Abstract
The oral part of the pontine reticular formation (PnO) contributes to the regulation of sleep, anesthesia, and pain. The role of PnO GABA in modulating these states remains incompletely understood. The present study used time to Loss and time to Resumption of Righting Response (LoRR and RoRR) as surrogate measures of loss and resumption of consciousness. This study tested three hypotheses: (1) pharmacologically manipulating GABA levels in rat PnO alters LoRR, RoRR, and nociception; (2) propofol decreases GABA levels in the PnO; and (3) inhibiting GABA synthesis in the PnO blocks hyperalgesia caused by sleep deprivation. Administering a GABA synthesis inhibitor (3-MPA) or a GABA uptake inhibitor (NPA) into rat PnO significantly altered LoRR caused by propofol. 3-MPA significantly decreased LoRR for propofol (−18%). NPA significantly increased LoRR during administration of propofol (36%). Neither 3-MPA nor NPA altered RoRR following cessation of propofol or isoflurane delivery. The finding that LoRR was decreased by 3-MPA and increased by NPA is consistent with measures showing that extracellular GABA levels in the PnO were decreased (41%) by propofol. Thermal nociception was significantly decreased by 3-MPA and increased by NPA, and 3-MPA blocked the hyperalgesia caused by sleep deprivation. The results demonstrate that GABA levels in the PnO regulate the time for loss of consciousness caused by propofol, extend the concept that anesthetic induction and emergence are not inverse processes, and suggest that GABAergic transmission in the PnO mediates hyperalgesia caused by sleep loss.
Keywords: consciousness, emergence, pain, microinjection, microdialysis
Introduction
Gamma-aminobutyric acid (GABA)-mediated transmission participates in regulating the three interacting states of sleep (Brown et al., 2012), anesthesia (Brown et al., 2010), and pain (Enna & McCarson, 2006). Drugs that enhance transmission at GABAA receptors are used clinically as sedative-hypnotics (Richey & Krystal, 2011) and general anesthetics (Franks, 2008; Brown et al., 2010). The GABA analogues pregabalin and gabapentin that are prescribed to treat chronic pain cause analgesia, in part, by increasing ambient levels of GABA (Maneuf et al., 2003; Tassone et al., 2007). Efforts to identify the mechanisms through which GABAergic transmission so powerfully modulates states of arousal and pain must contend with the fact that the effects of GABAergic transmission vary by brain region (Baghdoyan & Lydic, 2012; Vanini et al., 2012).
In the oral part of the pontine reticular formation (PnO), GABAergic transmission contributes to the regulation of sleep and wakefulness (reviewed in Steriade & McCarley, 2005; Brown et al., 2010). Pharmacologically enhancing GABAergic transmission in the PnO increases wakefulness and decreases sleep (Watson et al., 2008; Flint et al., 2010; Vanini et al., 2011; Vanini & Baghdoyan, 2013). Similarly, administration of drugs that decrease GABAergic transmission in the PnO decreases wakefulness and increases sleep (Marks et al., 2008; Watson et al., 2008; Flint et al., 2010).
The findings that increasing GABA levels in the PnO promotes wakefulness and decreasing GABA levels in the PnO promotes sleep (Watson et al., 2008) are consistent with parallel studies using the general anesthetic isoflurane (Vanini et al., 2008). The isoflurane studies showed that administering a GABA synthesis inhibitor into the PnO to decrease GABA levels caused a decrease in anesthetic induction time, and increasing GABA levels in the PnO with a GABA uptake inhibitor caused an increase in isoflurane induction time (Vanini et al., 2008). The foregoing data raised the questions of whether manipulating GABA levels in the PnO (1) alters the time to recovery from anesthesia caused by isoflurane, and (2) modulates the time required for anesthetic induction and recovery of wakefulness for non inhaled anesthetics.
The present experiments were designed to test three hypotheses that share the unifying goal of identifying brain regions and neurotransmitters regulating interacting states of arousal and pain. The first hypothesis was that manipulating GABA levels in the PnO alters recovery time from isoflurane anesthesia, induction and recovery time for the intravenous anesthetic propofol, and nociception. These experiments blocked GABA synthesis or GABA uptake while quantifying time to Loss of Righting Response (LoRR), time to Recovery of Righting Response (RoRR), and paw withdrawal latency in response to a thermal stimulus. The second hypothesis was that propofol decreases extracellular GABA levels in the PnO. The third hypothesis was that hyperalgesia caused by sleep deprivation (Arima et al., 2001; Edwards et al., 2008; Roehrs et al., 2012) is diminished by administration of a GABA synthesis inhibitor into the PnO. Preliminary reports from these experiments have appeared as abstracts (Vanini et al., 2009; Nemanis et al., 2011; Vanini & Nemanis, 2013).
Materials and Methods
Animals, Chemical Suppliers, and Drug Solutions
All procedures using rats were approved by the University Committee on Use and Care of Animals and followed the Guide for the Care and Use of Laboratory Animals (National Academies Press, 8th Edition, Washington, D.C., 2011). Adult (250 to 350g) male Sprague-Dawley rats (n=39) were purchased from Charles River Laboratories, Wilmington, MA, USA. Animals were housed in a 12-h light:dark cycle within the Unit for Laboratory Animal Medicine facility. Rats had free access to food and water. The GABA uptake inhibitor nipecotic acid (Krogsgaard-Larsen & Johnston, 1975), the GABA synthesis inhibitor 3-mercaptopropionic acid (Engel et al., 2001), high performance liquid chromatography (HPLC)–grade methanol, acetonitrile, sodium tetraborate decahydrate, ß-mercaptoethanol, and GABA were purchased from Sigma-Aldrich (St. Louis, MO, USA). The supplier for salts used to make Ringer’s solution (147.0 mM NaCl, 2.4 mM CaCl2, 4.0 mM KCL, 1.0 mM MgSO4, pH 6.0), o-phosphoric acid, and sodium phosphate dibasic was Thermo Fisher Scientific (Waltham, MA, USA). O-phthaldialdehyde was purchased from Mallinckrodt (St. Louis, MO). Propofol (1%) was obtained from APP Pharmaceuticals, LLC (Schamburg, IL, USA). Nipecotic acid (1.29 μg/100 nL; 10 nmol) and 3-mercaptopropionic acid (1.06 μg/100 nL; 10 nmol) were dissolved and diluted in Ringer’s immediately before each experiment.
Surgical Procedures and Conditioning
Rats were anesthetized with 3.0% isoflurane (Hospira, Inc., Lake Forest, IL, USA) in 100% O2. The delivered concentration of isoflurane was measured continuously by spectrometry (Cardiocap™/5; Datex-Ohmeda, Louisville, CO, USA). When anesthetized, rats were placed in a Kopf Model 962 stereotaxic frame fitted with a Model 906 rat anesthesia mask (David Kopf Instruments, Tujunga, CA, USA). Delivered isoflurane concentration was then reduced to 2.0%. Core body temperature was maintained at 37-38° C using a water-filled pad connected to a heat pump (Gaymar Industries, Orchard Park, NY, USA). The skull was exposed and a microinjection guide cannula (C313G2UP22; Plastics One, Roanoke, VA, USA) was aimed 3 mm above the PnO at stereotaxic coordinates: 8.4 mm posterior to bregma, 1.0 mm lateral to the midline, and 6.2 mm below the skull surface (Paxinos & Watson, 2007). For microdialysis experiments, one CMA/11 guide cannula (CMA, North Chelmsford, MA, USA) was aimed 1 mm above the PnO at 8.4 mm posterior to bregma, 1.0 mm lateral to bregma, and 8.4 mm ventral to the skull surface (Paxinos & Watson, 2007). The microinjection or microdialysis guide cannula and six anchor screws (MPX008002PC; Small Parts Inc., Miami Lakes, FL, USA) were fixed to the skull with dental acrylic (Lang Dental Manufacturing Company, Inc., Wheeling, IL, USA).
A second group of rats also was implanted with jugular vein catheters in order to administer propofol during subsequent microinjection or microdialysis experiments. A silicone catheter (0.020″ × 0.037″ × 0.0065″; Dow Corning, Midland, MI, USA) was inserted into the external jugular vein, sutured, and tunneled subcutaneously to exit between the scapulae, as described previously (Hambrecht-Wiedbusch et al., 2010; Gauthier et al., 2011). The catheter pedestal held a 22 G cannula (C313G3UPSPC; Plastics One, Roanoke, VA, USA) cemented with dental acrylic to a piece of polyethylene mesh (Robinson et al., 2001). Intravenous infusion of 0.2 ml sterile heparin-saline solution (100 U/ml) was performed daily to maintain patent catheters.
All rats received a single dose of gentamicin (5 mg/kg, intravenous) and analgesia was maintained with carprofen (5 mg/kg, subcutaneous) for a minimum of 24 h after surgery. Rats were given one week to recover from surgery, during which time they were conditioned to handling that simulated procedures for intracerebral microinjections. Rats implanted for microdialysis were conditioned to a Raturn® recording chamber (Bioanalytical Systems (BAS), West Lafayette, IN, USA) for one week before being used for an experiment. Rats used for nociceptive testing were conditioned to the IITC Model 336T Paw Stimulator Analgesia Meter with a heated glass plate (IITC Model 400 Heated Base; IITC Life Science Inc., CA, USA). In all experiments, microinjections of Ringer’s and drug (100 nL) into the same rat were made in random order and were separated by seven days. A microinjection volume of 100 nL is estimated to spread up to 0.8 to 1 mm in diameter within the first h post-injection (Vanini et al., 2007).
