Abstract
Intermittent systemic exposure to psychostimulants such as amphetamine leads to several forms of long-lasting behavioral plasticity including nonassociative sensitization and associative conditioning. In the nucleus accumbens (NAcc), the protein serine/threonine kinase cyclin-dependent kinase 5 (Cdk5) and its phosphorylation target, the guanine-nucleotide exchange factor kalirin-7 (Kal7), may contribute to the neuroadaptations underlying each of these forms of plasticity. Pharmacological inhibition of Cdk5 in the NAcc prevents the increases in dendritic spine density in this site and enhances the locomotor sensitization normally observed following repeated cocaine. Mice lacking the Kal7 gene display similar phenotypes suggesting that locomotor sensitization and increased NAcc spine density need not be positively correlated. As increases in spine density may relate to the formation of associative memories and both Cdk5 and Kal7 regulate the generation of spines following repeated drug exposure, we hypothesized that either inhibiting Cdk5 or preventing its phosphorylation of Kal7 in the NAcc may prevent the induction of drug conditioning. In the present experiments, blockade in rats of NAcc Cdk5 activity with roscovitine (40 nmol/0.5μl/side) prior to each of 4 injections of amphetamine (1.5 mg/kg; i.p.) prevented the accrual of contextual locomotor conditioning but spared the induction of locomotor sensitization as revealed on tests conducted one week later. Similarly, transient viral expression in the NAcc exclusively during amphetamine exposure of a threoninealanine mutant form of Kal7 [mKal7(T1590A)] that is not phosphorylated by Cdk5 also prevented the accrual of contextual conditioning and spared the induction of sensitization. These results indicate that signaling via Cdk5 and Kal7 in the NAcc is necessary for the formation of context-drug associations, potentially through the modulation of dendritic spine dynamics in this site.
Keywords: conditioning, dendritic spines, psychostimulants, learning, memory, roscovitine, sensitization
1. Introduction
Repeated intermittent amphetamine exposure can lead to several forms of behavioral plasticity including associative conditioning and nonassociative sensitization. The formation of associative memories can link contextual stimuli to unconditioned drug effects allowing these cues to elicit drug-like excitatory conditioned responses as demonstrated by the locomotor activating effects of amphetamine and contexts previously paired with amphetamine (Stewart, 1992; Stewart and Vezina, 1988). In the case of sensitization, drug-evoked behavioral and neurochemical responses become exaggerated with successive infusions of the drug (Vezina, 2004). This form of plasticity accrues independent of association formation as demonstrated by the ability of amphetamine infusions into the ventral tegmental area (VTA) to produce sensitization in the absence of drug conditioning (Singer et al, 2009; Vezina and Stewart, 1990). Although associative conditioning and nonassociative sensitization reflect distinct processes, drug-paired and drug-unpaired environments can come to control the expression of sensitized responding (Anagnostaras and Robinson, 1996; Anagnostaras et al, 2002; Stewart and Vezina, 1988, 1991; Wang and Hsiao, 2003). As both of these forms of plasticity are known to regulate drug-related behaviors and have been linked, separately and together, to addiction vulnerability in humans and animal models (Vezina and Leyton, 2009; Leyton and Vezina, 2013), it is important to elucidate their underlying neuronal mechanisms.
Drugs of abuse are typically administered in the presence of a large number of salient environmental stimuli, providing ample opportunity for the formation of drug-stimulus associations and the possibility for these associations to subsequently influence responding. Morphological changes in dendritic spines have long been thought to underlie aspects of this type of memory storage and have been observed following learning, the induction of long-term potentiation, and behavioral enrichment (Geinisman et al, 2001; Lamprecht and LeDoux, 2004; Leuner et al, 2003). In the nucleus accumbens (NAcc), exposure to sensitizing regimens of systemic amphetamine injections produces long lasting increases in dendritic spine density (Robinson and Kolb, 1997, 1999). Considering that these are not observed following repeated infusions of amphetamine into the VTA, it is likely that they reflect associative drug conditioning rather than nonassociative sensitization (Singer et al, 2009). Consistent with this possibility, Marie et al (2012) showed that the development of cocaine CPP correlates with increased dendritic spine density in the NAcc. Thus, preventing these increases in NAcc dendritic spine density normally observed in rats exposed to systemic amphetamine would be predicted to inhibit the development of conditioning while preserving the induction of sensitization. This reasoning provided the rationale for the present experiments to investigate the contribution to the induction of conditioning of proteins known to regulate dendritic spine dynamics.
Two such proteins, the proline-directed serine/threonine kinase cyclin-dependent kinase 5 (Cdk5) and its phosphorylation target, the guanine-nucleotide exchange factor kalirin-7 (Kal7), are known amongst other actions to regulate cytoskeletal stability related to dendritic spine formation and retraction (Penzes and Jones, 2008; Xie et al, 2007) and have been implicated in drug-induced spine proliferation in the NAcc. Preventing Cdk5 phosphorylation of Kal7 at its threonine 1590 (T1590) residue, for example, reduces spine maturity (Xin et al, 2008). As predicted, pharmacological inhibition of Cdk5 in the NAcc blocks cocaine-induced increases in dendritic spine density in this site (Norrholm et al, 2003) but enhances the induction of locomotor sensitization (Bibb et al, 2001; Taylor et al, 2007). Similarly, interfering with Kal7 function spares (Wang et al, 2013) or even enhances (Kiraly et al, 2010) locomotor sensitization while preventing the increases in dendritic spine density normally observed in the NAcc following cocaine exposure. However, the link between the actions of Cdk5 and Kal7 in the NAcc and the development of drug conditioning is not clear.
Pharmacological inhibition of Cdk5 in the lateral septum and hippocampus has been reported to block the acquisition of fear conditioning (Fischer et al, 2002) and when applied to the basolateral amygdala, to prevent the acquisition of cocaine CPP (Li et al, 2010). However, its effect in the NAcc on the development of drug conditioning has yet to be assessed and no experiments have yet been conducted with amphetamine. Strategies using transgenic mice or long-lasting viral-mediated gene transfer to target the NAcc have yielded a number of different results regarding the functions of Cdk5 and Kal7 in Pavlovian and instrumental conditioning. For example, mice with either Cdk5 knocked out (Hawasli et al, 2007) or subjected to transient p25 expression and elevated Cdk5 activity (Fischer et al, 2005) both show enhanced contextual fear conditioning with the latter effects possibly involving a Cdk5 substrate shift (Meyer et al, 2008). Selective Cdk5 knock-out in the NAcc has been reported to lower the threshold dose required for acquisition of cocaine CPP (Benavides et al, 2007) while decreased acquisition of cocaine CPP has been shown in Kal7 knock-out mice (Kiraly et al, 2010). Reducing Kal7 in the NAcc with lentiviral delivery of Kal7 shRNA decreased incentive motivation but had no effect on the acquisition of cocaine self-administration (Wang et al, 2013) while Cdk5 knock-out mice show similarly unaffected acquisition of instrumental responding but enhanced incentive motivation (Benavides et al, 2007). However, regardless of the outcome, the results obtained in these latter experiments are difficult to interpret because, unlike in pharmacology studies, the Cdk5 and Kal7 manipulation strategies used did not distinguish between acquisition and expression of conditioning, making it difficult to ascertain whether the results obtained were due to effects on one, the other, or a combination of the two. As with sensitization (Vezina, 2004), different neuronal mechanisms underlie the acquisition and expression of excitatory conditioning (Aujla and Benninger, 2004; Banasikowski et al, 2010; Cervo and Samanin, 1995) and these may be differentially affected by changes in Cdk5 and Kal7. Thus, the roles played by Cdk5 and Kal7 in the NAcc specifically in the development of drug conditioning remain unclear.
