Abstract
Background
Sensitive general cardiometabolic risk assessment tools of modifiable risk factors would be helpful and practical in a range of primary prevention interventions or for preventive health maintenance.
Purpose
To develop and validate a cumulative general cardiometabolic risk score that focuses on non–self-reported modifiable risk factors such as glycosylated hemoglobin (HbA1c) and BMI so as to be sensitive to small changes across a span of major modifiable risk factors, which may not individually cross clinical cut off points for risk categories.
Methods
We prospectively followed 2,359 cardiovascular disease (CVD)-free subjects from the Framingham offspring cohort over a 14–year follow-up. Baseline (fifth offspring examination cycle) included HbA1c and cholesterol measurements. Gender–specific Cox proportional hazards models were considered to evaluate the effects of non–self-reported modifiable risk factors (blood pressure, total cholesterol, high–density lipoprotein cholesterol, smoking, BMI, and HbA1c) on general CVD risk. We constructed 10–year general cardiometabolic risk score functions and evaluated its predictive performance in 2012–2013.
Results
HbA1c was significantly related to general CVD risk. The proposed cardiometabolic general CVD risk model showed good predictive performance as determined by cross-validated discrimination (male C-index=0.703, 95% CI=0.668, 0.734; female C-index=0.762, 95% CI=0.726, 0.801) and calibration (lack-of-fit χ2=9.05 [p=0.338] and 12.54 [p=0.128] for men and women, respectively).
Conclusions
This study presents a risk factor algorithm that provides a convenient and informative way to quantify cardiometabolic risk based on modifiable risk factors that can motivate an individual’s commitment to prevention and intervention.
Introduction
Cardiovascular disease (CVD), accounting for more than one third of total deaths in the U.S.,1 has constantly necessitated the development of powerful predictive models for the “modifiable” probability of future CVD events, especially with cardiovascular prevention strategies rapidly shifting toward modifiable behavioral interventions, such as direct interventions modifying diet or exercise to reduce risk.2 Related but slightly varying Framingham CVD risk score models assign weights to major CVD risk factors such as age, gender, total cholesterol, low–density lipoprotein (LDL) cholesterol, high–density lipoprotein (HDL) cholesterol, smoking status, blood pressure, and diabetes status.3–8
General assessment of modifiable cardiometabolic risk would be most helpful and practical in a range of primary prevention interventions (e.g., to quantify intervention efficacy) or for preventive health maintenance.9,10 To address the need for a practical general CVD risk assessment tool, D’Agostino et al.6 developed a model (hereafter D’Agostino model) assessing risk of developing any major atherosclerotic CVD event. We added BMI and glycosylated hemoglobin (HbA1c) to extend the function of their model by incorporating variables that can reflect change and relies less on self-reported measures prone to bias in community samples.11–13
The aim of this study is to develop and evaluate a cumulative general cardiometabolic risk score that focuses on non–self-reported modifiable risk factors such as HbA1c and BMI in order to be sensitive to small changes across a span of modifiable risk factors, which may not individually cross clinical cut off points for risk categories. Recognizing the important role of metabolic function and diabetes in overall cardiometabolic disease development, the proposed risk model includes the evaluation of HbA1c, a time-integrated marker of average blood glucose concentration that is increasingly used for management and screening of diabetes,14 on general CVD risk prediction. Elevated levels of cardiometabolic biomarkers are associated with increased risk of CVD and premature mortality.15–18 Additionally, HbA1c is highly associated with the risk of complications compared to single or episodic measures of glucose levels.19,20
Operationally, the proposed general cardiometabolic risk model will keep continuous risk factor measures as such to maximize their contribution to risk assessment. The rationale is that intervention and behavioral change strategies in community settings may produce small but persistent changes across risk factors, which may yield large population health effects.21 In these cases, small changes in the distribution of risk factors can have substantial effects on overall population health patterns.
Methods
Study Population
Study participants were selected from the Framingham offspring study, a community–based prospective study of general CVD risk factors.22,23 Time to general CVD was defined using standard Framingham heart study criteria for general CVD: diagnosed conditions that fall into coronary heart disease (coronary death, myocardial infarction, coronary insufficiency, and angina), cerebrovascular events (including transient ischemic attack, ischemic stroke, and hemorrhagic stroke), peripheral artery disease (intermittent claudication), and diagnosed heart failure.24,25 Permission to analyze the Framingham offspring database was obtained from the National Heart, Lung, and Blood Institute according to a research proposal approved by our IRB. Baseline was the fifth examination cycle (January 1991–June 1995), where HbA1c measurements were collected for 2,359 subjects. Participants free of general CVD at baseline with no missing observations on any modifiable risk factors were considered (see Appendix). Participants with diabetes were included if they did not have a general CVD event before baseline. The median follow-up time for a total of 2,359 subjects free of general CVD at baseline was 14.25 years (SD=3.5 years). A total of 342 general CVD events were observed during the follow-up period.
