Abstract
The mammalian (or mechanistic) target of rapamycin (mTOR) complex 1 (mTORC1) is a serine and threonine kinase that regulates cell growth, survival and proliferation. mTORC1 is a master controller of the translation of a subset of mRNAs. In the central nervous system (CNS), mTORC1 plays a crucial role in mechanisms underlying learning and memory by controlling synaptic protein synthesis. Here, we review recent evidence suggesting that the mTORC1 signaling pathway promotes neuroadaptations following exposure to a diverse group of drugs of abuse including stimulants, cannabinoids, opiates and alcohol. We further describe potential molecular mechanisms by which drug-induced mTORC1 activation may alter brain functions. Finally, we propose that mTORC1 is a focal point shared by drugs of abuse to mediate drug-related behaviors such as reward seeking and excessive drug intake, and offer future directions to decipher the contribution of the kinase to mechanisms underlying addiction.
Introduction
Accumulating evidence in the past decade suggest that the kinase mTORC1 (mammalian/mechanistic Target of Rapamycin in Complex 1) is a critical mediator of protein synthesis (Ma & Blenis 2009) including dendritic translation of synaptic proteins (Costa-Mattioli et al. 2009, Liu-Yesucevitz et al. 2011). Accordingly, mTORC1 plays a major role in molecular mechanisms underlying normal brain functions such as learning and memory (Costa-Mattioli et al. 2009), but also appears to be dysregulated in a number of neurological disorders including epilepsy, Parkinson’s disease and Alzheimer’s disease (Swiech et al. 2008, Hoeffer & Klann 2010, Dazert & Hall 2011, Maiese et al. 2013, Costa-Mattioli & Monteggia 2013).
Addiction is psychiatric disorder that manifests itself as compulsive drug seeking and taking despite detrimental negative consequences, resulting in major health and socioeconomic impacts world-wide (Koob & Volkow 2010, Spanagel 2009). Although drugs of abuse have diverse chemical structures and pharmacological site of actions, they share behavioral phenotypes. Acute drug use is reinforcing, however, prolonged repeated intake may lead to detrimental behaviors such as uncontrolled drug intake, craving, anxiety and relapse (Nestler 2005, Koob & Volkow 2010). The limbic system, consisting of the hippocampus, ventral tegmental area (VTA), striatum and the prefrontal cortex (PFC) plays a key role in circuits that underlie behavioral phenotypes induced by both acute and chronic exposure to drugs of abuse (Koob & Volkow 2010, Spanagel 2009). Recently, a series of studies raised the intriguing possibility that mTORC1 in the limbic system is a central molecular determinant of drug-induced neuroadaptations underlying common adverse behavioral phenotypes.
Here, we first briefly review the key features of mTORC1, its main upstream activators and downstream targets and functions. Next, we describe recent findings converging towards the possibility that neuronal mTORC1 is a focal point where signaling pathways altered by drugs of abuse merge and propose potential mechanisms that may underlie mTORC1’s function in the onset and maintenance of maladaptive neuroadaptations. Finally, we offer new directions of investigation to further decipher the contribution of neuronal mTORC1 kinase in drug-induced synaptic plasticity.
mTOR in Complex 1 (mTORC1)
Structural and functional features of the mTOR complexes
mTOR is a large ubiquitous and evolutionary conserved protein kinase, which belongs to the phosphoinositide 3-kinase (PI3K)-related kinase (PIKK) family (Zoncu et al. 2011). mTOR is an atypical kinase as it signals through two distinct multi-protein complexes mTORC1 and mTOR in complex 2 (mTORC2) (Ma & Blenis 2009, Zoncu et al. 2011). Both mTORC1 and mTORC2 protein complexes contain the DEP domain-containing mTOR-interacting protein (DEPTOR), and the mammalian lethal with SEC13 protein 8 (mLST8 also known as G protein beta-like, GβL) (Zoncu et al. 2011). However, the kinase also associates with distinct adaptor proteins that define the complex. Specifically, the regulatory-associated protein of mTOR (RAPTOR) and the 40 kDa Pro-rich Akt substrate (PRAS40) are specific to mTORC1, whereas the rapamycin-insensitive companion of mTOR (RICTOR), the mammalian stress-activated MAP kinase-interacting protein 1 (mSIN1) and the protein observed with RICTOR (PROTOR) are exclusively found in mTORC2 (Ma & Blenis 2009, Zoncu et al. 2011). Although mTOR bears the same kinase activity in both complexes, the unique multiprotein network encompassing the kinase results in distinct mTORC1 and mTORC2 substrates and cellular functions (Dowling et al. 2010, Sparks & Guertin 2010).
While the role of mTORC2 in addiction is likely to be of interest (Mazei-Robison et al. 2011), this review focuses solely on the role of mTORC1 in neuroadaptations induced by drugs of abuse.
Signaling pathways leading to mTORC1 activation
A wide variety of stimuli including nutrients, stress, energy or growth factors converge at the level of mTORC1 to sense and integrate the complex cellular environment to promote an appropriate cellular response. The main upstream activator of mTORC1 is the ras homolog enriched in brain protein (Rheb) (Hay & Sonenberg 2004, Zoncu et al. 2011). Rheb is a GTP binding protein and, as such, the enzyme cycles between a GTP-active and a GDP-inactive state. The direct interaction between GTP-bound Rheb and mTORC1 leads to the activation of the kinase (Laplante & Sabatini 2012). The cycling of Rheb between active GTP- and inactive GDP-bound states is controlled by a heterodimer composed of the tuberous sclerosis complex 1 (TSC1, also called hamartin) and the tuberous sclerosis complex 2 (TSC2, also called tuberin), which displays a GTPase-activating protein (GAP) activity (Hay & Sonenberg 2004, Kwiatkowski & Manning 2005). Therefore, inhibition of TSC1/TSC2 heterodimer increases the activity of Rheb which subsequently leads to activation of mTORC1 (Laplante & Sabatini 2012).
The activity of TSC2, and consequently the activity of mTORC1, is regulated by phosphorylation. For example, phosphorylation of TSC2 by AMP-activated protein kinase (AMPK) and glycogen synthase kinase 3β (GSK3β) results in GAP activation (and thus in mTORC1 inhibition) (Zoncu et al. 2011). In contrast, extracellular signal-regulated kinases 1/2 (ERK1/2) or its downstream kinase the 90 kDa ribosomal S6 kinase (RSK), as well as AKT (also known as PKB) phosphorylate TSC2 on other distinct sites resulting in the inhibition of TSC2 GAP activity leading to mTORC1 activation (Ma & Blenis 2009, Zoncu et al. 2011, Mendoza et al. 2011).
Downstream effectors and functions of mTORC1
The best-characterized substrates of mTORC1 are the S6 ribosomal kinase 1 (S6K1) and the eukaryotic translation initiation factor-4E binding protein (4E-BP) (Costa-Mattioli et al. 2009, Ma & Blenis 2009). Phosphorylation of S6K1 and 4E-BP controls the initiation and elongation of translation of a subset of mRNAs displaying a 5′ terminal oligopyrimidine (TOP) motif (Zoncu et al. 2011, Thoreen et al. 2012). For instance, mTORC1-induced phosphorylation of 4E-BP allows the interaction of the eukaryotic translation initiation factor 4E (eIF4E) to eIF4G at the 5’ cap structure of mRNAs, a process that is critical for translation initiation (Ma & Blenis 2009). Besides, mTORC1-induced phosphorylation of S6K1 affects translation initiation and elongation by binding or affecting the function of several proteins implicated in these processes such as eukaryotic elongation factor 2 kinase (eEF2K), eIF4B and the S6K1 Aly/REF-like target protein (SKAR) which interacts with the exon junction complex of spliced mRNA (Ma et al. 2008, Zoncu et al. 2011). mTORC1 activation also increases translational rate by stimulating the production of ribosomal proteins and translation factors (Liao et al. 2007), as well as by inducing the transcription of ribosomal RNAs (rRNAs) (Zoncu et al. 2011). Furthermore, mTORC1 activity has also been shown to control the transcription of a subset of metabolic genes by regulating the activity of several transcription factors such as the sterol regulatory element-binding proteins (SREBPs) (Lamming & Sabatini 2013, Laplante & Sabatini 2013) .