The present experiments assessed the contribution of Cdk5 and Kal7 in the NAcc to the induction of amphetamine-induced locomotor conditioning. Locomotor sensitization was also assessed to control for potential contributions to non-associative plasticity. The approach used targeted induction specifically by pharmacologically inhibiting Cdk5 or using a transient viral infection system to express exclusively during amphetamine exposure a threonine-alanine mutant form of Kal7 (mKal7) that is not phosphorylated by Cdk5. Our results indicate that Cdk5 and Kal7 signaling in the NAcc is necessary for the induction of excitatory contextual drug conditioning, possibly through a pathway involving Cdk5 phosphorylation of Kal7.
2. Materials and Methods
2.1. Animals
Male Sprague-Dawley rats (Harlan Sprague-Dawley, Madison, WI) weighing 250-275 g on arrival were used. Rats were individually housed in a reverse cycle room (12-hour light/12-hour dark; lights on at 2000 hours) with food and water available ad libitum. All procedures were performed during the dark phase of the light cycle. Following a 4-5 day acclimation period, all rats were anesthetized with a mix of ketamine (100 mg/kg, IP) and xylazine (10 mg/kg, IP), placed in a stereotaxic instrument with the incisor bar positioned 5.0 mm above the interaural line, and implanted with 22 gauge chronic bilateral guide cannulae angled at 10o to the vertical and aimed at the NAcc shell (A/P, +3.4; M/L, ± 0.8; DV, −7.5mm from bregma and skull; as per the angled brain atlas of Pellegrino et al, 1979) with tips positioned 1 mm (for the roscovitine experiment) or 4 mm (for the HSV-mKal7 experiment) above the final injection site. The NAcc shell was targeted because previous studies of the effects of NAcc roscovitine examined this subnucleus (Norrholm et al, 2003; Taylor et al, 2007) and it is uniquely innervated by the ventral hippocampus, a structure known to process contextual information (Moses et al, 2002). The cannulae (Plastics One, Roanoke, VA) were imbedded in a dental cement cap secured by six screws fastened to the skull. After surgery, 28 gauge obturators were placed into the guide cannulae (either flush for the HSV-mKal7 experiment or protruding 1mm beyond the guide cannula tips for the roscovitine experiment) and rats were returned to their home cage for 10-14 days of recovery. All surgical procedures were conducted using aseptic techniques according to an approved Institutional Animal Care and Use Committee protocol.
2.2. Locomotor Testing Chambers
A bank of 8 open field activity boxes (Med Associates, St. Albans, VT) was used to measure locomotor responding to saline and amphetamine. Each open field (43.2 × 43.2 × 30.5 cm) was constructed of acrylic walls, a wire mesh floor, a removable Plexiglas top, and was fitted with a 16 × 16 horizontal grid of infrared sensors positioned 3.5 cm above the floor. Separate interruptions of photocell beams were detected as ambulatory counts and recorded via an electrical interface by a computer situated in an adjacent room using Med Associates Open Field Activity Software (SOF-811).
2.3. Effect of Inhibiting Cdk5 in the NAcc on the Induction of Locomotor Conditioning and Sensitization by Amphetamine
2.3.1. Behavioral Procedures
In this experiment, rats were subjected to three phases: drug exposure, withdrawal, and testing.
The exposure phase used a discrimination learning paradigm that consisted of four 3-day conditioning blocks (Table 1). Injections were given on the first 2 days of each block (the first in the open field and the second in the home cage); rats were left undisturbed in the home cage on the third. For each block, rats in two groups (Paired-Veh and Paired-Ros) were administered amphetamine (1.5 mg/kg, IP) in the open field preceded 30 minutes earlier by bilateral infusion into the NAcc of vehicle (Veh; 0.5μl/side) or the Cdk5 inhibitor roscovitine (Ros; 40 nmol/0.5μl/side) and locomotor activity was recorded for 2 hours. The following day, these rats were administered saline (1.0 ml/kg, IP) in the home cage preceded by NAcc obturator movements to mimic NAcc microinjections (thereby reducing the total number of actual microinjections into tissue). Rats in two additional groups (Unpaired-Veh and Unpaired-Ros) were administered the same injections but in the reverse order: saline with obturator movements in the open field and amphetamine preceded by vehicle or roscovitine in the home cage. Rats in two final groups (Control-Veh and Control-Ros) received saline in both environments preceded either by NAcc vehicle, roscovitine, or obturator movements. No differences in open field locomotion were observed during the exposure phase between controls administered roscovitine in the open field or the home cage. The data for these animals was therefore combined.
Table 1. Induction of Conditioning and Sensitization: Roscovitine.
Outline of one 3-day conditioning block used to expose rats to their respective injections during the drug exposure phase of the roscovitine experiment. This 3-day block was repeated four times to complete the 12- day drug exposure phase. During exposure, saline (1.0 ml/kg) and amphetamine (1.5 mg/kg) were administered IP. Roscovitine (40 nmol/0.5μl/side) was administered bilaterally into the NAcc. NAcc microinjections and obturator movements preceded the IP injections by 30-minutes. Following a 1- week withdrawal period, rats were then tested for conditioning (following a saline injection; 1.0 ml/kg, IP) or for sensitization (following an amphetamine injection; 1.0 mg/kg, IP). Roscovitine was not administered on either test. Veh, vehicle. Ros, roscovitine. nc = n/group for conditioning. ns = n/group for sensitization.
| Group | Day 1 - Open Field | Day 2 - Home Cage | Day 3 - Home Cage | |||
|---|---|---|---|---|---|---|
| NAcc infusion |
Systemic injection |
NAcc infusion |
Systemic injection |
No NAcc infusion |
No Systemic injection |
|
|
Paired-Veh
nc=6, ns=6 |
Vehicle | Amphetamine | Obturator Movements |
Saline | Procedure and drug free | |
|
Paired-Ros
nc=7, ns=6 |
Roscovitine | Amphetamine Obturator |
Movements | Saline | ||
|
Unpaired-Veh
nc=5, ns=7 |
Obturator Movements |
Saline | Vehicle | Amphetamine | ||
|
Unpaired-Ros
nc=6, ns=6 |
Obturator Movements |
Saline | Roscovitine | Amphetamine | ||
|
Control-Veh
nc=7, ns=5 |
Vehicle or Obturator Movements |
Saline | Vehicle or Obturator Movements |
Saline | ||
|
Control-Ros
nc=6, ns=5 |
Roscovitine or Obturator Movements |
Saline | Roscovitine or Obturator Movements |
Saline | ||
Following the 12 days of exposure (4 × 3-day blocks), rats were afforded a 1-week withdrawal period during which they were left undisturbed in the home cage. Rats were then tested for conditioned locomotion for 1-hour in the open field following a saline injection (1.0 ml/kg, IP) or for locomotor sensitization for 2-hours following an amphetamine injection (1.0 mg/kg, IP). Roscovitine was not administered before either test. Thus, 12 separate groups of rats were tested in this experiment: 6 for conditioned locomotion and 6 for locomotor sensitization (Table 1).
2.3.2. Drugs and Microinjections
S(+)-amphetamine sulfate (Sigma-Aldrich Inc., Saint Louis, MO) was dissolved in sterile saline. The Cdk5 inhibitor (R)-roscovitine (Enzo Life Sciences Inc., Plymouth Meeting, PA) was dissolved in 1XPBS/50% DMSO vehicle. Doses refer to the weight of the salt and were selected based on effective doses administered in previous reports (Bibb et al, 2001; Singer et al, 2009; Taylor et al, 2007).
