Abstract
Background
The time that red cell units are stored before transfusion may be associated with postoperative complications, although the evidence is conflicting. However, the association between the length of red cell unit storage and postoperative delirium has not been explored. We hypothesized that the length of storage of transfused red cell units would be associated with delirium after cardiac surgery.
Methods
We conducted a case-control study in which patients undergoing coronary artery bypass, valve, or ascending aorta surgery with cardiopulmonary bypass at Johns Hopkins from 2005–2011 were eligible for inclusion. Patients were excluded if they did not receive red cell units, received >4 red cell units during hospitalization, received any transfusion after the first postoperative day, or received red cell units that were not exclusively stored for ≤14 days or >14 days. Eighty-seven patients met transfusion-related inclusion criteria and developed postoperative delirium. Controls who did not develop delirium were selected from the same source population of eligible patients and were matched 1:1 based on age (+/− 5 years), 2-2.5-year band of date of surgery, and surgical procedure. For each patient, we calculated the average storage duration of all transfused red cell units. The primary outcome was odds of delirium in patients who were transfused red cell units with exclusive storage duration >14 days compared to ≤14 days. Secondary outcomes were odds of delirium with each increasing day of average red cell unit storage duration. We used conditional multivariable regression to test our hypotheses.
Results
In conditional multivariable analysis of 87 case-control pairs, there was no difference in the odds of patients developing delirium if they were transfused red cell units with an exclusive storage age >14 days compared to ≤14 days (odds ratio [OR] 1.83; 95% CI, 0.73–4.58, P=0.20). Each additional day of average red cell unit storage beyond 14 days was associated with a 1.01–1.13 fold increase in the odds of postoperative delirium (OR, 1.07; P=0.03). Each additional day of average storage beyond 21 days was associated with a 1.02–1.23 fold increase in the odds of postoperative delirium (OR, 1.12; P=0.02).
Conclusion
Transfusion of red cell units that have been stored for more than 14 days is not associated with increased odds of delirium. However, each additional day of storage more than 14 days or 21 days may be associated with increased odds of postoperative delirium in patients undergoing cardiac surgery. More research is needed to further characterize the association between delirium and storage duration of transfused red cell units.
Introduction
Delirium is common after cardiac surgery, occurring in as many as 53% of patients.1 Characterized by acute decline in attention and cognition, delirium is associated with long-term consequences, including decreased cognitive function,2,3 reduced functional capacity,4,5 and increased mortality.6,7 Once delirium has developed, it is difficult to treat; thus, developing measures to prevent its occurrence is critical. Although postoperative delirium has many known risk factors, most cannot be modified (age, education, and baseline cognition), and so identifying modifiable risk factors is crucial. Transfusion of red cell units is a potentially modifiable risk factor that has been associated with postoperative delirium in observational data.8
Recent laboratory and clinical data have shown that increasing the duration of red cell unit storage may have adverse consequences for patients who receive them, although the evidence is conflicting.9 In retrospective analyses of patients undergoing cardiac surgery, transfusion of red cell units that have been stored for more than 14 days has been associated with postoperative morbidity and mortality,10 and such poor outcomes also have been reported in other surgical populations.11,12 However, other retrospective analyses of patients undergoing cardiac surgery have found no evidence of an association between length of storage of red cell units and postoperative outcomes.13–15 Similarly, a recent large retrospective study in noncardiac surgery patients found no evidence that increased mortality was associated with increasing storage duration of red cell units.16
The mechanism by which duration of red cell unit storage might lead to adverse outcomes is unclear, but potential explanations include reduced deformability of stored red blood cells (RBCs),9 reduction in 2,3-diphosphoglycerate (2,3-DPG) levels,17 and changes in availability of nitric oxide, 18 all potentially leading to a reduction in oxygen delivery.19 Similarly, the mechanism for the development of delirium is unclear, but since the brain is exquisitely sensitive to adequate oxygen delivery, impairment in oxygen delivery is a potential mechanism for subsequent delirium. Indeed, delirium after cardiac surgery has been associated with several factors that either predispose or reflect impaired oxygen delivery to the brain, including anemia,20,21 decreased perfusion pressure during bypass,22 and low cerebral oximetry values.23
However, it is unknown whether the storage age of RBCs is associated with the development of postoperative delirium. In this study, we examined whether the storage age of blood is associated with postoperative delirium in a cohort of patients who underwent cardiac surgery with cardiopulmonary bypass and were assessed prospectively for the development of delirium. We hypothesized that the length of storage of transfused red cell units would be associated with the development of postoperative delirium after cardiac surgery.
Methods
Patients
The population for this case-control study consisted of patients > 21 years old who underwent coronary artery bypass graft, cardiac valve repair or replacement, combined bypass and valve surgery, or surgery of the ascending aorta using cardiopulmonary bypass at Johns Hopkins Hospital from January 2005 to June 2011. These dates were chosen because of data availability, in particular the outcome diagnosis. This study was conducted with IRB approval and waived patient consent. We excluded patients who required insertion of a ventricular assist device or patients in whom an aortic anastomosis required deep hypothermic circulatory arrest (<25°C).
We included patients who met several a priori transfusion-related criteria, which were applied to selection of both cases and controls. We only included patients who received a transfusion with red cell units, and the transfusion could not occur after postoperative day 1. We also excluded patients who received >4 red cell units in total. Finally, we included patients only if they had received red cell units that had been stored for ≤14 days exclusively or >14 days exclusively, based on methodology of a previous study.10
We selected cases (patients who developed delirium) and matched controls (patients who did not develop delirium) from the same source population of patients who met the previously described criteria. In order to adjust for strong confounding variables, cases were matched 1:1 with controls by date of surgery (2005–6, 2007–2008, 2009–June 2011), initial type of surgical procedure, and age+/− 5 years. Patients could serve as controls for more than one case, and so there were 80 individual controls for 87 cases.
