Abstract
Aim
To assess the risk of developing cerebral palsy in relation to pregnancy disorders and preterm birth.
Method
By linking the Medical Birth Registry of Norway to other national registries, we identified all live births in Norway from 1967 through to 2001. Risks of cerebral palsy (CP) after preterm delivery and pregnancy disorders were estimated in different gestational age groups.
Result
In total, 1,764,509 children delivered at 23–43 weeks’ gestation were included. The prevalence of CP was 1.8 per 1000 births. Absolute risk of CP was 8.5% among children born at 23–27 weeks’ gestation, 5.6% at 28–30 weeks, 2.0% at 31–33 weeks, 0.4% at 34–36 weeks, and 0.1% thereafter. Placental abruption, chorioamnionitis, prolonged rupture of membranes, intrauterine growth restriction, pre-eclampsia, multiple births, placenta previa, bleeding, cervical conization, and congenital malformation were all associated with CP. Before 32 weeks’ gestation, absolute risk of CP was highest with chorioamnionitis (9.1%) and lowest with pre-eclampsia (3.1%). Among those born after 31 weeks, the absolute risk of CP was more consistently (but also more slightly) increased with a recorded pregnancy disorder.
Interpretation
Early delivery and pregnancy disorders were both strong risk factors for CP. The added risks with recorded pregnancy disorders varied within categories of gestational age.
The risk of developing cerebral palsy (CP) strongly increases with preterm birth,1–3 but the biological basis for this association is not clear. Preterm delivery exposes the fetus to extrauterine life before it may be ready, and this exposure may be particularly harmful to the developing brain. However, almost every preterm birth is the result of an underlying pathological process, and this underlying process may also contribute to the risk of developing CP. Numerous pregnancy disorders are associated with both preterm birth and CP,2–11 and it is unclear to what extent the association between preterm birth and CP can be attributed to the early delivery, to the pregnancy disorder, or to the combination of the two. Parents who deal with pregnancy complications or preterm birth are often concerned for their child’s outcome. A better understanding of the role of preterm birth and pregnancy disorders as risk factors of developing CP may be clinically useful to clinicians and parents.
We linked the Medical Birth Registry of Norway (MBRN)12,13 to other national registries for all live births in Norway from 1967 through to 2001 in order to describe the risk of developing CP in relation to pregnancy disorders and preterm birth.
METHOD
We performed a national cohort study with prospectively collected data from compulsory registries. Each Norwegian citizen is assigned a unique personal identification number. By using the personal identification number in an encrypted form, we linked information from the MBRN, Statistics Norway,14 and the National Insurance Scheme. MBRN provided information on maternal health, pregnancy disorders, delivery, and birth, while data on education, migration, and death were obtained from Statistics Norway.
We identified all live births from 1967 to 2001 registered in the MBRN. Gestational age was calculated from the first day of the last menstrual period. Children with missing data on gestational age, children born at less than 23 weeks’ or more than 43 weeks’ gestational age, and children with a birthweight more than 3 standard deviations from the mean of the sex-specific weight for gestational age,15 were excluded as they are likely to have an incorrectly registered gestational age. We also excluded children who died within the first year of life, since these children were not likely to have been diagnosed with CP. We also excluded children who were not registered as Norwegian residents. The cohort was followed through to 2005.
The study was approved by the Norwegian Data Inspectorate, the Norwegian Labour and Welfare Administration, the National Population Register, and the Norwegian Directorate of Health. This approval included a waiver of individual consent.
Pregnancy disorders included placental abruption, chorioamnionitis, placenta previa, multiple births, prolonged rupture of membranes, intrauterine growth restriction, congenital malformations, cervical conization, unspecified bleeding, and pre-eclampsia. We defined chorioamnionitis to include both recorded chorioamnionitis and fever or sepsis during labour without a specific diagnosis of chorioamnionitis. Chorioamnionitis is reported to the MBRN by obstetricians during admission at the maternity unit. More specific information on how chorioamnionitis was diagnosed is not available in this register. Pre-eclampsia included eclampsia and early and late pre-eclampsia. Prolonged rupture of membranes was defined as rupture more than 24 hours before birth. Intrauterine growth restriction was defined as birthweight below 2SDs from the mean according to sex and gestational age.15 Unspecified bleeding was defined as bleeding during pregnancy other than that caused by placenta previa or placental abruption. Since cervical conization was not registered in the MBRN before 1987, this variable was analysed in a sub-cohort of children born from 1987 through to 2001, using the same exclusion criteria as in the larger cohort. Congenital malformations are recorded at maternity units and, after 1999, also at neonatal departments. We also collected data on malformations that were diagnosed after the neonatal period from the Norwegian Insurance Scheme. Hip dysplasia was not included as a congenital malformation. Single mother at birth, parental level of education, sex, immigrant status, and maternal age were included as sociodemographic factors. Families were identified as immigrant if both parents were born outside Norway.
