Abstract
The dopamine (DA) terminal fields in the rat dorsal striatum (DS) and nucleus accumbens core (NAcc) are organized as patchworks of domains that exhibit distinct kinetics of DA release and clearance. The present study used fast-scan cyclic voltammetry recordings of electrically evoked DA overflow to test the hypothesis that nomifensine might exhibit domain-dependent actions within the NAcc, as we previously found to be the case within the DS. Within the NAcc, nomifensine preferentially enhanced evoked dopamine overflow in the slow compared to the fast domains. To seek a kinetic explanation for nomifensine’s selective actions, we quantified the apparent KM of DA clearance by numerically evaluating the derivative of the descending phase of the DA signal after the end of the stimulus. For comparison, we likewise quantified apparent KM in the domains of the DS. As expected because it is a competitive inhibitor, nomifensine significantly increased the apparent KM in both the fast and slow domains of both the NAcc and DS. However, our analysis also leads to the novel finding that nomifensine preferentially increases the apparent KM in the NAcc compared to the DS: apparent KM increased by ~500% in the NAcc and ~200% in the DS.
Keywords: Dopamine, Nomifensine, Preferential Action, Apparent KM, Voltammetry
INTRODUCTION
Dopamine (DA) is crucial to the motor, motivation, and reward related functions of the central nervous system (Johnson & Kenny, 2010; Jenkinson & Brown, 2011; Volkow et al., 2011). DA neurotransmission is initiated by the release of DA molecules and terminated by their reuptake via the dopamine transporter (DAT). The spatiotemporal dynamics of DA’s extracellular concentration, and therefore the extent and duration of DA receptor stimulation, are intricately determined by the kinetics of DA release and clearance as well as the mass transport of DA through the extracellular space (Wightman et al., 1988; Garris et al., 1994; Cragg & Rice, 2004; Taylor et al., 2012; Taylor et al., 2013). Because numerous drugs that affect DA release and clearance have therapeutic value (Gottwald & Aminoff, 2011; Volkow et al., 2012) and/or abuse potential (Phillips et al., 2003; Hollander & Carelli, 2007), it is highly significant to know how such drugs alter the spatiotemporal dynamics of DA concentrations in the extracellular space.
The psychostimulants cocaine and nomifensine are competitive DAT inhibitors and thus alter DA’s extracellular dynamics (Church et al., 1987; Koob & Bloom, 1988; Kuhar et al., 1991). That the actions of such drugs vary between brain regions has been a subject of intense interest. For example, their tendency to preferentially increase measures of extracellular DA in the nucleus accumbens (NAc) compared to the dorsal striatum (DS) conforms well with the central importance of the NAc in the mechanisms of substance abuse (Dichiara & Imperato, 1988; Carboni et al., 1989; Cass et al., 1992; Cass et al., 1993; Wu et al., 2001a). But, the present understanding of this preferential action remains somewhat incomplete because, first, the DS receives the densest dopaminergic innervation in the brain (Bjorklund & Dunnett, 2007), second, as yet there is no clear sign that DAT inhibitors exhibit greater potency in the NAc than DS (Boja & Kuhar, 1989; Izenwasser et al., 1990; Jones et al., 1995b) and, third, as yet there is no clear sign that competitive DAT inhibitors, such as cocaine and nomifensine, preferentially increase the DAT’s apparent KM in the NAc: rather, prior reports suggest that nomifensine acts preferentially in the DS and that cocaine acts non-preferentially (Wu et al., 2001a; Wu et al., 2001b).
We (Shu et al., 2013) have reported that the core of the NAc (NAcc), similarly to the DS (Moquin & Michael, 2009; Wang et al., 2010; Moquin & Michael, 2011), contains a patchwork of DA kinetic domains that respond in unique fashion to electrical stimulation of the medial forebrain bundle. The discovery of these domains leads us to hypothesize that the actions of nomifensine might be domain-dependent within the NAcc, as we previously found to be the case in the DS (Moquin & Michael, 2009; Taylor et al., 2012). So, the objectives of the present study were, first, to test the hypothesis that nomifensine exhibits domain-dependent actions in the NAcc and, second, to compare nomifensine’s actions in the NAcc with those in the DS (Taylor et al., 2013). We report herein for the first time a) that, within the NAcc, nomifensine preferentially promotes evoked DA release in the slow compared to the fast domains and b) that nomifensine preferentially increases the apparent KM of DA clearance in the NAcc compared to the DS.
METHODS AND MATERIALS
Chemicals were used as-received from the indicated suppliers and solutions were prepared with ultrapure water (NANOPure, Barnstead, Dubuque, IA, USA). Nomifensine maleate (Sigma-Aldrich, St Louis, MO) was dissolved in phosphate buffered saline (137 mM NaCl, 2.7 mM KCl, 1.47 mM KH2PO4, 10 mM Na2HPO4, pH 7.4). Dopamine hydrochloride (Sigma-Aldrich) was dissolved just before use in artificial cerebrospinal fluid (aCSF, 144 mM NaCl, 1.2 mM CaCl2, 2.7 mM KCl, 1.0 mM MgCl2, 2.0 mM NaH2PO4, pH 7.4) and stored under N2 to prevent DA oxidation. Paraformaldehyde (PFA, 4% in phosphate buffered saline) was prepared from resin (Sigma-Aldrich) and stored at 4°C before use. Isoflurane was from Baxter Healthcare (Deerfield, IL, USA).
Carbon fiber microelectrodes were constructed with 7-µm diameter carbon fibers (T650, Cytec Carbon Fibers, LLC, Piedmont, SC, USA). The fibers were trimmed to a length of 200 µm and immersed in reagent grade isopropyl alcohol (Sigma) for 20 min before use (Bath et al., 2000).
Background subtracted fast-scan cyclic voltammetry (FSCV) was performed with a potentiostat (University of Pittsburgh, Department of Chemistry Electronics Shop), a current amplifier (Keithley 428, Keithley Instruments, Inc., Cleveland, OH, USA), and software (TarHeel CV v4, courtesy of Prof. Michael Heien, Department of Chemistry and Biochemistry, University of Arizona, Tucson, USA (Heien et al., 2003). Between scans the applied potential was held at 0 V vs. Ag/AgCl. During the scans the voltage was swept linearly to 1.0 V, then to -0.5 V and back to 0 V at 400 V/s: the scan repetition frequency was 10 Hz.
Electrode calibration was performed using freshly prepared DA solutions before and after in vivo recordings. In vivo dopamine concentrations were determined by post-calibration.
Subjects were male Sprague-Dawley rats (n = 37, 250-275 g, Hilltop Laboratories, Scottsdale, PA, USA). All procedures involving animals were in accordance with NIH guidelines (publication 86-23) and approved by The Institutional Animal Care and Use Committee of the University of Pittsburgh.
Surgery was performed as described previously (Shu et al., 2013) under isoflurane anesthesia (5% by vol. initially and 2.5% for maintenance): isoflurane was vaporized (Cyprane Fortec, Fraser Harlake, Inc., Orchard Park, NY) in oxygen and delivered through a ventilator (Harvard Apparatus, Holliston, MA, USA). The anesthetized rats were maintained at 37°C with a homoeothermic blanket (Harvard Apparatus). Carbon fiber electrodes were first placed into the striatum (A/P +1.2 mm, M/L +1.4 mm, D/V 5.0 mm below dura) (Paxinos & Watson, 1998) and bipolar, stainless-steel stimulating electrodes (MS303/B, Plastics One, Inc., Roanoke, VA, USA) were placed as described before (Ewing et al., 1983; Kuhr et al., 1984; Michael et al., 1987) in the medial forebrain bundle (A/P −4.3 mm, M/L +1.2 mm, D/V from 7.5 below dura: the vertical coordinate was adjusted until evoked release was observed). Finally, without moving the stimulating electrode, the carbon fiber electrodes were slowly lowered into the NAcc (D/V 6.6-7.6 mm below dura).
