Abstract
Background
The brain undergoes dynamic and requisite changes into the early twenties that are associated with improved cognitive efficiency, particularly in prefrontal regions that are still undergoing neuromaturation. As alcohol consumption is typically initiated and progresses to binge drinking during this time, the objective of the present study was to investigate the impact of binge alcohol consumption on frontal lobe cortical thickness in emerging adults.
Methods
Twenty-three binge drinking (BD; 11 females, mean age 21.5 ± 1.4) and thirty-one light drinking (LD; 15 females, mean age 21.9 ± 1.6) emerging adults underwent high-resolution magnetic resonance imaging at 3 Tesla. Cortical surface reconstruction and thickness estimation were performed using Freesurfer for three a priori brain regions of interest: bilateral anterior cingulate cortex (ACC), posterior cingulate cortex (PCC) and parieto-occipital sulcus (POS). Cortical thickness measurements were then compared between BD and LD groups.
Results
Cortical thickness was significantly lower in BD than LD in the right middle ACC (mid-ACC; p≤0.05) and in the left dorsal PCC (dPCC; p≤0.01). No significant differences in cortical thickness were observed in the POS. Cortical thickness in the mid-ACC correlated negatively with higher quantity and frequency of drinks consumed (p<0.01), and positively with the number of days elapsed since most recent use (p<0.05). Furthermore, less cortical thickness in the mid-ACC in the BD group alone correlated with reported patterns of high quantity and frequency of alcohol consumption (p≤0.05).
Conclusions
Findings suggest that past and recent patterns of intermittent heavy alcohol consumption are associated with less frontal cortical thickness (i.e. ‘thinness’) of the right mid-ACC and left dPCC in emerging adults, but not the POS. While cortical thinness could have predated binge drinking, this pattern of maladaptive consumption may have acute neurotoxic effects that interfere with the finalization of neuromaturational processes in the vulnerable frontal cortex, resulting in increased microarchitectural pruning.
Keywords: cortical thickness, anterior cingulate cortex, binge alcohol drinking, young adults, synaptic pruning
Introduction
Binge drinking, as defined by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Survey on Drug Use and Health (NSDUH), is a pattern of consuming 4+ drinks (female), or 5+ drinks (male), over the course of 2 hours, on a single occasion. National rates of binge drinking among adolescents (aged 12–17) and emerging adults (aged 18–24) are approximately 23% and 79%, respectively (NSDUH; 2010). These alarmingly high rates of regular excessive use of alcohol have become a prominent public health concern, as adolescent heavy drinkers are more likely as adults to be diagnosed with an alcohol use disorder (AUD) (Hingson et al., 2006). Moreover, adolescent and young adult binge drinkers report low perceived risk associated with binge drinking (Patrick et al., 2010) and show elevated risky behaviors that reflect poor judgment, such as impaired driving, riding with an impaired driver, unsafe sex, illicit drug use, and attempting suicide (Silveri, 2012). In addition to disinhibiting deleterious behaviors, persistent binge drinking at a young age can adversely impact progressive brain development in adolescence and young adulthood (Squeglia et al., 2012; Lisdahl et al., 2013).
Adolescence is a critical neurodevelopmental period, with maturation of gray and white matter occurring between ages 4 and 20, and extending to age 25, which has been reported in magnetic resonance imaging (MRI) studies (Reiss et al., 1996; Giedd et al., 2009). Gray matter (GM) cortical thinning, reflecting synaptic pruning of excess neurons, is a normative maturational process occurring throughout adolescence and into early adulthood. Thinning principally begins in dorsal parietal regions (i.e. primary sensorimotor areas) and proceeds rostrally to frontal cortex (Sowell et al., 2001; Gogtay et al., 2004). GM pruning then extends caudally to parietal, occipital and temporal cortices (Gogtay et al., 2004). While this dynamic progression across the cortex has been established, there is non-linear maturation within distinct cortical regions. Within frontal cortex, synaptic pruning in the precentral gyrus and frontal pole are completed early in adolescence, followed by the superior and inferior frontal gyri maturing progressively in an anterior direction, and prefrontal cortical pruning and maturation occurring last (Gogtay et al., 2004). Parietal lobe maturation is similarly non-linear. Pruning within parietal cortex is completed early in the postcentral gyrus, and progresses laterally to the angular and supramarginal gyri. In addition, the occipital pole, containing primary visual cortex, and the temporal pole undergo GM pruning and maturation in early adolescence (Gogtay et al., 2004). In contrast, the rest of the temporal lobe initiates maturation processes in later adolescence. Some early maturation of entorhinal cortex occurs in inferior temporal lobe, but pruning of superior, middle and inferior temporal gyri characteristically progress slowly and in a lateral-to-medial direction (Gogtay et al., 2004). Collectively, temporal cortex and prefrontal cortex (PFC) are the last cortical regions to reach full GM maturation in early adulthood.
The temporal patterns of cortical maturation are aligned with cognitive development and behavioral changes that accompany the transition from adolescence into early adulthood. In general, GM thinning and white matter (WM) proliferation are developmental processes involving synaptic pruning and myelination that correspond to improvements in executive functioning (Kharitonova et al., 2013). In particular, these maturational processes support increased cortical efficiency and cognitive processing speed that lead to improved decision-making and risk evaluation (Reiss et al., 1996). Thus, these improved functions from late adolescence and into emerging adulthood are related to frontal lobe reorganization and refinement, as well as experience-dependent synaptic plasticity (Giedd et al., 1999; Sowell et al., 2001).
One frontal cortical region critically involved in executive functioning, reward processing, and regulating adaptive behaviors, is the cingulate cortex (Fujiwara et al., 2009). The anterior cingulate cortex (ACC), and in particular the middle anterior cingulate (mid-ACC) that mediates dorsal ACC activity (Torta and Cauda, 2011), is involved in regulating response selection (Botvinick et al., 2004), assessing risk and exerting cognitive control to make decisions (Christopolous et al., 2009), reward anticipation (Marsh et al., 2007), and error detection and conflict monitoring (Orr and Hester, 2012). The posterior cingulate cortex (PCC) also plays an important role in directing behavioral adaptations to changes in environment and exerting control over attentional focus and vigilance (Hayden et al., 2009), as well as functionally connecting with and coordinating other brain regions to meet task demands (Leech et al., 2012). Despite substantial roles in executive functioning, e.g., exerting control over impulsive and risky choices, ACC and PCC are among the last brain regions to reach full neuromaturation until early to mid-adulthood.
