Abstract
Prostaglandin E2 (PGE2) is an essential signaling molecule involved in the regulation of detrusor smooth muscle (DSM) function. However, the underlying regulatory mechanism by which PGE2 augments DSM cell excitability and contractility is not well understood. Here, we investigated whether PGE inhibits the large conductance voltage- and Ca2+-activated K+ (BK) channels in guinea pig DSM, thereby increasing DSM excitability and contractility. We used a multidisciplinary experimental approach including amphotericin–B perforated patch-clamp electrophysiology and live-cell Ca2+ imaging in native freshly-isolated DSM cells, isometric tension recordings of intact DSM strips, and pharmacological tools to investigate BK channel regulation by PGE2 in guinea pig DSM. PGE2 increased the spontaneous phasic contractions of isolated DSM strips in a concentration-dependent manner (10 nM-10 μM). BK channel inhibition with paxilline (1 μM) attenuated the PGE2-induced DSM phasic contractions, suggesting that BK channels are involved in the mechanism of PGE2-induced DSM contractions. PGE2 (10 μM) increased the intracellular Ca2+ levels in freshly-isolated DSM cells. PGE2 (10 μM) also caused an inhibition of the amplitude and frequency of spontaneous transient BK currents in DSM cells. Moreover, PGE2 (10 μM) did not affect the amplitude of whole cell steady-state BK currents in DSM cells. Our findings provide strong experimental evidence that PGE2 leads to an inhibition of the spontaneous transient BK currents, elevation of intracellular Ca2+ levels in freshly-isolated DSM cells, and augmentation of DSM phasic contractions. Thus, we have revealed a novel mechanism that BK channels mediate PGE2-induced contractions in guinea pig DSM.
Keywords: Prostaglandin E2, detrusor, patch-clamp, Ca2+ imaging
1. INTRODUCTION
Prostaglandin E2 (PGE2) is an essential signaling molecule differentially regulating smooth muscle function depending on the expression of E-prostanoid (EP) receptor subtypes-EP receptor subtype 1 (EP1), EP receptor subtype 2 (EP2), EP receptor subtype 3 (EP3), and EP receptor subtype 4 (EP4) (Narumiya et al., 1999). PGE2 is coupled to divergent signaling pathways for distinct cellular and physiological functions in smooth muscles, including detrusor smooth muscle (DSM) (Narumiya et al., 1999). PGE2 has been shown to be involved in the regulation of DSM contractility in both rodents and humans (Anderson, 1993; Creed and Callahan, 1989; Klausner et al., 2011; Kobayter et al., 2012; McCafferty et al., 2008). It is synthesized in the urothelial and muscle layers of the urinary bladder (Brown et al., 1980; Klausner et al., 2011; Masunaga et al., 2006; Park et al., 1999). Immunohistochemistry analyses revealed that EP1 and EP2 receptors are expressed in the urothelium, lamina propria, and DSM (de Jongh et al., 2007; Rahnama’i et al., 2010).
Differential effects of PGE2 on smooth muscle function have been reported (Anderson, 1993; Coleman and Sheldrick, 1989; Ishizuka et al., 1995; Kobayter et al., 2012; Zhu et al., 2002). PGE2 has either stimulatory or inhibitory effects depending on the species examined or the tissue or cell types being investigated. PGE2 increases the spontaneous activity of mouse DSM strips, whereas in human urethral smooth muscle it causes relaxation (Anderson, 1993; Kobayter et al., 2012). In mouse DSM, PGE2 has been reported to cause cell membrane depolarization, to increase Ca2+ oscillations, and to potentiate phasic contractions (Kobayter et al., 2012). In human airway smooth muscle, PGE2 has been demonstrated to induce either stimulatory or inhibitory effects on contractions depending on the concentration applied (Coleman and Sheldrick, 1989). In addition, PGE2 has been suggested to cause relaxation of coronary artery smooth muscle by activating the large conductance voltage- and Ca2+-activated K+ (BK) channels (Zhu et al., 2002).
Among all known ion channels, BK channels are the most critical regulators of DSM function in both rodents and humans (Hristov et al., 2011; Petkov, 2012; Petkov and Nelson, 2005). BK channels are activated by rapid and localized Ca2+ releases from sarcoplasmic reticulum ryanodine receptors, known as “Ca2+ sparks”, which elicit transient BK currents (TBKCs), also known as spontaneous transient outward currents (STOCs), in DSM cells (Herrera et al., 2001; Herrera and Nelson, 2002; Hristov et al., 2011; Petkov, 2012; Petkov and Nelson, 2005). In human DSM, direct inhibition of the BK channels with its selective blockers, iberiotoxin or paxilline, leads to membrane depolarization, activation of L-type voltage-gated Ca2+ (CaV) channels, and potentiation of DSM contractions (Hristov et al., 2011). By contrast, activation of BK channels with pharmacological openers such as NS1619, hyperpolarizes the membrane potential, increases BK channel open probability and limits the Ca2+ entry via CaV channels, thereby causing DSM relaxation (Hristov et al., 2011; Hristov et al., 2012; Malysz et al., 2013; Petkov, 2012). A recent report from our group revealed that activation of M3 muscarinic receptors leads to inhibition of BK channel activity via a Ca2+-dependent mechanism, thereby increasing DSM excitability and contractility in rat DSM (Parajuli and Petkov, 2013). Since PGE2 has been shown to depolarize the cell membrane potential and increase mouse DSM contractility (Kobayter et al., 2012), we hypothesize that BK channels play a critical role in mediating the PGE2 stimulatory effects on DSM excitability and contractility.
