Abstract
The extent to which environmental factors influence the ability of Anopheles mosquitoes to transmit malaria parasites remains poorly explored. Environmental variation, such as change in ambient temperature, will not necessarily influence the rates of host and parasite processes equivalently, potentially resulting in complex effects on infection outcomes. As proof of principle, we used Anopheles stephensi and the rodent malaria parasite, Plasmodium yoelii, to examine the effects of a range of constant temperatures on one aspect of host defense (detected as alterations in expression of nitric oxide synthase gene – NOS) to parasite infection. We experimentally boosted mosquito midgut immunity to infection through dietary supplementation with the essential amino acid L-Arginine (L-Arg), which increases midgut NO levels by infection-induced NOS catalysis in A. stephensi. At intermediate temperatures, supplementation reduced oocyst prevalence, oocyst intensity, and sporozoite prevalence suggesting that the outcome of parasite infection was potentially dependent upon the rate of NOS-mediated midgut immunity. At low and high temperature extremes, however, infection was severely constrained irrespective of supplementation. The effects of L-Arg appeared to be mediated by NO-dependent negative feedback on NOS expression, as evidenced by depressed NOS expression in L-Arg treated groups at temperatures where supplementation decreased parasite infection. These results suggest the need to consider the direct (e.g. effects of mosquito body temperature on parasite physiology) and indirect effects (e.g. mediated through changes in mosquito physiology / immunity) of environmental factors on mosquito-malaria interactions in order to understand natural variation in vector competence.
Keywords: mosquito, malaria, ambient temperature, arginine, nitric oxide, resistance, immunity
1. BACKGROUND
Transmission of malaria involves an intimate interaction between the mosquito vectors and the malaria parasites. When mosquitoes feed on a malaria parasite-infected vertebrate host, gametocytes localize to the posterior midgut and within minutes become gametes. Male gametes will then fertilize female gametes to form zygotes. Under standard laboratory conditions, zygotes become ookinetes within the first 12–24 hrs post-infection and traverse both the peritrophic matrix and the midgut epithelium from 24–32 hrs post-infection to establish as oocysts under the basal lamina [1]. Established oocysts mature by approximately 14 days postinfection, releasing sporozoites into the hemolymph. These sporozoites eventually invade the salivary glands and are transmitted through the bite of an infectious mosquito to the next vertebrate host [1]. Throughout this process, mosquitoes mount coordinated midgut [e.g. 2, 3–8] and hemolymph-mediated immune responses [e.g. 2, 9–11] involving midgut enzymes including peroxidases, oxidases, and nitric oxide synthase (NOS), as well as activation of immune pathways (Toll, IMD, MAPK) and the mosquito complement-like effector molecule thioester binding protein 1 (TEP1).
Current understanding of these physiological and molecular interactions between mosquitoes and malaria parasites derives largely from studies conducted under standardized laboratory settings. However, malaria transmission occurs across diverse environments [12, 13] and we expect the net outcome of insect-parasite interactions to depend on both genetic and environmental factors [14, 15]. Specifically, the effectiveness of mosquito immune responses toward malaria parasites will be dependent upon the rates of host enzymes involved in nitration and lysis of Plasmodium spp. parasites as well as the rates of Plasmodium ookinete formation and migration through the midgut epithelium [2]. Given the influence of temperature on enzyme kinetics and ectotherm physiology in general, changes in ambient temperature are expected to affect the rates of these mosquito and parasite processes. The net effect on overall mosquito vector competence will depend on the relative thermal sensitivity of both host and parasite traits [16].
Here we investigated how temperature and dietary supplementation influenced the outcome of the mosquito-malaria interaction. To do this we assessed how metrics of parasite fitness and one aspect of midgut-mediated immunity, the production of nitric oxide (NO) by the enzyme nitric oxide synthase (NOS), were affected by varying mean ambient temperature and providing a subset of mosquitoes with access to an essential amino acid, L-Arginine (L-Arg). NO is a free radical gas that can react with a variety of oxidants to generate cytotoxic reactive nitrogen species [RNS, 17, 18]. RNS are toxic to a wide diversity of pathogens [19] and are key to the mosquito midgut immune response toward Plasmodium parasites [2–4, 20]. NO-mediated defenses may also limit the number of sporozoites that successfully invade the salivary glands because NO can be produced and secreted into the hemolymph by circulating hemocytes [21, 22]. Further, NO production has been implicated in the response of other Anopheles species to infection with Plasmodium [2, 23–25], in the response of Rhodnius prolixus to infection with Trypanosma rangeli [26, 27], and of D. melanogaster to infection with Asobara rabid [28].
Both temperature and L-Arg supplementation have been shown to influence components of NOS-mediated immunity [4, 28–30]. The expression of NOS protein levels increase with warming temperatures and are maximal between 30°C and 34°C [29, 30], suggesting that higher NOS activity could enhance NO-mediated immunity at warmer temperatures. L-Arg is an essential amino acid used in mosquito reproduction [31], which can only be obtained through diet [32]. Malaria infection induces severe hypoargininemia in the vertebrate host [33], resulting in low L-Arg levels obtained by the mosquito in the bloodmeal. L-Arg is further limited because it is also utilized in midgut-mediated immune defenses against the parasite [4] and potentially in parasite development [34, 35]. Previous studies have shown that L-Arg supplementation result in enhanced production of NO, increasing both NO-mediated immunity and reducing Plasmodium prevalence and intensity [4].
Due to established effects of environmental temperature on P. yoelii development [36] and boosted immunity and resistance in response to L-Arg supplementation An. stephensi [4] and in D. melanogaster [28], we make the following predictions: (1) Overall, parasite establishment (oocyst prevalence) should begin declining at warmer than optimal temperatures for the parasite (26°C and 28°C); (2) oocyst intensities and sporozoite prevalence should be highest around the thermal optimum for parasite development (24°C); (3) in general, NOS expression and activity should increase with temperature [30]; and (4) overall, L-Arg supplementation should decrease oocyst prevalence, oocyst intensities, and sporozoite prevalence except at cool temperatures were NOS expression and NOS activity are lowest. We demonstrate that NO-dependent host immunity and parasite transmission potential are affected by changes in ambient temperature. Further, while the effects of supplementation were apparent at intermediate temperatures, high and low temperature extremes relieved the effects of supplementation on parasite infection and on negative feedback regulation of NOS expression, indicating that the upper and lower bounds for parasite transmission were set irrespective of boosted immune function.
