Abstract
Deterrence of synthetic cathinone abuse is hampered by the lack of a high-throughput immunoassay screen. The Randox Drugs of Abuse V biochip immunoassay (DOA-V) contains two synthetic cathinones antibodies: Bath Salt I (BSI) targets mephedrone/methcathinone and Bath Salt II (BSII) targets 3’,4’-methylenedioxypyrovalerone (MDPV)/3’,4’-methylenedioxy-α-pyrrolidinobutiophenone (MDPBP). We evaluated DOA-V synthetic cathinones performance and conducted a full validation on the original assay with calibrators reconstituted in water, and the new assay with calibrators prepared in lyophilized urine; both utilized the same antibodies and were run on the fully automated Evidence® Analyzer. 20,017 authentic military urine specimens were screened and confirmed by LC-MS/MS for 28 synthetic cathinones. Limits of detection (LOD) for the original and new assays were 0.35 and 0.18 (BSI), and 8.5 and 9.2µg/L (BSII), respectively. Linearity was acceptable (R2>0.98); however, a large negative bias was observed with in-house prepared calibrators. Intra-assay imprecision was <20% BSI-II, while inter-assay imprecision was 18–42% BSI and <22% BSII. Precision was acceptable for Randox controls. Cross-reactivities of many additional synthetic cathinones were determined. Authentic drug-free negative urine pH <4 produced false positive results for BSI (6.3µg/L) and BSII (473µg/L). Oxidizing agents reduced BSI and increased BSII results. Sensitivity, specificity, and efficiency of 100%, 52.1%, and 53.0% were obtained at manufacturer’s proposed cutoffs (BSI 5µg/L, BSII 30µg/L). Performance improved if cutoff concentrations increased (BSI 7.5µg/L, BSII 40µg/L); however, there were limited confirmed positive specimens. Currently, this is the first and only fully validated immunoassay for preliminary detection of synthetic cathinones in urine.
Keywords: synthetic cathinones, mephedrone, MDPV, Randox, immunoassay
Introduction
Designer drug intake grew rapidly around the world over the past several years [1–3]. These purported “legal highs” pose problems for law enforcement, military and public safety officials, and also toxicologists who must identify an unending variety of new drugs of abuse. Novel drugs are constantly being developed to circumvent legislative and regulatory efforts. These include new synthetic cathinones that are available online and in head shops or convenience stores.
Synthetic cathinones are novel stimulants intended to produce effects similar to those experienced with stimulants like methamphetamine, cocaine, or 3’, 4’-methylenedioxymethamphetamine (MDMA)[4–6]. Clinically reported symptoms of cathinone ingestion also include euphoria [5–7], agitation [6, 8–11], combative and violent behavior [8, 10], tachycardia [6, 8, 10], hallucinations [8–9], paranoia [8–10], and in some cases excited delirium [9] and death [11–15]. These drugs are sold online as “plant food,” “bath salts,” or “research chemicals” and are labeled “not for human consumption” in order to avoid potential regulation [7, 16–17]. Abuse is documented across population groups from mid-to-late adolescents to older adults [18]. In 2010, the American Association of Poison Control Centers reported 304 phone calls regarding “bath salt” consumption [19]. This number increased dramatically in 2011 to 6,138 calls, decreased to 2,656 in 2012, with only 833 calls as of October 31, 2013. Mephedrone (4-methylmethcathinone), 3’,4’-methylenedioxypyrovalerone (MDPV), and methylone (3’,4’-methylenedioxy-N-methylcathinone) are now Schedule I drugs in the U.S. based on the Controlled Substances Act [20–21]. As of November 28, 2012, at least 43 states banned cathinone derivatives [22].
Synthetic cathinones were identified in hair [23–26], whole blood (including post-mortem) [8, 11–13, 15, 23, 27–36], plasma [37], serum [14, 38–39], cerebral spinal fluid [39], and urine [8, 11, 14, 28, 30–32, 39–52]. Therefore, high-throughput screening methods and sensitive and specific gas chromatography and liquid chromatography tandem mass spectrometry (LC-MS/MS) confirmation methods for synthetic cathinones are needed for workplace (including military) and criminal justice programs. There are no government mandated screening and confirmation cutoffs for cathinone derivatives in any biological matrix.
Thus, there is a critical need for reliable screening assays that are rapid and cost efficient. Most amphetamine immunoassays do not readily detect a wide range of synthetic cathinones. Some cathinones, including MDPV, produced false-positive results in phencyclidine (PCP) immunoassays [9, 53–54]. Additionally, Swortwood et al. reported positive Immunalysis and Neogen methamphetamine tests with synthetic cathinone concentrations starting at 1250µg/L[55]. Greater cross-reactivity (2–25%) was observed with 40–450µg/L mephedrone, methcathinone, methylone, 4-methylethcathinone (4-MEC), flephedrone (4-fluoromethcathinone), butylone, and methedrone in the OraSure methamphetamine assay. However, with the continual development of novel cathinone derivatives, it is difficult to predict if immunoassays, not specifically designed for these drugs, have sufficient cross-reactivity for reliable screening for these compounds in urine. Recently, Randox developed a semi-quantitative screening system for synthetic cathinone detection in urine utilizing the Randox Drugs of Abuse V Biochip Array Technology (DOA-V). To date, this is the only commercially available immunoassay for urinary cathinone detection.
The objective of this study was to fully validate the DOA-V as a sensitive and specific assay for screening synthetic cathinones in urine.
Material and Methods
Authentic Specimens
Authentic human urine specimens (N=20,017) collected between July 2011 and June 2012 were randomly selected from negative specimens analyzed from five Department of Defense (DoD) forensic drug testing laboratories from service personnel serving around the world. Specimens were anonymized and shipped for analysis with the DOA-V synthetic cathinones assay between March and June 2012. These specimens, collected worldwide, had previously screened negative for amphetamines (d-amphetamine, d-methamphetamine, methylenedioxymethamphetamine, methylenedioxyamphetamine), benzoylecgonine, opioids (codeine, morphine, hydrocodone, hydromorphone, oxycodone, oxymorphone), cannabinoids and PCP. Specimens were stored at room temperature once received, generally for two to four weeks prior to screening; however, some specimens were stored longer (up to 9 months) due to delays in receiving urine specimens from the military drug testing facilities after collection, time required to obtain waste water discharge approval, and autoanalyzer performance and maintenance issues. Based on the manufacturer’s proposed cutoffs, a positive result for BSI (≥5µg/L) or BSII (≥30µg/L) was considered presumptive positive for synthetic cathinones. Presumptive positive specimens were stored at 4–7°C prior to liquid chromatography high resolution tandem mass spectrometry (LC-HRMS) confirmation.
Reagents and Consumables
Randox (Crumlin, UK) released the original DOA-V assay, controls (QC, level 1 and 2), and calibrators (BSI 0–20.7µg/L and BSII 0–1053µg/L), with calibrator reconstitution in water. In July 2012, a new Randox DOA-V assay was released, with identical antibodies, requiring calibrator reconstitution in lyophilized urine (BSI 0–32.2µg/L and BSII 0–1037µg/L). Additional Evidence Analyzer universal reagents such as buffer wash, displacement fluid and signal reagent also were obtained from Randox. Mephedrone HCl, MDPV HCl, methylone HCl, R(+) methcathinone HCl, and S(−) methcathinone HCl standards were purchased from Cerilliant (Round Rock, TX), and buphedrone, butylone, N-ethylcathinone, ethylone, 3-fluoromethcathinone, 4-fluoromethcathinone, 4-methyl-α-pyrrolidinobutiophenone (4-MPBP), 3’,4’-methylenedioxy-α-pyrrolidinobutiophenone (MDPBP), 3’,4’-methylenedioxy-α-pyrrolidinopropiophenone (MDPPP), MDPV, 4-methylethcathinone (4-MEC), methedrone, 3-methoxymethcathinone, methylone, naphyrone, pentedrone, and pentylone were acquired from Cayman Chemicals (Ann Arbor, MI). All standards were stored at −20°C. Intermediate (100mg/L) and working concentrations (0.01–10mg/L) were prepared with high performance liquid chromatography (HPLC)-grade methanol (Sigma-Aldrich, St. Louis, MO) and stored at 4–7°C prior to analysis. Pooled negative urine samples from 10 individuals were utilized to prepare synthetic cathinone and interference standards, and for negative control samples after chromatographic evaluation to ensure negativity. Interference standards were purchased from Cerilliant and Sigma-Aldrich and prepared in in-house certified negative urine.
Immunoassay Screening on the Evidence® Analyzer
DOA-V is a competitive binding immunoassay on a chemically-modified biochip. Free antigens (e.g., synthetic cathinones) in the specimen compete with horseradish peroxidase (HRP)-labeled analyte (conjugate) for binding sites on the immobilized polyclonal antibodies. Signal reagent, containing a 1:1 mixture of luminol/enhancer and peroxide solution, is added to the biochip and produces a chemiluminescent signal when HRP-labeled analyte binds to the antibody sites. Chemiluminescent signals are detected with digital imaging technology (charged coupled device (CCD) camera), with readings compared to calibrator signals, and signal input is inversely proportional to analyte concentration. The Randox Evidence® analyzer (EV 180–120) simultaneously runs multiple tests on one specimen (multiplexing) on one biochip, allowing laboratories to perform high-throughput analyses.