Bilateral NAcc roscovitine and vehicle microinjections were performed in freely moving rats using 1μl syringes (Hamilton, Reno, NV) connected to injection cannulae (28 gauge) via PE20 tubing. Injectors were inserted 1 mm beyond the guide cannula tips and 0.5 l of the solution was simultaneously infused into each hemisphere over a 30-second period. Following a diffusion time of 1 minute, injectors were removed and obturators replaced.
2.4. Effect of Transiently Expressing mKal7 in the NAcc on the Induction of Locomotor Conditioning and Sensitization by Amphetamine
2.4.1. Behavioral Procedures
In this experiment, rats were subjected to four phases: viral infection, drug exposure, withdrawal, and testing.
Replication-deficient herpes simplex virus (HSV) vectors were chosen to express exclusively during amphetamine exposure a serine-alanine mutant form of Kal7 (mKal7) in the NAcc that is not phosphorylated by Cdk5 as these viral vectors produce transient expression of the transgene lasting 4-5 days (Carlezon and Neve, 2003; Loweth et al, 2010; Neve et al, 1997; Singer et al, 2010). This allowed for selective disruption of Kal7 signaling in the NAcc only during the acquisition of conditioning. As expected, no evidence for mKal7 expression remained 8 days post infection, well before the tests for expression of locomotor conditioning and sensitization. Control rats were administered NAcc infusions of 1X PBS vehicle or HSV vectors to transiently express GFP (Mock).
The exposure phase used a discrimination learning paradigm that consisted of four 2-session conditioning days (Table 2) beginning the day after viral infection. This compacted drug exposure phase was designed to accommodate the transient infection afforded by the HSV vectors. On each day, rats were administered an injection in the open field in one session and in the home cage in the other. These sessions were separated by five hours and their order was counterbalanced on each day. Each day, rats in two groups (Paired-Mock and Paired-mKal7) were administered amphetamine (1.5 mg/kg, IP) in the open field and saline (1.0 ml/kg, IP) in the home cage. Rats in two additional groups (Unpaired-Mock and Unpaired-mKal7) were administered the same injections but in the reverse location: saline in the open field and amphetamine in the home cage. Rats in two final groups (Control-Mock and Control-mKal7) received saline in both environments. Locomotor activity was recorded for two hours following injections in the open field.
Table 2. Induction of Conditioning and Sensitization: mKal7.
Outline of one of the 4 conditioning days used to expose rats to their respective injections during transient HSV infection in the HSV-mKal7 experiment. One day before this 4-day exposure phase, rats received bilateral HSV-mKal7 or Mock (HSV-GFP or 1X PBS) infusions into the NAcc. During exposure, saline (1.0 ml/kg) and amphetamine (1.5 mg/kg) were administered IP. On each of the 4 conditioning days, rats received an injection in either the open field or the home cage in one session and an injection in the opposite location five hours later. Session order was counterbalanced each day. Following a 1-week withdrawal period, rats were then tested for conditioning (following a saline injection; 1.0 ml/kg, IP) and five days later for sensitization (following an amphetamine injection; 1.0 mg/kg, IP).
| Group | Day 1 | |
|---|---|---|
| Open Field | Home Cage | |
|
Paired-Mock
n=5 |
Amphetamine | Saline |
|
Paired-mKal7
n=6 |
Amphetamine | Saline |
|
Unpaired-Mock
n=5 |
Saline | Amphetamine |
|
Unpaired-mKal7
n=5 |
Saline | Amphetamine |
|
Control-Mock
n=5 |
Saline | Saline |
|
Control-Mock
n=5 |
Saline | Saline |
Following the 4 days of exposure, rats were left undisturbed in the home cage for 1 week of withdrawal. Rats were then tested for conditioned locomotion for 1-hour following a saline injection (1.0 ml/kg, IP). Five days later, the same rats were tested for locomotor sensitization for 2-hours following an amphetamine injection (1.0 mg/kg, IP). Thus, six separate groups of rats were tested in this experiment (Table 2).
2.4.2. Viral Vectors and Microinjections
Replication-deficient HSV vectors (p1005) were constructed as described previously (Neve et al, 1997). The average titer of the viral stocks used was 4.0 × 107 infectious units/ml. pEAK10.His.Myc Kal7 T1590A, a threonine-alanine mutant construct of Kal7 [mKal7(T1590A)] that is not phosphorylated by Cdk5, was generously supplied by Dr. Betty Eipper (University of Connecticut, Farmington, CT) and packaged into the HSV vectors by Dr. Rachael Neve (Massachusetts Institute of Technology, Cambridge, MA). Construct empty HSV-GFP (p1005) vectors were also provided by Dr. Neve. mKal7 was driven by the HSV IE 4/5 promoter and GFP by a CMV promoter.
After recovery from surgery, rats were transferred to a biosafety level 2 facility where they were administered bilateral infusions into the NAcc of HSV-mKal7 or control infusions of HSV-GFP or the 1X PBS vehicle (Mock). The mKal7 was infused as a 1:10 dilution of stock HSV-mKal7 in sterile 1X PBS. The latter two control infusions were used interchangeably as they did not differ in their behavioral effects. Microinjections were made in freely moving rats in a volume of 2 μl/side over 10 minutes through 28 gauge cannulae extending 4 mm beyond the guide cannula tips. Injection cannulae were connected via PE20 tubing to 10 μl syringes (Hamilton, Reno, NV) and left in place for 5 minutes after the injection to allow for diffusion. Rats were returned to the colony room the following day and the drug exposure phase initiated.
2.4.3. Immunofluorescence
Immunofluorescence was used in separate rats administered HSV-mKal7 or HSV-GFP to visualize the distribution of infected cells around the injection cannula tips (Figure 4c). Rats were anesthetized with ketamine (100 mg/kg, IP) and xylazine (10 mg/kg, IP) and perfused with saline followed by 4% paraformaldehyde (PF). Brains were harvested, stored in 4% PF for 48 hours, and transferred to a 25% sucrose solution for at least an additional 48 hours. 40 μm coronal sections were then obtained using a cryostat and transferred to 1X PBS for free-floating immunohistochemistry. Slices were washed multiple times with 0.3% Tween20 solution, blocked in 10% normal donkey solution (Jackson ImmunoResearch Laboratories, West Grove, PA), and then incubated overnight in this solution containing a GFP antibody fused to FITC (1:400, Abcam #ab6662) to amplify fluorescence. The next day, slices were again washed in 1X PBS and then mounted onto gelatin-coated slides and cover slipped for imaging. Examination of consecutive brain sections revealed that GFP-positive neurons were observed only in close proximity to the injection cannula tips in the NAcc shell (Figure 4c).
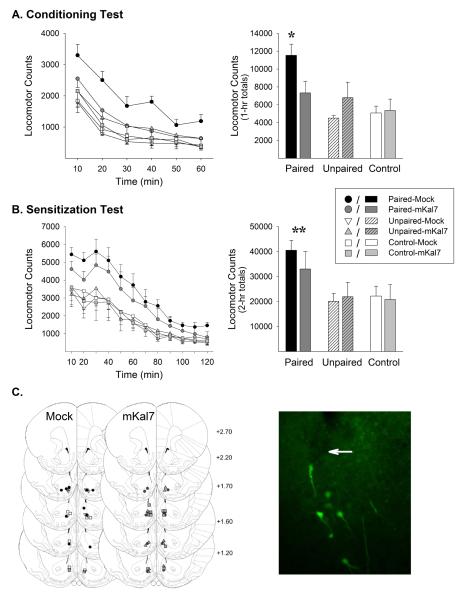
Figure 4.
