Abstract
Mephedrone (4-methylmethcathinone) is a synthetic cathinone designer drug that alters presynaptic dopamine (DA) activity like many psychostimulants. However, little is known about the postsynaptic dopaminergic impacts of mephedrone. The neuropeptide neurotensin (NT) provides inhibitory feedback for basal ganglia and limbic DA pathways, and postsynaptic D1-like and D2-like receptor activity affects NT tissue levels. This study evaluated how mephedrone alters basal ganglia and limbic system NT content and the role of NT receptor activation in drug consumption behavior. Four 25 mg/kg injections of mephedrone increased NT content in basal ganglia (striatum, substantia nigra and globus pallidus) and the limbic regions (nucleus accumbens core), while a lower dosage (5 mg/kg/injection) only increased striatal NT content. Mephedrone-induced increases in basal ganglia NT levels were mediated by D1-like receptors in the striatum and the substantia nigra by both D1-like and D2-like receptors in the globus pallidus. Mephedrone increased substance P content, another neuropeptide, in the globus pallidus, but not in the dorsal striatum or substantia nigra. Finally, the NT receptor agonist PD149163 blocked mephedrone self-administration, suggesting reduced NT release, as indicated by increased tissue levels, likely contributing to patterns of mephedrone consumption.
Keywords: Mephedrone, Neurotensin, Basal Ganglia, Limbic System, Dopamine, Stimulants
Introduction
Mephedrone (4-methylmethcathinone) is a synthetic cathinone designer stimulant that alters central dopamine (DA) and serotonin (5-HT) systems and is often a principal component of crystalline or powdered `bath salts' stimulant mixtures. `Bath salts' containing mephedrone are distributed worldwide through head shops, gas stations and the internet, and mephedrone itself is replacing methylenedioxymethamphetamine (MDMA) as the primary constituent of ecstasy tablets in some European countries. The rapid rise in mephedrone abuse led to legislative restriction throughout Europe in 2010 and Schedule I classification within the US in 2011 (for a review of the patterns, prevalence, and consequences of mephedrone abuse, see German et al. 2013).
The stimulant effects of mephedrone are likely related to drug-induced activation of DA systems, much like other stimulants including the amphetamines and cocaine (German et al. 2013, Fleckenstein et al. 2007, Chausmer & Katz 2001). To date, the reported effects of mephedrone include the disruption of DA reuptake, release, and synaptic vesicle sequestration. DA transporter (DAT) function is reduced following in vivo treatment with, or in vitro exposure of synaptosomes to, mephedrone (Baumann et al. 2012, Hadlock et al. 2011). In addition, mephedrone acts as a DAT substrate and is transported into presynaptic terminals, causing DA release through a presumed change in the DA concentration gradient (Baumann et al. 2012). Mephedrone administration increases extracellular DA when measured by in vivo striatal microdialysis, and DA release as measured by in vitro striatal tissue rotating disk electrode voltammetry or radiolabeled DA release from cells, further supporting the role of mephedrone as a DAT substrate (Baumann et al. 2012, Hadlock et al. 2011, Simmler et al. 2013). Further, mephedrone reduces in vitro DA uptake into synaptic vesicles through the vesicular monoamine transporter-2 (VMAT2) in a dose-dependent manner (Lopez-Arnau et al. 2012). Loss of DAT and VMAT2 transport function underlies the large quantities of unsequestered intracellular DA following mephedrone exposure, likely leading to aberrant DA signaling and stimulant-related behaviors.
