Highlights
-
•
Analysis of influenza entry pathways in polarized epithelial cells.
-
•
Clathrin-mediated endocytosis is critical for influenza entry in polarized MDCK cells.
-
•
Influenza virus may use multiple endocytic pathways in non-polarized cells.
Keywords: Influenza virus, Clathrin, Polarized cell, Virus entry
Abstract
In non-polarized cell culture models, influenza virus has been shown to enter host cells via multiple endocytic pathways, including classical clathrin-mediated endocytic routes (CME), clathrin- and caveolae-independent routes and macropinocytosis. However, little is known about the entry route of influenza virus in differentiated epithelia, in vivo site of infection for influenza virus. Here, we show that in polarized Madin–Darby canine kidney type II (MDCK II) cells, influenza virus has a specific utilization of the clathrin-mediated endocytic pathway and requires Eps15 for host cell entry.
1. Introduction
Most animal viruses take advantage of the diverse cellular endocytic pathways to gain access to the host cells [1], [2], [3]. After virus binding to host cell receptors and being endocytosed, viruses can initiate replication only once they penetrate or fuse with the endosomal membrane [4]. A role of dynamin-dependent endocytosis for influenza virus entry was first demonstrated by examining the effect of dominant-negative (DN) dynamin, a small GTPase mediating the scission of clathrin-coated vesicles from the plasma membrane, on influenza virus entry in Mv-1 lung cells [5]. Studies from our group also showed that the infectivity of influenza viruses in cells expressing a DN mutant of Eps15 (epidermal growth factor receptor pathway substrate 15), which inhibits clathrin-dependent endocytosis specifically, was not impaired in HeLa cells [6]. When chemical inhibitors as well as DN mutant of caveolin-1 were used to disrupt caveolae-dependent endocytosis in host cells, influenza infectivity was retained as compared to that in untreated cells [6]. These observations led to the realization that non-clathrin-dependent, non-caveolae-dependent endocytic pathways exist for influenza virus entry, in addition to the classical clathrin-dependent pathway. More recently, with detailed dissection of influenza entry pathways independent of dynamin using pharmacological inhibitors, de Vries et al. discovered that influenza virus can utilize a macropinocytosis-like route in many cell types [7]. With the recent advancements of live cell imaging, individual influenza particles can be tracked in real time without disruption of endocytic pathways, a novel technique in discovering redundant or parallel endocytic pathways. With the new techniques, epsin1 (Epn1), an adaptor protein that interacts with clathrin, AP2 adaptors, and Eps15 in clathrin-coated pits, was demonstrated to be an influenza cargo-specific adaptor for entry via the clathrin-mediated endocytic (CME) pathway in BSC-1 cells [8]. Examining the dynamics of the endocytic uptake also led to the conclusion that influenza viruses exploit different pathways with the same efficiency, and these non-classical, less-characterized pathways do not act as alternative pathways for influenza virus entry.
Polarized, simple epithelial cells have a plasma membrane that is separated by tight junctions into two clearly distinct domains: the apical domain facing the tract lumen and the basolateral surface facing the extracellular matrix [9]. Cellular actin and the microtubule network, as well as an array of cellular proteins, participate in the organization and maintenance of cell polarity. It is well recognized that influenza enters and buds from apical domain of polarized MDCK cells [10]. Previous studies from our laboratory have demonstrated that actin microfilaments play different roles in influenza virus infection in polarized epithelial cells compared to non-polarized cells [11]. In contrast to their dispensable role in viral infection of non-polarized cells, intact actin filaments are obligatory for influenza virus infection in polarized epithelial cells. Since there are significant differences between polarized and non-polarized cells with regard to receptor distribution, cytoskeletal structure, trafficking events, and mechanism of endocytosis, it is possible that our current knowledge of influenza virus entry in non-polarized cells, such as HeLa, MDCK, BSC-1 cells, does not completely apply to in vivo viral infection, which is initiated at the differentiated airway epithelial cells. Here we examined influenza virus entry pathways using pharmacological inhibitors and DN mutant proteins in fully polarized MDCK II cells, a well established and robust model for differentiated epithelia [12].