Histological examination was performed as described previously (Peters et al., 2004; Shu et al., 2013) to verify that microelectrodes were correctly placed in the NAcc.
Electrical stimulation (constant current, biphasic square wave, pulse intensity 250 µA, pulse width 2 ms) was supplied by a pair of stimulus isolators (NL800A, Digitimer, Ltd., Hertfordshire, England). Stimulation was performed at 60, 30 and 15 Hz: details of the train duration are provided in the Results section.
Experiment design: Once the recording and stimulating electrodes were in position, stimulus responses were recorded before and 30 min after a single dose of nomifensine (20 mg/kg i.p.) with no alterations in the electrodes, recording location, stimulating location, stimulation parameters, etc. As during our prior study in the DS (Taylor et al., 2012), we compared pre- and post-nomifensine responses in the same group of animals to assure the effect of the drug was established under the same recording and stimulating conditions.
Data Analysis: Overshoot duration was defined by the time needed after the end of the stimulus for the evoked response to reach its maximum amplitude. The overshoot concentration was the amount by which the dopamine concentration continued to increase after the end of the stimulus. We quantified the apparent KM of DA clearance by numerically evaluating the derivative of the descending phase of the response and finding the point at which the slope was one-half the corresponding apparent Vmax reported by Shu et al., 2013. Statistical analyses were by the t-test, two-way analysis of variance (ANOVA) and two-way ANOVA with a repeated measures design (IBM SPSS Statistics 20).
RESULTS
Evoked responses reported recorded within the NAcc were objectively identified as fast or slow domains according to the criterion established by Shu et al., 2013 (see Fig 1 of Shu et al., 2013: fast sites produce evoked DA within 200 ms of the start of a stimulus (60 Hz, 250 µA) but slow sites do not). The fast and slow profiles recorded during this study exhibit no statistically significant differences from those recorded in different animals and reported in Fig. 2 of Shu et al., 2013 (Supplementary Information, Fig. S1). The sites of all NAcc recordings reported herein were confirmed by histology (Supplementary Information, Fig. S2).
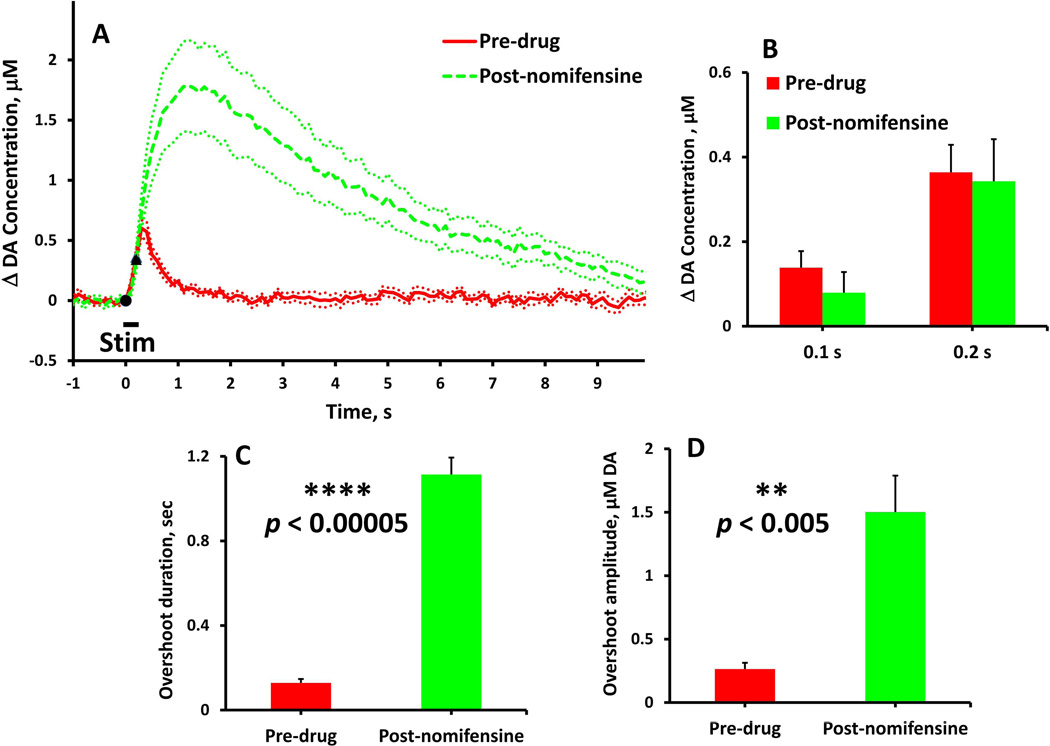
Figure 1.
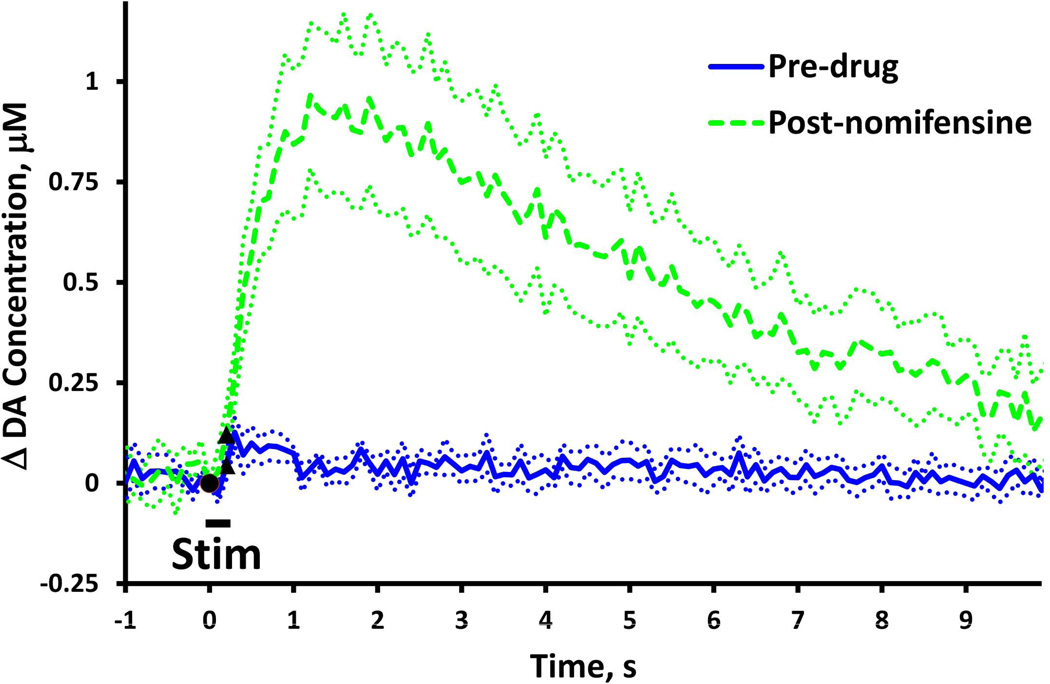
The effects of nomifensine on evoked DA overflow in fast NAcc domains (stimulus = 0.2 s, 60 Hz, 250 µA). In this and subsequent figures, solid and dash lines show the average of responses recorded in a group of rats and dotted lines show the SEM. Black symbols denote when the stimulus starts and stops. (A) Evoked responses (n=7) recorded before (solid) and after (dash) nomifensine administration. (B) Nomifensine did not significantly (two-way ANOVA, repeated measures design: nomifensine F(1,12) = 0.220, p > 0.5) affect the DA overflow during the 0.2 s stimulus. (C & D) Nomifensine significantly increased the duration and amplitude (paired-samples t-tests: **p < 0.005, **** p < 0.00005) of the stimulus overshoot.
