Abstract
Until recently, primary central nervous system lymphoma (PCNSL) was associated with a uniformly dismal prognosis. It is now reasonable to anticipate long-term survival and possibly cure for a significant proportion of patients diagnosed with PCNSL. Accumulated data generated over the past ten years has provided evidence that long-term progression-free survival (PFS) can reproducibly be attained in a significant fraction of PCNSL patients that receive dose-intensive chemotherapy consolidation, without whole brain radiotherapy. One consolidative regimen that has reproducibly demonstrated promise is the combination of infusional etoposide plus high-dose cytarabine (EA), administered in first complete remission after methotrexate, temozolomide and rituximab-based induction. Given evolving principles of management and the mounting evidence for reproducible improvements in survival rates in prospective clinical series, our goal in this review is to highlight and update principles in diagnosis, staging and management as well as to review data regarding the pathogenesis of central nervous system lymphomas, information that is likely to constitute a basis for the implementation of novel therapies that are requisite for further progress in this unique phenotype of non-Hodgkin lymphoma.
Keywords: Primary CNS Lymphoma, High-Dose Chemotherapy, Rituximab, Tumour Microenvironment
Introduction
Primary central nervous system lymphoma (PCNSL) is characterized by dissemination of aggressive non-Hodgkin lymphoma (NHL) within the brain, cranial nerves, leptomeninges, cerebrospinal fluid (CSF), intraocular structures and spinal cord. (Hochberg & Miller, 1988; Batchelor & Loeffler, 2006) In secondary central nervous system (CNS) lymphomas, CNS localization of lymphoma is accompanied by either concomitant or a history of systemic involvement. While PCNSL constitutes approximately 3% of all brain tumours, and 2–3% of all cases of NHL, according to the Surveillance, Epidemiology and End Results database (http://seer.cancer.gov/), its incidence appears to be increasing among persons age sixty-five years and older. (Villano et al., 2011)
Historically, PCNSL, known previously as reticulum cell sarcoma or microglioma, was associated with an extremely grave prognosis. (Norden et al., 2011) During the 1960’s, physicians lacked prospective data to guide management and whole brain irradiation (WBRT) was usually implemented as a first-line intervention, given its activity in yielding immediate responses in patients faced with a rapidly deteriorating course, resulting in median survival of 12 months. Treatment of CNS lymphomas became more effective in the 1970’s with recognition of the efficacy of high-dose methotrexate (HD-MTX). (Skarin et al., 1977; Ervin & Canellos, 1980)
Given that several recent phase I/II studies have demonstrated improvements in outcomes for patients with PCNSL, our goal is to review current information regarding disease biology, diagnosis, staging and strategies in therapeutic management. (Illerhaus et al., 2008; Wieduwilt et al., 2012; Bromberg et al., 2013; Korfel et al., 2013; Rubenstein et al., 2013a; Rubenstein et al., 2013b)
Aetiology
The best established risk factors for CNS involvement of NHL are acquired or congenital immunodeficiency states. PCNSL is an acquired immunodeficiency syndrome (AIDS)-defining illness associated with a low CD4 count (< 0.05 × 109 cells/l) and almost 100% association with Epstein Barr Virus (EBV). While only 20% of systemic AIDS-related lymphomas are associated with EBV, EBV infection of the malignant clone correlates with increased risk for CNS involvement. (Cingolani et al., 2000) Patients with severe-combined or common-variable immunodeficiency, ataxia-telangiectasia or Wiskott-Aldrich syndrome have about a 4% risk of PCNSL. Post-transplant lymphoproliferative disorder (PTLD) involving the brain develops in 1–2% of renal transplant recipients and 2–7% recipients of cardiac, lung and liver transplants. CNS PTLD is strongly associated with EBV in the setting of T-cell immunodeficiency caused by agents such as mycophenolate mofetil. (Schabet, 1999) By contrast, among patients without overt immunosuppression, EBV infection of CNS lymphoma is rarely detected. (Camilleri-Broet et al., 2006)
Molecular Pathogenesis and the Microenvironment
Like glioblastoma, PCNSL is a highly infiltrative neoplasm, particularly at relapse, prompting its description as a “whole brain disease.” (Lai et al., 2002) It is generally appreciated that the radiographic appearance of the tumour underestimates disease extent and, like malignant gliomas, PCNSL is essentially impossible to completely resect. (Lai et al., 2002) One of the characteristic histological features of PCNSL is that of angiotropism, in which lymphoma cells accumulate around small and medium-sized blood vessels, contributing to disruption of the blood-brain barrier, and enabling their detection by virtue of pathological contrast enhancement. PCNSL usually presents as a solitary mass with vasogenic oedema and mass effect. (Figures 1–3). The frequency of multiple lesions is increased two-fold among immunosuppressed patients. (Fine & Mayer, 1993)
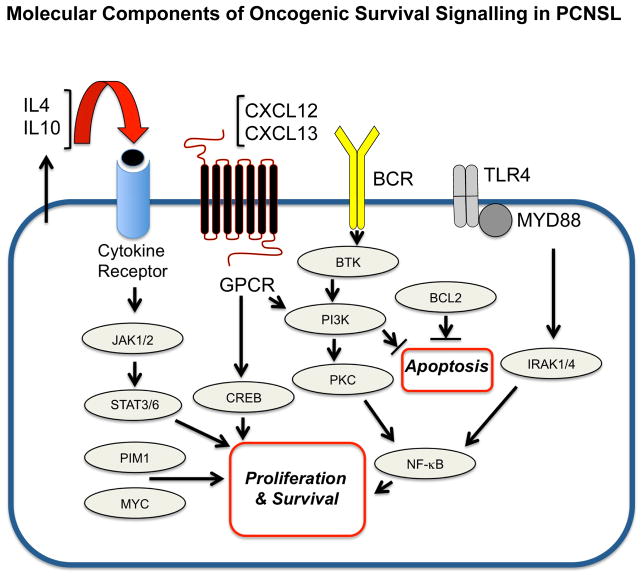
Figure 1. Molecular components of oncogenic survival signalling in primary central nervous system lymphoma.
Notably, activation of the TLR/MYD88 pathway may directly contribute to pro-survival signalling via NFκB as well as via enhanced secretion of IL10, which probably promotes pro-survival signals via the JAK/STAT pathway. GPCR, G protein-coupled receptor.
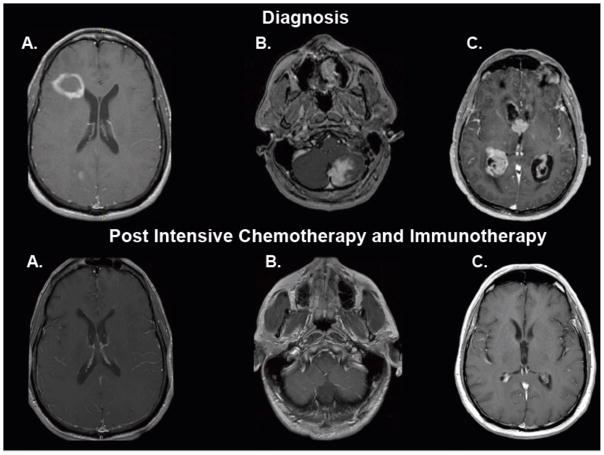
Figure 3. Distinct radiographic presentations of central nrvous system lymphomas and durable responses to intensive chemotherapy and immunotherapy, Without whole brain radiotherapy (T1 axial, post-gadolinium magnetic resonance imaging, at diagnosis and at restaging, at least two months after completion of therapy).
3A. Ring enhancing lesion in the right frontal lobe, adjacent to the lateral ventricle. 3B. Solid enhancing infiltrative mass involving the left cerebellum with evidence of dural attachment. 3C. Diffuse involvement of the ventricular system by nodular, avidly enhancing masses with extension into the surrounding parenchyma.
Approximately 95% of PCNSL tumours are large B-cell lymphoma; other histologies include T-cell (2%)(Shenkier et al., 2005), lymphoblastic, Burkitt, and marginal zone lymphoma. PCNSL is distinguished from dural-based marginal zone lymphomas as these have a distinct pathogenesis, are typically devoid of intraparenchymal extent and share radiographic features with meningioma. (Tu et al., 2005)
Nearly 20% of PCNSL cases present with intraocular disease with involvement of the retina, vitreous and uveal tract. It is important for the clinician to recognize that intraocular lymphoma (IOL) disseminates within CNS in greater than 80% of cases, and therefore, suspicion or detection of IOL mandates staging of the neuroaxis, including brain magnetic resonance imaging (MRI) and CSF evaluation. (Rubenstein et al., 2005) Between 50% to 80% of tumours express BCL6 by immunohistochemistry (Braaten et al., 2003) and greater than 95% stain positive for MUM1; thus the majority of PCNSL express an immunophenotype consistent with the activated B-cell subclass of large-cell lymphoma. (Camilleri-Broet et al., 2006) Between 56–93% of PCNSL cases express BCL2. (Braaten et al., 2003; Camilleri-Broet et al., 2006) In addition, there is reproducible evidence for distinct transcriptional features in PCNSL (Jordanova et al., 2002; Rubenstein et al., 2006; Booman et al., 2008; Tun et al., 2008) and, given that the disease requires a unique therapy, PCNSL is recognized as a distinct subtype of large B-cell lymphoma by the World Health Organization(Campo et al., 2011).
Genomic aberrations in PCNSL include losses on chromosome 6p21 containing human leucocyte antigen (HLA) loci. (Harada et al., 2001; Boonstra et al., 2003; Cady et al., 2008) Candidate tumour suppressor genes linked to deleted loci on chromosome 6q include PRDM1, a regulator of B-cell differentiation and tumour suppressor,(Courts et al., 2008) PTPRK, a protein tyrosine phosphatase involved in cell adhesion,(Nakamura et al., 2003) and TNFAIP3 (A20), a regulator of nuclear factor (NF)κB signalling. (Braggio et al., 2011) Aberrant activation of NFκB is supported by gain in DNA copy number for MALT1 (Schwindt et al., 2009) activating mutations of CARD11 (Montesinos-Rongen et al., 2010) and MYD88. The activating exchange of leucine to proline at position 265 of MYD88 may be enriched in PCNSL and has been demonstrated to occur in between 38% and 50% of cases. (Montesinos-Rongen et al., 2011; Gonzalez-Aguilar et al., 2012) In addition, CD79B, a component of the B-cell receptor signalling pathway, contains mutations in 20% of cases, suggesting that dysregulation of the B-cell receptor and NFκB pathways contribute to pathogenesis of PCNSL. (Montesinos-Rongen et al., 2012) Silencing of the cell cycle regulator CDKN2A occurs in 50% of CNS lymphoma and correlates with inferior outcome. (Schwindt et al., 2009; Gonzalez-Aguilar et al., 2012)
The basis for selective tropism and dissemination of lymphoma within the brain are problems fundamental to the pathogenesis of PCNSL. Expression of chemokine (C-X-C motif) ligand (CXCL)12 and CXCL13 within PCNSL has been documented (Smith et al., 2003; Smith et al., 2007; Fischer et al., 2009) and chemotactic responsiveness to these peptides by CNS lymphoma recently demonstrated, supporting their role as neurotropic factors. In addition, elevated CXCL13 concentration in CSF correlates with adverse prognosis, supporting its role as a survival factor in PCNSL. Measurement of CSF concentration of CXCL13, as well as interleukin 10 (IL10), may be useful in facilitating diagnosis of CNS lymphoma; bivariate upregulated expression of each peptide in CSF has diagnostic sensitivity at least two-fold greater than cytology and/or flow-cytometry. (Rubenstein et al., 2013c) In a multicentre study, the positive predictive value of bivariate elevation of IL10 plus CXCL13 in CSF was 95% in identification of newly diagnosed human immunodeficiency virus (HIV)-negative PCNSL (Rubenstein et al., 2013c).