2. Materials and methods
2.1. Virus preparation
For preparation of virus stocks for infection, approximately 103 plaque-forming units (PFU) of influenza virus A/WSN/33 (H1N1) virus were inoculated into 10-day-old specific-pathogen-free chicken embryos. At 48 h post inoculation, allantoic fluid from infected embryos was collected, clarified by centrifugation at 1800×g for 15 min at 4 °C, and used as a virus stock. The virus stocks were titered by plaque assay in MDBK (bovine kidney) cells and stored at −80 °C.
2.2. Cell culture
In order to obtain polarized MDCK II epithelial cell culture, MDCK II cells (provided by Dr. Colin Parrish, Cornell University) were grown in DMEM media supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin (Cellgro) on 0.4 μm semi-permeable Transwell filters (Corning). Polarity was monitored by measurement of the transepithelial electrical resistance (TEER) of the monolayer cultivated on the semi-permeable filter, using an EVOMX meter along with electrodes for cell culture inserts (World Precision Instruments). Before measurement, culture media was changed to fresh warm media for all filter inserts. After being confluent for 3–4 days on Transwell filters, MDCK II reached an average TEER of 230 ohms.cm2, which was consistent with observations in the literature [13]. MDCK cells (ATCC CCL34) that were not polarized displayed both fibroblast-like and epithelia-like morphology were used as control, due to their inability to form a tight monolayer. The measured TEER of MDCK-CCL34 cells grown under the same culture conditions of MDCK II cells on Transwell filters was 10-fold lower than the TEER of those MDCK II cells.
2.3. Chemical inhibitor treatments and virus infection
Different chemical inhibitors listed below were used to treat fully polarized MDCK II cells and non-polarized MDCK cells for 30 min before infection with influenza virus A/WSN/33 (H1N1), diluted in RPMI 1640 medium containing 0.2% bovine serum albumin (Sigma), 1 mM HEPES, pH 6.8. The inhibitor-treated cells were then incubated with influenza virus at an MOI of 1 (MDCK-CCL34) or an MOI of 5 (MDCK II) for 1 h at 37 °C in the presence of inhibitors. After virus adsorption, the inoculum was replaced with fresh media (DMEM supplemented with 2% fetal bovine serum) and inhibitor for the duration of the incubation period. At 5 h post infection, cells were fixed and analyzed.
2.4. Chemicals and plasmids
Chlorpromazine (CPZ), dynasore, 5-ethylisopropyl amiloride (EIPA), methyl-β-cyclodextrin (MBCD) and nystatin were purchased from Sigma–Aldrich. Dynamin-2 wild type (WT) and DN constructs were obtained from Dr. Mark McNiven of Mayo Clinic Cancer Center. Eps15 WT and DN plasmids were received from Dr. Jennifer Lippincott-Schwartz (NIH). Epsin1 WT and DN plasmids were provided by Dr. Xiaowei Zhuang of Harvard University. Caveolin-1 constructs were a gift from Dr. Ari Helenius (ETH Zürich).
2.5. Immunofluorescence microscopy
Cells were seeded on glass cover slips or Transwell filters. After infection and incubation, cells were fixed with 3% paraformaldehyde (PFA). For detection of influenza infection, we used mouse anti-nucleoprotein (NP) antibody (H16, L10-4R5, ATCC). The secondary antibodies AlexaFluor goat anti-mouse 488 purchased from Molecular Probes. Nuclei were stained with Hoechst 33258 (Molecular Probes). Cover slips with cells were mounted on glass slides using Mowiol. Cells on glass cover slips were examined on a Nikon Eclipse E600 fluorescence microscope equipped with a SensiCam EM camera (Cooke Corp). MDCK II cells on Transwell filters were imaged on a Leica SP5 confocal microscope.