Every Norwegian resident is insured through the National Insurance Scheme.16 This insurance programme provides a basic benefit for disabilities that cause substantial expenses, an attendance benefit for disabled persons needing special attention or nursing, and a disability pension for persons whose working capacity is reduced by more than 50%.17 The decision to grant benefits and pensions is based on information provided by the applicant and a medical examination, and benefits are granted irrespective of family income. CP cases were identified by the International Classification of Diseases codes 342.0–344.9 (9th revision) and G80–G83.9 (10th revision) in the insurance database. The date of diagnosis was not available in the dataset. A validation study has shown that the National Insurance Scheme has a sensitivity of 70% (mild cases tend to be underreported) and a specificity of 99% for the diagnosis CP.18 Data from the National Insurance Scheme were registered through 2005, leaving a follow-up time of 4 to 39 years.
Statistical analysis
We investigated the prevalence of CP in relation to gestational age at birth and pregnancy disorders, and in various time periods. We also evaluated the associations of pregnancy disorders and sociodemographic factors with preterm birth (<37wks) and CP. The year of birth was used as a continuous variable, while other variables were categorical.
Next, we studied the association between gestational age at birth and CP. gestational age was categorized as 23–27 weeks, 28–30 weeks, 31–33 weeks, 34–36 weeks, 37–41 weeks (reference), and 42–43 weeks. We calculated the odds ratios of CP according to gestational age and examined whether odds ratios changed after adjustment for sociodemographic factors and year of birth, and after additional adjustment for pregnancy disorders.
We also assessed whether the associations between pregnancy disorders and CP differed within the various gestational age categories. We estimated absolute risks of CP with each pregnancy disorder stratified in two preterm groups (23–31 weeks and 32–36 weeks), a term group (37–41 weeks), and a post-term group (42–43 weeks). We calculated odds ratios adjusted for sociodemographic factors and year of birth, with the reference being children born of pregnancies with no registered disorders. Risks were considered significantly different when 95% confidence intervals of odds ratios did not include 1.0. This corresponds to a two-sided hypothesis test with a significance level of 5%. Odds ratios with 95% confidence intervals were estimated by logistic regression models (PASW software, version 18.0; IBM SSPS Statistics, IBM Corp. NY, USA).
Persons missing relevant data were excluded from all adjusted analyses. Since the data were collected over several decades, we also conducted the main analyses separately in three time periods (1967–1978, 1979–1990, and 1991–2001). Since more than one fetus is affected by a pregnancy disorder in multiple births (and pregnancy disorders themselves can be exacerbated by multiple birth), we also repeated the analyses after excluding multiple births. As patent ductus arteriosus and undescended testicles may be normal findings among preterm children, we performed the analyses without including these in the congenital malformation variable.
RESULTS
There were 2,024,215 live births in Norway from 1967 to 2001. We excluded 122,701 (6.1%) with missing information on gestational age at birth, 64,424 (3.2%) who were not registered as residents of Norway, 38,266 (1.9%) born after 43 completed weeks of gestation, 18,997 (0.9%) with a presumed error in registered gestational age, 13,971 (0.7%) who died before 1 year of age, and 1347 (0.1%) with gestational age less than 23 weeks, leaving a cohort of 1,764,509 (87%) children. Of these, 92,320 (5.2%) were preterm. Sociodemographic data were available for more than 99% of the cases.
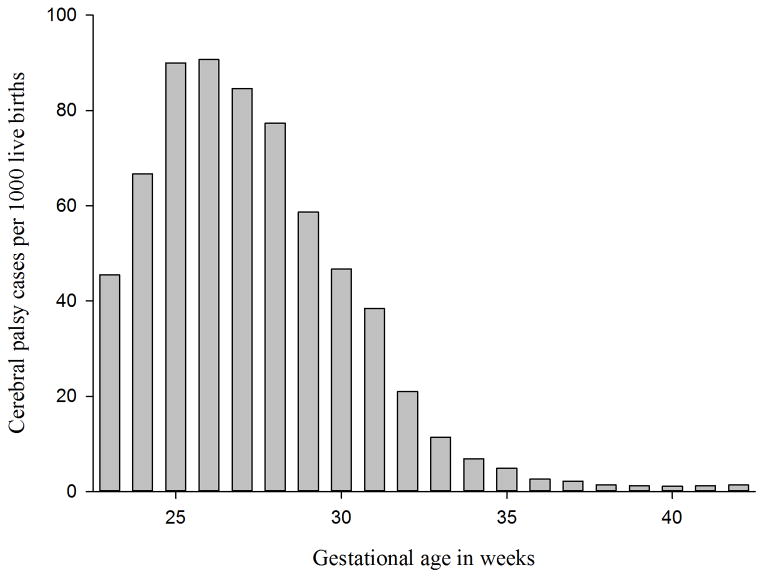
CP was recorded for 3151 of these children, with an overall prevalence of 1.8 per 1000 births. The prevalence was 1.9 per 1000 births in the period 1967–1978, 2.0 per 1000 births in the period 1979–1990, and 1.5 per 1000 in the period 1991–2001 (Fig. S1, online supporting information. The prevalence of CP increased with earlier delivery, ranging from 1.1 per 1000 at 40 weeks’ gestation to a maximum of 90.7 per 1000 at 26 weeks’ gestation (Fig. 1). Of all CP cases, 5% were born at 23–27 weeks’ gestation, 9% at 28–30 weeks, 10% at 31–33 weeks, 9% at 34–36 weeks, 57% at 37–41 weeks, and 11% at 42–43 weeks.