Transient expression of mKal7 in the NAcc during exposure blocked the induction of conditioned locomotion but spared the induction of locomotor sensitization. Time course (left) and session total locomotor counts (right) are shown for the 1-hour conditioning test (A) and 2-hour sensitization test (B). Data are shown as mean (±SEM). All rats were administered saline on the conditioning test and amphetamine (1.0 mg/kg, IP) on the sensitization test. The tests were conducted starting 1 week following the exposure phase. No evidence of mKal7 expression remained 8 days post-infection, well before both tests. Group names refer to treatments administered during the exposure phase. *, p<0.05, compared to all other groups. **, p<0.01, either Paired-Veh or Paired-Ros compared to remaining groups. C. Line drawings (Paxinos and Watson, 1997) depicting location of microinjection cannula tips in the NAcc shell for rats included in the data analyses (left). Numbers indicate mm from bregma. The photomicrograph to the right was obtained 4 days after infection with HSV-mKal7(T1590A)-GFP and illustrates GFP-positive neurons in close proximity to the injection cannula tip in the NAcc shell (arrow). n/group=5-6.
2.4.4. Immunoblotting
The extent of overexpression of Kal7 was determined with immunoblotting in separate rats administered HSV-mKal7 (n=4) or HSV-GFP (n=4). Brains were removed rapidly, sections (1 mm thick) obtained with a brain matrix, and 2-mm-diameter punches taken bilaterally around the injection cannula tips. Detailed procedures for immunoblotting were as described previously (Loweth et al, 2010; Singer et al, 2010). Briefly, following transfer and incubation in blocking solution, membranes were incubated with a primary antibody for kalirin (1:500; Sigma-Aldrich, St Louis, MO) or Tubulin (1:10 000; Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were then incubated in a horseradish peroxidase conjugated anti-rabbit or anti-mouse IgG and visualized using the ECL detection system (ECL Advanced; GE Healthcare, Waukesha, WI).
2.5. Histological Verification of Cannula Tip Placements
At the conclusion of the behavioral experiments, rats were anesthetized with ketamine (100 mg/kg, IP) and xylazine (10 mg/kg, IP) and perfused intracardially with 0.9% saline followed by 10% formalin for the roscovitine experiment or 4% PF for the HSV-mKal7 experiment. Brains were then harvested, stored in either 10% formalin or 4% PF for approximately 1 week, and 40 μm coronal slices subsequently obtained with a cryostat. Brain slices were mounted on gelatin-coated slides and stained with cresyl violet to verify cannula tip placements (Figure 2c). Only rats with bilateral cannula tips placed correctly in the NAcc shell were included in the behavioral analyses (Figures 2c and 4c). No consistent evidence for DMSO-induced toxicity at the injection cannula tips was detected.
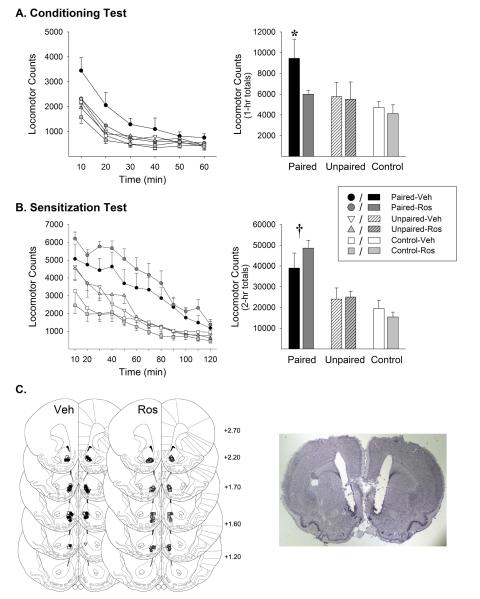
Figure 2.
NAcc roscovitine (Ros) administered during exposure blocked the induction of conditioned locomotion but spared the induction of locomotor sensitization. Time course (left) and session total locomotor counts (right) are shown for the 1-hour conditioning test (A) and 2-hour sensitization test (B). Data are shown as mean (±SEM). All rats were administered saline on the conditioning test and amphetamine (1.0 mg/kg, IP) on the sensitization test. Both tests were conducted 1 week following the exposure phase. Ros was not administered on either test. Group names refer to treatments administered during the exposure phase. *, p<0.05, compared to all other groups. †, p<0.05, either Paired-Veh or Paired-Ros compared to remaining groups. C. Line drawings (Paxinos and Watson, 1997) depicting location of microinjection cannula tips in the NAcc shell for rats included in the data analyses (left). Numbers indicate mm from bregma. The photomicrograph to the right shows a representative cresyl violet stained brain section with bilateral cannula tracks targeting the NAcc shell. n/group=5-7.
2.6. Statistical Analyses
The session total locomotor counts obtained during drug exposure were analyzed using three-way (two between one within) ANOVA with conditioning group and either roscovitine or HSV infection as the between factors and days as the within factor. Locomotor counts obtained on the tests for conditioning and sensitization were similarly analyzed with three-way (two between one within) ANOVA but with time as the within factor. Only statistically significant effects and interactions are reported. Post hoc comparisons were conducted using the LSD test. Immunoblotting data measuring Kal7 expression were analyzed using the t-test.
3. Results
3.1. Effect of Inhibiting cdk5 in the NAcc on the Induction of Locomotor Conditioning and Sensitization by Amphetamine
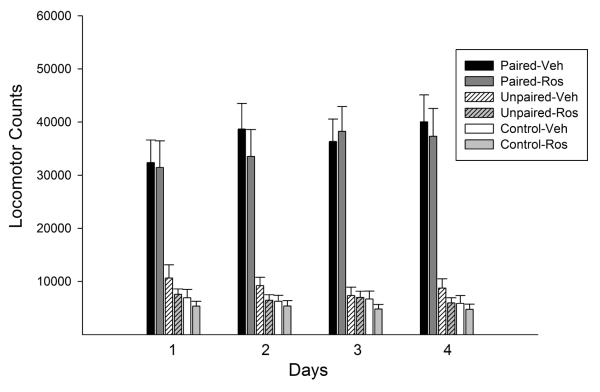
As expected, Paired rats administered amphetamine in the open field during the exposure phase displayed a greater locomotor response throughout this phase than Unpaired and Control rats administered saline. Like previous reports with cocaine (Bibb et al, 2001; Taylor et al, 2007), roscovitine spared the increase in amphetamine-induced locomotion over days although, unlike these reports, it did not enhance it (Figure 1). The ANOVA conducted on these data revealed a significant effect of conditioning (F(2,63)=67.75, p<0.001) and a significant conditioning × day interaction (F(6,189)=4.97, p<0.001). The significant interaction reflects the finding indicated by posthoc comparisons that rats in both the Paired-Veh and Paired-Ros groups showed significant increases in locomotion over days while the opposite was true for rats in the remaining groups. No significant differences were detected between the Paired-Veh and Paired-Ros groups on any day. While statistically significant, the increase in locomotion over days observed in the Paired groups was weak. This may reflect the sensitization of competing stereotypic behaviors that can sometimes occlude progressive enhancements in locomotion during exposure to amphetamine (Crombag et al, 1999; Stewart and Vezina, 1987). For this reason, a lower dose of amphetamine was used in the subsequent test for sensitization in which a robust sensitized response was observed (see below).
Figure 1.