2.6. Western blot
MDCK cells were infected with influenza virus after chemical treatments or mock treatment. Cells were then lysed with RIPA buffer (Millipore) with protease inhibitor cocktail (Roche). The lysate was clarified by centrifugation at 18,000×g at 4 °C and mixed with Laemmli buffer with 10% β-mercaptoethanol. Western blot bands were revealed with an antibody against influenza virus matrix (M1) protein (M2-1C6-4R3, ATCC) and anti-mouse secondary antibodies coupled to horseradish peroxidase.
2.7. Electroporation of plasmid DNA
To overcome the difficulty of recombinant protein expression in polarized cells, we used defined electroporation conditions on fully polarized MDCK II cells as described in [14]. 30 μg/ml of plasmid DNA was mixed with electroporation buffer (Eppendorf) and was delivered using an electrode (CUY512-5, Nepagene) connected to an ECM 830 electroporation system (BTX Instruments).
3. Results and discussion
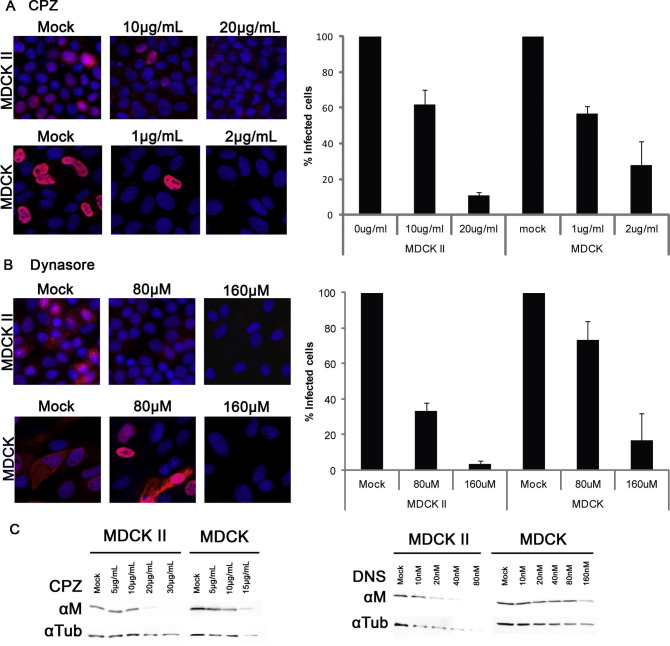
To examine the CME pathway in polarized MDCK cells, we first utilized pharmacological inhibitors that target the CME pathways in host cells. Chlorpromazine (CPZ), which prevents clathrin assembly, has been widely used to block CME including studies on the entry mechanism of influenza viruses in non-polarized cells [1]. Dynasore has an inhibitory effect on GTPase activity of dynamin with low cell toxicity [15]. Since the discovery of dynasore, it has been widely used as specific dynamin inhibitor for entry studies of various virus [16], [17], [18]. CPZ or dynasore were used to treat fully polarized MDCK II cells and non-polarized MDCK-CCL34 cells for 30 min before infection with influenza virus A/WSN/33 (H1N1). At 5 h post infection, cells were fixed and analyzed by immunofluorescence microscopy. MDCK-CCL34 cells were more sensitive to the toxicity of CPZ than MDCK II cells; thus lower concentrations of CPZ were used to treat MDCK-CCL34 cells. The quantity of inhibitors used in the treatment did not affect cell viability (data not shown). Treatment with CPZ on both MDCK-CCL34 and MDCK II caused a substantial reduction on the number of NP expressing cells after influenza infection (Fig. 1 A) However, dynasore treatment had a more obvious impact on polarized MDCK II cells than their non-polarized counterpart (Fig. 1B). This evidence indicates a potential role of dynamin in the entry pathway of polarized MDCK II cells, while non-polarized MDCK-CCL34 cells do not depend as much on dynamin usage for entry. The effect of CPZ and dynamin on the canine kidney cells were further confirmed by western blot analysis (Fig. 1C).