Evaluation of Modifiable Risk Factors
At each exam, Framingham participants underwent a physical examination and BMI was calculated based on standardized measurements of height and weight. Fasting blood samples were also collected from participants during each exam. Ethylenediaminetetraacetic acid (EDTA) plasma from these samples was used to obtain participants’ total and HDL cholesterol levels via the Abell–Kendall technique and the Lipid Research Clinics Program protocol, respectively.3,26 These samples were also assayed to measure plasma HbA1c, insulin, and lipid levels. Glucose and insulin levels were also measured through use of a 75–g oral glucose tolerance test for participants who were not previously known to have diabetes.27–29 Systolic and diastolic blood pressure were obtained using the average of two separate readings taken by a physician at least 5 minutes apart.30 Smoking status was defined as smoking cigarettes regularly in the last year before the examination with categories of yes, no, and unknown.
Statistical Analyses
Descriptive statistics for all continuous (mean and SD) and categorical (relative frequencies) study variables were calculated. We evaluated Framingham general CVD risk through gender–specific Cox proportional hazards models.31 The covariates included in the gender–specific Cox model include baseline age, BMI, smoking, systolic blood pressure, total cholesterol, HDL cholesterol, and HbA1c. A summary of the similarities and differences between the D’Agostino and proposed risk models is described in Table 1. Diastolic blood pressure was excluded from our model because of its strong collinearity with systolic blood pressure. Model diagnostics, including nonlinearity checks32 and testing of proportional hazards assumptions,33 were performed to ensure proper functional form of risk factors and validity of the proportional hazard assumptions.
Table 1.
Risk factors of the D’Agostino et al.6 and proposed risk scores
| D’Agostino risk score | Proposed risk score |
|---|---|
| Blood pressure | Blood pressure |
| log(systolic blood pressure), if treated (self-report) | Systolic blood pressure No self-report |
| log(systolic blood pressure) if not treated (self-report) | |
| Smoking in previous year (yes/no) | Smoking in previous year (yes/no) |
| Diabetes (any of below) | Diabetes |
| Blood glucose ≥126 mg/dL | Measured HbA1c levels (%) |
| Use of insulin medication (self-report) | No self-report |
| Use of oral hypoglycemic medication (self-report) | |
| log(total cholesterol, mg/dL) | Total cholesterol, mg/dL |
| log(HDL cholesterol, mg/dL) | HDL cholesterol, mg/dL |
| BMI, kg/m2 | |
| log(Age) | Age |
| STRATA: Gender | Strata: Gender |
HbA1C, glycosylated hemoglobin; HDL, high-density lipoprotein
Evaluation of Model Performance
We considered three metrics to evaluate the performance of the risk prediction model: discrimination, calibration, and reclassification. Discrimination quantifies the ability of the proposed prediction model to correctly distinguish between subjects who experienced CVD events and those who did not. For Cox models, the typical measure of discrimination is the C-index.34,35 A completely random prediction would yield a C-index of 0.5 and a perfect predictive model would yield a C-index of 1.0. We obtained the C-index for the proposed model and the D’Agostino general CVD risk model applied to this specific Framingham offspring sample. Additionally, we estimated the C-index for the proposed model exchanging HbA1c with self–reported diabetes diagnosis to assess the change in predictive performance. To avoid overestimation of the predictive ability36,37 of the models, we estimated a 10–fold cross-validated (CV) C-index and obtained its 95% CI using 10,000 bootstrap replicates.38,39
Calibration quantifies how the predicted outcome for each individual patient agrees with the actual outcome.40 We constructed ten equally sized groups based on their estimated risk score, ranging from low risk to high risk. For each subgroup, we computed the average model–based risk probabilities and observed risk probabilities using the Kaplan–Meier estimate of the decile. A model is well calibrated if the observed and predicted survival probabilities do not differ among subgroups. We summarized calibration statistics based on a modified Hosmer–Lemeshow χ2 statistic with nine degrees of freedom.41
A reclassification analysis was performed to evaluate the ability of HbA1c to reclassify risk using 10–year risk categories defined as low (<6%), intermediate (6% to <20%) or high (≥20%). We assessed the number of reclassified participants and estimated the net reclassification improvement (NRI).42 For discrimination, calibration, and reclassification measures, we compared the proposed cardiometabolic model with HbA1c to the proposed model replacing HbA1c with self–reported diabetes status and D’Agostino general CVD risk model. We performed a gender–specific Cox regression using the variables in the D’Agostino model applied to the offspring data to estimate parameter and baseline hazard estimates for the D’Agostino model. From these parameter and baseline hazard estimates, we calculated risk functions to estimate 10–year general CVD risk for use in discrimination and calibration analyses. Similar 10–year general CVD risk was estimated for the proposed cardiometabolic risk models. Analyses were performed in 2012–2013 using R, version 2.15.1. Type I error was set to 5%.