Despite recent progress elucidating the presynaptic effects of mephedrone on central DA systems, the postsynaptic effects of mephedrone are largely unknown. Consequently, this study investigated the effects of in vivo mephedrone administration upon basal ganglia and limbic neurotensin (NT) systems. Neurotensin is a neuropeptide that functions as an inhibitory feedback system for dopaminergic projections throughout the CNS (for review, see (Binder et al. 2001). NT is associated with midbrain structures involved in processing information related to motor, emotion, memory, motivation and executive functions (Quirion et al. 1985, Roberts et al. 1981, Binder et al. 2001). In a reciprocal fashion, DA receptor activation affects the activity of NT systems. Stimulation of D1 receptors with direct agonists or high doses of stimulants, such as methamphetamine (METH) or cocaine, increases NT tissue content within the dorsal striatum, substantia nigra, globus pallidus and nucleus accumbens of the limbic system (Merchant et al. 1989). Increased NT tissue content, as measured by NT-like immunoreactivity (NTLI), corresponds to decreased NT release, as well as NT accumulation and elevated synthesis within these basal ganglia regions (Frankel et al. 2005). In contrast, D2 receptor activation by direct agonists or low doses of stimulants increases NT release, reducing NTLI tissue levels and antagonizing the DA-enhancing effects of these stimulants (Wagstaff et al. 1994, Chartoff et al. 2004), likely through enhanced GABAergic activity (Ferraro et al. 1998).
In order to determine how mephedrone influences the NT system, this study evaluated NTLI content within the basal ganglia and limbic system of rats exposed to a broad range of mephedrone doses. The role of postsynaptic DA receptors was also assessed through pre-treatment with D1- and D2-like receptor antagonists. To determine the specificity of mephedrone's effects, changes in the NT system were compared to those of another neuropeptide, substance P (SP), by measuring SP-like immunoreactivity (SPLI) within the basal ganglia. Finally, the NT-1 receptor (NTR1) agonist PD146163 was used to assess the involvement of the NT system in mephedrone self-administration.
Materials and Methods
Animals, Reagents and Drug Treatment
Male Sprague-Dawley rats (250 – 325 g; Charles River Laboratories, Raleigh, NC) were housed in a temperature (22 °C) and light-controlled (14/10 light/dark cycle) environment with food and water ad libitum, according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All experiments were approved by the University of Utah Insitutional Animal Care and Use Committee. 4-Methylmethcathinone (mephedrone) was a kind gift from the National Institute on Drug Abuse and synthesized by Research Triangle Institute (Research Triangle Park, NC). S-(−)-eticlopride hydrochloride and R(+)-SCH-23390 hydrochloride were purchased from Sigma Aldrich (St. Louis, MO). All drug doses were calculated as free base and prepared in sterile saline solution (0.9% wt/vol NaCl, pH 7.4). All chemicals, unless otherwise noted, were purchased from Sigma-Aldrich (St. Louis, MO).
Antiserum was raised against the NT carboxy-terminus in New Zealand White rabbits and expresses no cross-reactivity with 1000-fold excess concentrations of other endogenous neuropeptides, including dynorphin, metenkephalin, cholecystokinin, substance P or substance K. The SP antiserum was also raised in New Zealand White rabbits as previously described (Letter et al. 1987). This antiserum recognizes the SP carboxy terminus and expresses no cross-reactivity with 1,000-fold excess concentrations with dynorhpin A, metenkephalin, neurotensin, or substance K.
For all experiments, rats were administered four subcutaneous injections of either saline (1.0 ml/kg) or mephedrone (1.0, 2.5, 5.0 or 25.0 mg/kg) per injection with 2-h intervals and sacrificed by decapitation 18 h following the last drug administration. These mephedrone dosages were chosen based on previously observed toxicity and self-administration. The 25 mg/kg/injection binge mephedrone dosage used in this study was chosen for its acute disruption of the DA and 5-HT systems within the striatum and hippocampus, respectively (Hadlock et al. 2011). At a 5 mg/kg/injection dosage, binge-treated rats received a total amount of mephedrone (6 mg) equivalent to that previously observed to be self-administered within a single session (6 mg; 25 infusions of 0.24 mg mephedrone/infusion) (Hadlock et al. 2011). For pre-treatment experiments, animals were given i.p. injections of either a D1-like (SCH-23390; 0.5 mg/kg) or D2-like (eticlopride; 0.5 mg/kg) antagonist 15 min prior to each mephedrone administration. These antagonist dosages were chosen based on previous studies demonstrating their effectiveness within the basal ganglia and limbic system (Alburges et al. 2011). Following sacrifice, brains were removed, flash frozen on dry ice, and stored at −80 °C until dissected. Brains were sliced in 1-mm thick coronal sections and the dorsal striatum, posterior caudate, globus pallidus, nucleus accumbens core, nucleus accumbens shell and core, substantia nigra, ventral tegmental area, and frontal cortex were dissected according to The Rat Brain in Stereotaxic Coordinates (Paxinos & Watson 1986). Dissected tissues were stored at −80 °C until neuropeptide analysis.