2. METHODS
2.1 Mosquito rearing and handling
We reared A. stephensi (Liston) under standard insectary conditions at 27± 1° C, 80% humidity, and a 12 h light: 12 h dark photo-period. We placed mosquito eggs into plastic trays (25 cm × 25 cm × 7 cm) filled with 1.5 L of water. To minimize any potential variation in emerging adult mosquito body size, we divided recently hatched larvae to ensure a density of 400 individuals per tray. Larvae were fed Liquifry for the first five days post-hatching, and then were fed Tetrafin fish flakes for the duration of the larval period. Pupae were collected from larval trays and placed into experimental cages approximately two weeks after egg hatch. Upon emergence, 2000 adult females each were divided into two cages of 1000 mosquitoes supplemented with either sugar cubes and water alone, or sugar cubes and the NOS enzyme substrate L-Arg (0.002%) in water [4]; this dietary supplement regime was continued throughout the entire course of the experiment (supplementary information).
2.2 Malaria parasite infections
Forty mice (female C57Bl / 6 laboratory mice, Charles River Laboratories) were inoculated with 105 Plasmodium yoelii parasites (clone 17XNL, from the WHO Registry of Standard Malaria Parasites, University of Edinburgh, UK). Four days after inoculation, each cage of 1000 mosquitoes (three days post-emergence) were fed on 20 anesthetized, infectious mice at 24°C ± 0.5°C and 80% ± 5% relative humidity for 30 min. Two hours post-infection, mosquitoes that had taken a full blood-meal from each dietary treatment were randomly allocated to one of five reach-in incubators set at the following temperatures: 20°C, 22°C, 24°C, 26°C, and 28°C ± 0.5°C with relative humidity 80% ± 5% and 12 L : 12 D cycle photoperiod. Two and three days later mosquitoes were provided with egg laying bowls. This experiment was replicated two times through time, with each temperature replicated in different incubators (supplementary information).
To estimate infection prevalence and intensity, we dissected both midguts and salivary glands under a standard dissecting microscope, with 25 mosquitoes randomly selected from each replicate per temperature treatment per dissecting time interval. We recorded the number of midguts with oocysts, oocysts per midgut, and salivary glands with sporozoites to estimate the prevalence of infected and infectious mosquitoes, as well as the intensity of infection, for each temperature treatment and replicate. Because the rate of parasite development is affected by ambient temperature [36], we dissected midguts from a small sample of mosquitoes to determine when parasites had reached a specific developmental stage in each temperature treatment (mature oocysts and no salivary gland sporozoites versus oocysts with salivary gland invasion). We began recording daily mosquito mortality post-infection and terminated counts after sporozoite dissections, which ran from day 15 to day 30 depending on the temperature treatment (supplementary information).
2.3 Gene expression assays: RNA collection, cDNA synthesis, and quantitative PCR
To monitor NOS expression patterns with temperature and L-Arg supplementation throughout the course of infection, we removed 10 mosquitoes at 12 hr, 24 hr, 48 hr, as well as 1–2 days post sporozoite release from each experimental group (n = 500 total). After removal, mosquitoes were killed rapidly using a 5–10s exposure to chloroform and immediately stored in RNAlater RNA stabilization reagent at −20°C until termination of the experiment (25 days). Messenger RNA was extracted using the Qiagen RNeasy Mini Kit for animal tissues (as per the manufacturer’s protocol) with the optional DNase step included. Mosquitoes were isolated individually in β-Mercaptoethanol and RLT lysis buffer. Standards for quantitative PCR (qPCR) were prepared by extracting mRNA from a pool of four mosquitoes. The concentration of mRNA in each sample was quantified with a Nanodrop and stored at −80 °C, and RNA integrity from a subset of individuals was assessed using an Agilent 2100 bioanalyzer (RNA integrity scores ranged from 7–9). 100 ng of RNA was converted to cDNA with a high-capacity cDNA reverse transcription kit (10 μL of mRNA suspended in water into a 10 μL reaction mix) as per the manufacturer’s protocol (Applied Biosystems) on a Mastercycler Gradient thermal cycler (Eppendorf) under the following reaction conditions: 10 min at 25°C, 120 min at 37°C, 5 min at 85°C, and held at 4°C until storage at −80°C.
We quantified our diluted cDNA (1:10 dilution of neat cDNA) from our experimental samples by comparing their threshold cycle numbers against a standard curve generated from 1:10 serial dilutions of our standard sample (cDNA from a pool of four mosquitoes) [30]. Three replicates of each cDNA standard spanning six orders of magnitude were included in each qPCR run. We quantified cDNA for our target gene (NOS) and a standard reference gene ribosomal protein S7 (rpS7) from individual mosquitoes using the standard curve for each assay. We normalized our target gene expression (see statistical analysis) against a single reference gene because rpS7 was not influenced in this study by experimental conditions (supplementary information), its abundance is strongly correlated with total amounts of mRNA (supplementary information), and it is used in wide diversity of expression studies on Anopheles as an internal control [e.g. 2, 9, 37–39]. Within and between plate replicates of samples were incorporated in each plate to confirm an absence of significant variation between and within assays. DNA contamination was confirmed to be undetectable using qPCR on RNA samples. All real-time quantification was performed using an Applied Biosystems 7500 Fast Real-Time PCR System and Sequence Detection software (version 1.4) with an initial denaturation of 95 °C for 20 s followed by 40 cycles of denaturation at 95 °C for 3 s and annealing / extension at 60 °C for 30 s. Two microliters of working concentration cDNA were included in each 25 μL volume PCR with the following components: 1.5 μL each of forward and reverse primer (final concentration of 300 nM), 12.5 μL of 2 × PerfeCTa™ qPCR FastMix™, Low Rox, 1 μL of MGB probe (final concentration of 200 nM), and 6.5 μL of sterile water. Primers and probes for NOS and rpS7 were designed using Primer Express 3.0 (Applied Biosystems) off of the following GenBank sequences for An. stephensi and An. gambiae: NOS (Accession number: AY583529) and rpS7 (Accession number: AF539918).