Results
Baseline Characteristics
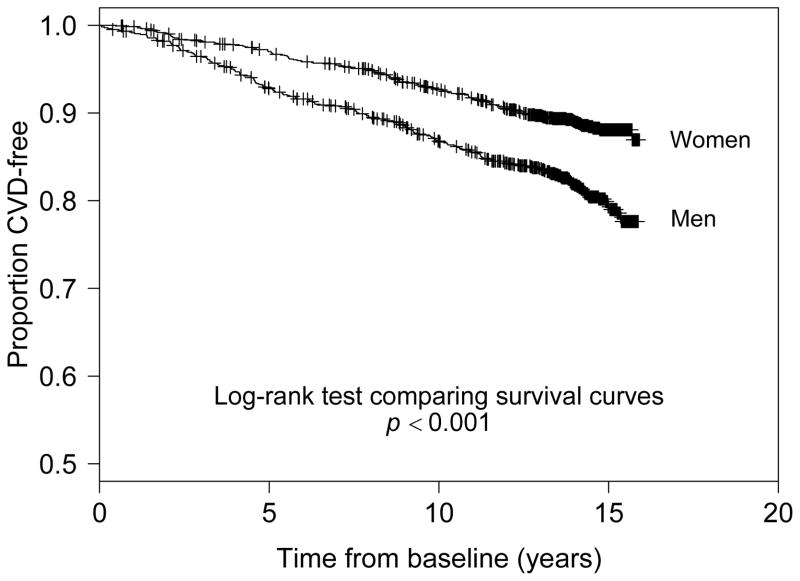
A total of 2,359 subjects free of general CVD at baseline were assessed over 14 years of follow-up. The mean (SD) age of the offspring study participants at baseline was 54.3 (9.7) years. The gender–specific subject characteristics at baseline are reported in Table 2. About 19% of male and female participants smoked regularly at baseline. Mean BMI at baseline for men was 28.8 kg/m2 and 26.6 kg/m2 for women. Mean HbA1c was similar for men (5.42%) and women (5.37%) at baseline. Average total and HDL cholesterol levels were higher in women whereas mean systolic blood pressure was higher in men. During follow-up, men experienced the highest general CVD cumulative incidence at 18.7% and women had a lower cumulative incidence of general CVD at 11.1%. Figure 1 shows the Kaplan–Meier survival curve of general CVD stratified by gender. An increased risk of general CVD appeared 2–3 years after baseline, after which there was a clear widening of survival curves between gender groups throughout the follow-up period (p<0.001).
Table 2.
Baseline characteristics of offspring Framingham cohort participants at exam 5
| Risk factor | Men (n=1,047) | Women (n=1,312) |
|---|---|---|
| BMI (kg/m2) | 28.2 (4.3) | 26.6 (5.4) |
| Age (years) | 54.5 (9.9) | 54.2 (9.8) |
| Smoked regularly in past year | 19.1% | 18.5% |
| Systolic blood pressure | 128.3 (16.7) | 123.3 (20.0) |
| Diastolic blood pressure | 76.8 (9.7) | 72.2 (10.0) |
| Total cholesterol, mg/dL | 200.9 (34.0) | 206.6 (38.3) |
| HDL cholesterol, mg/dL | 43.8 (11.5) | 56.4 (15.4) |
| HbA1c (%) | 5.42 (1.01) | 5.37 (0.85) |
| CVD during follow-up | 18.7% | 11.1% |
For continuous risk factors, the top number denotes the sample mean and the bottom its corresponding standard deviation.
Categorical risk factors are summarized by percentages.
CVD, cardiovascular disease; HbA1C, glycosylated hemoglobin; HDL, high-density lipoprotein
Figure 1.
Kaplan–Meier survival plots of time (years) to hard CVD outcome for age –gender strata categories.
Note: “+” denotes censoring.
CVD, cardiovascular disease
Proposed Cardiometabolic Risk Model
Multivariable gender–specific Cox model hazard ratios (HRs) for the proposed cardiometabolic risk model are presented in Table 3. Men had a 23% increased risk of general CVD for every percentage increase in HbA1c (HR=1.23; 95% CI=1.11, 1.36, p<0.0001). For women, a percentage increase in HbA1c led to a 14% higher general CVD risk (HR=1.146; 95% CI=1.00, 1.32, p=0.053).
Table 3.
Gender–specific Cox regression hazard ratios of general cardiovascular disease for the D’Agostino et al.6 and proposed models
| Men | ||||||
|---|---|---|---|---|---|---|
| D’Agostino et al. (2008) model | Cardiometabolic model (proposed) | |||||
| Risk factors | HR | 95% CI | p-value | HR | 95% CI | p-value |
| Total cholesterol | 2.302 | 0.948, 5.591 | 0.065 | 1.003 | 0.999, 1.007 | 0.163 |
| HDL cholesterol | 0.618 | 0.350, 1.093 | 0.098 | 0.989 | 0.976, 1.003 | 0.122 |
| Age | 26.235 | 9.684, 71.073 | <0.0001 | 1.063 | 1.045, 1.082 | <0.0001 |
| Smoking | 1.431 | 0.992, 2.065 | 0.055 | 1.353 | 0.939, 1.949 | 0.105 |
| SBP | 1.008 | 1.000, 1.016 | 0.059 | |||
| If treated | 3.513 | 1.118, 11.040 | 0.032 | |||
| If not treated | 3.327 | 1.047, 10.571 | 0.042 | |||
| Diabetes | 1.981 | 1.279, 3.066 | 0.002 | |||
| HbA1c | 1.231 | 1.114, 1.361 | <0.0001 | |||
| BMI | 1.033 | 0.997, 1.070 | 0.070 | |||
| Women | ||||||
| Total cholesterol | 2.168 | 0.833, 5.636 | 0.113 | 1.004 | 1.000, 1.008 | 0.069 |
| HDL cholesterol | 0.331 | 0.180, 0.608 | <0.001 | 0.979 | 0.967, 0.992 | 0.001 |
| Age | 38.860 | 11.512, 131.2 | <0.0001 | 1.069 | 1.048, 1.091 | <0.0001 |
| Smoking | 1.888 | 1.267, 2.815 | 0.002 | 1.948 | 1.316, 2.883 | 0.0008 |
| SBP | 1.012 | 1.003, 1.020 | 0.006 | |||
| If treated | 5.476 | 1.688, 17.767 | 0.005 | |||
| If not treated | 5.182 | 1.559, 17.216 | 0.007 | |||
| Diabetes | 1.666 | 0.899, 3.090 | 0.105 | |||
| HbA1c | 1.146 | 0.999, 1.315 | 0.053 | |||
| BMI | 1.022 | 0.991, 1.054 | 0.163 | |||
The HR provides an estimate of the relative risk of cardiovascular disease between two groups or for a one-unit increase in continuous risk factors.
