Abstract
To further studies of neonatal immune responses to pathogens and vaccination, we investigated the dynamics of B lymphocyte development and immunoglobulin (Ig) gene diversity. Previously we demonstrated that equine fetal Ig VDJ sequences exhibit combinatorial and junctional diversity levels comparable to those of adult Ig VDJ sequences. Herein, RACE clones from fetal, neonatal, foal, and adult lymphoid tissue were assessed for Ig lambda light chain combinatorial, junctional, and sequence diversity. Remarkably, more lambda variable genes (IGLV) were used during fetal life than later stages and IGLV gene usage differed significantly with time, in contrast to the Ig heavy chain. Junctional diversity measured by CDR3L length was constant over time. Comparison of Ig lambda transcripts to germline revealed significant increases in nucleotide diversity over time, even during fetal life. These results suggest that the Ig lambda light chain provides an additional dimension of diversity to the equine Ig repertoire.
Keywords: equine, immunoglobulin, lambda, light chain, diversity, development
1. Introduction
Immunoglobulins (Igs) are membrane-bound or secreted proteins produced by B lymphocytes that can bind pathogens to mediate clearance, killing, and neutralization. As a major component of the adaptive immune response, an individual Ig may achieve high affinity for a single antigen, but the spectrum of Ig antigen specificities that can be attained in a host is immense. Generation of the vast Ig repertoire is accomplished through a multifactorial process of combinatorial diversity (selection of single variable, diversity, and joining gene segments from the multiple germline loci and recombination to create one Ig transcript), junctional diversity from imprecise gene recombination and non-templated or palindromic nucleotide additions, and somatic hypermutation. Ig molecules are heterodimers of heavy and light chains in vertebrates other than sharks and, in some instances, camels (De Genst et al., 2006, Dooley and Flajnik, 2006). The sequence of both heavy and light chain Igs encode variable ‘complementarity-determining regions’ (CDRs), which together form the antigen-binding site, and are flanked by conserved framework regions (Near et al., 1990, Kabat and Wu, 1991, Kirkham et al., 1992). Clinically, the loss of Ig or B lymphocyte production results in humoral immunodeficiency (Park et al., 2008, Flaminio et al., 2009).
During B lymphocyte differentiation, heavy chain gene rearrangement precedes light chain gene rearrangement (Alt et al., 1981). Two Ig light chains, kappa and lambda, are used in mammalian species and until recently it was accepted that the lambda light chain gene locus only rearranged if the kappa rearrangement was unsuccessful, although the lambda gene locus may be active before kappa in pigs (Hieter et al., 1981, Sun et al., 2012). It is intriguing that the relative abundance of kappa (κ) and lambda (λ) light chain varies among species: 95% κ/ 5% λ in mice, 66% κ/ 34% λ in humans, 52% κ/ 48% λ in pigs, 9% κ/ 91% λ in cows, 8% κ/ 92% λ in horses, and lambda light chain is the only one expressed in chickens and ducks (Hood et al., 1967, Gibson 1974, Kessler et al., 1981, Reynaud et al., 1985, Magor et al., 1994, Arun et al., 1996). Subsequently, it has been suggested that the predominant light chain used may be attributed to the number of germline V genes present in that species (Almagro et al., 1998). Additional mechanisms have been shown to affect light chain usage in mice, such as higher recombination frequency at the Ig kappa locus, antigen selection, and increased self-reactivity in Ig kappa-deficient mice implies that Ig lambda may be subject to negative selection (Haughton et al., 1978, Ramsden and Wu, 1991, Knott et al., 1998). This striking lack of conservation in Ig light chain gene rearrangement order and range of usage is an interesting phenomenon of comparative immunology.
Recent studies have greatly advanced our knowledge of the equine Ig lambda genes in terms of genome organization and allelic diversity beyond the initial cDNA characterization (Home et al., 1992). Twenty-seven functional and 5 ORF IGLV gene segments have been annotated on EquCab2 chromosome 8 (NW_001867428), flanking a cluster of 7 IGLJ-IGLC pairs (Sun et al., 2010). The equine IGLV genes are divided into 11 subgroups based on sequence identity, and the expression of subgroup 8 IGLV genes is dominant in the horse spleen, complemented by lesser usage of subgroups 4 and 6 (Sun et al., 2010). Five of the 7 IGLJ-IGLC genes appear to be expressed although some individuals may lack particular genes; differences in Ig lambda gene content between individual genomes have also been reported in pigs (Sun et al., 2010, Hara et al., 2012, Wertz et al., 2013). Alleles of equine IGLV, IGLJ, and IGLC genes have been identified and they differ by up to 4% from the EquCab2.0 reference genome, which may encode distinct serological properties (Sun et al., 2010, Hara et al., 2012).
Early Ig repertoire studies reported restricted diversity during fetal life, for both Ig heavy and light chains. The murine and human fetal repertoire has been characterized by biased Ig gene segment usage and restricted CDR3 length (Schroeder and Wang, 1990, Shiokawa et al., 1999). Unlike humans and horses, fetal mice do not express TdT/DNTT and thus lack non-templated (N) additions (Li et al., 1993, Tallmadge et al., 2009). The human fetal Ig lambda repertoire is generated from distinct and non-random patterns of Ig lambda gene rearrangements in contrast to adults, and it expresses identical VλJλ junctions with unique Ig heavy chains (Lee et al., 2000). Analysis of fetal piglet Ig lambda repertoire revealed that 70% of the repertoire is derived from 3 IGLV genes, similar to the IGHV repertoire in adult pigs, although CDR3L diversity is very limited in contrast to the heavy chain (Sun et al., 1998, Butler et al., 2000, Wertz et al., 2013).
Because the equine placenta does not facilitate Ig or cell transfer to the fetus in utero, it is possible to investigate the intrinsic humoral system development in the fetus and pre-suckle neonate. Investigation of B lymphocyte markers and Ig heavy chain isotype expression paired with analysis of IGHV, IGHD, and IGHJ gene usage and diversity revealed that equine fetal B lymphocytes express mature and co-stimulatory B lymphocyte markers and most Ig heavy chain isotype transcripts (Tallmadge et al., 2009). In addition, a similar complement of Ig variable genes is used during equine fetal and post-natal life stages and Ig sequence diversity increases during fetal life (Tallmadge et al., 2013). Given the Ig VDJ diversity detected in equine fetal life and findings of other studies of the fetal Ig repertoire cited above, we hypothesized that little diversity would be present in equine fetal Ig lambda chain transcripts. Herein we describe the repertoire of expressed Ig lambda chain sequences spanning equine development from fetal life to adulthood.
2. Materials and methods
2.1 Tissue samples
These experiments were approved by the Cornell University Center for Animal Resources and Education and Institutional Animal Care and Use Committee for the use of vertebrates in research. Tissue samples from a healthy induced-abortion equine Thoroughbred x Warmblood fetus without uterine disease (102 days of gestation), a neonate Warmblood foal (pre-suckle, < 1-hour-old), a 2 month-old Warmblood foal, and a Thoroughbred adult horse were archived and available for this study from research investigations performed by us and other investigators over the years at Cornell University College of Veterinary Medicine. None of the donors were related or had history of infections, and were maintained in Cornell University research herds with open housing conditions and vaccinated as recommended by the American Association of Equine Practitioners. Tissue samples were collected within 1 hour of euthanasia, snap frozen in liquid nitrogen, and stored at −80°C until use.