Fig. 1.
Differential utilization of clathrin-mediated endocytosis pathway by influenza virus in non-polarized MDCK cells and polarized MDCK II cells. MDCK-CCL43 (MDCK) cells or MDCK II cells were pretreated with different concentrations of CPZ (A) or dynasore (B) for 30 min at 37 °C prior to infection with influenza virus at an MOI of 1 (MDCK) or an MOI of 5 (MDCK II). At 5 h post infection, cells were stained with mouse anti-NP antibody and AlexaFluor 568 secondary antibody against mouse for immunofluorescence microscopy. NP-positive cells were quantified from images from three independent experiments. Error bars represent the standard deviation of the mean. Viral infectivity in MDCK cells or MDCK II cells with mock treatments was normalized to 100%. (C) MDCK cells or MDCK II cells were infected with influenza virus after pretreatment with CPZ or dynasore (DNS). Viral M protein from whole cell lysate was analyzed by western blot with mouse anti-M1. Tubulin (Tub) was used as loading control.
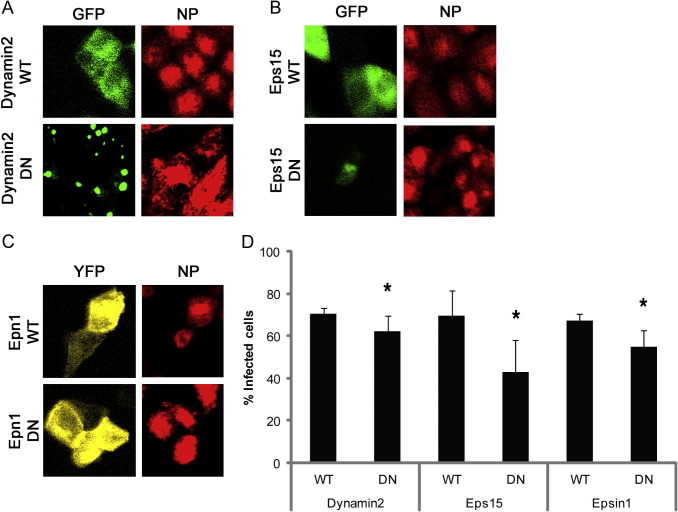
The pharmacological approaches mentioned above indicate an important role of CME in influenza virus infection in both polarized MDCK II cells and non-polarized MDCK-CCL34 cells. However, we wished to examine specific components of the CME pathway. Conventional transfection methods with cationic lipid or adenovirus transduction are either of low transfection efficiency or not ideal for our study. To overcome the difficulty of recombinant protein expression, we used electroporation on monolayer cells as described in [14]. We electroporated WT or DN constructs of dynamin 2 (Fig. 2 A), Eps15 (Fig. 2B), or Epn1 (Fig. 2C) into fully polarized MDCK II cells and infected these cells with influenza virus at a MOI of 5 after 18 h post electroporation. Cells were then fixed and stained for confocal microscopy after 5 h of infection. Expression of the DN form of dynamin 2 or Epn1 resulted in a statistically significant reduction of percentage of GFP or EYFP expressing cells that were positive for viral NP expression, compared to the control WT version of the respective construct (Fig. 2D). Unlike in non-polarized cells such as HeLa cells described in a previous study [6], Eps15-Δ95/295-GFP DN construct expression in MDCK II cells also significantly reduced the percentage of cells infected with influenza virus and expressing viral NP, comparing to its WT counterpart Eps15-DIIIΔ2-GFP expressing cells.
Fig. 2.
