Abstract
Converging results link histone acetylation dynamics to hippocampus-dependent memory, including evidence that histone deacetylase inhibitor (HDACi) administration enhances long-term memory. Previously we demonstrated that aging disrupts the coordinated epigenetic response to recent experience observed in the young adult hippocampus. Here we extended that work to test the cognitive effects of a novel, brain-penetrant HDACi (EVX001688; EVX) that we confirmed yields robust, relatively long lasting dose-dependent increases in histone acetylation in the hippocampus. In young rats, acute systemic EVX administration, scheduled to yield elevated histone acetylation levels during training in a contextual fear conditioning (CFC) task, had no effect on memory retention at 24 hours at any dose examined (10, 30, or 60 mg/kg). Pretraining injection of another HDACi, sodium butyrate, also failed to affect fear memory, and CFC training itself had no influence on hippocampal histone acetylation at 1 hour in mice or two strains of rats. EVX administration before water maze training in young rats yielded a modest effect such that the middle dose produced marginally better 24-hour retention than either the low or high dose, but only a small numerical benefit relative to vehicle. Guided by those findings, a final experiment tested the influence of pretraining EVX treatment on age-related spatial memory impairment. The results, revealing no effect on performance, are consistent with the idea that effective procognitive HDACi treatments in aging may require intervention aimed at restoring coordinated epigenetic regulation rather than bulk increases in hippocampal histone acetylation.
Introduction
Considerable research has focused on the role of chromatin regulation in learning and memory. Post-translational modifications of histones, most prominently acetylation, have been implicated in the molecular processes essential for learning and memory (Peixoto and Abel, 2013). A current perspective is that to-be-remembered events induce histone acetylation, leading to chromatin modifications permissive for the transcription of genes that mediate learning and memory related plasticity (Fischer et al., 2007; Miller et al., 2008; Levenson et al., 2004). Global, as well as gene-specific increases in H3 and H4 acetylation have been reported in response to training in many hippocampus-dependent tasks, including contextual fear conditioning (Vecsey et al., 2007; Levenson et al., 2004; Peleg et al., 2010) and object-in-place recognition memory procedures (Oliveira et al., 2007; Haettig et al., 2011). Histone acetylation is controlled by histone acetyltransferases (HATs) and histone deacetylases (HDACs), and pharmacological manipulation of these enzymes can affect the duration and magnitude of histone acetylation, with corresponding effects on learning and memory. For example, HDACi’s increase histone acetylation and reportedly improve long-term memory when delivered prior to learning (Levenson et al., 2004; Lattal et al., 2007; Fischer et al., 2007; Kilgore et al., 2010; Peleg et al., 2010). Evidence of this sort, however, has come predominantly from research using commercially available HDACi compounds where limited data are available concerning brain penetrance, in vivo histone selectivity and pharmacokinetics. Here we took advantage of a potent, and highly brain-penetrant HDACi, EVX001688 (EVX) as an independent experimental tool for evaluating the effects of manipulating hippocampal histone acetylation on memory.
Beyond documenting the cognitive effects of HDAC inhibition in young intact subjects, the accumulated evidence suggests that histone acetylation might comprise a tractable target for treating cognitive impairment associated with aging and neurodegenerative disease (Kilgore et al., 2010; Peleg et al., 2010; Gräff and Tsai, 2013; Stilling and Fischer, 2011; Fischer et al., 2007; Kazantsev and Thompson, 2008). We tested this proposal in the current experiments using a well-characterized rat model in which a substantial subpopulation of aged subjects exhibits spatial memory deficits indicative of hippocampal dysfunction (Gallagher et al., 1993). Memory impairment in this model is linked to disrupted epigenetic regulation in the hippocampus (Castellano et al., 2012), providing a particularly appropriate setting for evaluating the potential cognitive benefit of HDACi intervention.
Materials and Methods
Histone Deacetylase Enzyme Inhibition Assays
All enzymes and assay reagents were from BPS Bioscience. Enzyme assays for human recombinant HDAC enzymes 1–11 (Catalogue numbers 50001 to 50011) were incubated at 37°C in a 50-μl mixture of HDAC assay buffer (#50031), 5 μg bovine serum albumin, and HDAC substrate (#50037 or #50040) for either 30 min (HDAC 1–10) or 60 min (HDAC 11). HDAC class IIa substrate was used at 500 nM for HDAC 4, 5, 7, and 9 enzymes; and substrate 3 at 10 μM was used for the remaining enzymes. HDAC assay developer (50 μl of double strength, #50030) was added to each well and the plate was incubated at room temperature for 20 min. Fluorescence intensity was measured at an excitation of 360 nm and an emission of 460 nm using a BioTek Synergy™ 2 microplate reader.
HDAC activity assays were performed in duplicate at each concentration to generate a six-point inhibitor concentration-response-curve. The fluorescent intensity data were analyzed using GraphPad Prism software. In the absence of EVX, the fluorescent intensity (Ft) was defined as 100% activity. In the absence of HDAC, the fluorescent intensity (Fb) was defined as 0% activity. Percent activity in the presence of EVX was calculated according to the following equation: % activity = (F−Fb)/(Ft−Fb), where F= the fluorescent intensity in the presence of EVX. The values of % activity versus EVX concentration were then plotted using non-linear regression analysis of sigmoidal dose-response curve and the IC50 value was determined.
Subjects
A total of 138 Specific Pathogen-Free male Long-Evans (4–24 months; Charles River Laboratories), 19 male Sprague-Dawley rats (1 month; Harlan Laboratories) and 19 male C57BL/6 mice (9 months; Jackson Laboratories) were used. Rats were singly housed and mice were caged in groups of 5 in separate climate-controlled colonies on a 12:12 hr light:dark cycle. Animals with frank pathologies that might influence the principal outcome measures were excluded. Standard lab chow and water were available ad libitum. All subjects received extensive handling (5 min/day) during the week prior to behavioral testing. Live animal procedures were reviewed and approved by the Animal Care and Use Committee of the Intramural Research Program of the National Institute on Aging, Baltimore, MD.
Experimental Design
Dosing and behavioral testing parameters (i.e., time of day, age of animal, compound preparation) were controlled to allow comparisons within individual experiments (Table 1).
