Abstract
Our expectations about an event can strongly shape our subjective evaluation and actual experience of events. This ability, applied to the modulation of pain, has the potential to affect therapeutic analgesia substantially and constitutes a foundation for non‐pharmacological pain relief. A typical example of such modulation is the placebo effect. Studies indicate that placebo may be regarded as a reward, and brain activity in the reward system is involved in this modulation process. In the present study, we combined resting‐state functional magnetic resonance imaging (rs‐fMRI) measures, genotype at a functional COMT polymorphism (Val158Met), and personality measures in a model to predict the magnitude of placebo conditioning effect indicated by subjective pain rating reduction to calibrated noxious stimuli. We found that the regional homogeneity (ReHo), an index of local neural coherence, in the ventral striatum, was significantly associated with conditioning effects on pain rating changes. We also found that the number of Met alleles at the COMT polymorphism was linearly correlated to the suppression of pain. In a fitted regression model, we found the ReHo in the ventral striatum, COMT genotype, and Openness scores accounted for 59% of the variance in the change in pain ratings. The model was further tested using a separate data set from the same study. Our findings demonstrate the potential of combining resting‐state connectivity, genetic information, and personality to predict placebo effect. Hum Brain Mapp 35:4583–4593, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: fMRI, conditioning, resting state, regional homogeneity, placebo analgesia
INTRODUCTION
Belief is powerful medicine. Our expectations can significantly influence our experience of pain (Atlas et al., 2010; Keltner et al., 2006; Koyama et al., 2005; Porro et al., 2002). A typical example of such modulation is the placebo analgesia effect. Although the mechanism of placebo analgesia is still under investigation, the dominant theories invoke classical conditioning and expectancies as explanatory tools (Benedetti, 2008; Benedetti et al., 2003; Finniss et al., 2010; Jensen et al., 2012).
Although placebo effects are ubiquitous, not everyone responds to placebo in the same way. There is a profound individual difference in basal psychophysical pain responses (Coghill et al., 2003; Edwards et al., 2003; Yarnitsky et al., 1995), which may be further influenced by dopamine‐related genes (Kim et al., 2004; Mogil et al., 1996; Schmahl et al., 2012) and environmental influences (Taddio et al., 1995, 2002). The capacity to modulate pain also varies substantially across individuals (Tracey and Mantyh, 2007; Wager et al., 2011; Wanigasekera et al., 2012; Zubieta et al., 2006). This reflects a crucial difference in the ability to recruit self‐modulatory neurocircuitry that relieve noxious sensations. Understanding the biological basis of such individual differences is a crucial step towards personalized medicine.
Previous studies suggest that striatal dopamine release underlies placebo response in patients with Parkinson's disease (de la Fuente‐Fernandez et al., 2001). However, the role of the ventral striatum in the cognitive modulation of pain has not yet been established. Recent studies have examined a number of correlates of placebo response magnitude using task‐based fMRI (Wager et al., 2011), structural MRI (Schweinhardt et al., 2009; Stein et al., 2012), resting‐state fMRI (Hashmi et al., 2012; Kong et al., 2013a), positron emission tomography (PET) (Scott et al., 2007, 2008), genetics (Hall et al., 2012), and personality assessments (Geers et al., 2010; Morton et al., 2009; Pecina et al., 2012; Vase et al., 2005). Each of these studies has examined a single explanatory variable. Few studies have investigated predictors of placebo or related effects by combining different measurements. We reasoned that if reward is associated with motivated behavior, brain regions in reward circuitry, dopamine‐related genetic variation, and personality may all contribute to an individual's response to cue‐conditioned effects on pain. Another recent study showed that the analgesic effect of remifentanil is positively correlated with the trait reward responsiveness, and is predicted by the neuronal response to painful noxious stimuli before administration of the drug in key brain regions of the reward circuitry, including the nucleus accumbens and the ventral tegmental area (Wanigasekera et al., 2012). In light of the results from these experiments, we used three measures in our study [i.e., resting‐state brain activity, functional genetic variation (the COMT Val158Met polymorphism), and personality assessed by the Neuroticism–Extroversion–Openness (NEO) Personality Inventory] to predict the magnitude of a conditioned analgesia effect using a modified model applied in previous studies (Atlas et al., 2010; Keltner et al., 2006; Koyama et al., 2005; Ploghaus et al., 2001; Seymour et al., 2005). Specifically, we explored the association between pre‐test resting‐state regional activity and the amplitude of the cue effects, using regional homogeneity (ReHo). ReHo measures the similarity of the time series of a given voxel with its neighbors in a single region, providing information about local temporal synchrony in the brain (Liu et al., 2010; Zang, et al., 2004). To incorporate genetic variation, we focused on a common functional polymorphism in the COMT gene (Val158Met, rs4680), which has been associated with placebo response in previous studies, with dopaminergic tone (Hall et al., 2012; Leuchter et al., 2009; Meyer‐Lindenberg et al., 2005). In addition, we examined the “big five” personality traits to assess their association with conditioning response. Finally, we attempted to build a model to predict conditioning response by combining results obtained from ReHo, COMT genotype, and personality measurements. We randomly selected 80% of the data to build the model, and then used the remaining 20% of subjects to test the model.
EXPERIMENTAL PROCEDURES
We briefly describe the experimental procedures below (see Kong et al. 2013a for full details). In one of our previous studies, we used independent component analysis (ICA) and identified the association between the frontoparietal network during pre‐test resting state and conditioning analgesia effect (Kong et al., 2013a). ICA is a mathematic technique that maximizes statistical independence among its components. While ICA is used to spatially identify distinct resting state networks, ReHo provides a distinct method to investigate the regional synchronization of resting‐state signals. In the present study, we reanalyzed the data, focusing on building a model combining resting‐state regional coherence using ReHo (a resting‐state functional connectivity analysis method different from ICA analysis), COMT gene expression, and personality measurements to predict placebo conditioning effect. This result has not been reported before.
Subjects
Forty‐eight right‐handed healthy volunteers (29 females), aged 21–33 years (26.4 ± 3.6, mean ± SE) participated in the study. None of them reported neurological diseases, a history of any substance dependence, or a history of clinically significant head trauma. The Institutional Review Board at Massachusetts General Hospital approved the study and all subjects gave written informed consent.