Primers used in the quantification process were NOS forward 5′-GGTTCCCATCCGAAGCATT-3′, NOS reverse 5′-GCAACACAGGGCAGGTTACAT-3′, rpS7 forward 5′-CGTGAGGTCGAGTTCAACAACA-3′, and rpS7 reverse 5′-CGTGCTTGCCGGAGAACTT-3′. Probes used in quantification were NOS 5′ FAM-CCCCATTCGTCCTTG-MGB 3′ and rpS7 5′ FAM-CGATCATCATCTACGTGCC-MGB 3′ resulting in amplicon lengths of 133 and 127 base pairs for NOS and rpS7, respectively. We performed BLAST analysis to ensure our primers and probes targeted sequences from the genes of interest, and assay efficiencies were confirmed to be not significantly different at 96% for both NOS and rpS7 (Independent Samples t-test: n = 16, t1,14 = 0.211, p = 0.836). In addition to efficiency, we also calculated the average slopes, y-intercepts, and R2 values from eight standard / calibration curves for each assay: NOS slope = −3.42, y-intercept = 42.82, R2 = 0.996; rpS7 slope = −3.43, y-intercept = 40.89, R2 = 0.997). We did not determine a limit of detection (LOD) for our experimental samples because all of our positive samples fell well within (Cq < 33) the linear dynamic range for each assay (NOS1: range of Cq’s = 25–35; rpS7 range of Cq’s = 24–34).
2.4 Statistical analyses
Statistical analyses were performed with IBM SPSS Statistics 20.0 (IBM Corporation). Full factorial models from generalized linear model (GZLM) analysis were reduced through backward elimination of non-significant interactions. We assessed goodness of fit of the final models through model deviance, log likelihood values, and model residuals. Covariates included in GZLMs were centered on their grand mean, and the significance of pair-wise interactions was assessed with Bonferroni-adjusted post-hoc tests.
2.4.1 Malaria parasite infections
We used a binary logistic GZLM (logit function) to estimate how changes in ambient temperature and L-Arg supplementation influenced the mean probability of a mosquito being infected (presence of oocysts). To compare how the intensity of infection (number of oocysts per midgut) varied with treatment, we used GZLM analysis assuming a negative binomial distribution (log link function). Finally, to compare how experimental treatment affected the number of infectious mosquitoes (mosquitoes with sporozoites in their salivary glands), we performed a GZLM analysis on transformed data assuming a linear distribution. Full factorial analyses were performed for each response variable with temperature (20°C, 22°C, 24°C, 26°C, and 28°C), supplementation treatment (water or L-arginine), and replicate included as fixed factors.
2.4.2 NOS expression
To compare differences in NOS expression, we used GZLM analysis assuming a gamma distribution (log link function) to estimate how mean NOS cDNA varied with experimental treatment. We performed a full factorial analysis with ambient temperature (20°C, 22°C, 24°C, 26°C, and 28°C), supplementation treatment (water or L-Arg), sampling time point (12 hr, 24 hr, 48 hr post-infection, or sporozoite invasion), and replicate as fixed factors. To normalize our target gene expression for any inter-sample variation introduced through sampling, RNA extraction, or reverse transcription, we included rpS7 cDNA counts of each sample as a covariate in all models [30] to adjust the means predicted by the model for NOS expression by rpS7 expression. This normalization approach controls for inter-sample variation, is more conservative because it does not introduce artificial skew to the response variable as ratio data, and allows for parametric (more powerful) statistical analysis.
2.4.3 Mosquito survival
To assess the effects of temperature and L-Arg supplementation on mosquito mortality, we used an interval censored survival GZLM analysis (binomial distribution, complementary log-log function) to compare how the average probability of mosquito death varied with experimental treatment. Temperature (20°C, 22°C, 24°C, 26°C, or 28°C), supplementation treatment (water or L-Arg), and replicate were included as fixed factors, while number of days post-infection was included as a covariate in the model. We also used a Kaplan-Meier survival analysis (log rank, Mantel-Cox test) to determine if temperature, supplementation treatment, and replicate affected daily cumulative mosquito survival.
3. RESULTS
3.1 Malaria parasite infections
Changes in average ambient temperature and L-Arg supplementation significantly affected both the mean prevalence of mosquitoes infected with P. yoelii oocysts and sporozoites, as well as the mean number of oocysts per midgut (Table 1). L-Arg supplementation significantly reduced oocyst and sporozoite prevalence, and the intensity of oocysts per midgut (Figure 1). Overall, the probability of oocyst establishment in mosquito midguts was relatively unaffected by changes in ambient temperature, with significant reductions occurring only in mosquitoes maintained at 28°C; this effect of temperature was consistent whether mosquitoes received water or L-Arg supplementation (Figure 1A, both treatment groups: 20°C vs. 28°C, p < 0.0001; 22°C vs. 28°C, p = 0.001; 24°C vs. 28°C, p < 0.0001; and 26°C vs. 28°C, p < 0.0001).
Table 1.
Results from GZLM model analysis demonstrate significant effects of temperature and L-arginine supplementation on measures of vector competence and on the expression of nitric oxide synthase (NOS). Dashes indicate factors or interaction terms that were either backward eliminated from full models or were not included in the overall experimental design.
| oocyst prevalence (n = 500) |
oocyst intensity (n = 497) |
mosquitoes with sporozoites (n = 476) |
NOS expression (n = 485) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factors
|
Wald X2
|
d.f.
|
p-value | Wald X2
|
d.f.
|
p-value
|
Wald X2
|
d.f.
|
p-value | Wald X2
|
d.f. | p-value
|
| intercept | 15.54 | 1 | <0.0001 | 4145.89 | 1 | <0.0001 | 1122.16 | 1 | <0.0001 | 27312.86 | 1 | <0.0001 |
| temperature | 29.40 | 4 | <0.0001 | 324.98 | 4 | <0.0001 | 1509.75 | 4 | <0.0001 | 18.52 | 4 | 0.001 |
| supplementation | 18.05 | 1 | <0.0001 | 10.69 | 1 | 0.001 | 294.39 | 1 | <0.0001 | 12.27 | 1 | <0.0001 |
| sampling time point | – | – | – | – | – | – | – | – | – | 580.67 | 3 | <0.0001 |
| replicate | 0.92 | 1 | 0.338 | 30.02 | 1 | <0.0001 | 2.16 | 1 | 0.141 | 0.84 | 1 | 0.360 |
| centred rpS7 cDNA counts | – | – | – | – | – | – | – | – | – | 12.59 | 1 | <0.0001 |
| temperature × supplementation | 1.71 | 4 | 0.789 | 17.15 | 4 | 0.002 | 403.74 | 4 | <0.0001 | – | – | – |
| supplementation × sampling time point | – | – | – | – | – | – | – | – | – | 35.92 | 3 | <0.0001 |
| temperature × sampling time point | – | – | – | – | – | – | – | – | – | 33.32 | 12 | 0.001 |
Figure 1.