While it has been established that flow-cytometry is more sensitive than cytology in the detection of CNS lymphomas, (Quijano et al., 2009) recent evidence demonstrates that quantification of soluble CD19 in CSF may augment flow-cytometry in detection of secondary CNS lymphoma associated with diffuse large B-cell lymphoma or Burkitt lymphoma. (Muniz et al., 2014)
Transcriptional profile studies of PCNSL identified several potential mediators of disease pathogenesis, including upregulated MYC expression. (Rubenstein et al., 2006) Increased MYC in PCNSL was confirmed in the recent CALGB 50202 study. (Rubenstein et al., 2013b) High expression of miRNA’s involved in the MYC pathway, (Fischer et al., 2011) as well as MYC translocations (Cady et al., 2008) have also been demonstrated.
The JAK/STAT system is a candidate pro-survival pathway in PCNSL. Interleukin 4 (IL4), a B-cell growth factor that signals via the JAK/STAT pathway, is upregulated at the transcript and protein level within the vascular microenvironment in CNS lymphoma. (Rubenstein et al., 2006) Increased concentration of IL10 protein in the vitreous and CSF is associated with PCNSL and, in independent studies, correlated with adverse prognosis. (Roy et al., 2008; Sasayama et al., 2012) Intratumoural JAK1 transcripts are upregulated in PCNSL with demonstration of JAK1 activation. (Rubenstein et al., 2006; Sung et al., 2011) Elevated IL10 expression plus activation of JAK/STAT signalling in PCNSL are consistent with aberrant activation of the MYD88 pathway (Ngo et al., 2011).
While the brain is typically assumed to be an immunologically privileged site, histopathological evaluation of diagnostic specimens demonstrates a robust inflammatory response within PCNSL, with infiltrating activated macrophages and reactive T-cells. Importantly, there is evidence that reactive, perivascular T-cell infiltrates in PCNSL are associated with favourable outcome, perhaps supporting development of immunotherapies that potentiate T-cell-mediated immune surveillance. (Ponzoni et al., 2007)
Clinical Presentation
Among the immunocompetent, the median age of the PCNSL patient at diagnosis is 56 years with a male-to-female ratio of 1.2–1.7:1. Neurological symptoms of PCNSL typically reflect the neuroanatomical location of the lesion(s). Greater than 60% of patients present with constitutional, cognitive or motor symptoms; 20% present with seizures and 30% have visual symptoms. (Josephson et al., 2007) Leptomeningeal disease occurs in 15–20% of patients at presentation. (Fischer et al., 2008) IOL is manifest by non-specific symptoms of blurred vision, decreased visual acuity, eye pain, floaters and photophobia, usually with involvement of both eyes. The pace of neurological decline at presentation is variable; some patients exhibit chronic visual symptoms that antedate the diagnosis by years, while for others, disease progression is highly aggressive. Notably, in a recent retrospective series of patients with rapidly progressive neurological deterioration who presented for diagnostic brain biopsy, the most common aetiology was PCNSL (20%). (Josephson et al., 2007)
Diagnostic and Staging Evaluation of the Patient with Neurological Symptoms
As CNS and IOL patients typically present with nonspecific symptoms, establishing a diagnosis may be difficult and it is not uncommon for the interval between first onset of disease signs to extend from months to years before the diagnosis is established. The first-line test in diagnostic evaluation of suspected PCNSL is MRI-based examination of the brain, with gadolinium contrast. In 95% of cases, there is pathological enhancement that homogenously localizes to dominant tumour masses. Lesional necrosis is rare and is one of the radiographic features that may distinguish CNS lymphoma from glioblastoma. Among immunocompetent patients with newly-diagnosed PCNSL, lesions are solitary in 65% and multifocal in 35%. Cerebral hemisphere disease is the most common localization of lesions (38%) followed by the basal ganglia and thalamus (16%), corpus callosum (14%) ventricular region (12%) and cerebellum (9%).(Kuker et al., 2005) (Figure 3).
While glucocorticoids may produce rapid symptomatic improvement, with dramatic radiographic responses in 40% of patients, steroid-induced responses may increase the risk of a non-diagnostic brain or vitreal biopsy. (Porter et al., 2008) Steroid-induced diagnostic delays may extend for weeks to months, although on occasion, steroid-induced regressions of sentinel lesions may delay the diagnosis of PCNSL for years. (Pirotte et al., 1997) It is important to emphasize that, if possible, empiric dexamethasone be rapidly tapered or not administered until a diagnosis is established. If CNS lymphoma is confirmed, steroids should be tapered and discontinued as quickly as possible, unless there is symptomatic tumour-associated mass effect that can be mitigated by glucocorticoids.
The standard diagnostic approach for PCNSL is stereotactic brain biopsy, however in selected cases, subtotal or gross total resections, if safe, may be appropriate. Cytological and/or flow-cytometric analysis of meningeal lymphoma cells isolated from CSF or vitrectomy may also yield diagnostic material. In the setting of tumour-associated mass-effect, particularly in the posterior fossa, consultation with neurosurgery is advised to assess the safety of a diagnostic or staging lumbar puncture. CSF should be efficiently processed for cytology and flow-cytometric studies designed to identify, in most cases, a kappa or lambda-restricted B-cell lymphoma. Repeated CSF cytological or flow-cytometric studies infrequently improve diagnostic yield in PCNSL, supporting further development of innovative molecular diagnostic methods based upon proteomics or analysis of non-coding RNA’s. (Roy et al., 2008; Baraniskin et al., 2011; Rubenstein et al., 2013c)
Staging evaluation for the patient with presumptive PCNSL includes complete ophthalmological examination with slit lamp. Systemic staging is also indicated, given that between 4–12% of patients with presumptive PCNSL ultimately manifest extra-CNS disease. (Ferreri et al., 1996) Therefore, contrast-enhanced computerized tomography (CT) of the chest, abdomen and pelvis, as well as bone marrow biopsy are requisite; the value of positron emission tomography (PET) imaging has not been established. (Mohile et al., 2008) but may be useful in evaluation of possible concomitant testicular involvement. Serological testing for HIV, hepatitis B and C, plus quantification of serum lactate dehydrogenase (LDH) are standard-of-care at baseline. (Abrey et al., 2005)
Diagnostic and Staging Evaluation of Intraocular Lymphoma (IOL)
Given that nearly 80% of patients with IOL ultimately progress to CNS dissemination, MRI of the brain with gadolinium should be performed to evaluate idiopathic uveitis, in which lymphoma is a consideration. Additional diagnostic and staging tests for IOL include fluorescence angiography (Figure 1C) and optical coherent tomography. (Chan et al., 2011) Processing of diagnostic specimens from ocular lesions must be expedited to achieve the highest diagnostic yield by flow-cytometry or cytology. (Rubenstein et al., 2005) Identification of immunoglobulin gene rearrangements and/or quantitative determination of intraocular cytokine concentration of IL10 and IL6 may also be a useful adjunct to diagnosis. (Chan et al., 2002)
Prognostic Assessment in PCNSL
The International Extranodal Lymphoma Study Group (IELSG) identified five variables that correlate with prognosis in PCNSL, three are shared with systemic NHL: elevated LDH, age greater than 60 years, and Eastern Cooperative Oncology Group (ECOG) performance status greater than 1; CNS lymphoma-specific parameters include high CSF protein concentration and tumour location within the deep regions of the brain (periventricular, basal ganglia, brainstem and/or cerebellum). The presence of 0 – 1, 2 – 3, or 4 – 5 of adverse risk factors correlate with two-year survival rates of 80%, 48% or 15%.(Ferreri et al., 2003) Historically, age has been considered the most reproducible clinical prognostic factor, however there is a discrepancy regarding the cut-off point at which prognosis declines. While the IELSG considered age 60 years as the cut-off point above which prognosis declines, the Memorial Sloan-Kettering (MSK) prognostic index uses age 50 years. (Abrey et al., 2006) Notably, in the Cancer and Leukemia Group B (CALGB) 50202 trial, which evaluated intensive immunochemotherapy with dose-intensive consolidation without WBRT, patients older than 60 years did similarly well as younger patients,(Rubenstein et al., 2013b) an observation that suggests that the optimal cut-off point for age as a prognostic variable is largely treatment-dependent. (Wieduwilt et al., 2012)
Principles of Management
Surgery
Until recently, authorities have recommended against neurosurgical resection of CNS lymphoma based upon reports that surgical cytoreduction provides no survival benefit compared to biopsy alone and potentially increases risk of post-operative deficit. (DeAngelis et al., 1990; Bataille et al., 2000) Notably, retrospective analysis of the German PCNSL Study Group (SG)-1 Trial provided the first evidence that aggressive resection of CNS lymphoma correlated with improved PFS. (Weller et al., 2012) In our experience, maximum safe resection of lesions may provide immediate relief of mass effect, facilitate glucocorticoid taper, potentially eliminate drug-resistant tumour clones and provide substantial clinical benefit without contributing to neurological deficits, particularly when performed with modern neurosurgical mapping techniques. (Figure 4).
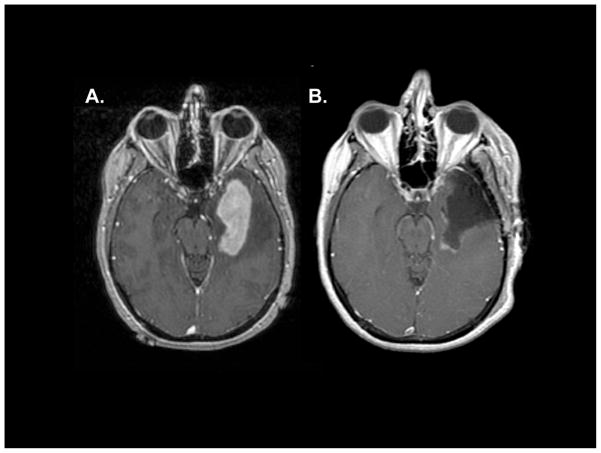
Figure 4. Example of safe surgical resection of primary central nervous system lymphoma.
A 54-year-old right-handed patient presented with a generalized seizure and a magnetic resonance imaging scan that demonstrated a homogeneous contrast-enhancing lesion involving the left temporal lobe with significant vasogenic oedema. (3A). Safe resection of the contrast-enhancing mass was performed with assistance of awake speech mapping, without a post-operative neurological deficit. The diagnosis was large B-cell lymphoma and staging revealed primary central nervous system lymphoma (PCNSL). (3B). Subsequently, the patient was treated with MT-R (high-dose methotrexate, temozolomide, rituximab) induction immuno-chemotherapy followed by EA (etoposide cytarabine) dose-intensive consolidation, without whole brain radiotherapy. She regained her pre-PCNSL functional and neurological status and continues to work full-time, seven years later, without evidence of disease. (Courtesy of Mitchel Berger, M.D. and Michael McDermott, M.D., UCSF).
Whole Brain Irradiation (WBRT)
WBRT is highly effective in the elicitation of immediate responses in CNS lymphoma and therefore brain radiotherapy has historically been of value. The impact of WBRT in the treatment of CNS lymphoma is however compromised by at least three important limitations: (1) Inadequate local control of lymphoma; (2) Dissemination of radiographically-occult lymphoma cells outside of the radiation field; (3) Deleterious effects of radiation on brain function. In one study, the use of WBRT as the sole treatment of PCNSL yielded a median survival of only 11.6 months with greater than 60% of patients experiencing lymphoma progression within the irradiated field. (Nelson et al., 1992) The archetypical features of delayed neurotoxicity caused by WBRT, incontinence, gait and memory disturbances, are most common in patients aged greater than 60 years, and many PCNSL survivors who experience this complication ultimately require custodial care. (Abrey et al., 1998) Whilst a preliminary analysis found that lower doses of WBRT were associated with neurotoxicity that is barely discernable (Correa et al., 2009), additional validation of these results are necessary, and, given the established deleterious neurocognitive effects of prophylactic cranial irradiation at 30 Gy (Sun et al., 2011), there is reason to be concerned that delayed neurotoxicity secondary to WBRT may be a continuous variable in terms of its relationship to dose. Finally, recent updates demonstrate that long-term outcomes of PCNSL patients treated with low-dose WBRT (23.4 Gy) in consolidation revealed significantly inferior outcome in the subgroup of patients older than 60 years. (Morris et al., 2013) For these reasons, there has been progressive interest in innovative strategies that defer or eliminate WBRT as a component of induction therapy.
