Abstract
Radiolabelled antiCD-20 antibodies have demonstrated single agent activity in relapsed diffuse large B-cell lymphoma (DLBCL). The S0433 clinical trial enrolled patients with newly diagnosed, advanced stage or bulky stage II, histologically confirmed DLBCL. Patients received six cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone), two cycles of CHOP, then iodine-131 tositumomab radioimmunotherapy consolidation 30–60 days after completion of chemotherapy. The primary endpoint was two-year progression-free survival (PFS). Eighty-four eligible patients were enrolled, and 56 patients completed the entire course of protocol treatment. Of the 84 patients evaluable for treatment response, 72 (86%, 95% confidence interval [CI]: 76%–92%) achieved a partial response (n=21) or a confirmed (n=41) or unconfirmed (n=10) complete response to therapy. With a median follow-up of 3.9 years, the 2-year PFS estimate is 69% and the 2-year overall survival estimate is 77%. Rituximab levels at time of radioimmunotherapy did not correlate with toxicity or outcome. Twenty percent of patients had double hit features (MYC+; BCL2+) by immunohistochemistry, and had inferior outcome. These current results suggest that the incorporation of novel agents earlier in therapy may ultimately have greater impact in DLBCL, as early progressions, deaths and declining performance status during CHOP chemotherapy limited the number of patients who ultimately could benefit from radioimmunotherapy consolidation.
Keywords: lymphoma, chemotherapeutic approaches, diffuse large B cell lymphoma, radioimmunotherapy, pharmacotherapeutics
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma (NHL) in the United States, and is curable with standard R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) chemotherapy. Despite the improvements in outcome of DLBCL with the routine incorporation of rituximab, high-risk subgroups defined by the International Prognostic Index (IPI) continue to have inferior outcome.(Ziepert, et al. 2010) For younger patients, consolidation with autologous stem cell transplantation (ASCT) in first remission prolongs progression-free survival (PFS), and possibly improved overall survival (OS) for the highest risk patients. (Stiff, et al. 2013) However, as the incidence of DLBCL increases with age, new therapies with minimal additive toxicity are clearly needed, particularly for patients unfit for ASCT.
Radioimmunotherapy with conjugated anti-CD20 antibodies has demonstrated single-agent activity in DLBCL. In patients with relapsed DLBCL, a phase 2 trial of ibritumomab tiuxetan demonstrated a response rate of over 50%. (Morschhauser et al. 2007) We have previously demonstrated the safety of sequential chemotherapy with CHOP followed by consolidative radioimmunotherapy with the iodine-131 (131I) tositumomab regimen in patients with follicular lymphoma, demonstrating high response rates, and conversion from partial response to complete response after consolidative radioimmunotherapy. (Press, et al. 2006)
We therefore designed the S0433 clinical trial, which incorporated consolidative radioimmunotherapy with the 131I tositumomab regimen after standard R-CHOP chemotherapy for patients with newly diagnosed DLBCL. The objective of trial S0433 was to estimate the 2-year PFS, in hope of increasing the durable remission rate in patients with aggressive lymphomas, without serious additive toxicity.
Methods
Study registration
This Phase II multicentre trial was initiated in November 2005. The protocol was approved by the Institutional Review Board at each site, and written consent was obtained from all patients prior to enrollment. The study was registered with ClinicalTrials.gov prior to enrolling patients (ClinicalTrials.gov Identifier: NCT00107380).
Eligibility
The original trial design limited patient inclusion to those > 60 years of age; after 17 (20% of total) patients were enrolled, an amendment expanded the study inclusion criteria to include patients > 18 years of age after a competing study for younger patients completed accrual. Eligible patients had a previously untreated diagnosis (World Health Organization classification) of CD20-positive advanced stage (Stage III, IV, or bulky [>10 cm] Stage II) DLBCL with bidimensionally measurable disease; all pathology was confirmed by central review. Eligible patients had adequate performance status (Zubrod 0–2) and adequate renal, hepatic, haematologic, and cardiac function. Baseline laboratory parameters included circulating lymphoid cells <20×109/l. Patients were excluded if they had a previous diagnosis of malignancy (with the exception of basal cell or squamous cell of the skin, in situ cervical cancer, adequately treated Stage I or II cancer for which patient was in complete remission, or any other cancer from which patient had been disease-free for at least 5 years); or clinical evidence of central nervous system (CNS) involvement by lymphoma. Pregnant or nursing female patients, patients known to be human immunodeficiency virus (HIV) positive or with a history of solid organ transplantation, and patients requiring continuous supplemental oxygen therapy, were also excluded.
Baseline Studies
Baseline evaluation included a history and physical examination, radiographic imaging (computerized tomography of the chest, abdomen, and pelvis), routine laboratory studies, bone marrow evaluation and an electrocardiogram.
Protocol Treatment
Patients were treated with standard R-CHOP chemotherapy as follows: every 21 days for the first 6 cycles (Coiffier, et al. 2002); rituximab was omitted from cycles 7 and 8 to limit antigen binding competition as observed in murine studies.(Gopal, et al. 2008) 131I tositumomab (Bexxar; supplied by GlaxoSmithKline, Research Triangle Park, NC) was administered 30–60 days after cycle 8 of CHOP, as described. (Press, et al. 2003) Intrathecal methotrexate was allowed at physician discretion for CNS prophylaxis. Patients were removed early from the protocol treatment for progressive disease, unacceptable toxicity, failure to meet criteria for tositumomab administration following completion of CHOP chemotherapy, or patient preference.
Criteria for response and toxicity
Restaging for response determination was performed within 4 weeks of the last cycle of CHOP (approximately Day 169), and then again at 12 weeks post-131I tositumomab treatment using the same imaging techniques used for baseline measurements. Clinical responses were coded according to International Workshop NHL criteria.(Cheson et al. 1999) National Cancer Institute Common toxicity criteria version 3.0 was used to grade toxicities (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf[ctep.cancer.gov]). Patients were followed prospectively and evaluated at 1 year, then every 6 months for 2 years, then annually for a maximum of 5 years.