In addition to its role in promoting protein synthesis and transcription, mTORC1 is a major regulator of cell autophagy, a cellular process aimed to recycle intracellular organelles following their lysosomal degradation (Harris & Rubinsztein 2012, Wong & Cuervo 2010). mTORC1 activity negatively regulates autophagy by phosphorylating substrates such as unc-51-like kinase 1 (ULK1), autophagy-related 13 (ATG13) or death-associated protein 1 (DAP1) (Hosokawa et al. 2009, Koren et al. 2010). Together, these processes allow the cell to control its metabolism, growth capacity, as well as to sustain cell division and accordingly dysregulated mTORC1 signaling has been highlighted in a number of disorders such as cancer, metabolic disorders, neurological diseases, and immunological diseases (Dazert & Hall 2011, Laplante & Sabatini 2012).
mTORC1 in the central nervous system
In agreement with its role in the regulation of cell growth, neuronal mTORC1 impacts the early steps of neuronal development (Swiech et al. 2008, Jaworski & Sheng 2006), and mTORC1 is thought to be of potential therapeutic interest in the regeneration of neurons after spinal cord injury (Liu et al. 2010, Park et al. 2010, Lu et al. 2012). In the adult brain, mTORC1 is localized in the cell body but also at the dendrites of neurons where it has been shown to contribute to the induction of the late phase of long-term potentiation (Cammalleri et al. 2003, Stoica et al. 2011, Tang et al. 2002). Therefore mTORC1 appears to be a critical mediator of synaptic plasticity mainly due to its capacity to promote local dendritic protein synthesis (Gobert et al. 2008, Swiech et al. 2008, Hoeffer & Klann 2010, Stoica et al. 2011) and thus the kinase participates in neural processes that require synaptic protein production such as learning, and memory. Malfunction of this pathway plays a role in the development of neurodegenerative diseases (Hoeffer & Klann 2010, Swiech et al. 2008) as well as psychiatric disorders including addiction (Costa-Mattioli & Monteggia 2013, Dayas et al. 2012, Jernigan et al. 2011).
Rapamycin: a pharmacologic tool to study mTORC1 function in the brain
Rapamycin was first isolated from a bacteria strain of the Streptomyces genus, which was collected in the Easter Island soil and initially described as an antifungal agent (Vezina et al. 1975). The name “rapamycin” originates “rapa-” which stands for “Rapa Nui”, the native name of the Easter Island, and “–mycin” for its antibiotic properties (Vezina et al. 1975). The immunosuppressant properties of rapamycin were discovered later on and in 1999 the drug was approved by the Food and Drug Administration (FDA) as treatment to prevent renal allograft rejection (Hartford & Ratain 2007).
Rapamycin acts as an allosteric specific inhibitor of mTORC1, which, in complex with FKBP12 protein (FK 506-binding protein of 12 kDa), binds to the FRB (FKBP12-rapamycin binding) domain of the kinase (Dowling et al. 2010, Yip et al. 2010). This interaction is thought to modify mTOR conformation, which, in turn not only weakens the integrity of the kinase complex but also prevents the association of its catalytic site with its substrates (Yip et al. 2010). Recently, an X-ray structure of the N-terminus-truncated mTOR bound to mLST8 revealed an intrinsically active but poorly accessible kinase catalytic cleft whose access is further restricted by the binding of rapamycin-FKBP12 complex to the FRB domain of mTOR (Yang et al. 2013). Rapamycin is a highly valuable pharmacological tool to address the consequence of mTORC1 activation in the brain. Rapamycin crosses the blood-brain barrier, and acutely, the inhibitor does not affect the activity of mTORC2 or other kinases (Davies et al. 2000, Dowling et al. 2010). In addition, systemic administration of rapamycin does not induce rodent behaviors such as anxiety or general locomotion (Blundell et al. 2008, Lin et al. 2014, Neasta et al. 2010). It should be noted that prolonged treatment with rapamycin was also shown to inhibit mTORC2 activity in non-neuronal tissue (Sarbassov et al. 2006, Lamming et al. 2012), an effect that was connected with insulin resistance (Lamming et al. 2012). However, it is not clear whether prolonged treatment with rapamycin inhibits mTORC2 in all brain structures. For instance, Mazei-Robison et al. (2011) found no modification of mTORC2 signaling in the VTA of mice that were administered with rapamycin daily during 6 days (Mazei-Robison et al. 2011).
mTORC1 regulates autophagy in neurons
Autophagy is required for proper cell homeostasis by mediating the turnover of organelles or by clearing misfolded proteins and malfunctioning of this catabolic process has been associated with several neurodegenerative disorders such as Huntington's, Alzheimer’s and Parkinson’s diseases (Harris & Rubinsztein 2012, Wong & Cuervo 2010). Similar to its function in non-neuronal cells, mTORC1 also regulates autophagy in neurons (Harris & Rubinsztein 2012). How regulation of autophagy by mTORC1 impacts normal brain functions is still poorly defined but has been recently related to neurotransmission (Hernandez et al. 2012, Torres & Sulzer 2012), and perhaps to synaptic plasticity (Shehata et al. 2012). From a therapeutic point of view, mTORC1 inhibitors have been reported to improve the symptoms of several neurodegenerative disorders, in part, by increasing the clearance of misfolded toxic proteins (Spilman et al. 2010, Dehay et al. 2010). Therefore, activation of autophagy in neurons using mTORC1 blockers is considered a promising strategy to fill unmet medical needs for patients affected by a number of neurodegenerative diseases (Harris & Rubinsztein 2012, Wong & Cuervo 2010).
mTORC1 and synaptic transmission
While the role of mTORC1 in the mechanisms that underlie neuronal development and synaptic plasticity has been well studied, less is known regarding its potential function in basic synaptic transmission. Recent studies addressed this question by the use of transgenic mice in which mTORC1 signaling was altered, together with rapamycin treatment to inhibit the kinase activity. In FKBP12-deficient mice (i.e., conditional knockout), in which mTOR interaction with RAPTOR is enhanced leading to an increased activity of mTORC1, Hoeffer et al. (2008) found no change in basal synaptic transmission in hippocampal slices (Hoeffer et al. 2008). In contrast, two recent studies reported that mTORC1 activity regulates several features of synaptic transmission at both pre- and postsynaptic levels (Hernandez et al. 2012, Weston et al. 2012). Although the molecular mechanism underlying this process remains largely unidentified, it may rely on autophagy (Hernandez et al. 2012) and/or synapse formation (Weston et al. 2012, Jaworski & Sheng 2006). Importantly, mTORC1 appears to regulate synaptic function in glutamatergic, GABAergic and dopaminergic neurons suggesting that mTORC1 is a universal controller of neurotransmission (Weston et al. 2012, Hernandez et al. 2012).
mTORC1 in learning and memory
Long-lasting forms of synaptic plasticity and memory require de novo protein synthesis (Costa-Mattioli et al. 2009), including specific synthesis at dendrites (Sutton & Schuman 2006). Since mTORC1 controls synaptic protein translation (Hoeffer & Klann 2010), the kinase has been suggested to play an important role in various learning and memory processes. For example, local inhibition of mTORC1 in the rat medial PFC (mPFC) by rapamycin caused a deficit in long-term retention of trace fear memory examined days after conditioning training, whereas short-term trace fear memory and object recognition memory were not affected (Sui et al. 2008). Likewise, rapamycin leads to impairment of spatial memory retrieval but not acquisition in mice when given systemically (Deli et al. 2012) and was shown to impair novel object recognition when infused into the basolateral amygdala or dorsal hippocampus of rats (Jobim et al. 2012b, Myskiw et al. 2008). Moreover, mTORC1 activation was also implicated in the consolidation and reconsolidation of fear- and drug-related memories (Blundell et al. 2008, Gafford et al. 2011, Glover et al. 2010, Mac Callum et al. 2013, Slipczuk et al. 2009, Stoica et al. 2011, Zhu et al. 2011, Lin et al. 2014). For example, mTORC1 inhibition in the hippocampus or the amygdala of rats was shown to impair consolidation and reconsolidation of long-term memory in an inhibitory avoidance-learning task (Bekinschtein et al. 2007, Jobim et al. 2012a, Slipczuk et al. 2009). In addition, mTORC1 inhibition during memory consolidation attenuated long-term taste memory in a conditioned taste aversion (CTA) paradigm, and novel-taste learning induced a correlative activation of mTORC1 in the gustatory cortex (Belelovsky et al. 2009). Taken together, these studies suggest that mTORC1 is critical for memory consolidation, reconsolidation, storage and/or retrieval processes, whereas its role in initial learning (acquisition) is yet to be fully characterized.
