Abstract
Tick borne diseases continue to emerge and have a great impact on public health and agriculture. In addition, many of the tick borne diseases agents, which are classified as Biosafety Level 4 (BSL-4) viruses, have the potential to be used as biothreat agents. In spite of the known importance of these pathogens, there is an acute shortage of facilities and trained personnel to study the pathogenesis of tick-borne diseases and to assess vaccine as well as other therapeutic interventions against tick-borne diseases as they are transmitted in nature. We, at the Galveston National Laboratory, have developed facilities and protocols to safely work with BSL4 virus infected ticks. This capability adds tremendous value to the Nation's training and research endeavors. In this report we describe the procedures and protocols to establish tick work in a BSL4 laboratory.
Introduction
Numerous pathogens responsible for both human and domestic animal diseases are transmitted by ticks. Many of these pathogens have emerged and/or reemerged over the past 40 years and have a great impact on public health and agriculture. In addition, many of these agents have the potential to be used as bioterrorism agents. Significantly, many of the NIAID category A-C agents are transmitted to humans by tick vectors. For example, a number of Biosafety Level 4 (BSL4) viruses including Crimean-Congo hemorrhagic fever virus, Kyasanur Forest disease virus, Alkhumra hemorrhagic fever virus, and Omsk hemorrhagic fever virus are transmitted by ticks. In nature, ticks not only transmit these viruses but also become persistently infected and therefore also serve as a reservoir. In order to accurately model the transmission, perpetuation of the virus through life stages, and early aspects of the pathogenesis as seen in nature, work with the live ticks as well as a tick-host transmission model is required. Therefore, protocols need to be in place to safely work with ticks on and off their host in a BSL4 laboratory. Herein, the biggest concern lies in the accidental release of a tick. There are no clear guidelines to work with ticks at BSL4, most likely due to lack of suitable facilities and comprehensive experience, as well as the extensive amount of approvals from regulatory bodies required. Hitherto, the Galveston National Laboratory (GNL) Insectaries Services core (GIS) is the only academic facility in the world that currently conducts tick work at BSL4. In this report we discuss the standard operating procedures or guidelines that we had developed for tick work in our BSL4 laboratory. These procedures were developed based on current arthropod containment guidelines (2003; Scott, 2005; Tabachnick, 2006; Prevention, 2009) and our experience working with ticks (Bouchard and Wikel, 2004).
Facility and Equipment Requirements
BSL4 laboratories can be categorized in suit laboratories and glove box laboratories. The structural requirements to contain ticks are fundamentally very different between the two types of laboratories. Here, we will only discuss the requirements for suit labs. The Central responsibility for working with ticks in a BSL4 laboratory is to ensure that at all times all ticks are safely contained and do not escape. This is both a biosafety issue, and a biocontainment issue. For tick work, a suite within the BSL4 should be dedicated with equipment or supplies only meant for tick research studies. Arthropod security is based on multiple layers of containment. For the storage of ticks these are: 1 = storage vials, 2 = humidified desiccator, 3 = environmental chamber, 4 = tick suite, 5 = BSL4 laboratory. Non-ventilated plexi-glass glove boxes with microscope-video outputs are recommended while manipulating ticks outside of the primary containment (storage vials). Feeding, inoculation, and dissection can all be performed within this, but appropriate training and practice is required to develop the skills to work within the box while wearing a positive pressure suit.
The tick suite should have minimal equipment and supplies to reduce potential hiding places for a loose arthropod. Sticky pads and Vaseline barriers should be placed in front of the door and within the non-ventilated plexi-glass glove box. Experimental animals should be housed within this suite in individually ventilated micro-isolator cages. No glass is allowed and items that contain the ticks such as desiccators and vials have to be made out of plastic that does not easily break. The work area should be free of clutter, and all work surfaces should be painted white.
All work involving infectious material should be in strict compliance with Environmental Health and Safety, Biosafety Committee and Institutional Animal Care and Use Committee approved guidelines and protocols. All personnel involved in tick studies at BSL4 must first complete all institutional required biosafety training (BSL2 –BSL4), animal training (ABSL2- ABSL4), and tick containment training. Only if a person has demonstrated a significant amount of independent training hours will they be allowed to work with ticks at BSL4.
Personal Protective Equipment
Personnel working in the tick-BSL4 suite are required to don a white Delta suit (Honeywell Safety, France) and not a blue Dover suit (ILC Dover, Delaware) to allow easy visualization if a tick crawls on the suit while working. Since all institutionally approved suit (outer) gloves are colored, all personnel should also don an extra pair of white gloves over the regular outer suit gloves to ensure easy visualization of ticks. These gloves should also have long cuffs that reach over the duct-taped cuff area of the suit in order to avoid the possibility that ticks can hide in duct tape crevasses. Double sided sticky tape will be taped at the end of the cuff as a barrier to the ticks. This will ensure that no ticks will get under the crevices of the duct tape on the cuffs of the glove. These white gloves are removed when leaving the tick room and will be inspected before discarding. It is recommended to use magnifying loupes when counting or handling ticks.
