Abstract
Objectives
Adherence is critical for maximizing the effectiveness of preexposure prophylaxis (PrEP) in preventing HIV infection. Strategies for promoting adherence to HIV treatment, and their potential application to PrEP adherence, have received considerable attention. However, adherence promotion strategies for prevention medications have not been well characterized and may be more applicable to PrEP. We aimed to identify adherence support interventions that have been effective in other prevention fields and could be applied in the HIV prevention context to support pill taking among PrEP users.
Methods
To identify adherence support interventions that could be evaluated and applied in the PrEP context, we conducted a systematic review across the following prevention fields: hypertension, latent tuberculosis infection, hyperlipidemia, oral contraceptives, osteoporosis, malaria prophylaxis, and post-exposure prophylaxis for HIV infection. We included randomized controlled trials that evaluated the efficacy of interventions to improve adherence to daily oral medications prescribed for primary prevention in healthy individuals or for secondary prevention in asymptomatic individuals.
Results
Our searches identified 585 studies, of which 48 studies met the eligibility criteria and were included in the review; nine evaluated multiple strategies, yielding 64 separately tested interventions. Interventions with the strongest evidence for improving adherence included complex, resource-intensive interventions, which combined multiple adherence support approaches, and low-cost, low-intensity interventions that provided education or telephone calls for adherence support.
Conclusions
Our review identified adherence interventions with strong evidence of efficacy across prevention fields and provides recommendations for evaluating these interventions in upcoming PrEP studies.
Keywords: medication adherence, preventive therapy, preexposure prophylaxis (PrEP), antiretroviral medications, human immunodeficiency virus (HIV), review
Introduction
Despite decades of prevention efforts and breakthroughs, human immunodeficiency virus (HIV) infection is a global pandemic, with 2.5 million people newly infected in 2011.[1] Preexposure prophylaxis (PrEP) with once-daily oral emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) was shown to reduce the risk of HIV infection among men who have sex with men (MSM) and transgender women in the iPrEx trial,[2] heterosexual serodiscordant couples in the Partners PrEP study,[3] and heterosexual men and women in a CDC PrEP trial.[4] Based on these findings, the U.S. Food and Drug Administration (FDA) approved daily oral FTC/TDF for the prevention of HIV acquisition in July 2012.
Results from PrEP trials have highlighted the relationship between adherence and efficacy. In iPrEx, participants with drug detected in blood were estimated to have a substantially higher reduction in HIV risk than seen in the intention-to-treat analysis (92% vs. 44%).[2] Similarly, in the CAPRISA microbicide trial, self-reported high adherers had the highest level of protection, while low adherers experienced lower PrEP efficacy.[5] Two large PrEP studies in African women, FEM-PrEP[6] and VOICE,[7] were unable to demonstrate PrEP efficacy, due in large part to low adherence to study drug.[6],[7] Across trials, analyses limited to those with detectable levels of study drug demonstrated higher efficacy than analyses that did not take adherence into consideration. As such, adherence has become a well-recognized requisite to realizing PrEP protection.
As PrEP rolls out in the United States, research and demonstration projects are testing intervention approaches that have effectively supported adherence to antiretroviral therapy (ART) among HIV-infected individuals. Guiding these efforts are a number of systematic reviews of ART adherence interventions,[8–11] as well as reviews of interventions for adherence to medications used to treat chronic medical conditions.[12–14] Although lessons learned from the treatment of HIV[15] and other chronic diseases can provide useful guidance in designing interventions to support PrEP adherence, successful adherence support strategies in healthy or asymptomatic populations may differ from those that are successful in symptomatic populations. For example, while a number of adherence barriers and facilitators identified in iPrEx participants were similar to those in the HIV treatment setting,[16] other factors, including level of sexual activity,[17] perception of risk of HIV acquisition,[9, 18] and support from an HIV-positive partner,[19] may be unique to PrEP and may require tailored interventions in the prevention setting.
A number of studies have evaluated interventions specifically to improve adherence to medications used for preventing, rather than treating, health conditions. However, these adherence promotion strategies have not been synthesized in a comprehensive review. To address this gap in the literature, we conducted a systematic review across clinical prevention fields to identify adherence support interventions that could be applied in the PrEP context.
Methods
Data Sources and Study Selection
We only considered randomized controlled trials (RCTs) for inclusion in this review because they provide the strongest evidence for intervention efficacy. Specifically, we included RCTs that evaluated the efficacy of interventions to improve adherence to daily oral medications. We included medications prescribed for primary prevention in healthy individuals or for secondary prevention in asymptomatic individuals, groups that are likely to have similar perceptions of risk and thus comparable barriers and facilitators to adherence. Searches were conducted separately for each of the following prevention medication fields: oral contraceptives to prevent pregnancy, prophylaxis for latent tuberculosis, prophylaxis for malaria, post-exposure prophylaxis for HIV, medications to prevent osteoporosis, and medications to control hyperlipidemia or hypertension to prevent cardiovascular disease and stroke.
Our search included studies published at any time through December 31, 2011. Sources of data included the following online databases: PubMed; the Cochrane Central Register of Controlled Trials; and the World Health Organization’s Global Health Library, which includes a wide range of regional indices (AIM, LILACS, IMEMR, IMSEAR, and WPRIM). Search terms for adherence and each prevention medication field were based on National Library of Medicine Medical Subject Headings (MeSH) terms and relevant terms used in recent Cochrane reviews on related topics. Adherence outcomes can be measured with a variety of approaches, including direct measures (e.g., drug levels or treatment outcomes), indirect measures (e.g., electronic monitoring), subjective measures (e.g., self-report), and secondary measures (e.g., attending a follow-up appointment).[20] We included studies in this review that used at least one of any of these measures of adherence.
Two trained researchers independently reviewed each title and abstract to determine whether it met the inclusion criteria, with conflicts resolved by discussion or consultation with a third reviewer. We manually searched the reference lists of reviews and included papers to identify any articles missed by our search strategy.