Radioimmunoassay
Neuropeptide (NTLI or SPLI) concentrations within brain regions were determined by solid-phase radioimmunoassay (RIA) as previously described (Alburges et al. 2011, Alburges et al. 2009). Briefly, dissected tissue was homogenized in 300 μL 10 mM HCl, boiled for 10 min, then centrifuged at 17000 g for 30 min. A small amount of supernatant was collected for protein analysis using the Bradford assay. The remaining sample was lyophilized and stored at −80 °C until further use. For the RIA, lyophilized samples were reconstituted in assay buffer consisting of 300 μL phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10.1 mM Na2HPO4, and 1.8 mM KH2PO4 in dH20; pH 7.4), 0.1% (wt/vol) gelatin and 0.1% (wt/vol) Triton X-100. Nunc-Immunoplates (ISI Bio-Express; Kaysville, UT) were prepared for the assay by incubating 50 μL protein G solution (50 ng/100 mL in 0.1 mol/L NaHCO3; Invitrogen; Carlsbad, CA) per well overnight at 4 °C followed by three washes with wash buffer (150 mM K2HPO4, 20 mM Na2HPO4, 0.2 mM ascorbic acid, 0.2% (vol/vol) Tween-20 and 0.1% (wt/vol) sodium azide in dH20; pH 7.4). Neurotensin (25 μL; 1:20000 dilution) and substance P (25 μL) antisera were diluted in assay buffer (wash buffer containing 0.1% (wt/vol) gelatin), incubated in plate wells for 2 h at 25 °C to attach antibody to protein G surface, and then washed three times with wash buffer. 25 μL of samples or standards were added to wells and incubated for 3 h at 25 °C. Radiolabeled NT or SP ([125I]NT or [125I]SP; 6500 dpm per 25 μL diluted in assay buffer) was then added to each well and incubated for 2 h at 25 °C. Following incubation wells were washed with wash buffer and protein G was removed from wells, placed in polypropylene tubes and radioactivity was counted in a five-channel Packard Cobra II Auto-Gamma counter (Packard Instrument Co.; Meriden, CT). NT and SP concentrations were determined by comparing bound to free [125I]NT or [125I]SP in each sample to standard curves ranging from 1 to 125 pg protein per assay tube.
Self-Administration and PD149163 Treatment
Rats were trained to self-administer mephedrone as previously described(Hadlock et al. 2011). Briefly, rats were first food trained to teach lever-pressing behavior by restricting them to approximately 90% of their free-feeding food quantity and exposing them to overnight 14-h food training sessions. During food training sessions, rats were placed into operant chambers with two randomly programmed retractable levers - an “active” lever that delivered a food-pellet (45 mg rodent grain food fellets; Bio-Serv, Frenchtown, NJ) and an “inactive” lever that delivered no reward. Rats were food trained for 5 days on an escalating reward ratio (FR1-FR5). Following food training, rats were anaesthetized (90 mg/kg ketamine and 7 mg/kg xylazine, i.p.) and catheters were implanted proximal to the scapula and fed into the right jugular vein. Catheter patency was maintained by daily infusions of 0.1 ml antibiotic solution cefazolin (10.0 mg/ml) dissolved in heparinized saline along with 0.05 ml heparin and 0.05 ml heparinized glycol to lock the catheter. Following a 3 d recovery period, animals were placed in operant chambers (4 h/session, 1 session/day, 29 °C room temperature) and allowed to self-administer mephedrone. Each active lever press delivered a mephedrone infusion (0.24 mg in 10 μl over 5 sec, FR1) while an inactive lever press delivered nothing. Levers were retracted following a press (active or inactive) and remained so for an additional 20 s. Levers were counterbalanced within each group and all presses recorded by computers running Graphic State software (Coulbourn Instruments, Allentown, PA). Mephedrone self-administration continued for 7 d. On self-administration day six, rats were pre-treated with the neurotensin-1 receptor agonist PD149163 (0.5 mg/kg, i.p., 15 min prior to self-administration)and allowed to self-administer mephedrone as before.