Thermal Pain Stimulation
Thermal pain stimuli were delivered to the skin of the right volar forearm using a TSA‐2001 Thermal Sensory Analyzer with a 3 cm × 3 cm probe (Medoc Advanced Medical Systems, Rimat Yishai, Israel). All stimuli were initiated from a baseline temperature of 32°C and increased to a target temperature. Each stimulus was presented for 12 s, including 2.5 s to ramp up to the target temperature and 2.5 s to ramp down to baseline. After each stimulus, subjects rated their pain according to the Gracely Sensory scale (Gracely et al., 1978), which asks subjects to self‐report the sensory intensity of pain on a scale of 0–20 with 13 verbal descriptors. This scale has been used in several brain imaging studies on pain and placebo effects from our lab (Kong et al., 2006a, b, 2008, 2009a, b).
Experimental Procedure
At the beginning of the study, subjects were told that the aim of the experiment was to investigate the brain's response to different levels of thermal pain. Subjects were then familiarized with the visual presentation paradigm, including a pre‐stimulus cue, a pain stimulus symbol, and a post‐stimulus rating scale. In addition, subjects were told that the pre‐stimulus cue (text saying either “HIGH” or “LOW”) would indicate the level of the subsequent pain stimulus.
All subjects who participated in the fMRI study also participated in an unrelated behavioral study to investigate the acute analgesic effect of different treatments (real and sham acupuncture treatment, and placebo pill) (Kong et al., 2013a). An ascending series of heat stimuli (starting at 38°C and increasing at the rate of 1°C) were applied to both the arms in the first session. The baseline temperature for the ascending series (32°C) was systemically increased to target temperatures in order to obtain subjective pain tolerance levels or to a maximum of 52°C. Temperatures that elicited subjective intensity ratings in the LOW pain range (∼5 with a verbal descriptor of weak on the 0–20 Sensory Box Scale) and HIGH pain range (∼15 with verbal descriptor of intense on the 0–20 Sensory Box Scale) were selected for each subject and used in the treatment study as well as the present MRI study.
The present study was separated by at least two weeks from the behavioral study to avoid potential influence. Thus, at the time of this MRI study on cue effects, subjects were familiar with the pain rating scales and heat pain administration. Right before the fMRI scan, a brief pain sensitivity test was performed to further confirm the subjective high and low temperatures applied in this study and adjustments were made where needed.
During fMRI scanning, an initial resting‐state fMRI scan was collected (data analyzed for this report), followed by three different series of pseudo‐randomized pain sequences applied on the right distal forearm during fMRI scanning. Subjects were instructed to focus on a small black fixation cross in the center of the screen in front of them. They were told “During the scan, please keep your eyes open and focus on the black screen. You can blink normally. But please keep your eyes open and do not fall asleep.” The first scan was a contextual learning scan where subjects were presented with a pre‐stimulus cue, indicating (without deception) whether they would be administered a LOW or HIGH pain stimulus. The duration of the cue was 2 s and the time before onset of the pain stimulus varied among 4, 6, 8, and 10 s. The duration of the pain stimulus was always 12 s and the intensity of the stimulus for this first sequence always corresponded to the pre‐stimulus cue. After a delay of 4, 6, or 8 s, the Sensory Box Scale was displayed on the screen for 8 s and subjects rated the intensity of their subjective pain by moving a cursor along the scale. The interval between the end of the rating task and onset of the next stimulus cue ranged from 8 to 14 s, with an average of 12 s. In total, this learning sequence included four LOW and four HIGH pain stimuli.
The initial contextual learning and conditioning scan was followed by two test scans in which the LOW cue was sometimes followed by the HIGH pain stimulus (HP) (the LC condition), creating a condition in which subjects were expected to report less pain in response to a suggested low stimulus, and sometimes followed by the LOW pain stimulus. Both test scans included nine stimuli, three of which stimuli were cued as HIGH pain and six of which were cued as LOW pain stimuli. Following all HIGH pain cues, a high pain stimulus was delivered (the HC condition). Following three of the six LOW cues, a HIGH pain stimulus was delivered (the LC condition) instead of a LOW pain stimulus. The order of stimuli was randomized. All other timing aspects of the two test scans were identical to the first contextual learning/conditioning scan (Fig. 1A). The subjective pain ratings and fMRI signal changes evoked by the different cues (LC or HC with identical HIGH heat pain stimuli) in runs two and three were the primary outcomes of this study. Finally, an overall evaluation of personality traits was performed using the scores of the five dimensions of the Neuroticism–Extroversion–Openness (NEO) Personality Inventory‐Revised (Costa and McCrae, 1997). The NEO measures the Big Five personality traits: Neuroticism, Extraversion, Openness to Experience, Agreeableness, and Conscientiousness.
Figure 1.
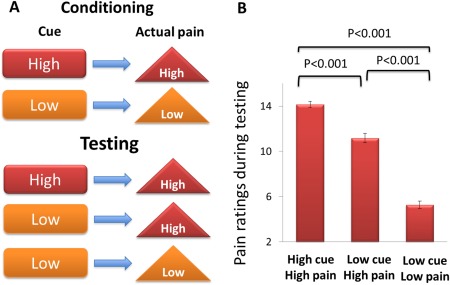
The experimental paradigm (A) and behavioral results (B). The conditioning analgesia effect is defined by the high‐cue high pain versus low‐cue high pain contrast. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
fMRI Scanning
Measurements of brain activity were obtained using a 3 T Siemens MRI System equipped for Echo Planar Imaging (EPI). A 12‐channel head coil was used as the RF signal receiver. Subjects were scanned with a high‐resolution MPRAGE sequence and then a 6‐min resting‐state fMRI. During the resting‐state fMRI scan, subjects were asked to look at a dark screen. The scan acquisition included 47 slices acquired using interleaved scanning with slice thickness of 3 mm without gaps, TR = 3000 ms, TE = 30 ms, flip angle = 90°, and a 3 × 3 mm in‐plane spatial resolution. The scan time of the resting‐state fMRI was approximately 6 min.
Following this resting fMRI, three functional runs were collected during pain administration (see Kong et al. (2012)). In the scanner, cushions and earplugs were used to reduce head movement and dampen scanner noise. Visual presentation was performed using E‐prime 2.0 software (Psychology Software Tools, Inc., USA) projected onto a screen in front of the subject.
