formation. Indeed, the biofilm mass of iron replete cells increased by almost 77% when compared with that of iron deplete cells (Figure 4B, right).
Figure 4.
Effect of iron on adhesion and biofilm formation in C. albicans. (A) Microscopic observations were made using Zeiss AxioImager Fluorescence Microscope at 40X magnification and show that WT cells have enhanced cell-to-cell adhesion in the presence of iron. (B) Left: Quantification of microscopic observations (total number of single cells/total number of cells in clumps*100) shows that WT cells are significantly more self-adherant in the presence of iron (red bars). Middle: Iron replete (red bars) WT cells sediment faster than iron deplete cells, as seen in significantly greater reductions in cell OD, after resuspension in buffer and being left undisturbed. Right: Iron replete (red bars) cells show significantly higher biofilm mass on polystyrene surface. All statistical significance are shown at p<0.05.
Iron affects various classes of genes involved in adhesion
To explain our observed phenotype and identify genes in adhesion related clusters affected by the presence of iron, we filtered Table S2 for genes with the word “adhesion” in the gene descriptions provided in CGD and identified 37 genes with adhesion-related functions (Table 2). Almost 60% percent of the adhesion-related genes in Table 2 indicated activation under iron replete conditions, based on their positive ΔOCC scores. A further search of CGD identified the majority of these genes as adherence-induced, or as required for adherence to silicone (used as a standard indicator of the adherence abilities of C. albicans cells) or polystyrene surfaces. Most genes fell into 3 broad categories: transcription related genes, cell-surface adhesions genes, and a general category comprised of genes encoding enzymes and other proteins.
Table 2.
Adhesion-related genes affected by iron at the chromatin level.
| ORF | CORR | ΔOCC | NAME | DESCRIPTION |
|---|---|---|---|---|
| Transcription-related genes | ||||
| orf19.4225* | 0.98 | 80.14 | LEU3 | Zinc-finger Transcription factor (TF); required for yeast cell adherence to silicone substrate |
| orf19.2287 | 0.98 | 61.5 | RPA12 | Putative DNA-directed RNA polymerase I; induced upon adherence to polystyrene |
| orf19.6182* | 0.99 | 44.51 | ZCF34 | TF with zinc cluster DNA-binding motif; required for yeast cell adherence to silicone |
| orf19.6925 | 0.81 | 24.18 | HTB1 | Putative histone H2B; induced upon adherence to polystyrene |
| orf19.6824 | 0.85 | 18.25 | TRY6 | Transcriptional regulator of yeast form adherence; required for yeast cell adherence to silicone |
| orf19.5312 | 0.8 | −7.53 | MET4 | Putative transcription coactivator; required for yeast cell adherence to silicone |
| orf19.5975* | 0.84 | −17.32 | TRY4 | Putative zinc finger DNA-binding TF; required for yeast cell adherence to silicone |
| Cell-surface adhesin genes | ||||
| orf19.3988 | 0.71 | 40.71 | - | Putative adhesin-like protein |
| orf19.2765 | 0.91 | 40.68 | PGA62 | Adhesin-like cell wall protein |
| orf19.1779* | 0.74 | 26.31 | MP65 | Cell surface mannoprotein; adhesion; adhesin motif; O-glycosylation |
| orf19.4886 | 0.8 | 22.59 | - | Putative adhesin-like protein; Hap43p–repressed gene |
| orf19.3618* | 0.67 | 1.69 | YWP1 | Secreted yeast cell wall protein; mutation causes increased adhesion |
| orf19.207* | 0.84 | −1.74 | PGA55 | Putative GPI-anchored protein; adhesin-like protein |
| orf19.5124* | 0.72 | −10.13 | RBR3 | Cell wall adhesin-like protein |
| orf19.4072* | 0.99 | −40.27 | IFF6 | Putative GPI-anchored adhesin-like protein |
| orf19.1490 | 0.91 | −40.86 | MSB2 | Adhesin-like protein; mucin family |
| orf19.4555* | 0.76 | −75.56 | ALS4 | GPI-anchored ALS family protein; role in adhesion |
| Genes encoding enzymes and other proteins | ||||
| orf19.2028* | 1 | 62.94 | MXR1 | Putative methionine sulfoxide reductase; possibly adherence-induced |
| orf19.5645 | 0.98 | 61.58 | MET15 | Sulfhydrylase; possibly adherence-induced |
| orf19.3265* | 0.97 | 57.12 | TRM1 | Putative tRNA methyltransferase; induced upon adherence to polystyrene |
| orf19.4099* | 0.95 | 53.92 | ECM17 | Enzyme of sulfur amino acid biosynthesis; possibly adherence-induced |
| orf19.3802 | 0.94 | 52.41 | PMT6 | Protein mannosyltransferase, required for adhesion to endothelium |
| orf19.4548* | 0.98 | 48.62 | MAK32 | Downregulated upon adherence to polystyrene |
| orf19.7115 | 0.98 | 47.21 | SAC7 | Putative GTPase activating protein (GAP) for RHO1; downregulated upon adherence to polystyrene |
| orf19.593 | 0.99 | 43.84 | FGR32 | Protein similar to S. cerevisiae Swa2p; induced upon adherence to polystyrene |
| orf19.946* | 0.94 | 41.14 | MET14 | Putative adenylylsulfate kinase; possibly adherence-induced |
| orf19.6399* | 0.84 | 4.67 | ATS1 | Induced upon adherence to polystyrene |
Orfs are sorted by ΔOCC scores under each gene sub-category. Positive ΔOCC scores reflecting potential activation at the chromatin level under iron replete conditions are highlighted in bold text. Orfs with potential CBC-regulatory elements upstream of their ATG are marked as.