Data Extraction and Quality Assessment
Study features were abstracted from each article, and authors were contacted for full text or clarification on methods or results as necessary. The assessment of methodological quality of each study was based on the Cochrane Collaboration’s bias assessment tool, which uses a set of criteria to judge the risk of bias introduced by the following study features: sequence generation; allocation concealment; blinding of participants, study personnel, and outcome assessors; completeness of outcome data; selective outcome reporting; and other potential threats to validity. Two reviewers working independently abstracted data and assessed the risk of bias for each study, with conflicts resolved by discussion or consultation with a third reviewer.
Data Synthesis and Analysis
Because of the heterogeneous nature of the study populations, outcomes, and types of interventions yielded by these searches, we determined that it was inappropriate to conduct a meta-analysis or other quantitative analyses. We summarized study characteristics and grouped interventions into categories that reflected the main component of the intervention evaluated. Studies that tested multiple interventions separately were included in multiple intervention categories, while studies testing combined interventions were categorized as multi-modal. To determine which interventions should be applied in the PrEP context, we identified studies that provided the strongest evidence of an effect, as well as those that showed benefit across clinical fields.
Results
Description of Studies
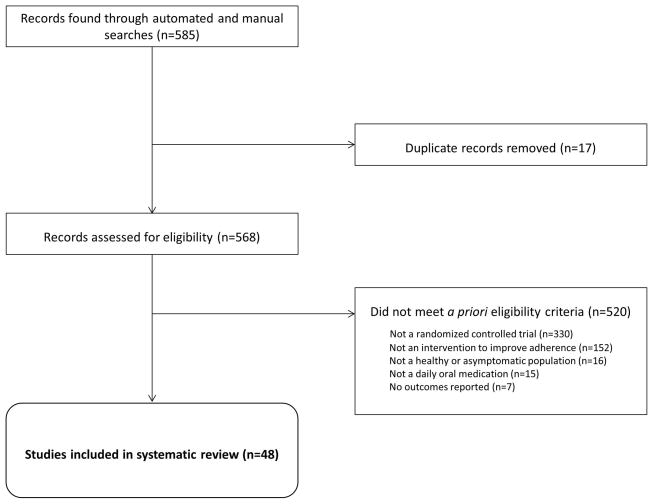
Our searches identified 585 studies, of which 48 studies met the eligibility criteria and were included in the review (Figure 1).[21–69] The studies were published from 1979 through 2011. After duplicate studies were removed, the most common reason for exclusion from the review was using a non-RCT study design. Of the 48 studies, nine evaluated multiple strategies, yielding 64 separately tested interventions. Most of the interventions (40/64; 63%) were tested in the United States. All studies were published in English with the exception of four Spanish-language articles.
Figure 1. Identified studies of interventions to support adherence to daily oral medications prescribed in healthy or asymptomatic populations.
Eligibility criteria were as follows: 1) randomized controlled trial; 2) at least one adherence outcome; 3) designed to support adherence to a daily oral medication in healthy or asymptomatic populations; 4) conducted in the following clinical fields: hypertension, latent tuberculosis infection, hyperlipidemia, oral contraceptives to prevent pregnancy, osteoporosis, malaria prophylaxis, and post-exposure prophylaxis for HIV infection.
Interventions were tested among individuals using preventive medication for hypertension (28), latent tuberculosis (15), hyperlipidemia (7), pregnancy (6), osteoporosis (6), malaria (1), and HIV infection (1). While some studies focused on marginalized populations, such as homeless individuals,[35, 37, 43] injection drug users,[36] Latino adolescents,[39] or low-income women,[25, 42] the majority included all individuals who had been prescribed or were eligible for the prevention medication under study. Several studies evaluated interventions among patients with asymptomatic disease that had not been successfully controlled, such as hypertension, but only one[48] was specifically targeted to individuals who had demonstrated difficulty with adherence.
Methodology and Study Quality
Adherence outcomes were measured using a variety of strategies, including self-report, pharmacy refill, visit attendance, pill count, medical records, direct observation, measurement of clinical outcomes, drug level testing, and electronic monitoring devices, such as Medical Electronic Monitoring System (MEMS) caps. Almost half of interventions (26/64; 41%) were evaluated using direct measures of clinical outcomes or drug level testing, while less than one fifth (10/64; 16%) relied exclusively on self-reported adherence. Half of interventions (32/64; 50%) were evaluated using multiple types of adherence measures. Duration of follow-up for adherence assessment ranged from three weeks to two years, with a mean duration of follow-up of eight months.
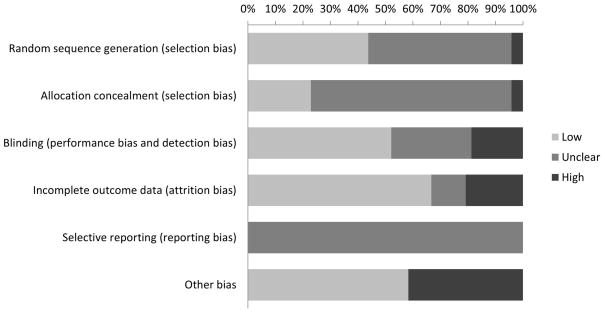
Approximately half of included studies (28/48; 58%) met at least one criterion for risk of bias. Given the nature of the interventions evaluated in some of the studies, it was not possible to blind participants, investigators, and/or outcome assessors. Thus, risk of bias most frequently resulted from lack of blinding (9/48; 19%), as well as loss to follow-up that resulted in incomplete outcome data (10/48; 21%; Figure 2).
Figure 2. Risk of bias among included studies (N=48).
“Other bias” included bias potentially introduced by using different adherence measures across intervention groups, not accounting for clustering in analysis, contamination of the control group, not accounting for baseline differences between the intervention and control group, and using different outcome measures over the course of study follow-up.
Intervention Types
Interventions were grouped into 10 categories (see Table 1) and are further described in the supplemental materials. Multi-modal approaches combined up to five components in one intervention. Interventions that provided information or education were the most frequently identified, and included printed materials such as brochures, magazines, or leaflets;[27, 50, 54, 57, 63, 67] videotapes;[57] personalized educational sessions with a nurse;[46, 51] or group sessions.[66, 67] Counseling or motivational interviewing interventions were provided by trained counselors,[39, 70] nurses,[33, 45] or an “expert system” computer program.[21] Counseling approaches included tailored adherence guidance based on the transtheoretical model,[21] providing an opportunity for patients to talk about problems with their medication,[45] identifying and reducing barriers to adherence,[33, 39] and reducing risk behavior,[21, 70] and ranged from one to 12 sessions. Counseling provided by peers[25, 31, 36] was included in the peer-based intervention category, which also included peer health advisers who directly observed doses taken at a clinic,[35] peer health advisers who accompanied patients to their visits,[37] and support provided by a friend or family member designated by the patient.[27]
Table 1.