Interestingly, mice with an increased mTORC1 activity (FKBP12-deficient mice) displayed enhanced long-term contextual fear memory retention, as well as compulsive-like perseverative behaviors in several tasks including the marble burying assay, object recognition task, Morris water maze, and Y maze reversal task (Hoeffer et al. 2008). However, no difference between FKBP12-deficient and wild type mice was found in short-term contextual memory and in short- and long-term cued memory (Hoeffer et al. 2008). Together, these findings suggest that over activation of the mTORC1 pathway may result in persistent memory and perseverative behaviors.
While the abovementioned studies were conducted using acute rapamycin treatments, it was recently reported that chronic rapamycin, given via food supplementation, enhanced spatial learning and memory as measured in Morris water maze and passive avoidance (Halloran et al. 2012, Spilman et al. 2010). In line with this finding, lifelong rapamycin administration was shown to ameliorate age-dependent spatial learning and memory deficits by reducing interleukin-1 beta and enhancing NMDA signaling (Majumder et al. 2012). Thus, these studies raise the possibility that long-term chronic inhibition of mTORC1 leads to cognitive enhancement and/or repair, in contrast to the memory impairing effect of acute inhibition of the kinase.
Drugs of abuse and mTORC1
Rodent models of drug-induced modifications of behavior
Repeated exposure to drugs of abuse promotes modifications of animal behavior that model certain aspects of addiction (Steketee & Kalivas 2011). For instance, recurring exposure of rodents to drugs of abuse results in sensitization, a progressive enhancement of responsiveness to the drug (Vanderschuren & Pierce 2010). Sensitization is thought to be related in part to adaptive changes in gene expression and synaptic organization induced by repeated activation of the reward/reinforcement system by drugs of abuse (Sanchis-Segura & Spanagel 2006, Vanderschuren & Pierce 2010). Conditioned placed preference (CPP) measures the preference of an animal to a context that was previously associated with a reward and is used to evaluate the reinforcing properties of a drug and reward-seeking behavior (Tzschentke 2007, Sanchis-Segura & Spanagel 2006). Similarly to sensitization, induction and expression of CPP induced by repeated administration of a drug are thought to depend on drug-induced neuroadaptations in relevant brain areas such as the reward circuit (Hyman et al. 2006).
Activation of mTORC1-mediated signaling pathway by drugs of abuse
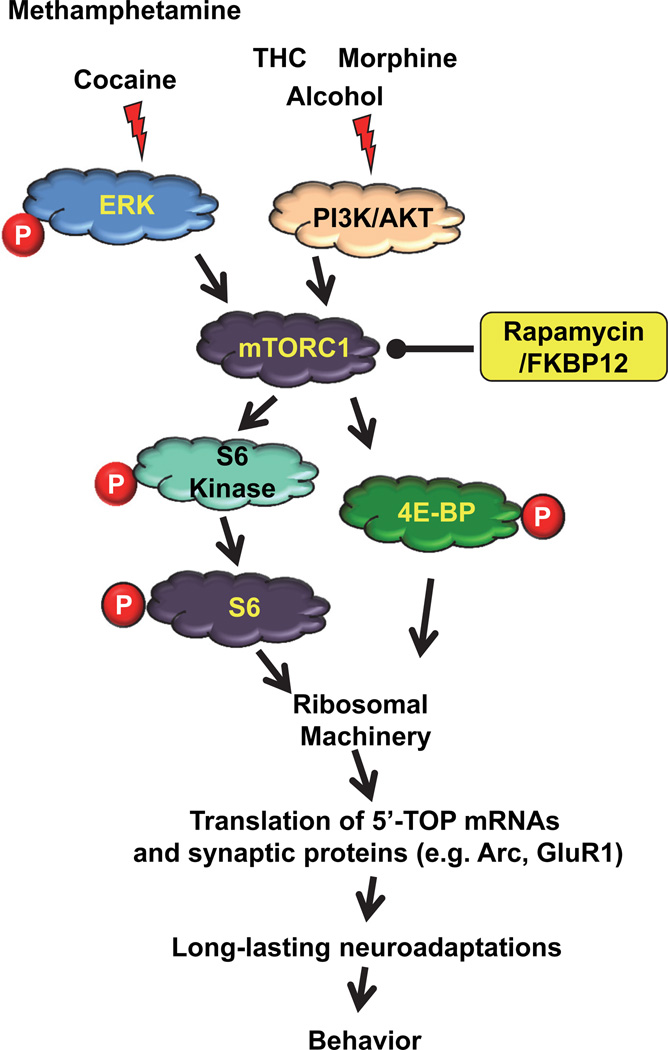
As described above, mTORC1 is known for its role in promoting neuroadaptations that underlie learning and memory processes by stimulating mRNA translation into proteins (Costa-Mattioli et al. 2009, Hoeffer & Klann 2010). Since neural circuitry and molecular mechanisms of learning and memory share similarities with those underlying drug addiction (Hyman et al. 2006), an attractive corollary would be that mTORC1 also orchestrates key signaling events leading to drug-induced maladaptive neuroadaptations. Recently, several studies have evaluated this possibility by examining mTORC1 activity in the brain limbic system following acute exposure to drugs of abuse. For instance, Wu et al. (2011) reported an enhancement in the phosphorylation level of the ribosomal protein S6, a substrate of S6K1, within the NAc, cortex and VTA of rats 1 hour after a single systemic administration of cocaine (Wu et al. 2011). Importantly, this increase was completely abolished by a systemic pre-treatment with the selective mTORC1 inhibitor, rapamycin, indicating that mTORC1 is activated in several brain nuclei that compose the limbic circuit. The activation of mTORC1 appears to be transient, at least in the NAc, as S6K1 and S6 were not phosphorylated 24 hours following acute administration of cocaine (Bailey et al. 2012). In line with this observation, Puighermanal et al. (2009) found that a single systemic administration of Tetrahydrocannabinol (THC) also led to a rapid increase (within 30 min) of mTORC1 activity in the hippocampus before returning to the basal level 4 to 6 hours after drug treatment (Puighermanal et al. 2009). Interestingly, THC-mediated stimulation of mTORC1 is not restricted to hippocampus as an increased activity of the kinase was also detected in the striatum, the frontal cortex and the amygdala (Puighermanal et al. 2013). We showed that a single systemic administration of a non-hypnotic dose of alcohol induced a rapid activation of mTORC1 signaling in the NAc as reflected by an enhancement of the phosphorylation level of its two substrates S6K1 and 4E-BP (Neasta et al. 2010). In contrast to these previous findings and ours, Gonçalves et al. (2012) observed a decrease in mTORC1 activity in the hippocampus of mice 24 hours after an acute dose of methamphetamine (Goncalves et al. 2012), however, the effect of this drug would need to be further evaluated in other brain structures. Nevertheless, at this point, it is striking to note that 3 different classes of drugs of abuse, namely cocaine, THC and alcohol with distinct primary cellular targets in the brain (Hyman et al. 2006) appear to have similar actions on the mTORC1-mediated signaling pathway in the limbic system. This observation therefore raises the possibility that some detrimental behaviors arising following repeated exposure to drugs of abuse may be mediated, at least in part, through mTORC1 activation in key addiction-related brain regions. In agreement with this hypothesis, we observed that excessive alcohol drinking, a hallmark of alcohol abuse (Spanagel 2009), triggers mTORC1 activation in the NAc which lasts at least 24 hours after alcohol withdrawal (Neasta et al. 2010). Likewise, phosphorylation of S6K1 was detected in the NAc 3 days after the last of 5 administrations of methamphetamine (Narita et al. 2005). Similarly, Puighermanal et al. (2013) reported a small but not significant increase of mTORC1 activity in the hippocampus of mice that were chronically administered THC when assessed 3 days after the last drug treatment (Puighermanal et al. 2013). Finally, a 5-day morphine treatment (via subcutaneous pellet) was recently reported to activate mTORC1 signaling in rat VTA (but not in the NAc) as assessed 24 hours after the last morphine pellet implantation (Mazei-Robison et al. 2011). In contrast, Bailey et al. (2012) did not observe mTORC1 activation in the NAc or the PFC of mice when assessed 24 hours after the last cocaine administration (Bailey et al. 2012). Nevertheless, together the studies above suggest that a variety of drugs produce acute but also long-lasting activation of the mTORC1 signaling pathway (Figure 1).