PFS was calculated from the first dose of study drug to the first documentation of disease progression or death due to any cause, whichever occurred first. Patients who were alive and progression-free at the time of final data analysis were censored at last assessment.
Correlative laboratory studies
Rituximab levels and toxicity
We utilized ELISA-0145-004, a sandwich enzyme-linked immunosorbent assay (ELISA) for the determination of Rituximab in human serum after cycle 8 of CHOP. The association between rituximab levels and toxicity were evaluated by comparing the proportion of patients with grade 3, 4 or 5 toxicities for patients with rituximab levels above versus below the median rituximab concentration using chi-square analysis.
Immunohistochemistry
We evaluated MYC and BCL2 protein expression and germinal center B-cell-like (GCB) vs. non-GCB status using immunohistochemistry techniques on patient samples obtained at baseline. Studies of MYC expression were performed and scored (40% cut-off; based on minimum 100 cell count) as described.(Johnson, et al. 2012) Cell of origin was determined using the Hans algorithm based on stains for CD10 (Biocare [Concord, CA], clone 56C6), BCL6 (Cell Marque [Rocklin, CA], clone GI191/A8), and MUM1 (Dako [Glostrup, Denmark], clone MUM1p).(Hans, et al. 2004)
Statistical analysis
For the primary objective, a modified intent-to-treat analysis was performed, excluding patients determined to be ineligible. Patients who did not receive any treatment were excluded from evaluation of adverse events. The primary endpoint of this study was the 2-year PFS rate. The planned statistical design anticipated that 80 patients accrued over 15 months with 18 months of additional follow-up would be sufficient to estimate the 2-year PFS to within +/− 0.12. The design also specified an adjustment for important prognostic groups. We assumed that 2-year PFS was approximately 60% for patients older than 60 years and for younger patients (≤60 years old) with high IPI scores (2 or higher), and approximately 85% for patients ≤60 years with low IPI scores (0 or 1).(Pfreundschuh et al. 2006) If 10% of patients were in the younger patient group (≤60 years old) with low IPI, the weighted 2-year PFS for R-CHOP would be 63%, compared to an alternative 20% greater (83%). Under these assumptions, an estimated 2-year PFS of 73% or greater (based on the 95% upper bound on the confidence interval [CI] using a one-arm survival design) would be sufficient to warrant further investigation.(Lawless 1982) The target value was adjusted based on the observed frequency of prognostic groups. Eighty patients would also be sufficient to estimate the probability of any particular toxicity to within +/− 0.11. Survival was estimated according to the method of Kaplan and Meier.(Kaplan and Meier 1958) Analyses of survival differences by prognostic factors were performed using Cox regression.(Cox 1972) Landmark survival analyses were conducted for the subset of patients completing all therapy, to avoid selection bias.(Anderson, et al. 1983) Data as of August 20, 2013 were included in the analysis.
Results
Patient characteristics
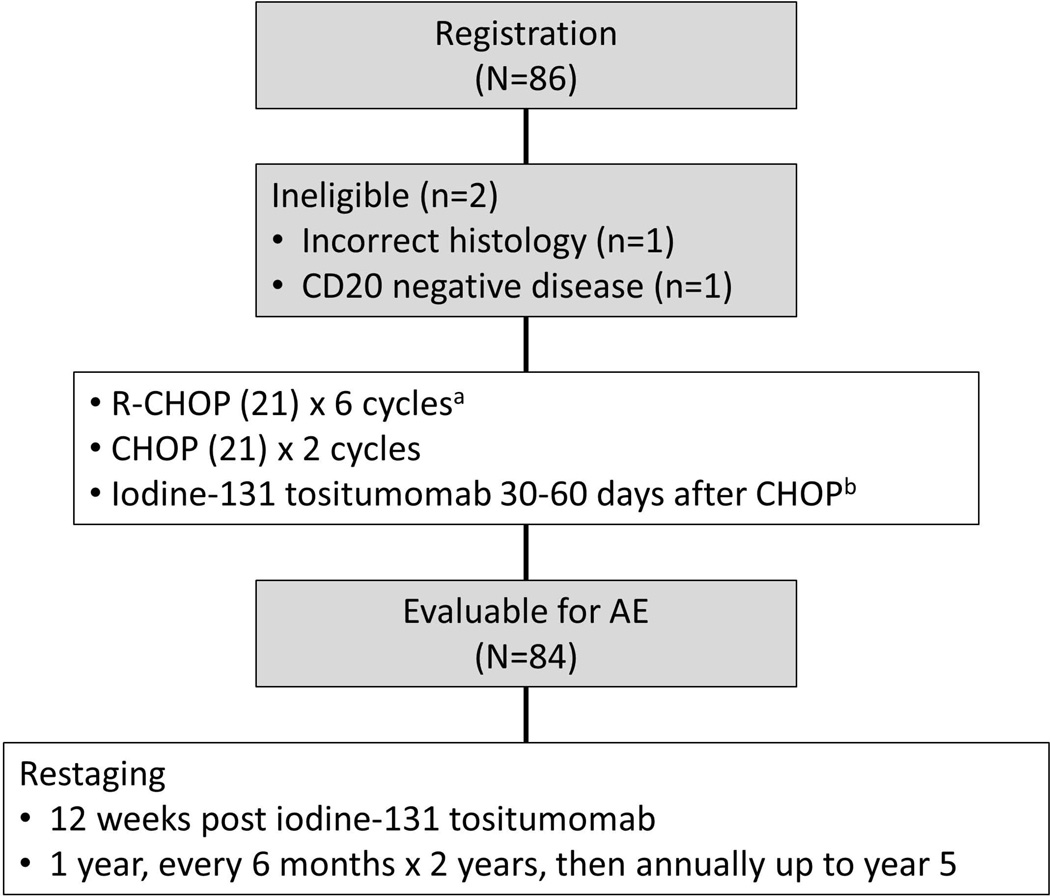
Between November 2005 and March 2010, 86 patients were registered (Figure 1). Two patients were determined to be ineligible before treatment (one incorrect histology; one CD20-negative disease). Baseline clinical characteristics are detailed in Table I.