Sources of Data
Data were obtained from several databases. First, characteristics of blood were determined using TMWare, a software package that is used by the Johns Hopkins Blood Bank to manage blood products. Data were extracted manually from this database for the purpose of this study and then the accuracy of the extraction was checked by another author. Second, patient and surgical characteristics were obtained using the Johns Hopkins-specific data that were included in the Society for Thoracic Surgeons database and were prospectively collected using standard Society for Thoracic Surgeons methods and definitions. Third, data on medications were obtained by retrospective chart review of the medication administration record. Infusions were recorded as the maximum dose, and opioid administration IV or by mouth were considered as morphine equivalents. Finally, neurological outcomes were obtained from a prospective database that has been developed and maintained for the purpose of assessing neurological outcomes after cardiac surgery. The methods for collecting these data and for defining the neurological outcomes in this database are described in the following section. Although data in each of these databases were collected prospectively, none of the utilized databases were established specifically for the purpose of this study.
Outcome Definition
On a daily basis, clinical nurses identified possible cases of neurological problems (stroke, delirium, confusion, agitation, change in mental status, seizure, coma, or slowness to awaken after surgery) in patients after cardiac surgery and communicated this information to a charge nurse or nurse practitioner. Using methodology previously described,6,24 on every day of every patient’s postoperative hospital recovery, the same research coordinator queried the charge nurse (for patients in the intensive care unit [ICU]) and reviewed daily patient progress sheets (prepared by nurse practitioners for patients on the floor) for evidence of any neurologic problems that were communicated to them by the clinical nurses. In the majority of patients, research staff discussed and clarified the type of neurological injury in person with the specific nursing staff, if any neurological injury was reported. Based on the records in this prospectively collected database, delirium was defined for the purposes of this study as the presence of any of the following observations by clinical nurses after clarification by the research coordinator: delirium, confusion, agitation, or change in mental status.6,24 We excluded patients with evidence of stroke, seizure, or coma. Patients who died within the first 2 days after surgery were excluded from analysis because of inadequate opportunity for evaluation.
Blood Management
No standard transfusion algorithm was used, although clinical practice is to minimize red cell unit transfusions in accord with anesthesia and surgical guidelines.25,26 Red-cell units were distributed to patients according to standard blood bank protocol in which the oldest available matching unit was provided for each request. All red cell units were leukoreduced after 2001. Characteristics of red cell units were obtained from the electronic blood bank record, which is used clinically to manage blood bank operations, and data were extracted retrospectively for the purposes of this study.
Statistical Analysis
In our analysis, we considered exposure to red cell units of various storage duration in two main ways. The primary a priori outcome analysis was to consider storage duration as a binary variable and to analyze patients according to whether they received red cell units that had been stored for exclusively ≤14 days or >14 days. Because many patients were transfused with red cell units of various storage duration, the second a priori analysis was to evaluate the average storage time of all red cell units received by a patient and to analyze patients according to the average storage time of all transfused red cell units.
For univariable comparisons of characteristics between patients, categorical data were compared by Chi square test, and continuous variables, which were normally distributed, were compared by t-tests. Univariable and multivariable conditional logistic regression was then used to investigate the association between postoperative delirium and storage duration of transfused red cell units. We considered storage duration as a binary variable (exclusively ≤14 vs. >14 days and average of all units ≤21 days vs. >21 days) or as a continuous variable, with a spline term at 14 days or 21 days. By using a spline term, we could determine if the risk of delirium increased with each day of additional storage after a certain cutoff, without considering the effect of storage age before the cutoff. Before data collection, we chose 14 days as a cutoff using results from published studies.10 We also chose 21 days a priori as a cutoff because of evidence suggesting that storage times longer than 14 days might lead to even more morbidity,27 and to allow comparison of results with the results from the large ongoing, multicenter Red Cell Storage Duration Study (RECESS) which uses a similar storage age cutoff of 21 days.28
The variables included in the final multivariable model either were chosen a priori (age, number of red cell units, cardiopulmonary bypass time, emergency status) or were considered a priori and also confirmed through forward and backward stepwise selection and Akaike information criteria (cerebrovascular disease). Goodness of fit of the models was examined using Hosmer and Lemeshow goodness of fit tests. Transformations or spline terms for continuous variables were considered for inclusion in the model through visual inspection of graphs of the log odds of delirium. Likelihood ratio testing was used to determine the contribution of transformations or spline terms, with the result that the simplest model assuming a linear relationship was chosen. The potential effect of number of red cell units, patient age, and history of cerebrovascular disease on the association between length of red cell unit storage and delirium were all investigated by using interaction terms in multivariable conditional logistic regression models, using the same variables included in the final model.
We also conducted a post hoc sensitivity analysis, after examining the data, in which we changed how we calculated the storage duration of red cell units that a patient received. Instead of using the average age of all transfused red cell units, we used the oldest age of any transfused red cell unit. We then repeated all of the statistical analyses as described above, considering storage duration of the oldest unit of transfused blood.
Stata 12.0 (College Station, TX) was used for statistical analysis. SAS (Cary, NC) was used to create Figure 2.
Figure 2.
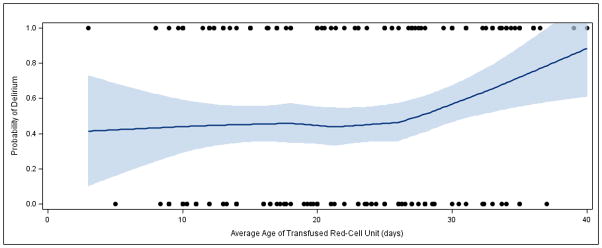
Lowess-smoothed curve showing estimated probability of delirium by average age of transfused red cell units. Dots on the top and bottom of the graph indicate actual values (1 = delirious, 0 = not delirious) in association with age of stored blood.