Differential alterations in cortical thickness, a surface-based representative measure of the cortical gray matter ribbon, in frontal regions such as the rostral anterior cingulate, frontal pole, and medial orbital frontal gyrus have been reported in binge drinking adolescents, and were related to poor attention, inhibition, and visuospatial performance (Squeglia et al., 2012). Reduced frontal lobe cortical thickness, including inferior frontal, superior frontal, and lateral frontal gyri, has also been reported in abstinent alcoholic adults (Fortier et al., 2011) and in medial frontal, inferior frontal and precentral gyri in alcohol dependent adult inpatients (Momenan et al., 2012), suggesting that long-term alcohol consumption may have toxic effects on frontal cortical tissue. Furthermore, relapse-vulnerable alcohol-dependent adults have also shown less cortical thickness in frontal tissue, including the ACC, insula, and middle and superior frontal gyri, suggesting that suboptimal morphology in brain regions related to reward may result in dysfunctional executive ‘top down’ control over reward processing and behavior that elevate risk of relapse to alcohol use (Sinha and Li, 2007; Durazzo et al., 2011).
It is not well known how binge drinking, relative to occasional light drinking, during emerging adulthood affects frontal lobe cortical thickness. Characterizing the effects of regular binge drinking on brain morphology in young emerging adults will provide a better understanding of measurable changes in the brain that result from early stages of regular, heavy use. Accordingly, the present study measured bilateral cortical thickness of ACC and PCC, and parietal occipital sulcus (POS) in emerging adult binge drinkers and age-matched light drinkers. It was hypothesized that binge drinkers would exhibit altered cortical thickness relative to light drinkers in frontal regions critical for executive function and reward processing, such as the ACC and PCC, but not the POS.
ACC and POS were chosen as a priori regions of interest (ROI) based our magnetic resonance spectroscopy (MRS) data, which reported lower inhibitory γ amino-butyric acid (GABA) and neuronal health marker N-acetyl-aspartate (NAA) in ACC, but not parieto-occipital cortex (POC), of binge drinkers, compared to light drinkers (Silveri et al., 2014). POS was selected in the current study as a comparison region, to examine specificity of altered cortical thickness in vulnerable frontal lobe regions. PCC was also included as an a priori ROI given its functional role in regulating attentional focus, vigilance and task management (Hayden et al., 2009; Leech et al., 2012). Given that ACC has already exhibited compromised neurochemical levels in binge drinkers, versus light drinkers, the rationale for conducting these ROI analyses is that group differences in cortical thickness isolated in these frontal regions may further refine our understanding of the nature of neurobiological correlates of alcohol consumption behaviors and consequences. Whole-brain analyses between binge and light drinkers were performed to explore regions demonstrating alterations in adult alcohol-dependent populations (Fortier et al., 2011; Momenan et al., 2012).
Materials and Methods
Participants
Fifty-four emerging adults, aged 18 to 24 years old, recruited from the Boston metropolitan area, were included in this study. Participants completed an alcohol use questionnaire using the Time Line Follow Back protocol (TLFB) and blood alcohol levels were estimated for each day of drinking over the past 30 days. Participants also were administered the Alcohol Use Disorders Identification Test (AUDIT) to gauge binge drinking, dependence symptoms and alcohol-related problems. Collectively, the TLFB and AUDIT were used to establish quantity and frequency of alcohol consumption during the participants’ most current episode of drinking (‘recent’ drinking) and during the three months (‘past’ drinking) prior to scanning. Via these reports, participants who met NIAAA and NSDUH criteria of consuming 4+(female)/5+(male) drinks in a single episode, on at least three occasions per month, were categorized as binge drinkers (BD). Participants reporting alcohol consumption of 1–2 drinks per week, and no binge pattern of consumption (past 3.5 years prior to study participation), were categorized as light drinkers (LD). Notably, nondrinkers were not excluded from the current study. These criteria yielded study groups of 23 BD (age = 21.5 ± 1.4; 11 female) and 31 age-matched LD (age = 21.9 ± 1.6; 15 female). Group demographics, including education, body mass index (BMI), socioeconomic status (SES), age at first alcohol use, recent and past quantity and frequency of episodic alcohol consumption, are provided in Table 1.
Table 1.
Demographic and Alcohol Use Measures
| Demographic Measures | BD (n=23) | LD (n=31) | p |
|---|---|---|---|
| % Female | 52% | 45% | ns |
| Age | 22.0 ± 1.2 | 21.5 ± 1.6 | ns |
| Education | 15.0 ± 1.2 | 14.6 ± 1.5 | ns |
| College Attendance (current, completed 4 year degree, some attendance) | 91.3%, 8.7%, 4.3% | 58.1%, 35.5%, 3.2% | - |
| SES | 54.6 ± 9.7 | 51.0 ± 11.4 | ns |
| Handedness | 22R, 1L | 31R, 0L | - |
| BMI | 24.0 ± 2.7 | 22.7 ± 3.3 | ns |
| % FH+ for alcoholism | 26% | 23% | ns |
| FH density | 0.13 ± 0.2 | 0.13 ± 0.3 | ns |
| Age of onset of alcohol use | 17.4 ± 1.5 | 18.1 ± 2.1 | ns (.06) |
| Recent drinking patterns (last use) | |||
| Days since last use | 6.1 ± 4.8 | 12.1 ± 14.2 | 0.05 |
| Alcoholic drinks most recently consumed | 5.2 ± 2.2 | 2.2 ± 1.2 | 0.000 |
| Past drinking patterns (last 3 months) | |||
| Days per week of drinking episodes | 1.8 ± 0.7 | 0.8 ± 0.7 | 0.000 |
| Alcoholic drinks consumed per week | 11.2 ± 13.7 | 1.7 ± 1.8 | 0.000 |
| Drinking episodes per month | 7.6 ± 3.8 | 3.0 ± 2.8 | 0.000 |
| Alcoholic drinks consumed per episode | 4.9 ± 1.9 | 1.8 ± 1.1 | 0.000 |
| Rate of drinking per episode (drinks/hour) | 2.7 ± 1.4 | 1.1 ± 0.3 | 0.000 |
| Clinical Measures | BD (n=23) | LD (n=31) | p |
|---|---|---|---|
| BIS | |||
| Cognitive | 14.2 ± 3.2 | 14.7 ± 4.4 | ns |
| Motor | 21.6 ± 3.6 | 20.0 ± 3.4 | ns |
| Non-planning | 20.4 ± 4.5 | 20.9 ± 4.7 | ns |
| Total Impulsivity | 56.4 ± 8.1 | 55.7 ± 10.4 | ns |
| YAACQ | |||