Figure 1.
CONSORT diagram illustrating patient flow and study schema on Southwest Oncology Group protocol S0433. AE, adverse event; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone chemotherapy; R-CHOP, CHOP with rituximab; IT, intrathecal.
Table I.
Characteristics of study participants treated on Southwest Oncology Group Protocol S0433.
| Characteristic | N=84 Eligible | |
|---|---|---|
| Age, median (range) | years | 64 (29–85) |
| Gender, n (%) | Female | 45 (54%) |
| Race, n (%) | White | 78 (93%) |
| Ethnicity, n (%) | Hispanic | 3 (4%) |
| IPI Risk level, n (%) | Low | 20 (24%) |
| Low-Intermediate | 27 (32%) | |
| High-Intermediate | 27 (32%) | |
| High | 10 (12%) | |
| Immunohistochemistry, n (%)* | BCL2+ | 57 (86%) |
| MYC+ | 15 (23%) | |
| BCL2+MYC+ | 13 (20%) |
successfully completed on N=66 subjects
IPI, International Prognostic Index
Treatment
Fifty-six patients (67%) completed the entire course of protocol treatment (RCHOP + radioimmunotherapy) as planned. Reasons for early termination included: toxicity (n=8 due to left ventricular ejection fraction changes and neuropathy; infection, backache, and neuropathy; drop in ejection fraction; infection and neuropathy; thrombocytopenia; fatigue; pancytopenia and cirrhosis); refusal unrelated to adverse events (n=8), progressive disease (n=4); death (n=3), institutional error (n=3) and physician decision (n=2). Patients who did not receive the full course of RCHOP/131I tositumomab were more likely to have high-intermediate/high IPI risk disease compared to those who did receive the entire course of planned therapy (64% vs. 34%; p=0.008).
Safety
Five patients experienced Grade 5 toxicities: cardiac ischaemia (n=2), febrile neutropenia (n=1), renal failure (n=1) and secondary malignancy (acute myeloid leukaemia, n=1). Forty-six additional patients (55%) had at worst Grade 4 toxicity, mostly haematological, as detailed in Table II. Common non-haematological adverse events, occurring in at least 10% of treated patients, are detailed in Table II.
Table II.
Adverse Events on Southwest Oncology Group Protocol S0433. Any Grade 4 or Grade 5 and Common (in 10% of patients or more) Grade 3 Events.
| Adverse Events | n, % of Patients (total n=84) |
||
|---|---|---|---|
| (grade ≥ 3) | Grade 3 | Grade 4 | Grade 5 |
| Cardiovascular | 6 (7%) | 1 (1%) | 2* (2%) |
| Flu-like symptoms | 11 (13%) | 1 (1%) | -- |
| Dehydration | -- | 1 (1%) | -- |
| Other Gastrointestinal Event | -- | 1 (1%) | -- |
| Haematological | |||
| Haemoglobin | 12 (14%) | -- | -- |
| Leucocytes | 23 (27%) | 24 (29%) | -- |
| Lymphopenia | 24 (29%) | 15 (18%) | -- |
| Neutrophils | 11 (13%) | 44 (52%) | -- |
| Platelets | 12 (14%) | 17 (20%) | -- |
| GI Haemorrhage: rectum | -- | 1 (1%) | -- |
| Infection | 20 (24%) | 1 (1%) | 1* (1%) |
| Pneumonitis | -- | 1 (1%) | -- |
| Hyperglycaemia | -- | 1 (1%) | -- |
| Hyperuricaemia | -- | 1 (1%) | -- |
| Hypokalaemia | -- | 1 (1%) | -- |
| Neurophathy | 9 (11%) | -- | -- |
| Renal Failure | -- | -- | 1* (1%) |
| Secondary Malignancy | 1 (1%) | -- | 1* (1%) |
2 cardiac ischaemia, 1 febrile neutropenia, 1 renal failure, 1 acute myeloid leukaemia GI, gastrointestinal
Efficacy
Of the 84 patients evaluable, 72 (86%, 95% CI: 76%–92%) achieved an objective response to therapy, including partial response (n=21) or a confirmed (n=41) or unconfirmed (n=10) complete response based upon prespecified criteria.(Cheson, et al. 1999) One additional patient maintained stable disease. Among the 56 patients that received all treatment, including 131I tositumomab, 55 (98%, 95% CI: 90%–100%) achieved a partial or complete response to therapy.