2.2 RACE library construction, Ig lambda chain amplification, and cloning
RNA was isolated from snap-frozen tissues following homogenization by QIAshredder (Qiagen, Valencia, CA) as directed by the RNeasy kit (Qiagen) including on-column digestion of contaminating genomic DNA. RNA was quantified with a Nanodrop (Thermo Fisher Scientific, Inc., Waltham, MA) and one microgram was used to generate a 5′-rapid amplification of cDNA ends (RACE) library with the SMARTer™ RACE cDNA Amplification Kit (Clontech Laboratories, Inc., Mountain View, CA). The same RACE libraries were used to assess Ig heavy chain diversity (Tallmadge et al., 2013). The gene-specific primer was designed to amplify all immunoglobulin lambda light chain constant genes. Amplification reactions contained 1X iProof high fidelity buffer, 1.5 mM MgCl2, 0.2 mM dNTPs, the provided universal forward primer with 0.5 μM gene-specific reverse primer 5′ CTCTGAGGGGGACAGTTTCTTCTCCAC 3′ (Integrated DNA Technologies, Coralville, IA), and 0.02 U iProof DNA polymerase (error rate of 4.4 × 10−7, Bio-Rad Laboratories, Hercules, CA). Thermal cycling parameters were 5 cycles of 98 °C for 30 seconds and 72°C for 1 minute; 5 cycles of 98 °C for 30 seconds, 70 °C for 30 seconds and 72°C for 1 minute; and 27 cycles of 98 °C for 30 seconds, 68 °C for 30 seconds and 72°C for 1 minute. Amplification products were run on 1% agarose gels and stained with GelGreen nucleic acid stain (Phenix Research Products, Candler, NC) for visualization. PCR products of approximately 750 bp were excised from the gel, purified with GeneJET gel extraction kit (Thermo Fisher Scientific, Inc.), and cloned with the CloneJET PCR cloning kit (Thermo Fisher Scientific, Inc.). Individual colonies were expanded in LB broth with ampicillin and plasmid DNA was purified with the GeneJET plasmid miniprep kit (Thermo Fisher Scientific, Inc.).
2.3 Amplification of Ig lambda genes from genomic DNA
Genomic DNA was isolated from liver samples of each horse as directed by the DNeasy Blood & Tissue Kit (Qiagen). Gene-specific primers were used in PCR reactions with 50 ng genomic DNA, 1X iProof GC buffer, 1.5 mM MgCl2, 0.2 mM dNTPs, 0.5 μM primers, and 0.02 U iProof DNA polymerase (Bio-Rad). Thermal cycling parameters included initial denaturation at 98 °C for 3 minutes; 35 cycles of 98 °C for 10 seconds, 60 °C for 10 seconds, and 72°C for 1 minute; followed by final extension at 72°C for 10 minutes. Primers to amplify IGLJ1 to IGLC1, IGLJ4 to IGLC4, and IGLJ7 to IGLC7 were published by Sun and colleagues (2010). The IGLC4 locus was also amplified from genomic DNA with primers 5′ TGAGAAGGATTTGGGCGGAG 3′ and 5′ GACTTGACGGTGAGCTGGAA 3′, generating a 320 base pair product. Primer sequences were designed for VL15 (5′ CAAAGGAAGCAGCTGACACG 3′ and 5′ GGGGCTGTGATTTGCATGTG 3′, amplified a 705 base pair product), VL17 (5′ CAAAGGAAGCAGCTGACGTG 3′ and 5′ CTCAGCTTTCCGTGAGGGTT 3′, amplified a 855 base pair product), and VL26 (5′ GGGCTTTGGAGACCTGAGAC 3′ and 5′ GAGGGCACAGCAGGTTTTTG 3′, amplified a 869 base pair product). PCR products were visualized, cloned, and sequenced as described above.
2.4 Sequence analysis
Clones were sequenced at the Cornell University Institute of Biotechnology, Ithaca, NY. Sequence content analysis was performed with Geneious software version 6.1.6 (Biomatters, Ltd., Auckland, New Zealand; Drummond et al., 2009). Only unique sequences were included in analyses. Sequences are available through GenBank with accession numbers KF985041 - KF985160 and KF992229 - KF992247. Shapiro-Wilk Normality test revealed that, in most cases, data were not normally distributed and hence all comparisons were made with the Wilcoxon-Mann-Whitney Rank Sum test (GraphPad Prism 6.0.2, GraphPad Software, Inc., La Jolla, CA). The level of amino acid polymorphism was calculated from the variability index described by Wu and Kabat (1970) and plotted against the amino acid position. Variability is determined by the number of different amino acids at a given residue divided by the frequency of the most common amino acid at that residue. Framework and CDR regions were identified per IMGT guidelines designation (Lefranc et al., 2003). IGLV, IGLJ, and IGLC gene usage was determined by comparing the expressed sequences obtained with germline sequence by BLAST and manually reviewing each match with the NCBI Splign tool and multiple alignments. Germline comparisons were initially performed with the equine reference genome sequence (EquCab2.0), and then to germline sequences obtained in this study or available in the GenBank nucleotide database. Bias of IGLV, IGLJ, and IGLC gene usage was assessed with the chi-square test (GraphPad Prism 6.0.2). Phylogenetic analysis based on nucleotide sequences of framework regions 1, 2, and 3 was performed with MEGA version 5.2 (Tamura et al., 2011), using p-distances and the neighbor-joining method (Saitou and Nei 1987). To determine the level of support for each node, bootstrap re-sampling was performed with 1,000 replications. Human IGKV1-12 (V01577) was included as an outgroup.
2.5 Immunoglobulin gene name nomenclature
The name of Ig lambda light chain variable, joining, and constant gene segments were assigned according to guidelines set forth by IMGT, the international ImMunoGeneTics information system (www.imgt.org). IGLV genes are named according to subgroup, determined by Sun and colleagues (2010), followed by a number corresponding to location in the equine Ig lambda locus, such that “Vλ1” is renamed “ IGLV1-38” to designate subgroup 1 and gene position 38, per the human IGLV nomenclature system (Lefranc, 2001). Similarly, consistent with the nomenclature of human Ig lambda genes, IGLJ and IGLC genes are designated IGLJ1 through IGLJ7 and IGLC1 through IGLC7 rather than the original “Jλ1” and “Cλ1” assignment (Lefranc 2001, Sun et al., 2010, Hara et al., 2012). Alleles are designated by the addition of “*01” after the gene name, as directed by the WHO-IUIS Nomenclature Subcommittee for immunoglobulins and T cell receptors (2008). Supplemental table 1 lists the correspondence between gene names assigned by Sun and colleagues (2010) and the new designations.
3. Results
Herein, we investigated the patterns of Ig lambda light chain gene usage and nucleotide diversity from fetal spleen, neonatal mesenteric lymph node (MLN), foal MLN, and adult horse MLN tissue. Fetal spleen was sampled rather than fetal MLN because spleen is a better developed and more accessible lymphoid organ at this stage. Ig lambda transcripts were amplified from RACE libraries, cloned, and 30 unique sequences were obtained from each sample (Supplemental table 2).