Intervention types identified by searches (N=64).
| Intervention type | Description | n (%) |
|---|---|---|
| Information or education-based | Printed materials; videotapes; individual sessions with a nurse; group sessions | 12 (19) |
| Telephone-based | Reminder calls; support for adherence or side effects | 8 (13) |
| Provision of feedback about medication adherence or clinical outcomes | Data shared with subject by physician or nurse on adherence or changes in clinical outcomes | 7 (11) |
| Multi-modal | Combination of intervention approaches | 7 (11) |
| Peer-based | Peer health advisers or counselors; designated friend or family member for support | 6 (9) |
| Incentives or contracts | Contracts or cash incentives for kept appointments or pills taken | 6 (9) |
| Changes in the structure of pill delivery | Blister packs; multiple pill packs at a time; digital timepiece on pill vial; directly observed therapy | 6 (9) |
| Counseling or motivational interviewing | Provided by trained counselors, nurses, or an “expert” computer program | 5 (8) |
| Short message service (SMS) or interactive voice response system (IVRS) | Adherence reminders; computer system to provide adherence support or collect clinical information | 4 (6) |
| Self-monitoring of clinical outcomes | Taking blood pressure at home | 3 (5) |
Pill delivery interventions included the provision of blister packs for pills,[30, 47] multiple packs at a time,[23] a digital timepiece on the medication vial that showed the last time it was opened,[32] and directly observed therapy.[36, 42] Telephone-based interventions involved calls to remind subjects about their scheduled visits,[50] to remind subjects to take their medication as prescribed,[26, 50, 64] to remind patients about their next prescription refill,[65] to provide adherence support,[22, 27, 59] or to provide counseling about side effects as needed.[62] All of the self-monitoring interventions involved hypertensive subjects taking their own blood pressure at home.[27, 30, 68] Interventions using incentives or contracts were primarily tested in individuals prescribed medication for latent tuberculosis infection,[31, 35–37, 43] with the exception of one tested in patients with hypertension,[30] and most tested the effect of small cash incentives for kept appointments, adherence, or directly observed therapy in homeless populations.
Interventions that provided feedback on medication adherence or clinical outcomes included sharing adherence data from MEMS caps with subjects,[40, 44] providing information on bone improvement among patients with osteoporosis,[38, 56, 60] and providing information on blood pressure among patients with hypertension.[32, 61] Both of the short message service (SMS) interventions were daily adherence reminders sent to patients’ mobile phones,[34, 58] while both of the interactive voice response system (IVRS) interventions included calls to or from a computer system to provide adherence support[49, 52] or collect clinical information (i.e., blood pressure measurements) from patients.[49]
The duration and intensity of interventions varied widely, ranging from low-cost, low-intensity interventions, such as a single educational packet over the course of a year,[54] to more intensive interventions, such as twice-weekly directly observed therapy.[35, 36] Of the 62 interventions for which the site of the intervention could be determined, 24 (29%) were clinic-based, 22 (35%) were home-based, and 16 (26%) involved some combination of delivery at the clinic and delivery in the home.
Intervention Effects
Overall, two-thirds of interventions (39/64; 61%) reported a statistically significant improvement in at least one adherence measure that was sustained until the end of the pre-specified follow-up period (Table 2). One self-monitoring intervention resulted in a small but statistically significant difference; the authors believed this slight difference was not clinically significant, so this intervention was not categorized as having had an effect.[68] Positive results were most frequently reported for multi-modal interventions (7/7; 100%), followed by counseling (4/5; 80%), telephone-based interventions (6/8; 75%), and education-based interventions (9/12; 75%). Positive results were more frequently reported for home-based interventions (15/22; 68%) or those that combined delivery in the home and clinic (11/16; 69%) than for interventions delivered in a clinic setting (11/24; 46%).
Table 2.
Percentage of each type of intervention reporting a statistically significant improvement in adherence by outcomes measured.a
| Type of intervention | Total |
Adherence measure
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Visit attendance |
Self-report | Pharmacy refill |
Pill count | Medical records |
Direct observation |
Clinical outcomes |
Drug level testing |
Electronic monitoring |
||
| NPR/NA (%) | NPR/NA (%) | NPR/NA (%) | NPR/NA (%) | NPR/NA (%) | NPR/NA (%) | NPR/NA (%) | NPR/NA (%) | NPR/NA (%) | NPR/NA (%) | |
| Total | 39/64 (61) | 4/6 (67) | 17/31 (55) | 7/9 (78) | 9/13 (69) | 4/6 (67) | 0/1 (0) | 11/20 (55) | 3/7 (43) | 3/13 (23) |
|
| ||||||||||
| Education | 9/12 (75) | 1/1 (100) | 4/6 (67) | 1/1 (100) | 3/3 (100) | - | - | 3/5 (60) | 1/1 (100) | 1/1 (100) |
|
| ||||||||||
| Telephone-based | 6/8 (75) | 1/1 (100) | 2/4 (50) | 3/4 (75) | 2/2 (100) | - | - | 1/1 (100) | 1/1 (100) | - |
|
| ||||||||||
| Feedback on adherence or clinical outcomes | 2/7 (29) | - | 1/2 (50) | - | 1/2 (50) | - | - | 1/4 (25) | - | 0/2 (0) |
|
| ||||||||||
| Multi-modal | 7/7 (100) | - | 2/2 (100) | 1/1 (100) | 1/1 (100) | 2/2 (100) | - | 2/2 (100) | 1/1 (1) | 2/2 (100) |
|
| ||||||||||
| Peer-based | 3/6 (50) | 1/2 (50) | 1/3 (33) | 1/1 (100) | 0/1 (0) | 1/2 (50) | - | 0/1 (0) | 0/2 (0) | 0/1 (0) |
|
| ||||||||||
| Incentives or contracts | 3/6 (50) | 1/2 (50) | 1/2 (50) | - | 0/1 (0) | 1/2 (50) | 0/1 (0) | 1/1 (100) | 0/1 (0) | 0/1 (0) |
|
| ||||||||||
| Structure of pill delivery | 2/6 (33) | - | 1/5 (20) | - | 1/2 (50) | - | - | 1/3 (33) | 0/1 (0) | 0/2 (0) |
|
| ||||||||||
| Counseling | 4/5 (80) | - | 4/4 (100) | - | - | - | - | 0/1 (0) | - | 0/1 (0) |
|
| ||||||||||
| SMS or IVRS | 2/4 (50) | - | 0/1 (0) | 1/1 (100) | 1/1 (100) | - | - | 1/1 (100) | - | 0/2 (0) |
|
| ||||||||||
| Self-monitoring of clinical outcomes | 1/3 (33) | - | 1/2 (50) | 0/1 (0) | - | - | - | 1/1 (100) | - | 0/1 (0) |
NPR = number of interventions reporting a positive resultstatistically significant improvement infor the given adherence measure; NA = number of interventions analyzing the given adherence measure; SMS = short messaging service; IVRS = interactive voice response system. A dash indicates that there were no studies of that type using a given adherence measure. Ns may not add up to row totals because of studies using multiple adherence measures.