Study Design and Procedures
Quantification of recovery time from isoflurane anesthesia
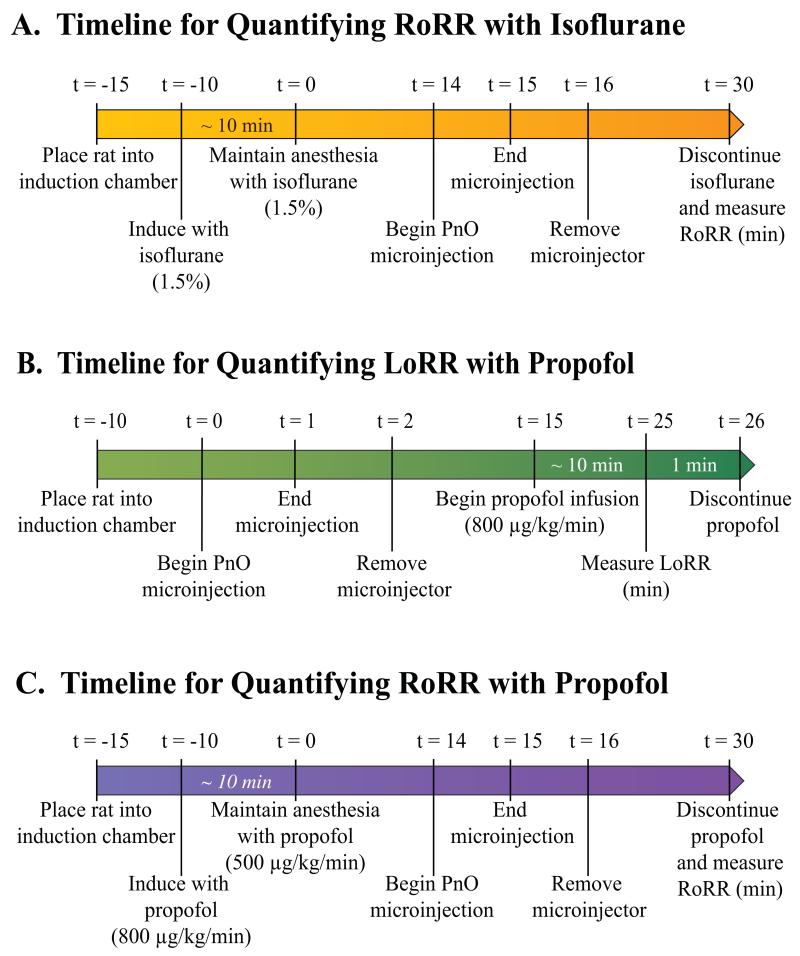
Loss and Resumption of Righting Response are widely used in rodents as surrogate measures of loss and resumption of consciousness, respectively (Tung et al., 2005; Alkire et al., 2007; Franks, 2008; Vanini et al., 2008; Hudetz et al., 2011). Figure 1A illustrates the experimental design used to quantify time to RoRR after discontinuation of isoflurane delivery. All studies began at approximately the same time each day (between 13:00 and 14:00). After a 5-min period (Fig. 1A, t = −10 min) of acclimation to the induction chamber, 1.5% isoflurane in oxygen was delivered at 2 L/min. After induction, (Fig. 1A, t = 0 min) rats were fitted with an anesthesia mask, the oxygen flow rate was reduced to 0.5 L/min, and the concentration of delivered isoflurane was maintained at 1.5%. Fourteen min after the loss of righting response, Ringer’s (vehicle control), nipecotic acid, or 3-mercaptopropionic acid was microinjected into the PnO. Injection duration was one min (Fig. 1A, t = 14 to t = 15 min). Isoflurane administration was discontinued 15 min after the end of the microinjection (Fig. 1A, t = 30 min; total anesthesia time = 30 min). Upon cessation of isoflurane delivery, the anesthesia mask was removed and rats were placed in a supine position. RoRR was quantified as the time (min) from cessation of isoflurane administration to resumption of a prone position. Core body temperature was maintained in all experiments during anesthesia as described above.
Figure 1.
Timelines for quantifying changes in recovery and induction time caused by manipulating GABA levels in the pontine reticular nucleus, oral part (PnO). Elapsed time (t) in min is indicated above each time line. After induction of anesthesia with isoflurane (A) rats received a microinjection of Ringer’s (vehicle control), nipecotic acid (NPA), or 3-mercaptopropionic acid (3-MPA) into the PnO. Anesthesia was maintained for 30 min after induction. Isoflurane delivery then was discontinued, rats were placed in a supine position, and recovery from anesthesia was quantified as the time to Resumption of Righting Response (RoRR). For quantification of induction time with propofol (B), rats received a microinjection of Ringer’s or drug (NPA or 3-MPA) during wakefulness. Fifteen min after the end of the microinjection, continuous intravenous infusion of propofol began and the time to induction was quantified as the time to Loss of Righting Response (LoRR). For quantification of recovery time after propofol anesthesia (C), the timeline and procedures were the same as in the isoflurane experiments.
Quantification of induction time with propofol
After a 10-min acclimation period to a Plexiglas chamber, Ringer’s, nipecotic acid, or 3-mercaptopropionic acid was microinjected (Fig. 1B, t = 0) into the PnO. Fifteen min after microinjection, induction of anesthesia began (Fig. 1B, t = 15) by continuous intravenous administration of propofol (800 μg/kg/min). Induction time was quantified as the time (min) to loss of righting response.
Quantification of recovery time from propofol anesthesia
Rats were conditioned to a Plexiglas induction chamber (Fig. 1C, t = −15) prior to induction of anesthesia by continuous administration of propofol (800 μg/kg/min). Propofol was delivered using a CMA/400 syringe pump (CMA, North Chelmsford, MA, USA) connected to the implanted intravenous line (Fig. 1C, t = −10). For all experiments using propofol, rats remained in a Plexiglas chamber during periods of induction, maintenance, and emergence. After induction of anesthesia (Fig. 1C, t = 0 min), propofol flow rate was reduced (500 μg/kg/min) and maintained for 30 min to ensure that the concentration of propofol in the brain had equilibrated (Dutta et al., 1997). As illustrated by figure 1C, 14 min after propofol-induced loss of consciousness, each rat received a microinjection of Ringer’s, nipecotic acid, or 3-mercaptopropionic acid into the PnO (Fig. 1C, t = 14 to t = 15 min). Fifteen min after the microinjection, intravenous propofol delivery was discontinued (Fig. 1C, t = 30 min) and rats were placed in a dorsal recumbent position. Recovery time (min) was quantified by measuring the time to RoRR.
In vivo microdialysis, intravenous propofol administration, and quantification of PnO GABA levels
Each rat was used for only one microdialysis experiment. A CMA/11 microdialysis probe (cuprophane membrane with a 1 mm length, 0.24 mm diameter, and 6 kDa cutoff) was aimed for the PnO and perfused with Ringer’s solution at a flow rate of 2.0 μl/min using a CMA/400 syringe pump. Microdialysis sample collection (14 μL) began 1 h after probe insertion to allow extracellular GABA levels to stabilize (Watson et al., 2008). Each experiment consisted of collecting 5 sequential samples during wakefulness followed by 5 samples during anesthesia. Procedures and doses for anesthesia with propofol were the same as described above for determining time to LoRR and RoRR. Samples were collected on ice for subsequent quantification of GABA. The amount of GABA recovered by each dialysis probe in vitro was calculated before and after each experiment. Mean ± SEM recovery for all the probes used was 6.7 ± 0.5%.
The methods used to quantify extracellular GABA levels have been described previously (Vanini et al., 2008; Vanini et al., 2011; Vanini et al., 2012). Briefly, dialysis samples obtained from the PnO were analyzed using an ESA HPLC system (Chelmsford, MA, USA) and EZChrom Elite chromatography data system (Scientific Software, Inc., Pleasanton, CA, USA). Each sample was mixed in an autosampler with a derivatization solution (o-phthaldialdehyde, ß-mercaptoethanol, borate buffer, and methanol), and then injected into a Shiseido CAPCELL PAK C-18 separation column (JM Science Inc., Grand Island, NY, USA). The detection limit for GABA was 11 fmol/10 μL. Standard curves were generated before and after each experiment, were used to calculate the amount of GABA in each sample, and to ensure that the sensitivity of the detection system remained unchanged during the analysis.
Nociceptive testing, sleep deprivation, and PnO drug administration
Effects of drugs and arousal state on thermal nociception were measured using the Hargreaves’ paw withdrawal latency (PWL) method (Hargreaves et al., 1988). To test for nociception, a thermal light source was focused onto the plantar surface of a hind paw and then activated. The light was then switched from idle to active intensity (40%) and a timer was simultaneously activated with onset of the thermal stimulus. The rat responded by moving its paw away from the stimulus. Immediately upon paw withdrawal, the thermal source and the timer were deactivated and the latency to withdrawal in s was recorded. On experiment days, rats were habituated to the individual experiment chambers for 20 min prior to testing. Immediately after the habituation period, baseline PWL was determined by 5 measurements taken over 20 min. These 5 measurements were averaged for each animal to obtain the baseline latency.
These experiments quantified whether nociception was increased or decreased, respectively, by PnO administration of a drug that inhibits GABA uptake (nipecotic acid) or a drug that decreases GABA synthesis (3-mercaptopropionic acid). Rats received, in random order, a microinjection of Ringer’s (vehicle), 3-mercaptopropionic acid, or nipecotic acid. Thereafter, four PWL measurements were taken at 20, 30, 60, 90, and 120 min post-microinjection and averaged for each time point.