Table 1.
| Experiment | Strain of Animals (age) |
|---|---|
| 1. Effects of EVX on histone acetylation and locomotor activity | Long-Evans (4 mos) |
| 2. Effects of EVX on LTM for CFC | Long-Evans (5 mos) |
| 3. Effects of NaB on LTM for CFC | Long-Evans (5 mos) |
| 4. Effects of CFC training on histone acetylation | Long-Evans (6 mos) Sprague-Dawley (1 mos) C57Bl/6 (9 mos) |
| 5. Effects of EVX on LTM in WM task in young rats | Long-Evans (6 mos) |
| 6. Effects of EVX on LTM in WM task in aged rats | Long-Evans (24 mos) |
EVX, EVX001688; CFC, contextual fear conditioning; LTM, long term memory; NaB, sodium butyrate; WM, water maze
HDACi Administration
EVX solution was freshly prepared just prior to administration by dissolving the compound in 60% Solutol (BASF) and 40% ethanol, subsequently diluted 1:1 with water, yielding a 10, 30 or 60 mg/ml solution, depending on dosage. Vehicle injections comprised equivalent volumes of Solutol/ethanol/water. Sodium butyrate (Sigma) was freshly prepared in sterile saline to create a 1 g/ml solution. Compounds were administered 80 min before open field testing, and 90 min before CFC and water maze training.
Open Field
Animals were placed in a circular arena (diameter = 2 m) in a novel environment 80 min after intraperitoneal (i.p.) injection of 10, 30, or 60 mg/kg doses of EVX. Subjects were allowed to freely locomote for 10 min and distance traveled as well as percent time immobile were quantified from digitized video recordings using ANY-maze software (Stoelting Co., Wood Dale, IL).
Contextual Fear Conditioning
On the basis of pilot data and published results (Peleg et al., 2010; Levenson et al., 2004), CFC protocols were configured for each animal model in order to yield reliable retention without ceiling effects, providing sufficient parametric space to detect improvement following HDACi treatment. Specifically, training in Long-Evans rats consisted of a single 4-min exposure to the conditioning context, including 0.5 mA (1 sec duration) shocks at minutes 2 and 3. Training in Sprague-Dawley rats consisted of a single 7-min exposure to the conditioning context with 0.5 mA (1 sec duration) shocks at minutes 2, 4, and 6. Training in C57Bl/6 mice consisted of a single 3.5-min exposure to the conditioning context with a single 1 mA shock (2 sec duration) at minute 3. In experiments examining the effects of HDACi administration on long-term memory (Experiments 2 and 3, Table 1), retention was tested 24 hr later; animals were reintroduced to the conditioning context for the same duration as initial training and freezing behavior was measured using automated video tracking hardware (Freeze Frame, Med Associates, Inc., St. Albans, VT). No shock was delivered during test trials. In order to examine the effects of CFC training on hippocampal histone acetylation (Experiment 4), memory was tested at 1 hr post-training, just before sacrifice. Freezing behavior was defined as lack of movement for at least 0.75 sec, and percent freezing for both the training and test trials was calculated relative to the pre-shock interval from training trials (2 min for rats, and 3 min for mice).
Water Maze Assessments
Standard Background Training
Rats subsequently used to test the effects of EVX on spatial learning and memory (Experiments 5 and 6) were pretrained in a standard place version of the Morris water maze (Gallagher et al., 1993). Training was conducted across 8 consecutive days and each day included three 90 sec trials with a 60 sec inter-trial interval. A probe trial, in which the platform was retracted to the bottom of the maze for 30 sec and then raised to allow the animal to escape, was provided every sixth trial (i.e., the last trial every other day). Performance was assessed using learning index scores, calculated as a weighted average proximity to the escape platform during probe trials (Gallagher et al., 1993). Animals matched according to this index were assigned to treatment groups for subsequent testing.
Redundant Place-Cue Training
Approximately two weeks after standard background training, rats were tested on a redundant place-cue task (RPC) in a new spatial environment (Castellano et al., 2012). Training consisted of 15 trials with an escape platform held in a constant location (15 sec intertrial interval), and a probe trial 24 hr after the first training trial. The escape location was cued by a black platform extending 3 cm above the water surface on trials 1–9, 11, 13, and 15. On trials 10, 12, and 14, the cued platform was removed but the hidden base platform just beneath the surface of the water was still accessible. Subjects that failed to escape within a 60 sec cut-off were guided to the platform. Performance during training trials was measured by path length (the total distance swum from start location to platform). During the probe trial 24 hr later, the platform was removed from the maze and rats swam for 60 sec. Probe trial performance was measured by proximity to escape platform location.
Immunoblotting
Animals were sacrificed and hippocampal subregions were microdissected on ice using a dissecting microscope. Samples were stored at −80°C. Hippocampal subregions were homogenized in a hypotonic buffer (10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, and 2X Protease Inhibitor; Pierce). After 30 min incubation on ice, samples were centrifuged at 1,000g at 4°C for 15 min. The supernatant (cytosolic fraction) was reserved and the nuclear pellet was washed with hypotonic buffer and subsequently centrifuged at 1,000g at 4°C for 15 min. The nuclear pellet was resuspended in 5% SDS and stored at −80°C. Samples were normalized for total protein concentration using a Protein Assay kit (Pierce) and separated by SDS-PAGE (NuPage 3–8% Tris-Acetate; Invitrogen). Gels were transferred to nitrocellulose membranes using the iBlot system (Life Technologies). The primary antibodies were anti-acetyl H3 (1:2500; Millipore 06-599), anti-acetyl H4 (1:2500; Millipore 06-866), anti-H3 phospho S10 (1:1000; Abcam ab 5176), anti-acetyl-α-tubulin (1:500; Abcam ab24610) and anti-β-actin (1:1000; Biovision 3662-100). The histone acetylation antibodies used here recognize a number of acetylated lysine sites, including K9 and K14 on H3, and K5, K8, K12, and K16 on H4. Accordingly, our approach surveyed multiple modifications implicated in the regulation of learning and memory. β-actin was used as a protein loading control. Direct comparisons revealed no statistically significant differences in levels of β-actin across experimental conditions (all p-values > .05). Immunoreactivity was detected with Alexa 488 and Alexa 633 conjugated secondary antibodies (1:2500; Invitrogen). All groups and conditions were represented within each blot for each hippocampal subfield. Immunoblots were scanned at a resolution of 100 μm/pixel on a Typhoon Trio Plus Scanner (GE Healthcare) and bands were analyzed using ImageQuant TL image analysis software (GE Healthcare). Competition assays using excess peptide completely eliminated signal at the appropriate molecular weight for all antibodies.
Statistical Analysis
Parametric statistics (analysis of variance, ANOVA) were used to compare relevant behavioral outcome measures and histone acetylation levels across dosage and age. Least Significant Difference (LSD) post-hoc tests were used to compare outcomes between treatment groups.