Resting‐State MRI Analysis
The first 10 volumes of each functional time series were discarded because of instability of the initial MRI signal and adaptation of participants to the circumstance, leaving 114 volumes in total. The remaining fMRI images were slice time corrected, head‐motion corrected, normalized to the standard SPM8 Montreal Neurological Institute (MNI) template, and then re‐sampled to 3‐mm cubic voxels. After linear detrending, data was filtered using typical temporal bandpass (0.01–0.08 Hz).
Regional homogeneity was calculated using REST software (Song et al., 2011) (http://restfmri.net/forum/index.php). ReHo analysis was performed for each participant by calculating the Kendall's coefficient of concordance (KCC) of the time series of a given voxel with those of its nearest neighbors (26 voxels) in a voxel‐wise analysis:
where W ranges from 0 to 1; , where rij is the rank of the ith time point in the jth voxel; = (n + 1)k/2 is the mean of the Ri, n is the number of time points of each voxel time series (here n = 170); and k is the number of time series within the measured cluster (here k = 27, the central voxel plus its 26 neighbors). The intracranial voxels were extracted to make a mask. For standardization purposes, each individual subject's ReHo map was divided by its own mean ReHo within the mask. The resulting fMRI data were then spatially smoothed with a Gaussian kernel of 6 × 6 × 6 mm3 full‐width at half‐maximum.
To explore the association and conditioning cue effect as indicated by pain rating changes evoked by high and low cues, a regression analysis was performed on the individual normalized ReHo maps in a voxel‐by‐voxel manner using conditioning effect as a covariate. A threshold of family‐wise error (FWE) corrected threshold of P < 0.05 after small volume correction (svc) was set. The independent region of interest (ROI) for svc were the bilateral ventral striatum (±13, 18, −1, radius of the sphere = 4 mm) obtained from other research group (Schweinhardt et al., 2009), see Figure 2A. These coordinates were selected based on a previous study showing gray matter density in ventral striatum was correlated with “dopamine‐related personality” and placebo responses (Schweinhardt et al., 2009). For non‐region of interest brain regions, we used a voxel‐wise threshold P < 0.005 uncorrected and P < 0.05 FWE corrected at cluster level. All coordinates were reported in MNI coordinates, as used by SPM.
Figure 2.
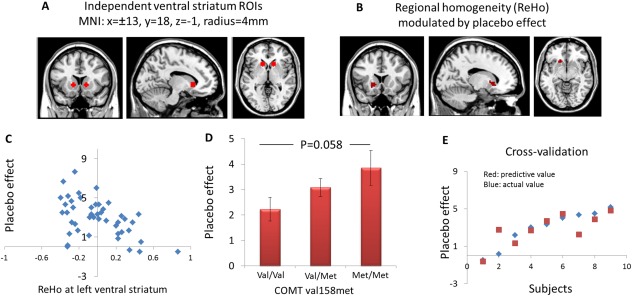
(A) The bilateral ventral striatum ROIs using independent coordinates identified in a previous study. (B) Regional homogeneity (ReHo) at left ventral striatum showed a significant negative correlation with placebo effect, i.e., subjective pain rating changes evoked by different cues (high cue minus low cue). (C) Scatter plots depict the relationships between ReHo in the left ventral striatum and the placebo effect. The y axis of the scatter graph indicates the subjective pain rating changes, the x axis of the scatter graph indicates the ReHo values. (D) The correlation between placebo effect and COMT polymorphism. (E) The relationship between predicted placebo scores (in red) and observed placebo scores (in blue) from cross‐validation analysis. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Genotyping
DNA was isolated from saliva samples by using Oragene‐DNA kits (DNA Genotek). Genotyping at the rs4680 single nucleotide polymorphism (Val158Met) was performed at the Psychiatric and Neurodevelopmental Genetics Unit at the MGH Center for Human Genetic Research using Sequenom's iPLEX Gold® chemistry and the MassARRAY® platform (Sequenom) with primers that have been previously described (Loggia et al., 2011; Roffman et al., 2013). The major steps in this process included the following: DNA amplification by PCR, post‐PCR removal of phosphate groups from the unincorporated dNTPs using shrimp alkaline phosphatase (SAP) to deactivate them, single‐base extension reactions for allele differentiation, salt removal using an ion‐exchange resin, SpectroCHIP® plating, and mass correlated genotype calling via MALDI‐TOF mass spectrometry. Previous studies have shown that individuals homozygous for the Val allele of COMT val158met gene have low levels of tonic extracellular dopamine, Met/Met individuals have a relative high level of extracellular dopamine, whereas Val/Met individuals have intermediate dopamine levels (Mannisto and Kaakkola, 1999). Here, we focused on the COMT val158met genotype.
RESULTS
Of the 48 subjects enrolled in the study, 2 subjects were dropped due to abnormal brain structures observed during MRI scanning. Behavioral data of the remaining 46 subjects showed that the subjective pain ratings for the low‐cue low pain condition averaged 5 ± 2.3 (mean ± SD), the low‐cue high pain condition (LC) averaged 11 ± 2.9, and the high‐cue high pain condition (HC) averaged 14 ± 2.0 during the test sequences (sequences 2 and 3). A one‐way mixed model was applied with mean pain ratings as a response, subject as a random effect, and conditions (low‐cue low pain, low‐cue high pain and high‐cue high pain) as a fixed effect for the two test sequences. The result showed that the contrasts low‐cue low pain versus high‐cue high pain (t = −24.7, P < 0.001), low‐cue low pain versus low‐cue high pain (t = −16.2, P < 0.001), and low‐cue high pain versus high‐cue high pain (t = 1.08, P < 0.001) were all highly significant (see Fig. 1B). These results indicate that the subjective pain ratings were significantly different depending on the preceding cues even when identical pain stimuli were applied. The differences between the low‐cue high pain condition and high‐cue high pain condition across different individuals ranged from −0.5 to 7.6 (median = 3.0), indicating significant interindividual variability. In order to explore whether or not trial‐to‐trial rating variability during the conditioning phase predicts placebo effect in the test phase, we used a median split (top half versus bottom half) to categorize subjects into a high variability group and a low variability group. Rating variability is calculated using the standard deviation (SD) of the four low pain ratings and the SD of the four high pain ratings in the conditioning phase. We found no significant difference in placebo effect between the high variability group and low variability group (low pain trials, t = −0.24, P = 0.81; high pain trials, t = 0.43, P = 0.67).