3.1 Equine Ig lambda light chain constant gene usage and diversity from germline over developmental stages
Ig lambda gene usage and identity to germline were determined by comparing the expressed sequences with the Ig lambda locus of the EquCab2.0 equine reference genome annotated by Sun and colleagues (2010). The Ig lambda joining and constant genes were investigated from fetal sequences first because IGLJ and IGLC genes exist as pairs in the equine genome and little nucleotide diversity was expected. Germline gene usage of IGLC1, IGLC4, and IGLC5 was found among the expressed fetal sequences (Figure 1), but many sequences differed from the EquCab2.0 gene sequence by 5 to 7 nucleotides. The Ig lambda constant region sequences of fetus clones IGLVJ1 - IGLVJ10 best matched the reference genome IGLC1 gene with 5 nucleotide mismatches, and the joining region sequences best matched IGLJ1 accordingly. However, 2 variants of IGLJ1 were observed in these expressed sequences: 4 of the 10 fetus IGLVJ1 - IGLVJ10 clone sequences were identical to the genome IGLJ1 and 6 differed by one nucleotide. To determine whether these discrepancies reflected germline alleles or clonal sequences containing somatic mutations, the region encompassing IGLJ1 and IGLC1 was amplified from genomic DNA isolated from the liver of the donor fetus and sequenced (data not shown). One IGLC1 sequence (IGLC1*01) was obtained from fetal liver genomic DNA that shared 100% identity with 9 of the fetal expressed sequences, and was 1 nucleotide different from fetus IGLVJ3 sequence (Table 1). Two IGLJ1 sequences were obtained from fetal liver genomic DNA; one matched the EquCab2.0 reference genome and the second differed from the genome by one nucleotide and was 100% identical to the 6 expressed IGLJ1 variants (IGLJ1*01, Table 1). It was subsequently determined that this IGLJ1*01 sequence was identical to the 1-Jλ1 sequence determined by Sun et al. (2010), validating these new IGLJ1 and IGLC1 sequences as germline alleles (Table 1). The fetal clone sequences IGLVJ11 - IGLVJ17 best matched the reference genome IGLC4 gene with 7 mismatches. Amplification and sequencing of the IGLC4 gene from the donor’s liver genomic DNA identified new IGLJ4 and IGLC4 alleles (IGLJ4*01, IGLC4*01), which matched the expressed sequences and differed from the reference genome IGLC4 gene (Table 1). The remaining fetal sequences, fetus IGLVJ18 – IGLVJ30, contained IGLJ5/IGLC5 sequences that were identical to the EquCab2.0 reference genome or the IGLC5b allele described by Hara et al. (2012), except for a single nucleotide variation in clone fetus IGLVJ18 constant region (Table 1).
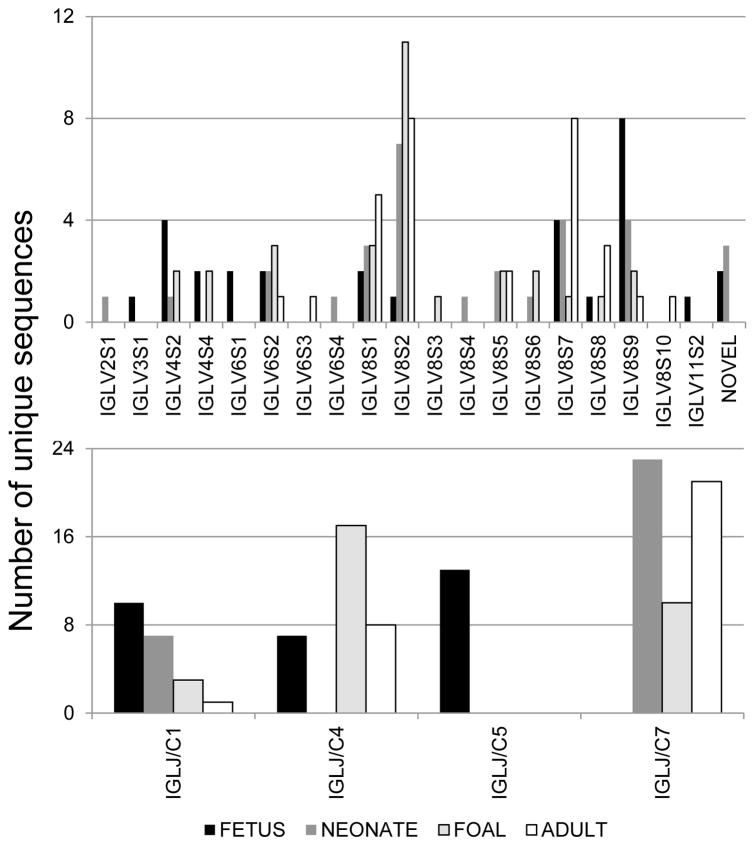
Figure 1. Equine immunoglobulin lambda light chain gene segment usage during fetal, neonatal, foal, and adult horse life stages.
The repertoire of expressed IGLV genes (top panel) and IGLJ/IGLC pairs (bottom panel) is shown for fetal (black bar), neonatal (dark gray), 2-month old foal (light gray with black border), and adult (white bar with black border). Chi-square tests revealed biased usage of both IGLV (p = 0.0442) and IGLJ/IGLC (p < 0.0001) genes over time.
Table 1.
Equine immunoglobulin lambda germline alleles expressed in lymphoid tissues.
| Ig lambda gene/allele | Identity to EquCab2.0 | RACE clones | RACE identity to germline | Reference, Genbank accession |
|---|---|---|---|---|
| IGLV8-122 | Fetus IGLVJ13 | 100 | Sun et al., 2010, NW_001867428 | |
| IGLV8-122*01 | 99.57 | Fetus IGLVJ26 Foal IGLVJ12 |
100 98.53 |
This study, KF992235 |
| IGLV8-122*02 | 99.86 | Neonate IGLVJ20, 21, 22 Adult IGLVJ5, 23 |
96.52 – 99.75 89.39 – 94.43 |
This study, KF992236 |
| IGLV8-122*03 | 96.73 | Foal IGLVJ2 Adult IGLVJ25 |
99.48 89.37 |
This study, KF992237 |
| IGLV8-122*04 | 98.87 | Adult IGLVJ6, 24 | 90.61 – 92.17 | This study, KF992238 |
| IGLV8-128 | Neonate IGLVJ1, 2, 8, 9, 10, 11, 12 Foal IGLVJ6, 7, 8, 9, 10, 22 Adult IGLVJ2, 10, 14, 15 |
97.57 – 98.38 93.98 – 96.56 89.84 – 97.67 |
Sun et al., 2010, NW_001867428 | |
| IGLV8-128*01 | 98.72 | Fetus IGLVJ18 Adult IGLVJ11, 12, 13 |
98.52 89.27 – 93.56 |
This study, KF992239 |
| IGLV8-128*02 | 97.16 | Foal IGLVJ1, 4, 5, 21, 23 | 95.80 – 99.75 | This study, KF992240 |
| IGLV8-128*03 | 96.30 | Adult IGLVJ1 | 88.68 | This study, KF992241 |
| IGLV8-26*01 | 98.54 | Neonate IGLVJ28 Foal IGLVJ29 |
98.28 95.56 |
This study, KF992242 |
| IGLV8-24 | Neonate IGLVJ3, 13, 14, 15 Foal IGLVJ24 Adult IGLVJ3, 4, 16, 17, 19 |
96.94 – 99.24 97.97 84.35 – 97.64 |
Sun et al., 2010, NW_001867428 | |
| IGLV8-24*01 | 99.86 | Fetus IGLVJ19, 20, 21 Adult IGLVJ18, 20, 21 |
100 88.59 – 93.80 |
This study, KF992243 |
| IGLV8-20 | Foal IGLVJ13 | 96.27 | Sun et al., 2010, NW_001867428 | |
| IGLV8-20*01 | 97.12 | Fetus IGLVJ27 | 99.75 | This study, KF992244 |
| IGLV8-20*02 | 97.17 | Adult IGLVJ7, 8, 26 | 94.99 –98.53 | This study, KF992245 |
| IGLV8-12 | Fetus IGLVJ22, 23, 25 Neonate IGLVJ16, 17, 18, 19 |
99.01 – 100 96.08 – 100 |
Sun et al., 2010, NW_001867428 | |
| IGLV8-12*01 | 98.25 | Fetus IGLVJ1, 2, 3, 24 | 99.73 – 100 | This study, KF992246 |
| IGLV8-12*02 | 99.42 | Foal IGLVJ11, 25 Adult IGLVJ22 |
96.28 – 99.00 91.85 |
This study, KF992247 |
| IGLJ1 | Fetus IGLVJ4, 5, 6, 10 | 100 | Sun et al., 2010, NW_001867428 | |