Transcription factor genes
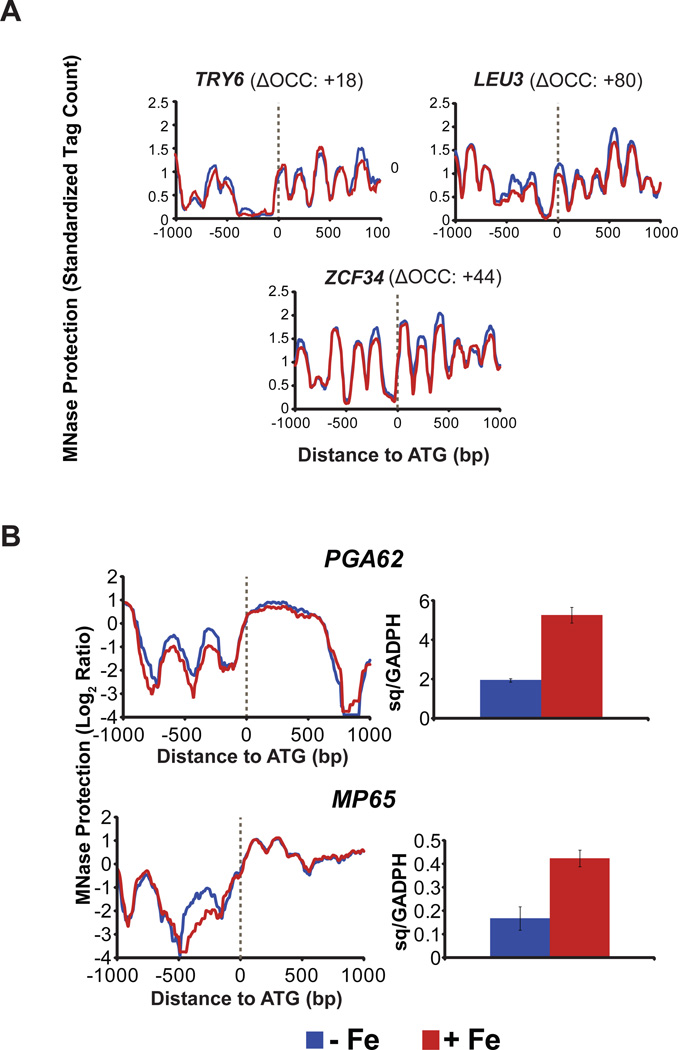
A large scale study, analyzing transcriptional factors regulating adherence, showed that C. albicans cells lacking LEU3, ZCF34, or TRY6 had a strong reduction in adherence to silicone (Finkel et al., 2012). Using these genes as representative genes, we analyzed them for their chromatin status in our data sets. Both LEU3 and ZCF34 had high +ΔOCC scores of 80.14 and 44.51, respectively, while TRY6 had a poor CORR score of 0.85 with a positive ΔOCC score (Table 2). Nucleosome maps of all three of these transcriptional factor genes (Figure 5A) showed a reduction in nucleosome occupancy, upstream of ATG, under iron replete conditions (red lines); and this, together with their positive ΔOCC scores, indicated activation at the chromatin level under replete conditions. Thus, iron is activating select transcription factors that are known to positively regulate adhesion.
Figure 5.
Iron activates adhesion-related genes at the chromatin level. (A) Reduced nucleosome occupancy is observed for adhesion-related transcription factor genes in the presence of iron, as seen in the loss of nucleosome positioning, upstream of ATG, under replete conditions (red lines), as compared to deplete cells (blue lines). (B) Decreased promoter occupancy in + Fe conditions (red lines, left), successfully predicted a corresponding increase in gene expression (red bars, right) by RT-qPCR for genes with a role in cell surface adhesion.
Cell-surface adhesion genes
Two candidate adhesion genes, MP65 and PGA62 that were affected by iron at the chromatin level (Table 2) in our study, have previously been shown to be involved in cell adhesion, based on phenotypic studies. Mp65, a cell surface mannoprotein, is the second highest among the top seven most abundantly secreted proteins in the Candida secretome (Sorgo et al., 2013), and plays an important role in cell wall integrity, adherence to epithelia, and biofilm formation (Sandini et al., 2011). Predicted GPI-anchored or PGA genes have been implicated in the control of cell surface and adherence properties of C. albicans (Plaine et al., 2008, Moreno-Ruiz et al., 2009, Gelis et al., 2012) and PGA62 encodes putative adhesion-like proteins (De Groot et al., 2003). We found that both of these genes showed strong chromatin activation in the presence of iron, based on their CORR/ΔOCC scores (Table 2). We further analyzed these two genes for their nucleosome tiling profiles and mRNA expression levels (Figure 5B). Both genes showed nucleosome signatures indicative of activation in the presence of iron (as seen in the decreased nucleosome occupancy upstream of ATG, under iron replete conditions, when compared to deplete conditions; Figure 5B, left) and correspondingly showed more than 2-fold increase in their mRNA levels, when cells are iron replete (Figure 5B, right).
Since Pga proteins represent a well characterized class of adhesion-related proteins based on protein sequence and structure (de Groot et al., 2013), we also looked for other PGA genes in our master list (Table S2) that may have failed to appear on our list of adhesion related genes (Table 2) because of lack of specific gene descriptors in CGD. Interestingly, we observed that two out of the five iron-regulated PGA genes identified from Table S2 had CORR/ΔOCC scores indicative of activation under iron replete conditions (Table 3). PGA18 with a high ΔOCC score (54.72) encodes for a protein with homology to a highly conserved domain of known C. albicans adhesions (de Groot et al., 2013). Thus we found that iron induces an active chromatin state for known gene categories involved in adhesion (adhesion-related transcription factors and PGA genes) as well as novel cell surface proteins with putative adhesion-like functions (Table 2).
Table 3.
PGA genes affected by iron at the chromatin level.
| ORF | CORR | ΔOCC | NAME | DESCRIPTION |
|---|---|---|---|---|
| orf19.301 | 0.98 | 54.72 | PGA18 | Putative GPI-anchored protein; regulated by Nrg1p, Tup1p |
| orf19.2765 | 0.91 | 40.68 | PGA62 | Adhesin-like cell wall protein; putative GPI-anchor; fluconazole-induced; regulated by iron; expression greater in high iron; induced during cell wall regeneration; Cyr1p or Ras1p downregulated; positively regulated by Tbf1p |
| orf19.207 | 0.84 | −1.74 | PGA55 | Putative GPI-anchored protein; adhesin-like protein; filament induced; regulated by Nrg1p, Tup1p; possibly transcriptionally regulated upon hyphal formation; mRNA binds to She3p and is localized to buds of yeast-form cells and hyphal tips |
| orf19.4651 | 0.83 | −3.36 | PGA53 | GPI-anchored cell surface protein of unknown function; greater mRNA abundance observed in a cyr1 homozygous null mutant than in wild type |
| orf19.5635 | 0.60 | −156.53 | PGA7 | Hyphal surface antigen precursor; possible GPI-anchor; induced by ciclopirox olamine, ketoconazole, or by Rim101p at pH 8; regulated during biofilm and planktonic growth; cell wall regeneration-induced; Hap43p-, fluconazole, biofilm-induced |
Orfs are sorted by ΔOCC scores. Positive ΔOCC scores reflecting potential activation at the chromatin level under iron replete conditions are highlighted in bold text.