The DOA-V biochip has 11 different immobilized polyclonal antibodies, two of which bind to synthetic cathinones. Bath Salt I (BSI) contains anti-methcathinone antibodies 100% cross-reactive to mephedrone, and Bath Salt II (BSII), anti-MDPV antibodies for the detection of MDPV/MDPBP. Other synthetic cathinones cross-react with BSI and BSII, including, but not limited to, methylone, flephedrone, naphyrone and pentedrone. DOA-V is specifically designed to run on the Evidence® paired with a computer and software for calibration, sample analysis, and historical data/result retrieval.
Instrument Calibration
DOA-V utilizes nine multi-analyte calibrator standards and an installation disk (CD-ROM). This disk contains product information, analyte concentration for each level, and four pre-defined performance parameters for each analyte. The software builds a derived calibration curve for competitive assays with pre-defined parameters (A, B, C, D), observed results and the following equation where y is the Relative Light Unit (RLU) and x is the concentration, µg/L:
The software constructs an optimal fit from the results with a 4-parameter curve fit method; a 4-parameter logistic (4PL) non-linear regression assuming a central turning point and two asymptotes at the terminal ends. A calibration curve for each analyte is displayed with a correlation coefficient (r). Target curve fit (r) is 0.949 or higher; FAIL is indicated if fit is lower, or if four or more calibrators are out of range. Calibrators were prepared each week, stored at 4–7°C, and were stable for up to seven days. Calibration was performed daily and repeated within day only when a new immunoassay lot was assayed or if quality control sample results were unacceptable. A typical analytical batch included one negative, two different positive quality controls (analyzed in duplicate) and 86 samples. On average, six batches were analyzed daily.
Validation Procedures
Limits of Detection (LOD)
Duplicates of 10 blank urine samples were assayed over three days (n = 60). Mean observed concentrations and standard deviations (SD) for BSI & BSII were calculated. LOD was defined as mean observed concentration + 3SD.
Linearity
Calibrator concentrations were 0–20.7µg/L (BSI) and 0–1053µg/L (BSII) for the original, and 0–32.2µg/L (BSI) and 0–1037µg/L (BSII) for the new assays. Mephedrone HCl (100mg/L) and MDPV HCl (100mg/L) stock solutions were diluted with methanol to intermediate and working solutions; certified negative urine samples were fortified with mephedrone HCl, or MDPV HCl to final concentrations. Mephedrone in-house prepared linearity samples were 0.25, 0.50, 1, 2.5, 5.0, 7.5, 10, 20, 30, 40, and 50µg/L (BSI), and for MDPV 10, 20, 30, 75, 135, 275, 555, 1035, 1050, 1100, and 1200µg/L (BSII). Each concentration was analyzed in triplicate per batch for five separately calibrated batches (n=15). Curve fit (r) for each antibody was derived from plotting mean RLUs (y) versus log mean concentration (x) for all 15 batches in GraphPad Prism, and linearity (R2) for assay calibrators and in-house standards. In addition, %bias was determined for each concentration as 100 × (group mean observed concentration – known concentration)/ known concentration. Acceptable %bias was ±20% of target.
Imprecision
Low, medium, and high mephedrone (1.0, 5.0, 15µg/L) and MDPV (30, 275, 555µg/L) in-house standards were analyzed in quadruplicate in each of five batches (n=20). Mean observed concentrations, SD, coefficient of variation (%CV) for intra- and inter-batch, and total imprecision were determined. Pooled intra-day, inter-day, and total imprecision were calculated for each concentration according to the procedure of Krouwer and Rabinowitz [56].
Cross-reactivity
Synthetic cathinones and structurally similar compounds were prepared at two concentrations based on the cross-reactivity profile provided by Randox in the current DOA-V product insert. Negative urine was fortified to final concentrations and analyzed in triplicate in one batch. Cross-reactivity (%) was determined as 100 × (BSI or II mean observed concentration)/target analyte concentration.
Interferences
Extensive exogenous and endogenous interferences were fortified in in-house certified negative urine (Supplemental Table 1). Additionally, in-house certified negative urine samples pH were adjusted to pH 4.0 and pH 8.0 and evaluated for interferences. To evaluate peroxide and bleach as potential adulterants, mephedrone HCl (10µg/L) and MDPV HCl (275µg/L) were mixed with negative urine containing either 10% bleach or 10% peroxide. Adulterant-free samples were included to establish baseline readings. A value <LOD was considered free from interference.
Carryover
Carryover was evaluated with in-house certified negative urine fortified at 250µg/L mephedrone and 2000µg/L MDPV. Fortified samples were analyzed followed by two negative urine samples. Negative samples with concentrations ≤LOD were considered without carryover.
Authentic Urine Specimen Analysis
Presumptive positive urine specimens based on the Randox recommended cutoffs for BSI (5µg/L) and BSII (30µg/L) were confirmed with a fully validated liquid chromatography tandem high resolution mass spectrometry (LC-HRMS) confirmation method for 28 synthetic cathinones in urine [40]. Limits of quantification were 0.5µg/L for all synthetic cathinones, except buphedrone ephedrine (1µg/L). In addition, presumptive negative specimens were selected at random from the original batches that provided presumptive positive specimens and analyzed by LC-HRMS. True positive (TP) specimens screened and confirmed positive, and true negative (TN) specimens were negative in both assays. False positive (FP) specimens screened positive but no synthetic cathinones were identified by confirmation, and false negative (FN) specimens screened negative but confirmed positive for one or more synthetic cathinones. Performance parameters were calculated as: sensitivity = 100 × (TP/TP+FN); specificity = 100 × (TN/TN+FP); and efficiency = 100 × (TP+TN/Total). Sensitivity, specificity, and efficiency of manufacturer’s proposed cutoff concentrations, as well as 7.5 and 10ug/L BSI cutoffs and 40 and 50ug/L BSII cutoffs also were evaluated.
Additionally, performance was evaluated around cutoff concentrations of 2.5, 5.0, 7.5, and 10µg/L for BSI and 20, 30, and 40µg/L for BSII, and ±25% and ±50% of each of these cutoffs. Samples were analyzed in triplicate in one batch. Cutoffs were evaluated by examining the overlap between mean cutoff concentrations and ±25% and ±50% of each of these cutoffs.
Results
The original and new DOA-V were evaluated with 10% quality control samples (Negative, Control 1, and Control 2) included in each analytical batch. All calibrations had r>0.994 and quality control results within acceptable ranges.
Limits of Detection
LODs for the original and new assays were 0.35 (BSI), 8.5 (BSII), 0.18 (BSI) and 9.2µg/L (BSII), respectively. LODs were 93–96% and 69–72% below the proposed cutoffs for BSI and BSII, respectively.
Linearity
Four PL curve fits for BSI-II were acceptable (R2>0.99) in the original and new assays. BSI assay calibrators (original and new) were linear at concentrations below the manufacturer’s proposed cutoff and upper limits of linearity (ULOL); R2 ≥0.98 (1.3–20.73µg/L) and R2≥0.99 (1–32.2µg/L), respectively. Mean %bias for BSI-II in the linear range was −19% to 19%. BSII assay calibrators (original and new) were linear (R2≥0.99) above the proposed cutoff to the ULOL (original: 32.9–1053µg/L, new: 43.1–1037µg/L), exhibiting mean %bias across the linear range of −17% to 15%.