All continuous factors for the D’Agostino et al.6 model were log transformed.
HbA1c, glycosylated hemoglobin; HDL, high-density lipoprotein; HR, hazard ratio of general cardiovascular disease; SBP, systolic blood pressure
For men and women, smoking led to a 35% and 95% increase in general CVD risk, respectively. Additionally, an increase in BMI was marginally associated with an increased risk of general CVD. HDL cholesterol was significantly associated with general CVD risk for female participants (HR=0.979, 95% CI=0.967, 0.992) as well as systolic blood pressure (HR=1.012, 95% CI= 1.003, 1.020). Nonlinearity checks showed that a linear functional form for all continuous risk factors was appropriate and proportional hazards testing suggested the assumption was appropriate (results not shown).
Comparison of Risk Factors Between Models
We compared the HRs of the risk factors between the proposed risk score and the gender–specific D’Agostino risk score (Table 3). Presence of diabetes led to a doubling of general CVD risk for men (p=0.002). Systolic blood pressure was positively related to CVD risk in both men and women. For both models, age was highly associated with general CVD risk.
Evaluation of the Model: Discrimination
Model performance evaluated by discrimination is presented in Table 4. Discrimination (10–fold CV C-index43) achieved by the D’Agostino risk model on the offspring sample was 0.687 for men and 0.748 for women. The CV C-index of the proposed cardiometabolic risk score with HbA1c was 0.703 for men and 0.762 for women. When HbA1c was replaced with self–reported diabetes diagnosis in the proposed model, the CV C-index decreased to 0.690 for men and 0.757 for women. The difference in C-index between the proposed cardiometabolic model with HbA1c versus diabetes self-report was not statistically significant (men=0.0131, 95% CI= −0.0006, 0.0187; women=0.0047, 95% CI= −0.0078, 0.0055).
Table 4.
Evaluation of model performance for the D’Agostino et al.6 and proposed cardiometabolic models
| Men (n =1,047) | |||
|---|---|---|---|
| D’Agostino et al. (2008) model | Cardiometabolic model (with diabetes diagnosis self-report) | Proposed cardiometabolic model (with HbA1c) | |
| Discrimination | |||
| C-index | 0.706 | 0.704 | 0.715 |
| CV C-index | 0.687 | 0.690 | 0.703 |
| 95% CI for CV C-index |
0.652, 0.730 | 0.659, 0.734 | 0.668, 0.742 |
| Calibration | |||
| χ2 | 5.016 | 8.912 | 9.051 |
| p-value for χ2 | 0.755 | 0.349 | 0.338 |
| Women (n=1,312) | |||
| Discrimination | |||
| C-index | 0.772 | 0.771 | 0.773 |
| CV C-index | 0.748 | 0.757 | 0.762 |
| 95% CI for CV C-index |
0.714, 0.791 | 0.724, 0.799 | 0.726, 0.801 |
| CALIBRATION | |||
| χ2 | 3.1026 | 12.674 | 12.547 |
| p-value for χ2 | 0.9277 | 0.1235 | 0.1284 |
C-index and CV C-index denote the estimated probability that the prediction model assigns a higher cardiometabolic risk to subjects that develop CVD compared to those who do not develop CVD.
Calibration quantifies how the predicted outcome for each individual patient agrees with the actual outcome; p-values >0.05 suggest good fit.
CV, cross-validated; CVD, cardiovascular disease
Evaluation of the Model: Calibration
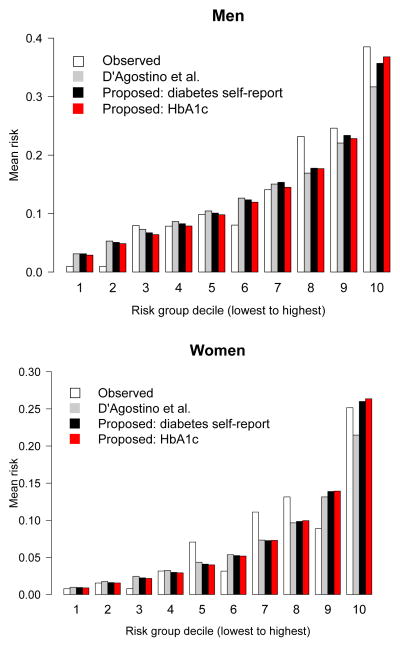
Figure 2 shows the calibration plot comparing predicted deciles of general CVD risk and observed risk for the D’Agostino model and proposed cardiometabolic risk models. Overall, the models demonstrate good calibration across all risk deciles. The calibration χ2 statistics for the two models are presented in Table 4. The goodness-of-fit χ2 statistic was lowest in the D’Agostino model (men, χ2=5.02; women, χ2 =3.10). The p-values for lack of fit were >0.05 for the all models, suggesting proper model fit.