Tick Handling
Ticks are best handled when cooled prior to use. Placing the vial in ice or on a chill table for a few minutes will slow the ticks' activity. Handling unfed nymphs and adults by the hind leg with fine forceps and minimal pressure is recommended. Engorged ticks of all life cycle stages are best handled with blunt-end forceps, as to prevent rupturing.
Storage of Ticks
Following engorgement, ticks may be housed in sterile clear plastic sample vials. Six 2mm holes cut into the non-autoclavable lightweight plastic lid, coupled with a piece of fine mesh approximately 4 cm2 in size, serves as a secure tick barrier while allowing sufficient air exchange.
Vials should be housed in plastic desiccators containing saturated salt solutions in the desiccator basins. The most critical parameter in maintaining a laboratory colony is relative humidity. Most Ixodid colonies require a relative humidity from 90-97%, depending upon species. It has been shown that saturated solutions of potassium nitrate (94%) and potassium sulfate (97%) produce a relative humidity within the ideal range for housing ticks. Rim around the top and lid of the desiccator should be coated with a thin film of silicone vacuum grease, to ensure a tight seal and as added security against tick escape.
Vials should be grouped by species and labeled accordingly. We have found that computer-printed labels (GA International Cryo-Labels) that do not fade, collect mold, or wrinkle easily under humid conditions, work best. Label information should include genus and species, stage, date of last feeding, whether or not the contents are infected with a pathogen, as well as any numeric inventory systems.
Desiccators are stored in environmental chambers with temperature and light/dark cycle controls. Most species can be continuously kept at 22° C, though when a colony is maintained at 15° C, the ticks' viability is prolonged. This phenomenon allows tick researchers the option of keeping a separate, long-term backup colony. It has been our experience that a constant 14:10 hour light/dark cycle is sufficient for most species. Infected tick colonies should be stored separately, and only in an approved chamber with clear markings.
Tick Infestation on Mice
Ticks typically crawl/wander on the host in order to find a suitable spot to attach. In order to contain the tick on the host, it is preferred to let the tick feed on the host in a feeding capsule.
Preparation of feeding capsule
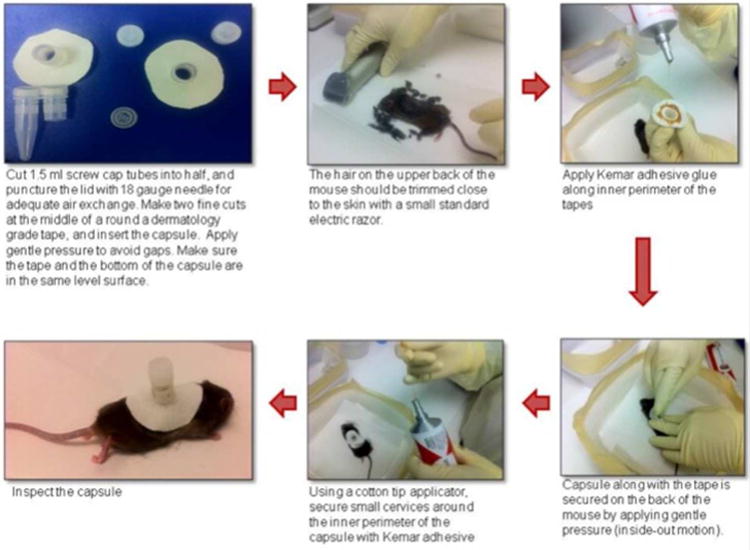
All procedures described under this section (Figure 1) can be conducted at ACL-2 facility. Tick feeding capsule can be prepared from the top half of 5ml screwcap cryovials. The lid is removed and punctured using a 26 gauge needle for adequate air exchange. The capsule is then inserted into the middle of a round dermatology grade tape and gentle pressure is applied along the rim to seal any gaps. Mice are anesthetized as per the approved IACUC protocol. Under sedated condition, the hair on the upper back of the mouse is trimmed close to the skin with a small standard electric razor. Apply adhesive glue (Kamar adhesive (Kamar Inc, Indiana) or Tag Cement (Neogen Corporation, Kentucky) along inner perimeter of the tape, and attach to the trimmed animal's upper back. Secure the capsule along with the tape on the back of the animal by applying gentle pressure (inside-out motion). Using a cotton tip applicator, secure small crevices around the inner perimeter of the capsule with school glue. Inspect the capsule, and allow the glue to dry.