Iron induces Msb2-mediated Cek1 phosphorylation
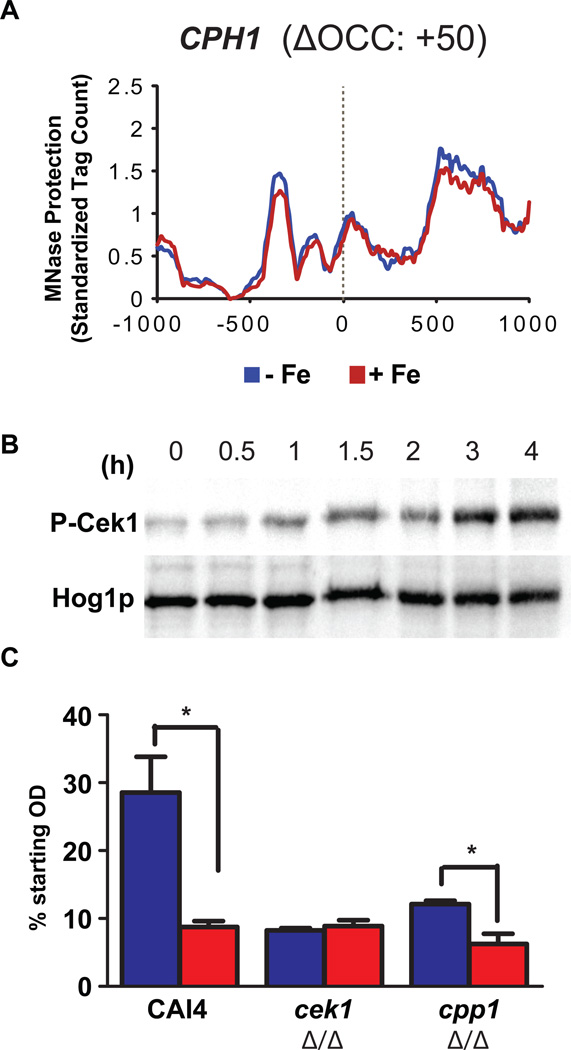
Cell adhesion is a function of cell surface properties and the Cek1 pathway majorly contributes towards maintenance of C. albicans cell surface (Li et al., 2009). Cek1 phosphorylation activates the transcription factor Cph1 (Liu et al., 1994) and cells lacking CPH1 have adhesion defects (Dieterich et al., 2002). We realized that the CPH1 gene description lacks any adhesion-related terms and hence CPH1 missed inclusion in our list of adhesion related genes affected by iron (Table 2). We therefore examined the nucleosome profile of CPH1 (Figure 6A) and observed lower nucleosome occupancy in the presence of iron (red line), with a positive ΔOCC score of 50, indicative of activation under iron replete conditions. This suggested that the Cek1 pathway may potentially be activated in response to iron, so we analyzed iron as a signal for the activation of this pathway. Iron alone induced Cek1 phosphorylation after only 1 h of exposure (Figure 6B), and overall cellular levels of Cek1 signaling increased so that the highest phosphorylation levels were observed after 3 h of iron exposure. Thus, iron can affect the expression of adhesion-related genes by not only inducing the activating Cek1 MAPK kinase upstream of the transcription factor Cph1, but also by inducing a state of active chromatin for the transcription factor gene itself. This suggests an iron-mediated feed forward activation mechanism for adhesion in C. albicans that may operate independent of the iron-mediated chromatin activation of various other classes of adhesion related genes (Table 2).
Figure 6.
Iron signals into Cek1 MAPK pathway. (A) Decreased nucleosome occupancy is observed for the Cek1 MAPK transcription factor gene, CPH1, in the presence of iron (red line), indicating chromatin activation under replete conditions. (B) Cek1 phosphorylation occurs after 1h of exposure to iron with maximal phosphorylation at 3h. Total cellular protein (20 µg) from cell lysates were immunoblotted with α-phospho p42/44 MAPK ERK1/2 Thr202/Tyr204 rabbit monoclonal antibody for detection of Cek1 phosphorylation (C) Iron replete (red bars) WT cells sediment faster than iron deplete cells (blue bars), as seen in significantly greater reductions in cell OD in the presence of iron, whereas cek1Δ/Δ cells are non-responsive to iron status. Cells lacking CPP1 have higher sedimentation levels as compared to WT cells even in the absence of iron, while they show maximal sedimentation under iron replete conditions as compared to all the 3 strains. Statistical significance is shown at p<0.05.
Using our sedimentation assay (as described for Figure 4B, middle) as a measure of cell-cell adhesion, we next compared cell sedimentation of two mutants that either lacked CEK1 or had a constitutively active Cek1 pathway (cells lacking phosphatase Cpp1 that is responsible for removing the phosphate group of Cek1, thereby causing constitutive activation of Cek1). As previously shown (Li et al., 2009), cek1Δ/Δ cells had higher sedimentation levels (Figure 6C) as compared to WT cells. However, the presence of iron did not further increase this already enhanced adhesiveness (Figure 6C) showing that loss of Cek1 signaling prevented further iron responsive adhesion of C. albicans. Furthermore, cells lacking cpp1Δ/Δ and with higher levels of basal Cek1 phosphorylation showed a two-fold higher sedimentation than WT in the absence of iron. In the presence of iron, these cells showed maximal sedimentation levels (highest reduction in OD) as compared to all conditions tested (Figure 6C). These results illustrate the contribution of Cek1 signaling as well as iron-mediated activation of numerous non-Cek1 related adhesion genes at the chromatin level (Table 2).