Figure 1.
A graphical model depicting the signaling cascades that are known to activate mTORC1 in response to exposure to drugs of abuse and a possible mechanism by which mTORC1 serves as a central player in drugs of abuse-dependent neuroadaptations that underlie phenotypes of drug addiction such as drug seeking and relapse.
Possible molecular mechanisms underlying mTORC1 activation by drugs of abuse
The potential role of PI3K and AKT kinases in THC, morphine and alcohol-mediated mTORC1 activation
mTORC1 is a downstream target of both ERK1/2 and AKT signaling (Mendoza et al. 2011). Acute exposure to psychostimulants, THC and opiates resulted in ERK1/2 activation in the NAc (Valjent et al. 2004, Valjent et al. 2001). In contrast, acute systemic administration and excessive intake of alcohol activate the PI3K/AKT signaling cascade within the NAc (Cozzoli et al. 2009, Ron & Messing 2011, Neasta et al. 2011). THC administration induced both ERK1/2 (Derkinderen et al. 2003) and PI3K/AKT (Ozaita et al. 2007) activation along with the phosphorylation of S6K1 in the hippocampus (Puighermanal et al. 2009). However, THC-induced phosphorylation of S6K1 on threonine 389 (a marker of mTORC1 activity) was not blocked by SL327, a selective inhibitor of ERK1/2 (Puighermanal et al. 2009). Although Puighermanal et al. (2009) did not test the consequence of PI3K and/or AKT inhibition on S6K1 phosphorylation, this finding implies that THC-induced mTORC1 activation is likely to result from the activation of the PI3K/AKT cascade. It is plausible however that ERK1/2 cooperates with PI3K/AKT/mTORC1 to activate S6K1 and to induce mRNA translation (Puighermanal et al. 2009). The contribution of the PI3K/AKT pathway to mTORC1 signaling has also been suggested for morphine. Specifically, Cui et al. (2010) showed that local infusion of the PI3K inhibitor, LY294002, within the hippocampus blocked mTORC1 activation observed following morphine place preference (Cui et al. 2010), although it is noteworthy to point out that the lack of mTORC1 activation could not be directly due to PI3K inhibition but rather to the fact that animals did not respond to the drug-paired context as they did not express morphine place preference. Finally, we generated indirect evidence suggesting that mTORC1 activation in response to alcohol exposure is the result of the activation of the PI3K/AKT pathway. Specifically, we showed that acute systemic administration and recurring cycles of voluntary consumption of alcohol led to the activation of the small G protein H-Ras, the upstream activator of the PI3K/AKT signaling, in the NAc (Ben Hamida et al. 2012, Neasta et al. 2011). The same alcohol exposure paradigms resulted in the activation of PI3K/AKT but not ERK1/2 in the NAc (Neasta et al. 2011). Further studies are needed however to confirm that H-Ras/PI3K/AKT cascade is indeed upstream of mTORC1 in the NAc in response to alcohol. Nevertheless, together these studies strongly suggest that the PI3K/AKT pathway is likely to contribute to mTORC1 activation in several limbic structures following exposure to THC, morphine and alcohol (Figure 1).
The potential role of ERK1/2 kinases in cocaine-mediated mTORC1 activation
Several studies suggest that ERK1/2 activation may be necessary for cocaine-mediated induction of mTORC1 signaling. First, acute administration of cocaine induces a rapid activation of both ERK1/2 (Valjent et al. 2000, Valjent et al. 2004, Girault et al. 2007) and mTORC1 (Wu et al. 2011) in limbic structures. Secondly, activation of both ERK1/2 (Girault et al. 2007, Lu et al. 2006) and mTORC1 (Bailey et al. 2012, Wang et al. 2010b, Wu et al. 2011) in the limbic system promote neuroadaptations that underlie certain behavioral alterations induced by repeated cocaine exposure. Finally, the activation of ERK1/2 (Lu et al. 2006, Miller & Marshall 2005) and mTORC1 (Wang et al. 2010b) is specifically localized to the NAc core of animals exposed to a cocaine-paired cue. This tight connection raises the possibility that ERK1/2 signaling activation is necessary for mTORC1 by cocaine (Figure 1).
The potential role of NMDA receptor in drug-mediated mTORC1 signaling
Several studies have shown that mTORC1 signaling can be triggered in response to activation of the NMDA receptor (Cammalleri et al. 2003, Gong et al. 2006) whose activity is an important mediator of cocaine and alcohol-dependent behaviors (Stuber et al. 2010, Wang et al. 2007, Wang et al. 2010a). Accordingly, Wang et al. (2010) proposed that cocaine-paired cue activates mTORC1 through NMDA receptor stimulation (Wang et al. 2010b). Furthermore, inhibition of the NMDA receptor with MK801, a potent noncompetitive antagonist, prevented the activation of mTORC1 in the hippocampus following THC administration (Puighermanal et al. 2009). In contrast, acute alcohol exposure, which has been shown to be a potent inhibitor of NMDA receptor function (Lovinger et al. 1989, Ron & Wang 2009) promotes mTORC1 activation (Neasta et al. 2010), and interestingly, the rapid antidepressant effect of ketamine, a noncompetitive NMDA receptor antagonist, is thought to be mediated through mTORC1 stimulation (Autry et al. 2011, Li et al. 2010). Therefore, it is likely that drugs of abuse activate mTORC1 through different but cross-talking mechanisms.
mTORC1 and drug-related behaviors
As mentioned above, sensitization and CPP paradigms were used to uncover the functional role of mTORC1 in alterations of animal behavior following repeated drug exposure (Table 1). Wu et al. (2011) showed that when rapamycin is administered to rats along with cocaine during the acquisition phase, animals did not express locomotor sensitization when tested after 2 weeks of withdrawal (Wu et al. 2011). In contrast, Bailey et al. (2012) did not observe that rapamycin affects the acquisition of cocaine sensitization in mice (Bailey et al. 2012). Therefore, the functional role of mTORC1 in the mechanisms that promote long-lasting neuroadaptations that are necessary for the acquisition of cocaine sensitization needs to be further clarified. However, systemic administration of rapamycin for 4 days (Wu et al. 2011) or only 1 hour (Bailey et al. 2012) prior to the challenge drug administration, was found to inhibit the expression of cocaine locomotor sensitization in both rats and mice. In line with these findings, we previously found that a single systemic administration of rapamycin attenuates the expression of alcohol-induced locomotor sensitization (Neasta et al. 2010), although rapamycin did not affect the locomotor hyperactivity typically observed following acute systemic administration of cocaine or alcohol (Bailey et al. 2012, Neasta et al. 2010), suggesting that mTORC1 activation is specifically implicated in neural plasticity underlying the expression of cocaine and alcohol locomotor sensitization, but not in the acute locomotor effect of these substances. Finally, systemic mTORC1 inhibition decreased the expression of cocaine and alcohol place preference but not the acquisition of cocaine place preference (Bailey et al. 2012, Neasta et al. 2010). Altogether, these data indicate that mTORC1 regulates certain but not all neural processes underlying drug-related behaviors and further suggest that local infusion of rapamycin in specific brain structures is necessary to clarify the contribution of the kinase to these processes.