Induction Chemotherapeutic Strategies
Studies performed by Canellos and colleagues demonstrated the feasibility and efficacy of systemic HD-MTX in recurrent CNS lymphomas. (Skarin et al., 1977; Ervin & Canellos, 1980) Subsequently, HD-MTX was incorporated more broadly in induction and salvage regimens and identified in a multivariate analysis as the most significant treatment-related prognostic variable related to survival for CNS lymphomas. (Blay et al., 1998).
The optimal dose of MTX during induction has not been defined. It is clear from our experience that systemic doses ≥ 1 g/m2 mediate lymphocytotoxic effects within brain parenchyma, in agreement with others. (Skarin et al., 1977). In an important study, Glantz et al (1998) demonstrated that intravenous MTX administered at 8 g/m2 over 4 h yields higher cytotoxic levels of MTX (greater than 1 μM) in serum and CSF than intrathecal MTX (12 mg) at 48 and 72 h post-infusion. In another influential analysis, investigators at MSK demonstrated that elimination of intrathecal MTX from induction therapy for PCNSL did not affect outcome if patients received HD-MTX at doses of 3.5 gm/m2.(Khan et al., 2002) These results indicate that high-dose intravenous MTX administered every two weeks for a minimum of six cycles can be used to treat aggressive lymphoma within the brain and leptomeningeal compartment, without intrathecal therapy. (Wieduwilt et al., 2012) One of the many therapeutic issues in PCNSL yet to be resolved is the optimal number of cycles of HD-MTX administered during induction. Given data that greater than four cycles of MTX may be necessary to obtain an effective remission before consideration of dose-intensive consolidation (Abrey et al., 2003), our approach has been to administer eight cycles of HD-MTX during induction, assuming a complete remission is attained by cycle six.
Prevention and Management of HD-MTX Toxicity
It is important for the haematologist to be skilled in the management of the toxicities of HD-MTX, in particular MTX nephropathy, caused by precipitation of MTX and 7-OH-MTX within renal tubules. Basic principles to prevent this life-threatening complication, which occurs in up to 5% of patients, include vigorous hydration, urine alkalinization, and avoidance of agents that interact with HD-MTX, such as penicillin derivatives. Third-space effusions must be drained before MTX administration and leukovorin rescue started at 24 h, with serial monitoring of MTX serum concentrations. Delayed MTX excretion mandates continued alkalinization and hydration as well as escalated leukovorin dosing. Additional interventions for delayed MTX clearance include carboxypeptidase-G2 (CPDG2, glucarpidase), a recombinant enzyme. Glucarpidase reduces toxic serum MTX concentrations within 15 min, via direct hydrolysis of MTX. (Green, 2012) It is also important to be aware of the risk of superimposed iodine contrast nephropathy with that of MTX nephropathy, which can be mitigated by providing an interval of at least two days between CT imaging and HD-MTX.
Combined-Modality Regimens
DeAngelis and colleagues pioneered combined modality therapy for PCNSL, consisting of HD-MTX plus procarbazine and vincristine, followed by WBRT and high-dose cytarabine (HD-Ara-C); implementation of this regimen in the multicentre setting, coordinated by RTOG, yielded a median progression-free survival of 24 months. (DeAngelis et al., 2002) (Table I). Because of this encouraging efficacy, combined-modality therapy became a widely adopted approach for PCNSL. (DeAngelis et al., 1992; Glass et al., 1994) In a large randomized phase II study that evaluated HD-MTX-based induction, with or without HD-Ara-C (2 g/m2) followed by consolidative WBRT: the median failure-free survival in patients who received HD-MTX in combination with HD-Ara-C induction was eight months; by contrast, the median failure-free survival of patients who received HD-MTX without cytarabine, was only four months. (Ferreri et al., 2009) In the SG-1 trial, a large randomized phase III trial involving 551 patients in which half the patients received WBRT as first-line consolidation, Thiel et al (2010) provided evidence that omission of WBRT from first-line chemotherapy does not compromise survival. While WBRT resulted in a modest improvement in PFS after MTX-based induction, this did not translate into improved overall survival, possibly because of severe neurotoxicity with WBRT, detected in nearly half of patients in the radiotherapy arm. (Thiel et al., 2010)
Table I.
High-dose methotrexate-based trials in PCNSL that yielded median progression-free survival ≥ 2-years.
| Regimen | Reference | Patients (n) | Median PFS | Median OS |
|---|---|---|---|---|
| MTX 2.5 g/m2, PCB, vincristine, IT-MTX, WBRT | DeAngelis et al (2002) | 98 | 24 | 37 |
| MTX 8 g/m2, TMZ, rituximab, etoposide, ARA-C | Wieduwilt et al (2012) | 31 | 24 | 66 |
| MTX 3.5 g/m2, rituximab, vincristine, PCB, ARA-C, rd-WBRT | Morris et al (2013) | 52 | 39 | 79 |
| MTX 8 g/m2, TMZ, Ritux, Etop, ARA-C | Rubenstein et al (2013b) | 44 | 48 | NR |
PCNSL, primary central nervous system lymphoma; PFS, progression-free survival; OS, overall survival; MTX, methotrexate; PCB, procarbazine; IT-MTX, intrathecal methotrexate; WBRT, whole brain radiotherapy; TMZ, temozolomide; ARA-C, cytarabine; rd-WBRT, reduced dose whole brain radiotherapy. NR, not reached. (Response criteria according to Abrey et al., 2005).
Dose-Intensive Chemotherapy Consolidation
Given the increased recognition of the delayed neurotoxicity caused by WBRT in CNS lymphoma survivors, during the past two decades there has been significant interest in the role of high-dose consolidation, including autologous stem cell rescue, in PCNSL, both at diagnosis and in the setting of relapse. Regimens that contain agents with good CNS penetration, such as carmustine, thiotepa, cyclophosphamide, busulfan, HD-Ara-C and etoposide, have been associated with the best results. (Alvarnas et al., 2000; Soussain et al., 2001; Illerhaus et al., 2008; Korfel et al., 2013) (Table II). Notably, results obtained using the BEAM regimen (carmustine, etoposide, cytarabine, melphalan) followed by autologous stem cell rescue were not promising, however in this trial a large proportion of patients had inadequate disease control before myeloablative therapy, possibly because of the abbreviated induction used in this trial. (Abrey et al., 2003)
Table II.
Chemotherapy regimens used in dose-intensive consolidative and preparative regimens that are effective in central nervous system lymphoma.
| Intensive Consolidation/Preparative Regimen | Reference |
|---|---|
| Carmustine, thiotepa, etoposide | Korfel et al (2013) |
| Infusional etoposide, high-dose cytarabine | Wieduwilt et al (2012); Rubenstein et al (2013b) |
| Thiotepa, busulfan, cyclophosphamide | Soussain et al (2001, 2008); Cote et al (2012) |
| Carmustine, thiotepa | Illerhaus et al (2008) |
| Cyclophosphamide, carmustine, etoposide | Alvarnas et al (2000) |
Soussain et al (2001) demonstrated the efficacy of dose-intensive chemotherapy and autologous stem cell transplant in recurrent CNS and IOL. Their data provided evidence that HD-Ara-C plus etoposide (EA) constitutes a highly potent salvage regimen when used in combination for recurrent/refractory CNS lymphomas: 12 of 14 patients attained responses, eight of which were complete responses (Soussain et al., 2001). After stem cell collection, responding CNS lymphoma patients received a myeloablative regimen consisting of thiotepa, busulfan and cyclophosphamide.
Beginning in 2001, investigators at the University of California, San Francisco (UCSF), began to pursue dose-intensive chemotherapy as first-line consolidation, without WBRT, after induction immunochemotherapy in patients with newly-diagnosed PCNSL. We developed a two-step regimen: the induction phase uses HD-MTX given every two weeks with oral temozolomide and rituximab (MT-R). MTX is administered at 8 g/m2 with dose reductions as appropriate and leucovorin rescue day 2. Intravenous rituximab is administered day 3, and weekly for six doses, an interval during which the blood-brain barrier may be most compromised. (Ott et al., 1991) Temozolomide is an alkylating agent with lipophilic properties that has established efficacy at relapse in CNS lymphoma, alone and in combination with rituximab (Reni et al., 2000; Wong et al., 2004; Reni et al., 2007). Importantly, temozolomide has a superior health-related quality of life and toxicity profile compared to procarbazine (Osoba et al., 2000a; Osoba et al., 2000b). Temozolomide is administered monthly in a five-day course at 150 mg/m2, beginning days 7–11. To consolidate response after induction with MT-R, PCNSL patients received intensive consolidation with non-cross-resistant agents: 96-h infusional etoposide (40 mg/kg IV) plus eight doses of HD-Ara-C (EA) (2 g/m2, every 12 h) (Damon et al., 2008; Damon et al., 2009; Linker et al., 2009). Notably, infusional etoposide is incorporated within the EPOCH regimen (etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone), which is active against large B-cell lymphoma (Wilson et al., 1993; Wilson et al., 2008). A number of studies provide evidence for activity of etoposide in brain tumours, including CNS lymphoid leukaemia (Relling et al., 1996). Notably, when given in combination with CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) in patients with aggressive lymphoma, etoposide was associated with a reduced risk of secondary CNS lymphoma (Boehme et al., 2007)
While the impact of HD-Ara-C in PCNSL was demonstrated in a randomized phase II study (Ferreri et al., 2009) the dose-intensity of cytarabine within EA is markedly greater than in previous studies in PCNSL and similar to schedules of HD-Ara-C used to treat Burkitt and mantle cell lymphoma and acute myeloid leukaemia. (Damon et al., 2008; Damon et al., 2009; Linker et al., 2009; Schaich et al., 2011; Schaich et al., 2013). Notably, the dose-intensity of EA is also approximately two-fold higher than doses of etoposide-cytarabine used as first-line salvage in the Soussain series. (Soussain et al., 2001)
One of the goals of the two-step MT-R EA programme was to develop an induction regimen that incorporates an alkylator, temozolomide (Reni et al., 2007) plus rituximab (Batchelor et al., 2011), and yet causes minimal myelosuppression, resulting in few treatment delays during the first weeks of treatment, the interval at which maximal lymphoma cytoreduction is achieved. Long-term follow-up of the first cohort of patients treated with this regimen demonstrates that combination EA is highly effective as consolidation after MT-R in newly diagnosed PCNSL. (Wieduwilt et al., 2012) Of the first 14 PCNSL patients who received MT-R followed by EA consolidation, 12 remain in remission, with a median follow-up of greater than 72 months. Based on promising institutional pilot data, the MT-R plus EA regimen was evaluated in CALGB 50202, which, for the first time, demonstrated the multicentre feasibility of high-dose chemotherapy in newly-diagnosed PCNSL. The rate of complete response to MT-R induction in CALGB 50202 was 0.66 and the two-year PFS was 0.57, exceeding other chemotherapy-alone studies thus far. Moreover, the median time to progression of all 50202 patients, four years, is two-times longer than achieved with combined-modality therapy in multicentre trials using standard-dose WBRT and appears to compare favourably to reduced-dose WBRT. (DeAngelis et al., 2002; Thiel et al., 2010) Also encouraging is the fact that the PFS curves reached a stable plateau, and outcome data was similar in patients older than 60 compared to younger patients. (Table I; Figure 5).