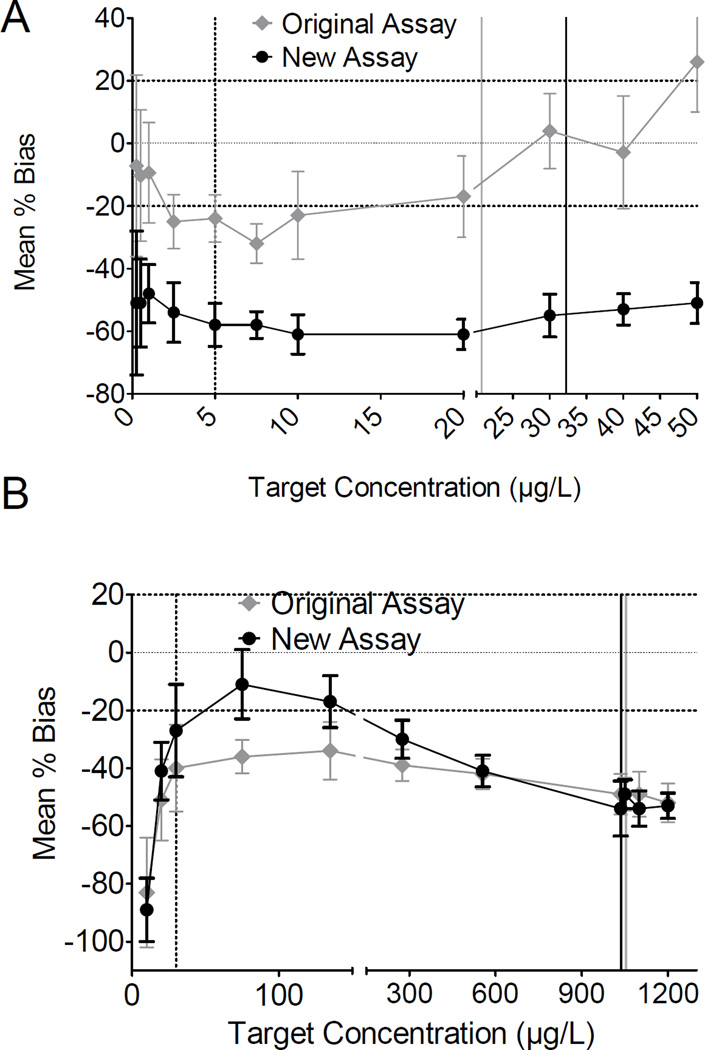
The in-house standard preparations for the original assays were linear (R2≥0.99) from below the manufacturer’s proposed cutoff (2.5µg/L, with the proposed 5µg/L cutoff) to the ULOL (20µg/L) for BSI. For BSII, linearity was documented between the manufacturer’s proposed cutoff (30µg/L) and ULOL (1050µg/L); however, BSI %bias was −32 to −17% (median: −24%, mean: −24%, SD: 5.4%) between the proposed cutoff (5µg/L) and 20µg/L. BSII %bias was −49 to −34% (median: −40%, mean: −41%, SD: 5.9%) from the 30µg/L proposed cutoff to 1050µg/L (highest calibrator). Figures 1A and B highlight the %bias for BSI and BSII across the linear ranges.
Figure 1.
Mean %bias of the Randox DOA-V synthetic cathinone biochip A) BSI and B) BSII assays across their linear ranges using DOA-V original and new assays. Randox proposed cutoffs were 5 and 30µg/L for BSI and BSII. The vertical line (····) represents the manufacturer’s proposed cutoffs for original and new assays and (—) highest calibrators (grey: original assay, black: new assay). Horizontal lines represent (····) ±20% acceptance criteria.
The new assays for BSI-II were linear (R2≥0.98) from below the manufacturer’s proposed cutoff to the ULOL (BSI) and from the manufacturer’s proposed cutoff to above the ULOL (BSII) for the in-house standard preparations (BSI: 2.5–30µg/L, BSII: 30–1050µg/L). BSI %bias ranged from −61 to −54% (median: −58%, mean: −58%, SD: 2.9%) between the proposed cutoff and 30µg/L (Figure 1A). BSII %bias for 30–1050µg/L was −54 to −11% (median: −30%, mean: −33%, SD: 16%) (Figure 1B).
Imprecision
Imprecision was assessed at 1, 5, and 15µg/L for BSI and 30, 275, and 555µg/L for BSII in the original assay. Intra-, inter-day and total imprecision are detailed in Table 1. BSI within-run imprecision (n=20) was <12% CV and BSII <17% CV. Inter-day imprecision (n=20) ranged from 18–41% and 17–40% CV for BSI and BSII, respectively. For BSI and BSII total imprecision (n=20) was 19–42% and 18–46% CV. Results for the new formulation also are in Table 1. BSI and BSII within-run imprecision (n=20) was respectively <7.2% and <8.3% CV for all concentrations. Inter-day imprecision (n=20) was 19–40% CV for BSI and 14–41% CV for BSII, and total imprecision (n=20) was 20–41% and 16–42% CV, respectively (Table 1). In-house precision was evaluated with fortified authentic urine samples prepared fresh each day, due to stability concerns with the large negative %bias, and quality control sample instability noted after approximately 6h.
Table 1.
Intra-, inter-, and total imprecision for Randox synthetic cathinone antibodies, BSI and BSII, for both original and new DOA-V assays at three concentrations (BSI 1, 5, 15µg/L and BSII 30, 275, 555µg/L).
| Randox Biochip Array Technology |
Intra-day imprecision (N=20) (CV%) |
Inter-day imprecision (N=20) (CV%) |
Total Imprecision (N=20) (CV%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low | Med | High | Low | Med | High | Low | Med | High | ||
| Original | BSI | 12 | 8.2 | 9.9 | 31 | 18 | 41 | 33 | 19 | 42 |
| BSII | 17 | 5.1 | 8.9 | 43 | 17 | 20 | 46 | 18 | 22 | |
| New | BSI | 7.2 | 7.0 | 4.9 | 40 | 31 | 19 | 41 | 32 | 20 |
| BSII | 7.6 | 5.3 | 8.3 | 41 | 15 | 14 | 42 | 16 | 16 | |
Cross-reactivity
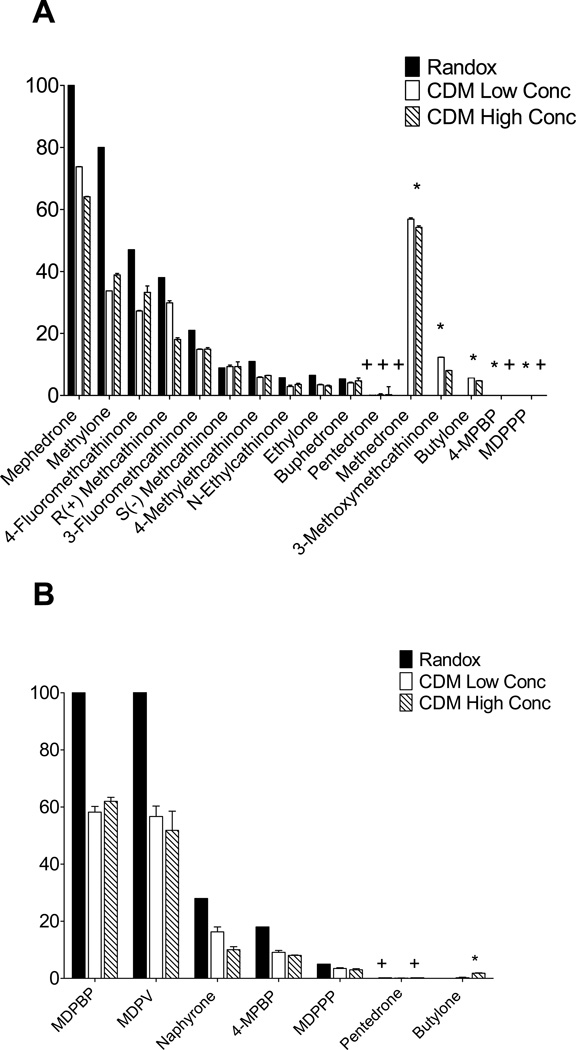
Cross-reactivity with the mephedrone antibody (BSI) varied (Figure 2A) and was lower than reported in the DOA-V product insert, especially for methylone. This study found that methedrone (4-methoxymethcathinone), 3-methoxymethcathinone and butylone cross-reacted with BSI, especially methedrone, with 57% (low) and 54% (high) cross-reactivity. 4-MPBP and MDPPP cross-reactivities were not evaluated by the manufacturer, but this study found less than 1% cross-reactivity with BSI. Pentylone, amphetamine, methamphetamine, ephedrine, pseudoephedrine, MDMA, MDEA, MDA, PMA, PMMA, MDPBP, MDPV, and naphyrone produced results <LOD.
Figure 2.
Randox and Chemistry and Drug Metabolism (CDM)-evaluated synthetic cathinone cross-reactivity with A) BSI (mephedrone) and B) BSII (MDPV). (*) represent no Randox cross-reactivity reported and (+) represent % cross-reactivity <1%.
MDPV cross-reactivities were much lower than the Randox reported cross-reactivity of 101% (Low: 37%, High: 42%). MDPBP cross-reactivities were 34% and 29% below reported 100% cross reactivity for low and high concentrations (Figure 2B). This study also found that naphyrone, 4-MPBP, MDPPP, pentedrone and butylone cross-reacted with BSII (Figure 2B). Butylone cross-reactivity with BSII was not tested by the manufacturer, but this study observed a low (<1.8%) cross-reactivity. No other tested substances demonstrated cross-reactivity with BSII >LOD including: mephedrone, methylone, 4-fluoromethcathinone, R(+) methcathinone, 3-fluoromethcathinone, 4-methylethcathinone, N-ethylcathinone, ethylone, buphedrone, methedrone, 3-methoxymethcathinone, pentylone, amphetamine, methamphetamine, ephedrine, pseudoephedrine, MDMA, MDEA, MDA, PMA, and PMMA.