Figure 1.
Prevalence of cerebral palsy according to gestational age among 1,764,509 live births.
Pregnancy disorders were recorded in 13% of all births: 42% of preterm births, 12% of term births, and 9% of post-term births. Pre-eclampsia, intrauterine growth restriction, unspecified bleeding, and multiple births were the most common disorders, and all pregnancy disorders and most of the sociodemographic factors were associated with both preterm birth and CP (Table I). Nearly half of children with CP (49%) were born after 36 completed weeks' gestation with no recorded pregnancy disorder.
Table I.
Odds ratios for preterm birth and cerebral palsy among 1 764 509 live births
| Preterm birth | Cerebral palsy | ||||||
|---|---|---|---|---|---|---|---|
| (n=92,320) | (n=3151) | ||||||
| Variable | n | No. of cases | Crude OR (95% CI) | Adjusted ORa (95% CI) | No. of cases | Crude OR (95% CI) | Adjusted ORa (95% CI) |
| Pregnancy disorders | |||||||
| Placental abruption | 7736 | 3133 | 12.7 (12.2–13.3) | 13.5 (12.8–14.2) | 129 | 9.8 (8.2–11.8) | 8.0 (6.6–9.6) |
| Chorioamnionitis | 4195 | 1224 | 7.5 (7.1–8.1) | 5.4 (5.0–5.8) | 69 | 9.5 (7.5–12.1) | 6.7 (5.2–8.7) |
| Placenta previa | 3656 | 1513 | 13.0 (12.2–13.9) | 15.2 (14.1–16.3) | 33 | 5.1 (3.6–7.2) | 3.9 (2.7–5.6) |
| Multiple births | 39,758 | 14,979 | 12.9 (12.6–13.2) | 13.0 (12.7–13.3) | 238 | 3.6 (3.1–4.1) | 2.8 (2.4–3.2) |
| Prolonged rupture of membranes | 15,717 | 4126 | 6.7 (6.5–6.9) | 6.8 (6.5–7.0) | 101 | 3.7 (3.0–4.5) | 3.1 (2.5–3.8) |
| Unspecified bleeding | 43,073 | 6093 | 3.1 (3.0–3.2) | 3.4 (3.3–3.5) | 185 | 2.5 (2.2–2.9) | 2.4 (2.1–2.8) |
| Pre-eclampsia | 50,209 | 8557 | 4.0 (3.9–4.1) | 3.6 (3.5–3.7) | 174 | 2.0 (1.7–2.3) | 1.7 (1.5–2.0) |
| Cervical conizationb | 4754 | 767 | 3.1 (2.8–3.3) | 3.0 (2.7–3.2) | 26 | 3.5 (2.4–5.2) | 2.5 (1.7–3.8) |
| Intrauterine growth restriction | 46,787 | 4457 | 2.0 (1.9–2.0) | 1.0 (0.9–1.0) | 287 | 3.7 (3.3–4.2) | 2.6 (2.3–2.9) |
| Congenital malformation | 38,840 | 3196 | 1.6 (1.6–1.7) | 1.5 (1.5–1.6) | 305 | 4.8 (4.3–5.4) | 4.5 (4.0–5.1) |
| No recorded pregnancy disorder | 1,538,233 | 53,296 | 1.0 (reference) | 1.0 (reference) | 1927 | 1.0 (reference) | 1.0 (reference) |
| Sociodemographic factors | |||||||
| Single mother at birth | |||||||
| Yes | 167,949 | 11,551 | 1.4 (1.4–1.4) | 1.4 (1.3–1.4) | 381 | 1.3 (1.2–1.5) | 1.1 (1.0–1.3) |
| No | 1,588,738 | 80,254 | 1.0 (reference) | 1.0 (reference) | 2759 | 1.0 (reference) | 1.0 (reference) |
| Maternal education | |||||||
| <11y | 457,918 | 27,653 | 1.2 (1.2–1.2) | 1.2 (1.1–1.2) | 984 | 1.2 (1.1–1.3) | 1.1 (1.0–1.2) |
| 11–14y | 803,971 | 40,511 | 1.0 (reference) | 1.0 (reference) | 1423 | 1.0 (reference) | 1.0 |
| >14y | 489,562 | 23,116 | 0.9 (0.9–1.0) | 0.9 (0.9–0.9) | 726 | 0.8 (0.8–0.9) | 0.9 (0.9–1.0) |