In this study, we sought to elucidate whether BK channels mediate the PGE2 stimulatory effects on guinea pig DSM excitability and contractility. For this purpose, we employed a multidisciplinary experimental approach including isometric DSM tension recordings, live-cell Ca2+ imaging, and perforated whole cell patch-clamp electrophysiology at both cellular (freshly-isolated single cells) and tissue levels (intact DSM strips). For the first time, our data revealed that PGE2 leads to inhibition of TBKCs, elevation of intracellular Ca2+ levels, and potentiation of guinea pig DSM contractility.
2. MATERIALS AND METHODS
2.1. DSM tissue collection and preparation
22 male Hartley Albino guinea pigs (Charles River Laboratories, Raleigh, NC) of average weight 473.7±16.3 g were euthanized by CO2 inhalation. The urinary bladders were dissected in accordance to the animal use protocol #1747, and reviewed and approved by the Institutional Animal Care and Use Committee at the University of South Carolina. Urinary bladders were cut open longitudinally from the lumen and stored in ice-cold dissection solution. DSM strips ~5-6 mm long and ~2-3 mm wide were dissected from the bladder wall and the urothelium and lamina propria were removed.
2.2. DSM single cell isolation
Guinea pig DSM single cells were enzymatically isolated using papain and collagenase as previously described (Hristov et al., 2013; Parajuli and Petkov, 2013). Single DSM cells were used for patch-clamp and live-cell Ca2+ imaging studies within 12 h after isolation.
2.3. Isometric DSM tension recordings
Isometric DSM tension recordings of freshly-isolated guinea pig DSM strips (~5-6 mm long and ~2-3 mm wide) were conducted as described previously (Hristov et al., 2013; Smith et al., 2013). Cumulative PGE2 concentrations (10 nM-10 μM) were applied at 15-min intervals into the baths in the presence or absence of paxilline (1 μM), a selective inhibitor of BK channels.
2.4. Live-cell Ca2+ imaging
Intracellular Ca2+ imaging experiments were performed using the ratiometric dye fura 2-AM in freshly-isolated DSM single cells as described previously (Hristov et al., 2013; Smith et al., 2013).
2.5. Patch-clamp recordings
The amphotericin-B perforated whole cell patch-clamp technique was used to record TBKCs and voltage-step depolarization-induced whole cell steady-state BK currents in freshly-isolated DSM single cells as described previously (Hristov et al., 2013; Parajuli and Petkov, 2013). TBKCs were recorded at a holding potential of −40 mV. Voltage-step-induced whole cell BK currents were recorded by holding the DSM cells at −70 mV and voltage-depolarization applied from −40 mV to +80 mV in increments of 20 mV with 200 ms duration, and then cells were repolarized back to −70 mV. All patch-clamp recordings were performed at room temperature (22-23° C).
2.6. Solutions and drugs
The Ca2+-free dissection solution, the extracellular bath solution used in the perforated patch-clamp and Ca2+ imaging experiments, and the patch-clamp pipette solution for perforated whole cell patch-clamp experiments were prepared as described previously (Hristov et al., 2013; Smith et al., 2013). Freshly-dissolved amphotericin-B (200 g/ml) in dimethyl sulfoxide (DMSO) was added to the pipette solution before the experiments and replaced every 1-2 h. PGE2 and fura 2-AM were purchased from Sigma-Aldrich (St. Louis, MO) and were dissolved in ethanol and DMSO as stock solutions, respectively. Paxilline was purchased from Tocris Bioscience (Bristol, UK) and was dissolved in DMSO. The DMSO concentration in the bath solution did not exceed 0.1%.
2.7. Data analysis and statistics
The effects of PGE2 on DSM contraction parameters or the amplitude and frequency of TBKCs were analyzed using MiniAnalysis software (Synaptosoft, Inc., Fort Lee, NJ) and then were normalized to control values and were expressed in percentages (%). The effect of PGE2 on voltage-step depolarization-evoked steady-state whole cell BK current was analyzed by Clampfit 10.2 software (Molecular Device, Union City, CA). The mean value of the last 50 ms of the 200 ms pulse from the average of 6-10 files recorded over 8-10 min (performed every 1 min) were calculated before and after the application of 10 μM PGE2. The last 5 min of at least 8-10 min stable voltage-clamp recordings prior to the application of PGE2 were used as a control and the last 5 min of continuous recordings of at least 10-15 min after the application of PGE2 were used to evaluate their effects on TBKCs from DSM cells. Statistical analysis was performed with GraphPad Prism 4.03 software (GraphPad Software, Inc., La Jolla, CA). The data are presented as mean±S.E.M. for the n (the number of cells or strips) isolated from N (the number of guinea pigs). Data were illustrated by using Corel Draw Graphic Suite X3 software (Corel Co., Mountain View, CA) and GraphPad Prism 4.03 software. Statistical analysis was performed using a two-tailed paired Student’s t-test or two-way ANOVA followed by Bonferroni post-hoc test. A P value <0.05 was considered to be statistically significant.