Interferences
All interference standards quantified below the respective LODs, with several exceptions (Table 2). L-Ascorbic acid produced a BSI result three times the LOD and hemoglobin four times the LOD; however, no results exceeded the 5µg/L Randox proposed cutoff. Drug-free certified negative urine adjusted to pH<4 produced false positive results greater than 20% for BSI (6.3µg/L) and BSII (473µg/L). Oxidizing agents (10% bleach or 10% peroxide) reduced BSI and increased BSII concentrations compared to non-adulterated specimens containing 10µg/L mephedrone and 275µg/L MDPV. The addition of bleach or peroxide to 10µg/L mephedrone reduced immunoassay results by 25 and 31%, respectively. The addition of bleach or peroxide to 275µg/L MDPV produced results 64 and 5% above the concentration of MDPV alone, respectively.
Table 2.
Interferences producing a measured concentration >LOD of BSI (0.35µg/L) and/or BSII (8.46µg/L) with the Randox DOA-V original assay.
| Interference | Target Concentration |
BSI Measured Concentration (µg/L) |
BSII Measured Concentration (µg/L) |
|---|---|---|---|
| L-Ascorbic Acid | 4.0 g/L | 1.1 | - |
| Hemoglobin | 1.5 g/L | 1.5 | - |
| Urine, pH < 4 | - | 6.3 | 473 |
| Mephedrone (10µg/L) | 10 µg/L | 8.6 | - |
| Mephedrone (10µg/L, 10% Bleach) | 10 µg/L | 6.4 | 2.3 |
| Mephedrone (10µg/L, 10% Peroxide) | 10 µg/L | 5.9 | - |
| MDPV (275µg/L) | 275 µg/L | - | 157 |
| MDPV (275µg/L, 10% Bleach) | 275 µg/L | - | 258 |
| MDPV (275µg/L, 10% Peroxide) | 275 µg/L | - | 165 |
Carryover
No carryover was detected in negative urine specimens following a 250µg/L mephedrone or 2000µg/L MDPV specimen.
Authentic Urine Specimen Analysis
Based on BSI and BSII immunoassay screening, the overall presumptive positive rate was 0.53% (N=106) for one or more synthetic cathinones (Table 3). Among presumptive positives, 62.3% (N=66) were positive for BSI (mephedrone/methcathinone), 29.2% (N=31) for BSII (MDPV/MDPBP), and 8.5% (N=9) for both. Nine presumptive positive specimens were unavailable for confirmation due to inadequate specimen volume or laboratory error. Four specimens confirmed positive for methylone, α-PVP, pentedrone, pentylone, and/or pyrovalerone [40]. The presumptive positive confirmation rate was 4.1% (Table 3), yielding an overall positive rate for synthetic cathinones of 0.02% of 20,017 specimens.
Table 3.
Randox DOA-V Biochip Immunoassay synthetic cathinone presumptive positive results for 20,017 screened urine specimens, and presumptive positive rates of synthetic cathinones (N=106). Presumptive positive urine specimens (N=97) confirmed by liquid chromatography high resolution tandem mass spectrometry (LC-HRMS).
| Total # of Specimens (N=20,017) |
Randox Presumptive Positive Rate |
SC Presumptive Positive Rate |
Total # of Confirmed Specimens (N=97)* |
Positivity Rate (%)** |
|
|---|---|---|---|---|---|
| Total Presumptive Positive Specimens for Synthetic Cathinones | 106 | 0.53% | N/A | 4 | 4.1% |
| Presumptive Positive Specimens for BSI only | 66 | 0.33% | 62.3% | 2 | 3.3% |
| Presumptive Positive Specimens for BSII only | 31 | 0.15% | 29.2% | 1 | 3.6% |
| Total Presumptive Positive Specimens for BSI and BSII | 9 | 0.04% | 8.5% | 1 | 11.1% |
9 presumptive positive specimens were not available for confirmation
Positivity Rate (%) = 100 × (number of positive by LC-HRMS/ number of positive by Randox)
Abbreviations: SC (synthetic cathinones)
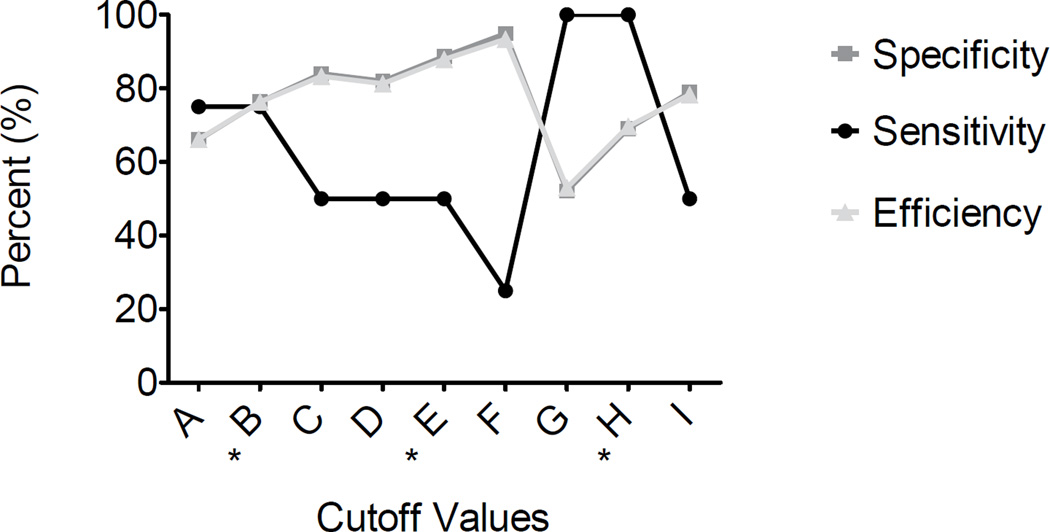
Only 4 of 97 presumptive positive specimens confirmed positive for one or more synthetic cathinones at the proposed cutoffs of 5µg/L (BSI) and 30µg/L (BSII). All 101 presumptive negative urine specimens confirmed negative, yielding a total of 4 TP, 93 FP, 101 TN, and 0 FN. Sensitivity, specificity, and efficiency were 100%, 52.1%, and 53.0%, respectively. Additional cutoffs were evaluated to test if sensitivity, specificity, and efficiency could be improved (Table 4). Cutoff values of 7.5µg/L for BSI only and 40µg/L for BSII only yielded sensitivities, specificities, and efficiencies of 75, 76, and 76%, and 50, 89, and 88%, respectively. Furthermore, sensitivity of the DOA-V assay improved when utilizing BSI and BSII cutoffs together (Figure 3). Optimal assay parameters were achieved when BSI 7.5µg/L and/or 40µg/L BSII cutoffs were applied, yielding sensitivity 100%, specificity 69% and efficiency 70%.
Table 4.
Evaluation of potential cutoffs for Randox DOA-V for the detection of synthetic cathinones in urine with confirmation results of authentic urine specimens (N=198).
| Cutoff (µg/L) | BSI | BSII | BSI & BSII | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 | 7.5† | 10 | 30 | 40† | 50 | 5, 30* | 7.5, 40† | 10, 50 | |
| TP | 3 | 3 | 2 | 2 | 2 | 1 | 4 | 4 | 2 |
| FP | 66 | 46 | 31 | 35 | 22 | 10 | 93 | 60 | 41 |
| TN | 128 | 148 | 163 | 159 | 172 | 184 | 101 | 134 | 153 |
| FN | 1 | 1 | 2 | 2 | 2 | 3 | 0 | 0 | 2 |
| Sensitivity (%) | 75.0 | 75.0 | 50.0 | 50.0 | 50.0 | 25.0 | 100 | 100 | 50 |
| Specificity (%) | 66.0 | 76.3 | 84.0 | 82.0 | 88.7 | 94.9 | 52.1 | 69.1 | 78.9 |
| Efficiency (%) | 66.2 | 76.3 | 83.3 | 81.3 | 87.9 | 93.4 | 53.0 | 69.7 | 78.3 |
Randox proposed cutoff concentrations for BSI and BSII
Optimal cutoffs for BSI only, BSII only, and BSI and BSII
Figure 3.
Sensitivity, specificity, and efficiency of Randox DOA-V for the detection of synthetic cathinones in authentic confirmed urine specimens (N=198) evaluated with different cutoffs: A) BSI (5µg/L), B*) BSI (7.5µg/L), C) BSI (10µg/L), D) BSII (30µg/L), E*) BSII (40µg/L), F) BSII (50µg/L), G) BSI (5µg/L) & BSII (30µg/L) (Randox proposed cutoffs), H*) BSI (7.5µg/L) & BSII (40µg/L), I) BSI (10µg/L) & BSII (50µg/L). Only four specimens were confirmed positive via LC-HRMS. * Represents optimal cutoff concentrations for BSI only (B), BSII only (E), and BSI & BSII (H).