Locomotor activity observed during the exposure phase: NAcc roscovitine. Data are shown as mean (+SEM) 2-hour total locomotor counts observed following amphetamine (Paired) or saline (Unpaired and Control) injections on each of the 4 exposure days. Amphetamine was administered at a dose of 1.5 mg/kg, IP, on each day. Roscovitine (Ros) was administered into the NAcc (40 nmol/0.5μl/side) 30 minutes before the IP injections. NAcc Ros produced no detectable effects in this phase. n/group=5-7.
Again as expected, repeated pairings of amphetamine and the open field in Paired-Veh rats led to a significant conditioned locomotor response relative to Unpaired-Veh and Control-Veh rats on the conditioning test conducted one week later. Roscovitine administered to the NAcc before each amphetamine injection during exposure blocked the induction of this conditioning as exemplified by the lack of a conditioned locomotor response in Paired-Ros rats on the test (Figure 2a). The ANOVA conducted on these data revealed significant effects of conditioning (F(2,31)=4.31, p<0.05) and time (F(5,155)=146.98, p<0.001) as well as significant conditioning × time (F(10,155)=4.00, p<0.001) and roscovitine × time (F(5,155)=5.39, p<0.001) interactions. Post-hoc LSD tests revealed that Paired-Veh rats displayed significantly greater locomotor responding overall compared to all other groups on this test (p<0.01-0.05). The remaining groups did not differ significantly from each other.
Unlike the effect on conditioning, roscovitine administered prior to the amphetamine injections during exposure had no effect on the induction of locomotor sensitization. On the test for sensitization also conducted one week after the last drug exposure injection, Paired-Veh and Paired-Ros rats showed a similar and significantly greater locomotor response than Unpaired and Control rats (Figure 2b). The lack of a sensitized locomotor response in Unpaired-Veh relative to Control-Veh rats is consistent with previous reports of context-specific sensitization, an effect thought to depend on conditioned inhibition of the expression of sensitization by drug-unpaired cues (Anagnostaras et al, 2002; Stewart and Vezina, 1991; Vezina and Leyton, 2009). Cdk5 inhibition did not affect the accrual of this type of learning as Unpaired-Ros rats similarly did not exhibit enhanced locomotion on the test for sensitization. The ANOVA conducted on these data revealed a significant effect of conditioning (F(2,29)=15.71, p<0.001) and time (F(11,319)=94.98, p<0.001) as well as a significant conditioning × time interaction (F(22,319)=6.14, p<0.001). Post-hoc LSD tests showed that rats in the Paired-Ros and Paired-Veh groups did not differ significantly from each other but did differ significantly from all remaining groups (p<0.05-0.001). These remaining groups did not differ significantly from one another.
3.2. Effect of Transiently Expressing mKal7 in the NAcc on the Induction of Locomotor Conditioning and Sensitization by Amphetamine
In this experiment, HSV vectors were used to express mKal7 in the NAcc exclusively during amphetamine exposure so as to selectively disrupt Kal7 during the acquisition of conditioning. As expected, the HSV vectors produced a transient increase in transgene expression that was observed 4 days and had dissipated 8 days post-infection (Carlezon et al, 1997; Neve et al, 1997). mKal7 infected rats showed a significant increase in kalirin protein expression in the NAcc (146.0±15.0%) compared to controls (100±2.7%) at day 4 post-infection (t6=1.94, p<0.05). No increase was detectable 8 days post infection.
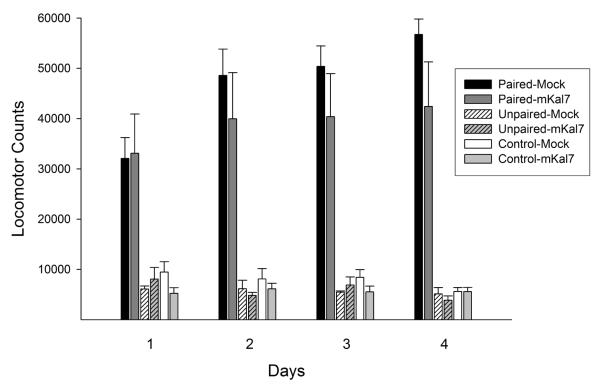
Paired rats administered amphetamine in the open field during the exposure phase again displayed a greater locomotor response throughout this phase than Unpaired and Control rats administered saline. As in the roscovitine experiment, mKal7 expression in the NAcc spared the increase in amphetamine-induced locomotion over days, although again and consistent with the findings of others (Wang et al, 2013), it did not enhance it (Figure 3). The ANOVA conducted on these data revealed significant effects of conditioning (F(2,25)=50.66, p<0.001) and day (F(3,75)=6.14, p<0.001) as well as significant conditioning × day (F(6,75)=12.34, p<0.001) and conditioning × day × infection (F(6,75)=2.59, p<0.05) interactions. Post hoc comparisons indicated that, unlike the remaining groups, rats in both the Paired-Mock and Paired-mKal7 groups showed significant increases in locomotion over days. No significant differences were detected between these two groups on any day.
Figure 3.
Locomotor activity observed during the exposure phase: NAcc mKal7. Data are shown as mean (+SEM) 2-hour total locomotor counts observed following amphetamine (Paired) or saline (Unpaired and Control) injections on each of the 4 exposure days. Amphetamine was administered at a dose of 1.5 mg/kg, IP, on each day. These 4 exposure days coincided with the 4 post-infection days when mKal7 was expressed in the NAcc. Expression of mKal7 produced no detectable effects in this phase. n/group=5-6.
In a manner strikingly similar to the results obtained with roscovitine, transient expression of mKal7 in the NAcc exclusively during exposure prevented the induction of locomotor conditioning as evidenced by the lack of a conditioned response in Paired-mKal7 rats on the test for conditioning conducted one week after exposure when mKal7 was no longer expressed (Figure 4a). The ANOVA conducted on these data revealed significant effects of conditioning (F(2,25)=7.55, p<0.01) and time (F(5,125)=102.74, p<0.001) as well as significant conditioning × infection (F(2,25)=3.89, p<0.05) and conditioning × time (F(10,125)=2.31, p<0.05) interactions. Post-hoc LSD tests showed that Paired-Mock rats displayed significantly greater locomotor responding on this test compared to all other groups (p<0.05). These did not differ significantly from each other.
Again in a manner strikingly similar to what was observed with roscovitine, transient expression of mKal7 in the NAcc during exposure did not affect the induction of locomotor sensitization. On the subsequent test for sensitization conducted when mKal7 was no longer expressed, Paired-Mock and Paired-mKal7 rats displayed a similar and significantly greater locomotor response than Unpaired and Control rats (Figure 4b). In addition, as with roscovitine, mKal7 expression during exposure did not affect the accrual of associations between contextual cues and the absence of amphetamine. Like Unpaired-Mock rats, Unpaired-mKal7 rats also did not exhibit enhanced locomotion on the test for sensitization. The ANOVA conducted on these data revealed significant effects of conditioning (F(2,25)=5.84, p<0.01) and time (F(11,275)=90.76, p<0.01) as well as a significant conditioning × time interaction. Post-hoc LSD tests showed that rats in the Paired-mKal7 and Paired-Mock groups did not differ from each other but did differ significantly from rats in the remaining groups (p<0.01). These did not differ significantly from one another.
Even at the time of maximal mKal7 expression in the exposure phase, it is likely that endogenous Kal7 remained in the NAcc rendering the serine-alanine nonphosphorylating Kal7 mutant a dominant negative that interfered with endogenous kinase function. Dominant-negative effects can be exerted by competition with endogenous kinase for upstream activators, downstream substrates, or subcellular regulatory pathways. Thus, it is possible that endogenous Kal7 was out-competed directly by mKal7 for activators and substrates. Alternatively, mKal7 may have also achieved these effects indirectly by occupying postsynaptic density (PSD) anchoring substrates such as PSD-95 (Penzes et al, 2001) that normally bind endogenous Kal7, thereby reducing PSD Kal7 levels available for Cdk5 phosphorylation or other signaling events.