DISCUSSION
While iron acquisition abilities of C. albicans is an important virulence factor, much less is known about how availability of environmental iron might modulate C. albicans transition from pathogenesis to commensalism. The human GI tract is iron rich owing to unabsorbed iron from daily dietary consumption, while the oral cavity is bathed in saliva where iron is abundant (Garhammer et al., 2004) and readily available (Mukherjee et al., 1997). We used a novel approach of MNase-seq to examine global chromatin changes that occur in response to iron that are driven either by a direct and immediate need to respond to excess iron, or are taking place in preparation for adaption to an iron rich environment. We found that analyses of C. albicans chromatin changes in response to iron not only confirmed regulation of iron homeostasis genes (Table 1), but also identified new roles for iron as it affects diverse biological functions including cell adhesion and biofilm formation. Therefore, MNase-seq is an important tool to complement mRNA expression studies as it provides a condition-specific genome-wide picture of chromatin-mediated regulation of future transcriptional changes.
The data presented here shows that iron positively affects adhesion (Table 2 and Figure 4A and B, left and middle) and biofilm formation (Figure 4B, right). Furthermore, we also show for the first time that iron signals into the MAPK Cek1 pathway (Figure 6B) that is known to affect cell adhesion through Cph1. Iron was previously shown to mediate cell flocculation and activate Hog1 MAPK as well (Kaba et al., 2013); it is likely that this was an induced stress response since Hog1 is the stress stress MAPK of C. albicans and iron is known to cause oxidative stress when present in excess. Thus our finding that cell adhesion is mediated by iron activation of Cek1 MAPK is mechanistically (Figure 7) different from the reported Hog1 MAPK stress response.
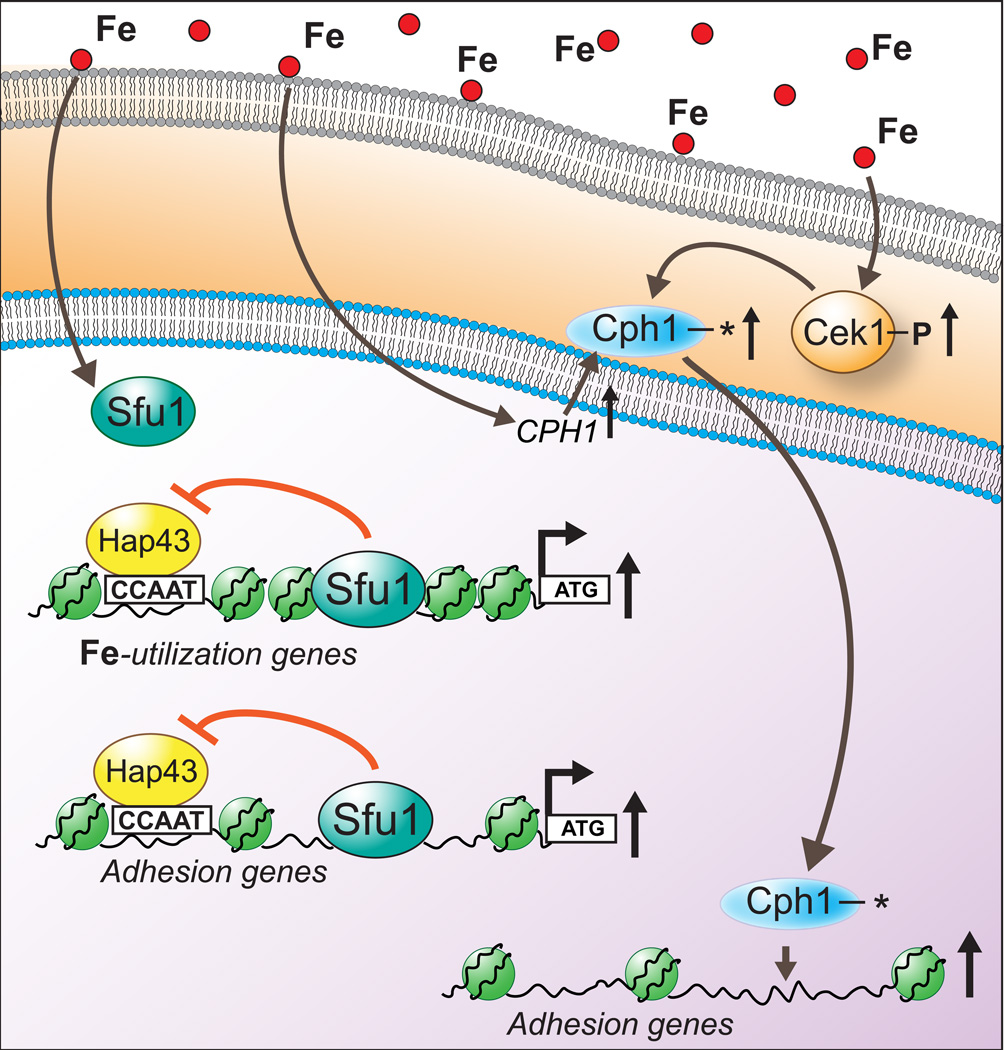
Figure 7.
Proposed model for iron-mediated control of adhesion in C. albicans. Under replete conditions, iron causes Cek1 phosphorylation and increases expression of its downstream transcriptional activator, CPH1, thereby allowing for a feed-forward loop for up-regulation of adhesion genes, one of the proposed target gene category for activated Cph1 (asterisk representing activation). Secondly, Sfu1 mediated de-repression (under iron replete conditions) of Hap43 repressed iron utilization genes, a known iron homeostasis regulatory event, is proposed for adhesion genes as well, owing to the identification of Hap43 target CCAAT upstream of ATG of certain iron-responsive adhesion genes identified in this study (Table 2). This model also shows a proposed mechanism of nucleosome-packing based repression for iron homeostasis genes, based on the distinct CORR profiles observed between genes involved in iron homeostasis versus genes involved in non-iron functions.