All intervention types were tested across multiple clinical fields with the exception of self-monitoring interventions. Interventions that were effective in improving adherence across multiple clinical fields included multi-modal interventions, counseling, incentives/contracts, education-based interventions, peer-based interventions, SMS and IVRS interventions, and telephone-based interventions. Education-based interventions were effective across the widest array of clinical fields.
Of the 25 interventions that did not result in a sustained improvement in adherence, several were conducted in individuals with high baseline adherence, potentially limiting their ability to show an effect of the intervention.[45, 57, 68] Of the four SMS/IVRS interventions, the two interventions that used daily text messages showed high acceptability among participants, but did not result in improved adherence; this lack of effect may have resulted from contamination of the control group or participants in the control arm using alternative reminder methods.[34, 58] On the other hand, the two IVRS interventions were both associated with improvements in adherence.[49, 52]
Strongest Evidence
Of the 39 interventions showing an improvement in adherence, 17 (44%) were associated with an improvement in at least one clinical outcome; of those 17, ten interventions (59%) were deemed to be at low risk of bias and thus provided the strongest evidence for approaches to improve prevention medication adherence. All were conducted in individuals with hypertension or hyperlipidemia; three were multi-modal,[32, 48, 69] three were education-based,[50, 63, 66] two provided feedback on adherence or clinical outcomes,[32, 61] one intervened on the method of pill delivery,[32] and one was telephone-based.[64] Of the 9 interventions presenting an absolute difference in adherence outcomes, effect sizes ranged widely (9%–73%), with most reporting effect sizes below 25 percent; one intervention presented relative results, with 40% lower odds of uncontrolled systolic blood pressure and 90% higher odds of adherence in the intervention arm compared with the control arm.
Of the 10 strongest-evidence interventions, the duration of follow-up was brief (4–6 months of follow-up), with the exception of one of the feedback interventions, which followed participants for 12 months. In that intervention, patients with hypertension were provided written treatment instructions by a physician along with a card on which blood pressure, prescription, and time of next visit were recorded, with the goal of improving continuation of treatment and blood pressure over the course of a year; the study found a 15% higher proportion continuing treatment and a 17% higher proportion with a 10% decrease in blood pressure in the intervention arm compared with the control arm.[61] Two of the multi-modal interventions – which used various combinations of skills training, information provision, self-monitoring, feedback on adherence and clinical outcomes, and other approaches – resulted in substantial increases in adherence (21%–73%) and reductions in blood pressure,[32, 48] although one the intervention yielding a 73% increase in adherence was conducted in a very small sample (N=15).[48]
Of note, the four strongest-evidence education-based and telephone-based interventions were relatively low-cost and low-intensity. In one study, three phone calls over the course of four months to discuss adherence and remind subjects about the next scheduled visit resulted in a 20% increase in the proportion of patients with hyperlipidemia who achieved control of lipid parameters.[64] Three of the strongest-evidence interventions were tested in the same RCT among hypertensive adults over age 50, in which participants were randomized to a) a group that used a medication vial equipped with a digital timepiece that provided feedback on the last time the cap was removed, b) a group that used the timepiece cap and cards to record blood pressure at study visits, or c) a group that used the cap and cards in addition to home blood pressure monitoring, with the self-monitoring group experiencing the most substantial improvement in adherence (22% higher than the control arm).[32]
Discussion
In this systematic review of interventions to improve adherence to prevention medications, we found evidence of interventions that demonstrated short-term improvements in medication adherence across a variety of clinical indications. The interventions with the strongest evidence for improving adherence were tested in individuals with hypertension or hyperlipidemia. These ranged from complex, resource-intensive interventions, which combined multiple adherence support approaches, to low-cost, low-intensity interventions that provided education or telephone calls for adherence support. Although some of the reviewed interventions or intervention components were not easily generalized to the PrEP context, the majority of the interventions, including those with the strongest evidence for adherence improvement, could be translated across clinical settings and could feasibly be evaluated among PrEP users.
Our review is consistent with previous reviews of medication adherence that were not specific to prevention medication. In a review by McDonald et al.,[13] and a more recent update of that review by Haynes et al.,[12] almost all of the interventions that improved adherence to long-term medications were complex and involved multiple components, including self-monitoring, reminders, counseling, education, and telephone calls.[12, 13] We found that all seven of the multi-modal interventions identified by our searches showed a benefit, and three were among the interventions with the strongest evidence for improvement in adherence. Unfortunately, because these studies did not test each intervention component separately, it is unknown whether all components were necessary to produce an effect on adherence. Furthermore, given their complexity, such interventions may be difficult to scale up outside of an experimental context. In Haynes et al., a variety of simple interventions were successful in improving adherence to short-term treatments but did not extend to chronic medications. Similarly, although we found that several simple interventions provided strong evidence for improving adherence, particularly those involving education and telephone support, duration of follow-up did not exceed six months in these studies and it is unknown whether their benefits would have been sustained beyond that time period.