Results
EVX is brain-penetrant and increases hippocampal histone acetylation in a dose-dependent manner
Here we used EVX as an independent experimental tool to test the effects of manipulating hippocampal histone acetylation on memory. HDAC enzyme inhibition assays showed that EVX inhibited class I (HDAC 1, 2, 3, and 8), class IIb (HDAC 6 and 10), and class IV (HDAC 11) enzymes at IC50 values ranging from 100–400 nM. Inhibition of class IIa (HDAC 4, 5, 7, and 9) enzymes occurred at IC50 values greater than 2 μM, consistent with EVX being a hydroxamic acid type HDACi (Table 2). EVX was found to be >1000 fold more potent against HDAC class 1 enzymes than sodium butyrate, suggesting good target selectivity. Furthermore, because hydroxamic acids bind in the Zn2+-containing reactive site of HDAC enzymes, we tested a set of four matrix metalloproteinases (MMPs), which also are Zn2+-dependent enzymes, for inhibition by EVX. Minimal inhibition at an EVX concentration of 20 μM was observed compared to the pan-MMP inhibitor actinonin (data not shown), again suggesting good target selectivity.
Table 2.
In vitro IC50 [μM] values of EVX against recombinant human HDAC 1–11 enzymes.
| HD1 | HD2 | HD3 | HD4 | HD5 | HD6 | HD7 | HD8 | HD9 | HD10 | HD11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EVX | 0.1 | 0.1 | 0.1 | >2 | >2 | 0.1 | >2 | 0.3 | >2 | 0.4 | 0.3 |
| NaB | 330 | 118 | 126 | NI | NI | NI | NI | 611 | NI | 543 | 565 |
NI, no inhibition up to 10 mM tested; HD, HDAC; NaB, sodium butyrate.
Pharmacodynamic studies documented a peak brain acetylation response between one and two hours after i.p. EVX injection in Long-Evans rats, and brain histone acetylation closely paralleled total drug levels in brain and plasma (data not shown). Based on this background, the experiments conducted here were arranged such that behavioral training and tissue collection for molecular analysis roughly overlapped with the peak histone acetylation response, at 90 minutes following injection (i.p.).
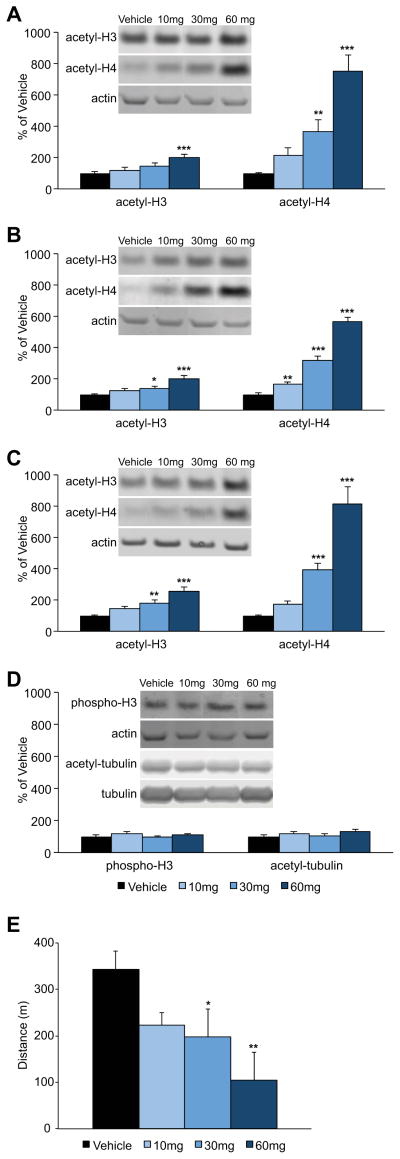
Before testing the potential memory effects of EVX, we first confirmed the influence of treatment on hippocampal histone acetylation in Long-Evans rats, using antibodies directed against multiple modification sites implicated in the epigenetic regulation of learning and memory. At 90 min post-injection, histone H3 and H4 acetylation levels increased in a dose-dependent manner in all subfields of the hippocampus (CA1: acetyl-H3 main effect of dose F(3,20)=5.982, p<.005; acetyl-H4, F(3,21)=25.568, p<.001; CA3: acetyl-H3, F(3,20)=13.084, p<.001; acetyl-H4, F(3,20)=167.263, p<.001; denate gyrus (DG): acetyl-H3, F(3,21)=16.833, p<.001; acetyl-H4, F(3,20)=50.998, p<.001). Pair-wise post-hoc comparisons documented that the 30 and 60 mg/kg doses significantly increased H3 and H4 acetylation relative to vehicle (all p-values, < .05, except CA1 acetyl-H3 vehicle versus 30 mg/kg) (Fig. 1A – C), and in many cases, differences in the magnitude of response between the low, middle and high dose groups were also statistically reliable. Importantly, the effects of EVX appeared relatively selective for histone acetylation since no overall treatment effect was detected in CA1 for either serine 10 phosphorylation on histone H3 (F(3,20)=1.157, p=.351), or α-tubulin acetylation (F(3,21)=1.788, p=.180; Fig. 1D). Open field activity, assessed during the 10 min just prior to sacrifice in the same animals, revealed a marked, dose-dependent decrement in exploration (main effect of dose F(3,16)=6.024, p=.006; pair-wise group contrasts compared to vehicle, all p-values < 0.05 except 10 mg dose, p = 0.057; Fig. 1E) consistent with the reported locomotor effects of other HDACi’s (Gundersen and Blendy, 2009).
Figure 1. EVX increases hippocampal histone acetylation in a dose-dependent manner.
Mean histone H3 and H4 acetylation normalized to β-Actin (±S.E.M.) in CA1 (A), CA3 (B) and dentate gyrus (C) 90 min after vehicle (n=8), 10 mg/kg (n=6), 30 mg/kg (n=6) or 60 mg/kg (n=5) EVX injection (i.p.). (D) Mean histone H3 serine 10 phosphorylation and α-tubulin acetylation in CA1, normalized to actin and tubulin, respectively. Representative western blots for individual young adult Long-Evans rats shown for all conditions. (E) Mean distance traveled (±S.E.M.) in an open field apparatus 80 min after vehicle (n=8), 10 mg/kg (n=4), 30 mg/kg (n=4) or 60 mg/kg (n=4) EVX injection (i.p.). *p<.05, **p<.01, and ***p<.001 post-hoc comparison with vehicle.