Figure 2.
Calibration plot comparing agreement between observed, D’Agostino et al.6 model, proposed model with self-reported diabetes status, and proposed model with HbA1c instead of self–reported diabetes status for 10–year predictions for general CVD risk.
Ten groups (equal number of subjects per group) were categorized using proposed risk scores that range from low risk to high risk. Kaplan–Meier estimates were used for observed bars. Men (top panel) and women (bottom panel).
CVD, cardiovascular disease; HbA1c, glycosylated hemoglobin
Comparison of Cardiometabolic Risk Score in Subjects With and Without a Self–Reported Diabetes Diagnosis
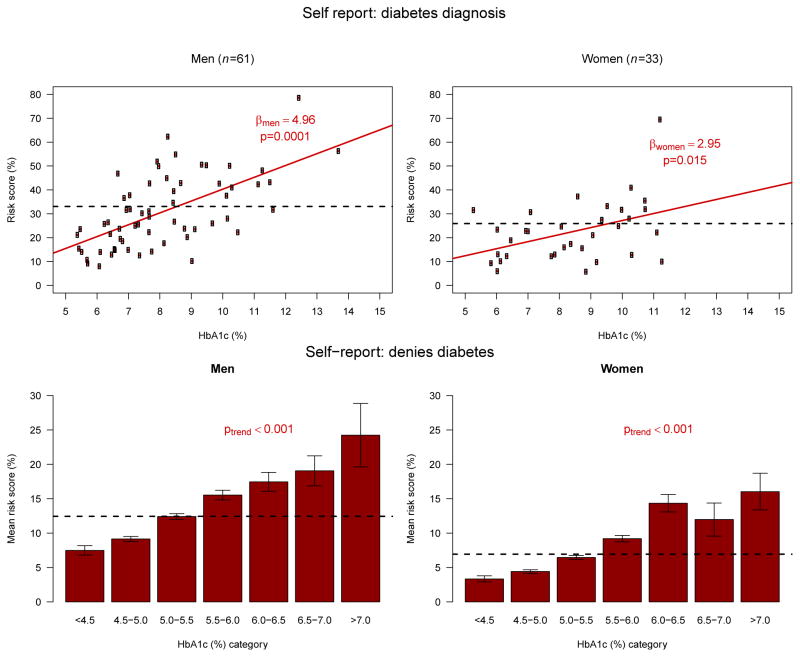
Figure 3 plots the estimated risk scores of the proposed model against HbA1c values for subjects with self–reported diabetes diagnosis (top panels). There was a significant dose–response relationship between HbA1c levels, with lower HbA1c values conferring less risk than the average risk score using the self–reported diabetes model, and higher HbA1c values conferring greater risk than the average risk score using the self–reported diabetes model, in both men and women. Additionally, for subjects who did not report a diabetes diagnosis (Figure 3, bottom panels), we observed a similar and significant trend.
Figure 3.
Comparison of cardiometabolic models with self–reported diabetes diagnosis versus Hba1c.
From a subset of responders who self-reported diabetes diagnosis (n=94), the average risk scores from the self–reported diabetes model stratified by gender is plotted as a black horizontal line for each group. For those with self–reported diabetes diagnosis (A, men; B, women), there is a significant dose–response relationship between HbA1c levels, with lower HbA1c values conferring less risk than the average, and higher HbA1c values conferring greater risk than the average. Bottom panels (C, D) present mean and SE of risk scores for each category of HbA1c. For those who deny a diabetes diagnosis (C, men; D, women), there is a significant dose-response relationship between HbA1c levels, with lower HbA1c values conferring less risk than the average, and higher HbA1c values conferring greater risk than the average.
HbA1c, glycosylated hemoglobin
Reclassification results for 10–year CVD risk are presented in Table 5. For ten subjects who experienced CVD events, classification using HbA1c in lieu of diabetes self-report improved, and for another ten it became worse with a net reclassification proportion of 0.00 (p=1.00). The net gain in reclassification proportion for subjects who did not experience an event was 0.013, with 65 subjects reclassified down and 38 reclassified up (p=0.008). The NRI was estimated as 0.013 and was not statistically significant (p=0.522).
Table 5.