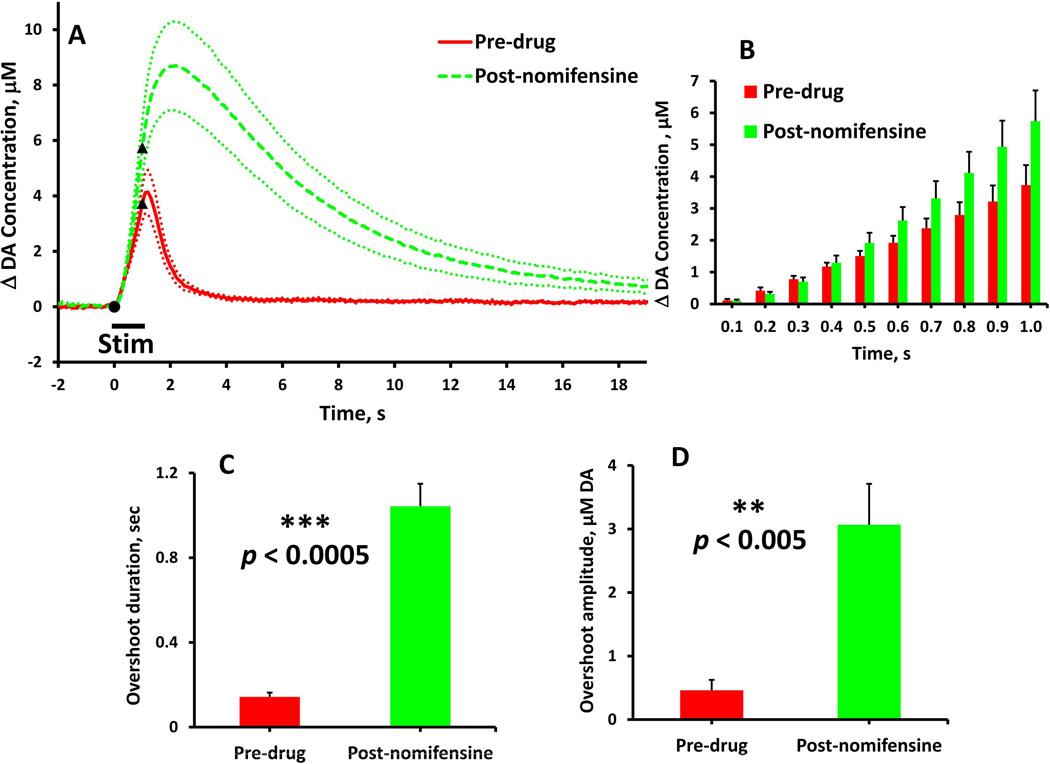
Figure 2.
The effects of nomifensine on evoked DA overflow in fast NAcc domains (stimulus = 1 s, 60 Hz, 250 µA). (A) Evoked responses (n = 7) recorded before (solid) and after (dash) nomifensine administration. (B) Nomifensine did not significantly (time 0.1 to 1.0 s, two-way ANOVA, repeated measures design: nomifensine F(1,12) = 2.190, p > 0.1) affect the rate of evoked DA overflow during the stimulus. (C & D) Nomifensine significantly increased the duration and amplitude (paired-samples t-tests: **p < 0.005, ***p < 0.0005) of the stimulus overshoot.
Format of the presentation of evoked responses
Evoked responses are reported in Figs. 1–4, Fig. 6, and Supplementary Figs. S1 and S3 in a consistent format (Fig.7 is in a different format, which is explained below). The solid and dash lines are the averages of responses recorded in groups of rats: the n values, the number of rats per group, are stated in the figure legends. In the main text, solid lines are pre-nomifensine responses from fast and slow domains, and dash lines are post-nomifensine responses: the dotted lines are the ±SEM intervals: black symbols denote when the stimulus begins and ends. The stimulus frequency and duration are stated in the text and in the figure legends: the stimulus current was fixed at 250 µA.
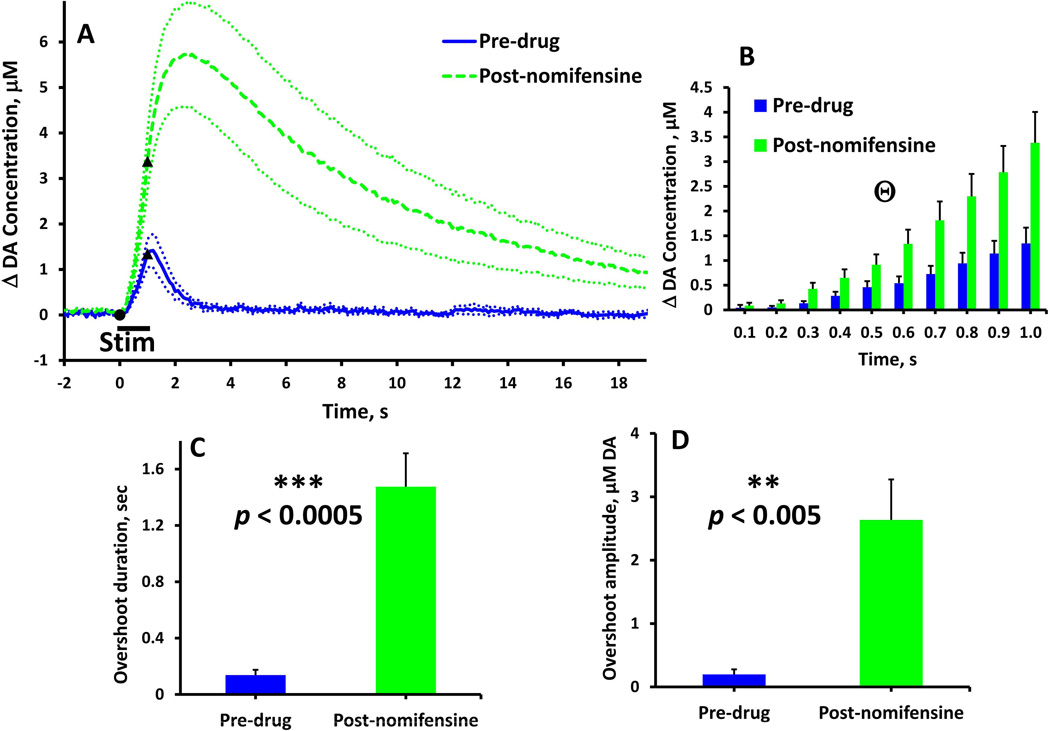
Figure 4.
Effect of nomifensine on evoked DA overflow in slow NAcc domains (stimulus = 1 s, 60 Hz, 250 µA). (A) Evoked responses (n = 8) recorded before (solid) and after (dash) nomifensine administration. (B) Nomifensine significantly (time 0.1 to 1.0 s, Θtwo-way ANOVA, repeated measures design: nomifensine F(1,14) = 6.738, p < 0.05; time & nomifensine interaction F(9,126) = 8.451, p < 0.01) increased the rate of DA overflow during the stimulus. (C & D) Nomifensine significantly increased the duration and amplitude (paired-samples t-tests: **p < 0.005, ***p < 0.0005) of the stimulus overshoot.