| Social-Interpersonal | 6.1 ± 4.4 | 1.9 ± 3.6 | 0.001 |
| Control | 6.7 ± 4.6 | 2.3 ± 3.4 | 0.000 |
| Self-Perception | 7.4 ± 4.5 | 2.4 ± 3.6 | 0.000 |
| Self-Care | 6.8 ± 4.7 | 2.2 ± 3.1 | 0.000 |
| Risk Behaviors | 6.6 ± 4.5 | 2.3 ± 3.2 | 0.000 |
| Academic/Occupational | 7.4 ± 5.0 | 2.5 ± 3.9 | 0.000 |
| Physical Dependence (PHYS- DEP) | 7.3 ± 5.2 | 2.5 ± 3.8 | 0.000 |
| Blackout Drinking (BLKOUT) | 4.9 ± 4.0 | 1.6 ± 2.6 | 0.001 |
| Total Score | 7.6 ± 5.2 | 2.5 ± 3.9 | 0.000 |
| POMS | |||
| Depression | 5.7 ± 7.4 | 5.3 ± 8.7 | ns |
| Vigor | 20.6 ± 4.1 | 16.8 ± 6.3 | 0.03 |
| Anger | 5.7 ± 5.5 | 3.6 ± 6.3 | ns |
| Confusion | 5.9 ± 2.2 | 6.0 ± 3.4 | ns |
| Tension | 7.6 ± 4.9 | 7.0 ± 4.0 | ns |
| Fatigue | 5.3 ± 4.7 | 4.2 ± 4.2 | ns |
| Total Mood Disturbance | 8.5 ± 20.0 | 7.6 ± 24.54 | ns |
Data represent mean values ± SD.
Abbreviations: BD, binge drinkers; LD, light drinkers; p, p-value; SES: socioeconomic status; BMI, body mass index; BIS, Barratt Impulsiveness Scale; YAACQ, Young Adult Alcohol Consequences Questionnaire; POMS, Profile of Mood States; ns, not statistically significant.
All participants were clinically evaluated using the Structured Clinical Interview for DSM-IV Non-Patient Edition (SCID-1/NP), and were free of Axis I diagnoses, neurological illness, and severe medical problems. Further exclusion criteria included past or present mental health disorders, current psychoactive substance use and/or dependence (including nicotine), current psychoactive medication use, prior episodes of loss of consciousness, or contraindications to MR scanning. Family history (FH) of alcoholism status was also evaluated, with those indicating a history of alcohol abuse or dependence in parents or grandparents meeting criteria for FH+ status, from which FH density of alcoholism was calculated (Zucker et al., 1994) (Table 1). Each participant also completed a clinical MRI scan, which was examined and interpreted by a clinical neuroradiologist. All study participants were free of any clinical brain abnormalities.
Procedure
The McLean Hospital Institutional Review Board approved the clinical research protocol. Following description of the study, participants provided informed consent, and were monetarily compensated for study participation. Under supervision by the study coordinator, participants provided a urine sample that was tested for psychoactive substances. Two participants testing positive for marijuana use were excluded. Females also underwent urine testing to confirm negative pregnancy status prior to scanning.
Clinical measures
All participants completed the Barratt Impulsivity Scale (BIS-11), Profile of Mood States (POMS) and Young Adult Alcohol Consequences Questionnaire (YAACQ).
Structural MR Imaging
Participants underwent high-resolution anatomical imaging on a 3 Tesla Siemens TIM Trio whole body MRI scanner (Siemens Medical Solutions USA Inc., Malvern, PA, USA) in the McLean Imaging Center, using a 12 channel head coil to acquire 1.0×1.0×1.3m3 magnetization-prepared, rapid acquisition with gradient echoes (MPRAGE) T1-weighted images. Imaging parameters were as follows: 128 slices, 2562 matrix, echo time (TE)=2.7ms; repetition time (TR)=2100ms; inversion time (TI)=1100ms; flip=12°. These imaging parameters were chosen to optimize signal contrast between white matter (WM) and gray matter (GM) as well as between GM and cerebro-spinal fluid (CSF), in order to facilitate cortical surface segmentation and reconstruction processes.
Cortical Thickness Processing and Analysis
Cortical surface reconstruction and thickness estimates were performed using the Freesurfer morphometric analysis suite (http://surfer.nmr.mgh.harvard.edu). Technical methodological details are described in previous publications (Dale et al., 1999; Fischl and Dale, 2000; Fischl et al., 2002; Segonne et al., 2004). Briefly, automated procedures included motion correction and averaging across 3-dimensional T1-weighted MR image volumes, stripping of non-brain tissue and skull (Segonne et al., 2004), linear Talairach atlas registration, voxel intensity classification and normalization, and cortical surface segmentation (Fischl et al., 2002). Freesurfer processing produces cortical models that include tessellation of the GM and WM boundary and surface deformation to optimally define GM/WM and GM/CSF borders (Dale et al., 1999). These models were subsequently registered to a spherical atlas that applies individual cortical folding patterns to closely align cortical geometry across all subjects, which facilitated accurate morphological matching of cortical vertices and regions at each point of the reconstructed surface. Each hemisphere was then automatically parcellated into 74 distinct cortical regions (Destrieux et al., 2010). Cortical thickness was calculated by an automated algorithm process measuring the closest distance between the GM/WM and GM/CSF boundaries at each vertex on the tessellated cortical surface (Fischl and Dale, 2000). The calculations were based on changes between spatial intensity gradients across tissue classes and do not rely on absolute signal intensity. Furthermore, representations of cortical thickness are not limited by voxel resolution of the original data, and therefore submillimeter microarchitectural differences can be detected between groups (Fischl and Dale, 2000). Automated cortical thickness measurements have been tested and validated against histological analysis (Rosas et al., 2002) and manual measurements (Salat et al., 2004). Statistical comparisons of whole-brain surface maps were conducted to assess BD and LD differences in cortical thickness at each vertex. Surface maps in each hemisphere were defined by statistical voxelwise thresholds of p=0.01, and were smoothed using a Gaussian kernel with a full-width half-maximum (FWHM)=10, which identified significant contiguous clusters of group differences in cortical thickness for an exploratory whole-brain analysis. In order to account and correct for multiple comparisons, a Monte Carlo simulation cluster analysis within the Freesurfer processing stream was performed with 10,000 iterations and a cluster threshold of p=0.05. Subsequent second-level analyses were conducted to measure cortical thickness differences in a priori regions of interest.