Eps15 is required for influenza virus infection on polarized MDCK II cells. MDCK II cells were polarized on Transwell filters for 3–4 days and then were electroporated with either wild type (WT) or dominant negative (DN) constructs of dynamin2 (A), Eps15 (B), or epsin1 (C). 18 h post electroporation, cells were infected with influenza virus at MOI of 10. At 5 h post infection, cells were fixed and stained with mouse anti-NP antibody for confocal microscopy. (D) Both transfected and infected cells were scored and quantified from images from three independent experiments. A student’s t-test was used to calculate the statistical significance between the percentage of infected cells expressing WT and DN proteins. ∗ indicates p < 0.05.
Recently influenza virus has been shown to be able to enter host cell via macropinocytosis pathway [7], [19]. To examine whether influenza virus can also utilize macropinocytosis in polarized MDCK II cells, we used 5-ethylisopropyl amiloride (EIPA), a macropinocytosis inhibitor that inhibits Na+/H+ exchanger activity, to treat the canine kidney cells before infecting with influenza virus [20]. By examination of viral protein M1 in whole cell lysate using western blot analysis, we found that neither MDCK-CCL34 cells nor MDCK II cells were significantly inhibited by EIPA, compared to leading control, regardless of drug treatment concentrations (Fig. 3 ). Our data suggest that similar to non-polarized MDCK cells, there exist redundant entry pathways (presumably CME) in polarized MDCK cells and macropinocytosis is not a preferential major pathway utilized by influenza virus in the cells used in this study.
Fig. 3.
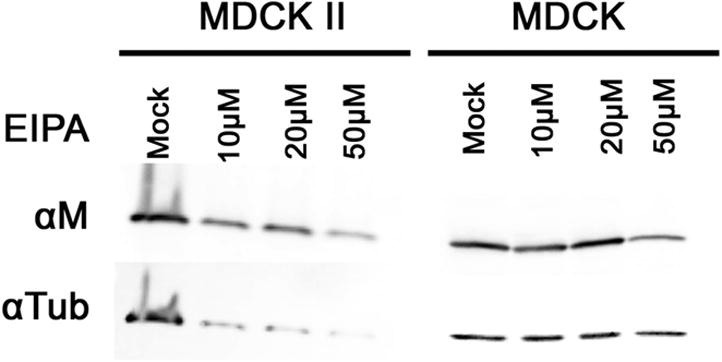
Macropinocytosis inhibitor does not affect influenza virus infection in MDCK II cells. Polarized MDCK II cells were treated with EIPA before infection with influenza virus. Viral protein M from whole cell lystate was analyzed by western blot with mouse anti-M1. Tubulin (Tub) was used as loading control.
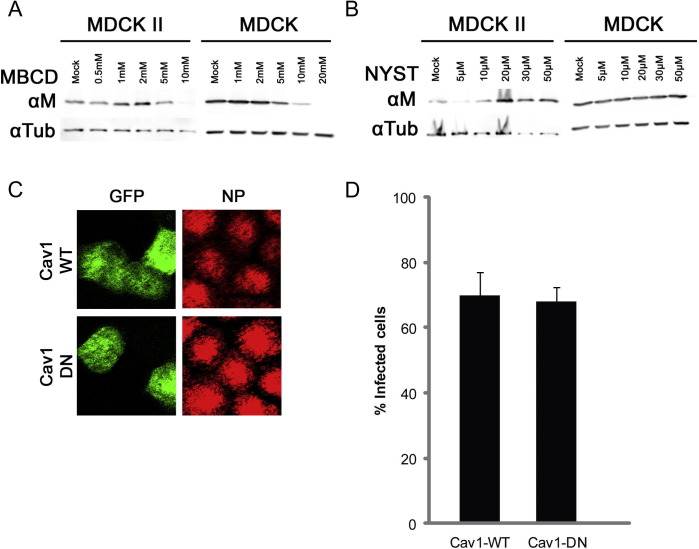
In non-polarized cell systems, influenza was shown to enter host cells via caveolae-independent entry routes [6]. To examine whether influenza virus enters polarized MDCK II cells via caveolae-independent entry pathway as in previous studies on non-polarized cells, we first treated the MDCK-CCL34 or MDCK II cells with different concentrations of caveolae-inhibiting drugs such as methyl-β-cyclodextrin (MBCD) and nystatin. As revealed by expression of viral protein M1 on western blot, treatment with high concentrations of MBCD were able to inhibit both polarized MDCK II cells and non-polarized MDCK-CCL34 cells (Fig. 4 A). In contrast, nystatin did not have an inhibitory effect on either cell type. To examine the caveolar-mediated pathway more specifically in polarized MDCK II cells, we electroporated GFP-tagged caveolin-1 WT or DN plasmids into polarized MDCK II cells before infection with influenza virus. Caveolin-1 is a major structural protein in the caveolae. Using confocal microscopy, both Cav1 WT and Cav1 DN expressing cells were able to be infected by influenza virus as shown by the positive signal of viral NP in the transfected cells (Fig. 4C and D). The discrepancy in influenza virus