4. Discussion
The present findings indicate that perturbing Cdk5 and Kal7 signaling in the NAcc exclusively during exposure to amphetamine prevents the development of conditioned locomotion but spares the development of locomotor sensitization by the drug. Therefore, Cdk5 and Kal7 signaling in the NAcc is necessary for the induction of excitatory contextual associative conditioning but not for nonassociative forms of plasticity such as sensitization.
These findings follow and are consistent with others showing that pharmacological inhibition of Cdk5 and knock out or knock down of the Kal7 gene both prevent psychostimulant-induced increases in dendritic spine density in the NAcc but spare the induction of locomotor sensitization (Bibb et al, 2001; Norrholm et al, 2003; Taylor et al, 2007; Kiraly et al, 2010; Wang et al, 2013) as well as others showing that amphetamine-induced changes in dendritic morphology in the NAcc correspond to associative drug conditioning rather than nonassociative drug sensitization (Singer et al, 2009). In addition, mKal7 induces the formation of filopodia-like spines (Xin et al, 2008) and the enlargement of these spines, a process linked to drug conditioning (Gipson et al, 2013), may also be regulated by Kal7 expression and its phosphorylation by Cdk5 (Xin et al, 2008). Considering the importance of dendritic spine proliferation in learning (Geinisman et al, 2001; Leuner et al, 2003; Lamprecht and LeDoux, 2004), these findings together suggest that Cdk5 inhibition and mKal7 expression in the NAcc prevented the induction of locomotor conditioning in the present experiments by preventing the neuroadaptations necessary to regulate spine dynamics in this site.
Although roscovitine has been used to selectively inhibit Cdk5 in a number of reports (see Introduction), this drug may also act on other substrates with lesser affinity. For example, roscovitine has been shown to slow the deactivation of N-type calcium channels (Buraei et al, 2007; Cho and Meriney, 2006), which may enhance psychostimulants responses (Kantor et al, 2004; Pierce et al, 1998). However, no evidence that roscovitine enhanced locomotor responding to amphetamine was found in the present experiments (see Figure 1). Similarly, roscovitine was found in experiments using different in vitro preparations to increase DA overflow and potentiate the ability of amphetamine to do so (Price et al, 2009). Yet again, our findings do not support this possibility in the NAcc as infusions of roscovitine into this site did not induce locomotion or enhance amphetamine-induced locomotion (Figure 1). Roscovitine may also inhibit casein kinase 1 (Bach et al, 2005; Fabian et al, 2005) but inhibition of casein kinase 1 in the NAcc reduces psychostimulant-induced locomotion (Bryant et al, 2009; Li et al, 2011) and no evidence for such an effect by NAcc roscovitine was obtained in the present experiments (see Figure 1). Nonetheless, it will be useful to confirm the present results in future experiments using more selective non-pharmacological approaches to inhibit Cdk5.
In the mKal7 experiment, the mutant transgene was transiently expressed in the NAcc during amphetamine exposure. mKal7 infected rats showed a significant increase in kalirin protein at day 4 post-infection with no increase detectable 8 days post-infection, well before the tests for the expression of locomotor conditioning and sensitization (see Results). In animals expressing mKal7, endogenous Kal7 likely remained available for phosphorylation. Therefore, the most likely explanation for our findings is that mKal7 acted as a dominant negative to interfere with normal Kal7 signaling during amphetamine exposure, either by preventing the interaction of endogenous Kal7 with Cdk5 (thereby impairing Kal7 phosphorylation by Cdk5, consistent with the findings obtained with roscovitine) or by preventing other protein-protein interactions (e.g., with scaffolding proteins; Penzes and Jones, 2008) known to be critical for Kal7 function. Although unequivocal in vivo evidence for a Cdk5-Kal7 signaling pathway remains lacking, the in vitro evidence reported by Xin et al (2008) and the similarity of the results obtained in the present experiments with roscovitine and mKal7 together support an important role for such a pathway in the NAcc in locomotor conditioning by amphetamine.
The present experiments assessed, in an anatomically and temporally specific manner, the contribution of Cdk5 and Kal7 to the induction of amphetamine-induced locomotor conditioning and sensitization by using a pharmacological Cdk5 inhibitor or a transient viral infection system to express mKal7 (for 4-5 days; no longer detectable 8 days post-infection) specifically in the NAcc and exclusively during amphetamine exposure. This permitted the unambiguous interpretation of the results obtained on the conditioning and sensitization tests as these were conducted days after dissipation of the pharmacological challenge and mutant protein expression: Cdk5 and Kal7 signaling in the NAcc is necessary for the induction of conditioned locomotion but not locomotor sensitization by amphetamine. In contrast, the results described in a number of recent reports using knock-out, knock-down or transgenic mice as well as long-lasting lentiviral-mediated gene transfer to manipulate Cdk5 and Kal7 are difficult to interpret because these manipulations spanned the induction and expression phases of conditioning and thus could not distinguish between the two. In these experiments, decreasing Cdk5 or Kal7 activity produced either no change (Benavides et al, 2007; Wang et al, 2013), a decrease (Kiraly et al, 2010), or an increase (Benavides et al, 2007; Hawasli et al, 2007) in conditioning, while subjecting mice to transient p25 expression and elevated Cdk5 activity increased conditioning (Fischer et al, 2005). A number of procedural differences between these different studies may have contributed to the different results obtained, including the subnucleus of the NAcc targeted for study (core: Benavides et al, 2007; Kiraly et al, 2010; Wang et al, 2013; cf, Norrholm et al, 2003; Taylor et al, 2007; shell), the type of conditioning assayed (appetitive: Benavides et al, 2007; Kiraly et al, 2010; fear: Fischer et al, 2005; Hawasli et al, 2007), and whether instrumental (Benavides et al, 2007; Wang et al, 2013) as opposed to Pavlovian conditioning was also tested. However, it is also possible that the different neuronal events underlying the induction and expression of conditioning were differentially affected by changes in Cdk5 and Kal7 in these experiments, rendering the effects ultimately observed difficult to interpret. Supporting this possibility, we recently found that, unlike the results obtained in the present experiments, inhibiting NAcc Cdk5 not during exposure but immediately before testing enhanced the expression of amphetamine-induced locomotor conditioning and sensitization (Singer et al, in review).
As expected, the locomotor sensitization observed in the present experiments was context-specific, observed in Paired but not Unpaired rats, an effect thought to depend on conditioned inhibition of the expression of sensitization by drug-unpaired cues in the latter rats (Anagnostaras et al, 2002; Stewart and Vezina, 1991; Vezina and Leyton, 2009). Interestingly, neither Cdk5 inhibition nor mKal expression in the NAcc during exposure affected this type of learning as expression of sensitization in these animals remained inhibited relative to rats in the Paired groups. These results suggest that Cdk5 and Kal7 signaling in the NAcc is not necessary for the accrual of associations between contextual cues and the absence of amphetamine but rather is specific to the formation of excitatory associations between these cues and the presence of the drug. While both associative in nature, excitatory conditioning and conditioned inhibition reflect different contingencies, produce different behavioral effects, are regulated differently (for a review and discussion, see Vezina and Leyton, 2009), and as suggested by the present results, are likely mediated by different neuronal mechanisms.