| IGLJ1*01 | 97.37 | Fetus IGLVJ1, 2, 3, 7, 8, 9 Neonate IGLVJ1, 2, 3, 4, 5, 6, 7 Foal IGLVJ1, 2, 3 Adult IGLVJ1 |
100 100 100 88.68 |
This study, KF992229 and Sun et al., 2010 1-J λ1 |
| IGLJ4*01 | 92.11 | Fetus IGLVJ11, 12, 13, 14, 15, 16, 17 | 100 | This study, KF992231 |
| 1-Jλ4/Jλ7 | 97.37 | Foal IGLVJ4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 Adult IGLVJ2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 20, 23, 24, 25, 30 |
100 85.71 – 100 |
Sun et al., 2010 |
| IGLJ7 | Neonate IGLVJ8, 13, 16, 20, 26, 27, 28, 29 Foal IGLVJ21, 22, 23, 24, 25, 26, 27, 28, 29, 30 Adult IGLVJ14, 15, 16, 17, 18, 19, 21, 22, 26, 27, 28, 29 |
97.14 – 100 97.37 – 100 85.29 – 100 |
Sun et al., 2010, NW_001867428 | |
| IGLJ7*01 | 94.74 | Neonate IGLVJ9, 10, 11, 12, 14, 15, 17, 18, 19, 21, 22, 23, 24, 25, 30 | 100 | This study, KF992233 |
| IGLC1 | Neonate IGLVJ1, 2, 3, 4, 5, 6, 7 Foal IGLVJ1, 2, 3 Adult IGLVJ1 |
100 100 100 |
Sun et al., 2010, NW_001867428 | |
| IGLC1*01 | 98.41 | Fetus IGLVJ1, 2, 3, 4, 5, 6, 7, 8, 9, 10 | 99.65 – 100 | This study, KF992230 |
| IGLC4*01 | 96.45 | Fetus IGLVJ11, 12, 13, 14, 15, 16, 17 | 98.59 – 100 | This study, KF992232 |
| 1-Cλ4 | 94.68 | Foal IGLVJ19, 20 | 100 | Sun et al., 2010 |
| 2-Cλ4-2 | 94.68 | Foal IGLVJ4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Adult IGLVJ2, 3, 4, 5, 6, 7, 8, 9 |
99.65 – 100 100 |
Sun et al., 2010 |
| IGLC5 | Fetus IGLVJ18, 19, 22, 24, 25, 26, 28, 30 | 99.30 – 100 | Sun et al., 2010, NW_001867428 | |
| IGLC5b | 98.44 | Fetus IGLVJ20, 21, 23, 27, 29 | 100 | Hara et al., 2012, JN228107 |
| IGLC7 | Neonate IGLVJ8, 13, 16, 20, 26, 27, 28, 29 Foal IGLVJ26 |
100 100 |
Sun et al., 2010, NW_001867428 | |
| IGLC7*01 | 99.65 | Neonate IGLVJ9, 10, 11, 12, 14, 15, 17, 18, 19, 21, 22, 23, 24, 25, 30 | 100 | This study, KF992234 |
| 1-Cλ7 | 99.65 | Adult IGLVJ10, 11, 12, 13, 15, 20, 23, 24, 25, 29, 30 | 98.94 – 100 | Sun et al., 2010 |
| IGLC6/7a3 | 99.37 | Foal IGLVJ21, 22, 23, 24, 25, 27, 28, 29, 30 Adult IGLVJ14, 16, 17, 18, 19, 21, 22, 26, 27, 28 |
99.65 – 100 100 |
Hara et al., 2012, JN228114 |
Two Ig lambda constant genes were identified from the neonatal expressed Ig lambda sequences: IGLC1 in 7 clones and IGLC7 in the other 23 clones (Figure 1). All 7 neonate IGLVJ1 – IGLVJ7 clone sequences were identical to IGLJ1*01 and reference genome IGLC1 (Table 1). The remaining 23 neonatal clones contained either the reference genome IGLJ7/IGLC7 pair or an allelic pair (IGLJ7*01/IGLC7*01) that was also amplified and sequenced from the donor’s liver genomic DNA (Table 1).
Three IGLC genes were identified among foal expressed Ig lambda clones: IGLC1 reference genome allele in 3 clones (foal IGLVJ1 – IGLVJ3), IGLC4 in 17 clones, and IGLC7 in 10 clones (Figure 1, Table 1). Two IGLC4 alleles were observed in the RACE clones that matched sequences 1-Cλ4 (foal IGLVJ19, 20) and 2-Cλ4–2 (foal IGLVJ4 - 18) described by Sun et al. (2010). The IGLC7 RACE sequences matched the EquCab2.0 reference genome IGLC7 (foal IGLVJ26) or the IGLC6/7a3 allele (foal IGLVJ21 - 25, 27 – 30) reported by Hara et al. (2012).
Similar to the lambda constant gene usage in the foal, the adult clone sequences contained 3 IGLC genes: IGLC1 in 1 clone (reference genome allele, adult IGLVJ1), IGLC4 in 8 clones (2-Cλ4–2 allele, adult IGLVJ2 - 9), and IGLC7 Cλ7 allele (adult IGLVJ10 – 13, 15, 20, 23 – 25, 29, 30) or IGLC6/7a3 allele (adult IGLVJ14, 16 – 19, 21, 22, 26 – 28)].
Of the total 120 expressed Ig lambda clone sequences, most lambda constant gene sequences were 100% identical to the respective germline IGLC gene segment (Supplemental table 2), although up to 4 nucleotide differences were observed between the expressed and germline IGLC sequences over 284 bases.
Although 2 or 3 different IGLC genes were expressed at each age, only IGLC1 was detected at all stages, although it was not the most frequently used gene at any stage (Figure 1). To determine whether a bias in IGLC gene usage was present, the chi-square test was applied and significance was found when all 4 ages were compared (p < 0.0001), as well as for each pair of comparisons (p < 0.02).
3.2 Equine Ig lambda light chain variable gene usage and diversity from germline over developmental stages
Ig lambda variable gene usage annotation revealed that 13 IGLV genes were identified from equine fetal Ig lambda light chain sequences, 14 IGLV from neonatal sequences, 11 from foal sequences, and 9 from adult sequences (Figure 1, Supplemental table 2). Of the 27 functional IGLV genes annotated in the genome, 18 were identified in our dataset. Additionally, the IGLV8-125 gene, originally predicted to be a pseudogene, was identified in functional Ig lambda adult IGLVJ30 sequence (pVλ74); expression of this IGLV gene has been reported in other horses as well (Hara et al., 2012). Five expressed sequences from the fetus (IGLVJ29, 30) and neonate (IGLVJ7, 29, 30) were not assigned a germline IGLV gene but rather designated as novel here because the best matches to the reference genome were <93% identical. Comparison of the 5 novel sequences with germline IGLV genes placed them in IGLV subgroups 4 (neonate IGLVJ7 and 30), 6 (neonate IGLVJ29), and 8 (fetus IGLVJ29 and 30). All other gene assignments exceeded 98% identity in fetal sequences and 96% in neonatal sequences.