infectivity in MDCK II cell after treatment with MBCD and with nystatin may be due to the discrete modes of action by the two drugs: MBCD inhibition of influenza virus entry in both MDCK-CCL34 and MDCK II cells was not by inhibiting caveolae-dependent endocytosis, by altering the structure of cholesterol-rich domain in plasma membrane. A similar observation was also described in SARS-coronavirus entry studies [21], [22]. Taken together, our data demonstrated that, similar to non-polarized MDCK cells, influenza virus does not enter polarized MDCK II through caveolae-dependent endocytosis.
Fig. 4.
Influenza virus does not enter polarized MDCK II cells via caveolae-mediated endocytosis. MDCK-CCL34 (MDCK) cells or MDCK II cells were either pretreated with MBCD (A) or nystatin (B) for 30 min before infected with influenza virus. Viral protein M was analyzed with mouse anti-M1 antibody on western blot from infected whole cell lysates. (C) Polarized MDCK II cells were infected with influenza virus after electroporation with caveolin-1 (Cav1) WT plasmid or Cav1-DN plasmid and were analyzed with confocal microscopy with mouse anti-NP antibody. (D) Both transfected and infected cells were quantified from confocal images from three independent experiments.
To our knowledge, this is the first time that endocytic pathways employed by influenza virus in a fully polarized epithelial cell system grown on Transwell filters have been examined. Polarized epithelial cells are usually refractory to transfection and more difficult to work with compared to non-polarized cells. Components in the CME pathway have shown to be regulators of cell polarity [23], [24], so to avoid DN cellular proteins disrupting formation of proper cellular polarity, we chose to introduce the DN constructs to the MDCK II cells after the cells had formed a tight monolayer and were fully polarized. Conventional transfection methods utilizing cationic lipid often yields low transfection efficiency in polarized epithelial cells (data not shown). Adenovirus transduction to introduce recombinant protein into polarized epithelia is another frequently used method. However, adenovirus can induce macropinocytosis and other undesirable signaling events after gaining access into host cell [25], [26], [27], [28].
Dynamin2 and Epn1 DN proteins had a small reduction on the percentage of MDCK II cells infected with influenza virus. This may be due to a masking effect by other endocytic pathways that the virus particles enter through while CME pathway was inhibited (at least partially) by dynamin 2 or Epn1 DN proteins. Unexpectedly, a large portion of polarized MDCK II cells expressing Eps15 DN were not able to be infected by influenza virus (Fig. 3D). Eps15 is an adaptor protein that interacts with Epsin1, AP180, and synaptojanin via the Eps homology (EH) domains and recruits cargo adaptor protein AP-2 to plasma membrane [29]. The inhibitory effect of Eps15-Δ95/295 DN protein on influenza virus entry in polarized MDCK II cells suggests that Eps15 plays an important role for influenza virus entry in the CME pathway. The requirement of functional Eps15 for influenza virus in clathrin-coated pits could involve a different adaptor protein that is associated with Eps15.