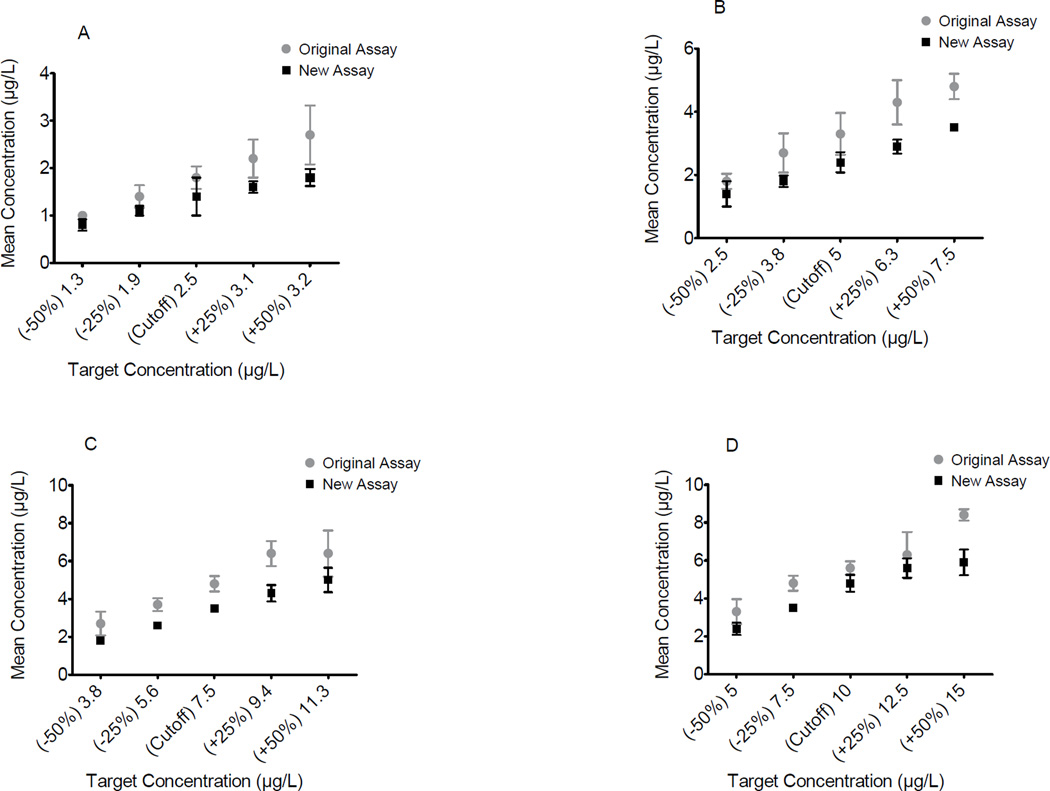
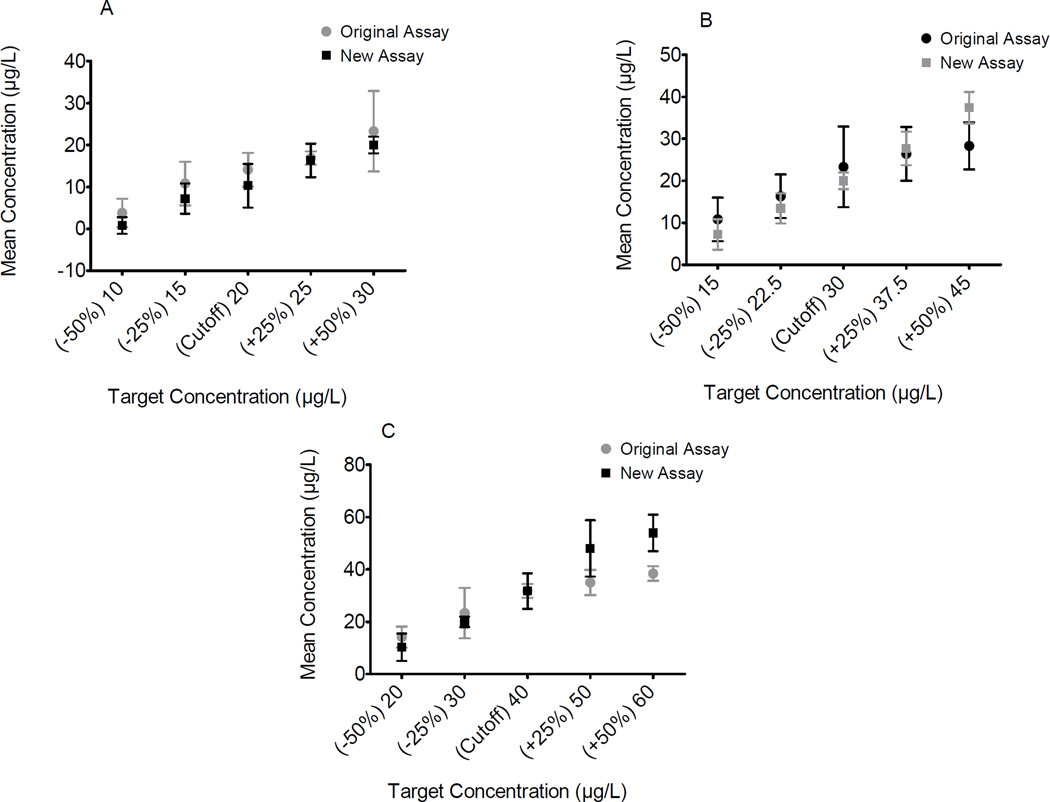
To evaluate the efficiency of the manufacturer’s proposed cutoffs four cutoff concentrations for BSI (2.5, 5, 7.5, 10µg/L) and three BSII cutoff concentrations (20, 30, 40µg/L) and ±25% and ±50% around each cutoff were evaluated (Figures 4 and 5). The consistent under recovery of expected concentrations at all proposed cutoffs and ±25% and ±50% of these concentrations limited evaluation of performance at these cutoffs. The manufacturer’s proposed BSI 5µg/L cutoff could be differentiated from samples fortified at ±50% of this concentration in both assays despite %bias −36 to −28% in the original and −54 to −45% in the new formulation (Figure 4B). Good separation was achieved for both BSI assays at ±25% above the proposed cutoff of 7.5µg/L (original %bias −43 to −28%, new %bias −56 to −52%) (Figure 4C). At the manufacturer’s BSII proposed 30µg/L cutoff, good separation was achieved at ±25% of the cutoff concentration for the new assay (%bias −52 to −17%) but not for the original assay (Figure 5B). At the proposed BSII 40µg/L cutoff, samples fortified at ±50% of this cutoff for the original assay (%bias −36 to −21%) (Figure 5C) were distinguished.
Figure 4.
Performance around cutoff concentrations of Randox synthetic cathinone BSI biochip for original and new DOA-V kits. Mephedrone fortified negative urine samples at cutoff A) 2.5, B) 5, C) 7.5, and D) 10µg/L, and ±25% and ±50% of cutoffs. Error bars represent ±2SD from mean observed concentration.
Figure 5.
Performance around cutoff concentrations of Randox synthetic cathinone BSII biochip for original and new DOA-V assays. MDPV fortified negative urine samples at A) 20, B) 30, and C) 40µg/L cutoffs, and ±25% and ±50% of these cutoffs. Error bars represent ±2SD from the mean observed concentration.
Discussion
The objectives of this study were to determine if the DOA-V provides a reliable screen for the detection of synthetic cathinones in urine and to evaluate the feasibility of this assay in a high-throughput laboratory setting. Based on observations, the DOA-V’s main limitation for the original and new assays was the high negative %bias. Concentrations for both mephedrone and MDPV were consistently lower and below acceptable criteria (±20%), despite daily preparation of fortified samples.
Due to low recovery in BSI (mephedrone) and BSII (MDPV) in the original assay and the high negative %bias for both BSI and BSII with the DOA-V new formulation, linearity, imprecision, and cutoff evaluation experiments were repeated with freshly fortified samples to ensure that the low recovery was not due to stability issues. Results did not improve and analyte recovery remained low.
The DOA-V demonstrated acceptable intra-assay imprecision for both formulations with three Randox control concentrations, but was unacceptable for some inter-assay and total imprecision measures. It is possible that the variability observed among the inter-day and total imprecision is due to different analysts preparing samples each day. Due to stability concerns, quality control samples were fortified each day to ensure that samples contained intact synthetic cathinones at the specified concentrations. This also could have increased imprecision to a small degree. It is more likely that the variability was related to the use of calibrators over several days that may have been less stable than indicated by the manufacturer’s instructions (stable for up to seven days when refrigerated). In addition, the high negative percent bias could have contributed to poor imprecision at lower quality control concentrations.
Method validation results demonstrated optimal performance of mephedrone in both DOA-V kits above the proposed Randox cutoff: 7.5µg/L, as it was determined that 95% of samples did not overlap with concentrations ±25% of the cutoff concentration. Optimal BSII (MDPV) performance was observed above the proposed Randox cutoff with the original assay (40µg/L). These results differed from what was observed for BSII with the new formulation. Noticeable separation of MDPV concentrations was demonstrated at both 30 and 20µg/L cutoffs..