The goal of our review was to identify interventions that may be useful for adherence support among PrEP users. Based on our findings, we recommend that PrEP researchers consider testing multi-modal intervention approaches, ideally with designs that allow for evaluation of the efficacy of individual components. Components of a multi-modal intervention to support PrEP adherence could include education about PrEP and the importance of adherence; counseling to improve adherence skills, such as incorporating pill taking into a daily routine and developing strategies for remembering doses when traveling;[9] SMS reminders or check-ins; and/or provision of feedback on medication adherence (e.g. providing results from drug level testing). We also recommend testing simpler interventions, specifically those that are education-based or provide telephone calls to support adherence to medication and remind users to attend scheduled visits. Based on recommendations in a review by Koenig et al., education-based interventions for PrEP users, in the form of either printed materials or brief discussion with a provider, could focus on improving users’ understanding and self-perception of HIV risk, information about the drug, the regimen’s requirements, potential side effects, and the signs and symptoms of acute HIV infection.[9] In addition to being less resource-intensive than complex interventions, such low-intensity interventions may be more feasible to scale up as PrEP is implemented in clinical care. Our review also found that several home-based interventions demonstrated positive results; home-based PrEP adherence strategies could be coupled with other home-based prevention strategies, such as home-based rapid HIV self-testing, which was recently approved by the FDA.[71] Finally, we recommend that adherence interventions be evaluated over a duration of follow-up that is sufficient to determine whether their benefits are sustained over time; these periods may vary given that PrEP should be used during periods of risk and is not viewed as a prevention approach required for a lifetime.
Current PrEP demonstration projects are evaluating a number of these interventions to promote PrEP adherence. These strategies include providing an educational handout with PrEP information and adherence guidance, telephone and SMS-based adherence support, drug detection feedback to participants and targeted interventions on the basis of drug detection, and various risk-reduction and adherence-promotion counseling approaches. However, most demonstration projects include adherence support for all participants and are not positioned to rigorously evaluate the efficacy of adopted approaches. There is a clear need for targeted research in this area. There are several limitations to our review. The diversity of study populations, intervention types, and adherence outcomes limited our ability to quantitatively analyze our results. Although we categorized interventions across clinical settings and intervention approaches, there may have been some overlap in intervention components such that some interventions could arguably have been classified as more than one type; regardless, we believe our categorizations provided a useful framework for descriptive analysis and recommendations. Because we did not restrict our searches to studies with a minimum sample size or a minimum duration of follow-up, the studies we included may have been limited in statistical power or their ability to identify long-term intervention effects. Although we did not exclude studies with risk of bias, some risks of bias may be difficult to avoid in the design of adherence interventions, such as lack of blinding. While we attempted to identify all eligible studies in the literature, it is possible that our search criteria missed some studies that should have been included. We were not able to include studies that have not been published; however, by searching the WHO Global Health Library, we hoped to alleviate the potential for publication bias by surveying studies published worldwide in non-English languages. Finally, the study populations included in the review may differ from populations that will be using PrEP, thus limiting the generalizability of our findings. However, we believe that the clinical settings we selected are analogous to PrEP with regards to the use of prevention medication in healthy or asymptomatic individuals, and that there are lessons to be learned from our findings that can be extrapolated to the PrEP context.
Despite these limitations, our systematic review identifies adherence interventions with strong evidence of efficacy across a range of prevention fields and provides recommendations for evaluating these potential adherence interventions in upcoming studies. Although adherence support strategies have been incorporated into PrEP trials to date,[72] almost all were implemented in such a way that their effect cannot be rigorously evaluated.[73] Several PrEP demonstration projects that are currently planned or underway are designed to evaluate the efficacy of interventions to support PrEP adherence using an RCT design, including studies in San Francisco, San Diego, and New York. In addition to the qualitative data from PrEP trials on facilitators and barriers to adherence, our review of the literature across prevention fields can provide guidance to these demonstration projects on intervention selection, study design, and outcome measurement. Improving adherence to PrEP will be critical for maximizing the public health impact of this important and novel strategy for HIV prevention.
Acknowledgments
Funding for the Prevention Umbrella for MSM in the Americas (PUMA) was by grant number R01AI083060 from the National Institute of Allergy and Infectious Diseases and the National Institute of Mental Health.