Data Analysis
Results are graphed as percentage difference from saline control (mean ± standard error of the mean) with absolute values of control samples reported in the figure legends. Data were analyzed in GraphPad Prism 5.01 (GraphPad Software; La Jolla, CA) using one-way ANOVA comparison between groups with Newman-Keuls multiple comparison post-tests and significance set at p ≤ 0.05.
Results
To determine if mephedrone alters the dorsal striatal NT system (the site of efferent NT cell bodies), striatal NTLI was measured 18 h following 4 injections of mephedrone (25 mg/kg/administration) or saline (1 ml/kg/administration) with 2-h intervals between each injection. The involvement of DA receptors in this NT response was assessed by pre-treating rats with saline, a D1-like (SCH-23390), or a D2-like (eticlopride) receptor antagonist 15 min (i.p.) prior to each mephedrone administration. SCH23390 alone did not alter striatal NTLI, though eticlopride increased striatal NTLI as previously reported (Alburges et al. 2011). Mephedrone-induced striatal NLTI increases were inhibited by the D1-like receptor blockade but not significantly altered by D2-like receptor blockade, since increased NTLI within the dorsal striatum of eticlopride-treated animals appeared additive (Fig. 1).
Figure 1.
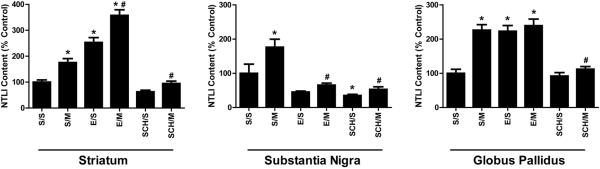
Effects of repeated high-dose mephedrone admnistration and the role of D1-like and D2-like receptors on NTLI within the (A) dorsal striatum (S/S = 577.2 ± 51.4 pg/mg protein), (B) substantia nigra (S/S = 1364.0 ± 393.1 pg/mg protein), (C) and globus pallidus (S/S = 1005.9 ± 128.1 pg/mg protein). Rats received either saline (S; 1 ml/kg/injection, s.c.), the D2-like antagonist eticlopride (E; 0.5 mg/kg/injection, i.p.) or the D1-like antagonist SCH-23390 (SCH; 0.5 mg/kg/injection, i.p.) 15 min prior to each of 4 injections (2-h intervals) of either saline (S; 1 ml/kg/injection, s.c.) or mephedrone (M; 25 mg/kg/injection, s.c.) and were sacrificed 18 h following the last injection. Results are expressed as percentages of the saline control group (S/S) and represent the mean ± SEM. S/S = saline/saline, S/M = saline/mephedrone, E/S = eticlopride/saline, E/M = eticlopride/mephedrone, SCH/S = SCH-23390/saline, SCH/M = SCH-23390/mephedrone. * p < 0.05 vs. S/S, # p < 0.05 vs S/M. N = 8 rats per treatment group
The impact of mephedrone on the NT systems associated with striatal efferent terminals, the substantia nigra (direct pathway) and globus pallidus (indirect pathway) were also evaluated as described for Fig. 1 (Fig. 2A and B, respectively). Mephedrone elevated NTLI in both structures, an effect that was blocked by SCH23990 (Fig. 2). Eticlopride pre-treatment did not affect NTLI within the substantia nigra but increased globus pallidus NTLI (Fig. 2B), as previously reported (Alburges et al. 2011). Mephedrone administration did not elevate substantia nigra NTLI in animals receiving eticlopride pre-treatment (Fig. 2A; E/M). Importantly, mephedrone did not further increase NTLI within the globus pallidus of eticlopride pre-treated animals (Fig. 2B; E/M).
Figure 2.