Reclassification of 10–year cardiometabolic risk among people who experienced a CVD event and those who did not
| Proposed cardiometabolic model with HbA1c | ||||
|---|---|---|---|---|
| Proposed model with diabetes self-report | <6% | 6% to <20% | ≥20% | Total |
| Participants who experience a CVD event | ||||
| <6% | 22 (88.0) | 3 (12.0) | 0 (0.0) | 25 |
| 6% to <20% | 5 (4.2) | 108 (90.0) | 7 (5.8) | 120 |
| ≥20% | 0 (0.0) | 5 (6.0) | 79 (94.0) | 84 |
| Total | 27 | 116 | 86 | 229 |
| Participants who do not experience a CVD event | ||||
| <6% | 940 (97.6) | 23 (2.4) | 0 (0.0) | 963 |
| 6% to <20% | 36 (4.0) | 858 (94.4) | 15 (1.6) | 909 |
| ≥20% | 0 (0.0) | 29 (12.9) | 196 (87.1) | 225 |
| Total | 976 | 910 | 211 | 2097 |
Columns and rows refer to categories of 10–year predicted risk.
Table is restricted to events occurring during the first 10 years of follow-up.
CVD, cardiovascular disease; HbA1c, glycosylated hemoglobin
Discussion
The Framingham risk score model is a widely used, practical model to evaluate CVD risk.6 We developed a general cardiometabolic risk score that is sensitive to modifiable and measurable non–self-reported risk factors that can be changed in an intervention. Focusing on modifiable risk factors such as HbA1c and BMI allows researchers and practitioners to better evaluate small changes in important risks and then communicate general cardiometabolic risk to individuals who may be involved in community–level primary prevention interventions. Small changes across multiple risk factors can substantially impact overall population patterns of health.44 We hope that this will serve to motivate an individual’s commitment to recommended behavioral changes or interventions that reduce risk. This innovative statistical and analytic tool utilizes new biomarkers and measures to optimize CVD or other risk prediction models. Of note, the use of HbA1c provides significantly better discriminatory power than self–reported diabetes diagnosis, and has a significant dose–response relationship with CVD risk even in those who deny a diabetes diagnosis. However, reducing HbA1c via medications may not reduce or even increase CVD risk.45 Behavioral risk reduction (eg. through diet and exercise) can lower HbA1c,46 which we and others find is associated with CVD risk.47
This study has several major strengths. The proposed prediction model expands the global CVD functions of the D’Agostino model, where Hba1c, now diagnostic for prediabetes and diabetes,48,49 is collected with BMI. Advances in cardiometabolic biomarkers have produced an abundance of data, providing a unique opportunity to study cardiometabolic risk in populations of individuals studied in a fuller context (e.g., workplace and home), and enhanced socioecologic validity (e.g., representative rather than convenience samples).
The use of the offspring cohort to develop the model allows us to generalize the risk score to a more general U.S. adult population that is typically found in intervention and primary care settings. We also demonstrated through discrimination analyses that inclusion of HbA1c and BMI leads to predictive performance that is in the same range of the D’Agostino model and of C-indexes from other Framingham risk scores.50,51 With calibration models, we showed that the generalized cardiometabolic model has good agreement between predicted and observed outcomes. Again, our goal here was not to improve on the excellent predictive ability of the earlier scales, but rather to introduce the ability to monitor small changes in important non–self-reported modifiable risk factors such as HbA1c.
This study has limitations. The study was restricted to Framingham offspring subjects with HbA1c at exam 5, which produced a smaller sample size with potentially more confounded data in the Framingham offspring cohort than the sample used in previous Framingham models, somewhat limiting generalizability. As a result, multiple risk factors in the both gender–specific risk models analyzed in this study, such as self–reported smoking status, did not show statistically significant relationships with general CVD risk as previous studies have shown.6 The lack of significance may be due to not having enough Framingham data in the analysis, or inherent self–report measure limitations, and may underestimate the true relationship between the risk factors and general CVD risk. The direction of the associations of the risk factors and general CVD risk are in line with previous published studies.3,6 Our emphasis is on cardiometabolic risk prediction. Often, measures of association do not meaningfully describe the ability of the variable to classify subjects.52 Future studies using measured risk factors (e.g., cotinine levels to assess smoking status and high–sensitivity C-reactive protein53–55) may identify models that are more predictive than those using self-reported measures. Unfortunately, we were limited by risk factors collected at Framingham offspring cohort exam 5, which did not include some risk factors such as C-reactive protein. The Framingham cohort is also predominantly white, which limits our generalizability to non-white cohorts. A possible solution to extend this work to non-white groups would be to consider adapting the probabilities to the demographics of the study population through recalibration.56 Future work should assess the impact of measurement error using self-reported measures, including diabetes, on estimates of association. Future studies should estimate predictive performance of the proposed model on an independent, more diverse population, and incorporate assessment of all risk factors, including health behaviors.
In conclusion, the study presents an algorithm that quantifies general CVD risk based on modifiable risk factors, including HbA1c and BMI, which can motivate an individual’s commitment to prevention and intervention. This model could be utilized in settings where measured biomarkers are already being collected: primary prevention efforts in communities and worksites, health outcomes research that use electronic medical records and administrative data, assessments in prediabetic patients, and primary care settings (e.g. managing patient care through annual check-ups). As preventive care in the U.S. and in other countries advances toward promising interventions, we hope that both medicine and public health will incorporate quantitative, modifiable risk factors into common practice.