Table 1. Summary of rapamycin effects on drug-related behaviors.
i.p. denotes intraperitoneal, ↓ denotes an inhibition.
| Substance of abuse |
Animal model |
Rapamycin administration |
Effect of rapamycin on drug-related behaviors | References |
|---|---|---|---|---|
| Alcohol | Mice | i.p. | ↓ Alcohol consumption | Neasta et al, 2010 |
| ↓ Expression of locomotor sensitization | ||||
| ↓ Expression of CPP | ||||
| Rats | i.p. | ↓ Alcohol seeking and consumption in operant procedure | ||
| Intra-NAc | ↓ Consumption in two-bottle choice procedure | |||
| Intra-NAc | ↓ Consumption in operant procedure | unpublished data | ||
| i.p. and intra-CeA | ↓ Reconsolidation of alcohol-associated memories | Barak et al, 2013 | ||
| Cocaine | Mice | i.p. | ↓ Expression of locomotor sensitization | Bailey et al, 2012 |
| ↓ Expression of CPP | ||||
| No change on acquisition of locomotor sensitization | ||||
| No change on acquisition of CPP | ||||
| Rats | i.p. | ↓ Acquisition of locomotor sensitization | Wu et al, 2011 | |
| ↓ Expression of locomotor sensitization | ||||
| i.p. | ↓ Reconsolidation of cocaine-associated memories | Lin et al, 2014 | ||
| Intra-NAc core | ↓ Cue-induced reinstatement of cocaine seeking | Wang et al, 2010 | ||
| Intra-NAc shell | No change on cue-induced reinstatement of cocaine seeking | |||
| Morphine | Rats | Intra-hippocampal | ↓ Acquisition of CPP | Cui et al, 2011 |
| i.p. | ↓ Reconsolidation of morphine-associated memories | Lin et al, 2014 | ||
| Methamphetamine | Rats | Intra-NAc | ↓ Acquisition of sensitization | Narita et al, 2005 |
| No change on acquisition of CPP |
As a complementary approach to systemic administration of rapamycin, local infusion of this inhibitor in relevant brain regions has been used to explore the functional relevance of the local increase in mTORC1 activity following exposure to drugs of abuse (Table 1). Specifically, intra-hippocampal infusion of rapamycin prior to morphine administration blocked the acquisition of CPP along with mTORC1 activation in rats (Cui et al. 2010). In contrast, intra-NAc infusion of rapamycin only affected the acquisition of sensitization elicited by methamphetamine but not the acquisition of methamphetamine CPP (Narita et al. 2005). Together, these data suggest that focal activation of mTORC1 rather than a global increase kinase activity is sufficient to elicit drug-related behaviors. Thus, in order to avoid discrepancy between studies, special attention must be paid to accurately locate the subregions where drugs trigger mTORC1 activation, and in parallel determine whether local administration of rapamycin is sufficient to inhibit both mTORC1 activation and drug-related behaviors.
Furthermore, mTORC1 also contributes to mechanisms that underlie alcohol and cocaine-seeking and intake. Specifically, systemic (Neasta et al. 2010) or intra-NAc (unpublished observation) administration of rapamycin reduces alcohol seeking and intake in a rat operant self-administration procedure. Likewise, a single systemic administration of rapamycin also reduced voluntary excessive alcohol drinking in the home cage of both mice and rats, and similar results were obtained when rapamycin was infused directly into the NAc of rats (Neasta et al. 2010). Finally, focal inhibition of mTORC1 within the NAc core was shown to suppress cue-induced reinstatement of cocaine seeking (Wang et al. 2010b). Together, these data suggest that mTORC1 plays an important role in a number of drug-related behaviors.
Finally, it is important to note that the inhibition of mTORC1 by rapamycin did not produce nonspecific modifications of rodent behavior. Specifically, we showed that rapamycin is neither rewarding nor aversive per se as reflected in lack of place preference or aversion to this drug in mice and rats (Neasta et al. 2010, Barak et al. 2013). Moreover, systemic mTORC1 inhibition did not affect the motivation of rats to obtain a natural reward, and did not affect taste palatability, locomotor activity and coordination in mice (Neasta et al. 2010, Barak et al. 2013). Together, these studies suggest that mTORC1 activation plays a unique role in drug intake and addiction-related behaviors, rather than in general reward or non-specific behaviors.
mTORC1 and drug-associated memory
The essential role of mTORC1 in mechanisms that underlie drug-associated memories is strengthened by evidence showing that exposure to a drug context or drug-related cues also activate this pathway. Specifically, Cui et al. (2010) reported that mTORC1 is activated in the hippocampus of animals exposed to a morphine-paired compartment following acquisition of morphine place preference (Cui et al. 2010). Although this study did not rule out the possibility that acquisition of morphine CPP induced a long-lasting activation of mTORC1 in the hippocampus, the data indicate that a mere re-exposure of animals to a context associated with the drug leads to mTORC1 activation. Consistent with this hypothesis, Wang et al. (2010) found that exposure of animals to a cocaine-related cue activated mTORC1 signaling pathway focally within the NAc core (Wang et al. 2010b). Recently, we found that mTORC1 is activated following retrieval of alcohol-associated memories in rats with a history of excessive alcohol intake, specifically in the central nucleus of the amygdala (CeA) and in the prelimbic (PrL) and orbitofrontal (OFC) regions of the prefrontal cortex (Barak et al. 2013). The alcohol-associated memories were retrieved after a period of abstinence by re-exposure of the rats to the alcohol-associated context (operant chambers), as well as presentation of a small, non-pharmacologically active quantity of alcohol, serving as an odor-taste cue (Barak et al. 2013). Interestingly, when the memory was retrieved solely by the odor-taste cue (i.e., in the home cage), mTORC1 was activated exclusively in the CeA, but not in the cortical regions (Barak et al. 2013), suggesting that activation of the kinase in the PrL and OFC plays a role in retrieval of alcohol-associated contextual and instrumental cues that are specific for the operant chamber. Importantly, countering this memory retrieval-induced mTORC1 activation, by systemic or CeA inhibition of mTORC1 immediately after memory retrieval, disrupted the reconsolidation of alcohol-associated memories, leading to a long-lasting suppression of relapse (Barak et al. 2013). Together, these data indicate that mTORC1 plays a crucial role in the retrieval and reconsolidation of alcohol and drug-related memories. Because relapse to drug seeking and consumption is frequently caused by the retrieval of drug-associated memories, these findings strongly suggest that the mTORC1 pathway contributes to the neurobiological processes underlying relapse after a period of abstinence.
mTORC1-dependent protein translation – a potential mechanism underlying the actions of drugs of abuse
As reviewed above, the best-characterized substrates of mTORC1 are S6K1 and 4E-BP, proteins that are part of the translation initiation machinery, the rate-limiting step of protein synthesis (Costa-Mattioli et al. 2009, Ma & Blenis 2009). In neurons, S6K1 and 4E-BP are expressed in the cell body and at the synapse (Schratt et al. 2004, Tang et al. 2002), where de novo protein synthesis is a key process that contributes to the molecular mechanisms underlying long-lasting neuroadaptations (Costa-Mattioli et al. 2009, Liu-Yesucevitz et al. 2011). Therefore, it is plausible that mTORC1 contributes to the mechanisms underlying drug effects, at least in part, by stimulating translation initiation of synaptic 5’ TOP mRNAs (Gobert et al. 2008, Thoreen et al. 2012). Accordingly, Puighermanal et al. (2009) data suggest that THC-mediated activation of the translation initiation machinery in the hippocampus relies, at least in part, on mTORC1 activation (Puighermanal et al. 2009). However, identification of proteins whose translation is controlled by mTORC1 following exposure to drugs of abuse is only now starting to unravel. We recently found that the protein levels of scaffolding protein Homer (Szumlinski et al. 2006) , whose translation was shown to be mTORC1-dependent (Schratt et al. 2004), was up-regulated in the NAc of rodents that consumed large amounts of alcohol (Neasta et al. 2010). Interestingly, the increase in the level of Homer was detected even 24 hours after withdrawal, and importantly, alcohol-mediated increase of Homer was not observed in animals that were pre-treated with rapamycin (Neasta et al. 2010). Other potential downstream effectors of mTORC1 are the subunits of the AMPA receptor. In fact, GluR1 and GluR2 translations were reported to depend on mTORC1 (Mameli et al. 2007, Slipczuk et al. 2009). Regarding GluR1, we observed that voluntary consumption of alcohol led to a robust increase in the immunoreactivty of GluR1, which was also maintained 24 hours after withdrawal (Neasta et al. 2010). Furthermore, more recently we found that retrieval of alcohol-associated memories in rats with a history of excessive alcohol intake resulted in an mTORC1-dependent increase in the protein levels of the activity-regulated cytoskeleton-associated protein (Arc) (Takei et al. 2004), in the amygdala, OFC and mPFC (Barak et al. 2013). Interestingly, retrieval of alcohol-associated memories produced an increased levels of the protein levels of GluR1 as well as postsynaptic protein 95 (PSD-95), whose translation was also shown to be mTORC1 dependent (Lee et al. 2005), in the amygdala and OFC (Barak et al. 2013). Together these studies imply that mTORC1 mediates drug-related maladaptive neuroadaptations, at least in part, by increasing the translation rate and thus the expression level of a subset of proteins that play a crucial role in synaptic plasticity (Figure 1). Identifying such downstream mTORC1 effectors is of particular interest not only to obtain new insights about the molecular basis of maladaptive neuroadaptations but also more generally to understand the means by which mTORC1 regulates neuronal function and plasticity.