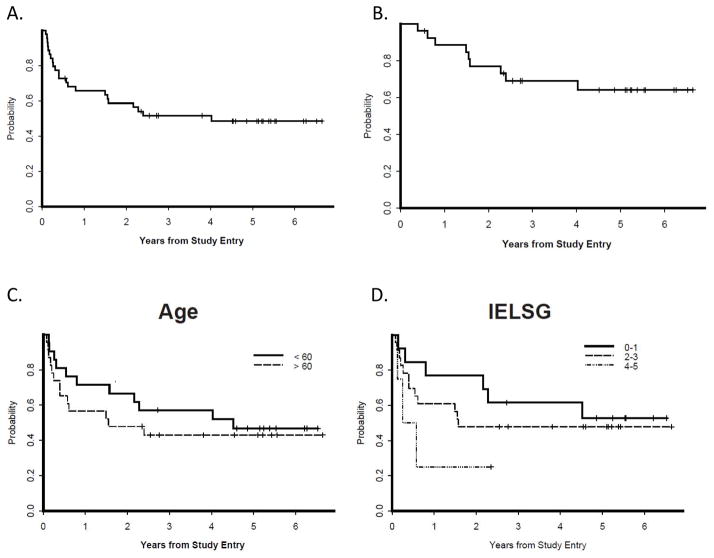
Figure 5. Outcomes with intensive chemotherapy and immunotherapy in newly-diagnosed primary central nervous system lymphoma, without whole brain radiotherapy: CALGB (Alliance) 50202).
Outcome for all CALGB 50202 patients; y-axis refers to cumulative probability of event. 4A. Progression-free survival (PFS) for all patients. The 2-year PFS was 59%. 4B. PFS for those patients who attained a complete response with MT-R (high-dose methotrexate, temozolomide, rituximab) induction and received EA (etoposide cytararbine) consolidation (n=27). 4C. PFS was similar for patients aged> 60 years (n=23) and for younger patients (n=21; p=0.48). 4D. There was a trend between shorter PFS and highest International Extranodal Lymphoma Study Group (IELSG) risk score of 4–5 (p=0.16).
Based upon the promising results of CALGB 50202, a successor randomized phase II trial, CALGB 51101, has been initiated and endorsed by major cooperative groups in the United States: Alliance, Southwestern Oncology Group (SWOG), and Eastern Cooperative Group (ECOG). In CALGB 51101, after remission induction therapy with MT-R, patients receive either consolidation with EA or myeloablative therapy and stem cell transplant with carmustine plus thiotepa, a regimen evaluated by the Freiburg group. (Illerhaus et al., 2008) Recently completed randomized control studies are comparing outcomes of high-dose chemotherapy versus WBRT, and, like CALGB 51101, the MATRIX/IELSG43 trial compares high-dose consolidation chemotherapy with conventional consolidative chemotherapy (dexamethasone, etoposide, ifosfamide and carboplatin) (Table III).
Table III.
Recently completed and ongoing randomized controlled trials for primary central nervous system lymphoma.
| Trial | Regimen | Status |
|---|---|---|
| G-PCNSL-SG1 | HD-MTX-based induction +/− WBRT consolidation | Thiel et al (2010) |
| IELSG-20 | HD-MTX +/− Ara-C - > WBRT consolidation | Ferreri et al (2009) |
| IELSG-32 | Myeloablative vs. WBRT consolidation | Accrual Complete |
| PRECIS | Myeloablative vs. WBRT consolidation | Active |
| Alliance 51101 | Intensive vs. Myeloablative consolidation (ASCT) | Active |
| MATRIX/IELSG43 | Intensive vs. Myeloablative consolidation (ASCT) | Active |
CALGB (Alliance) 51101 is comparing dose-intensive consolidation with infusional etoposide plus high-dose cytarabine (EA)(Rubenstein et al., 2013b) with high-dose chemotherapy (carmustine plus thiotepa) supported by autologous stem cell transplant. (Illerhaus et al., 2008) The MATRIX/IELSG43 is comparing high-dose chemotherapy (carmustine plus thiotepa) (Illerhaus et al., 2008) supported by autologous stem cell transplant with dose-intensive consolidation (dexamethasone, etoposide, Ifosfamide and carboplatin).
G-PCNSL-SG-1, German Primary Central Nervous System Lymphoma Study Group 1; IELSG, International Extranodal Lymphoma Study Group; PRECIS, Cranial Radiotherapy or Intensive Chemotherapy With Haematopoietic Stem Cell Rescue for Primary Central Nervous System Lymphoma in Young Patients; CALGB, Cancer and Leukemia Group B; HD-MTX, high-dose methotrexate; WBRT, whole brain radiotherapy, Ara-C, cytarabine; ASCT, autologous stem cell transplantation.
Neurocognitive Function
Given recent progress in outcomes in PCNSL, the issue of treatment-related neurotoxicity among survivors is receiving significant attention. Delayed cognitive dysfunction is recognized as a major complication of combined-modality therapy that includes WBRT. As above, while there is preliminary data that reduced-dose WBRT is associated with milder cognitive dysfunction among PCNSL survivors compared to standard-dose WBRT,(Correa et al., 2009) reduced doses of WBRT as consolidation may be associated with impairments of verbal memory and motor speed. PCNSL patients treated with HD-MTX-based therapies without consolidative WBRT do not appear to exhibit severe cognitive dysfunction as determined by post-treatment neuropsychological testing but nevertheless score lower than normative control subjects in evaluations of selective attention, motor speed, executive function, verbal learning and delayed recall. (Correa et al., 2012) Given that PCNSL is a highly infiltrative neoplasm associated with a spectrum of neurological symptoms, discernment of whether impairments of neurological function are caused by lymphoma or the consequence of the delayed neurotoxicity of agents, such as MTX, is a major challenge.
Management of Secondary CNS Lymphoma
CNS dissemination is one of the most devastating complications of relapsed aggressive systemic NHL. The natural history of secondary CNS lymphoma was defined in a retrospective analysis of SWOG 8516. Here it was recognized that CNS relapses occurred earlier than systemic relapses and median survival after diagnosis of secondary CNS lymphoma was only 2.2 months compared to 9 months with non-CNS relapse. Risk factors for CNS localization at recurrence of aggressive lymphomas include extranodal involvement, with testes a site of particular high-risk, as well as high International Prognostic Index score. The efficacy of intrathecal chemotherapy in prophylaxis of secondary CNS lymphoma could not be demonstrated in this analysis. (Bernstein et al., 2009)
Given the demonstration that higher sustained cytotoxic MTX levels in CSF are achieved after high-dose intravenous dosing compared to intrathecal administration,(Glantz et al., 1998) there is increasing interest in HD-MTX as prophylaxis for patients with systemic NHL with high-risk features of CNS relapse. Recent data substantiates evidence for the efficacy of this approach in preventing CNS relapse in patients at high-risk. (Abramson et al., 2010), and taken together, these data support our recommendation that HD-MTX be administered as prophylaxis of secondary CNS lymphoma in patients with aggressive lymphomas at high risk for CNS dissemination.
Therapeutic Options in Recurrent CNS Lymphomas
Dose-intensive chemotherapy with autologous stem cell transplant has become an attractive option in the management of relapsed CNS and IOL. (Soussain et al., 2001; Soussain et al., 2008; Bromberg et al., 2013) Recently, the Berlin group presented their experience using a salvage regimen consisting of HD-MTX-based chemotherapy plus other CNS-penetrant agents (ifosfamide, thiotepa, cytarabine and depocyt), followed by myeloablative therapy (carmustine, thiotepa, etoposide) and stem cell transplant. This approach yielded a PFS rate of 0.49 at two-years. (Korfel et al., 2013) An important consideration in the treatment of relapsed CNS lymphomas is whether the lymphoma is MTX-sensitive. In the setting of recurrent disease that is sensitive to HD-MTX, our approach is to administer repetitive cycles of HD-MTX, to achieve maximal cytoreduction, (six-to-eight cycles), followed by dose-intensive consolidation using non cross-resistant, CNS penetrant agents such as thiotepa. (Cote et al., 2012; Falzetti et al., 2012; Korfel et al., 2013) High-dose carmustine-based therapy without thiotepa has also been studied. (Alvarnas et al., 2000) (Table I)
For CNS lymphomas that have progressed within six months of dose-intensive consolidation, second-line high-dose chemotherapeutic salvage may not be a reasonable option. Such patients may be managed with additional HD-MTX, pemetrexed,(Raizer et al., 2012) WBRT or investigational agents.
Rituximab in CNS lymphomas
Because the blood-brain barrier normally excludes molecules that exceed 400 daltons, it is not surprising that most studies report that less than 1% of systemic rituximab penetrates the leptomeningeal compartment. (Rubenstein et al., 2003) While rituximab has become a cornerstone of therapy in systemic B-cell NHL, several studies demonstrated that the addition of rituximab to CHOP may not significantly decrease the rate of CNS recurrence of systemic large B-cell lymphoma compared to CHOP alone. (Feugier et al., 2004; Yamamoto et al., 2010; Tai et al., 2011) Nevertheless, intravenous rituximab may induce responses of contrast-enhancing lesions in CNS lymphoma, probably in foci in which there is disruption of the blood-brain barrier. (Batchelor et al., 2011)
Intraventricular Rituximab
We recently evaluated the safety and activity of intraventricular rituximab, both as monotherapy and in combination with intraventricular MTX in the setting of two phase I multicentre trials. These studies demonstrated that, when diluted in preservative-free normal saline and administered into ventricular CSF, 10 and 25 mg doses of rituximab are well-tolerated and can elicit responses within leptomeninges, intraocular compartments and in small parenchymal lesions. The efficacy of intraventricular rituximab was additive or synergistic with MTX. One of the key findings was that intraventricular rituximab/MTX appeared particularly useful in high burden leptomeningeal lymphoma. These studies also suggested that intraventricular rituximab overcomes resistance caused by the blood-brain barrier, in that CSF responses were documented in patients with baseline serum rituximab concentrations greater than 15 μg/ml. Notably, two patients achieved a first complete response of CNS lymphoma with intraventricular rituximab/MTX, including one with disease refractory to high-dose systemic and intrathecal MTX plus 20 previous intravenous infusions of rituximab. (Rubenstein et al., 2007; Rubenstein et al., 2013d) One mechanistic explanation for the efficacy of intraventricular rituximab is provided by the demonstration of activation of the complement cascade within CSF upon intra-CSF rituximab administration as well as pharmacokinetic evidence for penetration of rituximab into neural tissue. (Kadoch et al., 2013)
Given the evidence for activity of rituximab in CNS lymphomas, as monotherapy and in combination with MTX-based induction,(Shah et al., 2007) a number of protocols incorporate rituximab for this disease. While several studies demonstrate its activity at relapse, intraventricular rituximab should be considered investigational and the combination of intraventricular plus intravenous rituximab for recurrent CNS lymphoma is currently being studied in a phase I investigation (NCT01542918).
Therapy of Intraocular Lymphoma (IOL)
Most cases of IOL involve large B-cell NHL, either primary vitreoretinal lymphoma or uveal lymphomas, which are divided into primary neoplasms of the choroid, iris and ciliary body, or secondary choroidal lymphomas in patients with disseminated systemic NHL. Notably, between 65% and 90% of patients with primary vitreoretinal lymphoma ultimately develop CNS lymphoma, usually within 30 months. Conversely, IOL impacts between 15–25% of patients with PCNSL.
Therapy for primary vitreoretinal lymphoma can be divided into local approaches, such as ocular radiation and intravitreal therapy vs. systemic chemotherapy. External beam radiotherapy to the eyes using opposed lateral beams, is well tolerated, and associated with low rates of local recurrence. Complications of ocular radiotherapy are typically mild, including dry eye, cataract and mild radiation retinopathy (Berenbom et al., 2007) Intravitreal MTX and rituximab may be indicated in the setting of unilateral disease or prior ocular radiation. (Kitzmann et al., 2007; Itty & Pulido, 2009) Treatment-related complications of intravitreal MTX include vitreous haemorrhage, endophthalmitis, retinal detachment and hypotony. (Chan et al., 2011) Systemic therapeutic options for IOL are HD-MTX, (Batchelor et al., 2003) HD-Ara-C or trofasfamide. (Jahnke et al., 2009) Notably, in primary vitreoretinal lymphoma, implementation of HD-MTX plus binocular irradiation as induction provides local disease control and addresses the possibility of subclinical disease throughout the neuroaxis. (Stefanovic et al., 2010) Our approach to patients with primary IOL and/or concomitant PCNSL with IOL usually involves three steps: (1) HD-MTX-based induction (MT-R); (2) dose-intensive consolidation as used in CALGB 50202 (EA); (3) Binocular but not WBRT if there is persistence and/or recurrence of isolated IOL after completion of dose-intensive chemotherapy consolidation.