Supplementary Material
Acknowledgments
This study was supported by the Robert Wood Johnson Foundation Health and Society Scholars program at Harvard (Grant #69248), the Institute on Aging (Grant #U01AG027669), and the Work, Family, and Health Network (WorkFamilyHealthNetwork.org), which is funded by a cooperative agreement through the NIH and CDC: Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grant #U01HD051217;U01HD051218;U01HD051256;U01HD051276), National Institute on Aging (Grant #U01AG027669), Office of Behavioral and Science Sciences Research, National Heart, Lung, and Blood Institute (R01HL107240), and National Institute for Occupational Safety and Health (Grant #U01OH008788;U01HD059773). Orfeu M. Buxton received an investigator-initiated grant from Sepracor Inc. (now Sunovion; ESRC-0977, ClinicalTrials.gov Identifier NCT00900159). Grants from the William T. Grant Foundation, Alfred P. Sloan Foundation, and the Administration for Children and Families provided additional funding. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of these institutes and offices. Ralph B. D’Agostino was supported by Framingham study contract NHLBI NO1-HC-25195. Michael J. Pencia received support from the Framingham heart study. We thank the National Heart, Lung, and Blood Institute for providing Framingham data and are grateful to Framingham participants and staff.
Footnotes
No financial disclosures were reported by the other authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.11.003. S0735–1097(13):06029–4. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 4.Berry JD, Dyer A, Cai X, et al. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366(4):321–9. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113(6):791–8. doi: 10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]
- 6.D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation. 2008;117(6):743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 7.Pencina MJ, D’Agostino RB, Sr, Larson MG, Massaro JM, Vasan RS. Predicting the 30-year risk of cardiovascular disease: the Framingham heart study. Circulation. 2009;119(24):3078–84. doi: 10.1161/CIRCULATIONAHA.108.816694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Ruijter W, Westendorp RG, Assendelft WJ, et al. Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. BMJ. 2009;338:a3083. doi: 10.1136/bmj.a3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marma AK, Lloyd-Jones DM. Systematic examination of the updated Framingham heart study general cardiovascular risk profile. Circulation. 2009;120(5):384–90. doi: 10.1161/CIRCULATIONAHA.108.835470. [DOI] [PubMed] [Google Scholar]
- 10.Lloyd-Jones DM. Cardiovascular risk prediction: basic concepts, current status, and future directions. Circulation. 2010;121(15):1768–77. doi: 10.1161/CIRCULATIONAHA.109.849166. [DOI] [PubMed] [Google Scholar]
- 11.Keith SW, Fontaine KR, Pajewski NM, Mehta T, Allison DB. Use of self-reported height and weight biases the body mass index-mortality association. Int J Obes (Lond) 2011;35(3):401–8. doi: 10.1038/ijo.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2. 7 million participants. Lancet. 2011;378(9785):31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 13.Danaei G, Friedman AB, Oza S, Murray CJ, Ezzati M. Diabetes prevalence and diagnosis in U.S. states: analysis of health surveys. Popul Health Metr. 2009;7:16. doi: 10.1186/1478-7954-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pai JK, Cahill LE, Hu FB, Rexrode KM, Manson JE, Rimm EB. Hemoglobin a1c is associated with increased risk of incident coronary heart disease among apparently healthy, nondiabetic men and women. J Am Heart Assoc. 2013;2(2):e000077. doi: 10.1161/JAHA.112.000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu G, Qiao Q, Tuomilehto J, Eliasson M, Feskens EJ, Pyorala K. Plasma insulin and cardiovascular mortality in non-diabetic European men and women: a meta-analysis of data from eleven prospective studies. Diabetologia. 2004;47(7):1245–56. doi: 10.1007/s00125-004-1433-4. [DOI] [PubMed] [Google Scholar]
- 16.Kaptoge S, Di Angelantonio E, Lowe G, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375(9709):132–40. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapur S, Zava D. Cardiometabolic risk factors assessed by a finger stick dried blood spot method. J Diabetes Sci Technol. 2008;2(2):236–41. doi: 10.1177/193229680800200210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lakshmy R. Analysis of the use of dried blood spot measurements in disease screening. J Diabetes Sci Technol. 2008;2(2):242–3. doi: 10.1177/193229680800200211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(1S):S64–S71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. Geneva: WHO; Report No.: WHO/NMH/CHP/CPM/11.1. [Google Scholar]
- 21.Rose G, Katy-Tee K, Marmot M. The strategy of preventive medicine. Oxford: Oxford University Press, U.S; 2008. [Google Scholar]
- 22.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham offspring study. Design and preliminary data. Prev Med. 1975;4(4):518–25. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 23.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110(3):281–90. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 24.Kannel WB, D’Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159(11):1197–204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 25.Cupples LA, D’Agostino RB. Section 34: some risk factors related to annual incidence of cardiovascular disease and death in pooled repeated biennial measurements. In: Kannel WB, Wolf PA, Garrison RJ, editors. Framingham heart study: 30-year follow-up. Bethesda MD: USDHHS; 1987. [Google Scholar]