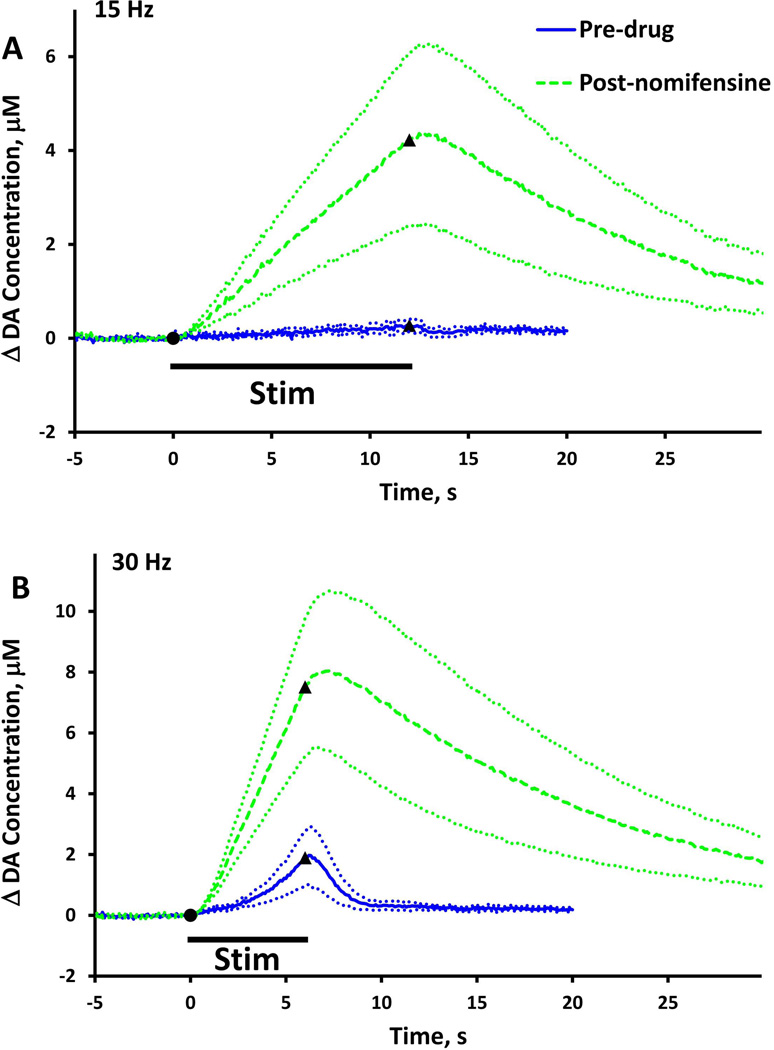
Figure 6.
Evoked responses (n=5) recorded in NAcc slow domains before (solid) and after (dash) nomifensine administration (A, stimulus = 180 pulses, 15 Hz, 250 µA: B, stimulus = 180 pulses, 30 Hz, 250 µA).
Figure 7.
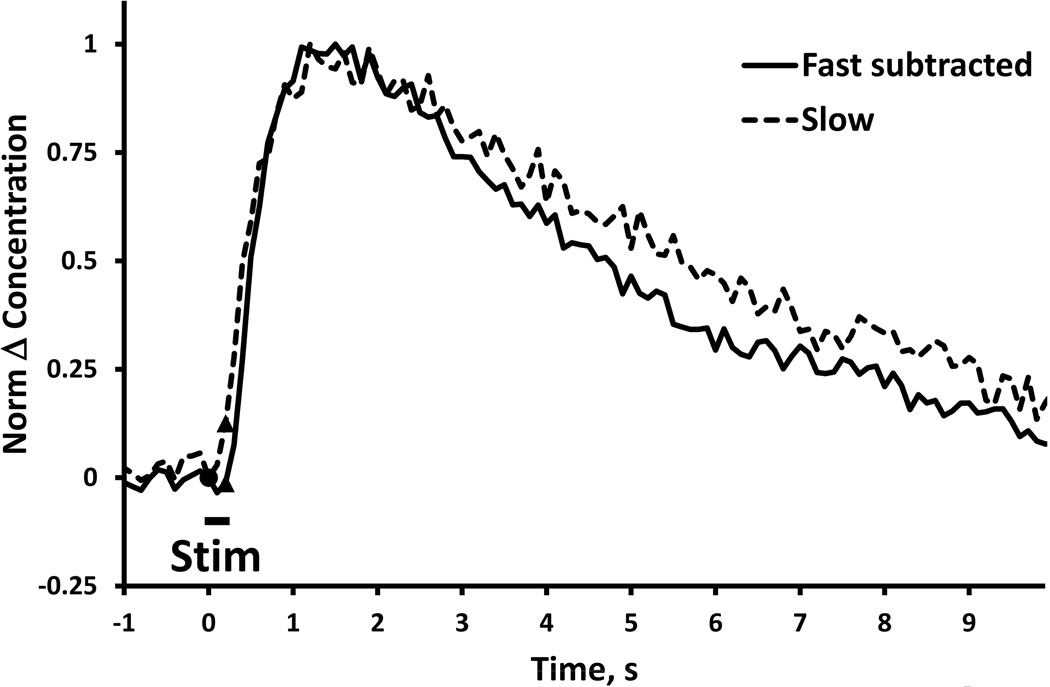
Comparison of “pure overshoots” observed in fast (solid) and slow (dash) NAcc domains after nomifensine administration (stimulus = 0.2 s, 60 Hz, 250 µA). The blue line was obtained from Fig. 1a by subtracting the pre-drug response from the post-nomifensine response (see also Fig. 9 of Taylor et al., 2012). The dash line is from Fig. 3. The two responses are normalized to their maximum amplitudes to enable comparison of their temporal features (SEMs omitted for clarity).
Domain-dependent effects of nomifensine: fast domains
Nomifensine affects evoked responses in the NAcc fast domains (Fig. 1, stimulus = 0.2 s, 60 Hz: Fig. 2, stimulus = 1 s, 60 Hz). Nomifensine did not significantly affect the response during the 0.2-s stimulus (Fig. 1b, two-way ANOVA, repeated measures design: nomifensine F(1,12) = 0.220, p > 0.5) but significantly increased both the duration (Fig. 1c, paired-samples ttest: p < 0.00005) and amplitude (Fig. 1d, paired-samples t-test: p < 0.005) of the overshoot. Nomifensine did not affect the response during the 1-s stimulus (Fig. 2b, time 0.1 to 1.0 s, two-way ANOVA, repeated measures design: nomifensine F(1,12) = 2.190, p > 0.1) but significantly increased both the duration (Fig. 2c, paired-samples t-test: p < 0.0005) and amplitude (Fig. 2d paired-samples t-test: p < 0.005) of the overshoot. Thus, in the NAcc fast domains, nomifensine primarily acts on the overshoot portion of the response to brief stimuli.
Domain-dependent effects of nomifensine: slow domains
Nomifensine affects evoked responses in the NAcc slow domains (Fig. 3, stimulus = 0.2 s, 60 Hz: Fig. 4, stimulus = 1 s, 60 Hz). Before nomifensine, the 0.2-s stimulus evoked no detectable DA overflow. However, after nomifensine the response exhibited a maximal overflow of ~1 µM DA and a duration of ~10 s. The onset of these responses was delayed with respect to the start of the stimulus: in four out of eight cases, the onset of the overflow began after the end of the stimulus. Nomifensine significantly increased the responses during the 1-s stimulus (Fig. 4b, time 0.1 to 1.0 s, two-way ANOVA, repeated measures design: nomifensine F(1,14) = 6.738, p < 0.05; time & nomifensine interaction F(9,126) = 8.451, p < 0.01) and significantly increased both the duration (Fig. 4c, paired-samples t-test: p < 0.0005) and amplitude (Fig. 4d, paired-samples t-test: p < 0.005) of the response overshoot. Thus, nomifensine acts on both DA release during the stimulus and the overshoot of the response in NAcc slow domains.