Resting‐state fMRI Results
The subjective pain rating changes (HC − LC) were used as an index of placebo analgesia effect (Kong et al., 2013a). Regression analysis between the subjective pain rating changes (HC − LC) and the pre‐test resting‐state fMRI regional homogeneity showed a significant negative association in the left ventral striatum ([−15 18 −3], z = 3.78, svc) (Fig. 2B), i.e., the higher the ReHo value in the left ventral striatum during pre‐test resting state, the less the expectancy/cue could modulate the pain experience as indicated by subjective pain rating changes. The correlation between conditioning effect and average ReHo values in the left ventral striatum ROI (independent ROI) was significant, r = −0.50, P < 0.001 (Fig. 2C). No significant association (positive or negative) was found in other brain regions.
COMT Genotype
The genotype information for two subjects was missing. The minor (Met) allele frequency of COMT val158met was 0.45 (val/val n = 13, val/met n = 24, and met/met n = 7) and the SNP was in Hardy–Weinberg Equilibrium (P = 0.20). The COMT rs4680/val158met genotypes were coded for each participant as follows: G → A, val → met, 1 = met/met, 0 = val/met; −1 = val/val. The conditioning effect was significantly associated with COMT Met alleles (r = −0.31, P = 0.04, Fig. 2D).
Personality and Conditioning Effect
The correlation between conditioning effect and each of the NEO dimensions (Neuroticism, Extraversion, Openness to Experience, Agreeableness, and Conscientiousness) was not significant, P values range from 0.2 to 0.5.
Regression Model
Finally, we attempted to build a regression model to identify the most significant predictors of conditioning effect. To do that, we used a stratified cross‐validation procedure in which the model was built on randomly selected 4/5 of the subjects and tested on the remaining 1/5 of the subjects. Model building was conducted by a backward selection regression in which the reduction in pain rating differences (HC − LC) was the dependent variable and independent variables (predictors) included ReHo, COMT genotype, and the five personality dimensions. Results showed that the most significant predictors were the ReHo in the left ventral striatum, rs4680/val158met, and Openness, accounting for 51% of the variance in conditioning placebo analgesia (P < 0.001, see Table 1). Using forward selection regression and stepwise selection regression also yielded similar results.
Table 1.
Regression model results
| Variable | Beta | T | P value |
|---|---|---|---|
| ReHo | −4.427 | −5.109 | <0.001 |
| COMT | 0.981 | 2.847 | 0.008 |
| Openness | −0.58 | −2.328 | 0.027 |
Following the model building, we used the remaining 1/5 data to validate the model. The predicted pain rating difference and observed pain rating difference are shown in Figure 2E. The correlation between predicted values and observed values was significant (r = 0.77, P = 0.015). The prediction model explained approximately 59% of the variance in the conditioning response for subjects (N = 9) in the test sample.
DISCUSSION
In this study, we explore the predictors of individual differences in conditioned cue effects using pre‐test resting‐state functional connectivity, COMT genotype, and personality. The results showed that baseline resting‐state ReHo activity in the left ventral striatum was significantly negatively correlated with conditioning effects. The conditioning effect was also significantly correlated with the number of Met alleles at rs4680 in COMT. Regression model building indicated that intrinsic ventral striatum activity, COMT genotype, and Openness were the three most important predictors of conditioning effect, accounting for 59% of the variance of conditioning responses in the independent test cohort of subjects.
Expectation has been shown to be critically involved in placebo and placebo‐related responses. Previous studies have identified brain correlates of placebo analgesia at the group level (Elsenbruch et al., 2012; Kong et al., 2006a; Lu et al., 2010). Only recently have researchers begun to investigate the neural correlates of individual differences in placebo analgesia. The idea that symptom reduction (reduced suffering/pain) can be regarded as a special case of reward has been suggested as a potential mechanism to explain how positive expectancy can produce pain relief (Benedetti, 2009; de la Fuente‐Fernandez et al., 2001; Flaten et al., 2011; Leknes et al., 2011; Petrovic et al., 2005; Rhudy et al., 2008; Scott et al., 2007; Seymour et al., 2005). Accumulating evidence suggests that the reward network may be crucial for the modulatory effect of expectancy cues (Atlas et al., 2010). Results from previous studies show that expectancy of pain relief can be regarded as a specific form of reward processing that recruits activity in reward regions such as the ventral striatum (Benedetti, 2009; de la Fuente‐Fernandex et al., 2002; Kim et al., 2006; Leknes et al., 2011; Petrovic et al., 2005; Schweinhardt et al., 2009; Scott et al., 2007). Dopamine activation in the striatum has been detected with positron emission tomography (PET) during receipt of a placebo in patients diagnosed with Parkinson's disease in a manner proportional to the anticipated improvement in motor control (Lidstone et al., 2010). Another PET study found that high placebo responses were associated with greater dopaminergic and opioid activity in the ventral striatum (Scott et al., 2008). Ventral striatal dopamine release accounted for 25% of the variance in placebo effects (Scott et al., 2008). Individual variations in ventral striatal response to reward expectation accounted for 28% of the variance in the magnitude of placebo analgesia (Scott et al., 2007), suggesting that the reward network may represent a basic component of analgesic expectancy. Our resting‐state findings further support the notion that conditioning/placebo effects are related to brain activity in reward processing circuitry.
Our study illustrates the feasibility of using resting‐state brain activity (e.g., ReHo) to investigate placebo effect to identify conditioning placebo responders. Analysis of ReHo has been successfully used to detect local abnormalities in subjects with different psychiatric disorders, (Cocchi et al., 2012; Lai and Wu, 2012; Liu et al., 2008; Paakki et al., 2010; Wu et al., 2011, 2009; Yao et al., 2009; Yuan et al., 2008) and to predict inhibition responses (Tian et al., 2012). Elucidating this linkage may enhance our understanding of the role of resting‐state BOLD spontaneous fluctuations as well as shed new light on individual differences in behavior and the prediction of behavioral responses. In recent years, spontaneous low‐frequency fluctuations in brain activity during rest, measured by fMRI, have been shown to reflect meaningful characteristics of underlying neurobiology (Biswal et al., 1995; Fox and Raichle, 2007; Raichle and Mintun, 2006). Since rs‐fMRI is non‐invasive and does not require the subject to perform cognitive tasks during image acquisition, its use is substantially simpler than task‐based neuroimaging approaches. For example, it can be implemented in populations that may have difficulties performing task‐based imaging studies, such as very young or elderly patients or patients suffering intense pain. Due to such simplicity and the reliability of rs‐fMRI data, this modality offers increased feasibility and potential for clinical application in the future.