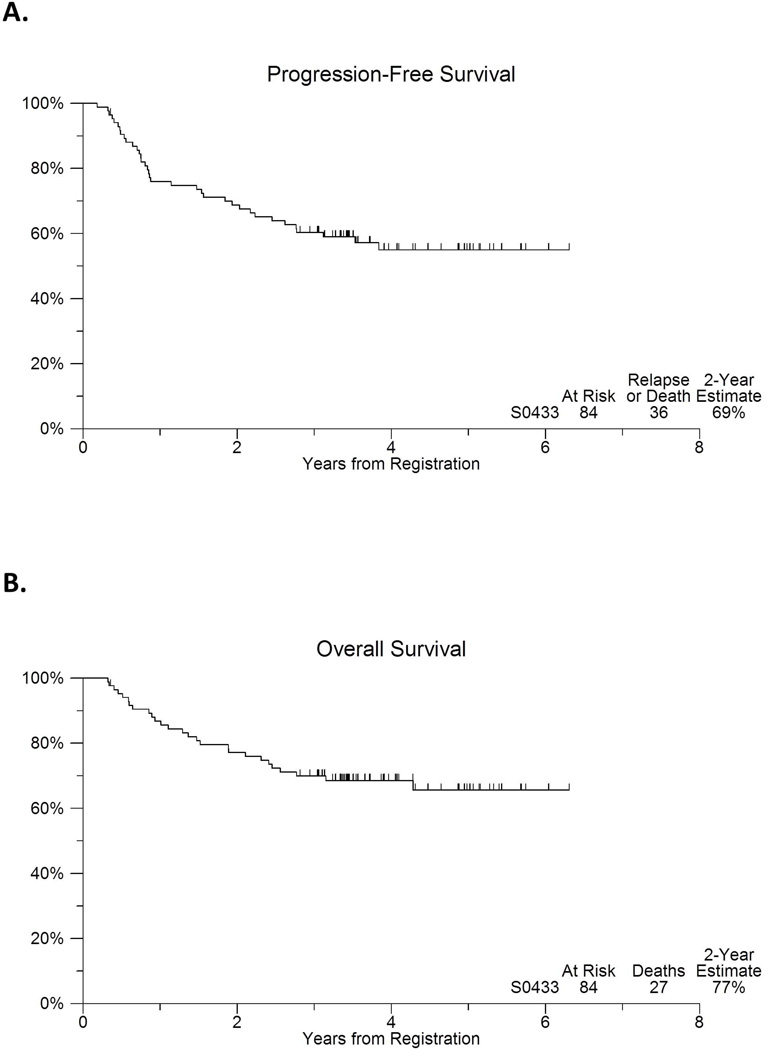
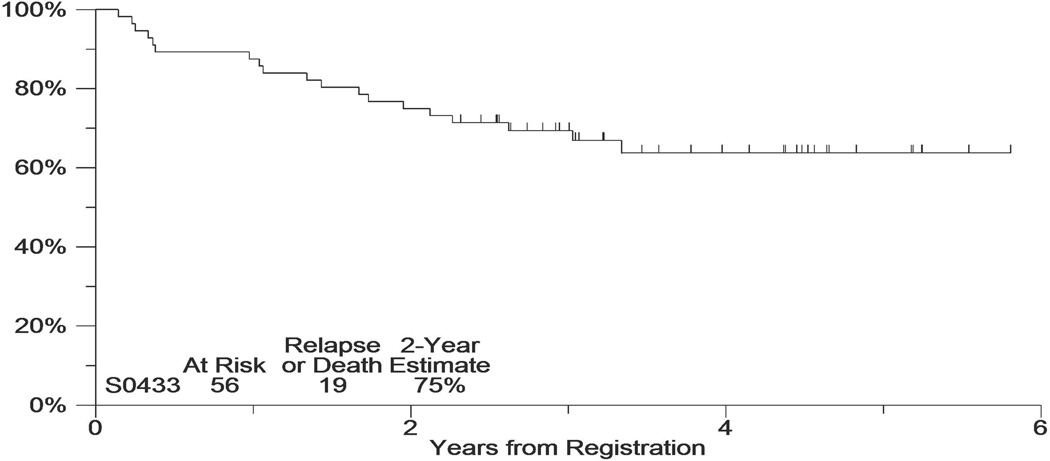
The median follow-up of the patients still alive is 3.9 years. Figure 2 includes the PFS and OS experience of this cohort. Eight patients (10%) were <60 years with low IPI. Therefore the weighted target PFS was 73%. The observed 2-year PFS estimate was 69% (95% CI: 59%–79%; Figure 2). The 2-year OS estimate was 77% (95% CI: 68%–86%). Among the 56 patients that received all CHOP-R/131I tositumomab, the 2-year PFS, beginning from 6 months after registration (i.e., after completion of tositumomab treatment using landmark analysis), was 75% (95%CI: 64%–86%; Figure 3).
Figure 2.
(A) Progression-free and (B) overall survival of 84 patients with advanced stage or bulky stage 2 confirmed diffuse large B cell lymphoma treated with 6 cycles of cyclophosphamide, doxorubicin, vincristine, prednisone chemotherapy (CHOP) with rituximab (R-CHOP), with two additional cycles of CHOP alone, followed by 131Itositumomab.
Figure 3.
Progression-free survival among patients who received 131I tositumomab (Progression-free survival started 6 months post-registration).
Correlative Laboratory Studies
Circulating rituximab levels at day 169
Serum rituximab levels following the eighth cycle of CHOP (drawn at day 169) were studied in 15 patients. Concentration values ranged from 19,100 ng/ml to 110,000 ng/ml, with a median concentration = 53,500 ng/ml. The proportion of patients with rituximab levels above the group median that experienced Grade 3–4 toxicities was 100% as compared to 86% Grade 3–5 toxicities among the patients with circulating rituximab levels below the group median at Day 169 (p=0.47).
MYC and BCL2 Immunohistochemistry
Baseline tissue was available for MYC and BCL2 immunohiistochemical analysis on 75 eligible patients; 79% were BCL2 protein positive, 19% were MYC protein positive. Of those with both MYC and BCL2 values (n=65), 13 (20%) were MYC/BCL2 double protein positive. Estimated 2-year PFS in double protein positive patients was 58% (95% CI: 27%, 80%); the 2-year PFS estimate in non-double positive patients was 73% (95% CI: 59%, 83%). Despite a trend, neither PFS nor OS were significantly associated with double protein positive status (p=0.34, and p=0.99, respectively).
GCB vs. non-GCB Status
Of the 22 patients evaluated for GCB status, 9 (41%) were GCB and 13 (59%) patients were non-GCB: 6 (67%) GCB and 10 (77%) non-GCB patients completed full protocol treatment. The estimated 2-year PFS in GCB patients and non-GCB patients was 67% (95% CI: 28%, 88%); and 85% (95% CI: 51%, 96%), respectively. Neither PFS nor OS were significantly associated with GCB vs. non-GCB status (p=0.97, and p=0.56, respectively).