Additional experiments determined whether hyperalgesia caused by sleep deprivation was diminished by PnO administration of a GABA synthesis inhibitor (3-mercaptopropionic acid). Rats received a microinjection of (1) Ringer’s and were allowed to sleep ad libitum during the intervals between PWL measurements, (2) Ringer’s and were sleep deprived by gentle handling (Tobler & Jaggi, 1987; Baracchi & Opp, 2008; Peterfi et al., 2010) during 4 h of PWL testing, or (3) 3-mercaptopropionic acid and were sleep deprived during 4 h of PWL testing. After the microinjection, four PWL measurements were taken at 20, 30, 60, 90, 120, 150, 180, 210 and 240 min post-microinjection.
All PWL measurements were obtained using bilateral stimulation by alternating the noxious thermal stimulus between right and left hind paws. Each experiment in the same rat was separated by a minimum of 5 days. The latency in s to paw withdrawal in response to the stimulus was expressed as percent change from pre-microinjection baseline values (%MPE, maximum possible effect) (Hayes et al., 1984) using the following equation: %MPE = (post injection PWL – baseline PWL)/(cutoff time – baseline PWL) × 100. A cut-off time of 15 s for the thermal stimulus was used to ensure no tissue damage. Positive %MPE values indicate a longer latency to paw withdrawal in response to the stimulus relative to baseline measures, consistent with decreased nociception. Negative %MPE values indicate shorter paw withdrawal latencies that are consistent with increased nociception.
Histological localization of microinjection and microdialysis sites
Upon completion of the experiments, animals were deeply anesthetized with isoflurane and decapitated. Brains were removed, frozen, and sectioned coronally at 40 μm. All tissue sections containing the pontine reticular formation were mounted serially on glass slides, dried, fixed with hot paraformaldehyde vapor, and stained with cresyl violet. Sections containing microinjection or microdialysis sites were digitized, and stereotaxic coordinates of microinjection and microdialysis sites were defined by comparison with a rat brain atlas (Paxinos & Watson, 2007).
Statistical Analysis
Statistical evaluation of the data was performed with input from the University of Michigan Center for Statistical Consultation and Research. Data analyses were performed using Statistical Analysis System (SAS) version 9.2 (SAS Institute, Inc., Cary, NC, USA) and Prism v6.0c for Mac OS × (GraphPad Software, Inc., La Jolla, CA, USA). All data were tested for normality. Data are reported as mean ± SEM, and a P value less than 0.05 was considered statistically significant.
Induction of and recovery from anesthesia
Because the data did not meet the assumptions of the underlying general linear model (i.e., normality was rejected), drug effects on LoRR and RoRR were evaluated by nonparametric statistics using Wilcoxon matched pairs signed rank tests. In addition, the magnitude of the treatment effect (effect-size) for the time to induction with propofol and isoflurane was quantified by computing Cohen’s d for each measure.
GABA measurement
GABA levels are reported as either fmol/10μL or normalized as percent change from average GABA levels during wakefulness (control). The differences in GABA levels as a function of arousal state were assessed by a linear mixed model allowing a random effect by rat and by condition nested within rat.
Nociception
Differences in %MPE (thermal nociception) as a function of time, drug, and time by drug interaction were evaluated by repeated measures, two-way ANOVA using a linear mixed model, controlling for random effects due to rat and experiment. Post hoc Tukey-Kramer procedure and t-test adjusted for multiple comparisons were used to evaluate differences in mean %MPE per time point. A mixed model was used to analyze changes in %MPE as a function of time and drug during sleep deprivation, allowing random intercepts and slopes per animal within each experiment. The differences in mean %MPE were determined by paired t-test or Kruskal-Wallis test and post hoc Dunn’s test.
Results
Inhibiting GABA Synthesis and GABA Uptake in the PnO Altered Induction Time but did not Change Recovery Time
Isoflurane
Previously published data showed that microinjection of the GABA synthesis inhibitor 3-mercaptopropionic acid into the PnO significantly decreased LoRR caused by isoflurane (Vanini et al., 2008). Consistent with evidence that GABAergic transmission in the pontine reticular formation promotes wakefulness (Vanini et al., 2011; Vanini & Baghdoyan, 2013), administration of the GABA uptake inhibitor nipecotic acid into the PnO increased LoRR with isoflurane (Vanini et al., 2008).
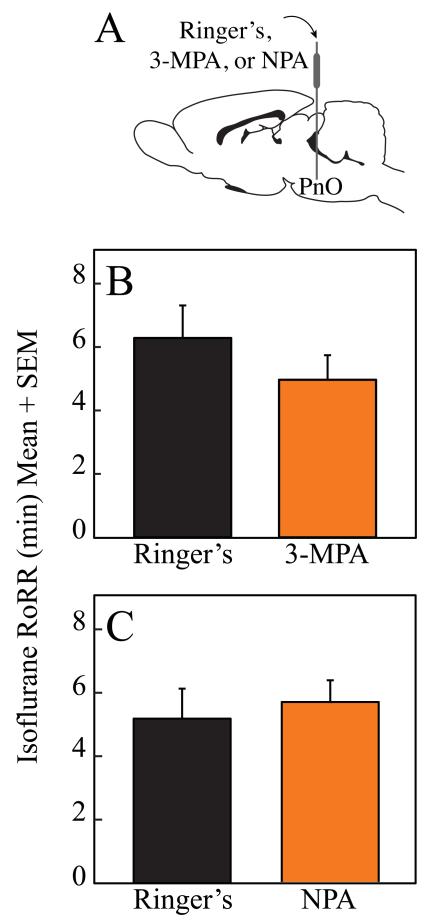
In contrast, figure 2 illustrates that microinjection of the GABA synthesis inhibitor, 3-mercaptopropionic acid (n = 9 rats) and the GABA reuptake inhibitor, nipecotic acid (n = 10 rats) into the PnO did not significantly alter RoRR after isoflurane anesthesia. In order to achieve statistical power for detecting a significant difference in time to recovery from isoflurane anesthesia, power calculations indicate that a minimum of 38 and 462 rats would be required for 3-mercaptopropionic acid and nipecotic acid, respectively.
Figure 2.
Pharmacologically increasing or decreasing endogenous GABA levels in the pontine reticular nucleus, oral part (PnO) did not alter recovery time. A schematic sagittal view of rat brain illustrates that a cannula inserted into the PnO was used for microinjection of Ringer’s or drug (A). Microinjection of either the GABA synthesis inhibitor 3-mercaptopropionic acid (3-MPA) (B) or the GABA uptake inhibitor nipecotic acid (NPA) (C) into the PnO did not alter the time to Resumption of Righting Response (RoRR) after isoflurane anesthesia.
Propofol
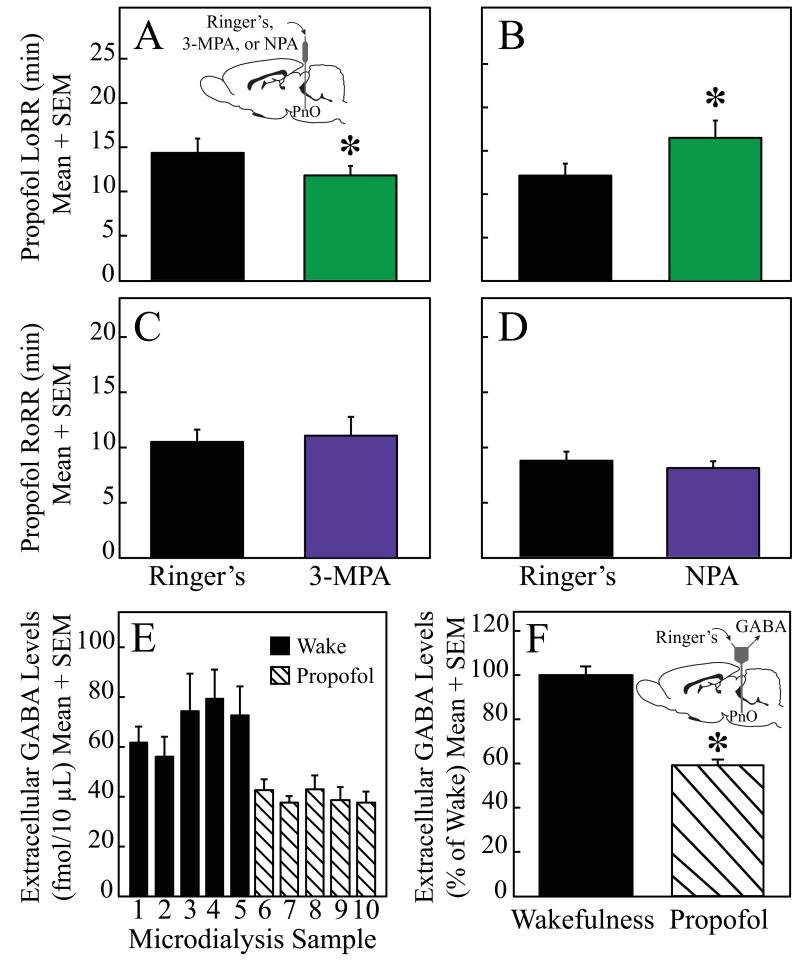
Figure 3 summarizes the effects of 3-mercaptopropionic acid and nipecotic acid on induction and recovery time caused by propofol. Microinjection of the GABA synthesis inhibitor 3-mercaptopropionic acid (n = 6 rats) into the PnO significantly (P = 0.03) decreased LoRR caused by propofol (Fig. 3A). The GABA uptake inhibitor nipecotic acid (n = 5 rats) significantly (P = 0.03) increased LoRR (Fig. 3B). Calculation of Cohen’s d revealed a large treatment effect on propofol-induced LoRR for both 3-mercaptopropionic acid (d = 0. 8) and nipecotic acid (d = 1.1). Also consistent with the results obtained with isoflurane was the finding that microinjection of 3-mercaptopropionic acid (n = 7 rats; fig. 3C) and nipecotic acid (n = 7 rats; fig. 3D) had no effect on RoRR after propofol anesthesia. Power calculations indicate that a minimum of 705 and 129 rats would be needed for the effects of 3-mercaptopropionic acid and nipecotic acid, respectively, to achieve statistical power for detecting a significant difference in the time to recovery from propofol anesthesia.