Figure 3.
Evoked responses (n=8) recorded in slow NAcc domains (stimulus = 0.2 s, 60 Hz, 250 µA) before (solid) and after (dash) nomifensine administration.
Domain-dependent effects of nomifensine: fast and slow comparisons
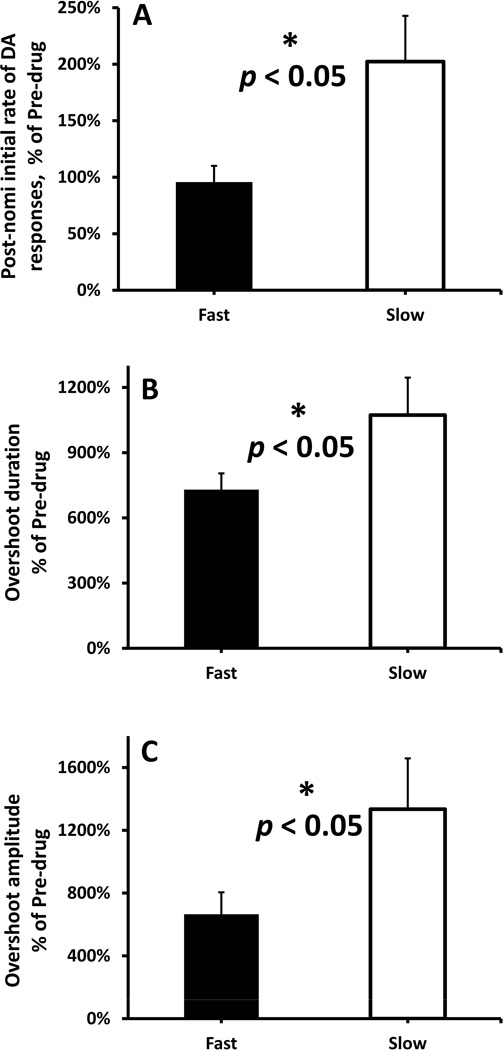
Within the NAcc, nomifensine preferentially increased three measures of evoked DA release in the slow compared to the fast domains (Fig. 5: the post-nomifensine values of initial rate of DA release and the duration and amplitude of the overshoot are normalized with respect to their pre-nomifensine values). Nomifensine preferentially increased the initial rate of DA release and the duration and amplitude of the overshoot in the slow (Fig. 5, white bars) compared to the fast domains (Fig. 5, black bars) (Fig. 5a-c, independent-samples t-test: p < 0.05). Thus, nomifensine preferentially enhances evoked DA release in the slow NAcc domains.
Figure 5.
The normalized effects of nomifensine on the evoked NAcc responses are domain-dependent (stimulus = 1 s, 60 Hz, 250 µA). (A) The initial rate of DA overflow (fast: 0-0.3s, slow: 0.3-0.5s), (B) the overshoot duration, and (C) the overshoot amplitude are reported here normalized with respect to their pre-nomifensine values. (A-C) Nomifensine preferentially increased the initial rate of DA release and the duration and amplitude of the overshoot in the slow (white bars) compared to the fast domains (black bars) (independent-samples t-tests: *p < 0.05).
The effects of nomifensine in slow domains during low frequency stimulation
We examined the effect of nomifensine on evoked responses in NAcc slow domains at lower stimulus frequency (Fig. 6). Upon stimulation at 15 Hz, a response was observed only after nomifensine administration (Fig. 6a). Hence, the NAcc slow domain exhibits the same “timing mismatch” phenomenon that we described previously in the slow domains of the DS (Taylor et al., 2013). Upon stimulation at 30 Hz, the pre-nomifensine response exhibited an initial delay of 1-2 s followed by short-term facilitation of evoked release, i.e. the response curved-upward as the stimulation continued (Fig. 6b, solid). Nomifensine eliminated the initial delay and the short-term facilitation but also increased the duration of the overshoot (Fig. 6b, dash). Thus, nomifensine exhibits an asymmetric effect on the temporal features of the DA response: nomifensine decreases the initial delay in the response when the stimulus begins but increases the overshoot when the stimulus ends.
Comparison of DA’s NAcc dynamics after nomifensine: 0.2 s responses
It is relevant (see Discussion) to compare the dynamics of the post-nomifensine fast and slow responses (Fig. 7). Fig. 7 compares the 0.2 s slow post-nomifensine response (dash) to the 0.2 s fast post-nomifensine response after subtraction of the fast pre-nomifensine response (solid), both normalized to their respective maximum amplitude. The intention of subtracting the fast pre-response from the fast-post response is to isolate the nomifensine-induced overshoot component: this subtraction was not necessary in the case of the slow response because the preresponse was non-detectable. The nomifensine-induced overshoots observed in fast and slow domains are essentially identical (Fig. 7).
Nomifensine’s impact on the apparent KM of the DAT
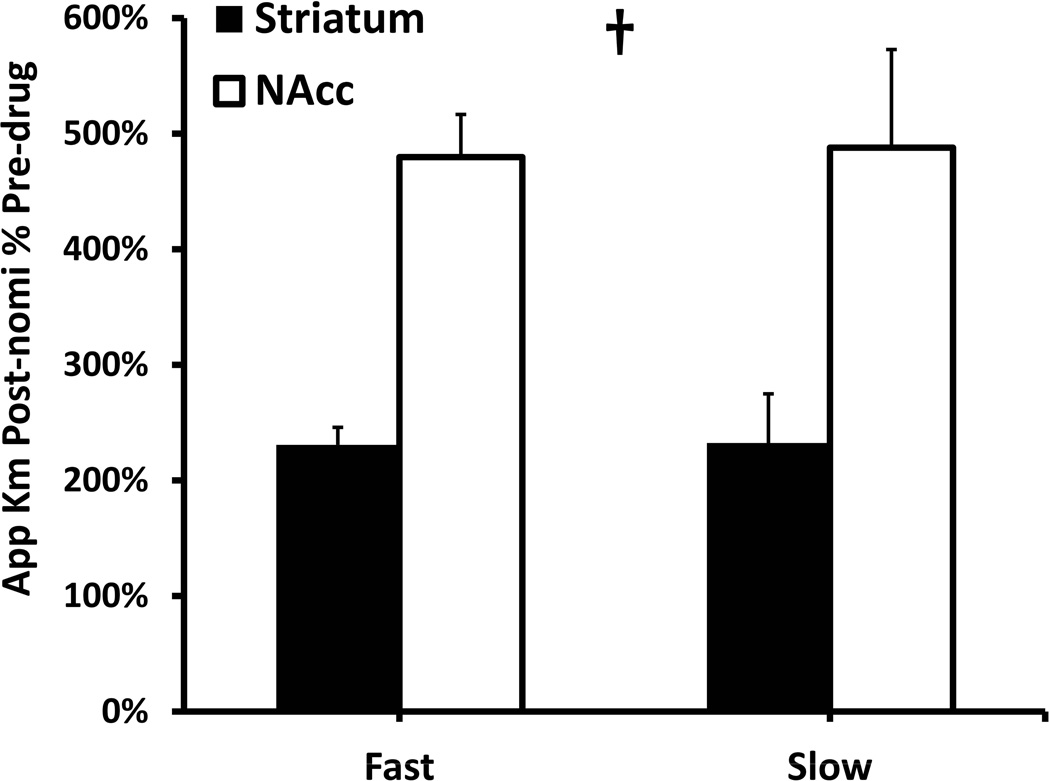
We quantified the apparent KM of DA clearance by numerically evaluating the derivative of the descending phase of the responses. For this purpose, we found it necessary to extend the stimulus duration to 3 s to ensure that the rate of clearance exceeded the one-half Vmax values (see Supplementary Information: the 3-s responses are reported in Fig. S3 and the individual apparent KM values are reported in Fig. S4). To gauge the effects of nomifensine, we normalized the post-nomifensine apparent KM values with respect to their pre-nomifensine magnitude (Fig. 8). Nomifensine significantly increased all four apparent KM values. The increase in apparent KM was significantly larger in the NAcc (~500%) than in the DS (~200%) but was not domain-dependent (two-way ANOVA: regions F(1,20) = 13.213, p < 0.002, domains F(1,20) = 0.932, p > 0.3). To our knowledge, this is the first report of a preferential kinetic action of a DAT inhibitor in the NAcc compared to the DS.