Discussion
In our clinical trial, a consolidation strategy utilizing 131I tositumomab after 8 cycles of CHOP chemotherapy (6 with rituximab) for DLBCL did not meet our predefined threshold (2 year PFS=73%) for future evaluation. Unlike our previous experiences in follicular lymphoma (Press, et al. 2003; Press et al. 2013), in this population of DLBCL, early progressions, deaths and declining performance status during R-CHOP chemotherapy prevented over one quarter of patients from receiving planned consolidation with radioimmunotherapy. For those selected patients who were able to received radioimmunotherapy, the outcome appeared quite favourable, however this group had lower IPI scores, and more favourable clinical characteristics compared to the group of patients who did not receive radioimmunotherapy.
Our results stand in contrast to the limited number of other trials that used radioimmunotherapy as consolidation after initial chemo-immunotherapy for DLBCL. Zinzani initially demonstrated feasibility of this concept in a pilot trial of 20 patients treated with six cycles of CHOP (no rituximab), followed by ibritumomab tiuxetan consolidation, demonstrating safety and efficacy.(Zinzani, et al. 2008) The same group then conducted a larger phase 2 trial in 55 high-risk elderly patients of an abbreviated (four cycles) course of R-CHOP followed by ibritumomab tiuxetan consolidation.(Zinzani, et al. 2010) Only seven patients (13%) did not receive planned radioimmunotherapy after abbreviated R-CHOP, all due to disease progression. The authors reported an estimated PFS of 85% at two years for the entire study population. Hamlin et al (2010) presented preliminary results of a single institution phase 2 study in elderly patients with DLBCL, utilizing six cycles of R-CHOP followed by ibritumomab tiuxetan consolidation.(Hamlin, et al. 2010) Of 63 patients enrolled, 19 (30%) did not receive planned radioimmunotherapy. Similar to our trial, the group of patients who were able to receive radioimmunotherapy had superior outcome. Finally, the Southwest Oncology Group (SWOG) has presented preliminary results of an approach for early stage DLBCL utilizing three cycles of CHOP (no rituximab), involved field radiation therapy, and ibritumomab tiuxetan consolidation, demonstrating higher response rates and longer PFS than historical controls without radioimmunotherapy.(Miller, et al. 2008)
All of these other trials have utilized ibritumomab tiuxetan instead of 131I tositumomab as the radioimmunotherapy consolidation strategy. Although there are clear differences between these agents, the clinical outcomes across various histologies of lymphomas have been remarkably similar when using either agent.(Schaefer-Cutillo and Friedberg 2008) Perhaps more importantly, these other studies utilized a shorter course of chemotherapy induction prior to consolidation. Although the pivotal studies of the R-CHOP regimen in advanced DLBCL utilized eight cycles of chemotherapy(Feugier et al. 2005), many experts now prefer six cycles as standard treatment, based in part upon the German experience demonstrating equivalence of 6 versus 8 cycles of R-CHOP given every 14 days.(Pfreundschuh, et al. 2008) Given that many patients in our study were not able to receive consolidation with radioimmunotherapy due to frailty and toxic effects of chemotherapy, a shorter course of induction therapy may have provided a more favorable outcome.
Preclinical studies in mice have suggested that rituximab concentrations can block the binding of a second anti-CD20 monoclonal antibody, and therefore circulating rituximab might impair the clinical efficacy of anti-CD20 radioimmunotherapy.(Gopal, et al. 2008) For this reason, we omitted rituximab for the seventh and eighth cycles of R-CHOP in our study. Despite this, rituximab levels performed in a subset of patients demonstrated wide variability of circulating rituximab prior to radioimmunotherapy administration. Targeting alternative antigens on DLBCL overcomes this theoretical concern, and a preliminary study of 64 patients with relapsed lymphoma demonstrated safety and efficacy of anti-CD22 fractionated radioimmunotherapy with epratuzumab tetraxetan.(Morschhauser, et al. 2010) Other alternative means of delivering radioimmunotherapy include pretargeting using streptavidin-biotin technology, which in model systems has demonstrated superior biodistribution, more complete tumor regressions, and longer survival compared with conventional radioimmunotherapy.(Green, et al. 2007) Finally, the Mayo Clinic group has demonstrated impressive results when combining a Toll-like receptor 9 agonist, which has demonstrated activity in combination with rituximab (Friedberg, et al. 2005; Friedberg, et al. 2009), with ibritumomab tiuxetan in patients with relapsed lymphoma.(Witzig, et al. 2013)
DLBCL is a heterogeneous disease, and gene expression profiling studies have identified at least three unique cell-of-origin profiles with differential therapeutic outcomes.(Lenz, et al. 2008) In our study, using immunohistochemistry as a surrogate for cell-or origin, patients with non-GCB lymphoma had equivalent outcome to patients with GCB histology. Recent data has suggested that perhaps the major unmet clinical need in treatment of DLBCL is to overcome the adverse prognostic impact of “double hit” histology, defined by dual overexpression of MYC and BCL2 in DLBCL.(Friedberg 2012) When measured by immunohistochemistry (double protein positive), up to 30% of newly diagnosed DLBCL can be identified, which portends a dismal prognosis after R-CHOP treatment.(Green, Young et al. 2012; Johnson, Slack et al. 2012) The majority of patients with double hit features are elderly, and new treatments are clearly indicated for this group.(Pfreundschuh 2012) In our study, the observed rates of PFS in the 20% of patients with double protein positivity appeared inferior, which was partly due to early progressions prior to planned radioimmunotherapy.