Results
Patient and Transfusion Characteristics
Of the 5,873 patients who underwent cardiac surgery during the period of study, 1,170 developed a neurologic injury, of which 629 were classified as the delirium type (Figure 1). Eighty-seven patients classified as having developed postoperative delirium met inclusion criteria and were matched 1:1 with 87 controls (with 7 controls being matched with more than 1 patient due to matching with replacement design). Thus, there were 87 case-control pairs comprising 167 individual patients in this study. The median day of delirium onset was on postoperative day 2 with an interquartile range of 1–2 and a range of 1–6. Baseline characteristics of the study patients are shown in Table 1. Patient demographics, comorbidities, and surgical characteristics were generally similar between patients who received red cell units stored ≤14 days and those who received units stored >14 days, and between patients who received red cell units with average storage duration ≤21 days and those who received units with average storage duration >21 days. However, patient age was significantly lower in the group that received red cell units with average storage duration ≤21 days (70.4 ± 9.9 y/o) compared to the group that received red cell units with average storage duration>21 days (73.8 ± 8.5 y/o, p=0.01). Sedative and opioid administration (propofol infusion, fentanyl infusion, midazolam bolus and infusion) were similar (median 0, IQR 0-0) among the delirious and non-delirious patients. Median postoperative morphine equivalents (mg) were also similar on the day before delirium (delirious patients; 6.25 mg, interquartile range [IQR] 0–15 mg vs. nondelirious patients; 10 mg, IQR 2.5–22.5 mg; P=0.12), and on the day of delirium onset (delirious patients; 5 mg, IQR 0–21.5 mg vs. nondelirious patients; 10 mg, IQR 2.5–20 mg, P=0.16).
Figure 1.
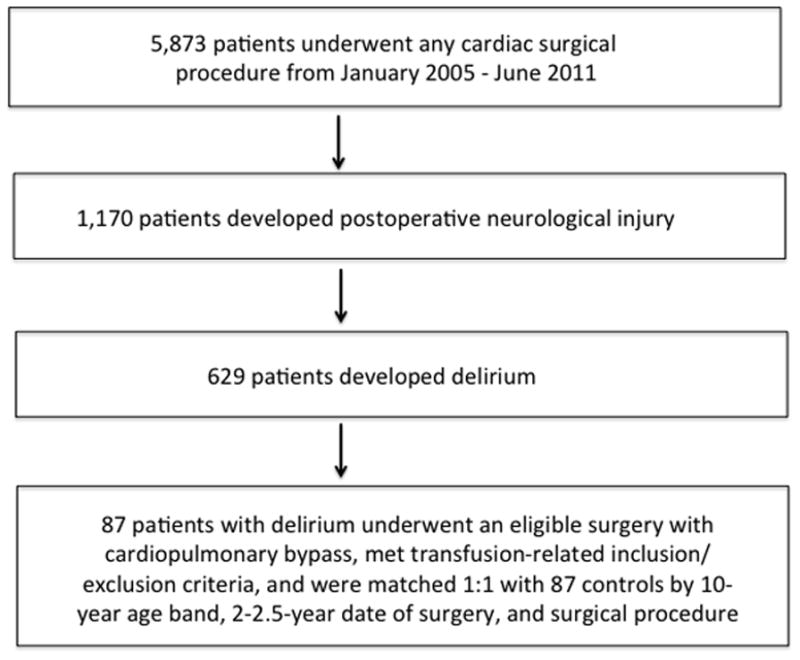
Eligible surgery: coronary artery bypass, valve, combined bypass/valve, or ascending aorta. Transfusion-related inclusion criteria: Transfusion with red cell units exclusively greater than or less than 14 days old. Exclusion criteria: transfusion after postoperative day 1, transfusion with >4 red cell units.
Table 1.
Baseline characteristics (using storage duration cutoffs of 14 days and 21 days)
| Transfused Blood with Storage Duration ≤14 Days vs. >14 Days |
Transfused Blood with Average Storage Duration ≤21 Days vs. >21 Days |
All Patients (N = 174) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| ≤14 Days (N = 45) | >14 Days (N = 129) | P Value | ≤21 Days (N = 85) | >21 Days (N = 89) | P Value | ||
| Demographics | |||||||
| Age (years; mean±SD) | 71.8±9.0 | 72.3±9.5 | 0.76 | 70.4±9.9 | 73.8±8.5 | 0.01 | 72.1±9. 3 |
| Male sex (%) | 62.2 | 58.9 | 0.70 | 62.4 | 57.3 | 0.50 | 59.8 |
| Race (%) | 0.3 | 0.27 | |||||
| White | 81.1 | 89.1 | 82.8 | 92.0 | 85.5 | ||
| Black | 18.9 | 10.9 | 17.2 | 8.0 | 14.5 | ||
| Transfused blood | |||||||
| Storage duration (days; mean±SD) | 11.0±2.3 | 25.7±6.5 | <0.001 | 14.3±4.2 | 29.1±4.6 | <0.001 | 21.9±8. 6 |
| No. of red cell units transfused per patient (%) | 0.06 | 0.30 | |||||
| 1 | 17.8 | 24.0 | 20.0 | 24.7 | 22.4 | ||
| 2 | 17.8 | 32.6 | 25.9 | 31.5 | 28.7 | ||
| 3 | 40.0 | 21.7 | 32.9 | 20.2 | 26.4 | ||
| 4 | 24.4 | 21.7 | 21.2 | 23.6 | 22.4 | ||
| Clinical features | |||||||
| Cerebrovascular diseasea (%) | 8.9 | 14.7 | 0.32 | 12.9 | 13.5 | 0.92 | 13.2 |
| Diabetesb (%) | 35.6 | 32.6 | 0.71 | 34.1 | 32.6 | 0.83 | 33.3 |
| NYHA class (%) | 0.73 | 0.07 | |||||
| I | 5.0 | 1.6 | 4.7 | 0 | 2.9 | ||
| II | 22.5 | 26.6 | 31.3 | 15.0 | 25.0 | ||
| III | 57.5 | 59.4 | 54.7 | 65.0 | 58.7 | ||
| IV | 15.0 | 12.5 | 9.4 | 20.0 | 13.5 | ||
| Surgical characteristics | |||||||
| CPB time (minutes; mean± SD) | 116.4±27.7 | 106.6±35.2 | 0.09 | 112.3±30.3 | 106.2±36.5 | 0.23 | 109.1± 33.7 |
| Surgery type (%) | 0.19 | 0.32 | |||||
| CAB | 37.8 | 55.0 | 44.7 | 56.2 | 50.6 | ||
| Valve | 31.1 | 26.4 | 28.2 | 27.0 | 27.6 | ||
| CAB + valve | 22.2 | 12.4 | 17.7 | 12.4 | 14.9 | ||
| Aortic | 8.9 | 6.2 | 9.4 | 4.5 | 6.9 | ||
| Emergency status (%) | 0.58 | 0.14 | |||||
| Elective | 55.6 | 55.8 | 52.9 | 58.4 | 55.8 | ||
| Urgent | 44.4 | 41.9 | 47.1 | 38.2 | 42.5 | ||
| Emergent | 0 | 2.33 | 0 | 3.4 | 1.7 | ||
| Lowest intraoperative hemoglobin (g/dL) | 8.16 | 8.20 | 0.81 | 8.26 | 8.12 | 0.28 | 8.19 |
NYHA indicates New York Heart Association; CPB, cardiopulmonary bypass; CAB, coronary artery bypass.