| Paternal education | |||||||
| <11y | 401,861 | 23,981 | 1.2 (1.2–1.2) | 1.1 (1.1–1.1) | 833 | 1.2 (1.1–1.3) | 1.1 (1.0–1.2) |
| 11–14y | 882,352 | 45,030 | 1.0 (reference) | 1.0 (reference) | 1582 | 1.0 (reference) | 1.0 (reference) |
| >14y | 452,356 | 21,256 | 0.9 (0.9–0.9) | 1.0 (0.9–1.0) | 652 | 0.8 (0.7–0.9) | 0.8 (0.8–1.0) |
| Sex | |||||||
| Female | 858,570 | 41,371 | 0.9 (0.8–0.9) | 0.9 (0.8–0.9) | 1347 | 0.8 (0.7–0.8) | 0.8 (0.7–0.9) |
| Male | 905,939 | 50,949 | 1.0 (reference) | 1.0 (reference) | 1804 | 1.0 (reference) | 1.0 (reference) |
| Immigrant | |||||||
| Yes | 41,621 | 3232 | 1.5 (1.5–1.6) | 1.4 (1.4–1.5) | 56 | 0.7 (0.6–1.0) | 0.8 (0.6–1.0) |
| No | 1,722,888 | 89,088 | 1.0 (reference) | 1.0 (reference) | 3095 | 1.0 (reference) | 1.0 (reference) |
| Maternal age | |||||||
| <18y | 21,621 | 1791 | 1.7 (1.6–1.7) | 1.6 (1.5–1.7) | 46 | 1.2 (0.9–1.6) | 1.0 (0.7–1.3) |
| 18–39 | 1,717,359 | 88,685 | 1.0 (reference) | 1.0 (reference) | 3061 | 1.0 (reference) | 1.0 (reference) |
| >39 | 25,528 | 1844 | 1.4 (1.4–1.5) | 1.3 (1.3–1.4) | 44 | 1.0 (0.7–1.3) | 0.8 (0.6–1.1) |
Adjusted for all variables in the table and year of birth.
Analysed in a sub-cohort, 1987–2001 (n=770,178), because of missing data before 1987. OR, odds ratio; CI, confidence interval.
Adjustment for sociodemographic factors and year of birth had no appreciable influence on the odds ratios of CP with early delivery (Table II). Additional adjustment for pregnancy disorders slightly weakened the odds of CP with preterm birth (Table II).
Table II.
Odds ratios for cerebral palsy according to gestational age at birth among 1,764,509 live births
| GA at birth (wk) | Absolute risk, % (No. with CP/No. in category) | Crude OR (95% CI) | Adjusted ORa (95% CI) | Adjusted ORb (95% CI) |
|---|---|---|---|---|
| 23–27 | 8.5 (142/1,673) | 74.0 (61.9–88.4) | 83.4 (69.4–100.4) | 58.9 (48.2–71.9) |
| 28–30 | 5.6 (268/4,746) | 47.7 (41.8–54.4) | 50.2 (43.8–57.4) | 37.1 (32.0–43.1) |
| 31–33 | 2.0(309/15,464) | 16.3 (14.4–18.4) | 16.5 (14.6–18.7) | 13.0 (11.3–14.9) |
| 34–36 | 0.4 (285/70,437) | 3.2 (2.9–3.7) | 3.3 (2.9–3.7) | 2.9 (2.5–3.3) |
| 37–41 | 0.1 (1807/1,442,598) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| 42–43 | 0.1 (340/229,591) | 1.2 (1.1–1.3) | 1.2 (1.1–1.3) | 1.2 (1.1–1.4) |
Adjusted for sociodemographic factors (single mother at birth, parental education, sex, immigrant status, and maternal age), and year of birth.
Adjusted for placental abruption, chorioamnionitis, prolonged rupture of membranes, intrauterine growth restriction, intrauterine growth restriction, pre-eclampsia, multiple births, placenta previa, unspecified bleeding, cervical conization, congenital malformation, sociodemographic factors, and year of birth. CI, confidence interval; CP, cerebral palsy; GA, gestational age; OR, odds ratio.