Figure 3.
Pharmacologically increasing or decreasing endogenous GABA levels in the pontine reticular nucleus, oral part (PnO) altered propofol induction time but not recovery time. Administration of 3-mercaptopropionic acid (3-MPA) and nipecotic acid (NPA) into the PnO significantly decreased (A) or increased (B), respectively, the time to Loss of Righting Response (LoRR). In contrast, 3-MPA (C) and NPA (D) had no effect on the time to Resumption of Righting Response (RoRR) after propofol anesthesia. Relative to wakefulness, extracellular GABA levels in the PnO were significantly decreased (E and F) during anesthesia with propofol.
Figures 3E-F plot mean extracellular GABA levels in the PnO during states of wakefulness and anesthesia with propofol. Relative to wakefulness, propofol caused a significant (F = 75.68; df = 1,2; P = 0.013) decrease (41%) in GABA levels. The graphs plot GABA levels averaged across experiments for all rats (n = 3).
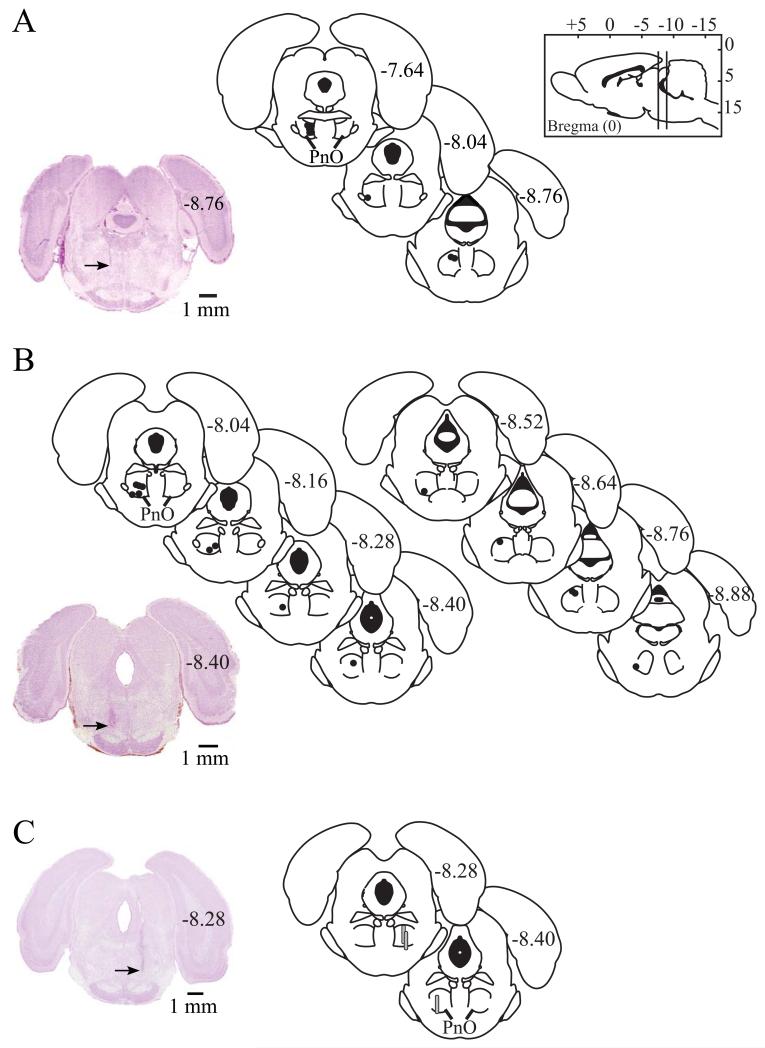
Figure 4A shows that microinjection sites from the isoflurane studies were all localized to the PnO, with average stereotaxic coordinates −8.0 ± 0.1 mm from bregma, 0.8 ± 0.2 mm from the midline, and −8.3 ± 0.1 mm from the skull surface (Paxinos & Watson, 2007). All microinjection sites for studies that measured time to induction and recovery from propofol anesthesia were localized to the PnO (fig. 4B). Average PnO stereotaxic coordinates for the figure 4B data were −8.3 ± 0.1 mm from bregma, 1.2 ± 0.1 mm from the midline, and −8.5 ± 0.1 mm from the skull surface (Paxinos & Watson, 2007). Histological analysis confirmed that all measures of extracellular GABA levels were obtained from the PnO (fig. 4C).
Figure 4.
Microinjection and microdialysis sites were localized to the oral pontine reticular nucleus (PnO). The sagittal drawing of the rat brain (top right) contains vertical lines that illustrate the anterior-to-posterior range of all microinjection and microdialysis sites. Microinjection sites from the isoflurane study (A) and the propofol study (B) are represented by black dots on coronal schematics modified from a rat brain atlas (Paxinos & Watson, 2007). Numbers on the right side of each schematic indicate mm posterior to bregma. The tissue sections at bottom left of parts A and B show representative brain stem sections stained with cresyl violet. Arrows on each section indicate a microinjection site in the left PnO. Coronal schematics (C) of rat brain stem modified from a rat brain atlas (Paxinos & Watson, 2007) show the location of each microdialysis membrane, drawn to scale as gray cylinders. Histological analysis also confirmed that GABA measures were obtained from the PnO. The coronal stained section to the right shows a representative microdialysis site in the right PnO. The arrow indicates the deepest portion of the microdialysis site.
Inhibition of GABA Synthesis and GABA Uptake Mechanisms in the PnO Altered Thermal Nociception
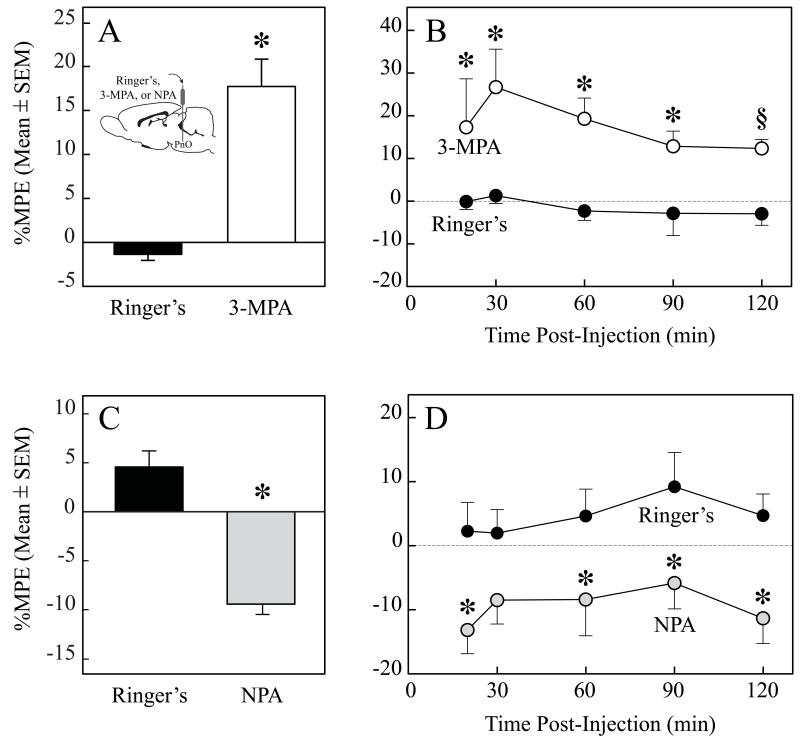
Figure 5A shows that the GABA synthesis inhibitor 3-mercaptopropionic acid significantly (t = 5.818; df = 24; P < 0.0001) decreased thermal nociception (i.e., increased %MPE). The graph plots %MPE averaged across the 2-h testing session for all rats (n = 5). Figure 5B illustrates the time course of %MPE during 2 h after microinjection of 3-mercaptopropionic acid. ANOVA revealed a significant (F = 11.49; df = 1,8; P = 0.0095) drug effect. Figure 5C summarizes the effect of PnO administration of a GABA uptake inhibitor. Nipecotic acid significantly (t = 5.650; df = 39; P < 0.0001) increased thermal nociception (i.e., decreased %MPE). The graph plots %MPE collapsed across time for all rats (n = 8). Figure 5D shows the time course data during 2 h post-microinjection. ANOVA revealed a significant (F = 8.72; df = 1,14; P = 0.0105) main drug effect.
Figure 5.
Pharmacologically increasing or decreasing endogenous GABA levels in the pontine reticular nucleus, oral part (PnO) altered thermal nociception. Microinjection of the GABA synthesis inhibitor 3-mercaptopropionic acid (3-MPA) significantly increased %MPE, reflecting decreased thermal nociception (A). Time course of thermal nociception during 2 h after a microinjection of 3-MPA (B). Administration of the GABA uptake inhibitor nipecotic acid (NPA) significantly decreased %MPE, indicating increased thermal nociception (C). Time course of the effect on thermal nociception during 2 h after microinjection of NPA (D). Time 0 on the abscissa indicates the end of the 1-min period during which Ringer’s or drug was microinjected into the PnO. Asterisks (*) indicate significant differences from control (Ringer’s). The § symbol in B indicates P = 0.0545.