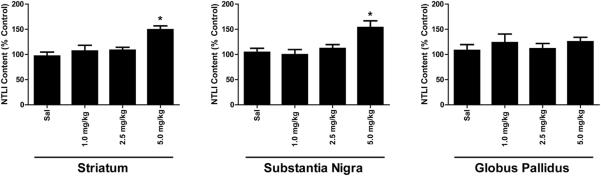
Effects of different low-dose mephedrone administrations on NTLI in basal ganglia regions. Rats received four injections (2-h intervals) of saline, 1.0, 2.5, or 5.0 mg/kg/injection of mephedrone and sacrificed 18 h following the last injection. S/S absolute values in the striatum = 157.7 ± 13.2 pg/mg protein; substantia nigra = 797.0 ± 66.5; globus pallidus = 956.7 ± 115.9. Results are percentages of control groups and represent the mean ± SEM. * p < 0.01 vs. all other corresponding groups.
The dose-response by basal ganglia structures to mephedrone was evaluated by measuring NTLI within the dorsal striatum, substantia nigra and globus pallidus 18 h after 4 injections of saline or mephedrone (Fig. 3; 1.0, 2.5, or 5.0 mg/kg/administration, 2 h intervals). Increased NTLI within the dorsal striatum and substantia nigra, but not the globus pallidus, was observed after the 5.0 mg dose of mephedrone but neither the 1.0 nor 2.5 mg/kg doses.
Figure 3.
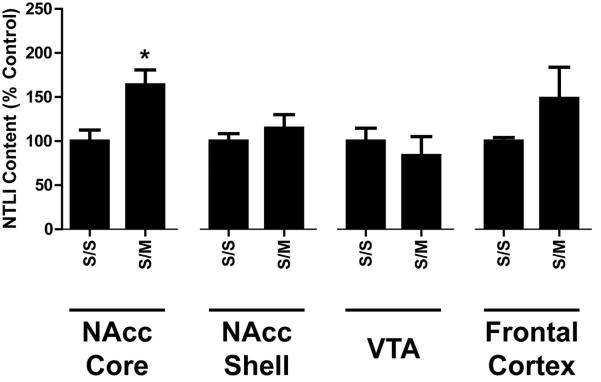
Effects of repeated high-dose mephedrone (4 × 25 mg/kg/injection) administration on limbic NTLI content. Rats were treated as described in Fig. 1 except they only received saline or mephedrone. S/S absolute values in the nucleus accumbens core = 386.8 ± 52.0 pg/mg protein; nucleus accumbens shell = 782.1 ± 71.2; ventral tegmental area = 1384.4 ± 218.0, frontal cortex = 40.4 ± 1.7. *p < 0.05 vs. corresponding saline (S) group.
For comparison, NTLI was measured within the limbic system structures (frontal cortex, and nucleus accumbens core and shell) following repeated 25 mg/kg mephedrone administrations (Fig. 4). The only significant effect of mephedrone on NTLI content within the limbic system was an increase in nucleus accumbens core.
Figure 4.
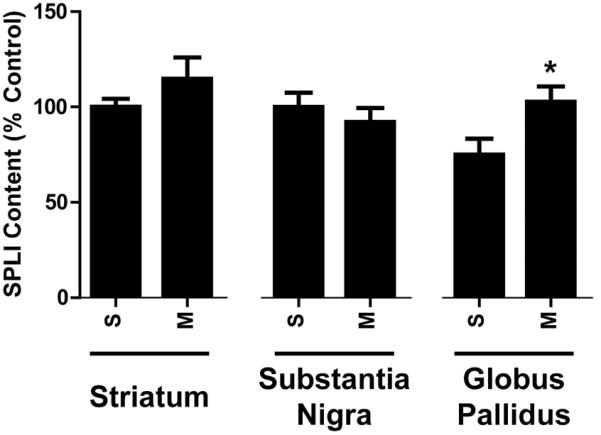
– Effects of repeated high-dose mephedrone (M; 25 mg/kg/injection) administration on SPLI content of basal ganglia structures. Rats were treated as described in Fig. 1 except they only received saline or mephedrone. S/S absolute values in the striatum = 4487.6 ± 207.7 pg/mg protein; substantia nigra = 4682.2 ± 381.166; globus pallidus = 2998.2 ± 361.0. *p < 0.05 vs. corresponding saline control (S) groups.