Positive correlation between experience of placebo analgesia and gray matter density in the bilateral ventral striatum has been found in a previous structural study (Schweinhardt et al., 2009). It seems surprising that we observed negative rather than positive correlations between the ventral striatum ReHo and conditioning effect. ReHo represents the temporal coherence of the spontaneous neural activity in the regional brain. Lower ReHo might be a sign of weak spontaneous local functionality at rest. Thus, the negative correlation between ReHo in ventral striatum and conditioning cue responses may reflect the ventral striatum's vulnerability to modulation by other regions (e.g., the prefrontal cortex). Moreover, this result may be consistent with previous results that suggest an association between the placebo effect and the gray matter density in the ventral striatum (Schweinhardt et al., 2009) and endogenous opioid release in ventral striatum during pain (Scott, et al., 2007, 2008). It worth noting that there have been no studies on investigating the association among gray matter density, endogenous opioid release, and resting‐state coherence. Thus, the association among ReHo, task evoked brain activity, or gray matter size/density remains unclear and further exploration is needed. Nevertheless, all these findings suggest that the ventral striatum plays a key role in modulating pain experience after expectation manipulation.
The ventral striatum is rich in neuromodulatory dopamine neurons projected from the midbrain. An animal study found that the ascending nociceptive control evoked by intense chemical or thermal noxious stimuli depends on both opioid and dopamine links in the nucleus accumbens (Gear et al., 1999). Dopamine is primarily catabolized by COMT, which is present in dopaminergic projection areas (e.g., ventral striatum and the prefrontal cortex), where it performs O‐methylation in the extracellular space. It is generally believed that the major controller of ventral striatal dopamine is re‐uptake into terminals through the dopamine transporter. The anticipation of therapeutic benefit itself is rewarding and may be linked to dopamine (DA) release in the reward system. Differences in COMT activity are related allelic variations at the Val158Met polymorphism in the COMT gene. Individuals homozygous for the Val allele have the highest levels of enzyme activity and, thus, a relative decrease in tonic extracellular dopamine; Met/Met individuals have the lowest enzyme activity and a relative increase in extracellular dopamine whereas Val/Met individuals have intermediate levels of both (Mannisto and Kaakkola, 1999). The COMT val158met polymorphism has been associated with placebo response in irritable bowel syndrome (Hall et al., 2012) and major depressive disorder (Leuchter et al., 2009). Our findings suggest that this COMT polymorphism is also related to placebo analgesia in healthy subjects.
Placebo effects have also been correlated with several personality traits, though findings have not been consistent (Beedie et al., 2008; Geers et al., 2010; Kelley et al., 2009; Morton et al., 2009; Pecina et al., 2012; Schweinhardt et al., 2009; Vase et al., 2005). We found that Openness was an important predictor in our final model, along with Reho and COMT. One neuropsychological model on Openness implicates that this personality trait is associated with dopaminergic system (DeYoung et al., 2005). Openness is found to be related to reward dependence (Fruyt et al., 2000). A previous study found positive correlations between Openness scores and integrity of white matter adjacent to the dorsolateral PFC (Xu and Potenza, 2012). These fiber tracts interconnect most cortical and subcortical regions including basal ganglia (Robbins, 2007). Openness correlated positively with orbitofrontal activity in both the sexes, which suggests that reward and emotional processing underlie individual differences in Openness (Sutin et al., 2009). Although speculative, Openness may modulate the conditioning placebo effect via PFC–striatum interactions.
In our previous study, using the same data set but different data analysis method, we found significant fMRI signal increases in the frontoparietal network, including the bilateral frontal and parietal cortex and the pMPFC/ACC during both anticipation and pain application (Kong et al., 2013a). This activation pattern of the frontoparietal network, observed during pain anticipation and administration of cue modulation, highly overlapped with the frontoparietal network identified during pre‐test resting state using ICA (Kong et al., 2013a). There is a large body of literature suggesting that the frontoparietal network plays an important role in endogenous pain modulation (Wager et al., 2011). The striatum has an intense connection with the frontal regions, forming the frontal–striatal network (Lidstone and Stoessl, 2007). We speculate that the reward system serves as the motivational input that triggers the frontoparietal network and initiates the descending pain modulatory system to modulate the pain network. Further research is needed to explore this speculation.
It is well known that many non‐pharmacological factors/methods can modify our pain experience (Tracey and Mantyh, 2007) in addition to placebo conditioning. These factors include attention (Legrain et al., 2002; Villemure and Bushnell, 2002), emotion (Ochsner et al., 2006; Porro et al., 2002), meditation (Zeidan et al., 2011), and acupuncture (Kong et al., 2005). It is not known if all of these different pain modulation methods share a common central pathway for pain modulation, or if each is associated with a specific network. Further study investigating the similarity and difference in brain networks between pain modulation methods will shed new light on our understanding of the pain regulation process.
Several limitations of the current study should be noted. First, our experimental design did not include a high‐cue low pain condition to balance the design. This condition was omitted to maintain a balance between sample size and total scan time (each additional condition required more scan time).
Second, the sample size was relatively small; although the robust behavioral conditioning effect and resting‐state fMRI results validate that the sample size was sufficient for detecting cue‐related effects.
Third, volunteers participated in a behavioral experiment 2 weeks before the present experiment. This may have potentially influenced their performance and neural responses during the rest. Additionally, participants may have anticipated future pain during the resting‐state MRI scanning and this may have made the ‘resting state' different from the ‘neutral resting state,' in which participants had not undergone any pain stimuli and did not anticipate future pain. However, the resting‐state scanning was collected at the very beginning of the experiment before application of pain stimuli. The thermal probe was not attached so participants knew they would not receive any pain stimulation during the scan. Most importantly, the resting state was collected at least two weeks after the previous behavioral study and we used a within study design to control for any potential influence from previous experience.