Summary and future directions
An intriguing paradox in the neurobiological basis of addiction is the fact that recurring exposure to chemically diverse substances such as drugs of abuse can induce similar debilitating behaviors. As reviewed here, recent findings suggest that mTORC1 kinase in the limbic system could be a focal point of the distinct signaling cascades altered by drugs of abuse following binding to their respective brain targets (Figure 1). This possibility stems from evidence showing that whereas substances of abuse as diverse as cocaine, THC and alcohol that acutely trigger disparate or intersecting signaling events, all appear to activate mTORC1 in several brain regions. Secondly, inhibiting drug-mediated mTORC1 stimulation with rapamycin blocked the development and expression of several drug-related behaviors in both mice and rats. Thus, and as proposed in other tissues (Zoncu et al. 2011), neuronal mTORC1 seems to be capable of being activated via diverse stimuli and it is tempting to speculate that mTORC1-mediated downstream responses, that need to be defined, are then common mechanisms of actions that are shared by several drugs of abuse. We therefore propose that mTORC1 is potentially a common focal point of neuronal signaling that governs drug-induced neuroplasticity (Figure 1). Importantly, once set up, these neuroadaptations appear to be labile or at least may be inhibited. This is suggested by the fact that, even after being acquired, drug-related behaviors are still sensitive to mTORC1 inhibition by rapamycin (Figure 1). Therefore, the molecular mechanisms that underlie the stabilization and/or the expression of these adverse behaviors probably rely, at least in part, on mTORC1 signaling 9Figure 1). This latter hypothesis obviously makes mTORC1 and its downstream effectors very valuable targets for the treatment of symptoms that are shared among drug and alcohol addiction.
However, many remaining questions need to be addressed. For example, it is plausible that activation of mTORC1 by drugs of abuse also affects neuronal function via translational-independent mechanisms, e.g., phosphorylation of relevant substrates and transcription. Global phosphoproteomics approaches were recently implemented to identify rapamycin-sensitive phosphorylation of proteins although these were carried out using non-neuronal cells (Hsu et al. 2011, Yu et al. 2011, Demirkan et al. 2011). Therefore, a global analysis aimed to identify mTORC1 substrates in neuronal cells, especially at the synaptic level, would be of great interest for a better understanding of mTORC1 function in the nervous system in general and in addiction in particular. Furthermore, as mentioned above, mTORC1 activity has been linked to rRNA transcription (Zoncu et al. 2011), and the expression of numerous metabolic genes is controlled by mTORC1 (Lamming & Sabatini 2013, Laplante & Sabatini 2013). These processes are important for non-neuronal cell growth and cycle, but could also be implicated in neuronal structural changes that are observed in several brain structures after repeated exposure to drugs (Russo et al. 2010). Thus far, only few studies have explored the role of mTORC1 in regulating the transcription of genes in neurons (Domanskyi et al. 2011, Jaworski & Sheng 2006). Therefore, the possible transcriptional contribution of mTORC1 in neuroplasticity induced by drugs of abuse needs further investigation.
Another critical question is whether mTORC1 activation is a direct consequence of the drug binding to its receptor, and an example to this possibility is the opioid peptide, DAMGO, activating mTORC1 in cells that heterologously express the mu opioid receptor (Polakiewicz et al. 1998). However, mTORC1 can also be activated in response to animal exposure to a drug-paired context (i.e., in absence of the drug) (Cui et al. 2010, Wang et al. 2010b, Barak et al. 2013) suggesting that, in certain cases, the binding of the drug to its target is not directly responsible of mTORC1 activation. Furthermore and as reviewed above, Puighermanal et al. (2009) proposed that binding of THC to the cannabinoid receptor inhibits GABAergic interneurons which consequently leads to an increased release of glutamate in the hippocampus, and that the subsequent activation of the NMDA receptor results in the activation of mTORC1 signaling (Puighermanal et al. 2009). In addition, dopamine neurotransmission has been linked to mTORC1 signaling in the striatum (Santini et al. 2009). As all drugs of abuse acutely increase extracellular dopamine levels in the NAc (Nestler 2005, Hyman et al. 2006), it would be of great interest to test whether drug-induced mTORC1 activation in the NAc depends on the dopaminergic system. Finally, the neurotrophin brain-derived neurotrophic factor (BDNF), which plays a crucial role in drug-induced synaptic plasticity in the limbic system (Autry & Monteggia 2012) regulates the translation of several synaptic proteins through an mTORC1-dependent mechanism (Takei et al. 2004, Schratt et al. 2004). Therefore, it would be also of interest to test whether activation of mTORC1 in certain limbic structures in response to drugs of abuse is a consequence of BDNF signaling.
In conclusion, the recent evidence indicating that mTORC1 kinase is an important contributor to mechanisms underlying drug-related behaviors open new areas of investigation in the neurobiology of addiction and its treatment. However, the fact mTORC1 controls a wide number of vital processes in all organs and that rapamycin and its derivatives are immunosuppressant and promote insulin resistance are obviously an issue for their long-term use to treat addiction. Therefore, identifying the downstream effectors and the detailed mechanisms by which mTORC1 specifically mediate drug-behavioral plasticity will offer potential new candidates and pharmaceutical strategies to help individual to cope with drug addiction.
Acknowledgements
The authors thank Drs. Vincent Warnault, Jacob Beckley, Emmanuel Darcq and Virginia Long for their contributions. This work was supported by NIAAA P50 AA012870 (DR).
References
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacological reviews. 2012;64:238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J, Ma D, Szumlinski KK. Rapamycin attenuates the expression of cocaine-induced place preference and behavioral sensitization. Addict Biol. 2012;17:248–258. doi: 10.1111/j.1369-1600.2010.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak S, Liu F, Ben Hamida S, Yowell QV, Neasta J, Kharazia V, Janak PH, Ron D. Disruption of alcohol-related memories by mTORC1 inhibition prevents relapse. Nat Neurosci. 2013;16:1111–1117. doi: 10.1038/nn.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Katche C, Slipczuk LN, Igaz LM, Cammarota M, Izquierdo I, Medina JH. mTOR signaling in the hippocampus is necessary for memory formation. Neurobiol Learn Mem. 2007;87:303–307. doi: 10.1016/j.nlm.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Belelovsky K, Kaphzan H, Elkobi A, Rosenblum K. Biphasic activation of the mTOR pathway in the gustatory cortex is correlated with and necessary for taste learning. J Neurosci. 2009;29:7424–7431. doi: 10.1523/JNEUROSCI.3809-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Hamida S, Neasta J, Lasek AW, Kharazia V, Zou M, Carnicella S, Janak PH, Ron D. The small G protein H-Ras in the mesolimbic system is a molecular gateway to alcohol-seeking and excessive drinking behaviors. J Neurosci. 2012;32:15849–15858. doi: 10.1523/JNEUROSCI.2846-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell J, Kouser M, Powell CM. Systemic inhibition of mammalian target of rapamycin inhibits fear memory reconsolidation. Neurobiol Learn Mem. 2008;90:28–35. doi: 10.1016/j.nlm.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammalleri M, Lutjens R, Berton F, King AR, Simpson C, Francesconi W, Sanna PP. Time-restricted role for dendritic activation of the mTOR-p70S6K pathway in the induction of late-phase long-term potentiation in the CA1. Proc Natl Acad Sci U S A. 2003;100:14368–14373. doi: 10.1073/pnas.2336098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Monteggia LM. mTOR complexes in neurodevelopmental and neuropsychiatric disorders. Nat Neurosci. 2013;16:1537–1543. doi: 10.1038/nn.3546. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Goulding SP, Zhang PW, et al. Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. J Neurosci. 2009;29:8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Zhang XQ, Xin WJ, Jing J, Liu XG. Activation of phosphatidylinositol 3-kinase/Akt-mammalian target of Rapamycin signaling pathway in the hippocampus is essential for the acquisition of morphine-induced place preference in rats. Neuroscience. 2010;171:134–143. doi: 10.1016/j.neuroscience.2010.08.064. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Smith DW, Dunkley PR. An emerging role for the Mammalian target of rapamycin in "pathological" protein translation: relevance to cocaine addiction. Front Pharmacol. 2012;3:13. doi: 10.3389/fphar.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazert E, Hall MN. mTOR signaling in disease. Curr Opin Cell Biol. 2011;23:744–755. doi: 10.1016/j.ceb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Dehay B, Bove J, Rodriguez-Muela N, Perier C, Recasens A, Boya P, Vila M. Pathogenic lysosomal depletion in Parkinson's disease. J Neurosci. 2010;30:12535–12544. doi: 10.1523/JNEUROSCI.1920-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deli A, Schipany K, Rosner M, Hoger H, Pollak A, Li L, Hengstschlager M, Lubec G. Blocking mTORC1 activity by rapamycin leads to impairment of spatial memory retrieval but not acquisition in C57BL/6J mice. Behav Brain Res. 2012;229:320–324. doi: 10.1016/j.bbr.2012.01.017. [DOI] [PubMed] [Google Scholar]