P values for categorical variables (number of red cell units transfused, NYHA class, surgery type, and emergency status) represent likelihood ratio testing for overall difference between groups)
Cerebrovascular disease is defined by history of stroke with deficit > 72 hours, transient ischemic attack with deficit < 24 hours, or unresponsive coma > 24 hours.
Diabetes refers to any history of diabetes regardless of length of disease or treatment.
In the study cohort, 433 red cell units were transfused. The range of storage duration was 3–40 days, and the average storage time was 21.9 ± 8.6 days. The average overall number of units per patient was 2.49 ± 1.07 (range, 1–4 units). For the 45 patients who received red cell units with exclusive storage time ≤14 days, the average storage time was 11.0 ± 2.3 days, and for the 129 patients who received red cell units with an exclusive storage age >14 days, the average storage time was 25.7 ± 6.5 days (P<0.001 vs. <14 day group). For the 85 patients who received red cell units with an average storage time ≤21 days, the average storage time was 14.3 ± 4.2 days, and for the 89 patients who received red cell units with an average storage age >21 days, the average storage time was 29.1 ± 4.6 days (P<0.001 vs. ≤21 day group).
Primary Analysis
Our primary analysis compared delirium in patients using a cutoff of 14 days for the storage of all transfused red cell units (Table 2). In univariable analysis, we found no significant difference in odds of delirium for patients who exclusively received red cell units stored >14 days compared to those patients who received all units stored ≤14 days (OR 1.88; 95%CI, 0.79–4.42; P=0.15). Similarly, after adjusting for potential confounders in multivariable analysis, we found no significant difference in the odds of delirium for patients who exclusively received red cell units stored >14 days compared to those patients who received units stored ≤14 days (OR 1.83; 95%CI, 0.73–4.58; P=0.20).
Table 2.
Odds of postoperative delirium for patients receiving red cell units stored for exclusively >14 days vs. ≤14 days
| Crude | Adjusted a | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Length of red cell unit storageb | ||||||
| ≤14 days | 1.0 | REFc | 1.0 | REFc | ||
| >14 days | 1.88 | 0.79–4.42 | 0.15 | 1.83 | 0.73–4.58 | 0.20 |
| Age | 0.99 | 0.88–1.12 | 0.88 | 1.00 | 0.87–1.15 | 0.99 |
| Cerebrovascular disease | 2.4 | 0.85–6.8 | 0.1 | 2.38 | 0.79–7.16 | 0.12 |
| Bypass time | 1.0 | 0.99–1.01 | 0.90 | 1.00 | 0.99–1.02 | 0.68 |
| Urgent or emergent status | 1.25 | 0.70–2.25 | 0.46 | 1.36 | 0.71–2.64 | 0.36 |
| No. of red cell units transfused | 0.40 | 0.43 | ||||
| 1 | 1.0 | REFc | 1.0 | REFc | ||
| 2 | 0.58 | 0.25–1.33 | 0.60 | 0.25–1.43 | ||
| 3 | 0.60 | 0.26–1.36 | 0.61 | 0.25–1.45 | ||
| 4 | 0.45 | 0.17–1.18 | 0.42 | 0.15–1.21 | ||
Variables in the adjusted model include average length of red cell unit storage, age, baseline cerebrovascular disease, bypass time, emergent status, and number of red cell units transfused.
Patients received red cell units that were stored for exclusively ≤14 days or >14 days
In the Length of red cell unit storage category, “REF” refers to the comparison group for odds ratio of delirium among patients transfused with red cell units with storage age of exclusively >14 days. In the No. of red cell units transfused category, “REF” refers to the comparison group for odds ratio of delirium among patients transfused with >1 red cell unit.
Secondary Analyses
We next determined the risk of delirium with each additional day of average storage duration of red cell units, based on our hypothesis that longer duration of storage would be associated with increased odds of delirium (Table 3). Based on the results of prior studies, we prespecified 14 days and 21 days of average red cell unit storage as important cutoffs to examine, after which we hypothesized the risk of delirium would increase. In multivariable analysis, we found that each additional day of average red cell unit storage beyond 14 days increased the odds of delirium in patients by an estimated 1.01–1.13 fold (OR, 1.07; P=0.03). Similarly, in multivariable analysis, we found that each additional day of average storage beyond 21 days increased the odds of delirium by an estimated 1.02–1.23 fold (OR, 1.12; P=0.02). The increase in the risk of delirium with increasing red cell unit storage is shown graphically in Figure 2. In this figure, we use nonparametric modeling to demonstrate that the risk of delirium increases in a linear manner after approximately 21 days of red cell unit storage. We also examined the importance of the 21-day cutoff by examining whether the risk of delirium increased when the average storage time of all transfused units was >21 days vs. ≤21 days. In univariable models, we found no increased risk (OR. 1.69; 95%CI, 0.85–3.36; P=0.13), while in multivariable models, we found that the odds of delirium was an estimated 1.01–4.97 fold higher (OR, 2.24; P=0.048) for patients receiving red cell units that had been stored for an average of >21 days vs. ≤21 days. However, the confidence intervals were wide with a P value that was only slightly less than 0.05, and thus these results should be interpreted cautiously.
Table 3.