Fourth, previous studies have found that the frequency of high activity COMT alleles may be modulated by ethnic differences (McLeod et al., 1994; Palmatier et al., 1999). In this study, there were 31 Caucasian, 6 Asian, 5 African American, 3 with more than one race, and 1 unknown race. However, when we added race into our model as a covariate, there was no significant change in the results. Future studies with larger sample size are needed to investigate the role of race to modulate the interaction between placebo analgesia and this gene or others. Finally, it is currently unknown whether different types of placebo effect share the same neural mechanisms. Future studies could test our findings on placebo effects in other domains and patient populations.
In summary, combining resting‐state fMRI, genotype, and personality, we found that dopamine‐related measurements including baseline ventral striatum coherence, functional variation at COMT, and openness to experience together could predict conditioning cue responses in healthy individuals. The elucidation of this mechanism may enhance our understanding of the individual variability of conditioning effects in response to pain. The substantial individual difference in placebo effect makes it challenging to control placebo responses in clinical trials or utilize it in clinical care. By identifying specific predictors of placebo response, our findings may inform the development of personalized approaches to analgesia.
There is no conflict of interest to claim for all authors.
REFERENCES
- Atlas LY, Bolger N, Lindquist MA, Wager TD (2010): Brain mediators of predictive cue effects on perceived pain. J Neurosci 30:12964–12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beedie C, Foad AJ, Coleman DA (2008): Identification of placebo responsive participants in 40km laboratory cycling performance. J Sports Sci Med:166–175. [PMC free article] [PubMed] [Google Scholar]
- Benedetti F (2008) Mechanisms of placebo and placebo‐related effects across diseases and treatments. Annu Rev Pharmacol Toxicol 48:33–60. [DOI] [PubMed] [Google Scholar]
- Benedetti F (2009) Placebo Effects: Understnding the Mechanism in Health and Disease. New York: Oxford University Press. [Google Scholar]
- Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I (2003) Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J Neurosci 23:4315–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Cocchi L, Bramati IE, Zalesky A, Furukawa E, Fontenelle LF, Moll J, Tripp G, Mattos P (2012) Altered functional brain connectivity in a non‐clinical sample of young adults with attention‐deficit/hyperactivity disorder. J Neurosci 32:17753–17761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill RC, McHaffie JG, Yen YF (2003) Neural correlates of interindividual differences in the subjective experience of pain. Proc Natl Acad Sci U S A 100:8538–8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT Jr, McCrae RR (1997) Stability and change in personality assessment: the revised NEO Personality Inventory in the year 2000. J Pers Assess 68:86–94. [DOI] [PubMed] [Google Scholar]
- de la Fuente‐Fernandex R, Schulzer M, Stoessl AJ (2002) The placebo effect in neurological disorders. Lancet Neurol 1:85–91. [DOI] [PubMed] [Google Scholar]
- de la Fuente‐Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ (2001) Expectation and dopamine release: mechanism of the placebo effect in Parkinson's disease. Science 293:1164–1166. [DOI] [PubMed] [Google Scholar]
- DeYoung CG, Peterson JB, Higgins DM (2005) Sources of openness/intellect: cognitive and neuropsychological correlates of the fifth factor of personality. J Pers 73:825–858. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Ness TJ, Weigent DA, Fillingim RB (2003) Individual differences in diffuse noxious inhibitory controls (DNIC): association with clinical variables. Pain 106:427–437. [DOI] [PubMed] [Google Scholar]
- Elsenbruch S, Kotsis V, Benson S, Rosenberger C, Reidick D, Schedlowski M, Bingel U, Theysohn N, Forsting M, Gizewski ER (2012) Neural mechanisms mediating the effects of expectation in visceral placebo analgesia: an fMRI study in healthy placebo responders and nonresponders. Pain 153:382–390. [DOI] [PubMed] [Google Scholar]
- Finniss DG, Kaptchuk TJ, Miller F, Benedetti F (2010) Biological, clinical, and ethical advances of placebo effects. Lancet 375:686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaten MA, Aslaksen PM, Lyby PS, Bjorkedal E (2011) The relation of emotions to placebo responses. Philos Trans R Soc Lond B Biol Sci 366:1818–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711. [DOI] [PubMed] [Google Scholar]
- Fruyt FD, De Wiele LV, Van Heeringen C (2000) Cloninger's psychobiological model of temperament and character and the five‐factor model of personality. Pers Indiv Differ:441–452. [Google Scholar]
- Gear RW, Aley KO, Levine JD (1999) Pain‐induced analgesia mediated by mesolimbic reward circuits. J Neurosci 19:7175–7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers AL, Wellman JA, Fowler SL, Helfer SG, France CR (2010) Dispositional optimism predicts placebo analgesia. J Pain 11:1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracely RH, McGrath PA, Dubner R (1978) Validity and sensitivity of ratio scales of sensory and affective verbal pain descriptors: manipulation of affect by diazepam. Pain 5:19–29. [DOI] [PubMed] [Google Scholar]
- Hall KT, Lembo AJ, Kirsch I, Ziogas DC, Douaiher J, Jensen KB, Conboy LA, Kelley JM, Kokkotou E, Kaptchuk TJ. (2012) Catechol‐O‐methyltransferase val158met polymorphism predicts placebo effect in irritable bowel syndrome. PLoS One 7:e48135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi JA, Baria AT, Baliki MN, Huang L, Schnitzer TJ, Apkarian AV (2012) Brain networks predicting placebo analgesia in a clinical trial for chronic back pain. Pain 153:2393–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Kaptchuk TJ, Kirsch I, Raicek J, Lindstrom KM, Berna C, Gollub RL, Ingvar M, Kong J (2012) Nonconscious activation of placebo and nocebo pain responses. Proc Natl Acad Sci U S A, 109:15959–15964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley JM, Lembo AJ, Ablon JS, Villanueva JJ, Conboy LA, Levy R, Marci CD, Kerr CE, Kirsch I, Jacobson EE, Riess H, Kaptchuk TJ (2009) Patient and practitioner influences on the placebo effect in irritable bowel syndrome. Psychosom Med 71:789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltner JR, Furst A, Fan C, Redfern R, Inglis B, Fields HL (2006) Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. J Neurosci 26:4437–4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Neubert JK, San Miguel A, Xu K, Krishnaraju RK, Iadarola MJ, Goldman D, Dionne RA (2004) Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain 109:488–496. [DOI] [PubMed] [Google Scholar]