Figure 8.
Comparison of apparent KM values obtained in fast and slow domains in the NAcc (white) and DS (black). The post-nomifensine apparent KM values are normalized with respect to the pre-drug values. The normalized values are region-dependent and domain-independent († two-way ANOVA: regions F(1,20) = 13.213, p < 0.002; domains F(1,20) = 0.932, p > 0.3).
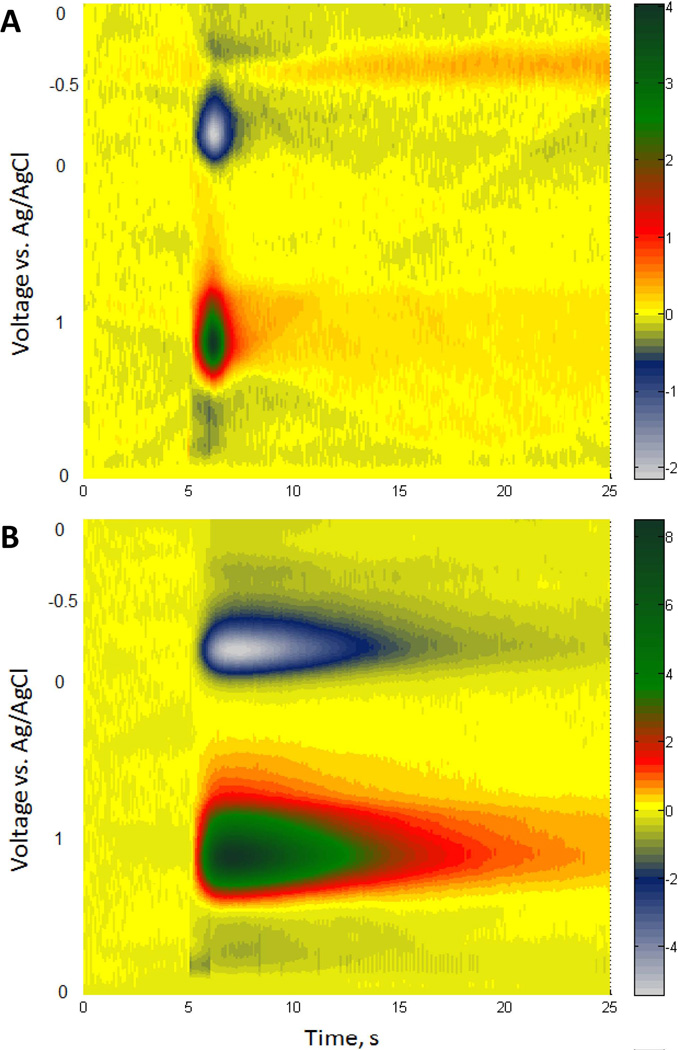
Color plots
In order to acquiesce to the demands of two reviewers of this report, we provide 3- dimensional color plots of the FSCV data for the 1-s stimulus responses recorded before and after administration of nomifensine in the fast domains of the NAcc (Fig. 9). These color plots, which provide no additional information over that which has already been stated above, show the FSCV data in a voltage-versus-time coordinate with current represented by a color scale. To prepare these plots, we normalized the background-subtracted FSCV currents with respect to each electrode’s post-calibration sensitivity factor and averaged the results across the group of animals (n=7). The plots show the dopamine oxidation peak, the quinone reduction peak, and background noise.
Figure 9.
Color plot (see text for explanation) of the FSCV data recorded during a 1-s stimulus (A) before and (B) after administration of nomifensine in fast NAcc domains.
DISCUSSION
The domain-dependent evoked DA responses (pre-nomifensine) recorded in the NAcc are not statistically different from those reported previously by Shu et al. (2013, see Supplementary Fig. S1), confirming that the NAcc domains are preserved across groups of animals. As in the DS (Taylor et al., 2012), nomifensine exerts robust and domain-dependent actions on evoked responses in the NAcc (Figs. 1–6). Together with our previous DS results (Taylor et al., 2012), the present study provides a comprehensive evaluation of both the region-dependent and domaindependent actions of a DAT inhibitor, for the first time.
Summary of nomifensine’s actions in the NAcc
When stimulation is delivered at 60 Hz in relatively brief trains (0.2 or 1 s, Figs. 1–4), nomifensine increases both the duration and amplitude of the response overshoot in both domains. In the case of the 0.2 s stimulus trains, the post-nomifensine maximum DA signal in the fast domain was more than 400% of the pre-nomifensine maximum amplitude and the overshoot duration was ~500% longer than the stimulus. In the slow domain, the pre-nomifensine response to the 0.2 s stimulus train was non-detectable (Fig. 3), so the post-nomifensine response is predominantly overshoot. Interestingly, the temporal features of the overshoots in the fast and slow domains are essentially identical (Fig. 7). Nomifensine significantly increased the DA concentrations observed during the 1-s stimulus trains in the slow domains, but not in the fast domains.
Nomifensine preferentially acts on NAcc slow domains compared to NAcc fast domains
With a 1-s stimulus, it was possible to quantify the initial rate of evoked DA release as well as the duration and amplitude of the overshoots in fast and slow domains under consistent experimental conditions. We normalized the post-nomifensine values with respect to their pre-nomifensine values (Fig. 5). All three measures of evoked DA release were significantly larger in the slow domain. We therefore conclude that nomifensine acts preferentially on evoked DA release in the NAcc slow domains compared to the NAcc fast domains. We report this phenomenon here for the first time.
Taylor et al. (2012) reported the domain-dependent actions of nomifensine on evoked DA release in the DS. There are notable differences between the NAcc and DS. First, nomifensine had a much larger effect on the initial rate of DA release in the DS slow domains (~800%) than the NAcc slow domains (~200%). In contrast with the NAcc, nomifensine’s actions on the duration and amplitude of the DS overshoot were largest in the fast domains. Thus, we report here for the first time that the domain-dependent actions of nomifensine are also region-dependent. These findings add to the view that the domains of the NAcc and DS are truly distinct from one other, reinforcing the concept that DA kinetics are both region and domain dependent.
Nomifensine’s actions on the apparent KM of DA uptake: considerations
As a competitive DAT inhibitor, nomifensine’s primary pharmacological action is to change the apparent KM of DA uptake. However, DAT inhibitors have additional secondary actions. For example, in anesthetized animals, DAT inhibition decreases the firing rate of DA neurons via indirect agonism of the D2 receptors (Studer & Schultz, 1987; Einhorn et al., 1988; Mercuri et al., 1991), while in non-anesthetized animals DAT inhibitors induce phasic DA events (Aragona et al., 2008) and burst firing of DA neurons (Koulchitsky et al., 2012). Here, however, we wish to evaluate nomifensine’s primary action by determining the apparent KM of DA clearance. To do so, however, requires consideration of how to go about extracting apparent KM information from evoked responses.