3. RESULTS
3.1. PGE2 increases the spontaneous phasic contractions of freshly-isolated DSM strips
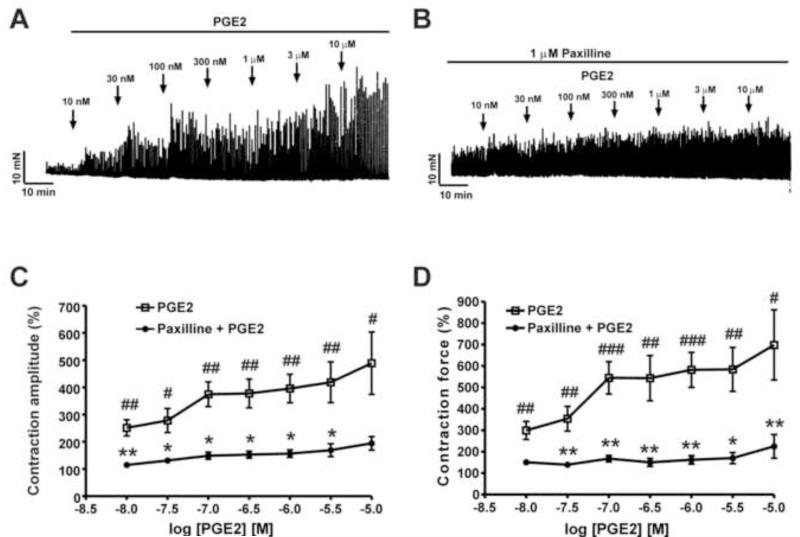
We sought to explore whether PGE2 regulates DSM spontaneous phasic contractions. As illustrated in Fig. 1, isolated DSM strips exhibiting spontaneous phasic contractions were exposed to cumulative concentrations of PGE2 (10 nM-10 μM). PGE2 (10 nM-10 μM) significantly increased the spontaneous phasic contraction amplitude and muscle force integral in a concentration-dependent manner (n=8, N=5; P<0.05; Fig. 1A, C, D). At the highest concentration used of 10 μM, PGE2 increased DSM spontaneous phasic contraction amplitude by 488.5±114.4% and the muscle force integral by 698.4±163.6% (n=8, N=5; P<0.05, Fig. 1).
Fig. 1. PGE2 significantly increases the spontaneous phasic contractions of guinea pig isolated DSM strips. A and B).
Representative original recordings showing the contractile effects of PGE2 (10 nM-10 μM) on spontaneous phasic contractions of isolated DSM strips in the absence (A) or presence (B) of paxilline (1 μM). C and D) Cumulative concentration-response curves for PGE2 on spontaneous phasic contraction amplitude and muscle force integral of DSM strips in the presence or absence of 1 μM paxilline (n=8, N=5; #P<0.05, ##P<0.01, and ###P<0.001 PGE2 vs. control, *P<0.05 and **P<0.01 vs. paxilline).
To further evaluate whether PGE2 stimulatory effects on DSM spontaneous phasic contractions were mediated by the BK channels, we investigated the effects of PGE2 (10 nM-10 μM) on DSM contractility in the presence of the BK channel selective inhibitor, paxilline (1 μM). As shown in Fig. 1B, we found that paxilline (1 μM) attenuated the PGE2 stimulatory effects on DSM phasic contraction amplitude and muscle force integral. Pretreatment of DSM strips with paxilline reduced the PGE2 (10 μM)-induced contraction amplitude and contraction muscle force integral from 488.5±114.4% to 194.2±24.7% and from 698.4±163.6% to 225.2±55.2%, respectively (n=8, N=5; P<0.05, Fig. 1C, D). We observed attenuation in the concentration-response curves for PGE2 effects on the spontaneous phasic contraction amplitude and the muscle force integral in the presence of paxilline (Fig. 1C, D). These data indicate that BK channels are involved in mediating the PGE2 stimulatory effects on DSM spontaneous phasic contractions.