- Kim H, Shimojo S, O'Doherty JP (2006) Is avoiding an aversive outcome rewarding? Neural substrates of avoidance learning in the human brain. PLoS Biol 4:e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Fufa DT, Gerber AJ, Rosman IS, Vangel MG, Gracely RH, Gollub RL (2005) Psychophysical outcomes from a randomized pilot study of manual, electro, and sham acupuncture treatment on experimentally induced thermal pain. J Pain 6:55–64. [DOI] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Polich G, Kirsch I, Laviolette P, Vangel M, Rosen B, Kaptchuk TJ (2008) A functional magnetic resonance imaging study on the neural mechanisms of hyperalgesic nocebo effect. J Neurosci 28:13354–13362. PMCID: PMC2649754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ (2006a) Brain activity associated with expectancy‐enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci 26:381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, White NS, Kwong KK, Vangel MG, Rosman IS, Gracely RH, Gollub RL (2006b) Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp 27:715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Jensen K, Loiotile R, Cheetham A, Wey HY, Tan Y, Rosen B, Smoller JW, Kaptchuk TJ, Gollub RL (2013a) Functional connectivity of the frontoparietal network predicts cognitive modulation of pain. Pain 154:459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- J Kong, R Spaeth, A Cook, I Kirsch, B Claggett, M Vangel, R Gollub, JS Smoller, TJ Kaptchuk (2013b) Are All Placebo Effects Equal? Placebo pills, sham acupuncture, cue conditioning and their Association. PLoS ONE 8:e67485. doi:67410.61371/journal.pone.0067485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Kaptachuk TJ, Polich G, Kirsch IV, Angel M, Zyloney C, Rosen B, Gollub R (2009a) Expectancy and treatment interactions: A dissociation between acupuncture analgesia and expectancy evoked placebo analgesia. Neuroimage 45:940–949. PMID: 19159691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Kaptchuk TJ, Polich G, Kirsch I, Vangel M, Zyloney C, Rosen B, Gollub RL (2009b) An fMRI study on the interaction and dissociation between expectation of pain relief and acupuncture treatment. Neuroimage 47:1066–1076. PMID: 19501656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T, McHaffie JG, Laurienti PJ, Coghill RC (2005) The subjective experience of pain: Where expectations become reality. Proc Natl Acad Sci U S A 102:12950–12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CH, Wu YT (2012) Frontal regional homogeneity increased and temporal regional homogeneity decreased after remission of first‐episode drug‐naive major depressive disorder with panic disorder patients under duloxetine therapy for 6 weeks. J Affect Disord 136:453–458. [DOI] [PubMed] [Google Scholar]
- Legrain V, Guerit JM, Bruyer R, Plaghki L (2002) Attentional modulation of the nociceptive processing into the human brain: selective spatial attention, probability of stimulus occurrence, and target detection effects on laser evoked potentials. Pain 99:21–39. [DOI] [PubMed] [Google Scholar]
- Leknes S, Lee M, Berna C, Andersson J, Tracey I (2011) Relief as a reward: hedonic and neural responses to safety from pain. PLoS One 6:e17870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuchter AF, McCracken JT, Hunter AM, Cook IA, Alpert JE (2009) Monoamine oxidase a and catechol‐o‐methyltransferase functional polymorphisms and the placebo response in major depressive disorder. J Clin Psychopharmacol 29:372–377. [DOI] [PubMed] [Google Scholar]
- Lidstone SC, Schulzer M, Dinelle K, Mak E, Sossi V, Ruth TJ, de la Fuente‐Fernandez R, Phillips AG, Stoessl AJ (2010) Effects of expectation on placebo‐induced dopamine release in Parkinson disease. Arch Gen Psychiatry 67:857–865. [DOI] [PubMed] [Google Scholar]
- Lidstone SC, Stoessl AJ (2007) Understanding the placebo effect: contributions from neuroimaging. Mol Imaging Biol 9:176–185. [DOI] [PubMed] [Google Scholar]
- Liu D, Yan C, Ren J, Yao L, Kiviniemi VJ, Zang Y (2010) Using coherence to measure regional homogeneity of resting‐state FMRI signal. Front Syst Neurosci 4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang K, Yu C, He Y, Zhou Y, Liang M, Wang L, Jiang T (2008) Regional homogeneity, functional connectivity and imaging markers of Alzheimer's disease: a review of resting‐state fMRI studies. Neuropsychologia 46:1648–1656. [DOI] [PubMed] [Google Scholar]
- Loggia ML, Jensen K, Gollub RL, Wasan AD, Edwards RR, Kong J (2011) The catechol‐O‐methyltransferase (COMT) val158met polymorphism affects brain responses to repeated painful stimuli. PLoS One 6:e27764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HC, Hsieh JC, Lu CL, Niddam DM, Wu YT, Yeh TC, Cheng CM, Chang FY, Lee SD (2010) Neuronal correlates in the modulation of placebo analgesia in experimentally‐induced esophageal pain: a 3T‐fMRI study. Pain 148:75–83. [DOI] [PubMed] [Google Scholar]
- Mannisto PT, Kaakkola S (1999) Catechol‐O‐methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev 51:593–628. [PubMed] [Google Scholar]
- HL McLeod, L Fang, X Luo, EP Scott, WE Evans (1994) Ethnic differences in erythrocyte catechol‐O‐methyltransferase activity in black and white Americans. J Pharmacol Exp Ther 270:26–29. [PubMed] [Google Scholar]
- Meyer‐Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney‐Leo A, Nussbaum R, Weinberger DR, Berman KF (2005) Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci 8:594–596. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Sternberg WF, Marek P, Sadowski B, Belknap JK, Liebeskind JC (1996) The genetics of pain and pain inhibition. Proc Natl Acad Sci U S A 93:3048–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton DL, Watson A, El‐Deredy W, Jones AK (2009) Reproducibility of placebo analgesia: Effect of dispositional optimism. Pain 146:194–198. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ludlow DH, Knierim K, Hanelin J, Ramachandran T, Glover GC, Mackey SC (2006) Neural correlates of individual differences in pain‐related fear and anxiety. Pain 120:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paakki JJ, Rahko J, Long X, Moilanen I, Tervonen O, Nikkinen J, Starck T, Remes J, Hurtig T, Haapsamo H, Jussila K, Kuusikko‐Gauffin S, Mattila ML, Zang Y, Kiviniemi V (2010) Alterations in regional homogeneity of resting‐state brain activity in autism spectrum disorders. Brain Res 1321:169–179. [DOI] [PubMed] [Google Scholar]