SPLI was measured within basal ganglia structures to assess the selectivity of repeated high-dose (25 mg/kg/injection) mephedrone administration upon neuropeptide systems. Mephedrone administration significantly increased pallidal SPLI content but in other structures SPLI content was unchanged (Fig. 5).
Figure 5.
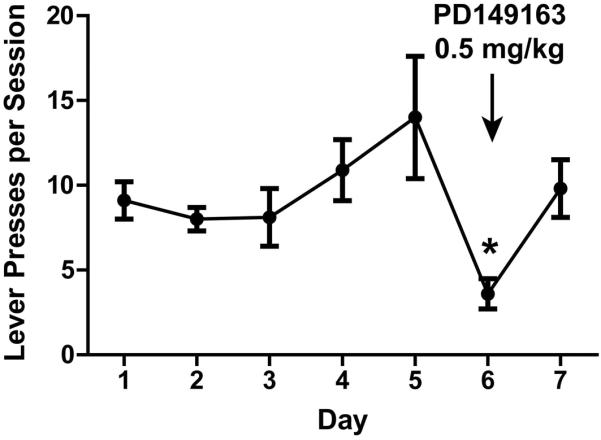
– NT involvement in mephedrone self-administration. As described in materials and methods, rats were food-trained, jugular catheters were implanted, and then allowed to self-administer mephedrone (0.24 mg mephedrone/infusion, FR1) for 7 d. On day 6, the neurotensin receptor-1 agonist PD149163 (0.5 mg/kg, i.p.) was given 15 min prior to the self-administration session. *p < 0.05 vs. day 5 lever presses.
Finally, rats readily self-administer mephedrone (Fig. 5), and the involvement of NT in consumption was assessed by treatment with PD149163 (0.5 mg/kg), a neurotensin-1 receptor (NTR1) agonist, prior to the sixth day of self-administration. The NTR1 agonist significantly reduced the rate of mephedrone lever-pressing (Fig. 5).
Discussion
Mephedrone users most frequently report consuming the drug in binge fashion, with multiple doses between 30 min and 2-h apart over a 9-h period (Addiction 2011). A binge-like paradigm of multiple mephedrone injections was therefore employed to determine any postsynaptic dopaminergic effects this drug may have. By measuring NTLI content within basal ganglia structures following a large (25 mg/kg/injection) binge regimen of mephedrone, with and without a pretreatment of either D1- or D2-like receptor antagonists, this study was able to determine whether mephedrone-induced dopaminergic disruption alters the basal ganglia NT system similar to other potent psychostimulants. Mephedrone increased dorsal striatal NTLI in a D1-like, but not D2-like, receptor-dependent mechanism (Fig. 1). In contrast, elevated nigral and pallidal NTLI following high-dose binge mephedrone treatment required activation of both D1- and D2-like receptors since SCH23390 pretreatment blocked the effects of mephedrone (Fig. 2A) and mephedrone did not increase NTLI beyond that of eticlopride pre-treated tissue (Fig. 2B). The differential role of DA receptors in mediating striatal and nigral NT responses to mephedrone was similar to that reported for METH (Castel et al. 1994, Gygi et al. 1994) and cocaine (Alburges et al. 2011), suggesting a shared DA-mediated postsynaptic mechanism within basal ganglia structures.
To test the effects of mephedrone on the NT system at dosages equivalent to and near those self-administered, binges of four 1.0, 2.5 or 5.0 mg/kg/injection were performed. Significant changes in NTLI were observed after four 5 mg/kg mephedrone administrations within the dorsal striatum and substantia nigra, but not the globus pallidus (Fig. 3). Based on these NTLI responses, the potency of mephedrone to affect this neuropeptide system is similar to that of METH (Letter et al. 1987). A previous study by Hadlock et. al. (2011) also reported that both mephedrone and METH disrupt DA uptake and induce DA release with similar potency, further suggesting similar pre- and postsynaptic mechanisms between these stimulants.