Pretraining EVX administration fails to affect long-term memory for contextual fear conditioning in young adult Long-Evans rats
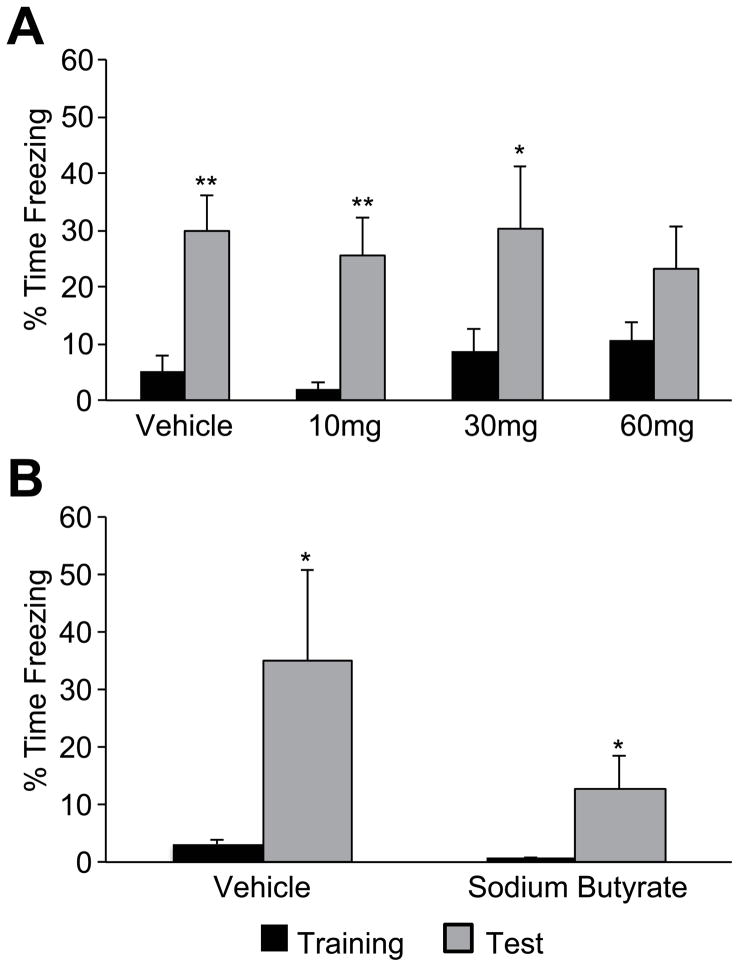
Guided by reports that non-selective compounds with HDACi activity can enhance long-term memory for contextual fear conditioning (CFC) (Bredy and Barad, 2008; Fischer et al., 2007; Kilgore et al., 2010; Vecsey et al., 2007; Levenson et al., 2004), here we tested the effects of systemic EVX, scheduling drug administration specifically to ensure that hippocampal histone acetylation was increased during CFC training, at 90 min post-injection. Despite the marked blunting of open field activity noted earlier (Fig. 1E), there was no difference in pre-shock freezing during training at any dose examined (main effect of dose F(3,28)=1.881, p=.156), despite an apparent numerical trend (Fig. 2A). Adopting the same design as previous experiments reporting improvements with pretraining HDACi administration (Levenson et al., 2004; Fischer et al., 2007; Peleg et al., 2010), memory for CFC was measured 24 hr after conditioning. With the exception of the 60 mg/kg dose, where baseline freezing was numerically elevated, all groups exhibited robust retention, displaying significantly increased freezing relative to pre-shock training levels (Fig. 2A) (Vehicle: F(1,7)= 20.491, p<.005; 10 mg/kg: F(1,7)=15.212, p<.01; 30 mg/kg: F(1,7)=6.280, p<.05; 60 mg/kg: F(1,7)=2.738, p=.146). The amount of freezing during the retention test, however, was equivalent across groups, indicating that EVX treatment failed to have any detectable benefit on long-term contextual fear memory (main effect of dose F(3,28)= .213, p=.886, Fig. 2A). As intended, the magnitude of the conditioned freezing response was substantially below maximum in all groups, discounting the possibility that ceiling effects masked enhanced retention induced by EVX.
Figure 2. Acute pretraining HDACi administration fails to affect contextual fear conditioning retention in young adult Long-Evans rats.
(A) Mean percent freezing (±S.E.M.) during training and 24 hr retention (Test) in animals injected (i.p.) with vehicle (n=8), 10 mg/kg (n=8), 30 mg/kg (n=8) or 60 mg/kg (n=8) EVX 90 min before CFC training. (B) Mean percent freezing (±S.E.M.) during training and the retention test after acute pretraining sodium butyrate (n=7) or vehicle (n=7) administration (1.2 g/kg, i.p.). *p<.05 and **p<.01, within group comparisons between training and test trials.
Pretraining sodium butyrate administration fails to affect long-term memory for contextual fear conditioning in young Long-Evans rats
The lack of an EVX effect on memory for CFC was unexpected, and next we sought to confirm the benefit reported earlier in rats and mice with a more commonly tested HDACi (Levenson et al., 2004; Peleg et al., 2010). Using the same design, here outbred Long-Evans rats received a 1.2 g/kg dose (i.p.) of sodium butyrate 1 hr prior to CFC training. The vehicle and drug groups displayed equivalent, low levels of freezing prior to shock, and significantly increased immobility when memory for the training context was assessed at 24 hr (Fig. 2B, main effect of trial: F(1,12)=7.456, p<.05; group by trial interaction: F(1,12)=1.495, p=.245). Sodium butyrate administration clearly failed to enhance retention, however, and instead there was a marked numerical trend toward worse memory in the treated group relative to controls (mean freezing vehicle=38.83 sec versus sodium butyrate=12.73 sec, F(1,12)=2.066, p=.176).
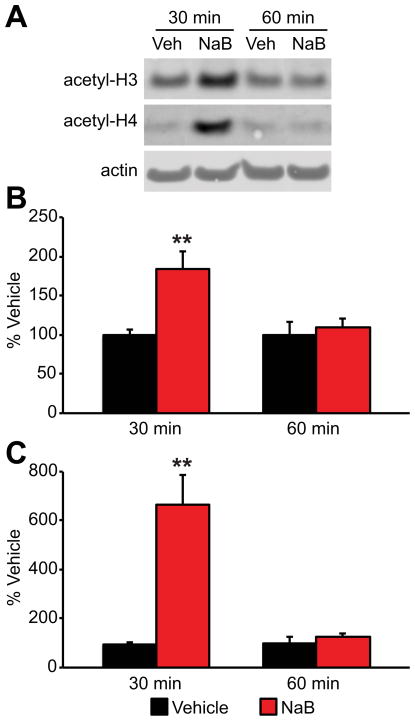
Although sodium butyrate is known to inhibit HDAC’s in cell culture preparations (Zhou et al., 2011; Biermann et al., 2011), and other investigations have reported that a single 1.2 g/kg dose 1 hr prior to CFC training can improve memory measured at 24 hr (Levenson et al., 2004; Peleg et al., 2010), few studies have directly confirmed the effects of systemic drug treatment on histone acetylation in brain. In order to address this issue, we measured histone acetylation in whole hippocampus of Long-Evans rats euthanized either 30 or 60 min after sodium butyrate administration (1.2 g/kg, i.p.). Interestingly, histone acetylation levels were only transiently elevated, increasing at 30 min relative to vehicle (acetyl-H3: F(1,7)=12.338, p=.01; acetyl-H4: F(1,7)=21.494, p<.01), but returning to baseline levels by 1 hr (acetyl-H3: F(1,6)=0.345, p=.579; acetyl-H4: F(1,6)=.964, p=.364) (Fig. 3). These findings are similar to the time-course of acetylation dynamics in brain reported previously for this compound (Schroeder et al., 2007; Dash et al., 2009), and they call into question the basis of the memory benefit observed when CFC training is scheduled 60 minutes after acute systemic sodium butyrate administration. Taken together the findings for acute EVX and sodium butyrate treatment indicate that substantially increasing hippocampal histone acetylation prior to or at the time of training is not sufficient to improve subsequent long-term memory for CFC.