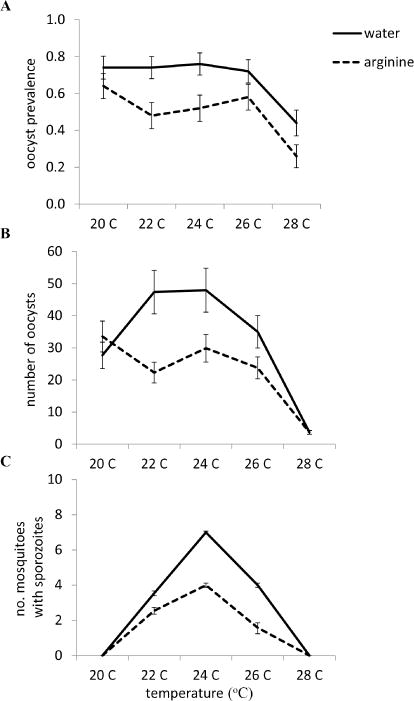
Both mean ambient temperature and supplementation treatment (water, solid lines; L-arginine, dotted lines) significantly affected the probability that a malaria infection will establish (mean oocyst prevalence, A), the intensity of malaria infection (the mean number of oocysts per midgut, B), and the probability of becoming infectious (mean sporozoite prevalence, C). Vertical bars represent standard errors of the mean.
Mean ambient temperature significantly influenced the number of oocysts that successfully established (Table 1); however, the effects of temperature were qualitatively different when mosquitoes were supplemented with water compared with mosquitoes supplemented with L-Arg, as indicated by the significant two-way interaction between temperature and supplementation treatment (temperature × supplementation, Figure 1B). For example, oocyst intensities were highest in control mosquitoes housed at 22°C and 24°C, while there is no significant increase in oocyst intensities in L-Arg supplemented mosquitoes housed at these temperatures. Oocyst intensities were significantly lower at 28°C relative to cooler temperatures in both water (20°C vs. 28°C, p < 0.001; 22°C vs. 28°C, p < 0.0001; 24°C vs. 28°C, p < 0.0001; 26°C vs. 28°C, p < 0.0001) and L-Arg supplemented mosquitoes (20°C vs. 28°C, p < 0.001; 22°C vs. 28°C, p < 0.0001; 24°C vs. 28°C, p < 0.0001; 26°C vs. 28°C, p < 0.0001; Figure 1B). We also observed a similar effect of temperature on the number of mosquitoes with sporozoites in their salivary glands (Figure 1C). In both L-Arg- and the control treatment group, there were significantly more infectious mosquitoes reared at 24°C relative to warmer and cooler temperatures (Figure 1C, both treatment groups: 20°C vs. 24°C, p < 0.0001; 22°C vs. 24°C, p < 0.0001; 24°C vs. 26°C, p < 0.0001; 24°C vs. 28°C, p < 0.0001). L-Arg supplementation, in general, decreased the number of infectious mosquitoes (except for those maintained at a cold and warm temperature extremes) compared with mosquitoes provided with water only (Figure 1C). We did observe a significant effect of experimental replicate on mean oocyst intensities, with mosquitoes experiencing on average 23 oocysts per midgut (SE ± 3.23) vs. 32 oocysts per midgut (SE ± 3.84) in the first and second experimental replicate, respectively. However, we did not observe any interactions between experimental treatment and replicate, demonstrating that there were no significant qualitative differences in temperature and L-Arg treatment on oocyst intensities across both experimental replicates.
3.2 NOS expression
GZLM analysis suggested that NOS expression was significantly affected by sampling time point, L-Arg supplementation, and temperature (Table 1). In general, NOS expression peaked 48 hr post-infection in both L-Arg and water supplemented individuals (6 hr vs. 48 hr, p < 0.0001; 12 hr vs. 48 hr, p < 0.0001; 48 hr vs. oocyst rupture, p < 0.0001), was on average lower in L-Arg supplemented mosquitoes, and significantly increased as warmer temperatures (28°C) relative to cooler temperatures (20°C vs. 28°C, p = 0.002; 24°C vs. 28°C, p = 0.001), which is consistent with previous work [29, 30].
Intriguingly, NOS expression was also significantly influenced by two, two-way interactions (Table 1) between sampling time point and supplementation (supplementation × sampling time point, Figure 2A) and sampling time point and temperature (temperature × sampling time point, Figure 2B). Both L-Arg and the control treatment group exhibited greater mean NOS expression at 24 hr relative to 12 hr post-infection, a period that spans ookinete maturation (12–20 hr) and transit across the midgut epithelium (20–24 hr) [40] (water: 12 hr vs. 24 hr, p = 0.003; L-Arg: 12 hr vs. 24 hr, p = 0.002); however, this effect was greater in mosquitoes supplemented with water only (Figure 2A). Across both supplementation treatment group NOS reached peak expression levels at 48 hr post-infection, a time consistent with early oocyst development for P. yoelii (water: 12 hr vs. 48 hr, p < 0.0001; L-Arg: 12 hr vs. 48 hr, p < 0.001 and 24 hr vs. 48 hr, p = 0.003), with substantial declines in NOS expression at later stages of oocyst development (both treatment groups: 48 hr vs. sporozoite invasion, p < 0.0001). The peak in NOS expression at 48 hr post-infection was greatest in mosquitoes maintained at 22°C (Figure 2B). Further, NOS expression increased earlier (24 hr post malaria infection) in mosquitoes maintained at warmer temperatures (24 hr: 26°C vs. 20°C, p = 0.005; 26°C vs. 24°C, p = 0.045; 28°C vs. 20°C, p = 0.011; 28°C vs. 22°C, p = 0.029; 28°C vs. 24°C, p = 0.016).
Figure 2.