CNS Lymphoma in the Immunocompromised Host
While HIV-associated PCNSL declined in incidence with advent of highly-active antiretroviral therapy (HAART), PCNSL continues to be a significant AIDS-defining illness that is aggressive and a major therapeutic challenge. The feasibility and efficacy of HD-MTX in HIV-associated PCNSL has been demonstrated. (Jacomet et al., 1997) Similarly, in the setting of CNS PTLD, reconstitution of immune function is a first principle in management, and can be achieved by reductions or cessation of immunosuppression. HD-MTX may be effective but its implementation needs to be balanced with risk of allograft failure. (Elstrom et al., 2006) Rituximab is also active in CNS complications of PTLD, via intravenous as well as intrathecal administration. (van de Glind et al., 2008)
Conclusions and Future Directions
Over the course of the past half-century, the haematology/oncology community has made significant progress in the treatment of PCNSL, a highly malignant brain tumour. Based upon the results of recent series that evaluate novel therapeutic approaches, approximately 40–50% of patients will exhibit long-term survival and a significant proportion may be cured. The vast majority of this progress has been achieved in the absence of randomized data. It is likely that the next five years of clinical trials will continue to focus on optimization of interventions based upon high-dose chemotherapy.
However, given that at least 40–50% of PCNSL patients develop disease refractory to the established armamentarium of agents, it is imperative that additional studies explore the potential efficacy of selective agents that target candidate resistance mechanisms for high-risk patients. For example, pharmacological agents that evaluate disruption of pathways involving the B-cell receptor, JAK-STAT, toll-like receptor, mTOR, and PIM kinases should be considered high priority in early phase investigation in CNS lymphomas. Another key target is MUM1/IRF4, targeted by the immunomodulatory class of agents, such as lenalidomide or pomalidomide, that are under evaluation in PCNSL. (Ponzoni et al., 2013) Transformative advances are needed in CNS lymphomas given its increasing predilection for an aging population among whom a major proportion cannot tolerate dose-intensive chemotherapy or WBRT.
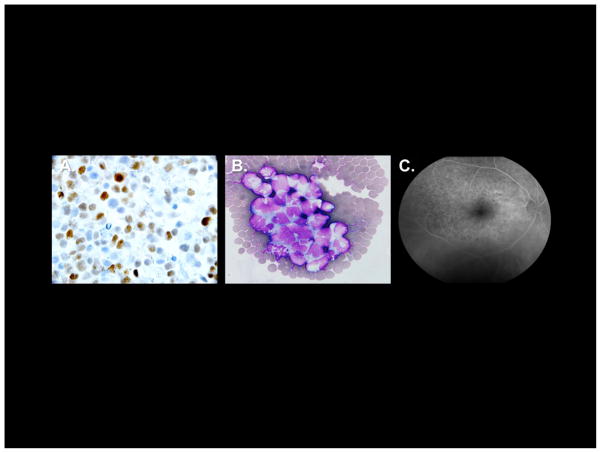
Figure 2. Pathological features of primary central nervous system lymphoma and intraocular lymphoma.
A. High expression of MUM1 by diffuse large B-cell lymphoma cells in a diagnostic specimen of primary central nervous system lymphoma, as demonstrated by immunohistochemistry 1000x). B. Cytological appearance of malignant diffuse large B-cell lymphoma in cerebrospinal fluid from recurrent central nervous system lymphoma. C. Fluorescein angiography showing classic “leopard spots” in Intraocular Lymphoma.
Acknowledgments
Supported by the National Institutes of Health, University of California San Francisco-Gladstone Institute of Virology & Immunology Center for AIDS Research (P30 AI027763), NIH R01CA139-83-01A1, and by the Leukemia & Lymphoma Society (JLR).
Footnotes
Authorship. Chia-Ching Wang, Julia Carnevale and James L. Rubenstein performed research and wrote the article.
Conflicts of Interest Disclosure: James L. Rubenstein receives research funding from Celgene and Genentech for a phase I clinical trial.
References
- Abramson JS, Hellmann M, Barnes JA, Hammerman P, Toomey C, Takvorian T, Muzikansky A, Hochberg EP. Intravenous methotrexate as central nervous system (CNS) prophylaxis is associated with a low risk of CNS recurrence in high-risk patients with diffuse large B-cell lymphoma. Cancer. 2010;116:4283–4290. doi: 10.1002/cncr.25278. [DOI] [PubMed] [Google Scholar]
- Abrey LE, DeAngelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1998;16:859–863. doi: 10.1200/JCO.1998.16.3.859. [DOI] [PubMed] [Google Scholar]
- Abrey LE, Moskowitz CH, Mason WP, Crump M, Stewart D, Forsyth P, Paleologos N, Correa DD, Anderson ND, Caron D, Zelenetz A, Nimer SD, DeAngelis LM. Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: an intent-to-treat analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:4151–4156. doi: 10.1200/JCO.2003.05.024. [DOI] [PubMed] [Google Scholar]
- Abrey LE, Batchelor TT, Ferreri AJ, Gospodarowicz M, Pulczynski EJ, Zucca E, Smith JR, Korfel A, Soussain C, DeAngelis LM, Neuwelt EA, O’Neill BP, Thiel E, Shenkier T, Graus F, van den Bent M, Seymour JF, Poortmans P, Armitage JO, Cavalli F. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23:5034–5043. doi: 10.1200/JCO.2005.13.524. [DOI] [PubMed] [Google Scholar]
- Abrey LE, Ben-Porat L, Panageas KS, Yahalom J, Berkey B, Curran W, Schultz C, Leibel S, Nelson D, Mehta M, DeAngelis LM. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:5711–5715. doi: 10.1200/JCO.2006.08.2941. [DOI] [PubMed] [Google Scholar]
- Alvarnas JC, Negrin RS, Horning SJ, Hu WW, Long GD, Schriber JR, Stockerl-Goldstein K, Tierney K, Wong R, Blume KG, Chao NJ. High-dose therapy with hematopoietic cell transplantation for patients with central nervous system involvement by non-Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 2000;6:352–358. doi: 10.1016/s1083-8791(00)70060-7. [DOI] [PubMed] [Google Scholar]
- Baraniskin A, Kuhnhenn J, Schlegel U, Chan A, Deckert M, Gold R, Maghnouj A, Zollner H, Reinacher-Schick A, Schmiegel W, Hahn SA, Schroers R. Identification of microRNAs in the cerebrospinal fluid as marker for primary diffuse large B-cell lymphoma of the central nervous system. Blood. 2011;117:3140–3146. doi: 10.1182/blood-2010-09-308684. [DOI] [PubMed] [Google Scholar]
- Bataille B, Delwail V, Menet E, Vandermarcq P, Ingrand P, Wager M, Guy G, Lapierre F. Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg. 2000;92:261–266. doi: 10.3171/jns.2000.92.2.0261. [DOI] [PubMed] [Google Scholar]
- Batchelor T, Loeffler JS. Primary CNS lymphoma. J Clin Oncol. 2006;24:1281–1288. doi: 10.1200/JCO.2005.04.8819. [DOI] [PubMed] [Google Scholar]
- Batchelor TT, Kolak G, Ciordia R, Foster CS, Henson JW. High-dose methotrexate for intraocular lymphoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9:711–715. [PubMed] [Google Scholar]
- Batchelor TT, Grossman SA, Mikkelsen T, Ye X, Desideri S, Lesser GJ. Rituximab monotherapy for patients with recurrent primary CNS lymphoma. Neurology. 2011;76:929–930. doi: 10.1212/WNL.0b013e31820f2d94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbom A, Davila RM, Lin HS, Harbour JW. Treatment outcomes for primary intraocular lymphoma: implications for external beam radiotherapy. Eye (Lond) 2007;21:1198–1201. doi: 10.1038/sj.eye.6702437. [DOI] [PubMed] [Google Scholar]
- Bernstein SH, Unger JM, Leblanc M, Friedberg J, Miller TP, Fisher RI. Natural history of CNS relapse in patients with aggressive non-Hodgkin’s lymphoma: a 20-year follow-up analysis of SWOG 8516 -- the Southwest Oncology Group. J Clin Oncol. 2009;27:114–119. doi: 10.1200/JCO.2008.16.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blay JY, Conroy T, Chevreau C, Thyss A, Quesnel N, Eghbali H, Bouabdallah R, Coiffier B, Wagner JP, Le Mevel A, Dramais-Marcel D, Baumelou E, Chauvin F, Biron P. High-dose methotrexate for the treatment of primary cerebral lymphomas: analysis of survival and late neurologic toxicity in a retrospective series. J Clin Oncol. 1998;16:864–871. doi: 10.1200/JCO.1998.16.3.864. [DOI] [PubMed] [Google Scholar]
- Boehme V, Zeynalova S, Kloess M, Loeffler M, Kaiser U, Pfreundschuh M, Schmitz N. Incidence and risk factors of central nervous system recurrence in aggressive lymphoma--a survey of 1693 patients treated in protocols of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL) Ann Oncol. 2007;18:149–157. doi: 10.1093/annonc/mdl327. [DOI] [PubMed] [Google Scholar]
- Booman M, Szuhai K, Rosenwald A, Hartmann E, Kluin-Nelemans H, de Jong D, Schuuring E, Kluin P. Genomic alterations and gene expression in primary diffuse large B-cell lymphomas of immune-privileged sites: the importance of apoptosis and immunomodulatory pathways. J Pathol. 2008;216:209–217. doi: 10.1002/path.2399. [DOI] [PubMed] [Google Scholar]
- Boonstra R, Koning A, Mastik M, van den Berg A, Poppema S. Analysis of chromosomal copy number changes and oncoprotein expression in primary central nervous system lymphomas: frequent loss of chromosome arm 6q. Virchows Arch. 2003;443:164–169. doi: 10.1007/s00428-003-0836-9. [DOI] [PubMed] [Google Scholar]
- Braaten KM, Betensky RA, de Leval L, Okada Y, Hochberg FH, Louis DN, Harris NL, Batchelor TT. BCL-6 expression predicts improved survival in patients with primary central nervous system lymphoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9:1063–1069. [PubMed] [Google Scholar]
- Braggio E, McPhail ER, Macon W, Lopes MB, Schiff D, Law M, Fink S, Sprau D, Giannini C, Dogan A, Fonseca R, O’Neill BP. Primary central nervous system lymphomas: a validation study of array-based comparative genomic hybridization in formalin-fixed paraffin-embedded tumor specimens. Clin Cancer Res. 2011;17:4245–4253. doi: 10.1158/1078-0432.CCR-11-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg JE, Doorduijn JK, Illerhaus G, Jahnke K, Korfel A, Fischer L, Fritsch K, Kuittinen O, Issa S, van Montfort C, van den Bent MJ. Central nervous system recurrence of systemic lymphoma in the era of stem cell transplantation - an International Primary Central Nervous System Lymphoma Study Group project. Haematologica. 2013;98:808–813. doi: 10.3324/haematol.2012.070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady FM, O’Neill BP, Law ME, Decker PA, Kurtz DM, Giannini C, Porter AB, Kurtin PJ, Johnston PB, Dogan A, Remstein ED. Del(6)(q22) and BCL6 rearrangements in primary CNS lymphoma are indicators of an aggressive clinical course. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:4814–4819. doi: 10.1200/JCO.2008.16.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri-Broet S, Criniere E, Broet P, Delwail V, Mokhtari K, Moreau A, Kujas M, Raphael M, Iraqi W, Sautes-Fridman C, Colombat P, Hoang-Xuan K, Martin A. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood. 2006;107:190–196. doi: 10.1182/blood-2005-03-1024. [DOI] [PubMed] [Google Scholar]
- Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117:5019–5032. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CC, Buggage RR, Nussenblatt RB. Intraocular lymphoma. Curr Opin Ophthalmol. 2002;13:411–418. doi: 10.1097/00055735-200212000-00012. [DOI] [PubMed] [Google Scholar]
- Chan CC, Rubenstein JL, Coupland SE, Davis JL, Harbour JW, Johnston PB, Cassoux N, Touitou V, Smith JR, Batchelor TT, Pulido JS. Primary vitreoretinal lymphoma: a report from an International Primary Central Nervous System Lymphoma Collaborative Group symposium. Oncologist. 2011;16:1589–1599. doi: 10.1634/theoncologist.2011-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani A, Gastaldi R, Fassone L, Pierconti F, Giancola ML, Martini M, De Luca A, Ammassari A, Mazzone C, Pescarmona E, Gaidano G, Larocca LM, Antinori A. Epstein-Barr virus infection is predictive of CNS involvement in systemic AIDS-related non-Hodgkin’s lymphomas. J Clin Oncol. 2000;18:3325–3330. doi: 10.1200/JCO.2000.18.19.3325. [DOI] [PubMed] [Google Scholar]
- Correa DD, Rocco-Donovan M, DeAngelis LM, Dolgoff-Kaspar R, Iwamoto F, Yahalom J, Abrey LE. Prospective cognitive follow-up in primary CNS lymphoma patients treated with chemotherapy and reduced-dose radiotherapy. J Neurooncol. 2009;91:315–321. doi: 10.1007/s11060-008-9716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa DD, Shi W, Abrey LE, Deangelis LM, Omuro AM, Deutsch MB, Thaler HT. Cognitive functions in primary CNS lymphoma after single or combined modality regimens. Neuro Oncol. 2012;14:101–108. doi: 10.1093/neuonc/nor186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote GM, Hochberg EP, Muzikansky A, Hochberg FH, Drappatz J, McAfee SL, Batchelor TT, LaCasce AS, Fisher DC, Abramson JS, Armand P, Chen YB. Autologous stem cell transplantation with thiotepa, busulfan, and cyclophosphamide (TBC) conditioning in patients with CNS involvement by non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2012;18:76–83. doi: 10.1016/j.bbmt.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Courts C, Montesinos-Rongen M, Brunn A, Bug S, Siemer D, Hans V, Blumcke I, Klapper W, Schaller C, Wiestler OD, Kuppers R, Siebert R, Deckert M. Recurrent inactivation of the PRDM1 gene in primary central nervous system lymphoma. J Neuropathol Exp Neurol. 2008;67:720–727. doi: 10.1097/NEN.0b013e31817dd02d. [DOI] [PubMed] [Google Scholar]
- Damon L, Damon LE, Gaensler K, Kaplan L, Martin T, 3rd, Rubenstein J, Linker C. Impact of intensive PBSC mobilization therapy on outcomes following auto-SCT for non-Hodgkin’s lymphoma. Bone marrow transplantation. 2008;42:649–657. doi: 10.1038/bmt.2008.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damon LE, Johnson JL, Niedzwiecki D, Cheson BD, Hurd DD, Bartlett NL, Lacasce AS, Blum KA, Byrd JC, Kelly M, Stock W, Linker CA, Canellos GP. Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:6101–6108. doi: 10.1200/JCO.2009.22.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelis LM, Yahalom J, Heinemann MH, Cirrincione C, Thaler HT, Krol G. Primary CNS lymphoma: combined treatment with chemotherapy and radiotherapy. Neurology. 1990;40:80–86. doi: 10.1212/wnl.40.1.80. [DOI] [PubMed] [Google Scholar]
- DeAngelis LM, Yahalom J, Thaler HT, Kher U. Combined modality therapy for primary CNS lymphoma. J Clin Oncol. 1992;10:635–643. doi: 10.1200/JCO.1992.10.4.635. [DOI] [PubMed] [Google Scholar]
- DeAngelis LM, Seiferheld W, Schold SC, Fisher B, Schultz CJ. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol. 2002;20:4643–4648. doi: 10.1200/JCO.2002.11.013. [DOI] [PubMed] [Google Scholar]
- Elstrom RL, Andreadis C, Aqui NA, Ahya VN, Bloom RD, Brozena SC, Olthoff KM, Schuster SJ, Nasta SD, Stadtmauer EA, Tsai DE. Treatment of PTLD with rituximab or chemotherapy. Am J Transplant. 2006;6:569–576. doi: 10.1111/j.1600-6143.2005.01211.x. [DOI] [PubMed] [Google Scholar]
- Ervin T, Canellos GP. Successful treatment of recurrent primary central nervous system lymphoma with high-dose methotrexate. Cancer. 1980;45:1556–1557. doi: 10.1002/1097-0142(19800401)45:7<1556::aid-cncr2820450707>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Falzetti F, Di Ianni M, Ballanti S, Iodice G, Reale A, Minelli O, Serio G, Martelli MF, Dammacco F, Vacca A, Ria R. High-dose thiotepa, etoposide and carboplatin as conditioning regimen for autologous stem cell transplantation in patients with high-risk non-Hodgkin lymphoma. Clin Exp Med. 2012;12:165–171. doi: 10.1007/s10238-011-0157-2. [DOI] [PubMed] [Google Scholar]
- Ferreri AJ, Reni M, Zoldan MC, Terreni MR, Villa E. Importance of complete staging in non-Hodgkin’s lymphoma presenting as a cerebral mass lesion. Cancer. 1996;77:827–833. doi: 10.1002/(sici)1097-0142(19960301)77:5<827::aid-cncr4>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Ferreri AJ, Blay JY, Reni M, Pasini F, Spina M, Ambrosetti A, Calderoni A, Rossi A, Vavassori V, Conconi A, Devizzi L, Berger F, Ponzoni M, Borisch B, Tinguely M, Cerati M, Milani M, Orvieto E, Sanchez J, Chevreau C, Dell’Oro S, Zucca E, Cavalli F. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:266–272. doi: 10.1200/JCO.2003.09.139. [DOI] [PubMed] [Google Scholar]
- Ferreri AJ, Reni M, Foppoli M, Martelli M, Pangalis GA, Frezzato M, Cabras MG, Fabbri A, Corazzelli G, Ilariucci F, Rossi G, Soffietti R, Stelitano C, Vallisa D, Zaja F, Zoppegno L, Aondio GM, Avvisati G, Balzarotti M, Brandes AA, Fajardo J, Gomez H, Guarini A, Pinotti G, Rigacci L, Uhlmann C, Picozzi P, Vezzulli P, Ponzoni M, Zucca E, Caligaris-Cappio F, Cavalli F. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet. 2009;374:1512–1520. doi: 10.1016/S0140-6736(09)61416-1. [DOI] [PubMed] [Google Scholar]
- Feugier P, Virion JM, Tilly H, Haioun C, Marit G, Macro M, Bordessoule D, Recher C, Blanc M, Molina T, Lederlin P, Coiffier B. Incidence and risk factors for central nervous system occurrence in elderly patients with diffuse large-B-cell lymphoma: influence of rituximab. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2004;15:129–133. doi: 10.1093/annonc/mdh013. [DOI] [PubMed] [Google Scholar]
- Fine HA, Mayer RJ. Primary central nervous system lymphoma. Ann Intern Med. 1993;119:1093–1104. doi: 10.7326/0003-4819-119-11-199312010-00007. [DOI] [PubMed] [Google Scholar]
- Fischer L, Martus P, Weller M, Klasen HA, Rohden B, Roth A, Storek B, Hummel M, Nagele T, Thiel E, Korfel A. Meningeal dissemination in primary CNS lymphoma: prospective evaluation of 282 patients. Neurology. 2008;71:1102–1108. doi: 10.1212/01.wnl.0000326958.52546.f5. [DOI] [PubMed] [Google Scholar]
- Fischer L, Korfel A, Pfeiffer S, Kiewe P, Volk HD, Cakiroglu H, Widmann T, Thiel E. CXCL13 and CXCL12 in central nervous system lymphoma patients. Clin Cancer Res. 2009;15:5968–5973. doi: 10.1158/1078-0432.CCR-09-0108. [DOI] [PubMed] [Google Scholar]
- Fischer L, Hummel M, Korfel A, Lenze D, Joehrens K, Thiel E. Differential micro-RNA expression in primary CNS and nodal diffuse large B-cell lymphomas. Neuro Oncol. 2011;13:1090–1098. doi: 10.1093/neuonc/nor107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz MJ, Cole BF, Recht L, Akerley W, Mills P, Saris S, Hochberg F, Calabresi P, Egorin MJ. High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: is intrathecal chemotherapy necessary? J Clin Oncol. 1998;16:1561–1567. doi: 10.1200/JCO.1998.16.4.1561. [DOI] [PubMed] [Google Scholar]
- Glass J, Gruber ML, Cher L, Hochberg FH. Preirradiation methotrexate chemotherapy of primary central nervous system lymphoma: long-term outcome. J Neurosurg. 1994;81:188–195. doi: 10.3171/jns.1994.81.2.0188. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Aguilar A, Idbaih A, Boisselier B, Habbita N, Rossetto M, Laurenge A, Bruno A, Jouvet A, Polivka M, Adam C, Figarella-Branger D, Miquel C, Vital A, Ghesquieres H, Gressin R, Delwail V, Taillandier L, Chinot O, Soubeyran P, Gyan E, Choquet S, Houillier C, Soussain C, Tanguy ML, Marie Y, Mokhtari K, Hoang-Xuan K. Recurrent mutations of MYD88 and TBL1XR1 in primary central nervous system lymphomas. Clin Cancer Res. 2012;18:5203–5211. doi: 10.1158/1078-0432.CCR-12-0845. [DOI] [PubMed] [Google Scholar]
- Green JM. Glucarpidase to combat toxic levels of methotrexate in patients. Ther Clin Risk Manag. 2012;8:403–413. doi: 10.2147/TCRM.S30135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K, Nishizaki T, Kubota H, Suzuki M, Sasaki K. Distinct primary central nervous system lymphoma defined by comparative genomic hybridization and laser scanning cytometry. Cancer Genet Cytogenet. 2001;125:147–150. doi: 10.1016/s0165-4608(00)00377-0. [DOI] [PubMed] [Google Scholar]
- Hochberg FH, Miller DC. Primary central nervous system lymphoma. J Neurosurg. 1988;68:835–853. doi: 10.3171/jns.1988.68.6.0835. [DOI] [PubMed] [Google Scholar]
- Illerhaus G, Muller F, Feuerhake F, Schafer AO, Ostertag C, Finke J. High-dose chemotherapy and autologous stem-cell transplantation without consolidating radiotherapy as first-line treatment for primary lymphoma of the central nervous system. Haematologica. 2008;93:147–148. doi: 10.3324/haematol.11771. [DOI] [PubMed] [Google Scholar]
- Itty S, Pulido JS. Rituximab for intraocular lymphoma. Retina. 2009;29:129–132. doi: 10.1097/IAE.0b013e318192f574. [DOI] [PubMed] [Google Scholar]
- Jacomet C, Girard PM, Lebrette MG, Farese VL, Monfort L, Rozenbaum W. Intravenous methotrexate for primary central nervous system non-Hodgkin’s lymphoma in AIDS. AIDS. 1997;11:1725–1730. doi: 10.1097/00002030-199714000-00009. [DOI] [PubMed] [Google Scholar]
- Jahnke K, Thiel E, Bechrakis NE, Willerding G, Kraemer DF, Fischer L, Korfel A. Ifosfamide or trofosfamide in patients with intraocular lymphoma. J Neurooncol. 2009;93:213–217. doi: 10.1007/s11060-008-9761-8. [DOI] [PubMed] [Google Scholar]
- Jordanova ES, Riemersma SA, Philippo K, Giphart-Gassler M, Schuuring E, Kluin PM. Hemizygous deletions in the HLA region account for loss of heterozygosity in the majority of diffuse large B-cell lymphomas of the testis and the central nervous system. Genes Chromosomes Cancer. 2002;35:38–48. doi: 10.1002/gcc.10093. [DOI] [PubMed] [Google Scholar]