High-dose, binge mephedrone administration (4 × 25 mg/kg/administration) only increased NTLI content within the nucleus accumbens core region of the limbic system. No NTLI changes were observed in other terminal (nucleus accumbens shell or frontal cortex) or cell body (VTA) sites of limbic NT projections (Binder et al. 2001). The preferential alteration of basal ganglia but not limbic system NTLI by mephedrone contrasts the significant increase in nucleus accumbens shell NTLI following METH self-administration and non-contingent treatment (Hanson et al. 2012). The distinct effects of mephedrone and METH on NT limbic systems may underlie differences in behavior instigated by these two drugs. Since the nucleus accumbens NT system has been linked to stimulant reward, sensitization, hyperactivity and reinstatement of drug consumption, the differences observed between METH and mephedrone-induced reinforcing behaviors (Hadlock et al. 2011) may be due to the different limbic responses.
To evaluate the selectivity of mephedrone's effects on the NT system, the response of substance P to repeated 25 mg/kg doses of mephedrone was assessed by measuring SPLI within basal ganglia structures (Fig. 5). Mephedrone increased pallidal, but not striatal nor nigral, SPLI levels, which contrasts increases in dorsal striatum and nigral SPLI observed following METH (Letter et al. 1987).
Finally, the role of the NT system in regulating mephedrone-associated consumptive behavior was assessed by treating rats with PD149163, a NTR1 agonist, during mephedrone self-administration (Fig. 5). Rats readily self-administer mephedrone in an escalating fashion as previously reported (Hadlock et al., 2011), and PD149163 pre-treatment significantly reduced lever pressing for mephedrone, suggesting a reduction in NTR1 activation may be involved in regulating consumption of this drug. A progressive increase in basal ganglia NT levels, which was observed following repeated low-dose mephedrone administration (Fig. 2) and corresponds with reduced neuropeptide release within these tissues (Frankel et al. 2005), may be closely tied to and explain the escalating progression of mephedrone self-administration observed over time (Fig. 5).
In summary, the findings reported herein suggest repeated doses (5 – 25 mg/kg) of mephedrone preferentially alter NT regulation of the DA system within the basal ganglia through a primarily D1-like receptor-dependent mechanism, while having minimal impact on the limbic NT or basal ganglia SP pathways. This pattern of mephedrone-induced change in neuropeptide systems has similarities and differences compared to those of other stimulants, such as METH, and may be tied to drug consumption behavior. The impact of mephedrone-induced alterations in neuropeptide function upon the short- and long-term behavioral effects of mephedrone warrants further investigation.
Acknowledgements
This work was funded by National Institutes of Health; National Institute on Drug Abuse (grant number DA031883, DA000378): This information is usually included already, but please add to the Acknowledgments if not.
Footnotes
ARRIVE guidelines have been followed: Yes
⇒ if No, skip complete sentence
⇒ if Yes, insert “All experiments were conducted in compliance with the ARRIVE guidelines.”
Conflicts of interest: None
⇒ if `none', insert “The authors have no conflict of interest to declare.”