Statistical Analyses
Demographic and clinical measures were analyzed using analyses of covariance (ANCOVAs) to compare groups. All ANCOVAs included sex as a covariate, due to previously characterized sex differences in brain structure and morphology (for review, see Ruigrok et al., 2014). Mean cortical thickness measures of parcellated regions in each hemisphere from Freesurfer were extracted for analysis of a priori ROI hypotheses (ACC, PCC, and POS), separately for the left hemisphere (LH) and the right hemisphere (RH). The parcellated anatomical structures of the ACC include the anterior cingulate gyrus and sulcus (ACC) and the middle anterior cingulate gyrus and sulcus (mid-ACC) (Destrieux et al., 2010). Parcellated PCC includes middle posterior cingulate gyrus (mid-PCC), dorsal posterior cingulate gyrus (dPCC) and ventral posterior cingulate gyrus (vPCC) (Destrieux et al., 2010). The POS is not further subparcellated. Correlations between cortical thickness, clinical measures, and self-reported alcohol use and consequences were examined using Pearson’s r correlation coefficients. Data were analyzed using SPSS 19.0 (SPSS, Chicago, IL, USA), with α=0.05.
Results
Demographic and alcohol consumption measures
There were no significant group differences observed for demographic variables (Table 1). Significant group differences were evident, however, on all quantity and frequency of alcohol consumption variables (Table 1). BD engaged in more frequent alcohol consumption behaviors per week, drinking approximately twice as often (F[1,51]=23.78, p≤0.001), at a higher rate (F[1,48]=36.37, p≤0.001), and consuming nearly 7 times as many drinks (F[1,51]=15.07, p≤0.001) as the age-matched LD group.
Alcohol use consequences and clinical measures
BD reported significantly greater adverse alcohol-use related consequences, relative to LD, on every domain of the YAACQ (Table 1), including greater likelihood of risky behaviors (i.e. drunk driving) (F[1,50]=16.38, p≤0.001), blackout drinking (F[1,50]=13.47, p≤0.001), impaired control over drinking (F[1,50]=16.11, p≤0.001), and diminished self-perception (F[1,50]=18.49, p≤0.001). No significant group differences were observed for BIS impulsiveness or POMS mood measures (Table 1), with the exception of BD reporting higher scores on the POMS Vigor subscale (F[1,43]=5.22, p≤0.03) relative to LD.
Regional measures of cortical thickness
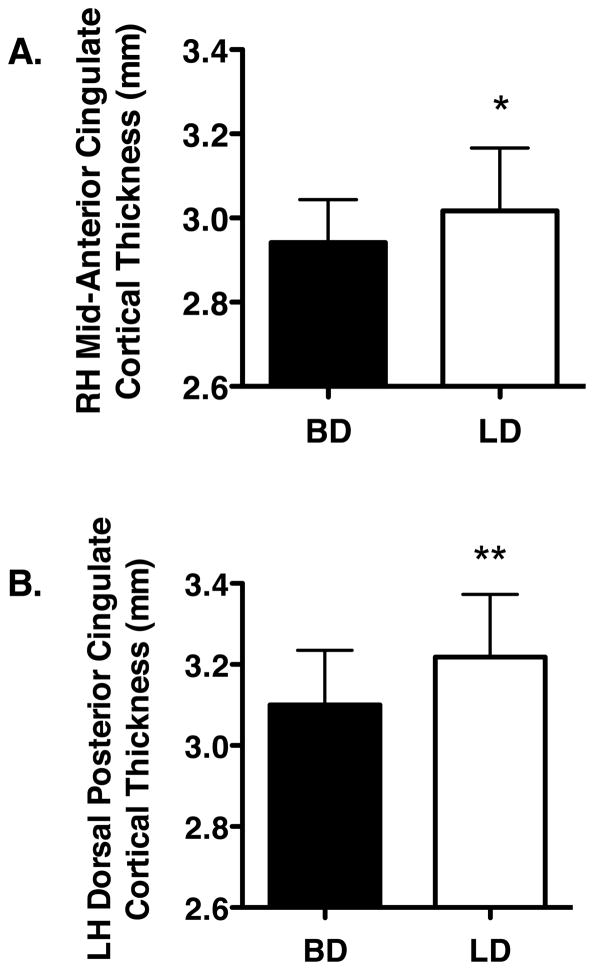
Regional a priori analyses of the ACC, PCC, and POS revealed less cortical thickness in BD than LD, in RH mid-ACC (F[1,51]=4.09, p≤0.05) and in LH dorsal PCC (dPCC) (F[1,51]=6.89, p≤0.01) (Figure 1). No other significant differences were observed between groups.
Figure 1.
Significant differences in cortical thickness values in binge (BD) and light drinkers (LD) are shown. A. Group differences in right hemisphere (RH) mid-anterior cingulate cortex thickness. B. Group differences in left hemisphere (LH) dorsal posterior cingulate cortex thickness. All values are the means ± SD. * p ≤ 0.05 and ** p ≤ 0.01.