Figure 3. Acute sodium butyrate (NaB) administration (1.2 g/kg, i.p.) transiently increases hippocampal histone acetylation.
(A) Representative western blots for individual young adult Long-Evans rats for histone H3 acetylation, histone H4 acetylation and β-Actin. (B) Mean hippocampal histone H3 acetylation normalized to β-Actin (±S.E.M.) 30 (vehicle n=4; NaB n=5) and 60 (vehicle n=4; NaB n=4) min after injection. (C) Mean hippocampal histone H4 acetylation normalized to β-actin (±S.E.M.) 30 and 60 min after injection. **p<.01, within time point treatment effect relative to vehicle.
Contextual fear conditioning fails to induce hippocampal histone acetylation in young Long-Evans rats, Sprague-Dawley rats, or C57BL/6 mice
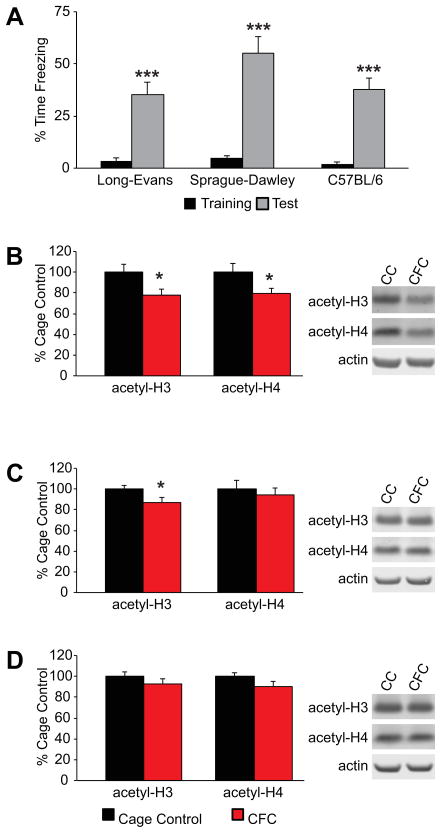
Prompted by our failure to detect a beneficial effect of multiple HDACi’s on memory for CFC, we revisited the related observation from earlier studies that fear conditioning itself induces a transient increase in hippocampal histone acetylation (Vecsey et al., 2007; Levenson et al., 2004; Levenson and Sweatt, 2005; Peleg et al., 2010). Adopting key procedural details from that work, here we examined histone H3 and H4 acetylation levels in the hippocampus 1 hr after CFC training in young Long-Evans rats, Sprague-Dawley rats, and C57BL/6 mice. All three strains displayed substantial contextual freezing at 1 hr post-shock (Long-Evans F(1,11)=19.650, p=.001; Spague-Dawley F(1,8)=76.105, p<.001; C57BL/6 F(1,8)=46.135, p<.001; Fig. 4A), confirming the efficacy of training. In contrast to previous reports, however, CFC in Long-Evans rats produced modest but significant decreases relative to home cage controls in both H3 (F(1,22)=6.221, p=.021) and H4 acetylation (F(1,22)=5.143, p=.034) (Fig. 4B). We also found a significant reduction in hippocampal H3 acetylation in Sprague-Dawley rats following CFC training (F(1,17)=5.776, p=.028), with no reliable change in H4 acetylation (F(1,16)=.265, p=.614) (Fig. 4C). There was no effect of training on H3 (F(1,15)=.684, p=.421) or H4 acetylation (F(1,15)=2.325, p=.148) in C57BL/6 mice relative to controls (Fig. 4D).
Figure 4. Contextual fear conditioning fails to increase hippocampal histone acetylation in young adult Long-Evans rats, Sprague-Dawley rats or C57Bl/6 mice.
(A) Mean percent freezing (±S.E.M.) during training and 1hr retention (Test), ***p≤.001 within group comparisons between training and test trials. Mean hippocampal histone H3 and H4 acetylation levels normalized to β-actin (±S.E.M.) in cage controls (CC) and 1 hr after CFC training in (B) Long-Evans rats (CC n=12, CFC n=12), (C) Sprague-Dawley rats (CC n=10, CFC n=9), and (D) C57Bl/6 mice (CC n=8, CFC n=9). *significant reductions (p<.05) in histone acetylation relative to respective cage controls. Representative western blots for individual subjects shown for all conditions (B–D).
Pretraining EVX administration influences spatial memory in young adult Long-Evans rats in a dose-dependent manner
The majority of available evidence concerning the memory effects of HDACi administration comes from experiments using a single behavioral task; contextual fear conditioning. Here we examined whether HDACi sensitivity extends to another assessment of hippocampal memory, a redundant place-cue (RPC) version of the Morris water maze (Castellano et al., 2012; Fletcher et al., 2006). This procedure is sensitive to aging, and we recently confirmed that RPC training significantly regulates histone acetylation in the young and aged rat hippocampus (Castellano et al., 2012). In the present experiments, animals were initially pretrained in the absence of pharmacological treatment on a standard 8-day, hidden platform version of the water maze, as described in detail elsewhere (Rapp and Gallagher, 1996; Gallagher et al., 1993). Performance improved rapidly over the course of pretraining (main effect of block F(3,105)=340.060, p<.005) and learning index scores for individual subjects (i.e., a measure of average proximity to the escape platform location) were used to configure groups matched according to spatial memory capacity for vehicle or EVX treatment during subsequent RPC training.