Administration of a GABA Synthesis Inhibitor into the PnO Decreased Hyperalgesia Caused by Sleep Deprivation
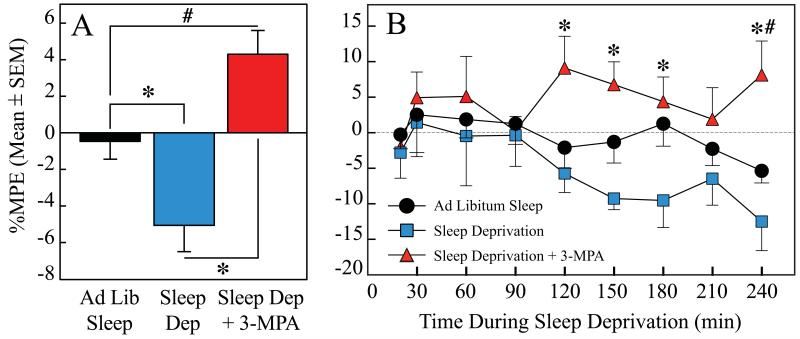
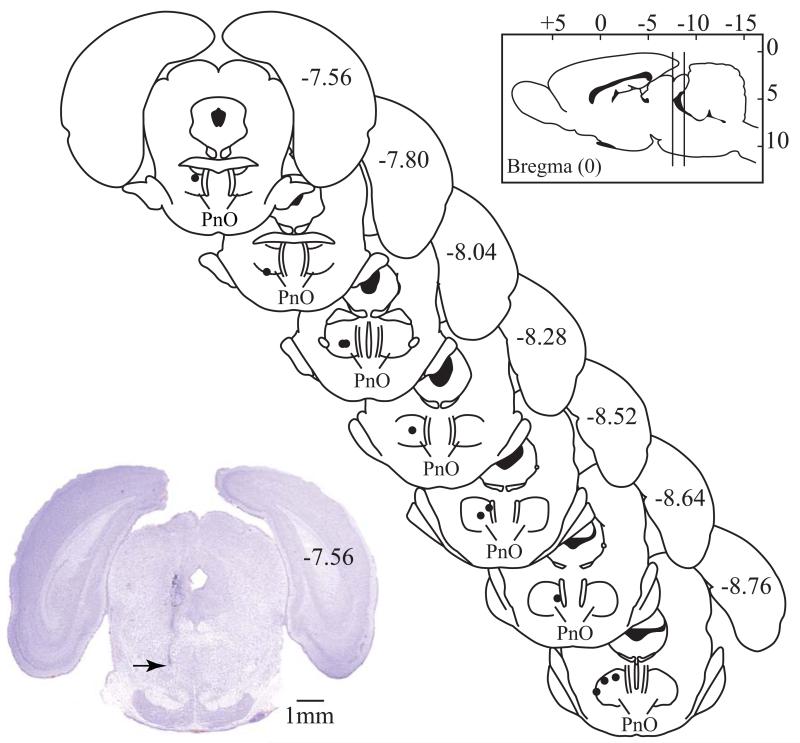
Figure 6A shows that relative to control, sleep deprivation significantly (P = 0.0080) decreased %MPE (i.e., increased thermal nociception). Administration of 3-mercaptopropionic acid into the PnO before the onset of sleep deprivation significantly (P < 0.0001) increased %MPE over control levels, preventing the hyperalgesia caused by sleep deprivation. The time course of %MPE during 4 h as a function of drug, sleep, and time after PnO microinjection is shown in Figure 6B. Repeated measures, two-way ANOVA indicated a significant (F = 6.04; df = 2,18; P = 0.0098) effect of treatment (i.e., sleep deprivation and sleep deprivation plus 3-MPA). There was no post-injection time effect, or treatment condition by time interaction. Post hoc tests revealed a significant increase in %MPE caused by 3-MPA. Regression analysis of %MPE after PnO microinjection of Ringer’s revealed that there was a significant (P = 0.0033) linear increase in thermal nociception during 4 h of sleep deprivation. The time spent awake accounted for a significant percent (76%) of the variance in %MPE. Data shown in Figure 6 summarize results from 7 rats that received all three treatments. Microinjection sites from all pain studies (Figs. 5 and 6) were localized within the PnO (Fig. 7), at average stereotaxic coordinates −8.3 ± 0.1 mm from bregma, 1.2 ± 0.1 mm from the midline, and −8.5 ± 0.1 mm from the skull surface (Paxinos & Watson, 2007).
Figure 6.
Microinjection of a GABA synthesis inhibitor into rat pontine reticular nucleus, oral part (PnO) reversed the hyperalgesia caused by sleep deprivation. A. Sleep deprivation significantly decreased %MPE (i.e., increased thermal nociception). PnO administration of 3-mercaptopropionic acid (3-MPA) significantly increased %MPE, preventing the increase in nociception caused by sleep deprivation. B. Time course of %MPE during 4 h as a function of drug, sleep, and time after PnO microinjection. Administration of 3-MPA caused a significant increase in %MPE during the 4-h testing period. Time 0 on the abscissa indicates the end of the 1-min period during which Ringer’s or 3-MPA was microinjected into the PnO. Asterisks (*) indicate significant differences from sleep deprivation. Pound symbol (#) indicates significant differences from ad libitum sleep.
Figure 7.
All microinjection sites for experiments that measured nociception were localized to the pontine reticular nucleus, oral part (PnO) and are represented by black dots on coronal brain stem plates (modified from Paxinos & Watson, 2007). The numbers on the right side of each plate indicate the distance (mm) from bregma. The vertical lines drawn on the sagittal schematic of the brain (Paxinos & Watson, 2007) (top right), indicate the anterior and posterior range of coordinates for all microinjection sites. The digitized coronal section (bottom left) stained with cresyl violet shows a representative microinjection site (arrow) in the PnO (Paxinos & Watson, 2007).
Discussion
The present data show that the time to loss of righting, but not time to resumption of righting caused by both isoflurane and propofol was significantly altered by manipulating GABA levels in the PnO. These results provide novel evidence that GABA in the PnO promotes wakefulness, and demonstrate in rat that induction of and emergence from anesthesia are not inverse processes. The present data extend previous findings (Kelz et al., 2008) by showing that the induction and emergence phases of anesthesia are differentially regulated by a specific brain region (PnO) and neurotransmitter (GABA). Measures of extracellular GABA levels during wakefulness and anesthesia revealed that propofol decreases GABA levels in the PnO. One implication of these data is that propofol causes loss of consciousness, in part, by decreasing extracellular GABA levels in the PnO. Manipulating GABA levels in the PnO altered nociception, and administering a GABA synthesis inhibitor into the PnO blocked the hyperalgesia caused by sleep deprivation. The results suggest that GABA in the PnO regulates nociception and mediates the hyperalgesia caused by sleep deprivation. The role of GABAergic transmission in the PnO in the regulation of anesthesia, sleep, and pain is discussed in the following sections.
Induction but not Emergence is Regulated by GABAergic Transmission in the PnO
Consistent with data from many laboratories demonstrating that GABAergic transmission in the PnO promotes wakefulness (Camacho-Arroyo et al., 1991; Xi et al., 1999; Sanford et al., 2003; Marks et al., 2008; Vanini et al., 2008; Watson et al., 2008; Brevig et al., 2010; Flint et al., 2010; Vanini et al., 2011; Watson et al., 2011; Vanini & Baghdoyan, 2013), the present data show that pharmacologically altering GABAergic transmission in the PnO modulates time to induction of general anesthesia. Microinjection of a GABA synthesis inhibitor (3-mercaptopropionic acid) into the PnO decreased induction time by the inhaled anesthetic isoflurane (Vanini et al., 2008) and the intravenous anesthetic propofol (fig. 3A). Microinjection of a GABA uptake inhibitor (nipecotic acid) into the PnO increased induction time by both isoflurane (Vanini et al., 2008) and propofol (fig. 3B). In contrast, administration of GABA synthesis and uptake inhibitors into the PnO did not change the time to recovery from either isoflurane (fig. 2) or propofol (figs. 3C and D) anesthesia. Thus, the effects of 3-mercaptopropionic acid and nipecotic acid on induction and emergence from anesthesia with the intravenous agent propofol paralleled the effects of these drugs on induction and emergence with the inhaled anesthetic isoflurane (Vanini et al., 2008). These data provide novel support for the conclusion that GABAergic transmission in the PnO regulates induction but not emergence from general anesthesia. The results are consistent with data that challenged the concept that emergence is the inverse process of the induction phase of anesthesia (Kelz et al., 2008). The lack of identity between the phases of induction and emergence is observed in flies, mice (Friedman et al., 2010), and now rats, indicating conservation across species.
Intravenous administration of propofol caused a significant decrease in extracellular GABA levels in the PnO (figs. 3E and F). These data support the interpretation that changes in propofol-induced LoRR and RoRR are a function of changes in endogenous GABA levels in the PnO, and that one mechanism by which propofol causes loss of consciousness is by decreasing GABAergic transmission in the PnO. The mechanisms by which propofol decreases extracellular GABA levels remain incompletely understood. Propofol enhances GABAergic transmission at GABAA receptors (Bali & Akabas, 2004) and can regulate neuronal GABA release (i.e., presynaptic inhibition) within the PnO. Extracellular glutamate levels in the PnO are greatest during wakefulness (Watson et al., 2011). Propofol decreases the release of glutamate and GABA, with a greater depression of glutamate release (Westphalen & Hemmings, 2003). Thus, one potential mechanism by which propofol eliminates consciousness is, in part, by decreasing the excitatory/inhibitory transmitter ratio in the PnO.
Additional support for the role of PnO GABA in regulating levels of arousal comes from studies of benzodiazepine site agonists. For example, administering the benzodiazepine site agonists zolpidem, diazepam, and eszopiclone directly into the PnO caused drug-specific changes in cortical electroencephalographic activity and increased acetylcholine release in the PnO (Hambrecht-Wiedbusch et al., 2010). Systemic administration of eszopiclone to awake rats significantly decreased acetylcholine release in the PnO and increased electroencephalographic power in the delta frequency (Hambrecht-Wiedbusch et al., 2010). These data suggest that different classes of clinically used sedative-hypnotics can exert their arousal-modulating effects by actions at GABAA receptors in the PnO.