- MA Palmatier, AM Kang, KK Kidd (1999) Global variation in the frequencies of functionally different catechol‐O‐methyltransferase alleles. Biol Psychiatry 46:557–567. [DOI] [PubMed] [Google Scholar]
- Pecina M, Azhar H, Love TM, Lu T, Fredrickson BL, Stohler CS, Zubieta JK (2012) Personality trait predictors of placebo analgesia and neurobiological correlates. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M (2005) Placebo in emotional processing – induced expectations of anxiety relief activate a generalized modulatory network. Neuron 46:957–969. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JN, Tracey I (2001) Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci 21:9896–9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porro CA, Baraldi P, Pagnoni G, Serafini M, Facchin P, Maieron M, Nichelli P (2002) Does anticipation of pain affect cortical nociceptive systems? J Neurosci 22:3206–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA (2006) Brain work and brain imaging. Annu Rev Neurosci 29:449–476. [DOI] [PubMed] [Google Scholar]
- Rhudy JL, Williams AE, McCabe KM, Russell JL, Maynard LJ (2008) Emotional control of nociceptive reactions (ECON): do affective valence and arousal play a role? Pain 136:250–261. [DOI] [PubMed] [Google Scholar]
- Robbins TW (2007) Shifting and stopping: fronto‐striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci 362:917–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffman JL, Brohawn DG, Nitenson AZ, Macklin EA, Smoller JW, Goff DC (2013) Genetic variation throughout the folate metabolic pathway influences negative symptom severity in schizophrenia. Schizophr Bull 39:330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahl C, Ludascher P, Greffrath W, Kraus A, Valerius G, Schulze TG, Treutlein J, Rietschel M, Smolka MN, Bohus M (2012) COMT val158met polymorphism and neural pain processing. PLoS One 7:e23658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinhardt P, Seminowicz DA, Jaeger E, Duncan GH, Bushnell MC (2009) The anatomy of the mesolimbic reward system: a link between personality and the placebo analgesic response. J Neurosci 29:4882–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK (2007) Individual differences in reward responding explain placebo‐induced expectations and effects. Neuron 55:325–336. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK (2008) Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry 65:220–231. [DOI] [PubMed] [Google Scholar]
- Seymour B, O'Doherty JP, Koltzenburg M, Wiech K, Frackowiak R, Friston K, Dolan R (2005) Opponent appetitive–aversive neural processes underlie predictive learning of pain relief. Nat Neurosci 8:1234–1240. [DOI] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF (2011) REST: A toolkit for resting‐state functional magnetic resonance imaging data processing. PLoS One 6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein N, Sprenger C, Scholz J, Wiech K, Bingel U (2012) White matter integrity of the descending pain modulatory system is associated with interindividual differences in placebo analgesia. Pain 153:2210–2217. [DOI] [PubMed] [Google Scholar]
- Sutin AR, Beason‐Held LL, Resnick SM, Costa PT (2009) Sex differences in resting‐state neural correlates of openness to experience among older adults. Cereb Cortex 19:2797–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddio A, Goldbach M, Ipp M, Stevens B, Koren G (1995) Effect of neonatal circumcision on pain responses during vaccination in boys. Lancet 345:291–292. [DOI] [PubMed] [Google Scholar]
- Taddio A, Shah V, Gilbert‐MacLeod C, Katz J (2002) Conditioning and hyperalgesia in newborns exposed to repeated heel lances. JAMA 288:857–861. [DOI] [PubMed] [Google Scholar]
- Tian L, Ren J, Zang Y (2012) Regional homogeneity of resting state fMRI signals predicts Stop signal task performance. Neuroimage 60:539–544. [DOI] [PubMed] [Google Scholar]
- Tracey I, Mantyh PW (2007) The cerebral signature for pain perception and its modulation. Neuron 55:377–391. [DOI] [PubMed] [Google Scholar]
- Vase L, Robinson ME, Verne GN, Price DD (2005) Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. Pain 115:338–347. [DOI] [PubMed] [Google Scholar]
- Villemure C, Bushnell MC (2002) Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain 95:195–199. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Leotti LA, Rilling JK (2011) Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J Neurosci 31:439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanigasekera V, Lee MC, Rogers R, Kong Y, Leknes S, Andersson J, Tracey I (2012) Baseline reward circuitry activity and trait reward responsiveness predict expression of opioid analgesia in healthy subjects. Proc Natl Acad Sci U S A 109:17705–17710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu QZ, Li DM, Kuang WH, Zhang TJ, Lui S, Huang XQ, Chan RC, Kemp GJ, Gong QY (2011) Abnormal regional spontaneous neural activity in treatment‐refractory depression revealed by resting‐state fMRI. Hum Brain Mapp 32:1290–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Long X, Zang Y, Wang L, Hallett M, Li K, Chan P (2009) Regional homogeneity changes in patients with Parkinson's disease. Hum Brain Mapp 30:1502–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Potenza MN (2012) White matter integrity and five‐factor personality measures in healthy adults. Neuroimage 59:800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Wang L, Lu Q, Liu H, Teng G (2009) Regional homogeneity in depression and its relationship with separate depressive symptom clusters: a resting‐state fMRI study. J Affect Disord 115:430–438. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D, Sprecher E, Zaslansky R, Hemli JA (1995) Heat pain thresholds: normative data and repeatability. Pain 60:329–332. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Zhang Z, Bai F, Yu H, Shi Y, Qian Y, Liu W, You J, Zhang X, Liu Z (2008) Abnormal neural activity in the patients with remitted geriatric depression: a resting‐state functional magnetic resonance imaging study. J Affect Disord 111:145–152. [DOI] [PubMed] [Google Scholar]
- Zang Y, Jiang T, Lu Y, He Y, Tian L (2004) Regional homogeneity approach to fMRI data analysis. Neuroimage 22:394–400. [DOI] [PubMed] [Google Scholar]
- Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC (2011) Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci 31:5540–5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Yau WY, Scott DJ, Stohler CS (2006) Belief or need? Accounting for individual variations in the neurochemistry of the placebo effect. Brain Behav Immun 20:15–26. [DOI] [PubMed] [Google Scholar]