In several previous reports, KM values were quantified by means of a numerical model that simulates the evoked responses (Wightman et al., 1988; Jones et al., 1995a; Wu et al., 2001a; Wu et al., 2001b). The model assumes that a gap, or a physical space, is interposed between the recording electrode and nearby DA terminals: such a gap is expected to cause diffusional distortion of the evoked responses. However, as we have explained before (Moquin & Michael, 2009; Taylor et al., 2012), the observed features of evoked responses in the DS cast doubt on the diffusion gap distortion model, which in turn, casts doubt on the kinetic values obtained via simulation. We now report that the same issue arises in the NAcc.
A diffusion gap is predicted to cause two types of temporal distortion of the evoked responses; an initial lag in the DA signal when the stimulus begins and an overshoot of the DA signal after the stimulus ends. A diffusion gap would necessarily cause these distortions to go hand-in-hand: if there is an initial lag, there must also be an overshoot, and vice versa. However, in both the DS and NAcc, responses with overshoot but without initial lag are entirely commonplace. In fast domains, for example, DAT inhibition leads to prominent overshoot but no obvious lag. According to the diffusion gap model, this should not be.
A modified diffusion gap model described by Venton et al., (2003) hypothesized that DAT inhibition increases the apparent dimension of the diffusion gap, invoking the concept that DAT inhibition extends DA’s lifetime in the extracellular space and so enables it to diffuse further. The evoked responses do not support this hypothesis:
First, both in the DS and the NAcc, DAT inhibition diminishes the initial lag in slow domains, this effect being most obvious at low stimulus frequencies (Fig. 6: see also Fig. 5 of Taylor et al., 2013). This observation directly contradicts the hypothesis that DAT inhibition increases the apparent diffusion gap. Moreover, the rapid onset of the post-nomifensine responses confirms the presence of DA terminals in close proximity to the recording electrode, which shows that there is no diffusion gap to cause diffusional distortion. Rather than diffusional distortion, we conclude that the temporal features of the evoked responses reflect the local activity of DA terminals in the immediate vicinity of the recording site.
Second, our findings do not provide clear evidence that DA’s diffusion distance post-nomifensine is domain dependent (Fig. 7). Originally, responses with prominent initial lag, were attributed to a poor choice of recording site with a large diffusion gap interposed between the electrode and its nearest-neighbor DA terminals (Kuhr et al., 1987; Engstrom et al., 1988; Wightman et al., 1988). Likewise, responses without lag were attributed to small gaps and minimal diffusional distortion. In that case, however, then the different gap sizes would necessarily produce distinct temporal profiles, regardless of experimental conditions. After nomifensine, however, this is simply not what is observed (see Fig. 7, above, and Fig. 9 of Taylor et al., 2012): the essentially identical appearance of the post-nomifensine overshoots would be impossible if the fast and slow responses were caused by different gaps.
Nomifensine’s actions on the apparent KM of DA uptake: the analysis
Because our findings do not confirm the presence of a diffusion gap, we chose to quantify apparent KM directly from the slope of the descending phase of the response. The advantage of slope analysis is that makes no mass transfer assumptions. However, the kinetic parameters obtained by slope analysis are clearly not the intrinsic, biophysical parameters of the DAT per se. Nevertheless, if it is to be assumed, as is generally the case, that the DA signal at the electrode is a useful analog of DA neurotransmission, then the apparent values are of great interest for the clear, simple, and logical reason that mass transport delivers DA molecules not only to the recording electrodes but also to their pre- and post-synaptic targets.
Nomifensine significantly increases the apparent KM in both the fast and slow domains of both the NAcc and DS: this is an expected result because nomifensine is a competitive inhibitor. However, nomifensine preferentially increases the apparent KM in the NAcc compared to the DS (Fig. 8), a phenomenon we report here for the first time. Interestingly, although the effect of nomifensine on apparent KM is clearly region-dependent, it is not domain-dependent: the proportional increase in apparent KM was similar in the fast and slow domains of each region (Fig. 8). Thus, the change in apparent KM may not by itself explain all the region and domain dependent actions of nomifensine on DA release. But, as mentioned above, DAT inhibitors have secondary pharmacological effects.
CONCLUSION
This study reaffirms the presence of a patchwork of DA kinetic domains within the NAcc and establishes that nomifensine’s actions within the NAcc are domain dependent. Nomifensine preferentially enhances three measures of DA release in the NAcc slow domains compared to the NAcc fast domains: the rate of initial release as well as the duration and amplitude of the overshoot. In this respect, the domain-dependent actions of nomifensine are distinct from those in the DS. Moreover, nomifensine preferentially increases the apparent KM of DA clearance in the NAcc compared to the DS, and this preferential effect was domain-independent. This first report of a preferential kinetic effect of a DAT inhibitor in the NAcc is in good accord with prior literature showing that DAT inhibitors preferentially affect the NAc compared to the DS.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the NIH (grant MH075989).
ABBREVIATIONS
- DA
dopamine
- NAc
nucleus accumbens
- NAcc
nucleus accumbens core
- DS
dorsal striatum
- DAT
dopamine transporter
- FSCV
fast-scan cyclic voltammetry
Footnotes
The authors declare no conflicts of interest, financial or otherwise.
REFERENCES
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008;28:8821–8831. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath BD, Michael DJ, Trafton BJ, Joseph JD, Runnels PL, Wightman RM. Subsecond adsorption and desorption of dopamine at carbon-fiber microelectrodes. Anal Chem. 2000;72:5994–6002. doi: 10.1021/ac000849y. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Boja JW, Kuhar MJ. [H-3] Cocaine Binding and Inhibition of [H-3] Dopamine Uptake Is Similar in Both the Rat Striatum and Nucleus Accumbens. Eur J Pharmacol. 1989;173:215–217. doi: 10.1016/0014-2999(89)90524-4. [DOI] [PubMed] [Google Scholar]
- Carboni E, Imperato A, Perezzani L, Dichiara G. Amphetamine, Cocaine, Phencyclidine and Nomifensine Increase Extracellular Dopamine Concentrations Preferentially in the Nucleus Accumbens of Freely Moving Rats. Neuroscience. 1989;28:653–661. doi: 10.1016/0306-4522(89)90012-2. [DOI] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA, Gillespie K, Curella P, Mayfield RD, Zahniser NR. Reduced Clearance of Exogenous Dopamine in Rat Nucleus-Accumbens, but Not in Dorsal Striatum, Following Cocaine Challenge in Rats Withdrawn from Repeated Cocaine Administration. J Neurochem. 1993;61:273–283. doi: 10.1111/j.1471-4159.1993.tb03565.x. [DOI] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA, Mayfield RD, Curella P, Zahniser NR. Differences in dopamine clearance and diffusion in rat striatum and nucleus accumbens following systemic cocaine administration. J Neurochem. 1992;59:259–266. doi: 10.1111/j.1471-4159.1992.tb08899.x. [DOI] [PubMed] [Google Scholar]
- Church WH, Justice JB, Byrd LD. Extracellular Dopamine in Rat Striatum Following Uptake Inhibition by Cocaine, Nomifensine and Benztropine. Eur J Pharmacol. 1987;139:345–348. doi: 10.1016/0014-2999(87)90592-9. [DOI] [PubMed] [Google Scholar]