GABAergic input to the PnO arises from multiple sources. GABAergic neurons that project to the PnO are localized to the lateral hypothalamus (Boissard et al., 2003; Rodrigo-Angulo et al., 2008), amygdala (Boissard et al., 2003), periaqueductal gray (Boissard et al., 2003; Sapin et al., 2009), and pontine reticular formation (Liang & Marks, 2009). Extracellular GABA originates from synaptic release (Mitchell & Silver, 2000; Hamann et al., 2002; Houston et al., 2012), as well as nonsynaptic release from neurons and glia (Timmerman & Westerink, 1997; Watson et al., 2006; Angulo et al., 2008; Halassa et al., 2009).
Substantial data support the conclusion that 3-mercaptopropionic acid decreases brain GABA levels. In vitro assays demonstrated that 3-mercaptopropionic acid inhibits glutamic acid decarboxylase (Engel et al., 2001; Monnerie & Le Roux, 2007). Whole-cell patch clamp recordings from CA3 neurons showed that 3-mercaptopropionic acid decreases the amplitude and frequency of GABAergic post-synaptic currents (Engel et al., 2001). Systemic administration of 3-mercaptopropionic acid selectively decreases GABA levels measured in different forebrain, cerebellum and brain stem regions (Van der Heyden et al., 1979; Alsip et al., 1984; DiMicco & Abshire, 1987; Kehr & Ungerstedt, 1988; Toth & Lajtha, 1988; Herbison et al., 1990; Alsip & DiMicco, 1992; Timmerman et al., 1992; Varga & Kunos, 1992). Microinjection or microdialysis delivery of 3-mercaptopropionic acid to the caudate region (Toth & Lajtha, 1988), medial preoptic area (Herbison et al., 1990), and substantia nigra (Van der Heyden et al., 1979) decreases GABA levels. Taken together, these studies support the conclusion that 3-mercaptopropionic acid inhibits glutamic acid decarboxylase and reduces extracellular brain GABA levels by decreasing newly synthesized GABA.
Microinjection of the GABA uptake inhibitor nipecotic acid into the PnO during isoflurane (Vanini et al., 2008) or propofol (fig. 3D) anesthesia did not significantly alter anesthesia recovery time. In view of evidence that general anesthesia decreases extracellular GABA levels in the PnO (Vanini et al., 2008) it could be argued that administration of nipecotic acid into the PnO during anesthesia does not alter endogenous GABA levels. Previously published data, however, demonstrate that delivery of nipecotic acid to the PnO during anesthesia increases extracellular GABA levels in the PnO (Watson et al., 2007; Vanini et al., 2008). Thus, there are now multiple lines of evidence to support the interpretation that increasing GABA levels in the PnO does not alter time to recovery from anesthesia.
GABAergic Transmission in the PnO Modulates Hyperalgesia Caused by Sleep Deprivation
The PnO is part of the ascending activating system that regulates sleep and wakefulness (Steriade & McCarley, 2005; Brown et al., 2012), processes nociceptive information (Porro et al., 1991; Knight et al., 2005; Ghazni et al., 2010), and coordinates autonomic and motor responses evoked by noxious stimuli (Price, 2000). Administering adenosine, acetylcholine, hypocretin, and opioid receptor agonists into the PnO alters nociception (Kshatri et al., 1998; Tanase et al., 2002; Wang et al., 2009; Watson et al., 2010). The present results show that thermal nociception was decreased by a GABA synthesis inhibitor (fig. 5A) and increased by a GABA uptake inhibitor (fig. 5C) delivered to the PnO. These data support the conclusion that GABAergic transmission in the PnO also modulates nociception.
Injection of pentobarbital into rat mesopontine tegmentum causes analgesia, atonia, and loss of consciousness (Devor & Zalkind, 2001; Namjoshi et al., 2009). Pentobarbital enhances transmission at GABAA receptors. The brain stem area in which pentobarbital administration causes an anesthesia-like state (Devor & Zalkind, 2001) is rostral and dorsal to the PnO region where GABA acts to promote wakefulness and increase nociception (figs. 4 and 7). These data further support the interpretation that the effects on arousal state and nociception brought about by activation of GABAA receptors vary as a function of brain region.
The finding that 4 h of total sleep deprivation increased thermal nociception (fig. 6) is consistent with evidence from studies in humans showing that sleep disruption increases pain perception (Arima et al., 2001; Roehrs et al., 2006; Edwards et al., 2008; Roehrs et al., 2012). Hyperalgesia caused by sleep deprivation was decreased by administration of a GABA synthesis inhibitor into the PnO (fig. 6). Nociceptive sensitivity varies as a function of arousal state (Callahan et al., 2008), and GABAergic transmission in the PnO regulates states of sleep and wakefulness (Vanini et al., 2011; Vanini & Baghdoyan, 2013). After PnO microinjection of the GABA synthesis inhibitor 3-mercaptopropionic acid, paw withdrawal latency was measured when rats were awake. No measures were obtained when rats showed behavioral signs of sleep onset. Thus, changes in paw withdrawal latency were likely caused by inhibition of GABA synthesis within PnO networks that process nociceptive information (fig. 7). The foregoing evidence supports the novel interpretation that GABAergic transmission within the PnO modulates nociception and mediates the increase in pain caused by sleep disruption. The present results encourage future studies designed to quantify the extent to which the results shown in figure 6 might reflect a net effect of hyperalgesia caused by sleep disruption and analgesia caused by 3-mercaptopropionic acid.
Unconsciousness and analgesia are two clinical endpoints used to operationally define the state of anesthesia. How states of consciousness are generated (Miller, 2005) and how anesthetics work (Kennedy & Norman, 2005; Brown et al., 2010) remain as major gaps in knowledge in anesthesiology and neuroscience. The data reported here help to bridge this gap by showing that GABAergic transmission in the PnO regulates the interacting states of wakefulness, sleep, anesthesia, and pain.
Limitations and Conclusions
An acknowledged limitation is that the present study did not determine whether the behavioral responses obtained after increasing or decreasing endogenous GABA can be attributed to the actions of GABA on one or a combination of GABAA and GABAB receptor subtypes. The present results encourage future studies using selective agonists and antagonists at GABAA and GABAB receptors in order to clarify the role of GABA receptor subtypes. Another limitation is that the neuronal networks by which GABA in the PnO regulates sleep, wakefulness, and nociception remain incompletely understood. Future studies are needed to identify the neuronal networks that are inhibited by GABAergic mechanisms within the PnO to alter states of behavioral arousal and pain.
The present results support the conclusions that GABA endogenous to the PnO promotes wakefulness (Vanini et al., 2008; Watson et al., 2008; Vanini et al., 2011), and that induction and emergence from propofol anesthesia are not inverse processes (Kelz et al., 2008; Friedman et al., 2010). GABAergic transmission in the PnO regulates the time to loss of consciousness caused by propofol and isoflurane, but not time to recovery of consciousness. The results also indicate that hyperalgesia caused by sleep disruption is modulated by GABAergic transmission in the PnO. These data have translational relevance for sleep disorders medicine, as well as for anesthesiology. A persisting clinical problem is that sleep disruption worsens pain and pain medications disrupt sleep (Gauthier et al., 2011). The rational development of drugs with fewer side effects will require data such as those presented here, that identify brain regions and neurotransmitter systems modulating altered states of consciousness such as anesthesia, sleep, and pain.
Acknowledgments
Supported by grants HL40881 (RL), HL65272 (RL), and MH45361 (HAB) from the National Institutes of Health, Bethesda, MD, and by the Department of Anesthesiology, University of Michigan, Ann Arbor, MI. For expert assistance the authors thank Sha Jiang, Mary A. Norat, and Melissa A. Teran from the Department of Anesthesiology, University of Michigan, Ann Arbor, MI, and Kathy Welch, M.A., M.P.H. (Center for Statistical Consultation and Research, University of Michigan).