- 26.Rutter MK, Meigs JB, Sullivan LM, D’Agostino RB, Sr, Wilson PW. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham offspring study. Circulation. 2004;110(4):380–5. doi: 10.1161/01.CIR.0000136581.59584.0E. [DOI] [PubMed] [Google Scholar]
- 27.Nathan DM, Singer DE, Hurxthal K, Goodson JD. The clinical information value of the glycosylated hemoglobin assay. N Engl J Med. 1984;310(6):341–6. doi: 10.1056/NEJM198402093100602. [DOI] [PubMed] [Google Scholar]
- 28.Meigs JB, Nathan DM, Cupples LA, Wilson PW, Singer DE. Tracking of glycated hemoglobin in the original cohort of the Framingham heart study. J Clin Epidemiol. 1996;49(4):411–7. doi: 10.1016/0895-4356(95)00513-7. [DOI] [PubMed] [Google Scholar]
- 29.Meigs JB, Nathan DM, Wilson PW, Cupples LA, Singer DE. Metabolic risk factors worsen continuously across the spectrum of nondiabetic glucose tolerance. The Framingham offspring study. Ann Intern Med. 1998;128(7):524–33. doi: 10.7326/0003-4819-128-7-199804010-00002. [DOI] [PubMed] [Google Scholar]
- 30.Lloyd-Jones DM, Evans JC, Larson MG, Levy D. Treatment and control of hypertension in the community: a prospective analysis. Hypertension. 2002;40(5):640–6. doi: 10.1161/01.hyp.0000035855.44620.da. [DOI] [PubMed] [Google Scholar]
- 31.Cox DR. Regression models and life tables. J Roy Statist Soc Ser B. 1972;34(2):187–220. [Google Scholar]
- 32.Grambsch PM, Therneau TM, Fleming TR. Diagnostic plots to reveal functional form for covariates in multiplicative intensity models. Biometrics. 1995;51(4):1469–82. [PubMed] [Google Scholar]
- 33.Grambsch PM, Therneau TM. Proportional hazard tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26. [Google Scholar]
- 34.Harrell FE. Regression modeling strategies. New York: Springer; 2001. [Google Scholar]
- 35.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23(13):2109–23. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 36.Hastie T, Tibshirani R, Friedman J. The elements of statistical learning. New York: Springer; 2003. [Google Scholar]
- 37.Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics. 2005;61(1):92–105. doi: 10.1111/j.0006-341X.2005.030814.x. [DOI] [PubMed] [Google Scholar]
- 38.Efron B, Gong G. A leisurely look at the bootstrap, the jackknife and cross-validation. Am Stat. 1983;37(1):36–48. [Google Scholar]
- 39.Verweij PJ, Van Houwelingen HC. Cross-validation in survival analysis. Stat Med. 1993;12(24):2305–14. doi: 10.1002/sim.4780122407. [DOI] [PubMed] [Google Scholar]
- 40.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 41.D’Agostino R, Nam BH. Evaluation of the performance of survival analysis models: discrimination and calibration measures. In: Balakrishnan N, Rao CR, editors. Handbook of statistics. Amsterdam: Elsevier; 2004. [Google Scholar]
- 42.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–72. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 43.Cooney MT, Dudina A, D’Agostino R, Graham IM. Cardiovascular risk-estimation systems in primary prevention: do they differ? Do they make a difference? Can we see the future? Circulation. 2010;122(3):300–10. doi: 10.1161/CIRCULATIONAHA.109.852756. [DOI] [PubMed] [Google Scholar]
- 44.Rose G. Strategy of prevention: lessons from cardiovascular disease. BMJ. 1981;282(6279):1847–51. doi: 10.1136/bmj.282.6279.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terry T, Raravikar K, Chokrungvaranon N, Reaven PD. Does aggressive glycemic control benefit macrovascular and microvascular disease in type 2 diabetes? Insights from ACCORD, ADVANCE, and VADT. Curr Cardiol Rep. 2012;14(1):79–88. doi: 10.1007/s11886-011-0238-6. [DOI] [PubMed] [Google Scholar]
- 46.American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(1S):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362(9):800–11. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.American Diabetes Association. Summary of revisions for the 2013 clinical practice recommendations. Diabetes Care. 2013;36(1S):S3. doi: 10.2337/dc13-S003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cook NR, Paynter NP, Eaton CB, et al. Comparison of the Framingham and Reynolds risk scores for global cardiovascular risk prediction in the multiethnic women’s health initiative. Circulation. 2012;125(14):1748–56. S1–S11. doi: 10.1161/CIRCULATIONAHA.111.075929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 51.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 52.Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159(9):882–90. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- 53.Rost NS, Wolf PA, Kase CS, et al. Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack: the Framingham study. Stroke. 2001;32(11):2575–9. doi: 10.1161/hs1101.098151. [DOI] [PubMed] [Google Scholar]
- 54.Wilson PW, Nam BH, Pencina M, D’Agostino RB, Sr, Benjamin EJ, O’Donnell CJ. C-reactive protein and risk of cardiovascular disease in men and women from the Framingham heart study. Arch Intern Med. 2005;165(21):2473–8. doi: 10.1001/archinte.165.21.2473. [DOI] [PubMed] [Google Scholar]
- 55.Wilson PW, Pencina M, Jacques P, Selhub J, D’Agostino R, Sr, O’Donnell CJ. C-reactive protein and reclassification of cardiovascular risk in the Framingham heart study. Circulation Circ Cardiovasc Qual Outcomes. 2008;1(2):92–7. doi: 10.1161/CIRCOUTCOMES.108.831198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.D’Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–7. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.