A recent trial in relapsed or refractory DLBCL comparing rituximab and BEAM (carmustine, etoposide, cytarabine, melphalan) chemotherapy conditioning to 131I tositumomab and BEAM chemotherapy conditioning prior to ASCT showed no improvement when radioimmunotherapy was added to the conditioning regimen.(Vose, et al. 2013) GlaxoSmithKline (Philadelphia, PA) has announced that, effective from February 2014, 131I tositumomab will no longer be commercially available in the United States. Ibritumomab tiuxetan remains commercially available, and indeed a phase 3 trial is planned to definitively evaluate the role of radioimmunotherapy consolidation in DLBCL. Based upon our results, favourable prognosis patients without double hit histology may benefit the most from this modality. For patients with high clinical risk features, or double hit features by immunohistochemistry, alternative approaches including the early incorporation of novel agents is preferable to a consolidation or maintenance approach, due to the high rate of early disease progression. Currently, SWOG is evaluating standard R-CHOP chemotherapy with vorinostat (Crump, et al. 2008) in advanced stages of DLBCL, and other groups are exploring lenalidomide (Chiappella et al. 2013) and ibrutinib (Younes, et al. 2013) in combination with R-CHOP. Moving forward, as our biological understanding of DLBCL improves, rational incorporation of novel approaches, including radioimmunotherapy, should be based upon biological disease signatures rather than empiricism.(Friedberg 2011)
Acknowledgments
Dr. Friedberg is a Scholar in Clinical Research of the Leukemia & Lymphoma Society. Correlative studies were supported in part by the University of Rochester SPORE in lymphoma CA 130805. This investigation was also supported in part by the National Cancer Institute, DHHS, PHS Cooperative Agreement grant award numbers CA32102, CA38926, CA11083, CA20319, CA35090, CA13612, CA46368, CA27057, CA35128, CA35119, CA46282, CA45450, CA12644, CA04919, CA073590, and in part by GlaxoSmithKline.
Footnotes
Presented in part at the American Society of Hematology Annual Meeting 2010, Orlando, FL.
Author contributions:
Jonathan W. Friedberg: designed reserch, performed research, analysed/interpreted data, wrote the manuscript
Joseph M. Unger: analysed and interpreted the data, performed statistical analysis, wrote the manuscript
W. Richard Burack: analysed and interpreted the data, wrote the manuscript
Ajay K. Gopal: performed research, analysed and interpreted the data
Robert N. Raju: performed research, analysed and interpreted the data
Auayporn P. Nademanee: performed research, analysed and interpreted the data
Mark S. Kaminski: performed research, analysed and interpreted the data
Hongli Li: analysed and interpreted the data, performed statistical analysis
Oliver W. Press: designed research, performed research, analysed/interpreted the data
Thomas P. Miller: designed research, analysed/interpreted the data
Richard I. Fisher: designed research, performed research, analysed/interpreted the data
References
- Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. Journal of Clinical Oncology. 1983;1(11):710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. Journal of Clinical Oncology. 1999;17(4):1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- Chiappella A, Tucci A, Castellino A, Pavone V, Baldi I, Carella AM, Orsucci L, Zanni M, Salvi F, Liberati AM, Gaidano G, Bottelli C, Rossini B, Perticone S, De Masi P, Ladetto M, Ciccone G, Palumbo A, Rossi G, Vitolo U. Lenalidomide plus cyclophosphamide, doxorubicin, vincristine, prednisone and rituximab is safe and effective in untreated, elderly patients with diffuse large B-cell lymphoma: a phase I study by the Fondazione Italiana Linfomi. Haematologica. 2013;98(11):1732–1738. doi: 10.3324/haematol.2013.085134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coiffier B, Lepage E, Brière J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C. CHOP Chemotherapy plus Rituximab Compared with CHOP Alone in Elderly Patients with Diffuse Large-B-Cell Lymphoma. New England Journal of Medicine. 2002;346(4):235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- Cox D. Regression models and life tables. Journal of the Royal Statistical Society. 1972;34:187–220. [Google Scholar]
- Crump M, Coiffier B, Jacobsen ED, Sun L, Ricker JL, Xie H, Frankel SR, Randolph SS, Cheson BD. Phase II trial of oral vorinostat (suberoylanilide hydroxamic acid) in relapsed diffuse large-B-cell lymphoma. Annals of Oncology. 2008;19(5):964–969. doi: 10.1093/annonc/mdn031. [DOI] [PubMed] [Google Scholar]
- Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, Fermé C, Christian B, Lepage E, Tilly H, Morschhauser F, Gaulard P, Salles G, Bosly A, Gisselbrecht C, Reyes F, Coiffier B. Long-Term Results of the R-CHOP Study in the Treatment of Elderly Patients With Diffuse Large B-Cell Lymphoma: A Study by the Groupe d'Etude des Lymphomes de l'Adulte. Journal of Clinical Oncology. 2005;23(18):4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- Friedberg JW. New strategies in diffuse large B-cell lymphoma: translating findings from gene expression analyses into clinical practice. Clinical Cancer Research. 2011;17(19):6112–6117. doi: 10.1158/1078-0432.CCR-11-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg JW. Double-hit diffuse large B-cell lymphoma. Journal of Clinical Oncology. 2012;30(28):3439–3443. doi: 10.1200/JCO.2012.43.5800. [DOI] [PubMed] [Google Scholar]