Correlations: cortical thickness, alcohol consumption behaviors, and clinical measures
All participants
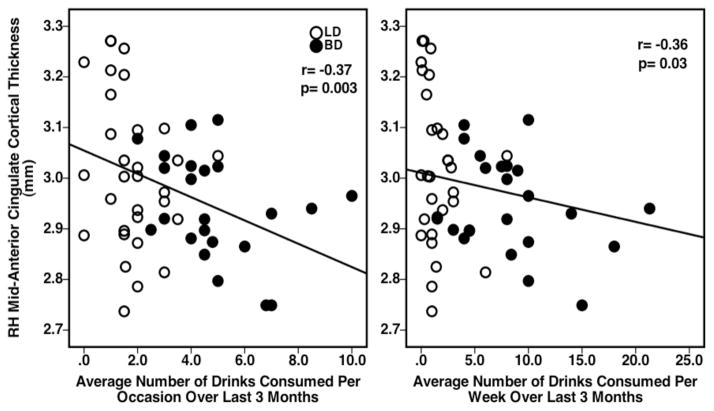
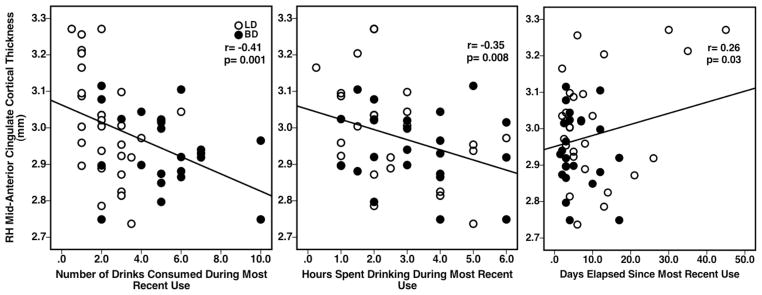
Significant associations observed between RH mid-ACC cortical thickness values and alcohol consumption measures included significant negative correlations between RH mid-ACC cortical thickness and recent number of drinks consumed (r=−0.41, p=0.001) and recent number of hours spent consuming alcohol (r=−0.35, p=0.008) (Figure 2). A positive correlation was observed between RH mid-ACC cortical thickness and number of days elapsed since the most recent use (r=0.26, p=0.030) (Figure 2). Inverse relationships were also observed between RH mid-ACC cortical thickness and past alcohol consumption behaviors over the last 3 months, including average number of drinks consumed per occasion (r=−0.37, p=0.003) and average number of drinks consumed per week (r=−0.36, p=0.003) (Figure 3). Correlations between RH mid-ACC cortical thickness and age of onset of alcohol use, average rate of drinking, YAACQ, POMS, or BIS scores, did not reach significance. There also were no significant associations observed for LH dPCC cortical thickness on any measures.
Figure 2.
Scatterplots representing individual BD and LD subject data and showing significant relationships between group RH mid-ACC cortical thickness values and reported alcohol consumption variables during most recent use.
Figure 3.
Scatterplots representing individual BD and LD subject data and showing significant relationships between group RH mid-ACC cortical thickness values and reported alcohol consumption variables averaged over the past 3 months of use.
Binge Drinkers
Given the significant differences in alcohol consumption behaviors between groups (Table 1), associations between mid-ACC cortical thickness and alcohol consumption measures were also examined in BD alone. Significant negative correlations were observed between mid-ACC cortical thickness and alcohol consumption behaviors reported by BD over the last 3 months, average number of drinks consumed per occasion (r=−0.35, p=0.053) and per week (r=−0.48, p=0.01) (Figure 4). No other significant associations were evident.
Figure 4.
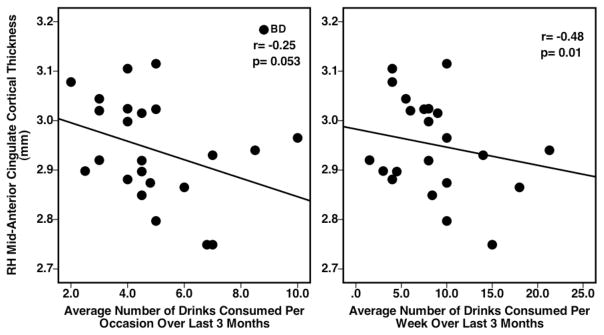
Scatterplots representing individual BD subject data and showing significant relationships between BD RH mid-ACC cortical thickness values and reported alcohol consumption variables averaged over the past 3 months of use.
Exploratory analyses: intra-hemisphere measures of cortical thickness
Vertex-based Freesurfer analyses revealed group differences in cortical thickness in a number of cortical clusters in each hemisphere (Table 2). Bilaterally, BD exhibited less cortical thickness, relative to LD, in superior frontal gyrus, lateral occipital sulcus, and insula. BD exhibited less cortical thickness in RH caudal anterior cingulate, rostral middle frontal gyrus, superior temporal gyrus, inferior temporal gyrus, isthmus cingulate, and inferior parietal sulcus. In LH, less cortical thickness was also observed in BD in precentral gyrus, posterior cingulate, supramarginal gyrus, superior parietal sulcus, pars orbitalis and pars triangularis. In contrast, BD showed greater cortical thickness, relative to LD, in RH supramarginal gyrus and in LH caudal anterior cingulate. Notably, intra-hemispheric differences in cortical thickness did not survive clusterwise Monte Carlo correction for multiple comparisons.
Table 2.
Vertex-based Cortical Thickness Cluster (p=0.01) Results
| Region | Maximum (log) p-value | Size (mm2) | Talairach Coordinates | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Left Hemisphere | |||||
|
| |||||
| LD > BD | |||||
| Precentral | −2.9 | 78.1 | −55.7 | −2.6 | 20.9 |
| Superior frontal | −2.8 | 54.0 | −11.9 | 15.1 | 39.1 |
| Pars triangularis | −2.6 | 20.6 | −42.6 | 26.7 | 5.4 |
| Posterior cingulate | −2.5 | 62.0 | −7.1 | −29.9 | 39.7 |
| Superior frontal | −2.5 | 33.9 | −17.0 | 43.3 | 36.6 |
| Superior parietal | −2.3 | 60.6 | −24.2 | −63.1 | 49.9 |
| Lateral occipital | −2.3 | 51.8 | −30.5 | −89.8 | 8.6 |
| Insula | −2.1 | 16.6 | −37.0 | −7.4 | 1.1 |
| Pars orbitalis | −2.1 | 8.7 | −42.4 | 28.2 | −10.5 |
| Supramarginal | −2.0 | 1.8 | −51.1 | −41.2 | 30.2 |
|
| |||||
| BD > LD | |||||
| Caudal anterior cingulate | 2.1 | 7.3 | −5.2 | 9.8 | 33.4 |
|
| |||||
| Right Hemisphere | |||||
|
| |||||
| LD > BD | |||||
| Lateral occipital | −3.8 | 125.6 | 39.6 | −82.1 | 2.5 |
| Superior frontal | −3.3 | 127.2 | 8.2 | 34.4 | 35.5 |
| Rostral middle frontal | −2.9 | 65.6 | 35.7 | 28.6 | 41.4 |
| Superior temporal | −2.5 | 30.9 | 52.2 | −10.2 | −4.3 |
| Inferior temporal | −2.5 | 61.8 | 46.2 | −36.3 | −20.0 |
| Isthmus cingulate | −2.3 | 12.1 | 5.5 | −42.8 | 30.8 |
| Caudal anterior cingulate | −2.2 | 16.2 | 9.7 | 15.5 | 33.4 |
| Inferior parietal | −2.1 | 8.0 | 45.2 | −65.6 | 29.3 |
| Insula | −2.0 | 2.2 | 33.3 | −21.6 | 11.8 |
| Rostral middle frontal | −2.0 | 2.8 | 23.6 | 51.7 | 20.0 |
| Insula | −2.0 | 1.0 | 36.2 | −17.7 | −4.2 |
|
| |||||
| BD > LD | |||||
| Supramarginal | 2.4 | 25.0 | 43.1 | −38.3 | 38.9 |
Abbreviations: BD, binge drinkers; LD, light drinkers.