We conclude that iron affects C. albicans adhesion through multiple mechanisms (Figure 7). Iron-mediated Cek1 phosphorylation (Figure 6B) and CPH1 promoter nucleosome occupancy decrease (Figure 6A) under iron replete conditions potentially affect cellular processes by exploitation of an existing MAPK signaling mechanism. We also suggest that some genes (LEU3 and ZCF34; Figure 5A) may become iron-responsive merely by virtue of sharing transcriptional motifs with known iron-regulated genes. For example, the transcription factor Sfu1 is recruited to the chromatin under iron replete conditions in order to relieve the Hap43-mediated repression of iron utilization genes (Chen et al., 2011). This recruitment would allow for concomitant expression of any gene along with iron utilization genes if both of these gene categories are similarly repressed through Hap-complex mediated repression mechanism under low iron conditions. This seems to be the case with adhesion genes losing nucleosome occupancy in response to iron as many of those genes (marked as * in Table 2) affected by iron have potential Hap43 binding sites (CCAAT motif) upstream of their ATG. Upon comparing the CORR/ΔOCC scores of all the genes across Tables 1 and 2, an interesting trend emerged that sheds further light on the mechanism of iron mediated chromatin changes. Most genes involved in iron homeostasis in Table 1 were poorly correlated and had substantial changes in their ΔOCC scores (Figure 3A and B). However, in many instances, genes involved in adhesion (Table 2) possessed significant ΔOCC scores despite being extremely well correlated (Figures 5A and B). From this, it appears that other iron-mediated chromatin remodeling mechanisms are at work, besides the two discussed above. Genes whose promoters show a low CORR between conditions may use SWI/SNF-like chromatin remodelers to reposition nucleosomes (Martens and Winston, 2002, Mao et al., 2006, Tolstorukov et al., 2013), resulting in more packed nucleosomes, as illustrated for iron-utilization genes in Figure 7. On the other hand, genes with promoters possessing only a significant ΔOCC may potentially be regulated directly by transcription factors competing with nucleosomes or by the lack of a stabilizing force like Tup1 (Rizzo et al., 2011). Interestingly, another study showed induction of adhesion genes along with iron utilization genes (an indicator of iron replete conditions) upon inhibition of rapamycin-sensitive Tor1 protein kinase signaling (Bastidas et al., 2009). Tor1 was shown to regulate the expression of adhesion related transcription factors as well as iron-related transcriptional corepressor, Tup1, providing another line of evidence for the intricate link between iron metabolism and adhesion.
We propose that iron replete conditions in the GI tract and the oral cavity may allow for enhanced colonization, without any increase in invasiveness, a situation perfectly suited for commensalism. C. albicans possesses many mechanisms to pursue a commensal lifestyle in the iron rich GI tract including Sfu1 that represses iron uptake under replete conditions and plays an important role in preventing iron toxicity (Chen et al., 2011); and commensal cells have been shown to exist primarily in the yeast form and express adhesins and other virulence factors (White et al., 2007, Rosenbach et al., 2010). Recently, Pande et al showed that passage of C. albicans through the murine gut triggers a phenotypic switch that promotes commensalism (Pande et al., 2013).
Since cells lacking the hyphae-promoting transcription factor Efg1 have enhanced colonization in the GI tract (Pierce et al., 2013), we also looked for hyphae related genes by sorting for genes with the word “hyphae” or filament” in Table S2 and identified 85 potentially iron-regulated hyphae-related genes (Table S3). Interestingly, many genes associated with yeast-to-hyphae transition or hyphal induction/maintenance are chromatin repressed (−ΔOCC scores) under iron replete conditions (marked as * in Table S3) while genes that are repressed during yeast-to-hyphae transition or hyphal growth are chromatin activated (+ΔOCC scores) in the presence of iron (marked as ^ in Table S3). This observation indicated that iron represses hyphal induction, which corroborates with the lack of germ tube formation in iron replete cells (Figure 4A), even though Cek1 MAPK that is known to signal hyphae formation (Puri et al., 2012) is activated in the presence of iron (Figure 6B). The lack of hyphae in response to iron also explains the absence of ALS3 and HWP1, two hyphal-specific genes with well important role in cell adhesion (de Groot et al., 2013), from our list of iron regulated adhesion genes (Table 2). Thus, our results uniquely identify adhesins relevant to C. albicans growth under iron replete conditions (Table 2).
Our results shed a new light to the evolving area of Candida commensalism as it is regulated by environmental iron. These iron riche niches potentially establish a reservoir of pathogen inoculum that is important since C. albicans has no known natural reservoir other than the human host. In fact, its presence in the GI tract as a commensal as well as the oral carriage of C. albicans without mucosal infection has long been known. The subsequent transition of C. albicans into a blood-borne pathogen or oral overgrowth into symptomatic thrush can then be initiated by potential changes in the host immune status. Thus C. albicans has evolved to exploit iron replete conditions for commensalism, rather than immediate virulence, with the aim of maintaining a permanent presence in its host.
EXPERIMENTAL PROCEDURES
Media and growth conditions
CAI4 was used as the WT strain in this study. Mutant strains used here (knock-out strains of CEK1 and CPP1, obtained from Malcolm Whiteway and Carol Kumamoto, respectively) have been described previously (Csank et al., 1997). 1.7g l−1 YNB media without iron, copper, and ammonium salt (4027-112; MP Biomedicals), 5g l−1 NH4SO4, 2% glucose, 0.79g l−1 amino acid supplement (Complete Supplement Mix: 4500-012, MP Biomedicals), and 2.5 µm CuSO4 was used as limited iron medium (LIM). LIM with 50 µm of iron chelator Bathophenanthriline-disulfonic acid (BPS) from Sigma (B1375) was used as deplete iron medium (DIM). DIM supplemented with 100 µm FeCl3.6H2O served as replete iron medium (RIM). All experiments were performed at 30˚C at 210 rpm in 10ml final volume in 50 ml centrifuge tubes, unless stated otherwise. YPD medium was used for biofilm propagation.