Odds of postoperative delirium for each day of additional red cell unit storage
| Crude | Adjusteda | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| ORb | 95% CI | P value | ORb | 95% CI | P value | |
| Average length of red cell unit storage | ||||||
| <14 days | 0.99 | 0.79–1.24 | 0.95 | 0.99 | 0.77–1.27 | 0.94 |
| >14 days | 1.06 | 1.00–1.12 | 0.04 | 1.07 | 1.01–1.13 | 0.03 |
| Average length of red cell unit storage | ||||||
| <21 days | 0.98 | 0.89–1.09 | 0.75 | 0.98 | 0.88–1.09 | 0.70 |
| >21 days | 1.10 | 1.01–1.20 | 0.02 | 1.12 | 1.02–1.23 | 0.02 |
Variables in the adjusted model include average length of red cell unit storage, age, baseline cerebrovascular disease, bypass time, emergent status, and number of red cell units transfused.
Odds ratio represents the increasing odds of delirium for each day of additional average red cell unit storage duration from 0–14 days (<14 day category) and from 14–42 days (>14 day category), or from 0–21 days (<21 day category) and from 21–42 days (>21 day category)
Sensitivity Analyses and Interactions
The aforementioned analyses regarding the effects of each additional day of storage used the average storage duration of all units that a patient received, but it is not clear whether the average storage duration or the storage duration of the oldest unit contributes more to posttransfusion morbidity. We conducted additional analyses post hoc using the oldest red cell unit that a patient received. In multivariable analysis, we found that for the oldest red cell unit, each additional day of storage beyond 14 days increased the odds of delirium in patients by 1.01–1.12 fold (OR, 1.06; P=0.02). Similarly, in multivariable analysis, we found that for the oldest red cell unit, each additional day of storage beyond 21 days increased the odds of delirium in patients by 1.03–1.20 fold (OR, 1.11; P=0.01)
When multivariable analysis was used to assess other risk factors for delirium (Table 2), there was no difference in the odds of delirium among patients with a history of cerebrovascular disease compared to no cerebrovascular disease (OR, 2.4; 95% CI, 0.79–7.16; P=0.12). No other patient comorbidity was significant, and no interaction between the storage age of blood and either the number of units transfused (P interaction= 0.59) or history of cerebrovascular disease (P interaction= 0.38) was found.
Discussion
The results of this study demonstrate that transfusion of red cell units with an exclusive storage age >14 days does not increase the odds of a patient developing delirium after cardiac surgery, the primary outcome of this study. However, for each day of average red cell unit storage duration more than 14 days or 21 days (prespecified secondary outcomes), there is a 1.01–1.13 fold and 1.02–1.23 fold respective increase in the odds of developing postoperative delirium. Of note, more than half of red cell units transfused into cardiac surgery patients at Johns Hopkins in 2011 had been stored for >21 days.
There are several explanations for the discrepancy between the results of our primary analysis (odds of delirium in patients receiving all red cell units stored for >14 days compared to ≤14 days) and our secondary analysis (the effect of each additional day of storage after 14 or 21 days). First, visual inspection of Figure 2 demonstrates a linear association between probability of delirium and storage age of red cell units, and statistical models that examine the effect of each additional day of storage would better capture this linear association. Second, the detrimental effects of red cell unit storage may only be evident after long storage duration, and the secondary analyses account for the fact that longer storage might lead to different biologic effects.
Our findings are consistent with mixed results in the literature. Several retrospective studies have shown an association between increased storage age of red cell units and postoperative morbidity, including renal failure,10 multiorgan failure,12 infections,27 and mortality.10,29 However, other retrospective studies have demonstrated no association between length of storage of red cell units and postoperative outcomes, including 30-day mortality,14 hospital and ICU length of stay,13,14 and morbidity15 (renal failures, pneumonia). These studies (and our study) are all generally limited by retrospective observational design, single-center data, and different definitions or cutoffs for storage age of red cell units. Because of the observational design of these studies, residual confounding may be an alternative explanation for the observed association.30 The few randomized trials have been limited by small size31 or restrictive transfusion criteria.32 Recently, the large multicenter ARIPI trial33 demonstrated that neonates randomized to transfusion of red cell units < 7 days old compared to usual practice experienced no difference in the composite outcome of major neonatal morbidity. Several large multicenter randomized trials are currently enrolling adult patients, including RECESS28 (cardiac surgery patients; NCT00991341), Canadian ABLE34 (ICU patients; ISRCTN44878718) and TRANSFUSE (critically ill patients in Australia and New Zealand; NCT01638416). However, to our knowledge, delirium has not been examined as an outcome in either observational trials or in the prospective randomized trials.
The mechanisms by which increased red cell unit storage duration may increase the incidence of complications, particularly delirium, are not known but may be related to reduced oxygen delivery by stored RBCs. The brain is exquisitely sensitive to adequate oxygen delivery, and reduced oxygen delivery during the perioperative period may impair neuronal function, through such mechanisms as alternation in neurotransmitters.35 Increased storage of red cell units may lead to reduced oxygen delivery in several ways. First, increased storage time causes morphologic changes in RBCs that result in osmotic fragility and reduced deformability,9 and subsequent decreased RBC microcirculation. Second, RBC metabolism during storage leads to decreased pH and degradation of 2,3-DPG, which causes an additional decrement in release of oxygen from hemoglobin at the tissue, although the 2-3-DPG has been shown to regenerate quickly. 9,36 Finally, through S-nitrosylation of hemoglobin and subsequent changes in local nitric oxide activity, RBCs may modulate vasodilation to enhance peripheral oxygen delivery. Storage of red cell units depletes S-nitrosylated hemoglobin and causes reduction in the ability of RBCs to induce vasodilation and augment oxygen delivery.18 During a period of hemodynamic instability, increased inflammation, and increased metabolic demand, reduced oxygen delivery may impair normal cerebral function. Indeed, acute reduction of hemoglobin concentration in healthy volunteers has been shown to decrease aspects of cognitive functioning in controlled experiments.37 Using an experimental paradigm of isovolemic hemoglobin reduction, subsequent experiments further demonstrated that either supplemental oxygen38 or administration of red cell units that had been stored for > 21 days39 were able to restore cerebral function, thus implying that stored red blood cells might be able to deliver adequate oxygen. However, the population studied was limited to healthy volunteers, with presumably little cerebrovascular disease, and no inflammatory response from surgery, so the vulnerability to decrements in oxygen delivery might be different in healthy patients compared to the postcardiac surgery population.