Discussion
Analyses of a priori regions of interest revealed significantly less cortical thickness in the RH mid-ACC and LH dPCC of emerging adult BD, relative to demographically-matched LD. Altered mid-ACC cortical thickness was most strongly predicted by recent number of drinks consumed, average number of drinks consumed per occasion and average number of drinks consumed per week. This is in contrast to other drinking measures reflecting more widespread effects of alcoholism, such as days per week of drinking, AUDIT score (reflecting abuse or dependence criteria), YAACQ total or subscale scores, or meeting criteria for abuse or dependence (one BD met for abuse, four BD met for subthreshold abuse, and no BD met for dependence). Thus, the current findings support that relationships observed with cortical thickness were related to criteria for a binge pattern of drinking, factors that could influence the vulnerable prefrontal cortices. Patterns of intermittent heavy alcohol exposure established by BD in the present study may therefore selectively contribute to anomalous synaptic pruning and dysfunctional programming of neuron populations in frontal regions involved in maintaining control over maladaptive behaviors, including excessive binge drinking. Given that episodic exposure may be neurotoxic to a frontal cortex that is still undergoing neuromaturation, emerging adult BD may be more vulnerable than LD to alterations in neuronal morphology, leading to frontal cortical thinness. ‘Cortical thinness’ is a descriptive term that confers directionality of an alteration in cortical thickness between populations. In contrast to cortical thinning, which is a continuous measure obtained from longitudinal data, cortical thinness is a relative indicator of less cortical thickness compared cross-sectionally between groups. To this end, ‘cortical thinness’ is applied as an operational definition of lower cortical thickness in binge drinkers relative to light drinkers. Interestingly, significant cortical differences were observed in BD, despite that they were clinically healthy. Apart from a potentially spurious difference in the POMS Vigor subscale, elevated in BD, there were no other significant clinical differences observed between groups.
The ACC is directly involved in assessing risk and detecting the likelihood of errors or other negative outcomes in order to prompt other prefrontal regions to establish control and minimize risk exposure (Brown and Braver, 2007). Thus, mid-ACC cortical thinness in BD may interfere with efficient detection of errors or negative outcomes during risky behaviors, delaying engagement of the frontal neural network and contributing to impulsive decision-making, possibly resulting in ‘just-one-more-drink’ binge alcohol consumption and diminished control over these behaviors. The dPCC has been proposed to be involved in maintaining cognitive control by adapting to alterations in task demands, and by flexibly managing externally directed behaviors and focused attention (Margulies et al., 2009; Leech et al., 2012). Furthermore, dPCC specifically encodes the reward value and size of potential outcomes in a current environment, as well as the variance associated with obtaining these rewards, which contributes to consequent adaptations in behavior (Pearson et al., 2011). Therefore, thinness of the dPCC in BD may contribute to dysfunctional assignment of greater reward value to habitual binge drinking and fixated attention on rapidly consuming available alcohol within the current environment. It is possible that in BD, the dPCC is less sensitive and more apt to override cognitive control mechanisms aimed at reducing excessive drinking behaviors or evaluating potential likely outcomes related to BD based on previous experiences. In the present study, BD reported significantly more alcohol use consequences, including elevated likelihood of engaging in risky behaviors, diminished self-control, and greater incidence of blackout drinking. Though the current investigation did not find significant associations between mid-ACC or dPCC thinness and alcohol consequences on the YAACQ, continued excessive episodic drinking in young adults can easily transition into habitual drinking patterns reinforced by alcohol dependence and characterized by more significant consequences such as alcohol-related accidents and injuries (Bonomo et al., 2004). Collectively, overly thinned mid-ACC and dPCC may be insufficient to promote optimal executive ‘top down’ control over maladaptive alcohol use behaviors that may escalate future drinking, which could eventually predict negative consequences.
Apart from cortical thickness differences measured in a priori frontal regions of interest, bilateral and asymmetric cortical thinness in BD was also observed in intra-hemisphere analyses, including superior and middle frontal gyri, lateral occipital sulcus, insula, superior temporal gyrus, precentral gyrus, supramarginal gyrus, superior and inferior temporal gyri, and precentral gyrus. Though these exploratory intra-hemisphere findings did not survive multiple comparisons corrections, cortical thinness observed in BD in these regions is consistent with previous reports indicating widespread patterns of less cortical thickness associated with binge drinking, and in alcohol-dependent adults and abstinent former alcoholics (Sinha and Li, 2007; Durazzo et al., 2011; Squeglia et al., 2012; Momenan et al., 2012). Notably, the present results are relevant given that alterations in cortical thickness were evident in regions that continue to undergo synaptic pruning during late adolescence, e.g., superior frontal gyrus and temporal gyri, as well as regions expected to have finalized neuromaturation, e.g., precentral and supramarginal gyri. Thus, repeated intermittent heavy alcohol exposure may have altered synaptic pruning processes by microstructurally eliminating or damaging subpopulations of neuronal connections in both mature and maturing brain regions. These speculations will be investigated further in larger-scale studies designed to specifically measure intra-hemisphere cortical differences between BD and LD.