Based on the evaluation of alternative cutoffs, poor performance around the manufacturer’s proposed cutoffs and good sensitivity, specificity, and efficiency observed at cutoff concentrations of 7.5 and 40µg/L suggest implementation of higher proposed cutoffs for synthetic cathinone detection in urine with the DOA-V assay. However, based on this method validation alone, it is hard to establish proposed cutoff values for the detection of mephedrone and MDPV for both the original and new DOA-V assays because of the high negative %bias. Additionally, limited specimens (N=4) were confirmed positive for synthetic cathinones in urine. More positive specimens are needed for accurate assessments of DOA-V assay parameters (sensitivity, specificity, and efficiency) utilizing alternative cutoff concentrations.
BSI and BSII cross-reactivities were consistently lower than Randox reported results, especially for methylone. This difference in cross-reactivity could be attributed to low analyte recovery observed in this validation. In addition, Randox cross-reactivities were assessed with nine concentrations in calibrator base (containing phosphate buffer) and not in authentic urine; therefore, matrix interferences may have contributed to the low cross-reactivities observed during our method validation. This validation also examined additional synthetic cathinone cross-reactivities that had not been previously reported. Five compounds exhibited cross-reactivity to mephedrone antibody, most notably methedrone (54–57%). Methedrone only differs from mephedrone by a methoxy group on the 4’ position. The 3’ methedrone regioisomer also demonstrated cross-reactivity with BSI, although to a lesser degree than methedrone. Butylone, a positional isomer of ethylone, had comparable cross-reactivity to the reported Randox cross-reactivity of ethylone. Both 4-MPBP and MDPPP exhibited cross-reactivity to mephedrone when present at high concentrations, albeit with minimal cross-reactivity (0.02%). Only one additional synthetic cathinone (butylone) demonstrated 1.8% cross-reactivity with BSII.
Based on our cross-reactivity data, it appears that 4-methylphenyl-based compounds, such as methylone and methedrone, are cross-reactive primarily to BSI, whereas MDPV-like compounds, such as MDPPP and naphyrone, are primarily cross-reactive with BSII. Those compounds containing mixed variations, like 4-MPBP, appear to have cross-reactivity to both BSI and BSII. It is hypothesized that newer synthetic cathinones and cathinones not analyzed during cross-reactivity experiments with similar structural features would behave in a similar manner. Increased BSI readings occurred when samples contained L-ascorbic acid (4 g/L) or hemoglobin (1.5 g/L), but concentrations were less than the 5µg/L proposed cutoff. Negative urine adjusted to pH 4 also interfered with BSI and BSII assays; concentrations were above the proposed cutoffs and yielded false positive screening results. The addition of oxidizing agents, bleach or peroxide, to mephedrone-fortified samples reduced concentrations as compared to mephedrone alone. In contrast, when these adulterants were added to MDPV-fortified urine, concentrations increased.
Another study limitation is the unknown stability of synthetic cathinones in authentic urine specimens. Specimens were stored at room temperature generally for two to four weeks prior to screening. Because urine specimens are rarely assayed immediately after collection, the stability of synthetic cathinones in urine is an important issue, but minimal data are available. Mephedrone urine concentrations decreased by 60% when samples were stored at room temperature for up to 14 days, but were stable at 4°C over the same time period [57]. MDPV was stable in urine for 14 days at room temperature and 4°C. Soh and Elliot reported that 4-MEC was not detectable in blood after 14 days at room temperature, with a corresponding 54% loss in plasma [58]. Two additional studies reported that substitutions on the benzene ring, nitrogen, and phenethylamine backbone affected synthetic cathinone stability [35, 59].
Cathinones including the pyrrolidinyl moiety, such as MDPV, MDPPP, MDPBP, α-PVP and 4-MPBP, are more stable. This enhanced stability may be due to steric hindrance by the bulky pyrrolidinyl group adjacent to the C=O. This may block the electrophilic carbon reducing its reactivity. Therefore, reduction of the ketone group to its corresponding alcohol may not occur as readily as for cathinones with the pyrrolidinyl substituent. Tsujikawa et al. also reported that tertiary amines, such as dimethylcathinone, are more stable [59]. However, these authors hypothesized the enhanced stability was attributed to sensitivity to oxidative deamination, as tertiary amines do not readily undergo oxidative deamination like secondary aliphatic amines. In addition, Zaitsu et al. found that methylone does not readily reduce to its corresponding alcohol like butylone and ethylone and suggested this may be due to the compound’s structural affinity differences to reductive enzymes [60]. Concheiro et al. previously documented short-term stability findings during confirmation method validation and showed that most synthetic cathinones were stable for 72h at 4°C, with the exception of benzedrone (27% loss) [40]. However, many synthetic cathinones were not stable for 24h at room temperature (20% to 67.6% loss). Only the metabolites buphedrone ephedrine, 4-methylephedrine, and 4-MEC metabolite, as well as the synthetic cathinones diethylcathinone, MDPPP, MDPBP, α-PVP, 4-MPBP and MDPV, were stable at room temperature for 24 h. There are no published long-term synthetic cathinone stability studies.
The majority of presumptive positive urine specimens (62.3%, N=66) screened positive on BSI, which targets mephedrone and methcathinone, with cross-reactivity to other cathinones; these were reported unstable at room temperature based on stability data [40]. The confirmation rate of presumptive positive specimens was only 4.1% by LC-HRMS, indicating a high false positive screening rate (95.9%). False positive results could be due to cross-reactivity with other cathinones not present in the confirmatory method (although this confirmation assay is the most comprehensive method to date and parent synthetic cathinones are well represented in urine), instability and degradation of the specimens over the interval between screening and confirmatory testing, or inappropriately low cutoff concentrations recommended by the manufacturer. Multiple cathinones exhibited cross-reactivity to mephedrone and MDPV in the screening assay; however, these cathinones were included in the confirmation method, and so should have been identified. The urine specimens were stored refrigerated (4°C) up to a year before confirmation analysis. The synthetic cathinone stability findings in urine at 4°C suggest that the high false positive screening rate and low confirmation rate could be due to analyte degradation, although it is not possible to definitively determine the reason unless new fresh urine specimens are analyzed.
Inclusion of only four metabolites in the confirmatory assay (due to lack of commercially available metabolite standards) could also contribute to the false positive screening rate. It is possible that metabolites present in urine but not included in the confirmation contributed to the low synthetic cathinones confirmation rate. However, this is unlikely, because for most synthetic cathinones, the parent drug is often detected in urine specimens in high concentrations urine [8, 11, 13–14, 28, 30–31, 39, 43], along with metabolites.
Little is known about the prevalence of synthetic cathinones despite the growing media attention and regulation of many of these cathinone derivatives. Kriikku et al. reported the prevalence of MDPV among drivers apprehended for driving under the influence of drugs (DUID) in Finland from August 2009 to August 2010. The study found that 5.7% (n=259) of all confirmed DUID cases, excluding alcohol-only cases (n=4570), tested positive for MDPV [33]. A recent study administered a self-report survey to 2349 students at a large university in the Southeastern United States and found only 25 (1.0%) students reporting at least one synthetic cathinone intake in their lifetime [61]. These authors hypothesized that the low prevalence of cathinone use suggests there may not be an epidemic of illicit use of these compounds. Similarly, the overall presumptive positive rate (0.53%) and presumptive positive confirmation rate (4.1%) were low in this study. However, due to storage conditions and stability issues with synthetic cathinones it is difficult to draw definitive conclusions.
Although the original DOA-V assay had a sensitivity of 100%, its specificity and efficiency were low (52.1% and 53.0%), potentially due to stability issues. As discussed previously, assay performance may be improved with alternative cutoffs. The Randox DOA-V Biochip Array Technology paired with the Randox Evidence® Analyzer is capable of testing 90 samples for 11 different analytes in approximately one to two hours. In particular, it is a sensitive assay capable of identifying urinary synthetic cathinones. To date, this is the only commercially available immunoassay capable of detecting synthetic cathinones in urine.
Several confirmation and screening methods utilizing LC-MSMS and GC-MS were previously published for the determination of synthetic cathinones in urine [28, 38, 41, 43–52, 62]. Previous analytical methods included no more than 12 synthetic cathinones, while this recently developed LC-HRMS confirmation method includes 24 synthetic cathinones and four metabolites [40]. It is difficult for immunoassay screening methods to detect new emerging synthetic cathinones, as they are limited to the antibody’s affinity and cross-reactivity to the target compound. LC-MSMS methods incorporating a wide range of cathinones in a rapid screening method is better suited to detect novel designer drugs compared to immunoassays for high-throughput laboratories, due to the long lead time to raise antibodies to new synthetic cathinones and to optimize new commercial immunoassays for newly introduced designer drugs. However, it is difficult for laboratories to conduct high throughput chromatographic assays.