There are several limitations to this study. First, a significant limitation was the lack of a uniform bedside tool to assess delirium. Instead, clinical nurses reported neurological injuries (including delirium, confusion, agitation, and change in mental status) to research coordinators, and the presence of any of these characteristics was considered evidence of delirium. Further, clinical nurses did not rely on a formal algorithm to define these neurological injuries and instead relied on clinical experience. Nurse identification of delirium has been shown to have low sensitivity and high specificity and often under-recognizes hypoactive delirium.40 Thus, we may not have recognized all patients with delirium, consistent with the overall low rate of delirium in our population, and our results might apply only to patients with hyperactive delirium, not hypoactive delirium. However, because nurses who identified delirium were not aware of the storage age of red cell units, we do not believe that our results are biased. In addition, clinical nurses cared for their patients over 8–12 hour shifts and so their judgment about cognitive status of their patients incorporated continuing assessments, which are important to diagnose a disease characterized by waxing and waning of symptoms. Further, patients were monitored during every day of their hospitalization, an approach that provides better sensitivity for delirium detection than one-time assessment, and the data on delirium assessments were collected prospectively using consistent methodology. Regarding transfusion, there was no standardized transfusion protocol to guide red cell unit administration. However, providers were unaware of the storage age of red cell units before administration, and we accounted for temporal trends in red cell unit management by matching cases and controls by band of surgery date. It is also noteworthy that our study population does not reflect the general cardiac surgery population, since we limited the study population in order to reduce bias in our analyses. We only examined transfused patients in order to reduce confounding if sicker patients were more likely to receive a transfusion and develop delirium. We also excluded patients who were transfused with red cell units after postoperative day 1, thereby ensuring that the transfusion of red cell units temporally preceded episodes of delirium. These exclusions may limit the generalizability of our results.
Results from this study demonstrate that transfusion of red cell units that have been stored for more than 14 days is not associated with increased odds of delirium. However, each day of additional storage of red cell units beyond 14 days or 21 days may be associated with increased odds of postoperative delirium in patients undergoing cardiac surgery. The observational design and retrospective analysis are limitations of this study. Thus, more research is needed to further characterize the risk of delirium based on storage age of transfused red cell units.
Acknowledgments
Funding: NIH KL-2 Clinical Research Scholars Program, NIH (RO3 AG042331), and the Jahnigen Career Development Award (CB); Charles A. Dana Foundation, New York, NY, American Society of Hematology Scholar Award (WS); Mid-Atlantic Affiliate of the American Heart Association and the NIH (RO1 HL092259) (CH), and the American Heart Association Scientist Development Grant (RG).
Footnotes
Reprints will not be available from the authors.
DISCLOSURES:
Name: Charles H. Brown IV, MD MHS
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Attestation: Charles H. Brown IV has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files
Conflicts of Interest: The author has no conflicts of interest to declare.
Name: Maura Grega, MSN
Contribution: This author helped conduct the study and write the manuscript
Attestation: Maura Grega approved the final manuscript
Conflicts of Interest: The author has no conflicts of interest to declare.
Name: Ola A Selnes, PhD
Contribution: This author helped write the manuscript
Attestation: Ola A Selnes approved the final manuscript
Conflicts of Interest: The author has no conflicts of interest to declare.
Name: Guy M. McKhann, MD
Contribution: This author helped write the manuscript
Attestation: Guy M. McKhann approved the final manuscript
Conflicts of Interest: The author has no conflicts of interest to declare.
Name: Ashish S. Shah, MD
Contribution: This author helped write the manuscript
Attestation: Ashish S. Shah approved the final manuscript
Conflicts of Interest: The author has no conflicts of interest to declare.
Name: Andrew LaFlam, BS
Contribution: This author helped conduct the study and write the manuscript
Attestation: Andrew LaFlam approved the final manuscript
Conflicts of Interest: The author has no conflicts of interest to declare.
Name: Steven M. Frank, MD
Contribution: This author helped write the manuscript
Attestation: Steven M. Frank approved the final manuscript
Conflicts of Interest: Steven M. Frank consulted for Haemonetics and consulted for CSL Behring I have consulted for the above companies, but there is no conflict of interest for this manuscript
Name: William J. Savage, MD, PhD
Contribution: This author helped conduct the study and write the manuscript
Attestation: William J. Savage approved the final manuscript
Conflicts of Interest: The author has no conflicts of interest to declare.
Name: Charles W. Hogue, MD
Contribution: This author helped write the manuscript
Attestation: Charles W. Hogue approved the final manuscript
Conflicts of Interest: Charles W. Hogue received research funding from Covidien, consulted for Ornim, and consulted for Merck Dr. Hogue has served as an advisor to Ornim and Merck. There is no conflict of interest with this manuscript.
Name: Rebecca F. Gottesman, MD, PhD
Contribution: This author helped design the study, analyze the data, and write the manuscript
Attestation: Rebecca F. Gottesman has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Conflicts of Interest: The author has no conflicts of interest to declare.
Contributor Information
Charles H. Brown, IV, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins Medical Institutions, Baltimore, Maryland.
Maura Grega, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins Medical Institutions, Baltimore, Maryland.
Ola A Selnes, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins Medical Institutions, Baltimore, Maryland.
Guy M. McKhann, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins Medical Institutions, Baltimore, Maryland.
Ashish S. Shah, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins Medical Institutions, Baltimore, Maryland.
Andrew LaFlam, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins Medical Institutions, Baltimore, Maryland.
Steven M. Frank, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins Medical Institutions, Baltimore, Maryland.
William J. Savage, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins Medical Institutions, Baltimore, Maryland (Current Affiliation: Department of Transfusion Medicine, Brigham and Women’s Hospital, Boston, Massachusetts).