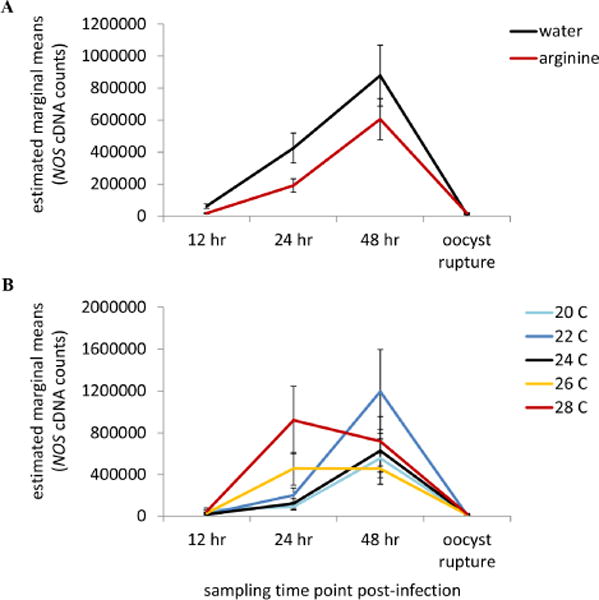
Supplementation treatment (A) and changes in mean ambient temperature (B) both significantly influenced the expression dynamics of nitric oxide synthase (NOS) throughout the course of malaria infection. A In both supplemenation treatment groups, NOS expression on average experienced a peak in expression at 48 hr post-infection; yet, this peak was significantly lower in L-arginine-fed mosquitoes (dotted line) relative to mosquitoes supplemented with wate (solid line). B Further, this peak in NOS expression at 48 hr post-infection is being driven by mosquitoes housed at 20°C (light blue line), 22°C (dark blue line), and 24°C (black line). Mosquitoes housed in warmer thermal environments (26°C, orange line and 28°C, red line) experienced relatively similar amounts of NOS expression at 24 hr as they did at 48 hr post-infection. Vertical bars represent standard errors of the mean.
3.3 Mosquito mortality
GZLM analysis (deviance / d.f. = 4.238; Likelihood Ratio X21,7 = 194.09, p < 0.0001) revealed that mean ambient temperature significantly affected the probability of mosquito death of both L-Arg- and water-supplemented mosquitoes (Wald X21,4 = 13.52, n = 1227, p = 0.009). There was no significant effect of supplementation treatment on the probability of mosquito mortality throughout the experiment (Wald X21,4 = 0.288, n = 1227, p = 0.591). Survival curves generated from Kaplan-Meier survival analysis illustrate that cumulative daily mosquito survival decreased, in general, for mosquitoes housed in standard laboratory conditions and warmer ambient temperatures relative to those mosquitoes housed at cooler temperatures (Figure 3, Table 2).
Figure 3.
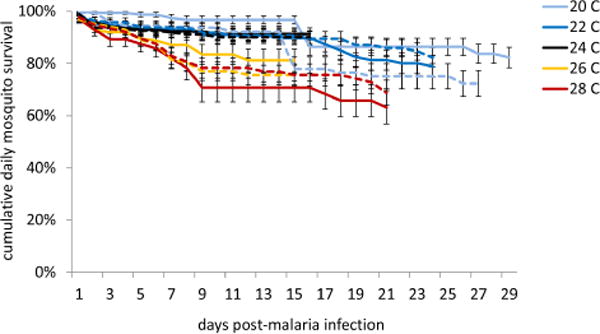
Mosquito survival curves were generated from Kaplan-Meier estimates of mosquito cumulative survival in each treatment group (water supplementation, solid lines; L-arginine supplementation, dashed lines). Temperature, but not L-arginine supplementation treatment, significantly influenced cumulative daily mosquito survival. Mosquitoes housed in warmer ambient temperatures (26°C, orange lines and 28°C, red lines) died significantly faster than mosquitoes housed at cooler temperatures (20°C, light blue lines; 22°C, dark blue lines; and 24°C, black lines)
Table 2.
Pairwise comparisons generated from Kaplan-Meier survival analyses comparing the effects of mean ambient temperature and supplementation treatment on cumulative daily mosquito survival. Significant differences between daily cumulative survival are highlighted in bold.
| Log Rank (Mantel-Cox) Pairwise Comparisons (N = 1778) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20°C | 22°C | 24°C | 26°C | 28°C | |||||||
| X2 | p-value | X2 | p-value | X2 | p-value | X2 | p-value | X2 | p-value | ||
|
|
|
|
|
|
|||||||
| water | 20°C | – | – | 0.488 | 0.485 | 0.911 | 0.34 | 13.33 | <0.0001 | 3.809 | 0.051 |
| 22°C | 0.488 | 0.485 | – | – | 0.058 | 0.81 | 11.985 | 0.001 | 10.935 | 0.001 | |
| 24°C | 0.911 | 0.34 | 0.058 | 0.81 | – | – | 9.139 | 0.003 | 7.336 | 0.007 | |
| 26°C | 13.33 | <0.0001 | 11.985 | 0.001 | 9.139 | 0.003 | – | – | 0.003 | 0.955 | |
| 28°C | 3.809 | 0.051 | 10.935 | 0.001 | 7.336 | 0.007 | 0.003 | 0.955 | – | – | |
|
| |||||||||||
| arginine | 20°C | – | – | 3.281 | 0.07 | 0.012 | 0.914 | 16.033 | <0.0001 | 23.74 | <0.0001 |
| 22°C | 3.281 | 0.07 | – | – | 0.079 | 0.778 | 3.215 | 0.073 | 9.488 | 0.002 | |
| 24°C | 0.012 | 0.914 | 0.079 | 0.778 | – | – | 3.913 | 0.048 | 12.474 | <0.0001 | |
| 26°C | 16.033 | <0.0001 | 3.215 | 0.073 | 3.913 | 0.048 | – | – | 1.935 | 0.164 | |
| 28°C | 23.74 | <0.0001 | 9.488 | 0.002 | 12.474 | <0.0001 | 1.935 | 0.164 | – | – | |
4. DISCUSSION
Both L-Arg supplementation and environmental temperature significantly influenced the probability of parasite establishment, the number of parasites establishing, transmission potential (number of mosquitoes with sporozoites in the salivary glands), and the expression of NOS. The main effects of temperature variation on P. yoelii infection, NOS expression (thermal maxima between 22°C and 24°C and around 30°C, respectively), and mosquito mortality closely matched previous observations [30, 36]. We demonstrated additionally that L-Arg supplementation significantly reduced all aspects of P. yoelii infection except at the upper and lower temperature extremes, where immunity was either not boosted, or the effects of L-Arg supplementation appeared to have no immunological effect.