- Josephson SA, Papanastassiou AM, Berger MS, Barbaro NM, McDermott MW, Hilton JF, Miller BL, Geschwind MD. The diagnostic utility of brain biopsy procedures in patients with rapidly deteriorating neurological conditions or dementia. J Neurosurg. 2007;106:72–75. doi: 10.3171/jns.2007.106.1.72. [DOI] [PubMed] [Google Scholar]
- Kadoch C, Li J, Wong VS, Chen L, Cha S, Munster PN, Lowell CA, Shuman MA, Rubenstein JL. Complement Activation and Intraventricular Rituximab Distribution in Recurrent Central Nervous System Lymphoma. Clin Cancer Res. 2013;20:1029–1041. doi: 10.1158/1078-0432.CCR-13-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan RB, Shi W, Thaler HT, DeAngelis LM, Abrey LE. Is intrathecal methotrexate necessary in the treatment of primary CNS lymphoma? J Neurooncol. 2002;58:175–178. doi: 10.1023/a:1016077907952. [DOI] [PubMed] [Google Scholar]
- Kitzmann AS, Pulido JS, Mohney BG, Baratz KH, Grube T, Marler RJ, Donaldson MJ, O’Neill BP, Johnston PB, Johnson KM, Dixon LE, Salomao DR, Cameron JD. Intraocular use of rituximab. Eye (Lond) 2007;21:1524–1527. doi: 10.1038/sj.eye.6702804. [DOI] [PubMed] [Google Scholar]
- Korfel A, Elter T, Thiel E, Hanel M, Mohle R, Schroers R, Reiser M, Dreyling M, Eucker J, Scholz C, Metzner B, Roth A, Birkmann J, Schlegel U, Martus P, Illerhaus G, Fischer L. Phase II study of central nervous system (CNS)-directed chemotherapy including high-dose chemotherapy with autologous stem cell transplantation for CNS relapse of aggressive lymphomas. Haematologica. 2013;98:364–370. doi: 10.3324/haematol.2012.077917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuker W, Nagele T, Korfel A, Heckl S, Thiel E, Bamberg M, Weller M, Herrlinger U. Primary central nervous system lymphomas (PCNSL): MRI features at presentation in 100 patients. J Neurooncol. 2005;72:169–177. doi: 10.1007/s11060-004-3390-7. [DOI] [PubMed] [Google Scholar]
- Lai R, Rosenblum MK, DeAngelis LM. Primary CNS lymphoma: a whole-brain disease? Neurology. 2002;59:1557–1562. doi: 10.1212/01.wnl.0000034256.20173.ea. [DOI] [PubMed] [Google Scholar]
- Linker CA, Owzar K, Powell B, Hurd D, Damon LE, Archer LE, Larson RA. Auto-SCT for AML in second remission: CALGB study 9620. Bone marrow transplantation. 2009;44:353–359. doi: 10.1038/bmt.2009.36. [DOI] [PubMed] [Google Scholar]
- Mohile NA, Deangelis LM, Abrey LE. The utility of body FDG PET in staging primary central nervous system lymphoma. Neuro Oncol. 2008;10:223–228. doi: 10.1215/15228517-2007-061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos-Rongen M, Schmitz R, Brunn A, Gesk S, Richter J, Hong K, Wiestler OD, Siebert R, Kuppers R, Deckert M. Mutations of CARD11 but not TNFAIP3 may activate the NF-kappaB pathway in primary CNS lymphoma. Acta Neuropathol. 2010;120:529–535. doi: 10.1007/s00401-010-0709-7. [DOI] [PubMed] [Google Scholar]
- Montesinos-Rongen M, Godlewska E, Brunn A, Wiestler OD, Siebert R, Deckert M. Activating L265P mutations of the MYD88 gene are common in primary central nervous system lymphoma. Acta Neuropathol. 2011;122:791–792. doi: 10.1007/s00401-011-0891-2. [DOI] [PubMed] [Google Scholar]
- Montesinos-Rongen M, Schafer E, Siebert R, Deckert M. Genes regulating the B cell receptor pathway are recurrently mutated in primary central nervous system lymphoma. Acta Neuropathol. 2012;124:905–906. doi: 10.1007/s00401-012-1064-7. [DOI] [PubMed] [Google Scholar]
- Morris PG, Correa DD, Yahalom J, Raizer JJ, Schiff D, Grant B, Grimm S, Lai RK, Reiner AS, Panageas K, Karimi S, Curry R, Shah G, Abrey LE, DeAngelis LM, Omuro A. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol. 2013;31:3971–3979. doi: 10.1200/JCO.2013.50.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniz C, Martin-Martin L, Lopez A, Sanchez-Gonzalez B, Salar A, Almeida J, Sancho JM, Ribera JM, Heras C, Penalver F, Gomez M, Gonzalez-Barca E, Alonso N, Navarro B, Olave T, Sala F, Conde E, Marquez JA, Cabezudo E, Cladera A, Garcia-Malo M, Caballero MD, Orfao A. Contribution of cerebrospinal fluid sCD19 levels to the detection of CNS lymphoma and its impact on disease outcome. Blood. 2014 doi: 10.1182/blood-2013-11-537993. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Kishi M, Sakaki T, Hashimoto H, Nakase H, Shimada K, Ishida E, Konishi N. Novel tumor suppressor loci on 6q22-23 in primary central nervous system lymphomas. Cancer Res. 2003;63:737–741. [PubMed] [Google Scholar]
- Nelson DF, Martz KL, Bonner H, Nelson JS, Newall J, Kerman HD, Thomson JW, Murray KJ. Non-Hodgkin’s lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int J Radiat Oncol Biol Phys. 1992;23:9–17. doi: 10.1016/0360-3016(92)90538-s. [DOI] [PubMed] [Google Scholar]
- Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH, Kohlhammer H, Xu W, Yang Y, Zhao H, Shaffer AL, Romesser P, Wright G, Powell J, Rosenwald A, Muller-Hermelink HK, Ott G, Gascoyne RD, Connors JM, Rimsza LM, Campo E, Jaffe ES, Delabie J, Smeland EB, Fisher RI, Braziel RM, Tubbs RR, Cook JR, Weisenburger DD, Chan WC, Staudt LM. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden AD, Drappatz J, Wen PY, Claus EB. Survival among patients with primary central nervous system lymphoma, 1973–2004. J Neurooncol. 2011;101:487–493. doi: 10.1007/s11060-010-0269-7. [DOI] [PubMed] [Google Scholar]
- Osoba D, Brada M, Yung WK, Prados M. Health-related quality of life in patients treated with temozolomide versus procarbazine for recurrent glioblastoma multiforme. J Clin Oncol. 2000a;18:1481–1491. doi: 10.1200/JCO.2000.18.7.1481. [DOI] [PubMed] [Google Scholar]
- Osoba D, Brada M, Yung WK, Prados MD. Health-related quality of life in patients with anaplastic astrocytoma during treatment with temozolomide. Eur J Cancer. 2000b;36:1788–1795. doi: 10.1016/s0959-8049(00)00165-9. [DOI] [PubMed] [Google Scholar]
- Ott RJ, Brada M, Flower MA, Babich JW, Cherry SR, Deehan BJ. Measurements of blood-brain barrier permeability in patients undergoing radiotherapy and chemotherapy for primary cerebral lymphoma. Eur J Cancer. 1991;27:1356–1361. doi: 10.1016/0277-5379(91)90009-3. [DOI] [PubMed] [Google Scholar]
- Pirotte B, Levivier M, Goldman S, Brucher JM, Brotchi J, Hildebrand J. Glucocorticoid-induced long-term remission in primary cerebral lymphoma: case report and review of the literature. J Neurooncol. 1997;32:63–69. doi: 10.1023/a:1005733416571. [DOI] [PubMed] [Google Scholar]
- Ponzoni M, Berger F, Chassagne-Clement C, Tinguely M, Jouvet A, Ferreri AJ, Dell’Oro S, Terreni MR, Doglioni C, Weis J, Cerati M, Milani M, Iuzzolino P, Motta T, Carbone A, Pedrinis E, Sanchez J, Blay JY, Reni M, Conconi A, Bertoni F, Zucca E, Cavalli F, Borisch B. Reactive perivascular T-cell infiltrate predicts survival in primary central nervous system B-cell lymphomas. British journal of haematology. 2007;138:316–323. doi: 10.1111/j.1365-2141.2007.06661.x. [DOI] [PubMed] [Google Scholar]
- Ponzoni M, Issa S, Batchelor TT, Rubenstein JL. Beyond high-dose methotrexate and brain radiotherapy: novel targets and agents for primary CNS lymphoma. Ann Oncol. 2013;25:316–322. doi: 10.1093/annonc/mdt385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter AB, Giannini C, Kaufmann T, Lucchinetti CF, Wu W, Decker PA, Atkinson JL, O’Neill BP. Primary central nervous system lymphoma can be histologically diagnosed after previous corticosteroid use: a pilot study to determine whether corticosteroids prevent the diagnosis of primary central nervous system lymphoma. Ann Neurol. 2008;63:662–667. doi: 10.1002/ana.21366. [DOI] [PubMed] [Google Scholar]
- Quijano S, Lopez A, Manuel Sancho J, Panizo C, Deben G, Castilla C, Antonio Garcia-Vela J, Salar A, Alonso-Vence N, Gonzalez-Barca E, Penalver FJ, Plaza-Villa J, Morado M, Garcia-Marco J, Arias J, Briones J, Ferrer S, Capote J, Nicolas C, Orfao A. Identification of leptomeningeal disease in aggressive B-cell non-Hodgkin’s lymphoma: improved sensitivity of flow cytometry. J Clin Oncol. 2009;27:1462–1469. doi: 10.1200/JCO.2008.17.7089. [DOI] [PubMed] [Google Scholar]
- Raizer JJ, Rademaker A, Evens AM, Rice L, Schwartz M, Chandler JP, Getch CC, Tellez C, Grimm SA. Pemetrexed in the treatment of relapsed/refractory primary central nervous system lymphoma. Cancer. 2012;118:3743–3748. doi: 10.1002/cncr.26709. [DOI] [PubMed] [Google Scholar]
- Relling MV, Mahmoud HH, Pui CH, Sandlund JT, Rivera GK, Ribeiro RC, Crist WM, Evans WE. Etoposide achieves potentially cytotoxic concentrations in CSF of children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:399–404. doi: 10.1200/JCO.1996.14.2.399. [DOI] [PubMed] [Google Scholar]
- Reni M, Ferreri AJ, Landoni C, Villa E. Salvage therapy with temozolomide in an immunocompetent patient with primary brain lymphoma. J Natl Cancer Inst. 2000;92:575–576. doi: 10.1093/jnci/92.7.575. [DOI] [PubMed] [Google Scholar]