Given the prevalence of IGLJ and IGLC germline alleles found above, we hypothesized that IGLV gene allelic variation may also be found between horses frequently. Thus, the most frequently detected IGLV genes were selected for germline donor amplification and sequencing (data not shown). Germline allelic variants were found for IGLV8-122, IGLV8-128, IGLV8-26, IGLV8-24, IGLV8-20, and IGLV8-12, and identity between the new alleles and the EquCab2.0 reference genome ranged from 96.73 to 99.86% (Table 1).
Figure 1 revealed that 9 IGLV genes were identified from only one developmental stage whereas 5 IGLV genes were identified in all stages. Given this distribution, a Chi-square test was performed to assess IGLV gene usage bias with respect to age, and significance was detected upon comparison of all 4 ages (p = 0.04), fetus versus foal (p = 0.02), and fetus versus adult (p < 0.01). Chi-square test significance was not detected for IGLV gene usage for fetus versus neonate, neonate versus foal, or foal versus adult comparisons (p > 0.15). The preferential usage of IGLV8-12 in fetal sequences (8 of 30 clones) compared to the preferential usage of IGLV8-128 in all later stages (≥7 of 30) contributed to these findings (p = 0.001).
Phylogenetic analysis was undertaken to confirm the germline assignments and to enumerate IGLV subgroups identified from expressed sequences, using the framework regions of 120 RACE sequences, 33 reference genome functional and ORF genes, and the IGLV8-125 functional allele. IGLV germline clustering was consistent with that previously determined (Sun et al., 2010) and all IGLV annotations assigned herein were consistent with the clustering observed in the phylogenetic tree (data not shown). Expressed sequences from IGLV subgroups 2, 3, 4, 6, 8, and 11 were detected in the entire dataset, although subgroup 8 accounted for 76% of the IGLV sequences. Sequences from IGLV subgroup 4 and 6 accounted for 9 and 10%, respectively. The remaining 5% of sequences were attributed to subgroup 1 or 2 IGLV genes. Within each age, subgroup 8 IGLV sequences were most prevalent, ranging from 60% of the fetal sequences to 93% of the adult sequences.
3.3 Combinatorial diversity of equine Ig lambda light chain sequences
Gene segment recombination pairs were plotted to appreciate the combinatorial diversity of the IGLV-IGLJ pairs during equine development (Figure 2). In total, 45 different IGLV-IGLJ recombination pairs were observed from 120 sequences. Twenty- eight combination pairs were only observed in one individual and 10 pairs were observed in 2 samples. Seven pairs were observed in samples from throughout development (fetus or neonate and foal and adult stages): IGLV6-101 to IGLJ7, IGLV8- 12 to IGLJ7, IGLV8-24 to IGLJ7, IGLV8-28 to IGLJ7, IGLV8-122 to IGLJ4, IGLV8-128 to IGLJ1, and IGLV8-128 to IGLJ7. Although these pairs were present across horses, most were only detected once in 30 clones. However, recombination pairs IGLV8-24 to IGLJ7 and IGLV8-28 to IGLJ7 were observed in up to 6 clones per stage.
Figure 2. Combinatorial diversity of equine immunoglobulin lambda light chain sequences.

IGLV-IGLJ/C recombination pairs are plotted for fetal (diamond), neonatal (triangle), foal (x), and adult (filled circle) stages. Plot indicates presence of recombination but not frequency.
3.4 Junctional diversity of equine Ig lambda light chain sequences
Pursuant to annotation of the discrete IGLV and IGLJ gene segments, the length of non-templated or palindromic V-J junction nucleotide additions was assessed (Table 2, Supplemental table 2). V-J junction insertions ranged from 0 to 3 nucleotides in fetal Ig lambda light chain sequences and increased significantly to 5 nucleotides in neonatal sequences (p = 0.03). No significant increase was observed in from neonatal junction lengths to foal sequences despite up to 8 nucleotides at the V-J junction (p = 0.08). A significant increase up to 27 nucleotides in junction length was detected between foal and adult sequences (p < 0.0001). Five adult sequences contained V-J junctions greater than 14 nucleotides, and each of these were only observed once.
Table 2.
Nucleotide length of Ig lambda light chain gene rearrangement junctions.
| IGLV-IGLJ junction | |||
|---|---|---|---|
| Median | Range | p-value* | |
| Fetus | 0 | 0–3 | |
| Neonate | 0 | 0–5 | 0.0361 |
| Foal | 1 | 0–8 | 0.0862 |
| Adult | 8 | 0–27 | < 0.0001 |
p-value compared to values with previous age (i.e., neonate vs. fetus; foal vs. neonate; adult vs. foal).
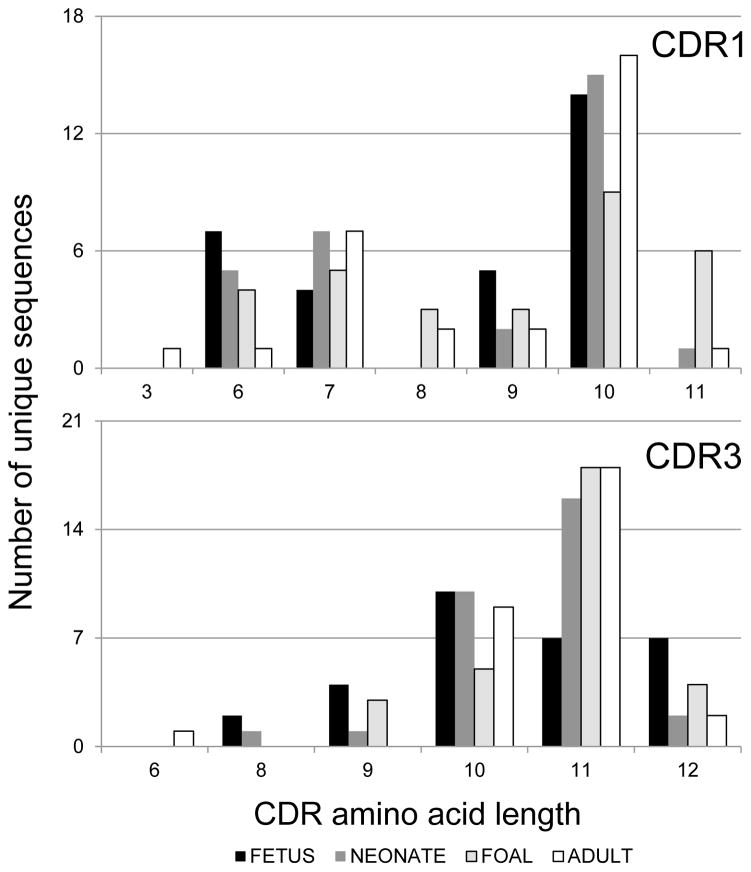
The length of each of the three CDRs was determined from the predicted translation of each equine Ig lambda light chain sequence as designated by the IMGT numbering system (Lefranc et al., 2003). CDR1L and CDR3L lengths varied between sequences within each age, whereas CDR2L had a length of 3 residues in all sequences (Figure 3, 4). CDR1L lengths varied from 6 to 10 residues in fetal sequences, 6 to 11 residues in neonatal and foal sequences, and 3 to 11 residues in adult sequences (Figure 3 top panel), and no statistical difference in CDR1L length was detected over time (p > 0.15). CDR3L lengths varied from 8 to 12 residues in fetal and neonatal sequences, from 9 to 12 residues in foal sequences, and from 6 to 12 residues in adult sequences (Figure 3 bottom panel). No statistical differences were detected for CDR3L amino acid length distribution with respect to age (p > 0.14).