- Cragg SJ, Rice ME. DAncing past the DAT at a DA synapse. Trends Neurosci. 2004;27:270–277. doi: 10.1016/j.tins.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Dichiara G, Imperato A. Drugs Abused by Humans Preferentially Increase Synaptic Dopamine Concentrations in the Mesolimbic System of Freely Moving Rats. P Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhorn LC, Johansen PA, White FJ. Electrophysiological Effects of Cocaine in the Mesoaccumbens Dopamine System - Studies in the Ventral Tegmental Area. J Neurosci. 1988;8:100–112. doi: 10.1523/JNEUROSCI.08-01-00100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom RC, Wightman RM, Kristensen EW. Diffusional distortion in the monitoring of dynamic events. Anal Chem. 1988;60:652–656. [Google Scholar]
- Ewing AG, Bigelow JC, Wightman RM. Direct Invivo Monitoring of Dopamine Released from 2 Striatal Compartments in the Rat. Science. 1983;221:169–171. doi: 10.1126/science.6857277. [DOI] [PubMed] [Google Scholar]
- Garris PA, Ciolkowski EL, Pastore P, Wightman RM. Efflux of dopamine from the synaptic cleft in the nucleus-accumbens of the rat-brain. J Neurosci. 1994;14:6084–6093. doi: 10.1523/JNEUROSCI.14-10-06084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald MD, Aminoff MJ. Therapies for Dopaminergic-Induced Dyskinesias in Parkinson Disease. Ann Neurol. 2011;69:919–927. doi: 10.1002/ana.22423. [DOI] [PubMed] [Google Scholar]
- Heien MLAV, Phillips PEM, Stuber GD, Seipel AT, Wightman RM. Overoxidation of carbon-fiber microelectrodes enhances dopamine adsorption and increases sensitivity. Analyst. 2003;128:1413–1419. doi: 10.1039/b307024g. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neurosci. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izenwasser S, Werling LL, Cox BM. Comparison of the Effects of Cocaine and Other Inhibitors of Dopamine Uptake in Rat Striatum, Nucleus-Accumbens, Olfactory Tubercle, and Medial Prefrontal Cortex. Brain Res. 1990;520:303–309. doi: 10.1016/0006-8993(90)91719-w. [DOI] [PubMed] [Google Scholar]
- Jenkinson N, Brown P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci. 2011;34:611–618. doi: 10.1016/j.tins.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13 doi: 10.1038/nn.2519. 635-U156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Garris PA, Kilts CD, Wightman RM. Comparison of Dopamine Uptake in the Basolateral Amygdaloid Nucleus, Caudate-Putamen, and Nucleus-Accumbens of the Rat. J Neurochem. 1995a;64:2581–2589. doi: 10.1046/j.1471-4159.1995.64062581.x. [DOI] [PubMed] [Google Scholar]
- Jones SR, Garris PA, Wightman RM. Different effects of cocaine and nomifensine on dopamine uptake in the caudate-putamen and nucleus accumbens. J Pharmacol Exp Ther. 1995b;274:396–403. [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and Molecular Mechanisms of Drug-Dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Koulchitsky S, De Backer B, Quertemont E, Charlier C, Seutin C. Differential effects of cocaine on dopamine neuroon firing in awake and anesthetized rats. Neuropsycopharm. 2012;37:1559–1571. doi: 10.1038/npp.2011.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The Dopamine Hypothesis of the Reinforcing Properties of Cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- Kuhr WG, Ewing AG, Caudill WL, Wightman RM. Monitoring the Stimulated Release of Dopamine with Invivo Voltammetry .1. Characterization of the Response Observed in the Caudate-Nucleus of the Rat. J Neurochem. 1984;43:560–569. doi: 10.1111/j.1471-4159.1984.tb00935.x. [DOI] [PubMed] [Google Scholar]
- Kuhr WG, Wightman RM, Rebec GV. Dopaminergic-Neurons - Simultaneous Measurements of Dopamine Release and Single-Unit Activity during Stimulation of the Medial Forebrain-Bundle. Brain Res. 1987;418:122–128. doi: 10.1016/0006-8993(87)90968-1. [DOI] [PubMed] [Google Scholar]
- Mercuri NB, Stratta F, Calabresi P, Bernardi G. Nomifensine but Not Amantadine Increases Dopamine-Induced Responses on Rat Substantia-Nigra Zona Compacta Neurons. Neurosci Lett. 1991;131:145–148. doi: 10.1016/0304-3940(91)90599-o. [DOI] [PubMed] [Google Scholar]
- Michael AC, Ikeda M, Justice JB., Jr Mechanisms contributing to the recovery of striatal releasable dopamine following MFB stimulation. Brain Res. 1987;421:325–335. doi: 10.1016/0006-8993(87)91302-3. [DOI] [PubMed] [Google Scholar]
- Moquin KF, Michael AC. Tonic autoinhibition contributes to the heterogeneity of evoked dopamine release in the rat striatum. J Neurochem. 2009;110:1491–1501. doi: 10.1111/j.1471-4159.2009.06254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moquin KF, Michael AC. An inverse correlation between the apparent rate of dopamine clearance and tonic autoinhibition in subdomains of the rat striatum: a possible role of transporter-mediated dopamine efflux. J Neurochem. 2011;117:133–142. doi: 10.1111/j.1471-4159.2011.07183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Peters JL, Miner LH, Michael AC, Sesack SR. Ultrastructure at carbon fiber microelectrode implantation sites after acute voltammetric measurements in the striatum of anesthetized rats. J Neurosci Meth. 2004;137:9–23. doi: 10.1016/j.jneumeth.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Shu Z, Taylor IM, Michael AC. The dopamine patchwork of the rat nucleus accumbens core. Eur J Neurosci. 2013;38:3221–3229. doi: 10.1111/ejn.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer A, Schultz W. The Catecholamine Uptake Inhibitor Nomifensine Depresses Impulse Activity of Dopamine Neurons in Mouse Substantia-Nigra. Neurosci Lett. 1987;80:207–212. doi: 10.1016/0304-3940(87)90655-0. [DOI] [PubMed] [Google Scholar]
- Taylor IM, Ilitchev AI, Michael AC. Restricted Diffusion of Dopamine in the Rat Dorsal Striatum. Acs Chem Neurosci. 2013;4:870–878. doi: 10.1021/cn400078n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor IM, Jaquins-Gerstl A, Sesack SR, Michael AC. Domain-dependent effects of DAT inhibition in the rat dorsal striatum. J Neurochem. 2012;122:283–294. doi: 10.1111/j.1471-4159.2012.07774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venton BJ, Zhang H, Garris PA, Phillips PE, Sulzer D, Wightman RM. Realtime decoding of dopamine concentration changes in the caudate-putamen during tonic and phasic firing. J Neurochem. 2003;87:1284–1295. doi: 10.1046/j.1471-4159.2003.02109.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn JH, Kollins SH, Wigal TL, Telang F, Fowler JS, Goldstein RZ, Klein N, Logan J, Wong C, Swanson JM. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol Psychiatr. 2011;16:1147–1154. doi: 10.1038/mp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Kollins SH, Wigal TL, Newcorn JH, Telang FW, Fowler JS, Logan J, Wong CT, Swanson JM. Methylphenidate-Elicited Dopamine Increases in Ventral Striatum Are Associated with Long-Term Symptom Improvement in Adults with Attention Deficit Hyperactivity Disorder. J Neurosci. 2012;32:841–849. doi: 10.1523/JNEUROSCI.4461-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Moquin KF, Michael AC. Evidence for coupling between steady-state and dynamic extracellular dopamine concentrations in the rat striatum. J Neurochem. 2010;114:150–159. doi: 10.1111/j.1471-4159.2010.06740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, May LJ. Real-Time Characterization of Dopamine Overflow and Uptake in the Rat Striatum. Neuroscience. 1988;25:513–523. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Kuhar MJ, Carroll FI, Garris PA. Preferential increases in nucleus accumbens dopamine after systemic cocaine administration are caused by unique characteristics of dopamine neurotransmission. J Neurosci. 2001a;21:6338–6347. doi: 10.1523/JNEUROSCI.21-16-06338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J Neurosci Methods. 2001b;112:119–133. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.