⇒ otherwise insert info unless it is already included
References
- Addiction E. M. C. f. D. a. D. Report on the risk assessment of mephedrone in the framework of the Council Decision on new psychoactive substances. The Publications Office of the European Union; Luxembourg: 2011. p. 193. [Google Scholar]
- Alburges ME, Frankel PS, Hoonakker AJ, Hanson GR. Responses of limbic and extrapyramidal substance P systems to nicotine treatment. Psychopharmacology. 2009;201:517–527. doi: 10.1007/s00213-008-1316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alburges ME, Hoonakker AJ, Horner KA, Fleckenstein AE, Hanson GR. Methylphenidate alters basal ganglia neurotensin systems through dopaminergic mechanisms: a comparison with cocaine treatment. Journal of neurochemistry. 2011;117:470–478. doi: 10.1111/j.1471-4159.2011.07215.x. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr., Partilla JS, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Kinkead B, Owens MJ, Nemeroff CB. Neurotensin and dopamine interactions. Pharmacological reviews. 2001;53:453–486. [PubMed] [Google Scholar]
- Castel MN, Morino P, Nylander I, Terenius L, Hokfelt T. Differential dopaminergic regulation of the neurotensin striatonigral and striatopallidal pathways in the rat. European journal of pharmacology. 1994;262:1–10. doi: 10.1016/0014-2999(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Szczypka MS, Palmiter RD, Dorsa DM. Endogenous neurotensin attenuates dopamine-dependent locomotion and stereotypy. Brain research. 2004;1022:71–80. doi: 10.1016/j.brainres.2004.06.061. [DOI] [PubMed] [Google Scholar]
- Chausmer AL, Katz JL. The role of D2-like dopamine receptors in the locomotor stimulant effects of cocaine in mice. Psychopharmacology. 2001;155:69–77. doi: 10.1007/s002130000668. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, O'Connor WT, Fuxe K, Soubrie P, Tanganelli S. The striatal neurotensin receptor modulates striatal and pallidal glutamate and GABA release: functional evidence for a pallidal glutamate-GABA interaction via the pallidal-subthalamic nucleus loop. J Neurosci. 1998;18:6977–6989. doi: 10.1523/JNEUROSCI.18-17-06977.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annual review of pharmacology and toxicology. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Frankel PS, Hoonakker AJ, Hanson GR, Bush L, Keefe KA, Alburges ME. Differential neurotensin responses to low and high doses of methamphetamine in the terminal regions of striatal efferents. European journal of pharmacology. 2005;522:47–54. doi: 10.1016/j.ejphar.2005.08.036. [DOI] [PubMed] [Google Scholar]
- German CL, Fleckenstein AE, Hanson GR. Life sciences. 2013. Bath salts and synthetic cathinones: An emerging designer drug phenomenon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gygi SP, Gibb JW, Hanson GR. Differential effects of antipsychotic and psychotomimetic drugs on neurotensin systems of discrete extrapyramidal and limbic regions. The Journal of pharmacology and experimental therapeutics. 1994;270:192–197. [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, et al. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. The Journal of pharmacology and experimental therapeutics. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson GR, Hoonakker AJ, Alburges ME, McFadden LM, Robson CM, Frankel PS. Response of limbic neurotensin systems to methamphetamine self-administration. Neuroscience. 2012;203:99–107. doi: 10.1016/j.neuroscience.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letter AA, Merchant K, Gibb JW, Hanson GR. Effect of methamphetamine on neurotensin concentrations in rat brain regions. The Journal of pharmacology and experimental therapeutics. 1987;241:443–447. [PubMed] [Google Scholar]
- Lopez-Arnau R, Martinez-Clemente J, Pubill D, Escubedo E, Camarasa J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methylone. British journal of pharmacology. 2012;167:407–420. doi: 10.1111/j.1476-5381.2012.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant KM, Gibb JW, Hanson GR. Role of dopamine D-1 and D-2 receptors in the regulation of neurotensin systems of the neostriatum and the nucleus accumbens. European journal of pharmacology. 1989;160:409–412. doi: 10.1016/0014-2999(89)90098-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Quirion R, Chiueh CC, Everist HD, Pert A. Comparative localization of neurotensin receptors on nigrostriatal and mesolimbic dopaminergic terminals. Brain research. 1985;327:385–389. doi: 10.1016/0006-8993(85)91542-2. [DOI] [PubMed] [Google Scholar]
- Roberts GW, Crow TJ, Polak JM. Neurotensin: first report of a cortical pathway. Peptides. 1981;2(Suppl 1):37–43. doi: 10.1016/0196-9781(81)90053-x. [DOI] [PubMed] [Google Scholar]
- Simmler L, Buser T, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener M, Liechti M. Pharmacological characterization of designer cathinones in vitro. British journal of pharmacology. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff JD, Bush LG, Gibb JW, Hanson GR. Endogenous neurotensin antagonizes methamphetamine-enhanced dopaminergic activity. Brain research. 1994;665:237–244. doi: 10.1016/0006-8993(94)91343-9. [DOI] [PubMed] [Google Scholar]