Table III provides absolute risks and odds ratios for infants born after pregnancy disorders at various gestational ages. Among the earliest deliveries (23–32 weeks’ gestation), infants with any registered pregnancy disorder were at no greater risk of developing CP than those with no registered disorder. Nonetheless, the risk of developing CP did vary with individual types of pregnancy disorders. At 31 weeks and earlier, risk was highest with chorioamnionitis (absolute risk 9.1%, odds ratio 1.7 [95% CI 1.2–2.3]) and lowest with pre-eclampsia (absolute risk 3.1%, odds ratio 0.6 [95% CI 0.4–0.9]), compared with preterm infants with no registered pregnancy disorder (absolute risk 5.7%, reference). Among those born after 31 weeks, the absolute risk of developing CP was more consistently (but also more slightly) increased if a pregnancy disorder was recorded. While the odds ratios for CP with pregnancy disorders appeared large, the excess risks were relatively small in absolute terms. After 31 weeks, pregnancy complications raised the absolute risk of developing CP by no more than 2%. These main results were stable over the time periods 1967–1978, 1979–1990, and 1991–2001 (Tables S I–S IX, online supporting information). Exclusion of multiple births resulted in a slight decrease in absolute risks of CP with placental abruption (from 7.3%–7.1% for children born at 23–31 weeks’ gestation and from 1.4% to 1.3% for children born at 32–36 weeks) and chorioamnionitis (from 9.1% to 8.6% for children born at 23–31 weeks’ gestation and from 2.1% to 1.9% for children born at 32–36 weeks). Exclusion of patent ductus arteriosus and undescended testicles did not substantially alter the estimates of absolute risks or odds ratios for CP with congenital malformation.
Table III.
Risk of cerebral palsy with pregnancy disorders and gestational age at birth among 1,764,509 live births
| Gestational age at birth | ||||||||
|---|---|---|---|---|---|---|---|---|
| 23–31 weeks (n= 9618) | 32–36 weeks (n=82,702) | 37–41 weeks (n=1,442,598) | 42–43 weeks (n=229,591) | |||||
| Variable | Absolute risk, % (No. with. CP/No. in category) | Adjusted ORa (95% CI) | Absolute risk, % (No. with. CP/No. children) | Adjusted ORa (95% CI) | Absolute risk, % (No. with. CP/No. in category) | Adjusted ORa (95% CI) | Absolute risk, % (No. with CP/No. in category) | Adjusted ORa (95% CI) |
| Placental abruption | 7.3 (52/715) | 1.3 (0.9–1.8) | 1.4 (34/2,418) | 2.5 (1.7–3.6) | 0.9 (37/4168) | 6.4 (4.6–9.0) | 1.4 (6/435) | 9.2 (4.0–20.8) |
| Chorioamnionitis | 9.1 (45/497) | 1.7 (1.2–2.3) | 2.1 (16/727) | 3.8 (2.2–6.6) | 0.3 (7/2417) | 2.7 (1.3–5.6) | 0.2 (1/554) | 1.3 (0.2–9.5) |
| Prolonged rupture of membranes | 6.6 (59/889) | 1.1 (0.8–1.5) | 0.9 (29/3,237) | 1.5 (1.0–2.3) | 0.1 (12/10,475) | 1.1 (0.6–1.9) | 0.1 (1/1116) | 0.8 (0.1–5.6) |
| Intrauterine growth restriction | 5.1 (23/448) | 1.1 (0.7–1.7) | 2.0 (79/4,009) | 3.9 (3.0–5.1) | 0.4 (156/37,106) | 2.8 (2.4–3.4) | 0.6 (29/5224) | 3.3 (2.2–4.9) |
| Pre-eclampsia | 3.1 (37/1,197) | 0.6 (0.4–0.9) | 0.6 (47/7,360) | 0.9 (0.7–1.3) | 0.2 (77/37,579) | 1.5 (1.2–1.9) | 0.3 (13/4073) | 2.1 (1.2–3.7) |
| Multiple births | 5.1 (106/2,071) | 0.9 (0.7–1.1) | 0.6 (74/12,908) | 1.0 (0.8–1.3) | 0.2 (55/24,126) | 1.4 (1.0–1.8) | 0.5 (3/653) | 1.7 (0.5–5.5) |
| Placenta previa | 7.6 (16/210) | 1.4 (0.8–2.3) | 0.7 (9/1,303) | 1.3 (0.7–2.5) | 0.4 (7/1984) | 2.0 (0.9–4.5) | 0.6 (1/159) | 3.3 (0.5–23.9) |
| Unspecified bleeding | 6.1 (83/1,360) | 1.1 (0.8–1.4) | 0.7 (32/4,733) | 1.2 (0.7–1.3) | 0.2 (63/32,797) | 1.5 (1.2–1.9) | 0.2 (7/4183) | 1.2 (0.5–2.4) |
| Cervical conizationb | 8.3 (14/169) | 1.4 (0.8–2.6) | 1.0 (6/598) | 1.4 (0.6–3.4) | 0.2 (6/3569) | 1.8 (0.8–4.0) | 0.0 (0/418) | – |
| Congenital malformation | 6.8 (33/483) | 1.3 (0.9–2.0) | 1.5 (40/2,713) | 2.6 (1.8–3.6) | 0.7 (201/30,655) | 5.7 (4.9–6.7) | 0.6 (31/4989) | 4.5 (3.1–6.5) |
| No recorded pregnancy disorder | 5.7 (193/3,380) | 1.0 (reference) | 0.4 (196/49,916) | 1.0 (reference) | 0.1 (1278/1,275,777) | 1.0 (reference) | 0.1 (260/209,160) | 1.0 (reference) |
| Any recorded pregnancy disorderd | 5.5 (340/6,238) | 1.0 (0.8–1.2)c | 0.8 (275/32,786) | 2.2 (1.8–2.6)c | 0.3 (529/166,821) | 3.2 (2.9–3.6)c | 0.4 (80/20,431) | 3.2 (2.5–4.2)c |
CP, cerebral palsy; –, not analysable.