Figure 1. Steps to prepare capsule for the tick infestation on mice.
Tick Infestation
All procedures with infected ticks should be handled within the non-ventilated plexiglass glove box. Carefully open the desiccators to collect tick vials. Transport the tick vials (within a closed secondary container) to the glove box. Procedures with ticks should be performed on a white sticky mat within the glove box. Chill the ticks along with the tick vial on chill table within the glove box before they are handled to ensure that they are immobilized or slowed down. Sticky tape barrier should be created along the perimeter of the tray. Opening and closing of vials containing ticks should be performed within a container with sidewalls to provide protection against a vial slipping from the work surface. Open the vial lid, and pick the exact number of ticks with blunt-end tweezers and transfer to 250μl PCR tubes. Document the change in tick numbers within the tick vial.
One day post feeding capsule attachment, anesthetize the mice and place the chilled PCR tubes with ticks inside the feeding capsule and close the lid. Lids of the PCR tubes must be opened prior to placing them inside the tick feeding capsule. Nymphs and adults may be hand-dropped into the feeding capsule. Close the feeding capsule lid tight, and secure the lid with masking tape. Document how many ticks (and what stage) were transferred to the capsule. Check the feeding capsule daily for its integrity.
Securing the cage
House mice individually in micro-isolator cages (Tecniplast, Buguggiate, Italy) with white paper chip bedding (Harlan Laboratories, Indianapolis, IN) to ensure better observation of escaped ticks. Provide nestlets (Ancare, Bellmore, NY) as enrichment. Avoid other enrichment, as this may dislodge the feeding capsule by contact with the enrichment object. The cages are kept in individually ventilated cage racks within the tick suite. The hermetic cage design maintains consistent negative pressure ensuring Biocontainment. As an additional layer of containment, a ring of sticky trap paste (Tangle Foot, Contech) should be applied one inch below the cage-top along the inner perimeter of the cages to prevent any tick escape. Furthermore, place a micro mesh over the exhaust and supply ports of the cage lids to prevent tick egress through the ventilation ports into the cage rack ventilation system.
Tick collection and storage
Anesthetize mice to check the status of feeding ticks. Use blunt-end tweezers and hold on to tick leg while picking fully-fed ticks from the capsule. Collect the ticks in appropriate storage vials. Accurate counting and recording should be maintained throughout this process. Storage vials should be labeled with the species, sex in the case of adult ticks, number of individuals for each life cycle stage, date of collection and name of the PI. Number of ticks collected should match the number of ticks dropped in the capsule. Carefully transport the storage vials (within a secondary closed container) to the desiccators housed inside the environmental chamber.
Handling tick egg mass/eggs
As soon as the fully engorged female lays eggs, separate one hundred eggs and allow hatching to larvae. Dispose of the remainder of the eggs as per BSL4 procedures. This significantly reduces the number of eggs/larvae handled at a given time.
Conclusion
Research on ticks transmitting BSL4 viruses is essential for better understanding of the role of ticks in the transmission of pathogens that causes disease and for the validation of vaccine and therapeutic targets. Standard operating procedures must be established to protect the personal conducting the research and eliminate the escape of ticks. The procedures recommended here will facilitate establishment of tick work in BSL4 laboratories. However extensive training of personnel to work with ticks in lower containment levels are highly recommended.
Acknowledgments
This work was funded by the UC7 (NIH/NIAID). We would like to thank Nicole Hauser for the help with tick rearing. We would also like to thank Drs. Aysen Gargili, Donald Bouyer, Stephen Wikel, Tom Ksiazek, and UTMB's Environmental Health Safety group for the suggestions with the tick work at BSL4.
References
- 1.Arthropod containment guidelines. A project of the American Committee of Medical Entomology and American Society of Tropical Medicine and Hygiene. Vector Borne Zoonotic Dis. 2003;3:61–98. [Google Scholar]
- 2.Bouchard k, Wikel s. Care, maintenance and experimental infestation of ticks in the laboratory setting. In: Marquardt WC, Black WC, Higgs S, Freier JE, James AA, Hagedorn HH, Kondratieff B, Hemingway J, Moore CG, editors. Biology of Disease Vectors. Second. Elsevier; 2006. pp. 705–712. [Google Scholar]
- 3.Scott TW. Containment of arthropod disease vectors. ILAR J. 2005;46:53–61. doi: 10.1093/ilar.46.1.53. [DOI] [PubMed] [Google Scholar]
- 4.Tabachnick WJ. Laboratory containment practices for arthropod vectors of human and animal pathogens. Lab Anim (NY) 2006;35:28–33. doi: 10.1038/laban0306-28. [DOI] [PubMed] [Google Scholar]