Figure 3. CDR1 and CDR3 length distribution of equine immunoglobulin lambda light chain sequences.
The length of complementarity-determining regions (CDR) 1 and 3 from unique sequences are plotted by age group. Fetal sequences are shown in black, neonatal sequences in dark gray, foal sequences in light gray with black border, and adult horse sequences in white with black border. No statistical differences were detected for CDR length distribution with respect to age.
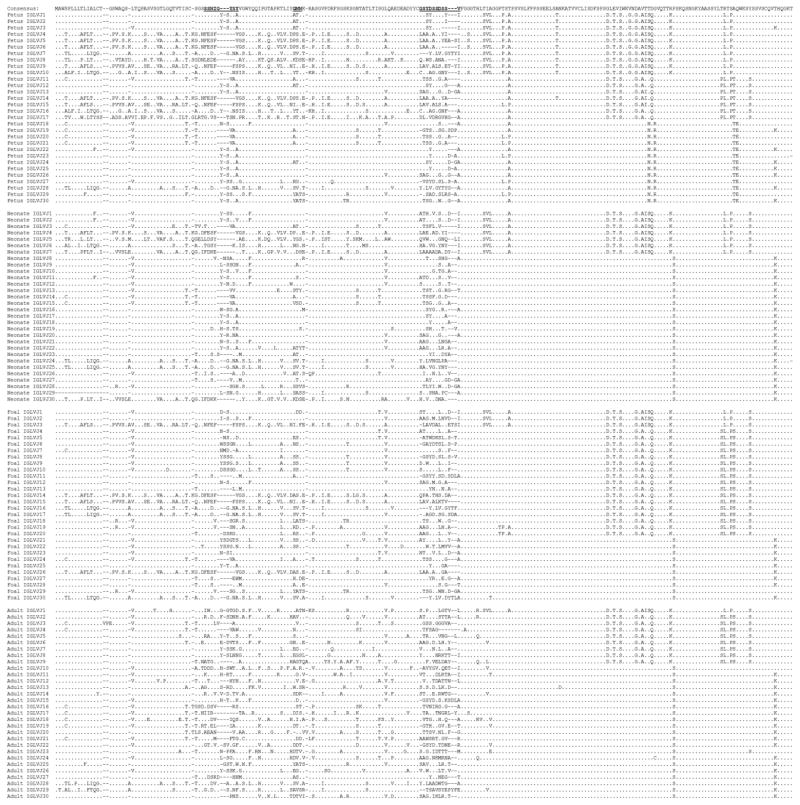
Figure 4. Amino acid alignment of equine immunoglobulin lambda light chain sequences.
Alignment of fetal, neonatal, foal, and adult horse Ig lambda light chain sequences. The 3 complementarity-determining regions are bolded and underlined. Residues identical to the consensus sequence are represented by dots and dashes represent gaps inserted to maximize the alignment.
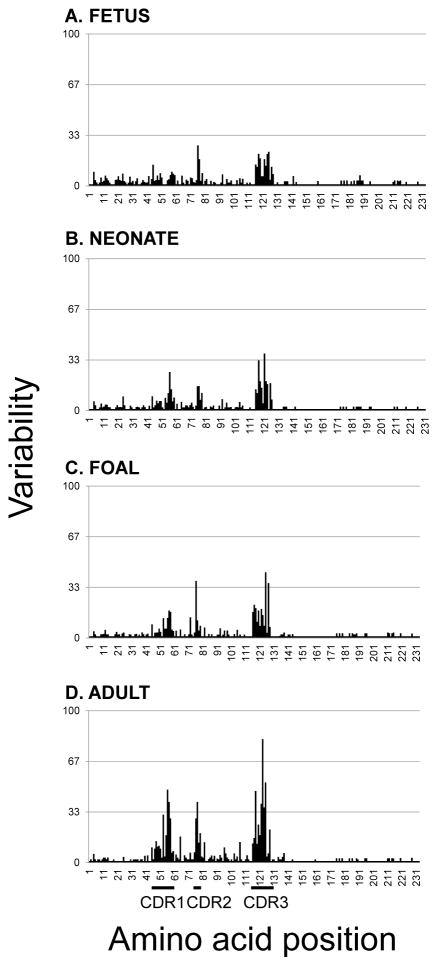
3.5 Variability of equine Ig lambda light chain sequences
Alignment of the predicted amino acid sequences revealed a considerable Ig lambda light chain repertoire in equine fetal life, given the divergent nature of the IGLV genes prior to contributions from somatic hypermutation (Figure 4). Length variations were evident in fetal life throughout the IGLV segment, particularly within the CDR motifs. Adult sequences exhibited polymorphic residues spanning the IGLV gene segment and not solely restricted to the CDRs. The variability index plot of fetal Ig lambda light chain sequences revealed concentrated areas of diversity clustering around CDR2L and CDR3L (Figure 5). CDR1L was first discernable at the neonatal stage and present at similar levels in foal sequences, and the CDR1L variability index more than doubled in adult sequences. Variability in CDR3L was appreciable during equine fetal life and steadily increased with age.
Figure 5. Variability plot of equine immunoglobulin lambda light chain sequences.
Variability plots of (A) fetal, (B) neonatal, (C) foal, and (D) adult horse Ig lambda light chain sequences. The variability index was determined per Wu and Kabat (1970).
4. Discussion
The analysis of Ig lambda light chain RACE clone sequences from equine fetal, neonatal, foal, and adult lymphoid tissue revealed substantial complexity of the Ig lambda light chain locus in terms of 1) abundance of IGLV, IGLJ, and IGLC germline alleles, 2) significant changes in the repertoire of genes used at discrete life stages, 3) presence of junctional diversity starting in mid-gestation fetal life, and 4) increases in variability throughout life.
Germline IGLJ and IGLC alleles have been reported among horses recently (Sun et al., 2010, Hara et al., 2012). Yet, we did not anticipate the abundance of IGLV germline alleles encountered in this study. Given the high level of sequence conservation of expressed IGLJ and IGLC sequences with germline, it is relatively easy to distinguish allelic variations from somatic mutations. However, it is more difficult to do the same for at least 27 germline IGLV genes, many of which share high levels of nucleotide identity and are likely to undergo somatic hypermutation in vivo. Sequencing each of the expressed 19 germline IGLV genes and the 5 novel IGLV genes from the 4 donor horses assayed would have likely identified additional alleles. The IGLV alleles determined in this work shared 96.73 to 99.86% identity with the EquCab2.0 reference genome. IGLJ and IGLC alleles described by others also differed from the reference genome by up to 4% (Sun et al., 2010, Hara et al., 2012). Therefore, we hypothesize that the data presented herein suggests that this germline-encoded diversity contributes another dimension to the potential Ig lambda repertoire at the species level, even before considering differential gene usage, combinatorial gene usage, or junctional diversity.