- Friedberg JW, Kim H, McCauley M, Hessel EM, Sims P, Fisher DC, Nadler LM, Coffman RL, Freedman AS. Combination immunotherapy with a CpG oligonucleotide (1018 ISS) and rituximab in patients with non-Hodgkin lymphoma: increased interferon-alpha/beta-inducible gene expression, without significant toxicity. Blood. 2005;105(2):489–495. doi: 10.1182/blood-2004-06-2156. [DOI] [PubMed] [Google Scholar]
- Friedberg JW, Kelly JL, Neuberg D, Peterson DR, Kutok JL, Salloum R, Brenn T, Fisher DC, Ronan E, Dalton V, Rich L, Marquis D, Sims P, Rothberg PG, Liesveld J, Fisher RI, Coffman R, Mosmann T, Freedman AS. Phase II study of a TLR-9 agonist (1018 ISS) with rituximab in patients with relapsed or refractory follicular lymphoma. British Journal of Haematology. 2009;146(3):282–291. doi: 10.1111/j.1365-2141.2009.07773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal AK, Press OW, Wilbur SM, Maloney DG, Pagel JM. Rituximab blocks binding of radiolabeled anti-CD20 antibodies (Ab) but not radiolabeled anti-CD45 Ab. Blood. 2008;112(3):830–835. doi: 10.1182/blood-2008-01-132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DJ, Pagel JM, Pantelias A, Hedin N, Lin Y, Wilbur DS, Gopal A, Hamlin DK, Press OW. Pretargeted radioimmunotherapy for B-cell lymphomas. Clinical Cancer Research. 2007;13(18 Pt 2):5598s–5603s. doi: 10.1158/1078-0432.CCR-07-1223. [DOI] [PubMed] [Google Scholar]
- Green TM, Young KH, Visco C, Xu-Monette ZY, Orazi A, Go RS, Nielsen O, Gadeberg OV, Mourits-Andersen T, Frederiksen M, Pedersen LM, Moller MB. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. Journal of Clinical Oncology. 2012;30(28):3460–3467. doi: 10.1200/JCO.2011.41.4342. [DOI] [PubMed] [Google Scholar]
- Hamlin PA, Jr, Rodriguez MA, Noy A, Portlock CS, Straus D, McLaughlin P, Pro B, Fayad L, Hagemeister F, Wegner B, Dumitrescu O, Tasker NP, Moskowitz CH, Zelenetz AD. Final Results of a Phase II Study of Sequential R-CHOP and Yttrium-90 Ibritumomab Tiuxetan (RIT) for Elderly High Risk Patients with Untreated Diffuse Large B-Cell Lymphoma (DLBCL) Blood (ASH Annual Meeting Abstracts) 2010;116(21):1793. [Google Scholar]
- Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, Pan Z, Farinha P, Smith LM, Falini B, Banham AH, Rosenwald A, Staudt LM, Connors JM, Armitage JO, Chan WC. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- Johnson NA, Slack GW, Savage KJ, Connors JM, Ben-Neriah S, Rogic S, Scott DW, Tan KL, Steidl C, Sehn LH, Chan WC, Iqbal J, Meyer PN, Lenz G, Wright G, Rimsza LM, Valentino C, Brunhoeber P, Grogan TM, Braziel RM, Cook JR, Tubbs RR, Weisenburger DD, Campo E, Rosenwald A, Ott G, Delabie J, Holcroft C, Jaffe ES, Staudt LM, Gascoyne RD. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. Journal of Clinical Oncology. 2012;30(28):3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Meier P. Nonparametric estimation from incomplete observations. Journal of the Americal Statistical Association. 1958;53:457–481. [Google Scholar]
- Lawless JF. Statistical Models and Methods for Lifetime Data. New York, NY: John Wiley and Sons; 1982. [Google Scholar]
- Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, Xu W, Tan B, Goldschmidt N, Iqbal J, Vose J, Bast M, Fu K, Weisenburger DD, Greiner TC, Armitage JO, Kyle A, May L, Gascoyne RD, Connors JM, Troen G, Holte H, Kvaloy S, Dierickx D, Verhoef G, Delabie J, Smeland EB, Jares P, Martinez A, Lopez-Guillermo A, Montserrat E, Campo E, Braziel RM, Miller TP, Rimsza LM, Cook JR, Pohlman B, Sweetenham J, Tubbs RR, Fisher RI, Hartmann E, Rosenwald A, Ott G, Muller-Hermelink HK, Wrench D, Lister TA, Jaffe ES, Wilson WH, Chan WC, Staudt LM, Lymphoma/Leukemia Molecular Profiling P. Stromal gene signatures in large-B-cell lymphomas. New England Journal of Medicine. 2008;359(22):2313–2323. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TP, Unger JM, Spier C, Stea B, Press OW, Friedberg JW, LeBlanc M, Fisher RI. Effect of Adding Ibritumomab Tiuxetan (Zevalin) Radioimmunotherapy Consolidation to Three Cycles of CHOP Plus Involved-Field Radiotherapy for Limited-Stage Aggressive Diffuse B-Cell Lymphoma (SWOG 0313) Blood (ASH Annual Meeting Abstracts) 2008;112(11):3598. [Google Scholar]
- Morschhauser F, Illidge T, Huglo D, Martinelli G, Paganelli G, Zinzani PL, Rule S, Liberati AM, Milpied N, Hess G, Stein H, Kalmus J, Marcus R. Efficacy and safety of yttrium-90 ibritumomab tiuxetan in patients with relapsed or refractory diffuse large B-cell lymphoma not appropriate for autologous stem-cell transplantation. Blood. 2007;110(1):54–58. doi: 10.1182/blood-2007-01-068056. [DOI] [PubMed] [Google Scholar]
- Morschhauser F, Kraeber-Bodere F, Wegener WA, Harousseau JL, Petillon MO, Huglo D, Trumper LH, Meller J, Pfreundschuh M, Kirsch CM, Naumann R, Kropp J, Horne H, Teoh N, Le Gouill S, Bodet-Milin C, Chatal JF, Goldenberg DM. High rates of durable responses with anti-CD22 fractionated radioimmunotherapy: results of a multicenter, phase I/II study in non-Hodgkin's lymphoma. Journal of Clinical Oncology. 2010;28(23):3709–3716. doi: 10.1200/JCO.2009.27.7863. [DOI] [PubMed] [Google Scholar]
- Pfreundschuh M. Growing importance of MYC/BCL2 immunohistochemistry in diffuse large B-cell lymphomas. Journal of Clinical Oncology. 2012;30(28):3433–3435. doi: 10.1200/JCO.2012.44.4729. [DOI] [PubMed] [Google Scholar]
- Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, Ma D, Gill D, Walewski J, Zinzani PL, Stahel R, Kvaloy S, Shpilberg O, Jaeger U, Hansen M, Lehtinen T, Lopez-Guillermo A, Corrado C, Scheliga A, Milpied N, Mendila M, Rashford M, Kuhnt E, Loeffler M. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7(5):379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, Reiser M, Nickenig C, Clemens M, Peter N, Bokemeyer C, Eimermacher H, Ho A, Hoffmann M, Mertelsmann R, Trumper L, Balleisen L, Liersch R, Metzner B, Hartmann F, Glass B, Poeschel V, Schmitz N, Ruebe C, Feller AC, Loeffler M, German High-Grade Non-Hodgkin Lymphoma Study G. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60) Lancet Oncology. 2008;9(2):105–116. doi: 10.1016/S1470-2045(08)70002-0. [DOI] [PubMed] [Google Scholar]
- Press OW, Unger JM, Braziel RM, Maloney DG, Miller TP, LeBlanc M, Gaynor ER, Rivkin SE, Fisher RI. A phase 2 trial of CHOP chemotherapy followed by tositumomab/iodine I 131 tositumomab for previously untreated follicular non-Hodgkin lymphoma: Southwest Oncology Group Protocol S9911. Blood. 2003;102(5):1606–1612. doi: 10.1182/blood-2003-01-0287. [DOI] [PubMed] [Google Scholar]
- Press OW, Unger JM, Braziel RM, Maloney DG, Miller TP, Leblanc M, Fisher RI, Southwest Oncology G. Phase II trial of CHOP chemotherapy followed by tositumomab/iodine I-131 tositumomab for previously untreated follicular non-Hodgkin's lymphoma: five-year follow-up of Southwest Oncology Group Protocol S9911. Journal of Clinical Oncology. 2006;24(25):4143–4149. doi: 10.1200/JCO.2006.05.8198. [DOI] [PubMed] [Google Scholar]
- Press OW, Unger JM, Rimsza LM, Friedberg JW, LeBlanc M, Czuczman MS, Kaminski M, Braziel RM, Spier C, Gopal AK, Maloney DG, Cheson BD, Dakhil SR, Miller TP, Fisher RI. Phase III randomized intergroup trial of CHOP plus rituximab compared with CHOP chemotherapy plus (131)iodine-tositumomab for previously untreated follicular non-Hodgkin lymphoma: SWOG S0016. Journal of Clinical Oncology. 2013;31(3):314–320. doi: 10.1200/JCO.2012.42.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer-Cutillo J, Friedberg JW. Non-myeloablative radioimmunotherapy for non-Hodgkin's lymphoma. Seminars in Hematology. 2008;45(2):110–117. doi: 10.1053/j.seminhematol.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Stiff PJ, Unger JM, Cook JR, Constine LS, Couban S, Stewart DA, Shea TC, Porcu P, Winter JN, Kahl BS, Miller TP, Tubbs RR, Marcellus D, Friedberg JW, Barton KP, Mills GM, LeBlanc M, Rimsza LM, Forman SJ, Fisher RI. Autologous transplantation as consolidation for aggressive non-Hodgkin's lymphoma. New England Journal of Medicine. 2013;369(18):1681–1690. doi: 10.1056/NEJMoa1301077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose JM, Carter S, Burns LJ, Ayala E, Press OW, Moskowitz CH, Stadtmauer EA, Mineshi S, Ambinder R, Fenske T, Horowitz M, Fisher R, Tomblyn M. Phase III randomized study of rituximab/carmustine, etoposide, cytarabine, and melphalan (BEAM) compared with iodine-131 tositumomab/BEAM with autologous hematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: results from the BMT CTN 0401 trial. Journal of Clinical Oncology. 2013;31(13):1662–1668. doi: 10.1200/JCO.2012.45.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzig TE, Wiseman GA, Maurer MJ, Habermann TM, Micallef IN, Nowakowski GS, Ansell SM, Colgan JP, Inwards DJ, Porrata LF, Link BK, Zent CS, Johnston PB, Shanafelt TD, Allmer C, Asmann YW, Gupta M, Ballas ZK, Smith BJ, Weiner GJ. A phase I trial of immunostimulatory CpG 7909 oligodeoxynucleotide and 90 yttrium ibritumomab tiuxetan radioimmunotherapy for relapsed B-cell non-Hodgkin lymphoma. American Journal of Hematology. 2013;88(7):589–593. doi: 10.1002/ajh.23460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes A, Flinn I, Berdeja JG, Friedberg JW, Alberti S, Thieblemont C, Morschhauser F, Hellemans P, Hall B, Smit J, Skee D, de Vries R, Todorovic M, Khan I, Fourneau N, Oki Y. Phase Ib study combining ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in patients with CD20-positive B-cell non-Hodgkin lymphoma (NHL) Journal of Clinical Oncology (ASCO Meeting Abstracts) 2013;31(15_suppl):8502. [Google Scholar]
- Ziepert M, Hasenclever D, Kuhnt E, Glass B, Schmitz N, Pfreundschuh M, Loeffler M. Standard International prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. Journal of Clinical Oncology. 2010;28(14):2373–2380. doi: 10.1200/JCO.2009.26.2493. [DOI] [PubMed] [Google Scholar]
- Zinzani PL, Tani M, Fanti S, Stefoni V, Musuraca G, Castellucci P, Marchi E, Farsad M, Fina M, Pellegrini C, Alinari L, Derenzini E, de Vivo A, Bacci F, Pileri S, Baccarani M. A phase II trial of CHOP chemotherapy followed by yttrium 90 ibritumomab tiuxetan (Zevalin) for previously untreated elderly diffuse large B-cell lymphoma patients. Annals of Oncology. 2008;19(4):769–773. doi: 10.1093/annonc/mdm560. [DOI] [PubMed] [Google Scholar]
- Zinzani PL, Rossi G, Franceschetti S, Botto B, Di Rocco A, Cabras MG, Petti MC, Stefoni V, Broccoli A, Fanti S, Pellegrini C, Montini GC, Gandolfi L, Derenzini E, Argnani L, Fina M, Tucci A, Bottelli C, Pileri S, Baccarani M. Phase II trial of short-course R-CHOP followed by 90Y-ibritumomab tiuxetan in previously untreated high-risk elderly diffuse large B-cell lymphoma patients. Clinical Cancer Research. 2010;16(15):3998–4004. doi: 10.1158/1078-0432.CCR-10-0162. [DOI] [PubMed] [Google Scholar]