- Demirkan G, Yu K, Boylan JM, Salomon AR, Gruppuso PA. Phosphoproteomic profiling of in vivo signaling in liver by the mammalian target of rapamycin complex 1 (mTORC1) PLoS One. 2011;6:e21729. doi: 10.1371/journal.pone.0021729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkinderen P, Valjent E, Toutant M, Corvol JC, Enslen H, Ledent C, Trzaskos J, Caboche J, Girault JA. Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus. J Neurosci. 2003;23:2371–2382. doi: 10.1523/JNEUROSCI.23-06-02371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanskyi A, Geissler C, Vinnikov IA, Alter H, Schober A, Vogt MA, Gass P, Parlato R, Schutz G. Pten ablation in adult dopaminergic neurons is neuroprotective in Parkinson's disease models. FASEB J. 2011;25:2898–2910. doi: 10.1096/fj.11-181958. [DOI] [PubMed] [Google Scholar]
- Dowling RJ, Topisirovic I, Fonseca BD, Sonenberg N. Dissecting the role of mTOR: Lessons from mTOR inhibitors. Biochim Biophys Acta. 2010;1804:433–439. doi: 10.1016/j.bbapap.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Gafford GM, Parsons RG, Helmstetter FJ. Consolidation and reconsolidation of contextual fear memory requires mammalian target of rapamycin-dependent translation in the dorsal hippocampus. Neuroscience. 2011;182:98–104. doi: 10.1016/j.neuroscience.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Glover EM, Ressler KJ, Davis M. Differing effects of systemically administered rapamycin on consolidation and reconsolidation of context vs. cued fear memories. Learn Mem. 2010;17:577–581. doi: 10.1101/lm.1908310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert D, Topolnik L, Azzi M, Huang L, Badeaux F, Desgroseillers L, Sossin WS, Lacaille JC. Forskolin induction of late-LTP and up-regulation of 5’ TOP mRNAs translation via mTOR, ERK, and PI3K in hippocampal pyramidal cells. J Neurochem. 2008;106:1160–1174. doi: 10.1111/j.1471-4159.2008.05470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves J, Baptista S, Olesen MV, Fontes-Ribeiro C, Malva JO, Woldbye DP, Silva AP. Methamphetamine-induced changes in the mice hippocampal neuropeptide Y system: implications for memory impairment. J Neurochem. 2012;123:1041–1053. doi: 10.1111/jnc.12052. [DOI] [PubMed] [Google Scholar]
- Gong R, Park CS, Abbassi NR, Tang SJ. Roles of glutamate receptors and the mammalian target of rapamycin (mTOR) signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J Biol Chem. 2006;281:18802–18815. doi: 10.1074/jbc.M512524200. [DOI] [PubMed] [Google Scholar]
- Halloran J, Hussong S, Burbank R, et al. Chronic inhibition of mTOR by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience. 2012;223:102–113. doi: 10.1016/j.neuroscience.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H, Rubinsztein DC. Control of autophagy as a therapy for neurodegenerative disease. Nat Rev Neurol. 2012;8:108–117. doi: 10.1038/nrneurol.2011.200. [DOI] [PubMed] [Google Scholar]
- Hartford CM, Ratain MJ. Rapamycin: something old, something new, sometimes borrowed and now renewed. Clin Pharmacol Ther. 2007;82:381–388. doi: 10.1038/sj.clpt.6100317. [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Hernandez D, Torres CA, Setlik W, et al. Regulation of presynaptic neurotransmission by macroautophagy. Neuron. 2012;74:277–284. doi: 10.1016/j.neuron.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Tang W, Wong H, et al. Removal of FKBP12 enhances mTOR-Raptor interactions, LTP, memory, and perseverative/repetitive behavior. Neuron. 2008;60:832–845. doi: 10.1016/j.neuron.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa N, Hara T, Kaizuka T, et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PP, Kang SA, Rameseder J, et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332:1317–1322. doi: 10.1126/science.1199498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jaworski J, Sheng M. The growing role of mTOR in neuronal development and plasticity. Mol Neurobiol. 2006;34:205–219. doi: 10.1385/MN:34:3:205. [DOI] [PubMed] [Google Scholar]
- Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, Karolewicz B. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2011;35:1774–1779. doi: 10.1016/j.pnpbp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobim PF, Pedroso TR, Christoff RR, Werenicz A, Maurmann N, Reolon GK, Roesler R. Inhibition of mTOR by rapamycin in the amygdala or hippocampus impairs formation and reconsolidation of inhibitory avoidance memory. Neurobiol Learn Mem. 2012a;97:105–112. doi: 10.1016/j.nlm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Jobim PF, Pedroso TR, Werenicz A, Christoff RR, Maurmann N, Reolon GK, Schroder N, Roesler R. Impairment of object recognition memory by rapamycin inhibition of mTOR in the amygdala or hippocampus around the time of learning or reactivation. Behav Brain Res. 2012b;228:151–158. doi: 10.1016/j.bbr.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren I, Reem E, Kimchi A. Autophagy gets a brake: DAP1, a novel mTOR substrate, is activated to suppress the autophagic process. Autophagy. 2010;6:1179–1180. doi: 10.4161/auto.6.8.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski DJ, Manning BD. Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum Mol Genet. 2005;14 Spec No. 2:R251–R258. doi: 10.1093/hmg/ddi260. [DOI] [PubMed] [Google Scholar]
- Lamming DW, Sabatini DM. A Central Role for mTOR in Lipid Homeostasis. Cell Metab. 2013;18:465–469. doi: 10.1016/j.cmet.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Katajisto P, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci. 2013;126:1713–1719. doi: 10.1242/jcs.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Huang CC, Wu MY, Hsu KS. Insulin stimulates postsynaptic density-95 protein translation via the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway. J Biol Chem. 2005;280:18543–18550. doi: 10.1074/jbc.M414112200. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao L, Pilotte J, Xu T, Wong CC, Edelman GM, Vanderklish P, Yates JR., 3rd BDNF induces widespread changes in synaptic protein content and up-regulates components of the translation machinery: an analysis using high-throughput proteomics. J Proteome Res. 2007;6:1059–1071. doi: 10.1021/pr060358f. [DOI] [PubMed] [Google Scholar]
- Lin J, Liu L, Wen Q, Zheng C, Gao Y, Peng S, Tan Y, Li Y. Rapamycin prevents drug seeking via disrupting reconsolidation of reward memory in rats. Int J Neuropsychopharmacol. 2014;17:127–136. doi: 10.1017/S1461145713001156. [DOI] [PubMed] [Google Scholar]