To maintain experimental feasibility across a large temperature range, we did not include supplementation groups often found in experiments that have explored the effects of L-Arg supplementation on insect resistance: for example, L-NAME, a non-oxidizable L-Arg analog that competes with L-Arg for the substrate binding site of NOS enzyme; D-NAME, the non-oxidizable enantiomer to L-Arg; and citrulline, produced along with NO from L-Arg oxidation). Thus, we cannot entirely rule out the possibility that the negative effects of L-Arg on parasite prevalence and intensity resulted from supplementing the host with additional nutrients. Further, L-Arg supplementation might also increase constitutively expressed levels of NO involved in cellular signaling; thus, supplementation with L-Arg could potentially modulate chemosensory signaling [41] and induction of humoral and cellular immune responses [42, 43].
Previous studies on An. stephensi found that infection with Plasmodium significantly increased NOS production, activity and NO levels in the midgut [4]. When mosquitoes were supplemented with L-Arg, both P. berghei and P. falciparum oocyst intensities were reduced due to a direct interaction between L-Arg and the NOS enzyme (both citrulline and D-NAME controls were equivalent to unsupplemented individuals, while L-NAME enhanced parasite infection [4, and also see 28]). Finally, catalytic activity of NOS and, hence, NO feedback to repress NOS mRNA expression in the A. stephensi midgut, was consistent with reductions in NOS expression seen here at temperatures associated with L-Arg-dependent inhibition of parasite development. It is likely, therefore, that L-Arg supplementation in our studies resulted in enhanced levels of NO that were toxic to developing parasites and that repressed NOS mRNA expression in a negative feedback loop. Repression of NOS mRNA levels would ultimately reduce NOS protein levels and, therefore, contribute to endogenous protection of mosquito cells from self-induced damage [44], which might explain why there is no effect of L-Arg supplementation on mosquito mortality. This reduction in NOS mRNA via negative feedback combined with endogenous protective mechanisms against nitrosative stress might represent a balance between parasite killing and NO-mediated reductions in survivorship. However, even at reduced NOS levels, NO synthesis would likely persist for hours, based on observed half-lives of approximately 2 hr for inducible NOS protein in a variety of mammalian cell types [45] and high catalytic activity of inducible NOS [46, 47].
Our observations of increased mosquito resistance with L-Arg supplementation are likely due to increased NO levels during the first three days of infection when parasites are invading and establishing as early oocysts. In particular, mature ookinetes in transit from the lumen to the outer surface of the midgut could become nitrated or damaged, consistent with previous observations of NO-mediated apoptosis of midgut ookinetes [48, 49], resulting in fewer robust oocysts that produce fewer sporozoites. Alternatively, it is also possible that sporozoites could be rapidly deactivated upon entering the hemolmyph by circulating hemocytes that also produce NO [21, 22, 24, 28].
While L-Arg supplementation tended to reduce malaria parasite infection, the effects were restricted to intermediate temperatures. At the highest temperature (28°C), P. yoelii infection was severely constrained irrespective of L-Arg supplementation. Whether this is an effect of temperature on the parasite, a result of earlier induction of NOS resulting in higher expression levels of NOS irrespective of L-Arg treatment [30], or the involvement of other immune mechanisms at 28°C is unclear. However, given that other Plasmodium species (notably the human malaria parasites) can be transmitted successfully at temperatures >30°C, it seems likely that the reduced parasite performance we observed at 28°C was a consequence of direct thermal sensitivity of P. yoelii or increased efficiency of rodent malaria specific immune responses (TOLL activation, TEP1 / APL1-C / LRIM-1 activity). At the lowest temperature (20°C), P. yoelii was able to establish following a blood meal and the lack of a supplementation effect could be because of limited NOS expression and/or inefficient conversion of L-Arg by the NOS enzyme at sub-optimal temperatures (6–7°C below standard rearing temperatures for A. stephensi). That said, failure of the parasite to produce sporozoites suggests again the potential for some direct negative effects of cooler temperatures on late stage parasite development that are independent of immunity. These results are consistent with a range of studies demonstrating thermally-induced shifts in the outcome of infection due to differences in the thermal performance of the invertebrate host and the parasite (e.g. [50–53]).
Overall we have shown that mosquito susceptibility to malaria parasite infection is affected by temperature and L-Arg supplementation. Given that the vast majority of studies examining mosquito-parasite interactions ignore any such environmental variation, this is an important insight. Further, we found that over a certain range of temperatures, addition of a key nutritional supplement to boost immunity reduced infection prevalence and intensity, while at higher and lower temperature extremes, boosting immunity had no effect on the parasite. These results indicate that susceptibility is modulated in part via host effects. Exact temperature responses depend on the specifics of the mosquito-parasite pairing. We used a rodent malaria parasite as a model, and the temperature dependencies of rodent malarias do not map directly to temperature dependencies of the human malarias [54–56], thus extrapolating to field environments is difficult. However, as it currently stands, we have very little understanding of the thermal performance curves for the four major human malaria species or the 20 or so key malaria vectors responsible for transmission worldwide, and whether or not the thermal performance of vector and parasite traits are affected by adaptation to local conditions [57]. Nonetheless, we would expect that human malaria parasites would exhibit similar qualitative responses, with extrinsic factors shaping the outcome of parasite infection success via direct and indirect effects. These insights are relevant for understanding natural variation in mosquito vector competence and for extending insights derived under abstracted laboratory environments to more realistic transmission conditions.
Supplementary Material
L-Arginine supplementation reduced malaria infection at intermediate temperatures.
This is potentially due to thermal influences on nitric oxide synthase immunity.
Infection was low at both temperature extremes irrespective of supplementation.
This could be due to the thermal sensitivity of the malaria parasite.
The direct / indirect effects of the environment on vector competence are important.
Acknowledgments
We thank members of the Thomas and Read lab groups for discussion and J. Teeple for insectary support. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences, the National Institute of Allergy and Infectious Diseases, and the National Institutes of Health. This project is funded, in part, under a grant with the Pennsylvania Department of Health using Tobacco Settlement Funds. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions. This research was funded by the following grants: NSF-NIH EID (EF-0914384) and NIH-R21 (AI096036-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
CCM conceived and designed this research, acquired the data, ran the gene expression assays, analyzed the data, and wrote / revised the manuscript.
SB participated in the design and execution of this experiment.
SL participated in the design of this experiment and in the writing / revising of the manuscript.
MBT participated in the design of this experiment and in the writing / revising of the manuscript.
All authors have read and approved the final manuscript.
Contributor Information
Courtney C. Murdock, Email: ccm15@psu.edu.
Simon Blanford, Email: stb13@psu.edu.
Shirley Luckhart, Email: sluckhart@ucdavis.edu.