MNase-seq
Protein-DNA cross-linking
Overnight cultures in LIM were diluted to OD 0.3 in 200ml RIM or DIM (in 500 ml flasks) and grown to OD 0.6 at 125 rpm at 30°C. Iron deprivation for MNase culture conditions was confirmed by lack of Cek 1 phosphorylation under iron deplete conditions (data not shown), as observed for culture conditions (detailed in real time qPCR section below) used for western blot experiments (Figure 6 B). 5.4 ml of formaldehyde was then added to the cultures, followed by 30 min incubation under same conditions. Finally 12.3 ml glycine was added, with an incubation of 5 min, followed by spinning of the contents, washing the pellets twice with ice cold PBS, and flash freezing with liquid nitrogen for storage at −80˚C.
MNase-Digestion and Sequencing
MNase-seq was performed as previously described (Rizzo et al., 2012) and the resulting 50 bp sequence reads were aligned to the Candida genome assembly 21 downloaded from CGD. Bowtie was used for the alignments allowing one mismatch (Langmead et al., 2009). All annotations for C. albicans features were downloaded from CGD under assembly 21. Chromatin data was visualized and extracted from aligned BAM files with the ArchTEx NGS data analysis platform with a 120 bp tag extension at 10 bp resolution (Lai et al., 2012). Data was either converted into normalized log2 space or standardized tag count space (standardized to 10 million sequenced tags). Shown profiles are an average between technical replicates for each condition.
Genome-wide Replicate Correlations
Normalized log2 occupancy was calculated for every non-repeating 1kb window at 10bp resolution for each sequencing run. Pearson correlation was calculated for each window relative to its corresponding window across all experiments. Frequency histogram was calculated as a sum of all correlation scores.
Heat Scatterplot
The normalized average log2 occupancy of every non-overlapping 500 bp windows at 10 bp resolution was calculated genome-wide for each sequencing experiment. Each technical replicate was averaged and plotted as replete versus deplete Fe conditions. The analysis was repeated for all promoter regions in C. albicans.
Chromatin Clustering
K-mediods clustering using the uncentered Pearson correlation and a K of 9 were used on log2 occupancy differences between iron replete and iron deplete conditions at a 1000 bp window at 10 bp resolution centered at ATG for all C. albicans genes. GO Terms and GO Slim Terms were downloaded from CGD. Hypergeometric testing with multiple testing correction was performed to identify enriched clusters.
ΔOCC Scores
The sum of aligned and standardized tags in the 500 bp window upstream of ATG for every gene in C. albicans was calculated for replete and deplete conditions. The average sum between technical replicates was generated for each gene and the difference between replete and deplete iron conditions was taken as the ΔOCC score for each gene.
CORR Scores
The average normalized log2 occupancy for each gene promoter (500bp upstream of ATG) was calculated between technical replicates for iron replete and deplete conditions. Pearson correlation was calculated between conditions for each gene’s promoter profile to generate the CORR score for each gene.
Cut-off generation
To identify significantly changing regions of the genome by ΔOCC and CORR, we estimated the replicate variance by 1 million simulations across the entire genome and used this empirical distribution to determine the p-values for each promoter, similar to the approach used previously (Trapnell et al., 2014) for RNA-seq. The p-values were then converted to FDR according to the method used by Benjamini and Hochberg (Benjamini and Hochberg, 1995).
The MNase-seq data has been submitted to Gene Expression Omnibus (GEO) with the accession number of GSE55819.
Real time qPCR
Overnight cultures were grown in DIM and diluted 100 fold into fresh DIM for another round of overnight growth. The cells were diluted next day into 10 ml of respective media in 50 ml centrifuge tubes to an OD of 0.3 and incubated for 4h. RNA isolation was performed using Qiagen RNAeasy kit. Transcript levels of selected genes were determined by qPCR, as described previously (Puri et al., 2010). Relative starting quantities of the mRNAs for the genes of interest and GADPH were calculated from the corresponding standard curves. Quantity of the interested genes was normalized to the quantity of GADPH for each respective condition. The results were expressed as average of triplicate samples ± SD.
Microscopy and sedimentation analysis
Cells were grown under iron replete and deplete conditions for 4h, as described above and observed microscopically using Zeiss AxioImager Fluorescence Microscope at 40X magnification. Percent clumping (total number of single cells/total number of cells in clumps*100) was calculated in triplicates from the microscopic observations. For sedimentation analysis, iron replete and deplete cells were spun down and resuspended in PBS to an OD of 0.9. 500 µl of cells were transferred to cuvettes, left undisturbed on bench, and absorbance was measured after 3h. Sedimentation was analyzed as percent of starting OD (OD after 3h/starting OD*100).
Biofilm studies
Iron replete and deplete cells were washed and resuspended in PBS to an OD of 0.9, and 2 ml was added to wells (in polystyrene plates) in triplicates, followed by incubation at 37˚C for 3h. The media in the wells was removed followed by gentle washing with PBS after which 1 ml of prewarmed (at 37˚C) YPD medium was added to each well. Plates were incubated at 37˚C for 48h to allow for biofilm formation. Biomass measurements were performed as described previously (Puri et al., 2012).
Protein phosphorylation
Cells were grown as described above for RNA isolation and were processed for immublotting to detect for Hog1 protein and phosphorylated Cek1 as described previously (Puri et al., 2012). For Cek1 phosphorylation, α-phospho p42/44 MAPK ERK1/2 Thr202/Tyr204 rabbit monoclonal (Signaling Technology) antibody was used as the primary antibody and for detection of Hog1 protein levels, Hog1 antibody (y-215: sc-9079 from Santa Cruz Biotechnology, Inc.) was used as primary antibody. Goat α-rabbit IgG-HRP (Jackson ImmunoResearch Laboratories, Inc.) was used as the secondary antibody in both cases.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by R01DE010641 and R01DE022720 (ME) funded by the National Institute of Dental and Craniofacial Research, National Institutes of Health; and NY State Department of Health (Grant C026714 to MJB); PhRMA predoctoral fellowship in Informatics (JMR).