- Liu-Yesucevitz L, Bassell GJ, Gitler AD, Hart AC, Klann E, Richter JD, Warren ST, Wolozin B. Local RNA translation at the synapse and in disease. J Neurosci. 2011;31:16086–16093. doi: 10.1523/JNEUROSCI.4105-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Lu Y, Lee JK, et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Lu P, Wang Y, Graham L, et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- Ma XM, Yoon SO, Richardson CJ, Julich K, Blenis J. SKAR links pre-mRNA splicing to mTOR/S6K1-mediated enhanced translation efficiency of spliced mRNAs. Cell. 2008;133:303–313. doi: 10.1016/j.cell.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Mac Callum PE, Hebert M, Adamec RE, Blundell J. Systemic inhibition of mTOR kinase via rapamycin disrupts consolidation and reconsolidation of auditory fear memory. Neurobiol Learn Mem. 2013 doi: 10.1016/j.nlm.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC, Wang S. mTOR: on target for novel therapeutic strategies in the nervous system. Trends Mol Med. 2013;19:51–60. doi: 10.1016/j.molmed.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder S, Caccamo A, Medina DX, Benavides AD, Javors MA, Kraig E, Strong R, Richardson A, Oddo S. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1beta and enhancing NMDA signaling. Aging Cell. 2012;11:326–335. doi: 10.1111/j.1474-9726.2011.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Balland B, Lujan R, Luscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- Mazei-Robison MS, Koo JW, Friedman AK, et al. Role for mTOR signaling and neuronal activity in morphine-induced adaptations in ventral tegmental area dopamine neurons. Neuron. 2011;72:977–990. doi: 10.1016/j.neuron.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci. 2011;36:320–328. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Myskiw JC, Rossato JI, Bevilaqua LR, Medina JH, Izquierdo I, Cammarota M. On the participation of mTOR in recognition memory. Neurobiol Learn Mem. 2008;89:338–351. doi: 10.1016/j.nlm.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Narita M, Akai H, Kita T, et al. Involvement of mitogen-stimulated p70-S6 kinase in the development of sensitization to the methamphetamine-induced rewarding effect in rats. Neuroscience. 2005;132:553–560. doi: 10.1016/j.neuroscience.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Neasta J, Ben Hamida S, Yowell Q, Carnicella S, Ron D. Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders. Proc Natl Acad Sci U S A. 2010;107:20093–20098. doi: 10.1073/pnas.1005554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neasta J, Ben Hamida S, Yowell QV, Carnicella S, Ron D. AKT signaling pathway in the nucleus accumbens mediates excessive alcohol drinking behaviors. Biol Psychiatry. 2011;70:575–582. doi: 10.1016/j.biopsych.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Ozaita A, Puighermanal E, Maldonado R. Regulation of PI3K/Akt/GSK-3 pathway by cannabinoids in the brain. J Neurochem. 2007;102:1105–1114. doi: 10.1111/j.1471-4159.2007.04642.x. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Kanter JL, He Z. PTEN/mTOR and axon regeneration. Exp Neurol. 2010;223:45–50. doi: 10.1016/j.expneurol.2009.12.032. [DOI] [PubMed] [Google Scholar]
- Polakiewicz RD, Schieferl SM, Gingras AC, Sonenberg N, Comb MJ. mu-Opioid receptor activates signaling pathways implicated in cell survival and translational control. J Biol Chem. 1998;273:23534–23541. doi: 10.1074/jbc.273.36.23534. [DOI] [PubMed] [Google Scholar]
- Puighermanal E, Busquets-Garcia A, Gomis-Gonzalez M, Marsicano G, Maldonado R, Ozaita A. Dissociation of the pharmacological effects of THC by mTOR blockade. Neuropsychopharmacology. 2013;38:1334–1343. doi: 10.1038/npp.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puighermanal E, Marsicano G, Busquets-Garcia A, Lutz B, Maldonado R, Ozaita A. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci. 2009;12:1152–1158. doi: 10.1038/nn.2369. [DOI] [PubMed] [Google Scholar]
- Ron D, Messing RO. Signaling Pathways Mediating Alcohol Effects. Curr Top Behav Neurosci. 2011 doi: 10.1007/7854_2011_161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Wang J. The NMDA Receptor and Alcohol Addiction. 2009 [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Santini E, Heiman M, Greengard P, Valjent E, Fisone G. Inhibition of mTOR signaling in Parkinson's disease prevents L-DOPA-induced dyskinesia. Sci Signal. 2009;2:ra36. doi: 10.1126/scisignal.2000308. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME. BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci. 2004;24:7366–7377. doi: 10.1523/JNEUROSCI.1739-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata M, Matsumura H, Okubo-Suzuki R, Ohkawa N, Inokuchi K. Neuronal stimulation induces autophagy in hippocampal neurons that is involved in AMPA receptor degradation after chemical long-term depression. J Neurosci. 2012;32:10413–10422. doi: 10.1523/JNEUROSCI.4533-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slipczuk L, Bekinschtein P, Katche C, Cammarota M, Izquierdo I, Medina JH. BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS One. 2009;4:e6007. doi: 10.1371/journal.pone.0006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Sparks CA, Guertin DA. Targeting mTOR: prospects for mTOR complex 2 inhibitors in cancer therapy. Oncogene. 2010;29:3733–3744. doi: 10.1038/onc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS One. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacological reviews. 2011;63:348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica L, Zhu PJ, Huang W, Zhou H, Kozma SC, Costa-Mattioli M. Selective pharmacogenetic inhibition of mammalian target of Rapamycin complex I (mTORC1) blocks long-term synaptic plasticity and memory storage. Proc Natl Acad Sci U S A. 2011;108:3791–3796. doi: 10.1073/pnas.1014715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Tye KM, Chen BT, Bonci A. Neuroplastic alterations in the limbic system following cocaine or alcohol exposure. Curr Top Behav Neurosci. 2010;3:3–27. doi: 10.1007/7854_2009_23. [DOI] [PubMed] [Google Scholar]
- Sui L, Wang J, Li BM. Role of the phosphoinositide 3-kinase-Akt-mammalian target of the rapamycin signaling pathway in long-term potentiation and trace fear conditioning memory in rat medial prefrontal cortex. Learn Mem. 2008;15:762–776. doi: 10.1101/lm.1067808. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Swiech L, Perycz M, Malik A, Jaworski J. Role of mTOR in physiology and pathology of the nervous system. Biochim Biophys Acta. 2008;1784:116–132. doi: 10.1016/j.bbapap.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Kalivas PW, Worley PF. Homer proteins: implications for neuropsychiatric disorders. Current opinion in neurobiology. 2006;16:251–257. doi: 10.1016/j.conb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci. 2004;24:9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012;485:109–113. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres CA, Sulzer D. Macroautophagy can press a brake on presynaptic neurotransmission. Autophagy. 2012;8:1540–1541. doi: 10.4161/auto.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pages C, Rogard M, Besson MJ, Maldonado R, Caboche J. Delta 9-tetrahydrocannabinol-induced MAPK/ERK and Elk-1 activation in vivo depends on dopaminergic transmission. Eur J Neurosci. 2001;14:342–352. doi: 10.1046/j.0953-816x.2001.01652.x. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Pierce RC. Sensitization processes in drug addiction. Curr Top Behav Neurosci. 2010;3:179–195. doi: 10.1007/7854_2009_21. [DOI] [PubMed] [Google Scholar]
- Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, Janak PH, Lovinger DM, Ron D. Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior. J Neurosci. 2007;27:3593–3602. doi: 10.1523/JNEUROSCI.4749-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S, Ron D. Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. J Neurosci. 2010a;30:10187–10198. doi: 10.1523/JNEUROSCI.2268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Luo YX, He YY, Li FQ, Shi HS, Xue LF, Xue YX, Lu L. Nucleus accumbens core mammalian target of rapamycin signaling pathway is critical for cue-induced reinstatement of cocaine seeking in rats. J Neurosci. 2010b;30:12632–12641. doi: 10.1523/JNEUROSCI.1264-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston MC, Chen H, Swann JW. Multiple roles for mammalian target of rapamycin signaling in both glutamatergic and GABAergic synaptic transmission. J Neurosci. 2012;32:11441–11452. doi: 10.1523/JNEUROSCI.1283-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E, Cuervo AM. Integration of clearance mechanisms: the proteasome and autophagy. Cold Spring Harb Perspect Biol. 2010;2:a006734. doi: 10.1101/cshperspect.a006734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, McCallum SE, Glick SD, Huang Y. Inhibition of the mammalian target of rapamycin pathway by rapamycin blocks cocaine-induced locomotor sensitization. Neuroscience. 2011;172:104–109. doi: 10.1016/j.neuroscience.2010.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Rudge DG, Koos JD, Vaidialingam B, Yang HJ, Pavletich NP. mTOR kinase structure, mechanism and regulation. Nature. 2013;497:217–223. doi: 10.1038/nature12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip CK, Murata K, Walz T, Sabatini DM, Kang SA. Structure of the human mTOR complex I and its implications for rapamycin inhibition. Mol Cell. 2010;38:768–774. doi: 10.1016/j.molcel.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YH, Yoon SO, Poulogiannis G, et al. Phosphoproteomic Analysis Identifies Grb10 as an mTORC1 Substrate That Negatively Regulates Insulin Signaling. Science. 2011;332:1322–1326. doi: 10.1126/science.1199484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu PJ, Huang W, Kalikulov D, et al. Suppression of PKR promotes network excitability and enhanced cognition by interferon-gamma-mediated disinhibition. Cell. 2011;147:1384–1396. doi: 10.1016/j.cell.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]