Matthew B. Thomas, Email: mbt13@psu.edu.
8. LITERATURE CITED
- 1.Vaughan JA. Population dynamics of Plasmodium sporogony. Trends in Parasitology. 2007;23(2):63–70. doi: 10.1016/j.pt.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Oliveira G, Lieberman J, Barillas-Mury C. Epithelial nitration by a peroxidase/NOX5 system mediates mosquito antiplasmodial immunity. Science. 2012;335:856–859. doi: 10.1126/science.1209678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson TML, Gow AJ, Luckhart S. Nitric oxide metabolites induced in Anopheles stephensi control malaria parasite infection. Free Radical Biology and Medicine. 2007;42(1):132–142. doi: 10.1016/j.freeradbiomed.2006.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luckhart S, et al. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(10):5700–5705. doi: 10.1073/pnas.95.10.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar S, Barillas-Mury C. Ookinete-induced midgut peroxidases detonate the time bomb in anopheline mosquitoes. Insect Biochemistry and Molecular Biology. 2005;35(7):721–727. doi: 10.1016/j.ibmb.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Price I, et al. In vivo, in vitro, and in silico studies suggest a conserved immune module that regulates malaria parasite transmission from mammals to mosquitoes. 2013:1095–8541. doi: 10.1016/j.jtbi.2013.05.028. (Electronic) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horton AA, et al. The mitogen-activated protein kinome from Anopheles gambiae: identification, phylogeny and functional characterization of the ERK, JNK and p38 MAP kinases. BMC Genomics. 2011;12 doi: 10.1186/1471-2164-12-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Surachetpong W, et al. MAPK ERK signaling regulates the TGF-beta 1-dependent mosquito response to Plasmodium falciparum. PLoS Pathogens. 2009;5(4):1–11. doi: 10.1371/journal.ppat.1000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garver LS, Dong YM, Dimopoulos G. Casper controls resistance to Plasmodium falciparum in diverse Anopheline species. PLoS Pathogens. 2009;5(3):1–12. doi: 10.1371/journal.ppat.1000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitri C, et al. Fine pathogen discrimination within the APL1 gene family protects Anopheles gambiae against human and rodent malaria species. PLoS Pathogens. 2009;5(9):1–10. doi: 10.1371/journal.ppat.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong YM, et al. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathogens. 2006;2(6):513–525. doi: 10.1371/journal.ppat.0020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanford JI, et al. Implications of temperature variation for malaria parasite development across Africa. Scientific Reports. 2013;3:1–11. doi: 10.1038/srep01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cator LJ, et al. Characterizing microclimate in urban malaria transmission settings: a case study from Chennai, India. Malaria Journal. 2013;12(84):1–10. doi: 10.1186/1475-2875-12-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulenburg H, et al. Introduction. Ecological immunology. Philosophical Transactions of the Royal Society. B-Biological Sciences. 2009;364(1513):3–14. doi: 10.1098/rstb.2008.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas MB, Blanford S. Thermal biology in insect-parasite interactions. Trends in Ecology & Evolution. 2003;18(7):344–350. [Google Scholar]
- 16.Murdock CC, et al. Rethinking vector immunology: the role of environmental temperature in shaping resistance. Nature Reviews Microbiology. 2012;10(12):869–876. doi: 10.1038/nrmicro2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colasanti M, et al. Cysteine protease as a target for nitric oxide in parasitic organisms. Trends in Parasitology. 2001;17(12):575–575. doi: 10.1016/s1471-4922(01)02191-2. [DOI] [PubMed] [Google Scholar]
- 18.Colasanti M, et al. Molecular bases for the anti-parasitic effect of NO. International Journal of Molecular Medicine. 2002;9(2):131–134. [PubMed] [Google Scholar]
- 19.James SL. Role of nitric oxide in parasitic infections. Microbiological Reviews. 1995;59(4):533–&. doi: 10.1128/mr.59.4.533-547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali M, et al. Naturally occurring triggers that induce apoptosis-like programmed cell death in Plasmodium berghei ookinetes. Plos One. 2010;5(9) doi: 10.1371/journal.pone.0012634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hillyer JF, Estevez-Lao TY. Nitric oxide is an essential component of the hemocyte-mediated mosquito immune response against bacteria. Developmental and Comparative Immunology. 2010;34(2):141–149. doi: 10.1016/j.dci.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Hillyer JF, Barreau C, Vernick KD. Efficiency of salivary gland invasion by malaria sporozoites is controlled by rapid sporozoite destruction in the mosquito haemocoel. International Journal for Parasitology. 2007;37(6):673–681. doi: 10.1016/j.ijpara.2006.12.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dimopoulos G, et al. Malaria infection of the mosquito Anopheles gambiae activates immune-responsive genes during critical transition stages of the parasite life cycle. EMBO Journal. 1998;17(21):6115–6123. doi: 10.1093/emboj/17.21.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrera-Ortiz A, et al. The effect of nitric oxide and hydrogen peroxide in the activation of the systemic immune response of Anopheles albimanus infected with Plasmodium berghei. Developmental and Comparative Immunology. 2011;35(1):44–50. doi: 10.1016/j.dci.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Vijay S, et al. Parasite killing in malaria non-vector mosquito Anopheles culicifacies species B: implication of nitric oxide synthase upregulation. Plos One. 2011;6(4) doi: 10.1371/journal.pone.0018400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitten MMA, et al. Role of superoxide and reactive nitrogen intermediates in Rhodnius prolixus (reduviidaeTrypanosoma rangeli interactions. Experimental Parasitology. 2001;98(1):44–57. doi: 10.1006/expr.2001.4615. [DOI] [PubMed] [Google Scholar]
- 27.Ascenzi P, Gradoni L. Nitric oxide limits parasite development in vectors and in invertebrate intermediate hosts. Iubmb Life. 2002;53(2):121–123. doi: 10.1080/15216540211472. [DOI] [PubMed] [Google Scholar]