In non-polarized cell systems, it is well documented that influenza virus can enter host cells through CME [6], [8]. However, other entry routes, such as clathrin, caveolae-independent routes and macropinocytosis, have also been described [6], [7], suggesting that influenza infectious entry utilizes different endocytic routes in different non-polarized epithelial cell types [30]. By using pharmacological and molecular approaches, our study demonstrates that influenza virus selectively enters polarized MDCK II cells via a clathrin-dependent route.
Acknowledgments
We thank Sandrine Belouzard for helpful advice during the course of this work. This work was supported by a research grant R01 AI48678 (to G.R.W.) from National Institutes of Health. Y.Z. was also supported by grant T32AI007618 (Training in Molecular Virology and Pathogenesis) from the National Institutes of Health.
References
- 1.Sieczkarski S.B., Whittaker G.R. Dissecting virus entry via endocytosis. J. Gen. Virol. 2002;83:1535–1545. doi: 10.1099/0022-1317-83-7-1535. [DOI] [PubMed] [Google Scholar]
- 2.Mercer J., Schelhaas M., Helenius A. Virus entry by endocytosis. Annu. Rev. Biochem. 2010;79:803–833. doi: 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
- 3.Mercer J., Helenius A. Gulping rather than sipping: macropinocytosis as a way of virus entry. Curr. Opin. Microbiol. 2012;15:490–499. doi: 10.1016/j.mib.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Marsh M., Pelchen-Matthews A. Endocytosis in viral replication. Traffic. 2000;1:525–532. doi: 10.1034/j.1600-0854.2000.010701.x. [DOI] [PubMed] [Google Scholar]
- 5.Roy A.M., Parker J.S., Parrish C.R., Whittaker G.R. Early stages of influenza virus entry into Mv-1 lung cells: involvement of dynamin. Virology. 2000;267:17–28. doi: 10.1006/viro.1999.0109. [DOI] [PubMed] [Google Scholar]
- 6.Sieczkarski S.B., Whittaker G.R. Influenza virus can enter and infect cells in the absence of clathrin-mediated endocytosis. J. Virol. 2002;76:10455–10464. doi: 10.1128/JVI.76.20.10455-10464.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Vries E., Tscherne D.M., Wienholts M.J., Cobos-Jimenez V., Scholte F., Garcia-Sastre A., Rottier P.J., de Haan C.A. Dissection of the influenza A virus endocytic routes reveals macropinocytosis as an alternative entry pathway. PLoS Pathog. 2011;7:e1001329. doi: 10.1371/journal.ppat.1001329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C., Zhuang X. Epsin 1 is a cargo-specific adaptor for the clathrin-mediated endocytosis of the influenza virus. Proc. Natl. Acad. Sci. U.S.A. 2008;105:11790–11795. doi: 10.1073/pnas.0803711105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuck S., Simons K. Polarized sorting in epithelial cells: raft clustering and the biogenesis of the apical membrane. J. Cell Sci. 2004;117:5955–5964. doi: 10.1242/jcs.01596. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez Boulan E., Sabatini D.D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc. Natl. Acad. Sci. U.S.A. 1978;75:5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun X., Whittaker G.R. Role of the actin cytoskeleton during influenza virus internalization into polarized epithelial cells. Cell. Microbiol. 2007;9:1672–1682. doi: 10.1111/j.1462-5822.2007.00900.x. [DOI] [PubMed] [Google Scholar]
- 12.Mostov K., Su T., ter Beest M. Polarized epithelial membrane traffic: conservation and plasticity. Nat. Cell Biol. 2003;5:287–293. doi: 10.1038/ncb0403-287. [DOI] [PubMed] [Google Scholar]
- 13.Shaw A.J. Oxford University Press; Oxford: 2002. Epithelial Cell Culture: A Practical Approach. [Google Scholar]