References
- 1.(UNAIDS) JUNPoHA. Global report: UNAIDS report on the global AIDS epidemic 2012. 2012. [Google Scholar]
- 2.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012 doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral Preexposure Prophylaxis for Heterosexual HIV Transmission in Botswana. The New England journal of medicine. 2012 doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 5.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure Prophylaxis for HIV Infection among African Women. The New England journal of medicine. 2012 doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marrazzo J, Ramjee G, Nair G, Palanee T, Mkhize B, Nakabiito C, et al. Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel int he VOICE study (MTN 003). 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. 2013. [Google Scholar]
- 8.Rueda S, Park-Wyllie LY, Bayoumi AM, Tynan AM, Antoniou TA, Rourke SB, et al. Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS. Cochrane Database Syst Rev. 2006;3:CD001442. doi: 10.1002/14651858.CD001442.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koenig LJ, Lyles C, Smith DK. Adherence to antiretroviral medications for HIV pre-exposure prophylaxis: lessons learned from trials and treatment studies. American journal of preventive medicine. 2013;44:S91–98. doi: 10.1016/j.amepre.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 10.Amico KR, Harman JJ, Johnson BT. Efficacy of antiretroviral therapy adherence interventions: a research synthesis of trials, 1996 to 2004. Journal of acquired immune deficiency syndromes. 2006;41:285–297. doi: 10.1097/01.qai.0000197870.99196.ea. [DOI] [PubMed] [Google Scholar]
- 11.Simoni JM, Pearson CR, Pantalone DW, Marks G, Crepaz N. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled trials. Journal of acquired immune deficiency syndromes. 2006;43 (Suppl 1):S23–35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane database of systematic reviews. 2008:CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 13.McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA : the journal of the American Medical Association. 2002;288:2868–2879. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- 14.Viswanathan M, Golin CE, Jones CD, Ashok M, Blalock SJ, Wines RC, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Annals of internal medicine. 2012;157:785–795. doi: 10.7326/0003-4819-157-11-201212040-00538. [DOI] [PubMed] [Google Scholar]
- 15.Stirratt MJ, Gordon CM. Adherence to biomedical HIV prevention methods: considerations drawn from HIV treatment adherence research. Current HIV/AIDS reports. 2008;5:186–192. doi: 10.1007/s11904-008-0027-z. [DOI] [PubMed] [Google Scholar]
- 16.Tangmunkongvorakul A, Chariyalertsak S, Amico KR, Saokhieo P, Wannalak V, Sangangamsakun T, et al. Facilitators and barriers to medication adherence in an HIV prevention study among men who have sex with men in the iPrEx study in Chiang Mai, Thailand. AIDS care. 2012 doi: 10.1080/09540121.2012.748871. [DOI] [PubMed] [Google Scholar]
- 17.Anderson PL, Lama JR, Buchbinder S, Guanira JV, Montoya-Herrera O, Casapia M, et al. Interpreting detection rates of intracellular FTC-DP and TFV-DP: the iPrEx trial. 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2011. [Google Scholar]
- 18.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ware NC, Wyatt MA, Haberer JE, Baeten JM, Kintu A, Psaros C, et al. What’s love got to do with it? Explaining adherence to oral antiretroviral pre-exposure prophylaxis for HIV-serodiscordant couples. Journal of acquired immune deficiency syndromes. 2012;59:463–468. doi: 10.1097/QAI.0b013e31824a060b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu YP, Roberts MC. A meta-analysis of interventions to increase adherence to medication regimens for pediatric otitis media and streptococcal pharyngitis. Journal of pediatric psychology. 2008;33:789–796. doi: 10.1093/jpepsy/jsn009. [DOI] [PubMed] [Google Scholar]
- 21.Johnson SS, Driskell MM, Johnson JL, Dyment SJ, Prochaska JO, Prochaska JM, et al. Transtheoretical model intervention for adherence to lipid-lowering drugs. Disease management : DM. 2006;9:102–114. doi: 10.1089/dis.2006.9.102. [DOI] [PubMed] [Google Scholar]
- 22.Salleras Sanmarti L, Alcaide Megias J, Altet Gomez MN, Canela Soler J, Navas Alcala E, Sune Puigbo MR, et al. Evaluation of the efficacy of health education on the compliance with antituberculosis chemoprophylaxis in school children. A randomized clinical trial. Tubercle and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 1993;74:28–31. doi: 10.1016/0962-8479(93)90065-6. [DOI] [PubMed] [Google Scholar]
- 23.Chin-Quee D, Otterness C, Wedderburn M, McDonald O, Janowitz B. One versus multiple packs for women starting oral contraceptive pills: a comparison of two distribution regimens. Contraception. 2009;79:369–374. doi: 10.1016/j.contraception.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Morisky DE, Malotte CK, Choi P, Davidson P, Rigler S, Sugland B, et al. A patient education program to improve adherence rates with antituberculosis drug regimens. Health education quarterly. 1990;17:253–267. doi: 10.1177/109019819001700303. [DOI] [PubMed] [Google Scholar]
- 25.Jay MS, DuRant RH, Shoffitt T, Linder CW, Litt IF. Effect of peer counselors on adolescent compliance in use of oral contraceptives. Pediatrics. 1984;73:126–131. [PubMed] [Google Scholar]
- 26.Guthrie RM. The effects of postal and telephone reminders on compliance with pravastatin therapy in a national registry: results of the first myocardial infarction risk reduction program. Clinical therapeutics. 2001;23:970–980. doi: 10.1016/s0149-2918(01)80084-9. [DOI] [PubMed] [Google Scholar]
- 27.Kirscht JP, Kirscht JL, Rosenstock IM. A test of interventions to increase adherence to hypertensive medical regimens. Health education quarterly. 1981;8:261–272. doi: 10.1177/109019818100800303. [DOI] [PubMed] [Google Scholar]
- 28.Deijen JB, Kornaat H. The influence of type of information, somatization, and locus of control on attitude, knowledge, and compliance with respect to the triphasic oral contraceptive Tri-Minulet. Contraception. 1997;56:31–41. doi: 10.1016/s0010-7824(97)00071-1. [DOI] [PubMed] [Google Scholar]
- 29.Magid DJ, Ho PM, Olson KL, Brand DW, Welch LK, Snow KE, et al. A multimodal blood pressure control intervention in 3 healthcare systems. The American journal of managed care. 2011;17:e96–103. [PubMed] [Google Scholar]