- 28.Kraaijeveld AR, et al. L-Arginine enhances immunity to parasitoids in Drosophila melanogaster and increases NO production in lamellocytes. Developmental and Comparative Immunology. 2011;35(8):857–864. doi: 10.1016/j.dci.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 29.Murdock CC, Moller-Jacobs LL, Thomas MB. Complex environmental drivers of immunity and resistance in malaria mosquitoes. Proceedings of the Royal Society B: Biological Sciences. 2013;280(1770) doi: 10.1098/rspb.2013.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murdock CC, et al. Complex effects of temperature on mosquito immune function. Proceedings of the Royal Society B-Biological Sciences. 2012;279(1741):3357–3366. doi: 10.1098/rspb.2012.0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uchida K. Balanced amino acid composition essential for infusion-induced egg development in the mosquito (Culex pipiens pallens) Journal of Insect Physiology. 1993;39(7):615–621. [Google Scholar]
- 32.Vrzal EM, Allan SA, Hahn DA. Amino acids in nectar enhance longevity of female Culex quinquefasciatus mosquitoes. Journal of Insect Physiology. 2010;56(11):1659–1664. doi: 10.1016/j.jinsphys.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Chau JY, et al. Malaria-associated L-Arginine deficiency induces mast cell-associated disruption to intestinal barrier defenses against non-typhoidal Salmonella bacteremia. Infection and Immunity. 2013;20:20. doi: 10.1128/IAI.00380-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vincendeau P, et al. Arginases in parasitic diseases. Trends in Parasitology. 2003;19(1):9–12. doi: 10.1016/s1471-4922(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 35.Rivero A. Nitric oxide: an antiparasitic molecule of invertebrates. Trends in Parasitology. 2006;22(8):352–352. doi: 10.1016/j.pt.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 36.Paaijmans KP, et al. Warmer temperatures reduce the vectorial capacity of malaria mosquitoes. Biology Letters. 2012;8(3):465–468. doi: 10.1098/rsbl.2011.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arrighi RBG, et al. The immunogenic properties of protozoan glycosylphosphatidylinositols in the mosquito Anopheles gambiae. Developmental and Comparative Immunology. 2009;33(2):216–223. doi: 10.1016/j.dci.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Coggins SA, Estevez-Lao TY, Hillyer JF. Increased survivorship following bacterial infection by the mosquito Aedes aegypti as compared to Anopheles gambiae correlates with increased transcriptional induction of antimicrobial peptides. Developmental and Comparative Immunology. 2012;37(3–4):390–401. doi: 10.1016/j.dci.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Dong Y, et al. Engineered Anopheles immunity to Plasmodium infection. PLoS Pathogens. 2011;7(12):1–12. doi: 10.1371/journal.ppat.1002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luckhart S, et al. Sustained activation of Akt elicits mitochondrial dysfunction to block Plasmodium falciparum infection in the mosquito host. PLoS Pathogens. 2013;9(2):e1003180. doi: 10.1371/journal.ppat.1003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muller U. The nitric oxide system in insects. Progress in Neurobiology. 1997;51(3):363–381. doi: 10.1016/s0301-0082(96)00067-6. [DOI] [PubMed] [Google Scholar]
- 42.Foley E, O’Farrell PH. Nitric oxide contributes to induction of innate immune responses to gram-negative bacteria in Drosophila. Genes & Development. 2003;17(1):115–125. doi: 10.1101/gad.1018503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nappi AJ, et al. Nitric oxide involvement in Drosophila immunity. Nitric Oxide-Biology and Chemistry. 2000;4(4):423–430. doi: 10.1006/niox.2000.0294. [DOI] [PubMed] [Google Scholar]
- 44.Peterson TML, Luckhart S. A mosquito 2-Cys peroxiredoxin protects against nitrosative and oxidative stresses associated with malaria parasite infection. Free Radical Biology and Medicine. 2006;40(6):1067–1082. doi: 10.1016/j.freeradbiomed.2005.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kolodziejski PJ, Koo JS, Eissa NT. Regulation of inducible nitric oxide synthase by rapid cellular turnover and cotranslational down-regulation by dimerization inhibitors. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(52):18141–18146. doi: 10.1073/pnas.0406711102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochemical Journal. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bredt DS, Snyder SH. Nitric oxide: a physiological messenger molecule. Annual Review of Biochemistry. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 48.Hurd H, Carter V, Nacer A. Interactions between malaria and mosquitoes: The role of apoptosis in parasite establishment and vector response to infection. Role of Apoptosis in Infection. 2005;289:185–217. doi: 10.1007/3-540-27320-4_9. [DOI] [PubMed] [Google Scholar]
- 49.Al-Olayan EM, Williams GT, Hurd H. Apoptosis in the malaria protozoan, Plasmodium berghei: a possible mechanism for limiting intensity of infection in the mosquito. International Journal for Parasitology. 2003;33(1):105–105. doi: 10.1016/s0020-7519(02)00087-5. [DOI] [PubMed] [Google Scholar]
- 50.Vale PF, Stjernman M, Little TJ. Temperature-dependent costs of parasitism and maintenance of polymorphism under genotype-by-environment interactions. Journal of Evolutionary Biology. 2008;21(5):1418–1427. doi: 10.1111/j.1420-9101.2008.01555.x. [DOI] [PubMed] [Google Scholar]
- 51.Mitchell SE, et al. Host-parasite and genotype-by-environment interactions: temperature modifies potential for selection by a sterilizing pathogen. Evolution. 2005;59(1):70–80. [PubMed] [Google Scholar]
- 52.Lazzaro BP, et al. Genotype-by-environment interactions and adaptation to local temperature affect immunity and fecundity in Drosophila melanogaster. PLoS Pathogens. 2008;4(3):1–9. doi: 10.1371/journal.ppat.1000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stacey DA, et al. Genotype and temperature influences pea aphid resistance to a fungal entomopathogen. Physiological Entomology. 2003;28:75–81. [Google Scholar]
- 54.Sato Y, et al. Effect of temperature to Plasmodium berghei and P. yoelii on mosquito stage in Anopheles stephensi. Japanese Journal of Parasitology. 1996;45(2):98–104. [Google Scholar]
- 55.Noden BH, Kent MD, Beier JC. The impact of variations in temperature on early Plasmodium falciparum development in Anopheles stephensi. Parasitology. 1995;111:539–545. doi: 10.1017/s0031182000077003. [DOI] [PubMed] [Google Scholar]
- 56.Okech BA, et al. Resistance of early midgut stages of natural Plasmodium falciparum parasites to high temperatures in experimentally infected Anopheles gambiae (Diptera : Culicidae) Journal of Parasitology. 2004;90(4):764–768. doi: 10.1645/GE-135R1. [DOI] [PubMed] [Google Scholar]
- 57.Sternberg ED, Thomas MB. Local adaptation to temperature and the implications for vector-borne diseases. Trends in Parasitology. 30(3):115–122. doi: 10.1016/j.pt.2013.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