The most unexpected finding of this work was the large number of IGLV genes used in fetal and pre-suckle neonatal lymphoid tissues (13 and 14, respectively). Fewer IGLV genes were detected in foal and adult lymphoid tissues (11 and 9, respectively), and the complement of genes used also differed with age. At the level of subgroups, genes from 6 IGLV subgroups were detected (IGLV2, 3, 4, 6, 8, and 11), which were more than previously reported for horses (Sun et al., 2010, Hara et al., 2012). Subgroup IGLV8 usage was dominant (76% of the dataset), followed by usage of subgroups IGLV4 and IGLV6; this pattern is consistent with that described in adult horse spleen (Sun et al., 2010). The less prevalent subgroups IGLV2, 3, and 11 were observed in fetal and neonatal stages exclusively. Given that Ig repertoire is initially populated by Ig genes present in the germline and then molded by adaptive responses to pathogens and environmental antigens, these findings should be substantiated with additional horses of various breeds, locations, and management conditions.
Three IGLV genes account for 53% of equine fetal Ig lambda repertoire: IGLV4-66, IGLV8-24, and IGLV8-12. In the neonate, 50% of the Ig lambda repertoire is comprised of IGLV8-128, IGLV8-24, and IGLV8-12. Ig heavy chain variable gene usage is also largely dominated by 3 genes in equine fetal life (IGHV2S2, IGHV2S3, and IGHV2S4), and those genes remain dominant throughout life (Tallmadge et al., 2013). The majority of the Ig repertoire in fetal and neonatal pigs is similarly composed of 3 variable genes for both heavy and lambda light chains, although IGLV gene usage is restricted to a single IGLV subgroup (Wertz et al., 2013).
The IGLV genes detected in expressed equine fetal and neonatal sequences represented genes spanning the length of the genomic Ig lambda locus (IGLV8-137 to IGLV2-41 upstream of the Jλ-Cλ cluster and IGLV11-33 to IGLV8-12 downstream of the Jλ-Cλ cluster), suggesting that the entire lambda locus is accessible during equine fetal life. Thus, our data does not support the positional hypothesis proposed by murine and human studies (Schroeder et al., 1987, Yancopoulos et al., 1984). Ig gene usage in fetal pigs also contradicts the positional hypothesis (Wertz et al., 2013).
The significant increases in IGLV-IGLJ junction nucleotide length were not reflected in significant increases in CDR3L amino acid length, perhaps due to compensatory differences in germline IGLV gene segment lengths or exonuclease activity in adult B lymphocytes. Deletions up to 21 nucleotides at the 3′ end of IGLV and up to 7 nucleotides at the 5′ end of IGLJ have been reported in adult equine IGLV sequences, which could account for constant CDR3L amino acid sequences despite longer IGLV-IGLJ nucleotide junctions (Sun et al., 2010). In adult horse spleen, the addition of N and P nucleotides at IGLV-IGLJ junction generates more diversity than the Ig kappa light chain (Sun et al., 2010). The diversity of the Ig kappa light chain has not yet been examined in equine fetal tissues, but we hypothesize that it would be less than the Ig lambda diversity described herein. The same pattern of increased number of non- templated insertions at the IGLV-IGLJ junction without a corresponding increase in CDR3L length has been observed in human neonatal B lymphocytes (Richl et al., 2008).
In sum, these analyses unveil significant contributions of the Ig lambda light chain to the total Ig repertoire that are most striking in equine fetal life, but also substantial in adulthood. The mechanisms underlying these contributions include the presence of germline alleles with up to 4% nucleotide diversity, significant changes in Ig lambda gene usage with respect to age, combinatorial diversity, additions of N and/or P nucleotides at IGLV-IGLJ junctions throughout life, and amino acid variability that increases with age, due in part to somatic hypermutation.
Supplementary Material
Highlights.
Biased usage of Ig lambda variable and constant genes was found in fetal life
Ig lambda sequence diversity increased significantly during fetal and post-natal life
No statistical differences in CDR length were detected with respect to age
Acknowledgments
The authors thank Steven C. Miller for excellent technical assistance. This study was partially supported by the Harry M. Zweig Memorial Fund for Equine Research and National Institutes of Health New Director’s Innovator Award DP2OD007216.
Abbreviations
- CDR
complementarity-determining region
- Ig
immunoglobulin
- IGLV
immunoglobulin lambda variable gene
- IGLJ
immunoglobulin lambda joining gene
- IGLC
immunoglobulin lambda constant chain gene
- ORF
open reading frame
- MLN
mesenteric lymph node
- RACE
rapid amplification of cDNA ends
- VDJ
immunoglobulin heavy chain variable, diversity, and joining regions
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almagro JC, Hernandez I, Ramirez MC, Vargas-Madrazo E. Structural differences between the repertoires of mouse and human germline genes and their evolutionary implications. Immunogenetics. 1998;47:355–363. doi: 10.1007/s002510050370. [DOI] [PubMed] [Google Scholar]
- Alt F, Rosenberg N, Lewis S, Thomas E, Baltimore D. Organization and reorganization of immunoglobulin genes in A-MULV-transformed cells rearrangement of heavy but not light chain genes. Cell. 1981;27:381–390. doi: 10.1016/0092-8674(81)90421-9. [DOI] [PubMed] [Google Scholar]
- Arun SS, Breuer W, Hermanns W. Immunohistochemical examination of light-chain expression (lambda/kappa ratio) in canine, feline, equine, bovine and porcine plasma cells. Zentralbl Veterinarmed A. 1996;43:573–576. doi: 10.1111/j.1439-0442.1996.tb00489.x. [DOI] [PubMed] [Google Scholar]
- Butler JE, Weber P, Sinkora M, Sun J, Ford SJ, Christenson RK. Antibody repertoire development in fetal and neonatal piglets. II. Characterization of heavy chain complementarity-determining region 3 diversity in the developing fetus. J Immunol. 2000;165:6999–7010. doi: 10.4049/jimmunol.165.12.6999. [DOI] [PubMed] [Google Scholar]
- De Genst E, Saerens D, Muyldermans S, Conrath K. Antibody repertoire development in camelids. Dev Comp Immunol. 2006;30:187–198. doi: 10.1016/j.dci.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Dooley H, Flajnik MF. Antibody repertoire development in cartilaginous fish. Dev Comp Immunol. 2006;30:43–56. doi: 10.1016/j.dci.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Flaminio MJ, Tallmadge RL, Salles-Gomes CO, Matychak MB. Common Variable Immunodeficiency in Horses is Characterized by B Cell Depletion in Primary and Secondary Lymphoid Tissues. J Clin Immunol. 2009;29:107–116. doi: 10.1007/s10875-008-9221-4. [DOI] [PubMed] [Google Scholar]
- Gibson D. Structural studies on normal horse immunoglobulin light chains. Detection of k-type N-terminal sequences. Biochemistry. 1974;13:2776–2785. doi: 10.1021/bi00710a018. [DOI] [PubMed] [Google Scholar]
- Hara S, Diesterbeck US, Konig S, Czerny CP. Transcriptional analysis of equine lambda-light chains in the horse breeds Rhenish-German Coldblood and Hanoverian Warmblood. Vet Immunol Immunopathol. 2012;145:50–65. doi: 10.1016/j.vetimm.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Haughton G, Lanier LL, Babcock GF, Lynes MA. Antigen-induced murine B cell lymphomas. II. Exploitation of the surface idiotype as tumor specific antigen. J Immunol. 1978;121:2358–2362. [PubMed] [Google Scholar]