The thinness of the RH mid-ACC and LH dPCC in BD is likely due to alterations in pruning, and possibly excessive pruning and neuronal loss related to the effects of intermittent heavy alcohol consumption during ongoing neuromaturation in the young adult brain. Evidence supports a role of glutamate-mediated synaptic plasticity in the molecular basis of synaptic pruning during cortical maturation in adolescence and young adulthood (Johnston, 1995; Zhang et al., 2013). Glutamate facilitates fast excitatory neurotransmission that is mediated by three classes of ionotropic receptors that include AMPA receptors, kainate receptors and NMDA receptors (NMDARs) (Dingledine et al., 1999). NMDAR activity is particularly critical for structural development of synapses and neurons and for establishing neural circuits during neurodevelopment, in addition to playing an important role in processes underlying synaptic refinement and pruning (Sceniak et al., 2012; Zhang et al., 2013). Consistent heavy, intermittent alcohol exposure, such as the patterns reported by BD in the present study, may generate a homeostatic potentiation of NMDAR signaling and excitatory glutamate neurotransmission that can produce persistent alterations in synapse numbers and dendritic spine morphology during adolescence and young adulthood (Carpenter-Hyland and Chandler, 2007). Furthermore, binge alcohol exposure has been proposed to directly damage brain cells during development and to contribute to neuronal loss and degeneration (West and Goodlett, 1990; Lundqvist et al., 1995), possibly via alcohol-induced neurotoxicity mediated by elevated glutamate receptor excitation (Collins et al., 1996). The stability of synaptic architecture is threatened by overstimulation of glutamate receptor activity, which may be a consequence of heavy binge alcohol consumption. Excessive activation of NMDARs, via binge drinking, may also cause destabilization, shrinkage, and/or collapse of dendritic spines that can leave the parent neuron vulnerable to future excitotoxic activation (Halpain et al., 1998). Alternatively, or in addition, maturation of the GABA system during adolescence (Silveri et al., 2013) may be delayed in reaching a typical neurobiological plateau in emerging adulthood when a BD pattern of consumption is established (Silveri et al., 2014).
While healthy neuromaturation is associated with longitudinal cortical thinning, binge alcohol exposure during this normative process may lead to excessive synaptic pruning, as well as morphological alterations in the remaining neurons. For instance, dendritic spines heavily impact synaptic connectivity and are critical to the molecular basis of learning and memory; changes in the structural features of dendritic spines, measured as an increase in the size of the spines, have been reported as a result of ethanol exposure (Holtmaat et al., 2005; Carpenter-Hyland and Chandler, 2007). It is possible that increased dendritic spine size could strengthen alcohol-induced associations across brain regions that serve to regulate addiction and relapse neurocircuitry (Carpenter-Hyland and Chandler, 2007). As such, neurocircuitry reinforced by heavy binge alcohol exposure and fortified by larger dendritic spines may eliminate opportunities for synaptic activity modification or reassignment during ongoing neuromaturation, thereby supporting persistent behavioral patterns of maladaptive alcohol use that could transition into long-term alcohol dependence.
A primary limitation of this cross-sectional study is the inability to distinguish whether regional frontal ‘cortical thinness’ measured in BD preceded heavy episodic consumption or whether thinness reflects a consequence of these behaviors and acute alcohol neurotoxicity. Further, reliance on self-reported patterns of past and recent alcohol consumption behaviors (Del Boca and Darkes, 2003), as well as on mood state and impulsivity measures, are also subject to their own limitations, such as social context and acceptability factors, individual recall and estimation ability, and willingness to respond accurately (Del Boca and Darkes, 2003). Participants provided self-report of alcohol consumption behaviors and patterns, and were assured responses would be kept completely confidential. As such, it is likely that this self-selected group was willing to provide accurate and comprehensive reports. Though sex was a non-significant covariate in current analyses (see also, Durazzo et al., 2011), normative cortical thinning is known to occur along different developmental curves for males and females (Giedd et al., 1999). Thus, future investigations should further explore the effects of binge alcohol consumption on sex-related differences in cortical and clinical measures.
In conclusion, ACC and PCC cortical thinness in BD may reflect early neurostructural consequences of excessive acute alcohol consumption that could precede more widespread cortical thinning observed in long-term alcohol dependent individuals (Momenan et al., 2012), thereby representing a neurobiological parallel and/or vulnerability marker for the continuum between binge drinking and alcohol dependence (Hermens et al., 2013). Alternatively, it is possible that binge alcohol consumption, established during late adolescence, a critical neurodevelopmental period lasting into emerging adulthood, may interfere with the finalization of neuromaturational processes, resulting in microarchitectural thinness in the frontal lobe.
Acknowledgments
This study was supported by NIDA K01 DA034028 (YM), NIAAA K01 AA014651 (MMS) and NIAAA R01 AA018153 (MMS) grants.
Footnotes
Financial Disclosures
There are no disclosures to declare.