Abbreviations
- GABA
gamma-aminobutyric acid
- HPLC
high performance liquid chromatography
- LoRR
loss of righting response
- 3-MPA
3-mercaptopropionic acid
- %MPE
percent of maximum possible effect
- NPA
nipecotic acid
- PnO
pontine reticular nucleus, oral part
- PWL
paw withdrawal latency
- RoRR
resumption of righting response
References
- Alkire MT, McReynolds JR, Hahn EL, Trivedi AN. Thalamic microinjection of nicotine reverses sevoflurane-induced loss of righting reflex in the rat. Anesthesiology. 2007;107:264–272. doi: 10.1097/01.anes.0000270741.33766.24. [DOI] [PubMed] [Google Scholar]
- Alsip NL, DiMicco JA. Time course of effects of 3-mercaptopropionic acid on GABA levels in different brain regions in guinea pigs: possible relationship with associated cardiovascular changes. Neurochem Res. 1992;17:443–448. doi: 10.1007/BF00969890. [DOI] [PubMed] [Google Scholar]
- Alsip NL, Simon JR, Fohl LD, DiMicco JA. Cardiovascular effects of 3-mercaptopropionic acid and levels of GABA in regions of the brain of guinea-pigs. Neuropharmacology. 1984;23:349–357. doi: 10.1016/0028-3908(84)90198-9. [DOI] [PubMed] [Google Scholar]
- Angulo MC, Le Meur K, Kozlov AS, Charpak S, Audinat E. GABA, a forgotten gliotransmitter. Prog Neurobiol. 2008;86:297–303. doi: 10.1016/j.pneurobio.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Arima T, Svensson P, Rasmussen C, Nielsen KD, Drewes AM, Arendt-Nielsen L. The relationship between selective sleep deprivation, nocturnal jaw-muscle activity and pain in healthy men. J Oral Rehabil. 2001;28:140–148. doi: 10.1046/j.1365-2842.2001.00687.x. [DOI] [PubMed] [Google Scholar]
- Baghdoyan HA, Lydic R. The Neurochemistry of Sleep and Wakefulness. In: Brady ST, Albers RW, Price DL, Siegel GJ, editors. Basic Neurochemistry. Elsevier; New York: 2012. pp. 982–999. [Google Scholar]
- Bali M, Akabas MH. Defining the propofol binding site location on the GABAA receptor. Mol Pharmacol. 2004;65:68–76. doi: 10.1124/mol.65.1.68. [DOI] [PubMed] [Google Scholar]
- Baracchi F, Opp MR. Sleep-wake behavior and responses to sleep deprivation of mice lacking both interleukin-1 beta receptor 1 and tumor necrosis factor-alpha receptor 1. Brain Behav Immun. 2008;22:982–993. doi: 10.1016/j.bbi.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissard R, Fort P, Gervasoni D, Barbagli B, Luppi PH. Localization of the GABAergic and non-GABAergic neurons projecting to the sublaterodorsal nucleus and potentially gating paradoxical sleep onset. Eur J Neurosci. 2003;18:1627–1639. doi: 10.1046/j.1460-9568.2003.02861.x. [DOI] [PubMed] [Google Scholar]
- Brevig HN, Watson CJ, Lydic R, Baghdoyan HA. Hypocretin and GABA interact in the pontine reticular formation to increase wakefulness. Sleep. 2010;33:1285–1293. doi: 10.1093/sleep/33.10.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–2650. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan BL, Gil AS, Levesque A. Modulation of mechanical and thermal nociceptive sensitivity in the laboratory mouse by behavioral state. J Pain. 2008;9:174–184. doi: 10.1016/j.jpain.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Camacho-Arroyo I, Alvarado R, Manjarrez J, Tapia R. Microinjections of muscimol and bicuculline into the pontine reticular formation modify the sleep-waking cycle in the rat. Neurosci Lett. 1991;129:95–97. doi: 10.1016/0304-3940(91)90728-c. [DOI] [PubMed] [Google Scholar]
- Devor M, Zalkind V. Reversible analgesia, atonia, and loss of consciousness on bilateral intracerebral microinjection of pentobarbital. Pain. 2001;94:101–112. doi: 10.1016/S0304-3959(01)00345-1. [DOI] [PubMed] [Google Scholar]
- DiMicco JA, Abshire VM. Evidence for GABAergic inhibition of a hypothalamic sympathoexcitatory mechanism in anesthetized rats. Brain Res. 1987;402:1–10. doi: 10.1016/0006-8993(87)91041-9. [DOI] [PubMed] [Google Scholar]
- Dutta S, Matsumoto Y, Gothgen NU, Ebling WF. Concentration-EEG effect relationship of propofol in rats. J Pharm Sci. 1997;86:37–43. doi: 10.1021/js960247n. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Almeida DM, Klick B, Haythornthwaite JA, Smith MT. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137:202–207. doi: 10.1016/j.pain.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel D, Pahner I, Schulze K, Frahm C, Jarry H, Ahnert-Hilger G, Draguhn A. Plasticity of rat central inhibitory synapses through GABA metabolism. J Physiol. 2001;535:473–482. doi: 10.1111/j.1469-7793.2001.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enna SJ, McCarson KE. The role of GABA in the mediation and perception of pain. Adv Pharmacol. 2006;54:1–27. doi: 10.1016/s1054-3589(06)54001-3. [DOI] [PubMed] [Google Scholar]
- Flint RR, Chang T, Lydic R, Baghdoyan HA. GABAA receptors in the pontine reticular formation of C57BL/6J mouse modulate neurochemical, electrographic, and behavioral phenotypes of wakefulness. J Neurosci. 2010;30:12301–12309. doi: 10.1523/JNEUROSCI.1119-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- Friedman EB, Sun Y, Moore JT, Hung HT, Meng QC, Perera P, Joiner WJ, Thomas SA, Eckenhoff RG, Sehgal A, Kelz MB. A conserved behavioral state barrier impedes transitions between anesthetic-induced unconsciousness and wakefulness: evidence for neural inertia. PLoS One. 2010;5:e11903. doi: 10.1371/journal.pone.0011903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier EA, Guzick SE, Brummett CM, Baghdoyan HA, Lydic R. Buprenorphine disrupts sleep and decreases adenosine concentrations in sleep-regulating brain regions of Sprague Dawley rat. Anesthesiology. 2011;115:743–753. doi: 10.1097/ALN.0b013e31822e9f85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazni NF, Cahill CM, Stroman PW. Tactile sensory and pain networks in the human spinal cord and brain stem mapped by means of functional MR imaging. AJNR Am J Neuroradiol. 2010;31:661–667. doi: 10.3174/ajnr.A1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology. 2009;57:343–346. doi: 10.1016/j.neuropharm.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–633. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- Hambrecht-Wiedbusch VS, Gauthier EA, Baghdoyan HA, Lydic R. Benzodiazepine receptor agonists cause drug-specific and state-specific alterations in EEG power and acetylcholine release in rat pontine reticular formation. Sleep. 2010;33:909–918. doi: 10.1093/sleep/33.7.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hayes RL, Katayama Y, Watkins LR, Becker DP. Bilateral lesions of the dorsolateral funiculus of the cat spinal cord: effects on basal nociceptive reflexes and nociceptive suppression produced by cholinergic activation of the pontine parabrachial region. Brain Res. 1984;311:267–280. doi: 10.1016/0006-8993(84)90089-1. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Heavens RP, Dyer RG. Endogenous release of gamma-aminobutyric acid from the medial preoptic area measured by microdialysis in the anaesthetised rat. J Neurochem. 1990;55:1617–1623. doi: 10.1111/j.1471-4159.1990.tb04947.x. [DOI] [PubMed] [Google Scholar]
- Houston CM, McGee TP, Mackenzie G, Troyano-Cuturi K, Rodriguez PM, Kutsarova E, Diamanti E, Hosie AM, Franks NP, Brickley SG. Are extrasynaptic GABAA receptors important targets for sedative/hypnotic drugs? J Neurosci. 2012;32:3887–3897. doi: 10.1523/JNEUROSCI.5406-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudetz AG, Vizuete JA, Pillay S. Differential effects of isoflurane on high-frequency and low-frequency gamma oscillations in the cerebral cortex and hippocampus in freely moving rats. Anesthesiology. 2011;114:588–595. doi: 10.1097/ALN.0b013e31820ad3f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehr J, Ungerstedt U. Fast HPLC estimation of gamma-aminobutyric acid in microdialysis perfusates: effect of nipecotic and 3-mercaptopropionic acids. J Neurochem. 1988;51:1308–1310. doi: 10.1111/j.1471-4159.1988.tb03101.x. [DOI] [PubMed] [Google Scholar]
- Kelz MB, Sun Y, Chen J, Cheng Meng Q, Moore JT, Veasey SC, Dixon S, Thornton M, Funato H, Yanagisawa M. An essential role for orexins in emergence from general anesthesia. Proc Natl Acad Sci U S A. 2008;105:1309–1314. doi: 10.1073/pnas.0707146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D, Norman C. What don’t we know? Science. 2005;309:75. doi: 10.1126/science.309.5731.75. [DOI] [PubMed] [Google Scholar]
- Knight YE, Classey JD, Lasalandra MP, Akerman S, Kowacs F, Hoskin KL, Goadsby PJ. Patterns of fos expression in the rostral medulla and caudal pons evoked by noxious craniovascular stimulation and periaqueductal gray stimulation in the cat. Brain Res. 2005;1045:1–11. doi: 10.1016/j.brainres.2005.01.091. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P, Johnston GA. Inhibition of GABA uptake in rat brain slices by nipecotic acid, various isoxazoles and related compounds. J Neurochem. 1975;25:797–802. doi: 10.1111/j.1471-4159.1975.tb04410.x. [DOI] [PubMed] [Google Scholar]
- Kshatri AM, Baghdoyan HA, Lydic R. Cholinomimetics, but not morphine, increase antinociceptive behavior from pontine reticular regions regulating rapid-eye-movement sleep. Sleep. 1998;21:677–685. doi: 10.1093/sleep/21.7.677. [DOI] [PubMed] [Google Scholar]