- 14.Deora A.A., Diaz F., Schreiner R., Rodriguez-Boulan E. Efficient electroporation of DNA and protein into confluent and differentiated epithelial cells in culture. Traffic. 2007;8:1304–1312. doi: 10.1111/j.1600-0854.2007.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macia E., Ehrlich M., Massol R., Boucrot E., Brunner C., Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Johannsdottir H.K., Mancini R., Kartenbeck J., Amato L., Helenius A. Host cell factors and functions involved in vesicular stomatitis virus entry. J. Virol. 2009;83:440–453. doi: 10.1128/JVI.01864-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vendeville A., Ravallec M., Jousset F.X., Devise M., Mutuel D., Lopez-Ferber M., Fournier P., Dupressoir T., Ogliastro M. Densovirus infectious pathway requires clathrin-mediated endocytosis followed by trafficking to the nucleus. J. Virol. 2009;83:4678–4689. doi: 10.1128/JVI.02401-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schelhaas M., Ewers H., Rajamaki M.L., Day P.M., Schiller J.T., Helenius A. Human papillomavirus type 16 entry: retrograde cell surface transport along actin-rich protrusions. PLoS Pathog. 2008;4:e1000148. doi: 10.1371/journal.ppat.1000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossman J.S., Leser G.P., Lamb R.A. Filamentous influenza virus enters cells via macropinocytosis. J. Virol. 2012;86:10950–10960. doi: 10.1128/JVI.05992-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mercer J., Helenius A. Virus entry by macropinocytosis. Nat. Cell Biol. 2009;11:510–520. doi: 10.1038/ncb0509-510. [DOI] [PubMed] [Google Scholar]
- 21.Li G.M., Li Y.G., Yamate M., Li S.M., Ikuta K. Lipid rafts play an important role in the early stage of severe acute respiratory syndrome-coronavirus life cycle. Microbes Infect. 2007;9:96–102. doi: 10.1016/j.micinf.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H., Yang P., Liu K., Guo F., Zhang Y., Zhang G., Jiang C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18:290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deborde S., Perret E., Gravotta D., Deora A., Salvarezza S., Schreiner R., Rodriguez-Boulan E. Clathrin is a key regulator of basolateral polarity. Nature. 2008;452:719–723. doi: 10.1038/nature06828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shivas J.M., Morrison H.A., Bilder D., Skop A.R. Polarity and endocytosis: reciprocal regulation. Trends Cell Biol. 2010;20:445–452. doi: 10.1016/j.tcb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amstutz B., Gastaldelli M., Kalin S., Imelli N., Boucke K., Wandeler E., Mercer J., Hemmi S., Greber U.F. Subversion of CtBP1-controlled macropinocytosis by human adenovirus serotype 3. EMBO J. 2008;27:956–969. doi: 10.1038/emboj.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashi S., Hogg J.C. Adenovirus infections and lung disease. Curr. Opin. Pharmacol. 2007;7:237–243. doi: 10.1016/j.coph.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sirena D., Lilienfeld B., Eisenhut M., Kalin S., Boucke K., Beerli R.R., Vogt L., Ruedl C., Bachmann M.F., Greber U.F., Hemmi S. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J. Virol. 2004;78:4454–4462. doi: 10.1128/JVI.78.9.4454-4462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wickham T.J., Mathias P., Cheresh D.A., Nemerow G.R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 29.Salcini A.E., Confalonieri S., Doria M., Santolini E., Tassi E., Minenkova O., Cesareni G., Pelicci P.G., Di Fiore P.P. Binding specificity and in vivo targets of the EH domain, a novel protein–protein interaction module. Genes Dev. 1997;11:2239–2249. doi: 10.1101/gad.11.17.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Conto F., Covan S., Arcangeletti M.C., Orlandini G., Gatti R., Dettori G., Chezzi C. Differential infectious entry of human influenza A/NWS/33 virus (H1N1) in mammalian kidney cells. Virus Res. 2010;155:221–230. doi: 10.1016/j.virusres.2010.10.008. [DOI] [PubMed] [Google Scholar]