The nature of the neuroadaptations underlying the induction of excitatory associative conditioning in the NAcc remains unknown. Changes in spines and dendritic morphology have been proposed to embody the neural representation of memory (Koleske, 2013; Lamprecht and LeDoux, 2004). According to this possibility, repeated psychostimulant exposure could increase Cdk5 in the NAcc via long-lasting increases in ΔFosB, a transcription factor for this protein (Hope et al, 1994), and lead to phosphorylation of Kal7 and other proteins (Barnett and Bibb, 2011) to produce stable changes in dendritic spine morphology. However, it is unlikely that such a static drug-induced change in dendritic spines mediated the excitatory associative conditioning observed in the present experiments as Paired and Unpaired rats were exposed to the same number of amphetamine exposure injections but only Paired rats showed a conditioned response when tested in the drug-paired context. Rather, the results reported here suggest that Cdk5 and Kal7 signaling, and perhaps other proteins, lead to neuroadaptations necessary for drug-paired cues, when present, to evoke changes in dendritic spines in the NAcc. In support of this possibility, rapid increases in spine tip diameter were recently reported in the NAcc following presentation of cocaine-paired cues (Gipson et al, 2013).
5. Conclusions
Repeated exposure to drugs of abuse such as amphetamine engages both associative and nonassociative processes which contribute to the generation of addictive behaviors. The present results demonstrate that Cdk5 and Kal7 signaling in the NAcc during initial drug exposure regulates the formation of associative contextual memories. In contrast, Cdk5 and Kal7 in the NAcc do not contribute to the formation of nonassociative locomotor sensitization by amphetamine. Perturbing Cdk5 and Kal7 signaling in the NAcc during amphetamine exposure may disrupt those drug-induced adaptations in dendritic spines that are necessary for conditioning to accrue.
HIGHLIGHTS.
Drug conditioning and sensitization are separate processes contributing to addiction
Cdk5 & Kal7 in the NAcc regulate contextual conditioning to drug-paired cues
Neither Cdk5 nor Kal7 in the NAcc regulate the induction of drug sensitization
Acknowledgements
This research was funded by National Institutes of Health grants R01 DA09397 (PV), T32 DA07255 (BFS, NMN, KRR), and F31 DA030021-01A1 (BFS). This work was also partially funded by the Chicago Biomedical Consortium with support from The Searle Funds at The Chicago Community Trust (BFS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
References
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: Modulation by associative learning. Behavioral Neuroscience. 1996;110:1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Schallert T, Robinson TE. Memory processes governing amphetamine-induced psychomotor sensitization. Neuropsychopharmacology. 2002;26:703–715. doi: 10.1016/S0893-133X(01)00402-X. [DOI] [PubMed] [Google Scholar]
- Aujla H, Beninger RJ. Intra-BLA or intra-NAc infusions of the dopamine D3 receptor partial agonist, BP 897, block intra-NAc amphetamine conditioned activity. Behavioral Neuroscience. 2004;118:1324–1330. doi: 10.1037/0735-7044.118.6.1324. [DOI] [PubMed] [Google Scholar]
- Bach S, Knockaert M, Reinhardt J, Lozach O, Schmitt S, Baratte B, et al. Roscovitine targets, protein kinases and pyridoxal kinase. Journal of Biological Chemistry. 2005;280:31208–31219. doi: 10.1074/jbc.M500806200. [DOI] [PubMed] [Google Scholar]
- Banasikowski TJ, Bespalov A, Drescher K, Behl B, Unger L, Haupt A, et al. Double dissociation of the effects of haloperidol and the dopamine D3 receptor antagonist ABT-127 on acquisition vs. expression of coaine-conditioned activity in rats. Journal of Pharmacology and Experimental Therapeutics. 2010;335:506–515. doi: 10.1124/jpet.110.171348. [DOI] [PubMed] [Google Scholar]
- Barnett DGS, Bibb JA. The role of Cdk5 in cognition and neuropsychiatric and neurological pathology. Brain Research Bulletin. 2011;85:9–13. doi: 10.1016/j.brainresbull.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides DR, Quinn JJ, Zhong P, Hawasli AH, DiLeone RJ, Kansy JW, et al. Cdk5 modulates cocaine reward, motivation, and striatal neuron excitability. Journal of neuroscience. 2007;27:12967–12976. doi: 10.1523/JNEUROSCI.4061-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, et al. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- Bryant CD, Graham ME, Distler MG, Munoz MB, Li D, Vezina P, et al. A role for caein kinase 1 epsilon in the locomotor stimulant respone to methamphetamine. Psychopharmacology. 2009;203:703–711. doi: 10.1007/s00213-008-1417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buraei Z, Schofield G, Elmslie KS. Roscovitine differentially affects CaV2 and Kv channels by binding to the open state. Neuropharmacology. 2007;52:883–894. doi: 10.1016/j.neuropharm.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Boundy VA, Haile CN, Lane SB, Kalb RG, Neve RL, et al. Sensitization to morphine induced by viral-mediated gene transfer. Science. 1997;277:812–814. doi: 10.1126/science.277.5327.812. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Neve RL. Viral-mediated gene transfer to study the behavioral correlates of CREB function in the nucleus accumbens of rats. Methods in Molecular Medicine. 2003;79:331–350. doi: 10.1385/1-59259-358-5:331. [DOI] [PubMed] [Google Scholar]
- Cervo L, Samanin R. Effects of dopaminergic and glutamatergic receptor antagonists on the acquisition and expression of cocaine conditioned place preference. Brain Research. 1995;673:242–250. doi: 10.1016/0006-8993(94)01420-m. [DOI] [PubMed] [Google Scholar]
- Cho S, Meriney SD. The effects of presynaptic calium channel modulation by reoscovitine on transmitter release at the adult frog neuromuscular junction. European Journal of Neuroscience. 2006;23:3200–3208. doi: 10.1111/j.1460-9568.2006.04849.x. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Mueller H, Browman KE, Badiani A, Robinson TE. A comparison of two behavioral measures of psychomotor activation following intravenous amphetamine or cocaine: Dose- and sensitization-dependent changes. Behavioral Pharmacology. 1999;10:205–213. doi: 10.1097/00008877-199903000-00009. [DOI] [PubMed] [Google Scholar]
- Fabian MA, Biggs WH, 3rd, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nature Biotechnology. 2005;23:329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J. Cyclin-dependent kinase 5 is required for associative learning. Journal of Neuroscience. 2002;22:3700–3707. doi: 10.1523/JNEUROSCI.22-09-03700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Pang PT, Lu B, Tsai L-H. Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron. 2005;48:825–838. doi: 10.1016/j.neuron.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Berry RW, Disterhoft JF, Power JM, Ven der Zee EA. Associative learning elicits the formation of multiple-synapse boutons. Journal of Neuroscience. 2001;21:5568–5573. doi: 10.1523/JNEUROSCI.21-15-05568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77:867–872. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Hayashi K, Chambon P, et al. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nature Neuroscience. 2007;10:880–886. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, et al. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–44. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Kantor L, Zhang M, Guptaroy B, Park YH, Gnegy ME. Repeated amphetamine couples norepinephrine transporter and calcium channel activities in PC12 cells. Journal of Pharmacology and Experimental Therapeutics. 2004;311:1044–1051. doi: 10.1124/jpet.104.071068. [DOI] [PubMed] [Google Scholar]