The RPC procedure was carried out in a novel spatial environment two weeks after training in the standard version of the water maze. With the escape platform held in a constant location throughout, testing consisted of 9 consecutive cued (visible platform) trials, followed by 6 alternating cued and non-cued (hidden platform) trials. Testing with a visible, fixed escape location is amenable to both cue-approach and allocentric spatial solutions, whereas maximally efficient escape on hidden platform trials requires an allocentric solution, relying on memory for extramaze spatial information. RPC training was conducted in a single relatively brief session 90 min after low (10 mg/kg), middle (30 mg/kg) or high dose (60 mg/kg) EVX administration or vehicle.
Despite the robust effect of EVX on motor activity noted earlier in the open field, the vehicle and EVX-injected groups displayed rapid and comparable improvement in the RPC task, measured as a decline in pathlength across the initial visible platform trials (Fig. 5A, main effect of trial F(8,216)=32.683, p<.001, trial by group interaction F(24,216)=1.513, p=.065). Performance was also comparable across groups on the subsequent interleaved hidden platform trials (main effect of group F(3,27)=.639, p=.596), and the alternating cued trials (main effect of group F(3,27)=1.904, p=.153). Spatial memory was measured 24 hr after training, calculated as proximity to the escape location during a single probe trial in which the platform was unavailable. Performance differed reliably across treatment groups (F(3,27)=4.547, p=.011), and the strongest numerical spatial bias (lowest proximity score) was observed among animals that received the middle dose of EVX prior to learning (Fig. 5B). Post-hoc comparisons confirmed that the difference between the low and middle dose groups was statistically significant (p=.001), whereas the benefit of the middle dose in comparison with the high dose and vehicle conditions was marginal (p=.071) and nonsignificant (p=.127), respectively. Indeed the low dose group scored nearly significantly worse than vehicle controls (p=.057), indicating that mild dose-dependent impairment may contribute to the overall profile. Together the results suggest that pretraining EVX administration at 30 mg/kg produces, at most, a modest benefit in spatial memory relative to a low dose, with no reliable benefit at any dose in comparison with vehicle treatment.
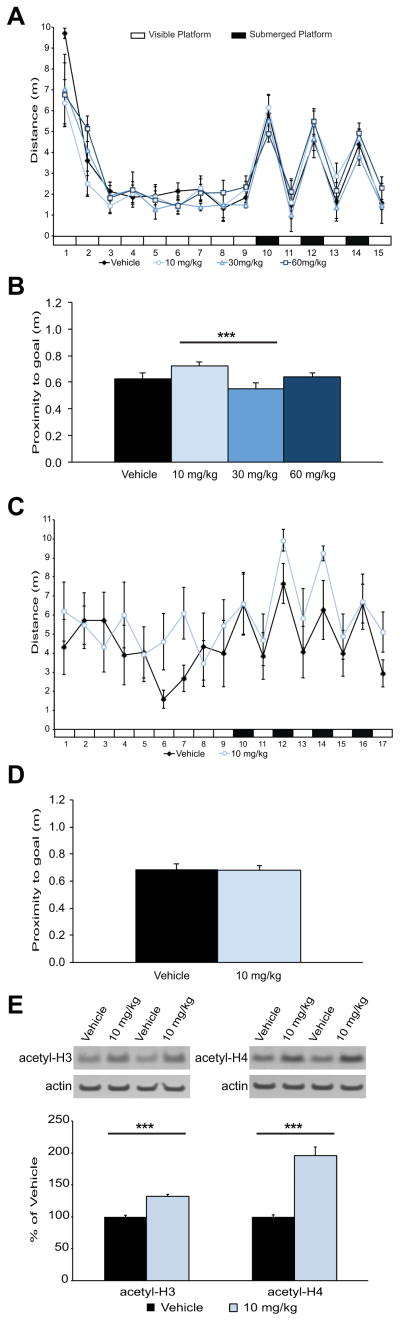
Figure 5. EVX administration only marginally improves spatial memory in young adult Long-Evans rats, and fails to benefit age-related spatial memory impairment.
(A) Mean pathlength (±S.E.M.) across training trials in the redundant place/cue task for young rats given vehicle (n=7), low (n=8), middle (n=8) or high (n=8) dose injection (i.p.) of EVX. (B) Mean proximity of young animals to the goal platform (±S.E.M.) during a probe test 24 hr after training. The improvement in long-term memory between the 10 mg/kg and 30 mg/kg was significant (***p=.001). (C) Mean pathlength (±S.E.M.) across training trials in the redundant place/cue task for aged rats given 10mg/kg (n=7) of EVX or vehicle (n=6). (D) Mean proximity of aged animals to the escape location (±S.E.M.) during the probe test 24h after drug administration. (E) Mean hippocampal histone H3 and H4 acetylation levels normalized to β-actin (±S.E.M) in aged animals 90 minutes after EVX (n=6) or vehicle (n=7) administration. ***p<.001, increases in histone acetylation relative to vehicle control levels.
Pretraining EVX administration fails to ameliorate hippocampal memory impairment in aged Long-Evans rats
Informed by the results from young rats, next we examined the effects of EVX administration on spatial memory impairment in a well-characterized Long-Evans rat model of cognitive aging. Age-related deficits revealed by this model are associated with disrupted epigenetic regulation in the hippocampus (Castellano et al., 2012), and are sensitive to rescue by pharmacological intervention targeting a variety of mechanisms (Koh et al., 2013; 2010). A pilot study in a small number of aged rats revealed that, consistent with results for exploratory activity in young subjects (Fig. 1E), the 30 mg/kg dose of EVX induced marked lethargy and disrupted swimming in the water maze to a degree incompatible with the reliable assessment of spatial learning. Swimming was grossly normal in aged rats administered the lower dose of EVX prior to RPC training (10 mg/kg; Fig. 5C), but, similar to young rats, there was no benefit on 24-hr retention of the escape location relative to vehicle-treated aged subjects (F(1,11)=.283, p=.605; Fig. 5D). One week after behavioral testing we confirmed in the same subjects that the EVX dose tested in this experiment significantly increased histone H3 and H4 acetylation in the aged hippocampus (Fig. 5E) 90 min after administration (acetyl-H3 F(1,11)=91.525, p<.001; acetyl-H4 F(1,11)=69.935, p<.001). Accordingly, the lack of an EVX effect on memory is not a consequence of failed efficacy for the intended drug target.