- Reni M, Zaja F, Mason W, Perry J, Mazza E, Spina M, Bordonaro R, Ilariucci F, Faedi M, Corazzelli G, Manno P, Franceschi E, Pace A, Candela M, Abbadessa A, Stelitano C, Latte G, Ferreri AJ. Temozolomide as salvage treatment in primary brain lymphomas. Br J Cancer. 2007;96:864–867. doi: 10.1038/sj.bjc.6603660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Josephson SA, Fridlyand J, Karch J, Kadoch C, Karrim J, Damon L, Treseler P, Kunwar S, Shuman MA, Jones T, Becker CH, Schulman H, Rubenstein JL. Protein biomarker identification in the CSF of patients with CNS lymphoma. J Clin Oncol. 2008;26:96–105. doi: 10.1200/JCO.2007.12.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Combs D, Rosenberg J, Levy A, McDermott M, Damon L, Ignoffo R, Aldape K, Shen A, Lee D, Grillo-Lopez A, Shuman MA. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood. 2003;101:466–468. doi: 10.1182/blood-2002-06-1636. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Treseler P, O’Brien JM. Pathology and genetics of primary central nervous system and intraocular lymphoma. Hematol Oncol Clin North Am. 2005;19:705–717. vii. doi: 10.1016/j.hoc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Fridlyand J, Shen A, Aldape K, Ginzinger D, Batchelor T, Treseler P, Berger M, McDermott M, Prados M, Karch J, Okada C, Hyun W, Parikh S, Haqq C, Shuman M. Gene expression and angiotropism in primary CNS lymphoma. Blood. 2006;107:3716–3723. doi: 10.1182/blood-2005-03-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Fridlyand J, Abrey L, Shen A, Karch J, Wang E, Issa S, Damon L, Prados M, McDermott M, O’Brien J, Haqq C, Shuman M. Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol. 2007;25:1350–1356. doi: 10.1200/JCO.2006.09.7311. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Gupta NK, Mannis GN, Lamarre AK, Treseler P. How I treat CNS lymphomas. Blood. 2013a;122:2318–2330. doi: 10.1182/blood-2013-06-453084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Hsi ED, Johnson JL, Jung SH, Nakashima MO, Grant B, Cheson BD, Kaplan LD. Intensive Chemotherapy and Immunotherapy in Patients With Newly Diagnosed Primary CNS Lymphoma: CALGB 50202 (Alliance 50202) J Clin Oncol. 2013b;31:3061–3068. doi: 10.1200/JCO.2012.46.9957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Wong VS, Kadoch C, Gao HX, Barajas R, Chen L, Josephson SA, Scott B, Douglas V, Maiti M, Kaplan LD, Treseler PA, Cha S, Hwang JH, Cinque P, Cyster JG, Lowell C. CXCL13 plus interleukin-10 are highly specific for the diagnosis of CNS lymphoma. Blood. 2013c;121:4740–4748. doi: 10.1182/blood-2013-01-476333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Li J, Chen L, Advani R, Drappatz J, Gerstner E, Batchelor T, Krouwer H, Hwang J, Auerback G, Kadoch C, Lowell C, Munster P, Cha S, Shuman MA, Damon LE. Multicenter phase 1 trial of intraventricular immunochemotherapy in recurrent CNS lymphoma. Blood. 2013d;121:745–751. doi: 10.1182/blood-2012-07-440974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasayama T, Nakamizo S, Nishihara M, Kawamura A, Tanaka H, Mizukawa K, Miyake S, Taniguchi M, Hosoda K, Kohmura E. Cerebrospinal fluid interleukin-10 is a potentially useful biomarker in immunocompetent primary central nervous system lymphoma (PCNSL) Neuro Oncol. 2012;14:368–380. doi: 10.1093/neuonc/nor203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabet M. Epidemiology of primary CNS lymphoma. J Neurooncol. 1999;43:199–201. doi: 10.1023/a:1006290032052. [DOI] [PubMed] [Google Scholar]
- Schaich M, Rollig C, Soucek S, Kramer M, Thiede C, Mohr B, Oelschlaegel U, Schmitz N, Stuhlmann R, Wandt H, Schafer-Eckart K, Aulitzky W, Kaufmann M, Bodenstein H, Tischler J, Ho A, Kramer A, Bornhauser M, Schetelig J, Ehninger G. Cytarabine dose of 36 g/m(2) compared with 12 g/m(2) within first consolidation in acute myeloid leukemia: results of patients enrolled onto the prospective randomized AML96 study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:2696–2702. doi: 10.1200/JCO.2010.33.7303. [DOI] [PubMed] [Google Scholar]
- Schaich M, Parmentier S, Kramer M, Illmer T, Stolzel F, Rollig C, Thiede C, Hanel M, Schafer-Eckart K, Aulitzky W, Einsele H, Ho AD, Serve H, Berdel WE, Mayer J, Schmitz N, Krause SW, Neubauer A, Baldus CD, Schetelig J, Bornhauser M, Ehninger G. High-dose cytarabine consolidation with or without additional amsacrine and mitoxantrone in acute myeloid leukemia: results of the prospective randomized AML2003 trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:2094–2102. doi: 10.1200/JCO.2012.46.4743. [DOI] [PubMed] [Google Scholar]
- Schwindt H, Vater I, Kreuz M, Montesinos-Rongen M, Brunn A, Richter J, Gesk S, Ammerpohl O, Wiestler OD, Hasenclever D, Deckert M, Siebert R. Chromosomal imbalances and partial uniparental disomies in primary central nervous system lymphoma. Leukemia. 2009;23:1875–1884. doi: 10.1038/leu.2009.120. [DOI] [PubMed] [Google Scholar]
- Shah GD, Yahalom J, Correa DD, Lai RK, Raizer JJ, Schiff D, LaRocca R, Grant B, DeAngelis LM, Abrey LE. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:4730–4735. doi: 10.1200/JCO.2007.12.5062. [DOI] [PubMed] [Google Scholar]
- Shenkier TN, Blay JY, O’Neill BP, Poortmans P, Thiel E, Jahnke K, Abrey LE, Neuwelt E, Tsang R, Batchelor T, Harris N, Ferreri AJ, Ponzoni M, O’Brien P, Rubenstein J, Connors JM. Primary CNS lymphoma of T-cell origin: a descriptive analysis from the international primary CNS lymphoma collaborative group. J Clin Oncol. 2005;23:2233–2239. doi: 10.1200/JCO.2005.07.109. [DOI] [PubMed] [Google Scholar]
- Skarin AT, Zuckerman KS, Pitman SW, Rosenthal DS, Moloney W, Frei E, 3rd, Canellos GP. High-dose methotrexate with folinic acid in the treatment of advanced non-Hodgkin lymphoma including CNS involvement. Blood. 1977;50:1039–1047. [PubMed] [Google Scholar]
- Smith JR, Braziel RM, Paoletti S, Lipp M, Uguccioni M, Rosenbaum JT. Expression of B-cell-attracting chemokine 1 (CXCL13) by malignant lymphocytes and vascular endothelium in primary central nervous system lymphoma. Blood. 2003;101:815–821. doi: 10.1182/blood-2002-05-1576. [DOI] [PubMed] [Google Scholar]
- Smith JR, Falkenhagen KM, Coupland SE, Chipps TJ, Rosenbaum JT, Braziel RM. Malignant B cells from patients with primary central nervous system lymphoma express stromal cell-derived factor-1. Am J Clin Pathol. 2007;127:633–641. doi: 10.1309/NUQHJ79BHWYD9TAF. [DOI] [PubMed] [Google Scholar]
- Soussain C, Suzan F, Hoang-Xuan K, Cassoux N, Levy V, Azar N, Belanger C, Achour E, Ribrag V, Gerber S, Delattre JY, Leblond V. Results of intensive chemotherapy followed by hematopoietic stem-cell rescue in 22 patients with refractory or recurrent primary CNS lymphoma or intraocular lymphoma. J Clin Oncol. 2001;19:742–749. doi: 10.1200/JCO.2001.19.3.742. [DOI] [PubMed] [Google Scholar]
- Soussain C, Hoang-Xuan K, Taillandier L, Fourme E, Choquet S, Witz F, Casasnovas O, Dupriez B, Souleau B, Taksin AL, Gisselbrecht C, Jaccard A, Omuro A, Sanson M, Janvier M, Kolb B, Zini JM, Leblond V. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Societe Francaise de Greffe de Moelle Osseuse-Therapie Cellulaire. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:2512–2518. doi: 10.1200/JCO.2007.13.5533. [DOI] [PubMed] [Google Scholar]
- Stefanovic A, Davis J, Murray T, Markoe A, Lossos IS. Treatment of isolated primary intraocular lymphoma with high-dose methotrexate-based chemotherapy and binocular radiation therapy: a single-institution experience. Br J Haematol. 2010;151:103–106. doi: 10.1111/j.1365-2141.2010.08321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A, Bae K, Gore EM, Movsas B, Wong SJ, Meyers CA, Bonner JA, Schild SE, Gaspar LE, Bogart JA, Werner-Wasik M, Choy H. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: neurocognitive and quality-of-life analysis. J Clin Oncol. 2011;29:279–286. doi: 10.1200/JCO.2010.29.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung CO, Kim SC, Karnan S, Karube K, Shin HJ, Nam DH, Suh YL, Kim SH, Kim JY, Kim SJ, Kim WS, Seto M, Ko YH. Genomic profiling combined with gene expression profiling in primary central nervous system lymphoma. Blood. 2011;117:1291–1300. doi: 10.1182/blood-2010-07-297861. [DOI] [PubMed] [Google Scholar]
- Tai WM, Chung J, Tang PL, Koo YX, Hou X, Tay KW, Quek R, Tao M, Lim ST. Central nervous system (CNS) relapse in diffuse large B cell lymphoma (DLBCL): pre- and post-rituximab. Annals of hematology. 2011;90:809–818. doi: 10.1007/s00277-010-1150-7. [DOI] [PubMed] [Google Scholar]
- Thiel E, Korfel A, Martus P, Kanz L, Griesinger F, Rauch M, Roth A, Hertenstein B, von Toll T, Hundsberger T, Mergenthaler HG, Leithauser M, Birnbaum T, Fischer L, Jahnke K, Herrlinger U, Plasswilm L, Nagele T, Pietsch T, Bamberg M, Weller M. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010;11:1036–1047. doi: 10.1016/S1470-2045(10)70229-1. [DOI] [PubMed] [Google Scholar]
- Tu PH, Giannini C, Judkins AR, Schwalb JM, Burack R, O’Neill BP, Yachnis AT, Burger PC, Scheithauer BW, Perry A. Clinicopathologic and genetic profile of intracranial marginal zone lymphoma: a primary low-grade CNS lymphoma that mimics meningioma. J Clin Oncol. 2005;23:5718–5727. doi: 10.1200/JCO.2005.17.624. [DOI] [PubMed] [Google Scholar]
- Tun HW, Personett D, Baskerville KA, Menke DM, Jaeckle KA, Kreinest P, Edenfield B, Zubair AC, O’Neill BP, Lai WR, Park PJ, McKinney M. Pathway analysis of primary central nervous system lymphoma. Blood. 2008;111:3200–3210. doi: 10.1182/blood-2007-10-119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Glind G, de Graaf S, Klein C, Cornelissen M, Maecker B, Loeffen J. Intrathecal rituximab treatment for pediatric post-transplant lymphoproliferative disorder of the central nervous system. Pediatr Blood Cancer. 2008;50:886–888. doi: 10.1002/pbc.21297. [DOI] [PubMed] [Google Scholar]
- Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer. 2011;105:1414–1418. doi: 10.1038/bjc.2011.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller M, Martus P, Roth P, Thiel E, Korfel A. Surgery for primary CNS lymphoma? Challenging a paradigm. Neuro Oncol. 2012;14:1481–1484. doi: 10.1093/neuonc/nos159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieduwilt MJ, Valles F, Issa S, Behler CM, Hwang J, McDermott M, Treseler P, O’Brien J, Shuman MA, Cha S, Damon LE, Rubenstein JL. Immunochemotherapy with intensive consolidation for primary CNS lymphoma: a pilot study and prognostic assessment by diffusion-weighted MRI. Clin Cancer Res. 2012;18:1146–1155. doi: 10.1158/1078-0432.CCR-11-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WH, Bryant G, Bates S, Fojo A, Wittes RE, Steinberg SM, Kohler DR, Jaffe ES, Herdt J, Cheson BD, Chabner BA. EPOCH chemotherapy: toxicity and efficacy in relapsed and refractory non-Hodgkin’s lymphoma. J Clin Oncol. 1993;11:1573–1582. doi: 10.1200/JCO.1993.11.8.1573. [DOI] [PubMed] [Google Scholar]
- Wilson WH, Dunleavy K, Pittaluga S, Hegde U, Grant N, Steinberg SM, Raffeld M, Gutierrez M, Chabner BA, Staudt L, Jaffe ES, Janik JE. Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center biomarkers. J Clin Oncol. 2008;26:2717–2724. doi: 10.1200/JCO.2007.13.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ET, Tishler R, Barron L, Wu JK. Immunochemotherapy with rituximab and temozolomide for central nervous system lymphomas. Cancer. 2004;101:139–145. doi: 10.1002/cncr.20339. [DOI] [PubMed] [Google Scholar]
- Yamamoto W, Tomita N, Watanabe R, Hattori Y, Nakajima Y, Hyo R, Hashimoto C, Motomura S, Ishigatsubo Y. Central nervous system involvement in diffuse large B-cell lymphoma. European journal of haematology. 2010;85:6–10. doi: 10.1111/j.1600-0609.2010.01438.x. [DOI] [PubMed] [Google Scholar]