Charles W. Hogue, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins Medical Institutions, Baltimore, Maryland.
Rebecca F. Gottesman, Department of Anesthesiology and Critical Care Medicine, Johns Hopkins Medical Institutions, Baltimore, Maryland.
References
- 1.Rudolph JL, Jones RN, Levkoff SE, Rockett CR, Inouye SK, Sellke FW, Khuri SF, Lipsitz LA, Ramlawi B, Levitsky S, Marcantonio ER. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119(2):229–236. doi: 10.1161/CIRCULATIONAHA.108.795260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, Jones RN. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30–39. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudolph JL, Marcantonio ER, Culley DJ, Silverstein JH, Rasmussen LS, Crosby GJ, Inouye SK. Delirium is associated with early postoperative cognitive dysfunction. Anaesthesia. 2008;63(9):941–947. doi: 10.1111/j.1365-2044.2008.05523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray A, Levkoff S, Wetle T, Beckett L, Cleary PD, Schor JD, Lipsitz LA, Rowe JW, Evans DA. Acute delirium and functional decline in the hospitalized elderly patient. J Gerontol. 1993;48(5):M181–186. doi: 10.1093/geronj/48.5.m181. [DOI] [PubMed] [Google Scholar]
- 5.Rudolph JL, Inouye SK, Jones RN, Yang FM, Fong TG, Levkoff SE, Marcantonio ER. Delirium: An Independent Predictor of Functional Decline After Cardiac Surgery. J Am Geriatr Soc. 2010;58(4):643–649. doi: 10.1111/j.1532-5415.2010.02762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gottesman R, Grega M, Bailey M, Pham LD, Zeger SL, Baumgartner WA, Selnes OA, McKhann GM. Delirium after coronary artery bypass graft surgery and late mortality. Ann Neurol. 2010;67(3):338–344. doi: 10.1002/ana.21899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr, Inouye SK, Bernard GR, Dittus RS. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 8.Marcantonio ER, Goldman L, Orav EJ, Cook EF, Lee TH. The association of intraoperative factors with the development of postoperative delirium. Am J Med. 1998;105(5):380–384. doi: 10.1016/s0002-9343(98)00292-7. [DOI] [PubMed] [Google Scholar]
- 9.Zubair A. Clinical impact of blood storage lesions. Am J Hematol. 2010;85(2):117–122. doi: 10.1002/ajh.21599. [DOI] [PubMed] [Google Scholar]
- 10.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red cell storage and complications after cardiac surgery. N Engl J Med. 2008;358(12):1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 11.Mynster T, Nielsen HJ. Storage time of transfused blood and disease recurrence after colorectal cancer surgery. Dis Colon Rectum. 2001;44:955–964. doi: 10.1007/BF02235483. [DOI] [PubMed] [Google Scholar]
- 12.Zallen G, Offner PJ, Moore EE, Blackwell J, Ciesla DJ, Gabriel J, Denny C, Silliman CC. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570–572. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 13.Vamvakas EC, Carven JH. Length of storage of transfused red cells and postoperative morbidity in patients undergoing coronary artery bypass graft surgery. Transfusion. 2000;40:101–109. doi: 10.1046/j.1537-2995.2000.40010101.x. [DOI] [PubMed] [Google Scholar]
- 14.Van De Watering L, Lorinser J, Versteegh M, Westendord R, Brand A. Effects of storage time of red blood cell transfusions on the prognosis of coronary artery bypass graft patients. Transfusion. 2006;46:1712–1718. doi: 10.1111/j.1537-2995.2006.00958.x. [DOI] [PubMed] [Google Scholar]
- 15.Yap CH, Lau L, Krishnaswamy M, Gaskel M, Yii M. Age of transfused red cells and early outcomes after cardiac surgery. Ann Thorac Surg. 2008;86:554–559. doi: 10.1016/j.athoracsur.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 16.Sessler DI, Kurz A. Erythrocyte Storage Duration Is Not Associated with Increased Mortality in Noncardiac Surgical Patients. Anesthesiology. 2013;118:151–58. doi: 10.1097/ALN.0b013e3182746ba4. [DOI] [PubMed] [Google Scholar]
- 17.Valeri CR, Collins FB. The physiologic effect of transfusing preserved red cells with low 2,3-diphosphoglycerate and high affinity for oxygen. Vox Sang. 1971;20(5):397–403. doi: 10.1111/j.1423-0410.1971.tb01807.x. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds JD, Hess DT, Stamler JS. The transfusion problem: role of aberrant S-nitrosylation. Transfusion. 2011;15;51(4):852–8. doi: 10.1111/j.1537-2995.2011.03097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lelubre C, Piagnerelli M, Vincent JL. Association between duration of storage of transfused red blood cells and morbidity and mortality in adult patients: myth or reality? Transfusion. 2009;49:1384–1394. doi: 10.1111/j.1537-2995.2009.02211.x. [DOI] [PubMed] [Google Scholar]
- 20.Joosten E, Lemiengre J, Nelis T, Verbeke G, Milisen K. Is Anaemia a Risk Factor for Delirium in an Acute Geriatric Population? Gerontology. 2006;52(6):382–5. doi: 10.1159/000095126. [DOI] [PubMed] [Google Scholar]