Discussion
Chromatin regulation in CNS plasticity
Considerable evidence suggests that chromatin dynamics regulate synaptic plasticity important for learning and memory. The best-studied example, experience-dependent histone acetylation, has been implicated in acquisition, retention, and extinction (Bousiges et al., 2010; Bredy et al., 2007; Vecsey et al., 2007; Levenson et al., 2004; Peleg et al., 2010) and corresponding results indicate that specific HDAC isoforms negatively regulate memory formation (McQuown et al., 2011; Guan et al., 2009; Gräff et al., 2012). Findings linking increased histone acetylation to improved memory have prompted the investigation of HDACi’s as memory-enhancers and potential therapeutics for cognitive dysfunction (for review see Fischer et al., 2010). Supportive observations indicate that HDACi administration improves long-term memory in normal intact subjects across multiple hippocampus-dependent tasks (Peleg et al., 2010; Levenson et al., 2004; Haettig et al., 2011; Fischer et al., 2007; Mahan et al., 2012). Furthermore, HDAC inhibition in models of impairment benefits synaptic plasticity (Guan et al., 2009; Vecsey et al., 2007; Ricobaraza et al., 2010) and rescues memory deficits (Kilgore et al., 2010; Peleg et al., 2010; Haettig et al., 2011). As outlined in the following sections, the novel findings reported here illuminate key open questions concerning the therapeutic potential of HDACi’s for neurocognitive disorders.
EVX is a potent brain-penetrant HDAC inhibitor
Many recent proof-of-concept studies linking HDAC inhibition to cognitive improvement have utilized drugs that have multiple off-target actions, such as valproic acid, or other compounds (e.g., sodium butyrate) with unconfirmed brain penetrance and efficacy in vivo in terms of histone acetylation capacity. By comparison, EVX inhibits class I, IIb and IV HDACs in the nanomolar range and elicits a potent histone acetylation response in brain after systemic administration. Acute peripheral injection of EVX in the present experiments produced dose-dependent increases in histone H3 and H4 acetylation across all subfields of the hippocampus. In agreement with evidence in other experimental settings (Gundersen and Blendy, 2009), HDACi treatment in the current investigation induced robust reductions in open field activity in young adult rats, and marked decline in water maze exploration among aged rats provided the intermediate dose of EVX (30 mg/kg). Although these effects were transient and we found no evidence of motor impairment when memory for fear conditioning or water maze retention was tested 24 hours after EVX administration, our findings underscore that non-specific performance factors should be considered when evaluating the potential cognitive benefits of HDACi treatment. The likely contribution of the many non-histone lysine acetylation targets of HDACi’s (Choudhary et al., 2009) also merits greater attention in this area of research.
Increased hippocampal histone acetylation is not sufficient to enhance, or a necessary correlate of, memory for contextual fear conditioning
Contextual fear conditioning has been widely used to examine the role of histone acetylation in hippocampus-dependent memory, taking advantage of the temporally discrete nature of training and the robust endurance of long-term memory for context. A number of laboratories have reported that CFC induces hippocampal histone acetylation and that pretraining HDACi treatment enhances long-term contextual fear memory (Levenson et al., 2004; Fischer et al., 2007; Peleg et al., 2010). Building on this literature, we evaluated the effect of 3 doses of EVX on long-term contextual fear memory. Contrary to expectations, although we directly confirmed that treatment induced marked, dose-dependent increases in hippocampal histone acetylation specifically at a time point corresponding to CFC training, EVX had no effect on 24-hour retention at any dose examined. The possibility that chronic EVX treatment or other delivery schedules might yield different outcomes, however, merits testing in future studies.
Prompted by our failure to detect the anticipated procognitive benefit of potent, brain-penetrant HDAC inhibition on memory for CFC, we revisited and extended key observations from the relevant prior literature in three ways. First we tested the effects on CFC of a more commonly used HDACi (i.e., sodium butyrate) in Long-Evans rats, taking the dose from earlier studies reporting robust memory enhancement with acute pretraining administration (Levenson et al., 2004; Peleg et al., 2010). Next we aimed to directly confirm the presumed target of sodium butyrate treatment in a time-course analysis of effects on hippocampal histone acetylation. Guided by evidence that fear conditioning itself induces histone modification in rats and mice, lastly we tested the effects of CFC training on histone acetylation in Long-Evans rats, Sprague-Dawley rats and C57BL/6 mice. Together the results suggest three significant conclusions. First, pharmacological induction of hippocampal histone acetylation at the time of learning is not sufficient to improve long-term memory for CFC. Second, sodium butyrate, as administered here and in previous studies (e.g., Levenson et al., 2004; Peleg et al., 2010) leads to a rapid and transient increase in hippocampal histone acetylation that returns to baseline at the time CFC training is typically conducted. Third, increased hippocampal histone acetylation is not an obligatory or necessary consequence of CFC training that yields robust memory. These findings call into question whether increased histone acetylation at the time of initial learning is the proximal basis for the memory enhancement reported in response to acute HDACi administration.
Dose-dependent EVX effects on long-term spatial memory in young adult rats
We recently reported that RPC training leads to bidirectional and region-specific regulation of histone acetylation across the principal subfields of the hippocampus (Castellano et al., 2012). Here we extended those findings and tested the potential enhancing effects of EVX on long-term memory in the water maze in young adult Long-Evans rats. The results revealed that spatial memory was numerically most robust among animals that received an intermediate dose of EVX prior to training. The absolute magnitude of enhancement was modest, however, and statistically significant only in comparison with performance in the low dose group. These findings suggest that maximizing the neurocognitive benefit of HDACi treatment may require careful dose titration, and that off-target effects or histone acetylation outside an optimal range may fail to improve or even impair memory. Indeed a narrow dose-response function may be among the factors accounting for our failure to detect HDACi effects on memory for CFC. The observation that HDACi administration can rescue impaired memory without enhancing performance in young intact subjects (Kilgore et al., 2010) suggests that baseline cognitive status may also critically influence the response to treatment.
Increased hippocampal histone acetylation following EVX administration fails to benefit age-related spatial memory impairment
Memory impairment in aged mice is associated with blunted behavioral regulation of hippocampal histone acetylation, and these deficits are reversed by pharmacological HDAC inhibition (Peleg et al., 2010). In a related study, we recently reported that behavioral training in young adults and aged rats with intact memory induces a coordinated pattern of dynamic chromatin modification across the principal cell fields of the hippocampus, including lysine- and region-specific, bidirectional changes in histone acetylation that are correlated with spatial memory capacity (Castellano et al., 2012). In the hippocampus of aged rats with documented deficits in spatial memory, by comparison, the pattern of epigenetic dynamics in response to recent experience is disrupted and entirely uncoupled from the status of memory. The significant implication based on those findings is that the development of effective drug intervention might profitably focus on the restoration of coordinated chromatin regulation, rather than non-selectively increasing histone acetylation at many lysine sites simultaneously. Consistent with that perspective, the present results document that marked, acute increases in hippocampal histone acetylation, pharmacologically induced specifically to overlap with the time of learning, are not sufficient to ameliorate age-related memory impairment. Future work advancing a detailed understanding of how experience influences chromatin remodeling should guide the development of promising treatment strategies in the emerging field of cognitive neuroepigenetics.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institute on Aging, and by NIH grants AG09973 and AG032845 (JFC). One author (HP) is an employee of EnVivo Pharmaceuticals, and the others declare no conflict of interest. The authors thank members of the Neurocognitive Aging Section of the Laboratory of Behavioral Neuroscience for helpful discussion.