- 30.Binstock ML, Franklin KL. A comparison of compliance techniques on the control of high blood pressure. American journal of hypertension. 1988;1:192S–194S. doi: 10.1093/ajh/1.3.192s. [DOI] [PubMed] [Google Scholar]
- 31.Morisky DE, Malotte CK, Ebin V, Davidson P, Cabrera D, Trout PT, et al. Behavioral interventions for the control of tuberculosis among adolescents. Public health reports. 2001;116:568–574. doi: 10.1016/S0033-3549(04)50089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKenney JM, Munroe WP, Wright JT., Jr Impact of an electronic medication compliance aid on long-term blood pressure control. Journal of clinical pharmacology. 1992;32:277–283. doi: 10.1002/j.1552-4604.1992.tb03837.x. [DOI] [PubMed] [Google Scholar]
- 33.Hanna KM. Effect of nurse-client transaction on female adolescents’ oral contraceptive adherence. Image--the journal of nursing scholarship. 1993;25:285–290. doi: 10.1111/j.1547-5069.1993.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 34.Ollivier L, Romand O, Marimoutou C, Michel R, Pognant C, Todesco A, et al. Use of short message service (SMS) to improve malaria chemoprophylaxis compliance after returning from a malaria endemic area. Malaria journal. 2009;8:236. doi: 10.1186/1475-2875-8-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tulsky JP, Pilote L, Hahn JA, Zolopa AJ, Burke M, Chesney M, et al. Adherence to isoniazid prophylaxis in the homeless: a randomized controlled trial. Archives of internal medicine. 2000;160:697–702. doi: 10.1001/archinte.160.5.697. [DOI] [PubMed] [Google Scholar]
- 36.Chaisson RE, Barnes GL, Hackman J, Watkinson L, Kimbrough L, Metha S, et al. A randomized, controlled trial of interventions to improve adherence to isoniazid therapy to prevent tuberculosis in injection drug users. The American journal of medicine. 2001;110:610–615. doi: 10.1016/s0002-9343(01)00695-7. [DOI] [PubMed] [Google Scholar]
- 37.Pilote L, Tulsky JP, Zolopa AR, Hahn JA, Schecter GF, Moss AR. Tuberculosis prophylaxis in the homeless. A trial to improve adherence to referral. Archives of internal medicine. 1996;156:161–165. [PubMed] [Google Scholar]
- 38.Schousboe JT, DeBold RC, Kuno LS, Weiss TW, Chen Y, Abbott TA. Education and phone follow-up in postmenopausal women at risk for osteoporosis. Dis Manage Health Outcomes. 2005;13:395–404. [Google Scholar]
- 39.Hovell MF, Sipan CL, Blumberg EJ, Hofstetter CR, Slymen D, Friedman L, et al. Increasing Latino adolescents’ adherence to treatment for latent tuberculosis infection: a controlled trial. American journal of public health. 2003;93:1871–1877. doi: 10.2105/ajph.93.11.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santschi V, Rodondi N, Bugnon O, Burnier M. Impact of electronic monitoring of drug adherence on blood pressure control in primary care: a cluster 12-month randomised controlled study. European journal of internal medicine. 2008;19:427–434. doi: 10.1016/j.ejim.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Roland ME, Neilands TB, Krone MR, Coates TJ, Franses K, Chesney MA, et al. A randomized noninferiority trial of standard versus enhanced risk reduction and adherence counseling for individuals receiving post-exposure prophylaxis following sexual exposures to HIV. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53:76–83. doi: 10.1093/cid/cir333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Westhoff C, Heartwell S, Edwards S, Zieman M, Cushman L, Robilotto C, et al. Initiation of oral contraceptives using a quick start compared with a conventional start: a randomized controlled trial. Obstetrics and gynecology. 2007;109:1270–1276. doi: 10.1097/01.AOG.0000264550.41242.f2. [DOI] [PubMed] [Google Scholar]
- 43.Tulsky JP, Hahn JA, Long HL, Chambers DB, Robertson MJ, Chesney MA, et al. Can the poor adhere? Incentives for adherence to TB prevention in homeless adults. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2004;8:83–91. [PubMed] [Google Scholar]
- 44.Wetzels GE, Nelemans PJ, Schouten JS, Dirksen CD, van der Weijden T, Stoffers HE, et al. Electronic monitoring of adherence as a tool to improve blood pressure control. A randomized controlled trial. American journal of hypertension. 2007;20:119–125. doi: 10.1016/j.amjhyper.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 45.Schroeder K, Fahey T, Hollinghurst S, Peters TJ. Nurse-led adherence support in hypertension: a randomized controlled trial. Family practice. 2005;22:144–151. doi: 10.1093/fampra/cmh717. [DOI] [PubMed] [Google Scholar]
- 46.Hacihasanoglu R, Gozum S. The effect of patient education and home monitoring on medication compliance, hypertension management, healthy lifestyle behaviours and BMI in a primary health care setting. Journal of clinical nursing. 2011;20:692–705. doi: 10.1111/j.1365-2702.2010.03534.x. [DOI] [PubMed] [Google Scholar]
- 47.Christensen A, Christrup LL, Fabricius PE, Chrostowska M, Wronka M, Narkiewicz K, et al. The impact of an electronic monitoring and reminder device on patient compliance with antihypertensive therapy: a randomized controlled trial. Journal of hypertension. 2010;28:194–200. doi: 10.1097/HJH.0b013e328331b718. [DOI] [PubMed] [Google Scholar]
- 48.Ruppar TM. Randomized pilot study of a behavioral feedback intervention to improve medication adherence in older adults with hypertension. The Journal of cardiovascular nursing. 2010;25:470–479. doi: 10.1097/JCN.0b013e3181d5f9c5. [DOI] [PubMed] [Google Scholar]
- 49.Friedman RH, Kazis LE, Jette A, Smith MB, Stollerman J, Torgerson J, et al. A telecommunications system for monitoring and counseling patients with hypertension. Impact on medication adherence and blood pressure control. American journal of hypertension. 1996;9:285–292. doi: 10.1016/0895-7061(95)00353-3. [DOI] [PubMed] [Google Scholar]
- 50.Marquez Contreras E, Vegazo Garcia O, Claros NM, Gil Guillen V, de la Figuera von Wichmann M, Casado Martinez JJ, et al. Efficacy of telephone and mail intervention in patient compliance with antihypertensive drugs in hypertension. ETECUM-HTA study. Blood pressure. 2005;14:151–158. doi: 10.1080/08037050510008977. [DOI] [PubMed] [Google Scholar]