Adjusted for all pregnancy disorders, sociodemographic factors (single mother at birth, parental education, sex, immigrant status, and maternal age), and year of birth.
Analysed in a sub-cohort, 1987–2001 (n = 770,178), because of missing data before 1987.
Adjusted for sociodemographic factors and year of birth.
Some pregnancies had more than one disorder.
DISCUSSION
In this national cohort of 1.7 million children, the risk of developing CP was higher in early deliveries and in pregnancies with recorded disorders. Early delivery is a well-known risk factor for CP.1–3 Similarly, several previous studies have shown associations between pregnancy disorders and CP,2–11 but those studies either did not consider a wide spectrum of pregnancy disorders or did not distinguish the risks at different gestational ages.
Children born after pregnancies complicated by placental abruption, chorioamnionitis, intrauterine growth restriction, and congenital malformation were at increased odds of developing CP in most gestational groups, while other pregnancy disorders were more likely to independently predict increased odds of developing CP in pregnancies after 31 weeks. This is not to suggest that the recorded pregnancy disorders did not contribute to the risk of developing CP in infants born at 31 weeks or earlier.
One must assume that all preterm births are caused by pathological processes, although these are often unknown and not recorded.19 Subclinical intrauterine infection is an example of such a process. Even though this pregnancy complication may account for more than half of the preterm births among the earliest deliveries,20 it is often not recognized. Preterm births without recorded pregnancy complications can therefore not be considered as births free of pregnancy complications. Our finding of similar risks of developing CP with or without a registered pregnancy disorder among children born before 32 weeks’ gestation suggests that the unrecorded disorders were as damaging as those that were identified and recorded. Unknown pregnancy pathologies in preterm births also help to explain the apparently reduced odds of CP with pre-eclampsia in early births. This finding suggests that pre-eclampsia is less harmful than the other recorded pregnancy disorders, and also less harmful than the unknown pathologies that have caused the pregnancies without recorded disorders to end early. Assuming that pre-eclampsia is harmful for fetuses, the low absolute risk of CP with pre-eclampsia among infants born before 32 weeks’ gestation suggests that the risk attributed to immaturity may be even lower. Although speculative, this may imply that both recorded and unrecorded pregnancy disorders have a large impact on the risk of developing CP. Therefore, we cannot determine to what extent the risk with early delivery may be attributed to the infant’s immaturity or to pathological conditions that may have caused the early delivery.
In contrast, infants born after 36 weeks with no recorded pregnancy disorders (the reference group) are mostly healthy, and pre-eclampsia in term infants is consistently associated with higher risk of CP. These findings are in accordance with previous studies,6,8 although a recent Norwegian study found that the risk of CP with pre-eclampsia was increased only for term children who were small for gestational age.10 This study had a smaller but more recent eligibility period, and the design differed from ours in exclusion criteria, possible confounders, estimation of gestational age, and outcome identification.
Several earlier studies have adjusted for gestational age in order to disentangle the effects of preterm birth and pregnancy disorders on CP. However, recent discussions of causal inference imply that this approach may result in biased estimates.21,22 The possible causal relations in this study are simplistically illustrated in a directed acyclic graph (Fig S2, online supporting information). Since pregnancy disorders may cause low gestational age, and both pregnancy disorders and low gestational age may cause CP, gestational age is a mediator in the causal pathways between pregnancy disorders and CP. Adjusting for gestational age may then introduce collider bias from unrecorded pregnancy disorders.21,22 This may seriously bias the estimates. Therefore, we have attempted to avoid collider bias by not adjusting for gestational age.
Strengths of our study include the national cohort design with a large sample size, minimal loss to follow-up, and prospectively collected data. Limitations include some missing data on gestational age (6.1%) and presumed underreporting of pregnancy disorders and diagnoses of CP. The prevalence of chorioamnionitis is particularly low in this cohort, despite a wide definition. The lower prevalence of CP in individuals born after 1991 probably reflects a shorter follow-up time than that of the others. Because of the missing dates of diagnosis, we were unable to perform analyses that could handle censored data, which would have resulted in a more accurate estimation of prevalence. Still, the prevalence of CP in this study was consistent with other, comparable studies.2,3,23 Moreover, the information in the insurance registry did not allow us to discern subtypes of CP. It is also probable that mortality among sick newborn infants selectively removed infants at high risk of CP This unavoidable bias in CP prevalence may help explain the declining prevalence of CP for gestational age below 26 weeks. Maternal smoking during pregnancy could not be adjusted for, since this information was not recorded in the birth registry before 1998. This potential confounder may be partly covered by adjustment for sociodemographic factors. Our data cover four decades, which raises the question of the validity of the results for contemporary settings. However, the risk estimates changed very little across the various time periods, suggesting that the results may be applicable to current practice.