3.2. PGE increases the intracellular Ca2+ levels in freshly-isolated DSM cells
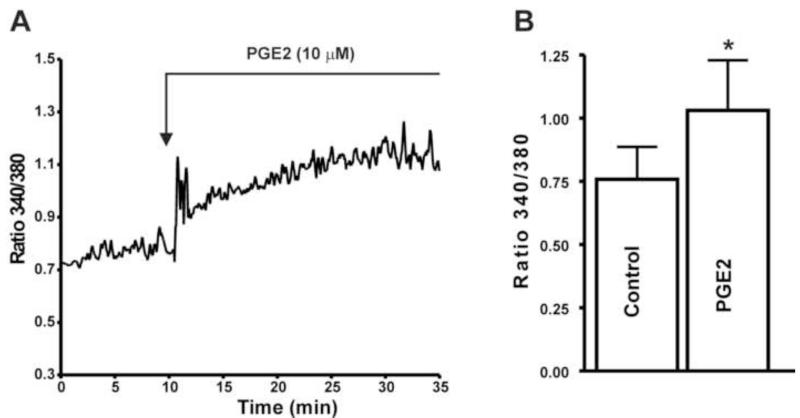
Since our data showed that PGE2 dramatically increased DSM spontaneous phasic contractions, we expect that PGE affects the intracellular Ca2+ levels in freshly-isolated DSM cells. Using the ratiometric dye fura 2-AM and live-cell Ca2+ imaging, we measured the intracellular Ca2+ levels in DSM cells as shown in Fig. 2A-B. In DSM cells, PGE2 (10 μM) significantly increased the fura 2 fluorescence ratio 340/340 of intracellular Ca2+ levels from its control value of 0.76±0.1 to 1.0±0.2 (P<0.05; n=6, N=6; Fig. 2).
Fig. 2. PGE2 increases the intracellular Ca2+ levels in native freshly-isolated guinea pig DSM cells. A).
A representative trace of fura 2 fluorescence ratio illustrating that PGE2 (10 μM) increases the intracellular Ca2+ levels in a DSM cell. B) Summary data depicting the DSM cell intracellular Ca2+ levels in the presence or absence of PGE2 (10 μM) (n=6, N=6; * P<0.05).
3.3. PGE2 inhibits the amplitude and frequency of TBKCs in freshly-isolated DSM cells
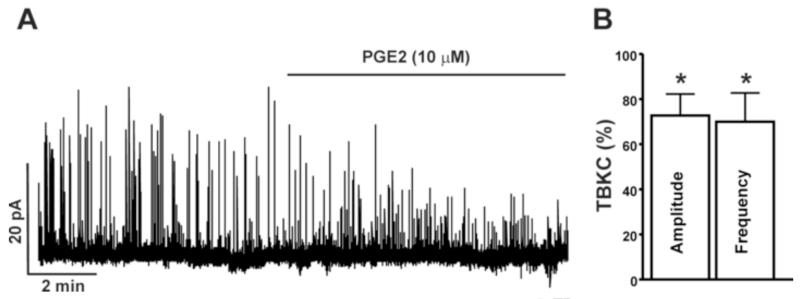
In the next experimental series, we studied the effect of PGE2 on the amplitude and frequency of TBKCs in DSM cells using the amphotericin-B perforated whole cell patch-clamp technique. The average cell capacitance of all 18 DSM cells isolated from 9 guinea pigs used in the present study was 35.16±4.4 pF, consistent with previous reports (Herrera et al., 2001; Herrera and Nelson, 2002). As shown in Fig. 3A-C, the treatment of DSM cells with PGE2 (10 μM) significantly inhibited the amplitude and frequency of TBKCs by 27.3±9.5% and 30.1±12.8%, respectively (n=12, N=7; P<0.05). These results indicate that PGE2 increases DSM contractility by inhibiting TBKCs in DSM cells. Next, we sought to elucidate whether PGE2 affects the steady-state whole cell BK currents in freshly-isolated DSM cells.
Fig. 3. PGE2 inhibits TBKCs in freshly-isolated guinea pig DSM cells. A).
A representative recording illustrating the PGE2 (10 μM) inhibitory effects on the TBKC amplitude and frequency in a freshly-isolated DSM cell. B) Summary data illustrating the inhibitory effect of PGE2 (10 μM) on the amplitude and frequency of TBKCs (n=12, N=7; *P<0.05). The average amplitude and frequency of TBKCs before PGE2 application (control) were taken to be 100% and data were normalized to controls.