- 21.Aldemir M, Ozen S, Kara IH, Sir A, Baç B. Predisposing factors for delirium in the surgical intensive care unit. Crit Care. 2001;5(5):265–70. doi: 10.1186/cc1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siepe M, Pfeiffer T, Gieringer A, Gieringer A, Zemann S, Benk C, Schlensak C, Beyersdorf F. Increased systemic perfusion pressure during cardiopulmonary bypass is associated with less early postoperative cognitive dysfunction and delirium. Eur J Cardiothorac Surg. 2011;40(1):200–7. doi: 10.1016/j.ejcts.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Schoen J, Meyerrose J, Paarmann H, Heringlake M, Hueppe M, Berger K-U. Preoperative regional cerebral oxygen saturation is a predictor of postoperative delirium in on-pump cardiac surgery patients: a prospective observational trial. Crit Care. 2011;15(5):R218. doi: 10.1186/cc10454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mckhann GM, Grega MA, Borowicz LM, Bechamps M, Selnes OA, Baumgartner WA, Royall RM. Encephalopathy and stroke after coronary artery bypass grafting: incidence, consequences, and prediction. Arch Neurol. 2002;59:1422–1428. doi: 10.1001/archneur.59.9.1422. [DOI] [PubMed] [Google Scholar]
- 25.Nuttall GA, Brost BC, Connis RT, Gessner JS, Harrison CR, Miller RD, Nickinovich DG, Nussmeier NA, Rosenberg AD, Spence R. Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105(1):198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 26.Ferraris VA, Ferraris SP, Saha SP, Hessel EA, Haan CK, Royston D, Bridges CR, Higgins RSD, Despotis G, Brown JR, Spiess BD, Shore-Lesserson L, Stafford-Smith M, Mazer CD, Bennett-Guerrero E, Hill SE, Body S Society of Thoracic Surgeons Blood Conservation Guideline Task Force, The Society of Cardiovascular Anesthesiologists Special Task Force on Blood Transfusion. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007;83(5 Suppl):S27–86. doi: 10.1016/j.athoracsur.2007.02.099. [DOI] [PubMed] [Google Scholar]
- 27.Vamvakas ECE, Carven JHJ. Transfusion and postoperative pneumonia in coronary artery bypass graft surgery: effect of the length of storage of transfused red cells. Transfusion. 1999;39:701–710. doi: 10.1046/j.1537-2995.1999.39070701.x. [DOI] [PubMed] [Google Scholar]
- 28.Steiner ME, Assmann SF, Levy JH, Marshall J, Pulkrabek S, Sloan SR, Triulzi D, Stowell CP. Addressing the question of the effect of RBC storage on clinical outcomes: The Red Cell Storage Duration Study (RECESS) (Section 7) Transfus Apher Sci. 2010;43:107–116. doi: 10.1016/j.transci.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Purdy FR, Tweeddale MG, Merrick PM. Association of mortality with age of blood transfused in septic ICU patients. Can J Anaesth. 1997;44(12):1256–1261. doi: 10.1007/BF03012772. [DOI] [PubMed] [Google Scholar]
- 30.Edgren G, Kamper-Jørgensen M, Eloranta S, Rostgaard K, Custer B, Ullum H, Murphy EL, Busch MP, Reilly M, Melbye M, Hjalgrim H, Nyrén O. Duration of red blood cell storage and survival of transfused patients. Transfusion. 2010;50:1185–1195. doi: 10.1111/j.1537-2995.2010.02583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hebert PC, Chin-Yee I, Fergusson D, Blajchman M, Martineau R, Clinch J, Olberg B. A pilot trial evaluating the clinical effects of prolonged storage of red cells. Anesth Analg. 2005;100:1433–8. doi: 10.1213/01.ANE.0000148690.48803.27. [DOI] [PubMed] [Google Scholar]
- 32.Wasser M, Houbiers JGA, D’Amaro J, Hermans J, Huysmans HA, Kinijnenburg GCV, Brand A. The effect of fresh versus stored blood on post-operative bleeding after coronary bypass surgery: a prospective randomized study. Br J Haematol. 1989;72:81–84. doi: 10.1111/j.1365-2141.1989.tb07656.x. [DOI] [PubMed] [Google Scholar]
- 33.Fergusson DA, Hébert P, Hogan DL, LeBel L, Rouvinez-Bouali N, Smyth JA, Sankaran K, Tinmouth A, Blajchman MA, Kovacs L, Lachance C, Lee S, Walker CR, Hutton B, Ducharme R, Balchin K, Ramsay T, Ford JC, Kakadekar A, Ramesh K, Shapiro S. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA. 2012;308:1443–1451. doi: 10.1001/2012.jama.11953. [DOI] [PubMed] [Google Scholar]
- 34.Lacroix J, Hébert P, Fergusson D, Tinmouth A, Blajchman MA, Callum J, Cook D, Marshall JC, McIntyre L, Turgeon AF for the ABLE study group. The Age of Blood Evaluation (ABLE) Randomized Controlled Trial: Study Design. Transfus Med Rev. 2011;25:197–205. doi: 10.1016/j.tmrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Maldonado J. Pathoetiological Model of Delirium: a Comprehensive Understanding of the Neurobiology of Delirium and an Evidence-Based Approach to Prevention and Treatment. Crit Care Clin. 2008;24:789–856. doi: 10.1016/j.ccc.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Beutler E, Wood L. The in vivo regeneration of red cell 2,3 diphosphoglyceric acid (DPG) after transfusion of stored blood. J Lab Clin Med. 1969;74:300–304. [PubMed] [Google Scholar]
- 37.Weiskopf RB, Kramer JH, Viele M, Neumann M, Feiner J, Watson J, Hopf H, Toy P. Acute severe isovolemic anemia impairs cognitive function and memory in humans. Anesthesiology. 2000;92(6):1646–52. doi: 10.1097/00000542-200006000-00023. [DOI] [PubMed] [Google Scholar]
- 38.Weiskopf RB, Feiner J, Hopf HW, Viele MK, Watson JJ, Kramer JH, Ho R, Toy P. Oxygen reverses deficits of cognitive function and memory and increased heart rate induced by acute severe isovolemic anemia. Anesthesiology. 2002;96(4):871–7. doi: 10.1097/00000542-200204000-00014. [DOI] [PubMed] [Google Scholar]
- 39.Weiskopf RB, Feiner J, Hopf H, Lieberman J, Finlay HE, Quah C, Kramer JH, Bostrom A, Toy P. Fresh blood and aged stored blood are equally efficacious in immediately reversing anemia-induced brain oxygenation deficits in humans. Anesthesiology. 2006;104:911–920. doi: 10.1097/00000542-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Inouye SK, Foreman MD, Mion LC, Katz KH, Cooney LM. Nurses’ recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med. 2001;161:2467–2473. doi: 10.1001/archinte.161.20.2467. [DOI] [PubMed] [Google Scholar]