REFERENCES
- Bastidas RJ, Heitman J, Cardenas ME. The protein kinase Tor1 regulates adhesin gene expression in Candida albicans . PLOS Pathog. 2009;5:e1000294. doi: 10.1371/journal.ppat.1000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun BR, Johnson AD. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science. 1997;277:105–109. doi: 10.1126/science.277.5322.105. [DOI] [PubMed] [Google Scholar]
- Buck MJ, Lieb JD. A chromatin-mediated mechanism for specification of conditional transcription factor targets. Nat Genet. 2006;38:1446–1451. doi: 10.1038/ng1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantero PD, Ernst JF. Damage to the glycoshield activates PMT-directed O-mannosylation via the Msb2-Cek1 pathway in Candida albicans . Mol Microbiol. 2011;80:715–725. doi: 10.1111/j.1365-2958.2011.07604.x. [DOI] [PubMed] [Google Scholar]
- Chen C, Pande K, French SD, Tuch BB, Noble SM. An iron homeostasis regulatory circuit with reciprocal roles in Candida albicans commensalism and pathogenesis. Cell Host Microbe. 2011;10:118–135. doi: 10.1016/j.chom.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csank C, Makris C, Meloche S, Schroppel K, Rollinghoff M, Dignard D, Thomas DY, Whiteway M. Derepressed hyphal growth and reduced virulence in a VH1 family-related protein phosphatase mutant of the human pathogen Candida albicans . Mol Biol Cell. 1997;8:2539–2551. doi: 10.1091/mbc.8.12.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot PW, Bader O, de Boer AD, Weig M, Chauhan N. Adhesins in human fungal pathogens: glue with plenty of stick. Eukaryot Cell. 2013;12:470–481. doi: 10.1128/EC.00364-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot PW, Hellingwerf KJ, Klis FM. Genome-wide identification of fungal GPI proteins. Yeast. 2003;20:781–796. doi: 10.1002/yea.1007. [DOI] [PubMed] [Google Scholar]
- Dieterich C, Schandar M, Noll M, Johannes FJ, Brunner H, Graeve T, Rupp S. In vitro reconstructed human epithelia reveal contributions of Candida albicans EFG1 and CPH1 to adhesion and invasion. Microbiology. 2002;148:497–506. doi: 10.1099/00221287-148-2-497. [DOI] [PubMed] [Google Scholar]
- Finkel JS, Xu W, Huang D, Hill EM, Desai JV, Woolford CA, Nett JE, Taff H, Norice CT, Andes DR, Lanni F, Mitchell AP. Portrait of Candida albicans adherence regulators. PLOS Pathog. 2012;8:e1002525. doi: 10.1371/journal.ppat.1002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan-Diez M, Arana DM, Serrano-Gomez D, Kremer L, Casasnovas JM, Ortega M, Cuesta-Dominguez A, Corbi AL, Pla J, Fernandez-Ruiz E. Candida albicans beta-glucan exposure is controlled by the fungal CEK1-mediated mitogen-activated protein kinase pathway that modulates immune responses triggered through dectin-1. Infect Immun. 2010;78:1426–1436. doi: 10.1128/IAI.00989-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garhammer P, Hiller KA, Reitinger T, Schmalz G. Metal content of saliva of patients with and without metal restorations. Clin Oral Invest. 2004;8:238–242. doi: 10.1007/s00784-004-0281-4. [DOI] [PubMed] [Google Scholar]
- Gelis S, de Groot PW, Castillo L, Moragues MD, Sentandreu R, Gomez MM, Valentin E. Pga13 in Candida albicans is localized in the cell wall and influences cell surface properties, morphogenesis and virulence. Fungal Genet Biol. 2012;49:322–331. doi: 10.1016/j.fgb.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Hameed S, Dhamgaye S, Singh A, Goswami SK, Prasad R. Calcineurin signaling and membrane lipid homeostasis regulates iron mediated multidrug resistance mechanisms in Candida albicans . PlOS One. 2011;6:e18684. doi: 10.1371/journal.pone.0018684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed S, Prasad T, Banerjee D, Chandra A, Mukhopadhyay CK, Goswami SK, Lattif AA, Chandra J, Mukherjee PK, Ghannoum MA, Prasad R. Iron deprivation induces EFG1-mediated hyphal development in Candida albicans without affecting biofilm formation. FEMS Yeast Res. 2008;8:744–755. doi: 10.1111/j.1567-1364.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285–297. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- Hsu PC, Chao CC, Yang CY, Ye YL, Liu FC, Chuang YJ, Lan CY. Diverse Hap43-independent functions of the Candida albicans CCAAT-binding complex. Eukaryot Cell. 2013;12:804–815. doi: 10.1128/EC.00014-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PC, Yang CY, Lan CY. Candida albicans Hap43 is a repressor induced under low-iron conditions and is essential for iron-responsive transcriptional regulation and virulence. Eukaryot Cell. 2011;10:207–225. doi: 10.1128/EC.00158-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung WH, Sham A, White R, Kronstad JW. Iron regulation of the major virulence factors in the AIDS-associated pathogen Cryptococcus neoformans . PLOS Biol. 2006;4:e410. doi: 10.1371/journal.pbio.0040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaba HE, Nimtz M, Muller PP, Bilitewski U. Involvement of the mitogen activated protein kinase Hog1p in the response of Candida albicans to iron availability. BMC Microbiol. 2013;13:16. doi: 10.1186/1471-2180-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight SA, Lesuisse E, Stearman R, Klausner RD, Dancis A. Reductive iron uptake by Candida albicans: role of copper, iron and the TUP1 regulator. Microbiology. 2002;148:29–40. doi: 10.1099/00221287-148-1-29. [DOI] [PubMed] [Google Scholar]
- Lai WK, Bard JE, Buck MJ. ArchTEx: accurate extraction and visualization of next-generation sequence data. Bioinformatics. 2012;28:1021–1023. doi: 10.1093/bioinformatics/bts063. [DOI] [PubMed] [Google Scholar]