3.4. PGE2 does not affect the steady-state whole cell BK currents in freshly-isolated DSM cells
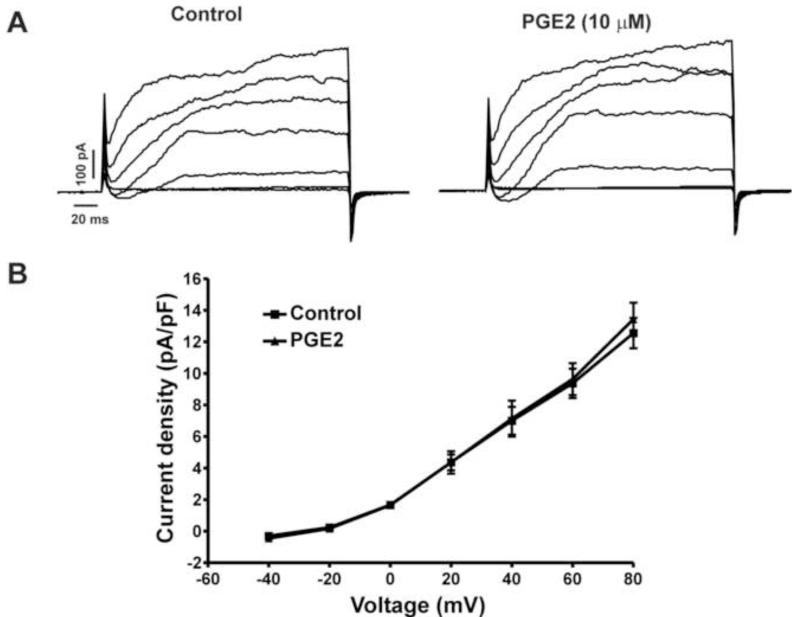
Previous studies from our group have shown that in animal and human DSM cells, the voltage-step-induced steady-state whole cell currents are primarily mediated by the BK channel activity (Hristov et al., 2011; Petkov and Nelson, 2005). Since PGE2 has shown statistically significant inhibitory effects on the amplitude and frequency of TBKCs, we sought to explore whether PGE2 also affects the voltage-step-induced steady-state BK currents in guinea pig DSM cells. We measured the effects of PGE2 (10 μM) on voltage-step depolarization-induced BK currents. PGE2 (10 μM) did not alter significantly the voltage-step depolarization-induced BK currents (n=6, N=6; P>0.05, Fig. 4). As shown in Fig. 4B, at the highest depolarization voltage applied (+80 mV), the steady-state whole cell BK currents were 12.5±0.9 pA/pF and 13.4±1.0 pA/pF in the absence and presence of PGE2 (10 μM), respectively (n=6, N=6; P>0.05; Fig. 4B). These results suggest that unlike the TBKCs, PGE2 does not affect the steady-state BK channel currents in freshly-isolated guinea pig DSM cells.
Fig. 4. PGE2 does not affect the steady-state whole cell BK currents in freshly-isolated guinea pig DSM cells. A).
Representative voltage-clamp recordings illustrating that PGE2 (10 μM) does not affect the steady-state BK currents at depolarization voltages from −40 mV to +80 mV. B) Current-voltage relationship curves depict the lack of PGE2 effect on the steady-state whole cell BK currents in DSM cells (n=6, N=6; P>0.05).
4. DISCUSSION
In the present study, for the first time, we have revealed a mechanism that BK channels mediate PGE2-induced excitability and contractility in guinea pig DSM. We provide evidence that PGE2 causes: 1) increase in DSM spontaneous phasic contractions in a BK channel-dependent manner, 2) elevation of the intracellular Ca2+ concentration in freshly-isolated DSM cells, and 3) inhibition of the amplitude and frequency of TBKCs in freshly-isolated DSM cells.
Our experimental data from isometric DSM tension recordings showed that PGE2 increased the spontaneous phasic contractions of isolated DSM strips (Fig. 1A). PGE2-induced stimulatory effects on spontaneous phasic contractions were significantly attenuated by the BK channel blocker paxilline, supporting that the observed PGE2 stimulatory effects on DSM contractility were mediated by the BK channels (Fig. 1B-E). A recent study in mouse DSM reported that PGE2 increased DSM contractions by activation of L-type CaV channels (Kobayter et al., 2012). The inhibition of BK channels indirectly activates the L-type CaV channels and increases the global Ca2+ concentration to promote DSM contractility (Petkov, 2012). Indeed, in the presence of paxilline, PGE2 had less pronounced stimulatory effects on DSM contractions as compared with PGE2-induced phasic contractions (Fig. 1).