Conclusion
This is the first method validation of the Randox Biochip Array Technology for the detection of synthetic cathinones in urine. Results demonstrated a large negative percent bias outside of acceptable limits for the detection of BSI (mephedrone) and BSII (MDPV). Cross-reactivity data in authentic urine were provided for many synthetic cathinones to suggest compounds that might or might not be detected by the assay. A smaller number of synthetic cathinone cross-reactivity data were provided in the package insert, but data were acquired with synthetic cathinones in calibrator base (phosphate buffer), rather than urine. The Randox DOA-V biochip array is currently the only immunoassay available for preliminary detection of synthetic cathinones in urine, and also is capable of high-throughput and requires only a small urine sample volume on the Evidence® Analyzer. This study provides critical data for scientists faced with the need to identify potential synthetic cathinone intake in clinical and forensic cases.
Supplementary Material
Acknowledgments
This work was funded by the Department of Defense Counter Narcotics Program and the Intramural Research Program of the National Institute for Drug Abuse (NIDA), National Institutes of Health (NIH), Department of Health and Human Services (DHHS). The assistance of the Forensic Toxicology Drug Testing Laboratory, Fort Meade, MD and Randox Toxicology Corporation in the conduct of this study is greatly appreciated. We acknowledge the staff of the Chemistry and Drug Metabolism Section, NIDA, NIH, and the Graduate Partnership Program, NIH
Footnotes
Disclaimer
The opinions or assertions herein are those of the authors and do not necessarily reflect the view of the Departments of the Army, Navy, or the Department of Defense.
References
- 1.Drug Enforcement Administration (DEA) National Forensic Laboratory Information System Special Report: Synthetic Cannabinoids and Synthetic Cathinones Reported in NFLIS, 2009–2010. 2011. [Google Scholar]
- 2.Peters FT, Meyer MR. In vitro approaches to studying the metabolism of new psychoactive compounds. Drug Test. Anal. 2011;3:483. doi: 10.1002/dta.295. [DOI] [PubMed] [Google Scholar]
- 3.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) Annual Report 2012: The State of the Drugs Problem in Europe. 2012. p. 89. [Google Scholar]
- 4.Baumann MH, Partilla JS, Lehner KR. Psychoactive "bath salts": not so soothing. Eur. J. Pharmacol. 2013;698:1. doi: 10.1016/j.ejphar.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zawilska BJ, Wojcieszak J. Designer cathinones--an emerging class of novel recreational drugs. Forensic Sci. Int. 2013;231:42. doi: 10.1016/j.forsciint.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Hill SL, Thomas SH. Clinical toxicology of newer recreational drugs. Clin. Toxicol. 2011;49:705. doi: 10.3109/15563650.2011.615318. [DOI] [PubMed] [Google Scholar]
- 7.Schifano F, Albanese A, Fergus S, Stair JL, Deluca P, Corazza O, Davey Z, Corkery J, Siemann H, Scherbaum N, Farre M, Torrens M, Demetrovics Z, Ghodse AH. Mephedrone (4-methylmethcathinone; 'meow meow'): chemical, pharmacological and clinical issues. Psychopharmacology. 2011;214:593. doi: 10.1007/s00213-010-2070-x. [DOI] [PubMed] [Google Scholar]
- 8.Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of "bath salts" and "legal highs" (synthetic cathinones) in the United States. Clin. Toxicol. 2011;49:499. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- 9.Penders TM, Gestring RE, Vilensky DA. Intoxication delirium following use of synthetic cathinone derivatives. Am. J. Drug Alcohol Abuse. 2012;38:616. doi: 10.3109/00952990.2012.694535. [DOI] [PubMed] [Google Scholar]
- 10.James D, Adams RD, Spears R, Cooper G, Lupton DJ, Thompson JP, Thomas SH. Clinical characteristics of mephedrone toxicity reported to the U.K. National Poisons Information Service. Emerg. Med. J. 2011;28:686. doi: 10.1136/emj.2010.096636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lusthof KJ, Oosting R, Maes A, Verschraagen M, Dijkhuizen A, Sprong AG. A case of extreme agitation and death after the use of mephedrone in The Netherlands. Forensic Sci. Int. 2011;206:e93. doi: 10.1016/j.forsciint.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Carbone PN, Carbone DL, Carstairs SD, Luzi SA. Sudden cardiac death associated with methylone use. Am. J. Forensic Med. Pathol. 2013;34:26. doi: 10.1097/PAF.0b013e31827ab5da. [DOI] [PubMed] [Google Scholar]
- 13.Pearson JM, Hargraves TL, Hair LS, Massucci CJ, Frazee CC, 3rd, Garg U, Pietak BR. Three fatal intoxications due to methylone. J.Anal. Toxicol. 2012;36:444. doi: 10.1093/jat/bks043. [DOI] [PubMed] [Google Scholar]
- 14.Murray BL, Murphy CM, Beuhler MC. Death following recreational use of designer drug "bath salts" containing 3,4-Methylenedioxypyrovalerone (MDPV) J. Med. Toxicol. 2012;8:69. doi: 10.1007/s13181-011-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rojek S, Klys M, Strona M, Maciow M, Kula K. "Legal highs"--toxicity in the clinical and medico-legal aspect as exemplified by suicide with bk-MBDB administration. Forensic Sci. Int. 2012;222:e1. doi: 10.1016/j.forsciint.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 16.Vardakou I, Pistos C, Spiliopoulou C. Drugs for youth via Internet and the example of mephedrone. Toxicol. Lett. 2011;201:191. doi: 10.1016/j.toxlet.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addiction. 2011;106:154. doi: 10.1111/j.1360-0443.2010.03130.x. [DOI] [PubMed] [Google Scholar]
- 18.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) Risk assessment report of a new pyschoactive substance- 4-methylmehtcathinone (mephedrone) 2011. [Google Scholar]
- 19.American Association of Poison Control Centers (AAPCC) Alerts: Bath Salts. 2013 Available at: http://www.aapcc.org/alerts/bath-salts/ [2 December 2013].
- 20.Synthetic Drug Abuse Prevention Act of 2012. 2012. [Google Scholar]
- 21.Drug Enforcement Administration (DEA) Schedules of Controlled Substances: Temporary Placement of Three Synthetic Cathinones Into Schedule I. Federal Register. 2011;76:204. [PubMed] [Google Scholar]
- 22.National Conference of State Legislatures. Substituted Cathinones (aka “Bath Salts”) Enactments. 2012 Available from: http://www.ncsl.org/issues-research/justice/substituted-cathinones-enactments.aspx [2 December 2013].