Our data cannot answer the question of whether prolongation of a complicated pregnancy may reduce the risk of CP. If an unknown factor causes both CP and preterm birth, then prolongation of pregnancy would not remove the effect of the unknown factor. It is even possible that the health of the mother or fetus may be endangered by the prolongation of a pathological pregnancy. Still, it is likely that preterm birth contributes some component of CP risk, and there is no reason to discard caution in considering the possible harm of elective delivery before term.
CP is a feared long-term consequence of complicated pregnancy and preterm delivery. Parents concerned about their child’s probability of developing CP will be most interested in absolute risk. The absolute risk of developing CP after preterm delivery ranged from 8.5% for the extremely preterm to 0.4% for the late preterm. Notably, after 31 weeks’ gestation the additional diagnosis of a pregnancy disorder added little or moderately to the absolute risk of CP. This may offer some reassurance for parents who are concerned about the effects of a registered pregnancy disorder on their child, particularly one born preterm.
Supplementary Material
Prevalence of cerebral palsy according to year of birth among 1 764 509 live births.
Possible causal relations between sociodemographic disorders, pregnancy disorders, gestational age, and cerebral palsy.
Tables SI: Odds ratios for preterm birth and cerebral palsy among 638,418 live births in the period 1967–1978.
Table SII: Odds ratios for preterm birth and cerebral palsy among 555,367 live births in the period 1979–1990.
Table SIII: Odds ratios for preterm birth and cerebral palsy among 570,724 live births in the period 1991–2001.
Table SIV: Odds ratios for cerebral palsy according to gestational age at birth among 638,418 live births in the period 1967–1978.
Table SV Odds ratios for cerebral palsy according to gestational age at birth among 555,367 live births in the period 1979–1990.
Table SVI Odds ratios for cerebral palsy according to gestational age at birth among 570,724 live births in the period 1991–2001.
Table SVII Absolute risks and odds ratios for cerebral palsy with pregnancy disorders and gestational age at birth among 638,418 live births in the period 1967–1978.
Table SVIII Absolute risks and odds ratios for cerebral palsy with pregnancy disorders and gestational age at birth among 555,367 live births in the period 1979–1990.
Table S IX Absolute risks and odds ratios for cerebral palsy with pregnancy disorders and gestational age at birth among 570,724 live births in the period 1991–2001
What this paper adds.
The paper provides absolute risk estimates of developing CP with preterm birth and pregnancy disorders.
Clinicians may use the results to provide more specific information about risk of CP with preterm birth and pregnancy disorders.
Placental abruption, chorioamnionitis, intrauterine growth restriction, and congenital malformation are associated with increased CP risk in most gestational ages.
Acknowledgments
The study was funded by the Western Norwegian Regional Health Authority and by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. These organisations had no role in study design, data analysis, and interpretation of the results.
ABBREVIATION
- MBRN
Medical Birth Registry of Norway
Footnotes
The authors have stated that they had no interests that might be perceived as posing a conflict or bias.
References
- 1.Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med. 2008;359:262–73. doi: 10.1056/NEJMoa0706475. [DOI] [PubMed] [Google Scholar]
- 2.Sukhov A, Wu Y, Xing G, Smith LH, Gilbert WM. Risk factors associated with cerebral palsy in preterm infants. J Matern Fetal Neonatal Med. 2011;25:53–57. doi: 10.3109/14767058.2011.564689. [DOI] [PubMed] [Google Scholar]
- 3.Thorngren-Jerneck K, Herbst A. Perinatal factors associated with cerebral palsy in children born in Sweden. Obstet Gynecol. 2006;108:1499–505. doi: 10.1097/01.AOG.0000247174.27979.6b. [DOI] [PubMed] [Google Scholar]
- 4.Blair E, Al Asedy F, Badawi N, Bower C. Is cerebral palsy associated with birth defects other than cerebral defects? Dev Med Child Neurol. 2007;49:252–8. doi: 10.1111/j.1469-8749.2007.00252.x. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert WM, Jacoby BN, Xing G, Danielsen B, Smith LH. Adverse obstetric events are associated with significant risk of cerebral palsy. Am J Obstet Gynecol. 2010;203:328, e1–5. doi: 10.1016/j.ajog.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenwood C, Yudkin P, Sellers S, Impey L, Doyle P. Why is there a modifying effect of gestational age on risk factors for cerebral palsy? Arch Dis Child Fetal Neonatal Ed. 2005;90:F141–6. doi: 10.1136/adc.2004.052860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulak W, Okurowska-Zawada B, Sienkiewicz D, Paszko-Patej G, Krajewska-Kulak E. Risk factors for cerebral palsy in term birth infants. Adv Med Sci. 2010;55:216–21. doi: 10.2478/v10039-010-0030-7. [DOI] [PubMed] [Google Scholar]