In other non-DSM smooth muscle tissues including that of the human airway and urethra, PGE2-induced responses were variable (Anderson, 1993; Coleman and Sheldrick, 1989). The diverse cellular and tissue-specific responses to PGE2 are suggested to be a result of the activation of multiple EP receptor subtypes as well as their variable distribution (Narumiya et al., 1999). Our experimental data demonstrate that activation of EP receptors with PGE2 leads to an inhibition of TBKC activity in guinea pig DSM, thereby increasing DSM excitability and contractility (Fig. 1). We observed PGE stimulatory effects on intracellular Ca2+ levels in DSM single cells and spontaneous phasic contractions of isolated DSM strips (Figs. 1 and 2). One of the prototypic signaling pathways involving PGE -induced elevation of intracellular Ca2+ levels and associated DSM contractility is thought to be a result of EP1 receptor activation (Bos et al., 2004). EP1 receptors couples to G q proteins and stimulates phospholipase C, which hydrolyzes phosphatidylinositol-4,5-bisphosphate (PIP2) to generate diacylglycerol and inositol-1,4,5-trisphosphate (Bos et al., 2004). The binding of inositol-1,4,5-trisphosphate to inositol-1,4,5-trisphosphate receptors leads to a release of Ca2+ from the sarcoplasmic reticulum, which elevates intracellular Ca2+ levels. Furthermore, diacylglycerol, another PIP2 product, may activate protein kinase-C (PKC). Recently, our group has reported that activation of PKC with phorbol 12-myristate 13-acetate (PMA) leads to an elevation of intracellular Ca2+ levels, potentiation of DSM phasic contractions, and inhibition of the amplitude and frequency of TBKCs of DSM cells (Hristov et al., 2013). Consistent with this mechanism, PGE2 most likely stimulates EP1 receptors and then activates PKC. PKC may cause a direct inhibition of ryanodine receptors or a reduction in sarcoplasmic reticulum Ca2+ load resulting in an inhibition of the amplitude and frequency of DSM TBKCs (Hristov et al., 2013; Petkov, 2012). In the present study, since activation of EP receptors with PGE leads to an elevation of intracellular Ca2+ levels, potentiation of DSM phasic contractions, and an inhibition of the amplitude and frequency of TBKCs consistent with PKC-induced effects on guinea pig DSM excitability and contractility, we suggest that PGE2 stimulatory effects on guinea pig DSM excitability and contractility may involve an EP1 receptor-PKC-BK channel-dependent mechanism.
Since our data on DSM contractility revealed that PGE2 stimulatory effects on DSM spontaneous phasic contractions are associated with reduction of TBKC channel activity, we further sought to elucidate whether the observed PGE2 effects on DSM contractions were associated with elevation of intracellular Ca2+ levels in DSM cells. In human DSM cells, BK channel activation with NS1619 leads to a reduction of intracellular Ca2+ levels indicating a relationship between BK channel activation and cytosolic Ca2+ level (Hristov et al., 2012). In DSM isolated strips, PGE2 caused membrane potential depolarization and enhanced nerve- and acetylcholine-induced contractions (Creed and Callahan, 1989). It was suggested that these effects were mediated by intracellular Ca2+ mobilization (Creed and Callahan, 1989). Furthermore, inhibition of DSM BK channel activity leads to membrane depolarization, increased intracellular Ca2+ levels, and promotion of DSM contractions (Petkov, 2012). Those previous reports are in support of the present findings as PGE2 significantly elevated the intracellular Ca2+ levels in DSM single cells and increased the spontaneous phasic contractions of isolated DSM strips. Our findings from Ca2+ imaging experiments further indicate that PGE2 stimulatory effects on DSM contractility are associated with elevation of intracellular Ca2+ levels in DSM cells (Figs. 1 and 2).
In the present study, we investigated the mechanism by which PGE2 causes stimulation of DSM cell excitability with the help of the perforated patch-clamp technique. The perforated mode of the patch-clamp technique has the advantage of preserving the intrinsic intracellular signaling pathways, thus maintaining the native physiological environment for studying DSM BK channels. To the best of our knowledge, this is the first time that patch-clamp electrophysiology and live-cell Ca2+ imaging has been performed on freshly-isolated native guinea pig DSM cells in order to evaluate the effect of PGE on intracellular Ca2+ levels and BK channel activity. Under these particular physiological conditions, PGE2 had a statistically significant inhibitory effect on the amplitude and frequency of TBKCs without affecting the amplitude of steady-state whole cell BK currents (Figs. 3 and 4). In DSM, Ca2+ release from sarcoplasmic reticulum ryanodine receptors activates TBKCs without influencing steady-state BK currents indicating the sarcoplasmic reticulum Ca2+ release via ryanodine receptors is critical in regulating BK channels (Herrera et al., 2000, 2001; Hristov et al., 2011; Petkov and Nelson, 2005). It has been shown that inhibition of ryanodine receptors with ryanodine eliminates the TBKCs without affecting the amplitude of steady-state BK currents (Hristov et al., 2011; Petkov and Nelson, 2005). In the present study, the observed PGE2 inhibitory effects on the amplitude and frequency of DSM TBKCs may be attributed to a direct inhibition of the ryanodine receptors or a reduction in sarcoplasmic reticulum Ca2+ load (Petkov, 2012).
Reports demonstrate that PGE2 concentrations are dramatically increased during bladder pathophysiological conditions (Aoki et al., 2009). Intravesical application of PGE2 has been shown to induce detrusor overactivity indicating a potential role of PGE2 in bladder pathophysiology (Schroder et al., 2004). Further, urinary levels of PGE2 were significantly increased in patients with overactive bladder symptoms indicating that PGE2 may be linked to bladder pathophysiology, thus urine PGE2 levels could be used as biomarkers of bladder dysfunction (Aoki et al., 2009; Kim et al., 2006; Rahnama’i et al., 2012). Urinary retention due to detrusor underactivity is another form of bladder dysfunction for which effective pharmacotherapies are lacking. A study on patients with detrusor underactivity, who were already receiving cholinergic stimulation treatment showed that PGE2 had an additive or synergistic effect on cholinergic stimulation-induced responses (Desmond et al., 1980). Another study on patients with detrusor underactivity suggested that PGE2 when combined with bethanechol may be considered for treatment of patients with detrusor underactivity (Hindley et al., 2004). As pharmacological activation of EP receptors with PGE2 increases DSM cell excitability and contractility, the present study suggests that EP receptor agonists, or agents that stimulate PGE2 production, may prove to be beneficial for the treatment of detrusor underactivity.