Supplementary Material: n/a
Disclosures: none
References
- Bonomo YA, Bowes G, Coffey C, Carlin JB, Patton GC. Teenage drinking and the onset of alcohol dependence: a cohort study over seven years. Addiction. 2004;99:1520–1528. doi: 10.1111/j.1360-0443.2004.00846.x. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Risk prediction and aversion by anterior cingulate cortex. Cogn Affect Behav Neurosci. 2007;7:266–277. doi: 10.3758/cabn.7.4.266. [DOI] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Chandler LJ. Adaptive plasticity of NMDA receptors and dendritic spines: implications for enhanced vulnerability of the adolescent brain to alcohol addiction. Pharmacol Biochem Behav. 2007;86:200–208. doi: 10.1016/j.pbb.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos GI, Tobler PN, Bossaerts P, Dolan RJ, Schultz W. Neural correlates of value, risk, and risk aversion contributing to decision making under risk. J Neurosci. 2009;29:12574–12583. doi: 10.1523/JNEUROSCI.2614-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MA, Corso TD, Neafsey EJ. Neuronal degeneration in rat cerebrocortical and olfactory regions during subchronic “binge” intoxication with ethanol: possible explanation for olfactory deficits in alcoholics. Alcohol Clin Exp Res. 1996;20:284–292. doi: 10.1111/j.1530-0277.1996.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction. 2003;98(Suppl 2):1–12. doi: 10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, Meyerhoff DJ. Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol Clin Exp Res. 2011;35:1187–1200. doi: 10.1111/j.1530-0277.2011.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fortier CB, Leritz EC, Salat DH, Venne JR, Maksimovskiy AL, Williams V, Milberg WP, McGlinchey RE. Reduced cortical thickness in abstinent alcoholics and association with alcoholic behavior. Alcohol Clin Exp Res. 2011;35:2193–2201. doi: 10.1111/j.1530-0277.2011.01576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara J, Tobler PN, Taira M, Iijima T, Tsutsui K. Segregated and integrated coding of reward and punishment in the cingulate cortex. J Neurophysiol. 2009;101:3284–3293. doi: 10.1152/jn.90909.2008. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, Lenroot RK. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J Am Acad Child Adolesc Psychiatry. 2009;48:465–470. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpain S, Hipolito A, Saffer L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J Neurosci. 1998;18:9835–9844. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Smith DV, Platt ML. Electrophysiological correlates of default-mode processing in macaque posterior cingulate cortex. Proc Natl Acad Sci USA. 2009;106:5948–5953. doi: 10.1073/pnas.0812035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermens DF, Lagopoulos J, Tobias-Webb J, De Regt T, Dore G, Juckes L, Latt N, Hickie IB. Pathways to alcohol-induced brain impairment in young people: a review. Cortex. 2013;49:3–17. doi: 10.1016/j.cortex.2012.05.021. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age of alcohol-dependence onset: associations with severity of dependence and seeking treatment. Pediatrics. 2006;118:e755–763. doi: 10.1542/peds.2006-0223. [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Johnston MV. Neurotransmitters and vulnerability of the developing brain. Brain Dev. 1995;17:301–306. doi: 10.1016/0387-7604(95)00079-q. [DOI] [PubMed] [Google Scholar]
- Kharitonova M, Martin RE, Gabrieli JD, Sheridan MA. Cortical gray-matter thinning is associated with age-related improvements on executive function tasks. Dev Cogn Neurosci. 2013;6C:61–71. doi: 10.1016/j.dcn.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Braga R, Sharp DJ. Echoes of the brain within the posterior cingulate cortex. J Neurosci. 2012;32:215–222. doi: 10.1523/JNEUROSCI.3689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, Thayer R, Squeglia LM, McQueeny TM, Tapert SF. Recent binge drinking predicts smaller cerebellar volumes in adolescents. Psychiatry Res. 2013;211:17–23. doi: 10.1016/j.pscychresns.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist C, Alling C, Knoth R, Volk B. Intermittent ethanol exposure of adult rats: hippocampal cell loss after one month of treatment. Alcohol Alcohol. 1995;30:737–748. [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Villringer A, Castellanos FX, Milham MP, Petrides M. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci U S A. 2009;106:20069–20074. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Blair KS, Vythilingam M, Busis S, Blair RJ. Response options and expectations of reward in decision-making: the differential roles of dorsal and rostral anterior cingulate cortex. Neuroimage. 2007;35:979–988. doi: 10.1016/j.neuroimage.2006.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momenan R, Steckler LE, Saad ZS, van Rafelghem S, Kerich MJ, Hommer DW. Effects of alcohol dependence on cortical thickness as determined by magnetic resonance imaging. Psychiatry Res. 2012;204:101–111. doi: 10.1016/j.pscychresns.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Orr C, Hester R. Error-related anterior cingulate cortex activity and the prediction of conscious error awareness. Front Hum Neurosci. 2012;6:177. doi: 10.3389/fnhum.2012.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Schulenberg JE. Alcohol use and heavy episodic drinking prevalence and predictors among national samples of American eighth- and tenth-grade students. J Stud Alcohol Drugs. 2010;71:41–45. doi: 10.15288/jsad.2010.71.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JM, Heilbronner SR, Barack DL, Hayden BY, Platt ML. Posterior cingulate cortex: adapting behavior to a changing world. Trends Cogn Sci. 2011;15:143–151. doi: 10.1016/j.tics.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119 (Pt 5):1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Ruigrok AN, Salimi-Khorshidi G, Lai MC, Baron-Cohen S, Lombardo MV, Tait RJ, Suckling J. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014;39C:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Sceniak MP, Berry CT, Sabo SL. Facilitation of neocortical presynaptic terminal development by NMDA receptor activation. Neural Dev. 2012;7:8. doi: 10.1186/1749-8104-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Silveri MM. Adolescent brain development and underage drinking in the United States: identifying risks of alcohol use in college populations. Harv Rev Psychiatry. 2012;20:189–200. doi: 10.3109/10673229.2012.714642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Sneider JT, Crowley DJ, Covell MJ, Acharya D, Rosso IM, Jensen JE. Frontal lobe gamma-aminobutyric acid levels during adolescence: associations with impulsivity and response inhibition. Biol Psychiatry. 2013;74:296–304. doi: 10.1016/j.biopsych.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Cohen-Gilbert JE, Crowley DJ, Rosso IM, Jensen JE, Sneider JT. Altered anterior cingulate neurochemistry in emerging adult binge drinkers with a history of alcohol-induced blackouts. Alcohol Clin Exp Res. 2014 doi: 10.1111/acer.12346. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Sorg SF, Schweinsburg AD, Wetherill RR, Pulido C, Tapert SF. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology (Berl) 2012;220:529–539. doi: 10.1007/s00213-011-2500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: 2011. NSDUH Series H-41 HHS Publication No. (SMA) 11–4658. [Google Scholar]
- Torta DM, Cauda F. Different functions in the cingulate cortex, a meta-analytic connectivity modeling study. Neuroimage. 2011;56:2157–2172. doi: 10.1016/j.neuroimage.2011.03.066. [DOI] [PubMed] [Google Scholar]
- West JR, Goodlett CR. Teratogenic effects of alcohol on brain development. Ann Med. 1990;22:319–325. doi: 10.3109/07853899009147914. [DOI] [PubMed] [Google Scholar]
- Zhang ZW, Peterson M, Liu H. Essential role of postsynaptic NMDA receptors in developmental refinement of excitatory synapses. Proc Natl Acad Sci U S A. 2013;110:1095–1100. doi: 10.1073/pnas.1212971110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RA, Ellis DA, Fitzgerald HE. Developmental evidence for at least two alcoholisms. I. Biopsychosocial variation among pathways into symptomatic difficulty. Ann N Y Acad Sci. 1994;708:134–146. doi: 10.1111/j.1749-6632.1994.tb24706.x. [DOI] [PubMed] [Google Scholar]