- 51.Amado Guirado E, Pujol Ribera E, Pacheco Huergo V, Borras JM. Knowledge and adherence to antihypertensive therapy in primary care: results of a randomized trial. Gaceta sanitaria/SESPAS. 2011;25:62–67. doi: 10.1016/j.gaceta.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 52.Stacy JN, Schwartz SM, Ershoff D, Shreve MS. Incorporating tailored interactive patient solutions using interactive voice response technology to improve statin adherence: results of a randomized clinical trial in a managed care setting. Population health management. 2009;12:241–254. doi: 10.1089/pop.2008.0046. [DOI] [PubMed] [Google Scholar]
- 53.Nielsen D, Ryg J, Nielsen W, Knold B, Nissen N, Brixen K. Patient education in groups increases knowledge of osteoporosis and adherence to treatment: a two-year randomized controlled trial. Patient education and counseling. 2010;81:155–160. doi: 10.1016/j.pec.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 54.Guilera M, Fuentes M, Grifols M, Ferrer J, Badia X. Does an educational leaflet improve self-reported adherence to therapy in osteoporosis? The OPTIMA study. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2006;17:664–671. doi: 10.1007/s00198-005-0031-8. [DOI] [PubMed] [Google Scholar]
- 55.Bednarek PH, Nichols MD, Carlson N, Edelman AB, Creinin MD, Truitt S, et al. Effect of “observed start” vs. traditional “Sunday start” on hormonal contraceptive continuation rates after medical abortion. Contraception. 2008;78:26–30. doi: 10.1016/j.contraception.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 56.Delmas PD, Vrijens B, Eastell R, Roux C, Pols HA, Ringe JD, et al. Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis. The Journal of clinical endocrinology and metabolism. 2007;92:1296–1304. doi: 10.1210/jc.2006-1526. [DOI] [PubMed] [Google Scholar]
- 57.Riesen WF, Noll G, Dariolo R. Impact of enhanced compliance initiatives on the efficacy of rosuvastatin in reducing low density lipoprotein cholesterol levels in patients with primary hypercholesterolaemia. Swiss medical weekly. 2008;138:420–426. doi: 10.4414/smw.2008.12120. [DOI] [PubMed] [Google Scholar]
- 58.Hou MY, Hurwitz S, Kavanagh E, Fortin J, Goldberg AB. Using daily text-message reminders to improve adherence with oral contraceptives: a randomized controlled trial. Obstetrics and gynecology. 2010;116:633–640. doi: 10.1097/AOG.0b013e3181eb6b0f. [DOI] [PubMed] [Google Scholar]
- 59.Waalen J, Bruning AL, Peters MJ, Blau EM. A telephone-based intervention for increasing the use of osteoporosis medication: a randomized controlled trial. The American journal of managed care. 2009;15:e60–70. [PubMed] [Google Scholar]
- 60.Clowes JA, Peel NF, Eastell R. The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. The Journal of clinical endocrinology and metabolism. 2004;89:1117–1123. doi: 10.1210/jc.2003-030501. [DOI] [PubMed] [Google Scholar]
- 61.Takala J, Niemela N, Rosti J, Sievers K. Improving compliance with therapeutic regimens in hypertensive patients in a community health center. Circulation. 1979;59:540–543. doi: 10.1161/01.cir.59.3.540. [DOI] [PubMed] [Google Scholar]
- 62.Schectman G, Hiatt J, Hartz A. Telephone contacts do not improve adherence to niacin or bile acid sequestrant therapy. The Annals of pharmacotherapy. 1994;28:29–35. doi: 10.1177/106002809402800104. [DOI] [PubMed] [Google Scholar]
- 63.Marquez Contreras E, Martel Claros N, Gil Guillen V, Martin De Pablos JL, De la Figuera von Wichmann M, Casado Martinez JJ, et al. Non-pharmacological intervention as a strategy to improve antihypertensive treatment compliance. Aten Primaria. 2009;41:501–510. doi: 10.1016/j.aprim.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marquez Contreras E, Casado Martinez JJ, Corchado Albalat Y, Chaves Gonzalez R, Grandio A, Losada Velasco C, et al. Efficacy of an intervention to improve treatment compliance in hyperlipidemias. Atencion primaria/Sociedad Espanola de Medicina de Familia y Comunitaria. 2004;33:443–450. doi: 10.1016/S0212-6567(04)79430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sclar DA, Chin A, Skaer TL, Okamoto MP, Nakahiro RK, Gill MA. Effect of health education in promoting prescription refill compliance among patients with hypertension. Clinical therapeutics. 1991;13:489–495. [PubMed] [Google Scholar]
- 66.Marquez Contreras E, Casado Martinez JJ, Lopez de Andres M, Cores Prieto E, Lopez Zamorano JM, Moreno Garcia JP, et al. Therapeutic compliance in dyslipidemias. A trial of the efficacy of health education. Atencion primaria/Sociedad Espanola de Medicina de Familia y Comunitaria. 1998;22:79–84. [PubMed] [Google Scholar]
- 67.Marquez Contreras E, Casado Martinez JJ, Celotti Gomez B, Gascon Vivo J, Martin De Pablos JL, Gil Rodriguez R, et al. Treatment compliance in arterial hypertension. A 2-year intervention trial through health education. Aten Primaria. 2000;26:5–10. doi: 10.1016/S0212-6567(00)78597-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Onzenoort HA, Verberk WJ, Kessels AG, Kroon AA, Neef C, van der Kuy PH, et al. Assessing medication adherence simultaneously by electronic monitoring and pill count in patients with mild-to-moderate hypertension. American journal of hypertension. 2010;23:149–154. doi: 10.1038/ajh.2009.207. [DOI] [PubMed] [Google Scholar]
- 69.Pladevall M, Brotons C, Gabriel R, Arnau A, Suarez C, de la Figuera M, et al. Multicenter cluster-randomized trial of a multifactorial intervention to improve antihypertensive medication adherence and blood pressure control among patients at high cardiovascular risk (the COM99 study) Circulation. 2010;122:1183–1191. doi: 10.1161/CIRCULATIONAHA.109.892778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roland ME, Elbeik TA, Kahn JO, Bamberger JD, Coates TJ, Krone MR, et al. HIV RNA testing in the context of nonoccupational postexposure prophylaxis. The Journal of infectious diseases. 2004;190:598–604. doi: 10.1086/421278. [DOI] [PubMed] [Google Scholar]
- 71.Consumer Health Information USFaDA. First Rapid Home-Use HIV Kit Approved for Self Testing. 2012. [Google Scholar]
- 72.Muchomba FM, Gearing RE, Simoni JM, El-Bassel N. State of the science of adherence in pre-exposure prophylaxis and microbicide trials. Journal of acquired immune deficiency syndromes. 2012;61:490–498. doi: 10.1097/QAI.0b013e31826f9962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Amico KR, Mansoor LE, Corneli A, Torjesen K, van der Straten A. Adherence Support Approaches in Biomedical HIV Prevention Trials: Experiences, Insights and Future Directions from Four Multisite Prevention Trials. AIDS and behavior. 2013 doi: 10.1007/s10461-013-0429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]