CONCLUSIONS
Our findings provide evidence that PGE2 leads to inhibition of TBKC activity, elevation of intracellular Ca2+ levels in DSM cells, and increase in DSM spontaneous phasic contractions. Therefore, we have revealed a novel mechanism by which BK channels mediate PGE2-induced stimulatory effects on guinea pig DSM excitability and contractility.
ACKNOWLEDGEMENTS
We would like to thank Drs. Kiril Hristov, John Malysz, Wenkuan Xin, and Mr. Vitor Fernandes for the critical evaluation of the manuscript.
GRANTS
This study was supported by a grant from the National Institutes of Health R01 DK084284 to Georgi V. Petkov and by a fellowship from the Urology Care Foundation Research Scholars Program and the Allergan Foundation to Shankar P. Parajuli.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson KE. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol Rev. 1993;45:253–308. [PubMed] [Google Scholar]
- Aoki K, Hirayama A, Tanaka N, Yoneda T, Yoshida K, Fujimoto K, Hirao Y. A higher level of prostaglandin E2 in the urinary bladder in young boys and boys with lower urinary tract obstruction. Biomed Res. 2009;30:343–347. doi: 10.2220/biomedres.30.343. [DOI] [PubMed] [Google Scholar]
- Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol. 2004;36:1187–1205. doi: 10.1016/j.biocel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Brown WW, Zenser TV, Davis BB. Prostaglandin E2 production by rabbit urinary bladder. Am J Physiol. 1980;239:F452–458. doi: 10.1152/ajprenal.1980.239.5.F452. [DOI] [PubMed] [Google Scholar]
- Coleman RA, Sheldrick RL. Prostanoid-induced contraction of human bronchial smooth muscle is mediated by TP-receptors. Br J Pharmacol. 1989;96:688–692. doi: 10.1111/j.1476-5381.1989.tb11869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed KE, Callahan SM. Prostaglandins and neurotransmission at the guinea pig and rabbit urinary bladder. Pflugers Arch. 1989;413:299–302. doi: 10.1007/BF00583544. [DOI] [PubMed] [Google Scholar]
- de Jongh R, van Koeveringe GA, van Kerrebroeck PE, Markerink-van Ittersum M, de Vente J, Gillespie JI. The effects of exogenous prostaglandins and the identification of constitutive cyclooxygenase I and II immunoreactivity in the normal guinea pig bladder. BJU Int. 2007;100:419–429. doi: 10.1111/j.1464-410X.2007.07011.x. [DOI] [PubMed] [Google Scholar]
- Desmond AD, Bultitude MI, Hills NH, Shuttleworth KE. Clinical experience with intravesical prostaglandin E2. A prospective study of 36 patients. Br J Urol. 1980;52:357–366. doi: 10.1111/j.1464-410x.1980.tb03060.x. [DOI] [PubMed] [Google Scholar]
- Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol. 2000;279:R60–68. doi: 10.1152/ajpregu.2000.279.1.R60. [DOI] [PubMed] [Google Scholar]
- Herrera GM, Heppner TJ, Nelson MT. Voltage dependence of the coupling of Ca2+ sparks to BKCa channels in urinary bladder smooth muscle. Am J Physiol Cell Physiol. 2001;280:C481–490. doi: 10.1152/ajpcell.2001.280.3.C481. [DOI] [PubMed] [Google Scholar]
- Herrera GM, Nelson MT. Differential regulation of SK and BK channels by Ca2+ signals from Ca2+ channels and ryanodine receptors in guinea-pig urinary bladder myocytes. J Physiol. 2002;541:483–492. doi: 10.1113/jphysiol.2002.017707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindley RG, Brierly RD, Thomas PJ. Prostaglandin E2 and bethanechol in combination for treating detrusor underactivity. BJU Int. 2004;93:89–92. doi: 10.1111/j.1464-410x.2004.04563.x. [DOI] [PubMed] [Google Scholar]
- Hristov KL, Chen M, Kellett WF, Rovner ES, Petkov GV. Large-conductance voltage- and Ca2+-activated K+ channels regulate human detrusor smooth muscle function. Am J Physiol Cell Physiol. 2011;301:C903–912. doi: 10.1152/ajpcell.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristov KL, Parajuli SP, Soder RP, Cheng Q, Rovner ES, Petkov GV. Suppression of human detrusor smooth muscle excitability and contractility via pharmacological activation of large conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol. 2012;302:C1632–1641. doi: 10.1152/ajpcell.00417.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristov KL, Smith AC, Parajuli SP, Malysz J, Petkov GV. Large conductance voltage and Ca2+-activated K+ channel regulation by protein kinase C in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol. 2013;306:460–470. doi: 10.1152/ajpcell.00325.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka O, Mattiasson A, Andersson KE. Prostaglandin E2-induced bladder hyperactivity in normal, conscious rats: involvement of tachykinins? J Urol. 1995;153:2034–2038. doi: 10.1016/s0022-5347(01)67397-x. [DOI] [PubMed] [Google Scholar]