- Hieter PA, Korsmeyer SJ, Waldmann TA, Leder P. Human immunoglobulin kappa light-chain genes are deleted or rearranged in lambda-producing B cells. Nature. 1981;290:368–372. doi: 10.1038/290368a0. [DOI] [PubMed] [Google Scholar]
- Home WA, Ford JE, Gibson DM. L chain isotype regulation in horse. I. Characterization of Ig lambda genes. J Immunol. 1992;149:3927–3936. [PubMed] [Google Scholar]
- Hood L, Gray WR, Sanders BG, Dreyer WJ. Light chain evolution. Cold Spring Harbor Symposia on Quantitative Biology. 1967;32:133–146. [Google Scholar]
- Kabat EA, Wu TT. Identical V region amino acid sequences and segments of sequences in antibodies of different specificities. Relative contributions of VH and VL genes, minigenes, and complementarity-determining regions to binding of antibody-combining sites. J Immunol. 1991;147:1709–1719. [PubMed] [Google Scholar]
- Kessler S, Kim KJ, Scher I. Surface membrane kappa and lambda light chain expression on spleen cells of neonatal and maturing normal and immune-defective CBA/NB mice the kappalambda ratio is constant. J Immunol. 1981;127:1674–1678. [PubMed] [Google Scholar]
- Kirkham PM, Mortari F, Newton JA, Schroeder HW., Jr Immunoglobulin VH clan and family identity predicts variable domain structure and may influence antigen binding. EMBO J. 1992;11:603–609. doi: 10.1002/j.1460-2075.1992.tb05092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott J, Bona C, Kaushik A. The primary antibody repertoire of kappa-deficient mice is characterized by non-stochastic Vlamda1 + V(H) gene family pairings and a higher degree of self-reactivity. Scand J Immunol. 1998;48:65–72. doi: 10.1046/j.1365-3083.1998.00369.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Monson NL, Lipsky PE. The V lambda J lambda repertoire in human fetal spleen evidence for positive selection and extensive receptor editing. J Immunol. 2000;165:6322–6333. doi: 10.4049/jimmunol.165.11.6322. [DOI] [PubMed] [Google Scholar]
- Lefranc MP. Nomenclature of the human immunoglobulin lambda (IGL) genes. Exp Clin Immunogenet. 2001;18:242–254. doi: 10.1159/000049203. [DOI] [PubMed] [Google Scholar]
- Lefranc MP, Pommie C, Ruiz M, Giudicelli V, Foulquier E, Truong L, Thouvenin-Contet V, Lefranc G. IMGT unique numbering for immunoglobulin and T cell receptor variable domains and Ig superfamily V-like domains. Dev Comp Immunol. 2003;27:55–77. doi: 10.1016/s0145-305x(02)00039-3. [DOI] [PubMed] [Google Scholar]
- Li YS, Hayakawa K, Hardy RR. The regulated expression of B lineage associated genes during B cell differentiation in bone marrow and fetal liver. J Exp Med. 1993;178:951–960. doi: 10.1084/jem.178.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magor KE, Higgins DA, Middleton DL, Warr GW. cDNA sequence and organization of the immunoglobulin light chain gene of the duck, Anas platyrhynchos. Dev Comp Immunol. 1994;18:523–531. doi: 10.1016/s0145-305x(06)80006-6. [DOI] [PubMed] [Google Scholar]
- Near RI, Ng SC, Mudgett-Hunter M, Hudson NW, Margolies MN, Seidman JG, Haber E, Jacobson MA. Heavy and light chain contributions to antigen binding in an anti-digoxin chain recombinant antibody produced by transfection of cloned anti-digoxin antibody genes. Mol Immunol. 1990;27:901–909. doi: 10.1016/0161-5890(90)90157-u. [DOI] [PubMed] [Google Scholar]
- Park MA, Li JT, Hagan JB, Maddox DE, Abraham RS. Common variable immunodeficiency a new look at an old disease. Lancet. 2008;372:489–502. doi: 10.1016/S0140-6736(08)61199-X. [DOI] [PubMed] [Google Scholar]
- Ramsden DA, Wu GE. Mouse kappa light-chain recombination signal sequences mediate recombination more frequently than do those of lambda light chain. Proc Natl Acad Sci U S A. 1991;88:10721–10725. doi: 10.1073/pnas.88.23.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud CA, Anquez V, Dahan A, Weill JC. A single rearrangement event generates most of the chicken immunoglobulin light chain diversity. Cell. 1985;40:283–291. doi: 10.1016/0092-8674(85)90142-4. [DOI] [PubMed] [Google Scholar]
- Richl P, Stern U, Lipsky PE, Girschick HJ. The lambda gene immunoglobulin repertoire of human neonatal B cells. Mol Immunol. 2008;45(2):320–327. doi: 10.1016/j.molimm.2007.06.155. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–25. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Schroeder HW, Jr, Wang JY. Preferential utilization of conserved immunoglobulin heavy chain variable gene segments during human fetal life. Proc Natl Acad Sci U S A. 1990;87:6146–6150. doi: 10.1073/pnas.87.16.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder HW, Jr, Hillson JL, Perlmutter RM. Early restriction of the human antibody repertoire. Science. 1987;238:791–793. doi: 10.1126/science.3118465. [DOI] [PubMed] [Google Scholar]
- Shiokawa S, Mortari F, Lima JO, Nunez C, Bertrand FE, 3rd , Kirkham PM, Zhu S, Dasanayake AP, Schroeder HW., Jr IgM heavy chain complementarity-determining region 3 diversity is constrained by genetic and somatic mechanisms until two months after birth. J Immunol. 1999;162:6060–6070. [PubMed] [Google Scholar]
- Sun J, Hayward C, Shinde R, Christenson R, Ford SP, Butler JE. Antibody repertoire development in fetal and neonatal piglets. I. Four VH genes account for 80 percent of VH usage during 84 days of fetal life. J Immunol. 1998;161:5070–5078. [PubMed] [Google Scholar]
- Sun X, Wertz N, Lager K, Sinkora M, Stepanova K, Tobin G, Butler JE. Antibody repertoire development in fetal and neonatal piglets. XXII. lambda Rearrangement precedes kappa rearrangement during B-cell lymphogenesis in swine. Immunology. 2012;137:149–159. doi: 10.1111/j.1365-2567.2012.03615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Wang C, Wang Y, Zhang T, Ren L, Hu X, Zhang R, Meng Q, Guo Y, Fei J, Li N, Zhao Y. A comprehensive analysis of germline and expressed immunoglobulin repertoire in the horse. Dev Comp Immunol. 2010;34:1009–1020. doi: 10.1016/j.dci.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Tallmadge RL, McLaughlin K, Secor E, Ruano D, Matychak MB, Flaminio MJ. Expression of essential B cell genes and immunoglobulin isotypes suggests active development and gene recombination during equine gestation. Dev Comp Immunol. 2009;33:1027–1038. doi: 10.1016/j.dci.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Tallmadge RL, Tseng CT, King RA, Felippe MJ. Developmental progression of equine immunoglobulin heavy chain variable region diversity. Dev Comp Immunol. 2013;41:33–43. doi: 10.1016/j.dci.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5 molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz N, Vazquez J, Wells K, Sun J, Butler JE. Antibody repertoire development in fetal and neonatal piglets. XII. Three IGLV genes comprise 70% of the pre-immune repertoire and there is little junctional diversity. Mol Immunol. 2013;55:319–328. doi: 10.1016/j.molimm.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Lefranc MP WHO-IUIS Nomenclature Subcommittee for immunoglobulins Tcell receptors . WHO-IUIS Nomenclature Subcommittee for immunoglobulins and T cell receptors report. Dev Comp Immunol; August 2007; 13th International Congress of Immunology; Rio de Janeiro, Brazil. 2008. pp. 461–463. [DOI] [PubMed] [Google Scholar]
- Yancopoulos GD, Desiderio SV, Paskind M, Kearney JF, Baltimore D, Alt FW. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984;311:727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.