- 23.Wikstrom M, Thelander G, Nystrom I, Kronstrand R. Two fatal intoxications with the new designer drug methedrone (4-methoxymethcathinone) J. Anal. Toxicol. 2010;34:594. doi: 10.1093/jat/34.9.594. [DOI] [PubMed] [Google Scholar]
- 24.Martin M, Muller JF, Turner K, Duez M, Cirimele V. Evidence of mephedrone chronic abuse through hair analysis using GC/MS. Forensic Sci. Int. 2012;218:44. doi: 10.1016/j.forsciint.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Rust KY, Baumgartner MR, Dally AM, Kraemer T. Prevalence of new psychoactive substances: A retrospective study in hair. Drug Test. Anal. 2012;4:402. doi: 10.1002/dta.1338. [DOI] [PubMed] [Google Scholar]
- 26.Shah SA, Deshmukh NI, Barker J, Petroczi A, Cross P, Archer R, Naughton DP. Quantitative analysis of mephedrone using liquid chromatography tandem mass spectroscopy: application to human hair. J. Pharm. Biomed. Anal. 2012;61:64. doi: 10.1016/j.jpba.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 27.Torrance H, Cooper G. The detection of mephedrone (4-methylmethcathinone) in 4 fatalities in Scotland. Forensic Sci. Int. 2010;202:e62. doi: 10.1016/j.forsciint.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Dickson AJ, Vorce SP, Levine B, Past MR. Multiple-drug toxicity caused by the coadministration of 4-methylmethcathinone (mephedrone) and heroin. J. Anal. Toxicol. 2010;34:162. doi: 10.1093/jat/34.3.162. [DOI] [PubMed] [Google Scholar]
- 29.Adamowicz P, Tokarczyk B, Stanaszek R, Slopianka M. Fatal mephedrone intoxication--a case report. J. Anal. Toxicol. 2013;37:37. doi: 10.1093/jat/bks085. [DOI] [PubMed] [Google Scholar]
- 30.Wyman JF, Lavins ES, Engelhart D, Armstrong EJ, Snell KD, Boggs PD, Taylor SM, Norris RN, Miller FP. Postmortem tissue distribution of MDPV following lethal intoxication by "bath salts". J. Anal. Toxicol. 2013;37:182. doi: 10.1093/jat/bkt001. [DOI] [PubMed] [Google Scholar]
- 31.Cawrse BM, Levine B, Jufer RA, Fowler DR, Vorce SP, Dickson AJ, Holler JM. Distribution of methylone in four postmortem cases. J. Anal. Toxicol. 2012;36:434. doi: 10.1093/jat/bks046. [DOI] [PubMed] [Google Scholar]
- 32.Pedersen AJ, Reitzel LA, Johansen SS, Linnet K. In vitro metabolism studies on mephedrone and analysis of forensic cases. Drug Test. Anal. 2013;5:430. doi: 10.1002/dta.1369. [DOI] [PubMed] [Google Scholar]
- 33.Kriikku P, Wilhelm L, Schwarz O, Rintatalo J. New designer drug of abuse: 3,4-Methylenedioxypyrovalerone (MDPV). Findings from apprehended drivers in Finland. Forensic Sci. Int. 2011;210:195. doi: 10.1016/j.forsciint.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Maskell PD, De Paoli G, Seneviratne C, Pounder DJ. Mephedrone (4-methylmethcathinone)-related deaths. J. Anal. Toxicol. 2011;35:188. doi: 10.1093/anatox/35.3.188. [DOI] [PubMed] [Google Scholar]
- 35.Sorensen LK. Determination of cathinones and related ephedrines in forensic whole-blood samples by liquid-chromatography-electrospray tandem mass spectrometry. J. Chromatogr. B. 2011;879:727. doi: 10.1016/j.jchromb.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 36.Ammann J, McLaren JM, Gerostamoulos D, Beyer J. Detection and Quantification of New Designer Drugs in Human Blood: Part 1 – Synthetic Cannabinoids. J. Anal.Toxicol. 2012;36:372. doi: 10.1093/jat/bks048. [DOI] [PubMed] [Google Scholar]
- 37.Derungs A, Schietzel S, Meyer MR, Maurer HH, Krahenbuhl S, Liechti ME. Sympathomimetic toxicity in a case of analytically confirmed recreational use of naphyrone (naphthylpyrovalerone) Clin. Toxicol. 2011;49:691. doi: 10.3109/15563650.2011.592838. [DOI] [PubMed] [Google Scholar]
- 38.Wood DM, Davies S, Puchnarewicz M, Button J, Archer R, Ovaska H, Ramsey J, Lee T, Holt DW, Dargan PI. Recreational use of mephedrone (4-methylmethcathinone. 4-MMC) with associated sympathomimetic toxicity. J. Med. Toxicol. 2010;6:327. doi: 10.1007/s13181-010-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thornton SL, Gerona RR, Tomaszewski CA. Psychosis from a bath salt product containing flephedrone and MDPV with serum, urine, and product quantification. J. Med. Toxicol. 2012;8:310. doi: 10.1007/s13181-012-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Concheiro M, Anizan S, Ellefsen K, A M. Huestis. Simultaneous quantification of 28 synthetic cathinones and metabolites in urine by liquid chromatography-high resolution mass spectrometry. Anal. Bioanal. Chem. 2013;405:9437. doi: 10.1007/s00216-013-7386-z. [DOI] [PubMed] [Google Scholar]
- 41.Marinetti LJ, Antonides HM. Analysis of synthetic cathinones commonly found in bath salts in human performance and postmortem toxicology: method development, drug distribution and interpretation of results. J. Anal. Toxicol. 2013;37:135. doi: 10.1093/jat/bks136. [DOI] [PubMed] [Google Scholar]
- 42.Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of "bath salts" containing 3,4-methylenedioxypyrovalerone. Ann. Emerg. Med. 2012;60:103. doi: 10.1016/j.annemergmed.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Ojanpera IA, Heikman PK, Rasanen IJ. Urine analysis of 3,4-methylenedioxypyrovalerone in opioid-dependent patients by gas chromatography-mass spectrometry. Ther. Drug Monit. 2011;33:257. doi: 10.1097/FTD.0b013e318208b693. [DOI] [PubMed] [Google Scholar]
- 44.Meyer M. Detection of Beta-keto Amphetamines in Urine within the Systematic Toxicological Analysis (STA) using GC-MS. TIAFT Bulletin. 2010;40:36. [Google Scholar]
- 45.Zaitsu K, Katagi M, Kamata HT, Kamata T, Shima N, Miki A, Tsuchihashi H, Mori Y. Determination of the metabolites of the new designer drugs bk-MBDB and bk-MDEA in human urine. Forensic Sci. Int. 2009;188:131. doi: 10.1016/j.forsciint.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Meyer MR, Wilhelm J, Peters FT, Maurer HH. Beta-keto amphetamines: studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2010;397:1225. doi: 10.1007/s00216-010-3636-5. [DOI] [PubMed] [Google Scholar]
- 47.Katagi M. Metabolism and Forensic Toxicological Analyses of the Extensively Abused Designer Drug Methylone. TIAFT Bulletin. 2010;40:30. [Google Scholar]
- 48.Kamata HT, Shima N, Zaitsu K, Kamata T, Miki A, Nishikawa M, Katagi M, Tsuchihashi H. Metabolism of the recently encountered designer drug, methylone, in humans and rats. Xenobiotica. 2006;36:709. doi: 10.1080/00498250600780191. [DOI] [PubMed] [Google Scholar]
- 49.Strano-Rossi S, Cadwallader AB, de la Torre X, Botre F. Toxicological determination and in vitro metabolism of the designer drug methylenedioxypyrovalerone (MDPV) by gas chromatography/mass spectrometry and liquid chromatography/quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2010;24:2706. doi: 10.1002/rcm.4692. [DOI] [PubMed] [Google Scholar]
- 50.Meyer MR, Du P, Schuster F, Maurer HH. Studies on the metabolism of the α-pyrrolidinophenone designer drug methylenedioxy-pyrovalerone (MDPV) in rat and human urine and human liver microsomes using GC–MS and LC–high-resolution MS and its detectability in urine by GC–MS. J. Mass Spec. 2010;45:1426. doi: 10.1002/jms.1859. [DOI] [PubMed] [Google Scholar]
- 51.Grueninger D, Englert R. Determination of the amphetamine-like designer drugs methcathinone and 4-methylmethcathinone in urine by LC-MS/MS. Ann. Toxicol. Anal. 2011;23:7. [Google Scholar]
- 52.Bell C, George C, Kicman AT, Traynor A. Development of a rapid LC-MS/MS method for direct urinalysis of designer drugs. Drug Test. Anal. 2011;3:496. doi: 10.1002/dta.306. [DOI] [PubMed] [Google Scholar]
- 53.Kasick DP, McKnight CA, Klisovic E. "Bath salt" ingestion leading to severe intoxication delirium: two cases and a brief review of the emergence of mephedrone use. Am. J. Drug Alcohol Abuse. 2012;38:176. doi: 10.3109/00952990.2011.643999. [DOI] [PubMed] [Google Scholar]
- 54.Macher AM, Penders TM. False-positive phencyclidine immunoassay results caused by 3,4-methylenedioxypyrovalerone (MDPV) Drug Test. Anal. 2013;5:130. doi: 10.1002/dta.1371. [DOI] [PubMed] [Google Scholar]
- 55.Swortwood MJ, Hearn WL, Decaprio AP. Cross-reactivity of designer drugs, including cathinone derivatives, in commercial enzyme-linked immunosorbent assays. Drug Test. Anal. 2013;405:1383. doi: 10.1002/dta.1489. [DOI] [PubMed] [Google Scholar]
- 56.Krouwer JS, Rabinowitz R. How to improve estimates of imprecision. Clin. Chem. 1984;30:290. [PubMed] [Google Scholar]
- 57.Johnson RD, Botch-Jones SR. The stability of four designer drugs: MDPV, mephedrone, BZP and TFMPP in three biological matrices under various storage conditions. J. Anal. Toxicol. 2013;37:51. doi: 10.1093/jat/bks138. [DOI] [PubMed] [Google Scholar]
- 58.Soh YNA, Elliott S. An investigation of the stability of emerging new psychoactive substances. Drug Testing and Analysis. 2013 doi: 10.1002/dta.1576. [DOI] [PubMed] [Google Scholar]
- 59.Tsujikawa K, Mikuma T, Kuwayama K, Miyaguchi H, Kanamori T, Iwata YT, Inoue H. Degradation pathways of 4-methylmethcathinone in alkaline solution and stability of methcathinone analogs in various pH solutions. Forensic Sci. Int. 2012;220:103. doi: 10.1016/j.forsciint.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 60.Zaitsu K, Katagi M, Tatsuno M, Sato T, Tsuchihashi H, Suzuki K. Recently abused β-keto derivatives of 3,4-methylenedioxyphenylalkylamines: a review of their metabolisms and toxicological analysis. Forensic Toxicol. 2011 Jul;29:73. [Google Scholar]
- 61.Stogner JM, Miller BL. Investigating the 'bath salt' panic: the rarity of synthetic cathinone use among students in the United States. Drug Alcohol Rev. 2013;32:545. doi: 10.1111/dar.12055. [DOI] [PubMed] [Google Scholar]
- 62.O'Byrne PM, Kavanagh PV, McNamara SM, Stokes SM. Screening of stimulants including designer drugs in urine using a liquid chromatography tandem mass spectrometry system. J. Anal. Toxicol. 2013;37:64. doi: 10.1093/jat/bks091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.