References
- Biermann J, Boyle J, Pielen A, Lagrèze WA. Histone deacetylase inhibitors sodium butyrate and valproic acid delay spontaneous cell death in purified rat retinal ganglion cells. Mol Vis. 2011;17:395–403. [PMC free article] [PubMed] [Google Scholar]
- Bousiges O, de Vasconcelos AP, Neidl R, Cosquer B, Herbeaux K, Panteleeva I, Loeffler J-P, Cassel J-C, Boutillier A-L. Spatial memory consolidation is associated with induction of several lysine-acetyltransferase (histone acetyltransferase) expression levels and H2B/H4 acetylation-dependent transcriptional events in the rat hippocampus. Neuropsychopharmacology. 2010;35:2521–2537. doi: 10.1038/npp.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy TW, Barad M. The histone deacetylase inhibitor valproic acid enhances acquisition, extinction, and reconsolidation of conditioned fear. Learn Mem. 2008;15:39–45. doi: 10.1101/lm.801108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem. 2007;14:268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JF, Fletcher BR, Kelley-Bell B, Kim DH, Gallagher M, Rapp PR. Age-related memory impairment is associated with disrupted multivariate epigenetic coordination in the hippocampus. PLoS One. 2012;7:e33249. doi: 10.1371/journal.pone.0033249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary C, Kumar C, Gnad F, Nielsen M, Rehman M, Walther T, Olsen J, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- Dash PK, Orsi SA, Moore AN. Histone deactylase inhibition combined with behavioral therapy enhances learning and memory following traumatic brain injury. Neuroscience. 2009;163:1–8. doi: 10.1016/j.neuroscience.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Mungenast A, Tsai L-H. Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol Sci. 2010;31:605–617. doi: 10.1016/j.tips.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai L-H. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- Fletcher BR, Calhoun ME, Rapp PR, Shapiro ML. Fornix lesions decouple the induction of hippocampal arc transcription from behavior but not plasticity. J Neurosci. 2006;26:1507–1515. doi: 10.1523/JNEUROSCI.4441-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gräff J, Rei D, Guan J-S, Wang W-Y, Seo J, Hennig KM, Nieland TJF, Fass DM, Kao PF, Kahn M, et al. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature. 2012;483:222–226. doi: 10.1038/nature10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräff J, Tsai L-H. Histone acetylation: molecular mnemonics on the chromatin. Nat Rev Neurosci. 2013;14:97–111. doi: 10.1038/nrn3427. [DOI] [PubMed] [Google Scholar]
- Guan J-S, Haggarty SJ, Giacometti E, Dannenberg J-H, Joseph N, Gao J, Nieland TJF, Zhou Y, Wang X, Mazitschek R, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen BB, Blendy JA. Effects of the histone deacetylase inhibitor sodium butyrate in models of depression and anxiety. Neuropharmacology. 2009;57:67–74. doi: 10.1016/j.neuropharm.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haettig J, Stefanko DP, Multani ML, Figueroa DX, McQuown SC, Wood MA. HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner. Learn Mem. 2011;18:71–79. doi: 10.1101/lm.1986911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazantsev AG, Thompson LM. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov. 2008;7:854–868. doi: 10.1038/nrd2681. [DOI] [PubMed] [Google Scholar]
- Kilgore M, Miller CA, Fass DM, Hennig KM, Haggarty SJ, Sweatt JD, Rumbaugh G. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2010;35:870–880. doi: 10.1038/npp.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh MT, Haberman RP, Foti S, McCown TJ, Gallagher M. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology. 2010;35:1016–1025. doi: 10.1038/npp.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh MT, Rosenzweig-Lipson S, Gallagher M. Selective GABA(A) α5 positive allosteric modulators improve cognitive function in aged rats with memory impairment. Neuropharmacology. 2013;64:145–152. doi: 10.1016/j.neuropharm.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal KM, Barrett RM, Wood MA. Systemic or intrahippocampal delivery of histone deacetylase inhibitors facilitates fear extinction. Behav Neurosci. 2007;121:1125–1131. doi: 10.1037/0735-7044.121.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nat Rev Neurosci. 2005;6:108–118. doi: 10.1038/nrn1604. [DOI] [PubMed] [Google Scholar]
- Mahan AL, Mou L, Shah N, Hu J-H, Worley PF, Ressler KJ. Epigenetic modulation of homer1a transcription regulation in amygdala and hippocampus with pavlovian fear conditioning. J Neurosci. 2012;32:4651–4659. doi: 10.1523/JNEUROSCI.3308-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T, Mullican SE, Jones S, Rusche JR, Lazar MA, et al. HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci. 2011;31:764–774. doi: 10.1523/JNEUROSCI.5052-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Campbell SL, Sweatt JD. DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity. Neurobiol Learn Mem. 2008;89:599–603. doi: 10.1016/j.nlm.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AMM, Wood MA, McDonough CB, Abel T. Transgenic mice expressing an inhibitory truncated form of p300 exhibit long-term memory deficits. Learn Mem. 2007;14:564–572. doi: 10.1101/lm.656907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto L, Abel T. The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology. 2013;38:62–76. doi: 10.1038/npp.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL, Gogol-Doering A, Opitz L, et al. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328:753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- Rapp P, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Natl Acad Sci U S A. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricobaraza A, Cuadrado-Tejedor M, Marco S, Pérez-Otaño I, García-Osta A. Phenylbutyrate rescues dendritic spine loss associated with memory deficits in a mouse model of Alzheimer disease. Hippocampus. 2010 doi: 10.1002/hipo.20883. [DOI] [PubMed] [Google Scholar]
- Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry. 2007;62:55–64. doi: 10.1016/j.biopsych.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Stilling RM, Fischer A. The role of histone acetylation in age-associated memory impairment and Alzheimer’s disease. Neurobiol Learn Mem. 2011;96:19–26. doi: 10.1016/j.nlm.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, Cabrera SM, McDonough CB, Brindle PK, Abel T, et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci. 2007;27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou VW, Goren A, Bernstein BE. Charting histone modifications and the functional organization of mammalian genomes. Nat Rev Genet. 2011;12:7–18. doi: 10.1038/nrg2905. [DOI] [PubMed] [Google Scholar]