- Liang CL, Marks GA. A novel GABAergic afferent input to the pontine reticular formation: the mesopontine GABAergic column. Brain Res. 2009;1297:32–40. doi: 10.1016/j.brainres.2009.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneuf YP, Gonzalez MI, Sutton KS, Chung FZ, Pinnock RD, Lee K. Cellular and molecular action of the putative GABA-mimetic, gabapentin. Cell Mol Life Sci. 2003;60:742–750. doi: 10.1007/s00018-003-2108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks GA, Sachs OW, Birabil CG. Blockade of GABA, type A, receptors in the rat pontine reticular formation induces rapid eye movement sleep that is dependent upon the cholinergic system. Neuroscience. 2008;156:1–10. doi: 10.1016/j.neuroscience.2008.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. What is the biological basis of consciousness? Science. 2005;309:79. doi: 10.1126/science.309.5731.79. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. GABA spillover from single inhibitory axons suppresses low-frequency excitatory transmission at the cerebellar glomerulus. J Neurosci. 2000;20:8651–8658. doi: 10.1523/JNEUROSCI.20-23-08651.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnerie H, Le Roux PD. Reduced dendrite growth and altered glutamic acid decarboxylase (GAD) 65- and 67-kDa isoform protein expression from mouse cortical GABAergic neurons following excitotoxic injury in vitro. Exp Neurol. 2007;205:367–382. doi: 10.1016/j.expneurol.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Namjoshi DR, McErlane SA, Taepavarapruk N, Soja PJ. Network actions of pentobarbital in the rat mesopontine tegmentum on sensory inflow through the spinothalamic tract. J Neurophysiol. 2009;102:700–713. doi: 10.1152/jn.90933.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemanis K, Vanini G, Baghdoyan HA, Lydic R. GABAergic transmission in Sprague-Dawley rat pontine reticular formation (PRF) modulates time required for the general anesthetic propofol to cause loss of wakefulness; SLEEP Meeting, 2011; Minneapolis. 2011; SLEEP 34 (Abstr Suppl):0010, 2011. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates Sixth Ed. Academic Press; Burlington, MA: 2007. [Google Scholar]
- Peterfi Z, McGinty D, Sarai E, Szymusiak R. Growth hormone-releasing hormone activates sleep regulatory neurons of the rat preoptic hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2010;298:R147–156. doi: 10.1152/ajpregu.00494.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porro CA, Cavazzuti M, Galetti A, Sassatelli L. Functional activity mapping of the rat brainstem during formalin-induced noxious stimulation. Neuroscience. 1991;41:667–680. doi: 10.1016/0306-4522(91)90358-u. [DOI] [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Richey SM, Krystal AD. Pharmacological advances in the treatment of insomnia. Curr Pharm Des. 2011;17:1471–1475. doi: 10.2174/138161211796197052. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Rodrigo-Angulo ML, Heredero S, Rodriguez-Veiga E, Reinoso-Suarez F. GABAergic and non-GABAergic thalamic, hypothalamic and basal forebrain projections to the ventral oral pontine reticular nucleus: their implication in REM sleep modulation. Brain Res. 2008;1210:116–125. doi: 10.1016/j.brainres.2008.02.095. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29:145–151. doi: 10.1093/sleep/29.2.145. [DOI] [PubMed] [Google Scholar]
- Roehrs TA, Harris E, Randall S, Roth T. Pain sensitivity and recovery from mild chronic sleep loss. Sleep. 2012;35:1667–1672. doi: 10.5665/sleep.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford LD, Tang X, Xiao J, Ross RJ, Morrison AR. GABAergic regulation of REM sleep in reticularis pontis oralis and caudalis in rats. J Neurophysiol. 2003;90:938–945. doi: 10.1152/jn.00993.2002. [DOI] [PubMed] [Google Scholar]
- Sapin E, Lapray D, Berod A, Goutagny R, Leger L, Ravassard P, Clement O, Hanriot L, Fort P, Luppi PH. Localization of the brainstem GABAergic neurons controlling paradoxical (REM) sleep. PLoS One. 2009;4:e4272. doi: 10.1371/journal.pone.0004272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCarley RW. Brain Control of Wakefulness and Sleep. Kluwer Academic/Plenum Press; New York: 2005. [Google Scholar]
- Tanase D, Baghdoyan HA, Lydic R. Microinjection of an adenosine A1 agonist into the medial pontine reticular formation increases tail flick latency to thermal stimulation. Anesthesiology. 2002;97:1597–1601. doi: 10.1097/00000542-200212000-00036. [DOI] [PubMed] [Google Scholar]
- Tassone DM, Boyce E, Guyer J, Nuzum D. Pregabalin: a novel gamma-aminobutyric acid analogue in the treatment of neuropathic pain, partial-onset seizures, and anxiety disorders. Clin Ther. 2007;29:26–48. doi: 10.1016/j.clinthera.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Timmerman W, Westerink BH. Brain microdialysis of GABA and glutamate: what does it signify? Synapse. 1997;27:242–261. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Timmerman W, Zwaveling J, Westerink BH. Characterization of extracellular GABA in the substantia nigra reticulata by means of brain microdialysis. Naunyn Schmiedebergs Arch Pharmacol. 1992;345:661–665. doi: 10.1007/BF00164580. [DOI] [PubMed] [Google Scholar]
- Tobler I, Jaggi K. Sleep and EEG spectra in the Syrian hamster (Mesocricetus auratus) under baseline conditions and following sleep deprivation. J Comp Physiol A. 1987;161:449–459. doi: 10.1007/BF00603970. [DOI] [PubMed] [Google Scholar]
- Toth E, Lajtha A. 3-Mercaptopropionic acid administration into the caudate-putamen of the rat provokes dyskinesia. Pharmacol Biochem Behav. 1988;29:525–528. doi: 10.1016/0091-3057(88)90014-7. [DOI] [PubMed] [Google Scholar]
- Tung A, Herrera S, Szafran MJ, Kasza K, Mendelson WB. Effect of sleep deprivation on righting reflex in the rat is partially reversed by administration of adenosine A1 and A2 receptor antagonists. Anesthesiology. 2005;102:1158–1164. doi: 10.1097/00000542-200506000-00015. [DOI] [PubMed] [Google Scholar]
- Van der Heyden JA, Venema K, Korf J. In vivo release of endogenous GABA from rat substantia nigra measured by a novel method. J Neurochem. 1979;32:469–476. [PubMed] [Google Scholar]
- Vanini G, Baghdoyan HA. Extrasynaptic GABAA receptors in rat pontine reticular formation increase wakefulness. Sleep. 2013;36:337–343. doi: 10.5665/sleep.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanini G, Lydic R, Baghdoyan HA. GABA-to-ACh ratio in basal forebrain and cerebral cortex varies significantly during sleep. Sleep. 2012;35:1325–1334. doi: 10.5665/sleep.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanini G, Nemanis K. Nociception caused by sleep deprivation is reduced by decreasing GABA levels in rat pontine reticular formation; SLEEP Meeting, 2013; Baltimore. 2013; Sleep 36 (Abstr Suppl):0281, 2013. [Google Scholar]
- Vanini G, Teran MA, Zhou M, Baghdoyan HA, Lydic R. GABAergic transmission in rat pontine reticular formation (PRF) does not modulate recovery time from isoflurane anesthesia; 39th Annual Meeting; Chicago. 2009; Society for Neuroscience Abstract Viewer/Itinerary Planner, Online: Program No. 375.13, 2009. [Google Scholar]
- Vanini G, Torterolo P, McGregor R, Chase MH, Morales FR. GABAergic processes in the mesencephalic tegmentum modulate the occurrence of active (rapid eye movement) sleep in guinea pigs. Neuroscience. 2007;145:1157–1167. doi: 10.1016/j.neuroscience.2006.12.051. [DOI] [PubMed] [Google Scholar]
- Vanini G, Wathen BL, Lydic R, Baghdoyan HA. Endogenous GABA levels in the pontine reticular formation are greater during wakefulness than during rapid eye movement sleep. J Neurosci. 2011;31:2649–2656. doi: 10.1523/JNEUROSCI.5674-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanini G, Watson CJ, Lydic R, Baghdoyan HA. Gamma-aminobutyric acid-mediated neurotransmission in the pontine reticular formation modulates hypnosis, immobility, and breathing during isoflurane anesthesia. Anesthesiology. 2008;109:978–988. doi: 10.1097/ALN.0b013e31818e3b1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga K, Kunos G. Inhibition of baroreflex bradycardia by ethanol involves both GABAA and GABAB receptors in the brainstem of the rat. Eur J Pharmacol. 1992;214:223–232. doi: 10.1016/0014-2999(92)90122-k. [DOI] [PubMed] [Google Scholar]
- Wang W, Baghdoyan HA, Lydic R. Leptin replacement restores supraspinal cholinergic antinociception in leptin-deficient obese mice. J Pain. 2009;10:836–843. doi: 10.1016/j.jpain.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CJ, Lydic R, Baghdoyan HA. Sleep and GABA levels in the oral part of rat pontine reticular formation are decreased by local and systemic administration of morphine. Neuroscience. 2007;144:375–386. doi: 10.1016/j.neuroscience.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CJ, Lydic R, Baghdoyan HA. Sleep duration varies as a function of glutamate and GABA in rat pontine reticular formation. J Neurochem. 2011;118:571–580. doi: 10.1111/j.1471-4159.2011.07350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CJ, Soto-Calderon H, Lydic R, Baghdoyan HA. Pontine reticular formation (PnO) administration of hypocretin-1 increases PnO GABA levels and wakefulness. Sleep. 2008;31:453–464. doi: 10.1093/sleep/31.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CJ, Venton BJ, Kennedy RT. In vivo measurements of neurotransmitters by microdialysis sampling. Anal Chem. 2006;78:1391–1399. doi: 10.1021/ac0693722. [DOI] [PubMed] [Google Scholar]
- Watson SL, Watson CJ, Baghdoyan HA, Lydic R. Thermal nociception is decreased by hypocretin-1 and an adenosine A1 receptor agonist microinjected into the pontine reticular formation of Sprague Dawley rat. J Pain. 2010;11:535–544. doi: 10.1016/j.jpain.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphalen RI, Hemmings HC., Jr. Selective depression by general anesthetics of glutamate versus GABA release from isolated cortical nerve terminals. J Pharmacol Exp Ther. 2003;304:1188–1196. doi: 10.1124/jpet.102.044685. [DOI] [PubMed] [Google Scholar]
- Xi MC, Morales FR, Chase MH. Evidence that wakefulness and REM sleep are controlled by a GABAergic pontine mechanism. J Neurophysiol. 1999;82:2015–2019. doi: 10.1152/jn.1999.82.4.2015. [DOI] [PubMed] [Google Scholar]