- Lan CY, Rodarte G, Murillo LA, Jones T, Davis RW, Dungan J, Newport G, Agabian N. Regulatory networks affected by iron availability in Candida albicans . Mol Microbiol. 2004;53:1451–1469. doi: 10.1111/j.1365-2958.2004.04214.x. [DOI] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Williams D, Lowman D, Monteiro MA, Tan X, Kruppa M, Fonzi W, Roman E, Pla J, Calderone R. The Candida albicans histidine kinase Chk1p: signaling and cell wall mannan. Fungal Genet Biol. 2009;46:731–741. doi: 10.1016/j.fgb.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kohler J, Fink GR. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266:1723–1726. doi: 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- Mao X, Cao F, Nie X, Liu H, Chen J. The Swi/Snf chromatin remodeling complex is essential for hyphal development in Candida albicans . FEBS Lett. 2006;580:2615–2622. doi: 10.1016/j.febslet.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Martens JA, Winston F. Evidence that Swi/Snf directly represses transcription in S. cerevisiae . Gene Dev. 2002;16:2231–2236. doi: 10.1101/gad.1009902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance RA, Widdowson EM. The absorption and excretion of iron following oral and intravenous administration. J Physiol. 1938;94:148–154. doi: 10.1113/jphysiol.1938.sp003669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miret S, Simpson RJ, McKie AT. Physiology and molecular biology of dietary iron absorption. Annual review of nutrition. 2003;23:283–301. doi: 10.1146/annurev.nutr.23.011702.073139. [DOI] [PubMed] [Google Scholar]
- Moreno-Ruiz E, Ortu G, de Groot PW, Cottier F, Loussert C, Prevost MC, de Koster C, Klis FM, Goyard S, d'Enfert C. The GPI-modified proteins Pga59 and Pga62 of Candida albicans are required for cell wall integrity. Microbiology. 2009;155:2004–2020. doi: 10.1099/mic.0.028902-0. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Crawford JM, McClear N, Tsang A. A longitudinal study of unsaturated iron-binding capacity and lactoferrin in unstimulated parotid saliva. Biol Trace Elem Res. 1997;57:1–8. doi: 10.1007/BF02803864. [DOI] [PubMed] [Google Scholar]
- Pande K, Chen C, Noble SM. Passage through the mammalian gut triggers a phenotypic switch that promotes Candida albicans commensalism. Nat Genet. 2013;45:1088–1091. doi: 10.1038/ng.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier B, Mercier A, Durand M, Peter C, Jbel M, Beaudoin J, Labbe S. Expression of Candida albicans Sfu1 in fission yeast complements the loss of the iron-regulatory transcription factor Fep1 and requires Tup co-repressors. Yeast. 2007;24:883–900. doi: 10.1002/yea.1539. [DOI] [PubMed] [Google Scholar]
- Pierce JV, Dignard D, Whiteway M, Kumamoto CA. Normal adaptation of Candida albicans to the murine gastrointestinal tract requires Efg1p–dependent regulation of metabolic and host defense genes. Eukaryot Cell. 2013;12:37–49. doi: 10.1128/EC.00236-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaine A, Walker L, Da Costa G, Mora-Montes HM, McKinnon A, Gow NA, Gaillardin C, Munro CA, Richard ML. Functional analysis of Candida albicans GPI-anchored proteins: roles in cell wall integrity and caspofungin sensitivity. Fungal Genet Biol. 2008;45:1404–1414. doi: 10.1016/j.fgb.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S, Hohle TH, O'Brian MR. Control of bacterial iron homeostasis by manganese. P Natl Acad Sci USA. 2010;107:10691–10695. doi: 10.1073/pnas.1002342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S, Kumar R, Chadha S, Tati S, Conti HR, Hube B, Cullen PJ, Edgerton M. Secreted aspartic protease cleavage of Candida albicans Msb2 activates Cek1 MAPK signaling affecting biofilm formation and oropharyngeal candidiasis. PLOS One. 2012;7:e46020. doi: 10.1371/journal.pone.0046020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo JM, Bard JE, Buck MJ. Standardized collection of MNase-seq experiments enables unbiased dataset comparisons. BMC Mol Biol. 2012;13:15. doi: 10.1186/1471-2199-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo JM, Mieczkowski PA, Buck MJ. Tup1 stabilizes promoter nucleosome positioning and occupancy at transcriptionally plastic genes. Nucleic Acids Res. 2011;39:8803–8819. doi: 10.1093/nar/gkr557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbach A, Dignard D, Pierce JV, Whiteway M, Kumamoto CA. Adaptations of Candida albicans for growth in the mammalian intestinal tract. Eukaryot Cell. 2010;9:1075–1086. doi: 10.1128/EC.00034-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandini S, Stringaro A, Arancia S, Colone M, Mondello F, Murtas S, Girolamo A, Mastrangelo N, De Bernardis F. The MP65 gene is required for cell wall integrity, adherence to epithelial cells and biofilm formation in Candida albicans . BMC Microbiol. 2011;11:106. doi: 10.1186/1471-2180-11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RP, Prasad HK, Sinha I, Agarwal N, Natarajan K. Cap2-HAP complex is a critical transcriptional regulator that has dual but contrasting roles in regulation of iron homeostasis in Candida albicans . J Biol Chem. 2011;286:25154–25170. doi: 10.1074/jbc.M111.233569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgo AG, Heilmann CJ, Brul S, de Koster CG, Klis FM. Beyond the wall: Candida albicans secret(e)s to survive. FEMS Microbiol Lett. 2013;338:10–17. doi: 10.1111/1574-6968.12049. [DOI] [PubMed] [Google Scholar]
- Sutak R, Lesuisse E, Tachezy J, Richardson DR. Crusade for iron: iron uptake in unicellular eukaryotes and its significance for virulence. Trends Microbiol. 2008;16:261–268. doi: 10.1016/j.tim.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Tolstorukov MY, Sansam CG, Lu P, Koellhoffer EC, Helming KC, Alver BH, Tillman EJ, Evans JA, Wilson BG, Park PJ, Roberts CW. Swi/Snf chromatin remodeling/tumor suppressor complex establishes nucleosome occupancy at target promoters. P Natl Acad Sci USA. 2013;110:10165–10170. doi: 10.1073/pnas.1302209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Cacchiarelli D, Grimsby J, Pokharel P, Li S, Morse M, Lennon NJ, Livak KJ, Mikkelsen TS, Rinn JL. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol. 2014;32:381–386. doi: 10.1038/nbt.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SJ, Rosenbach A, Lephart P, Nguyen D, Benjamin A, Tzipori S, Whiteway M, Mecsas J, Kumamoto CA. Self-regulation of Candida albicans population size during GI colonization. PLOS Pathog. 2007;3:e184. doi: 10.1371/journal.ppat.0030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc B Met. 1995;57:289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.