- 8.Mann JR, McDermott S, Griffith MI, Hardin J, Gregg A. Uncovering the complex relationship between pre-eclampsia, preterm birth and cerebral palsy. Paediatr Perinat Epidemiol. 2011;25:100–10. doi: 10.1111/j.1365-3016.2010.01157.x. [DOI] [PubMed] [Google Scholar]
- 9.O’Callaghan ME, Maclennan AH, Gibson CS, et al. Epidemiologic associations with cerebral palsy. Obstet Gynecol. 2011;118:576–82. doi: 10.1097/AOG.0b013e31822ad2dc. [DOI] [PubMed] [Google Scholar]
- 10.Strand KM, Heimstad R, Iversen AC, et al. Mediators of the association between pre-eclampsia and cerebral palsy: population based cohort study. BMJ. 2013;347:f4089. doi: 10.1136/bmj.f4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA. 2000;284:1417–24. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 12.Norwegian Institute of Public Health. [Accessed 27th January 2014];Medical Birth Registry of Norway. http://www.fhi.no/eway/default.aspx?pid=240&trg=Main_6664&Main_6664=6898:0:25,7840:1:0:0:::0:0.
- 13.Irgens LM. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand. 2000;79:435–9. [PubMed] [Google Scholar]
- 14. [Accessed 27th January 2014];Statistics Norway. http://www.ssb.no/en/
- 15.Skjaerven R, Gjessing HK, Bakketeig LS. Birthweight by gestational age in Norway. Acta Obstet Gynecol Scand. 2000;79:440–9. [PubMed] [Google Scholar]
- 16.The Norwegian Labour and Welfare Administration. [Accessed 27th January 2014];Membership in The National Insurance Scheme. http://www.nav.no/English/Membership+in+The+National+Insurance+Scheme.
- 17.Norwegian Ministry of Labour. [Accessed 27th January 2014];The Norwegian Social Insurance Scheme. http://www.regjeringen.no/upload/AD/publikasjoner/veiledninger_brosjyrer/2010/DNT_2010_eng.pdf.
- 18.Moster D, Lie RT, Irgens LM, Bjerkedal T, Markestad T. The association of Apgar score with subsequent death and cerebral palsy: A population-based study in term infants. J Pediatr. 2001;138:798–803. doi: 10.1067/mpd.2001.114694. [DOI] [PubMed] [Google Scholar]
- 19.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onderdonk AB, Hecht JL, McElrath TF, Delaney ML, Allred EN, Leviton A. Colonization of second-trimester placenta parenchyma. Am J Obstet Gynecol. 2008;199:52 e1–10. doi: 10.1016/j.ajog.2007.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanderweele TJ, Mumford SL, Schisterman EF. Conditioning on intermediates in perinatal epidemiology. Epidemiology. 2012;23:1–9. doi: 10.1097/EDE.0b013e31823aca5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilcox AJ, Weinberg CR, Basso O. On the pitfalls of adjusting for gestational age at birth. Am J Epidemiol. 2011;174:1062–8. doi: 10.1093/aje/kwr230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Surveillance of Cerebral Palsy in Europe (SCPE) Dev Med Child Neurol. 2000;42:816–24. doi: 10.1017/s0012162200001511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Prevalence of cerebral palsy according to year of birth among 1 764 509 live births.
Possible causal relations between sociodemographic disorders, pregnancy disorders, gestational age, and cerebral palsy.
Tables SI: Odds ratios for preterm birth and cerebral palsy among 638,418 live births in the period 1967–1978.
Table SII: Odds ratios for preterm birth and cerebral palsy among 555,367 live births in the period 1979–1990.
Table SIII: Odds ratios for preterm birth and cerebral palsy among 570,724 live births in the period 1991–2001.
Table SIV: Odds ratios for cerebral palsy according to gestational age at birth among 638,418 live births in the period 1967–1978.
Table SV Odds ratios for cerebral palsy according to gestational age at birth among 555,367 live births in the period 1979–1990.
Table SVI Odds ratios for cerebral palsy according to gestational age at birth among 570,724 live births in the period 1991–2001.
Table SVII Absolute risks and odds ratios for cerebral palsy with pregnancy disorders and gestational age at birth among 638,418 live births in the period 1967–1978.
Table SVIII Absolute risks and odds ratios for cerebral palsy with pregnancy disorders and gestational age at birth among 555,367 live births in the period 1979–1990.
Table S IX Absolute risks and odds ratios for cerebral palsy with pregnancy disorders and gestational age at birth among 570,724 live births in the period 1991–2001