- Kiraly DD, Ma X-M, Mazzone CM, Xin X, Mains RE, Eipper BA. Behavioral and morphological responses to cocaine require kalirin7. Biological Psychiatry. 2010;68:249–255. doi: 10.1016/j.biopsych.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleske AJ. Molecular mechanisms of dendrite stability. Nature Reviews Neuroscience. 2013;14:536–550. doi: 10.1038/nrn3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R, LeDoux J. Structural plasticity and memory. Nature Reviews Neuroscience. 2004;5:45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. Journal of Neuroscience. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M, Vezina P. Striatal ups and downs: Their roles in vulnerability to addictions in humans. Neuroscience and Biobehavioral Reviews. 2013;37:1999–2014. doi: 10.1016/j.neubiorev.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Herrera S, Bubula N, Nikitina E, Palmer AA, Hanck DA, et al. Casein kinase 1 enables nucleus accumbens amphetamine-induced locomotion by regulating AMPA receptor phoshphorylation. Journal of Neurochemistry. 2011;118:237–247. doi: 10.1111/j.1471-4159.2011.07308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Xue Y, Wang J, Fang Q, Li Y, Zhu W, et al. Basolateral amygdala cdk5 activity mediates consolidation and reconsolidation of memories for cocaine cues. Journal of Neuroscience. 2010;30:10351–10359. doi: 10.1523/JNEUROSCI.2112-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Singer BF, Baker LK, Wilke G, Inamine H, Bubula N, et al. Transient overexpression of α-Ca2+/calmodulin-dependent protein kinase II in the nucleus accumbens shell enhances behavioral responding to amphetamine. Journal of Neuroscience. 2010;30:939–949. doi: 10.1523/JNEUROSCI.4383-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie N, Canestrelli C, Noble F. Transfer of neuroplasticity from nucleus accumbens core to shell is required for cocaine reward. Plos One. 2012;7:e30241. doi: 10.1371/journal.pone.0030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DA, Richer E, Benkovic SA, Hayashi K, Kansy JW, Hale CF, et al. Striatal dysregulation of Cdk5 alters locomotor responses to cocaine, motor learning, and dendritic morphology. Proceedings of the National Academy of Science. 2008;105:18561–18566. doi: 10.1073/pnas.0806078105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses SN, Sutherland RJ, McDonald RJ. Differential involvement of amygdala and hippocampus in responding to novel objects and contexts. Brain Research Bulletin. 2002;58:517–527. doi: 10.1016/s0361-9230(02)00820-1. [DOI] [PubMed] [Google Scholar]
- Neve RL, Howe JR, Hong S, Kalb RG. Introduction of the glutamate receptor subunit 1 into motor neurons in vitro and in vivo using a recombinant herpes simplex virus. Neuroscience. 1997;79:435–447. doi: 10.1016/s0306-4522(96)00645-8. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Bibb JA, Nestler EJ, Ouimet CC, Taylor JR, Greengard P. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3rd Edtion Academic Press; New York, NY: 1997. [Google Scholar]
- Pellegrino LJ, Pellegrino AS, Cushman AJ. A stereotaxic atlas of the rat brain. Plenum Press; New York, NY: 1979. [Google Scholar]
- Penzes P, Johnson RC, Sattler R, Zhang X, Huganir RL, Kambampati V, et al. The neuronal Rho-GEF Kalirin-7 interacts with PDZ domain-containing proteins and regulates dendritic morphogenesis. Neuron. 2001;29:229–242. doi: 10.1016/s0896-6273(01)00193-3. [DOI] [PubMed] [Google Scholar]
- Penzes P, Jones KA. Dendritic spine dynamics - a key role for kalirin-7. Trends in Neurosciences. 2008;31:419–427. doi: 10.1016/j.tins.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Quick EA, Reeder DC, Morgan ZR, Kalivas PW. Calcium-mediated second messengers modulate the expression of behavioral sensitization to cocaine. Journal of Pharmacology and Experimental Therapeutics. 1998;286:1171–1176. [PubMed] [Google Scholar]
- Price DA, Sorkin A, Zahniser NR. Cyclin-dependent kinase 5 inhibitors: Inhibition of dopamine transporter activity. Molecular Pharmacology. 2009;76:812–823. doi: 10.1124/mol.109.056978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. Journal of Neuroscience. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine and cocaine. European Journal of Neuroscience. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Singer BF, Forneris J, Vezina P. Inhibiting cyclin-dependent kinase 5 in the nucleus accumbens enhances the expression of amphetamine-induced locomotor conditioning and sensitization. (in review) [DOI] [PMC free article] [PubMed]
- Singer BF, Loweth JA, Neve RL, Vezina P. Transient viral-mediated overexpression of α-calcium/calmodulin-dependent protein kinase II in the nucleus accumbens shell leads to long-lasting functional upregulation of α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptors: dopamine type-1 receptor and protein kinase A dependence. European Journal of Neuroscience. 2010;31:1243–1251. doi: 10.1111/j.1460-9568.2010.07155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer BF, Tanabe LM, Gorny G, Jake-Matthews C, Li Y, Kolb B, et al. Amphetamine-induced changes in dendritic morphology in rat forebrain correspond to associative drug conditioning rather than nonassociative drug sensitization. Biological Psychiatry. 2009;65:835–840. doi: 10.1016/j.biopsych.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. Neurobiology of conditioning to drugs of abuse. In: Kalivas PW, Samson HH, editors. The Neurobiology of Drug and Alcohol Addiction. New York Academy of Sciences; New York: 1992. pp. 335–346. [DOI] [PubMed] [Google Scholar]
- Stewart J, Vezina P. Environment-specific enhancement of the hyperactivity induced by systemic or intra-VTA morphine injections in rats pre-exposed to amphetamine. Psychobiology. 1987;15:144–153. [Google Scholar]
- Stewart J, Vezina P. Conditioning and behavioral sensitization. In: Kalivas PW, Barnes CD, editors. Sensitization in the Nervous System. Telford Press; Caldwell, NJ: 1988. pp. 207–224. [Google Scholar]
- Stewart J, Vezina P. Extinction procedures abolish conditioned stimulus control but spare sensitized responding to amphetamine. Behavioural pharmacology. 1991;2:65–71. [PubMed] [Google Scholar]
- Taylor JR, Lynch WJ, Sanchez H, Olausson P, Nestler EJ, Bibb JA. Inhibition of Cdk5 in the nucleus accumbens enhances the locomotor-activating and incentive-motivational effects of cocaine. Proceedings of the National Academy of Sciences. 2007;104:4147–4152. doi: 10.1073/pnas.0610288104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neuroscience and Biobehavioral Reviews. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Vezina P, Leyton M. Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology. 2009;56:160–168. doi: 10.1016/j.neuropharm.2008.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Stewart J. Amphetamine administered to the ventral tegmental area but not to the nucleus accumbens sensitizes rats to systemic morphine: Lack of conditioned effects. Brain Research. 1990;516:99–106. doi: 10.1016/0006-8993(90)90902-n. [DOI] [PubMed] [Google Scholar]
- Wang X, Cahill ME, Werner CT, Christoffel DJ, Golden SA, Xie Z, et al. Kalirin-7 mediates cocaine-induced AMPA receptor and spine plasticity, enabling incentive sensitization. Journal of Neuroscience. 2013;33:11012–11022. doi: 10.1523/JNEUROSCI.1097-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-C, Hsiao S. Amphetamine sensitization: Nonassociative and associative components. Behavioral Neuroscience. 2003;117:961–969. doi: 10.1037/0735-7044.117.5.961. [DOI] [PubMed] [Google Scholar]
- Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, et al. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–656. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin X, Wang Y, Ma X, Rompolas P, Keutmann HT, Mains RE, et al. Regulation of Kalirin by Cdk5. Journal of Cell Science. 2008;121:2601–2611. doi: 10.1242/jcs.016089. [DOI] [PMC free article] [PubMed] [Google Scholar]