- Kim JC, Park EY, Seo SI, Park YH, Hwang TK. Nerve growth factor and prostaglandins in the urine of female patients with overactive bladder. J Urol. 2006;175:1773–1776. doi: 10.1016/S0022-5347(05)00992-4. discussion 1776. [DOI] [PubMed] [Google Scholar]
- Klausner AP, Johnson CM, Stike AB, Speich JE, Sabarwal V, Miner AS, Cleary M, Koo HP, Ratz PH. Prostaglandin E2 mediates spontaneous rhythmic contraction in rabbit detrusor muscle. Can J Urol. 2011;18:5608–5614. [PubMed] [Google Scholar]
- Kobayter S, Young JS, Brain KL. Prostaglandin E2 induces spontaneous rhythmic activity in mouse urinary bladder independently of efferent nerves. Br J Pharmacol. 2012;165:401–413. doi: 10.1111/j.1476-5381.2011.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malysz J, Rovner ES, Petkov GV. Single-channel biophysical and pharmacological characterizations of native human large-conductance calcium-activated potassium channels in freshly isolated detrusor smooth muscle cells. Pflugers Arch. 2013;465:965–975. doi: 10.1007/s00424-012-1214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masunaga K, Yoshida M, Inadome A, Iwashita H, Miyamae K, Ueda S. Prostaglandin E2 release from isolated bladder strips in rats with spinal cord injury. Int J Urol. 2006;13:271–276. doi: 10.1111/j.1442-2042.2006.01274.x. [DOI] [PubMed] [Google Scholar]
- McCafferty GP, Misajet BA, Laping NJ, Edwards RM, Thorneloe KS. Enhanced bladder capacity and reduced prostaglandin E2-mediated bladder hyperactivity in EP3 receptor knockout mice. Am J Physiol Renal Physiol. 2008;295:F507–514. doi: 10.1152/ajprenal.00054.2008. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Parajuli SP, Petkov GV. Activation of muscarinic M3 receptors inhibits large-conductance voltage- and Ca2+-activated K+ channels in rat urinary bladder smooth muscle cells. Am J Physiol Cell Physiol. 2013;305:C207–214. doi: 10.1152/ajpcell.00113.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Yang T, Arend LJ, Schnermann JB, Peters CA, Freeman MR, Briggs JP. Obstruction stimulates COX-2 expression in bladder smooth muscle cells via increased mechanical stretch. Am J Physiol. 1999;276:F129–136. doi: 10.1152/ajprenal.1999.276.1.F129. [DOI] [PubMed] [Google Scholar]
- Petkov GV. Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol. 2012;9:30–40. doi: 10.1038/nrurol.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov GV, Nelson MT. Differential regulation of Ca2+-activated K+ channels by beta-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol. 2005;288:C1255–1263. doi: 10.1152/ajpcell.00381.2004. [DOI] [PubMed] [Google Scholar]
- Rahnama’i MS, van Kerrebroeck PE, de Wachter SG, van Koeveringe GA. The role of prostanoids in urinary bladder physiology. Nat Rev Urol. 2012;9:283–290. doi: 10.1038/nrurol.2012.33. [DOI] [PubMed] [Google Scholar]
- Rahnama’i MS, van Koeveringe GA, Essers PB, de Wachter SG, de Vente J, van Kerrebroeck PE, Gillespie JI. Prostaglandin receptor EP1 and EP2 site in guinea pig bladder urothelium and lamina propria. J Urol. 2010;183:1241–1247. doi: 10.1016/j.juro.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Schroder A, Newgreen D, Andersson KE. Detrusor responses to prostaglandin E2 and bladder outlet obstruction in wild-type and Ep1 receptor knockout mice. J Urol. 2004;172:1166–1170. doi: 10.1097/01.ju.0000134186.58854.2c. [DOI] [PubMed] [Google Scholar]
- Smith AC, Hristov KL, Cheng Q, Xin W, Parajuli SP, Earley S, Malysz J, Petkov GV. Novel role for the transient potential receptor melastatin 4 channel in guinea pig detrusor smooth muscle physiology. Am J Physiol Cell Physiol. 2013;304:C467–477. doi: 10.1152/ajpcell.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Han G, White RE. PGE2 action in human coronary artery smooth muscle: role of potassium channels and signaling cross-talk. J Vasc Res. 2002;39:477–488. doi: 10.1159/000067201. [DOI] [PubMed] [Google Scholar]