Abstract
Prior work established that a deficiency in the cysteine protease dipeptidyl peptidase I (DPPI) improves survival following polymicrobial septic peritonitis. To test whether DPPI regulates survival from severe lung infections, DPPI −/− mice were studied in a Klebsiella pneumonia lung infection model, finding that survival in DPPI −/− mice is significantly better than in DPPI +/+ mice 8 d after infection. DPPI −/− mice have significantly fewer bacteria in the lung than infected DPPI +/+ mice, but no difference in lung histopathology, lung injury, or cytokine levels. To explore mechanisms of enhanced bacterial clearance in DPPI −/− mice, we examined the status of pulmonary collectins, finding that levels of surfactant protein D, but not of surfactant protein A, are higher in DPPI −/− than in DPPI +/+ BAL fluid, and that DPPI −/− BAL fluid aggregate bacteria more effectively than control BAL fluid. Sequencing of the amino terminus of surfactant protein D revealed two or eight additional amino acids in surfactant protein D isolated from DPPI −/− mice, suggesting processing by DPPI. These results establish that DPPI is a major determinant of survival following Klebsiella pneumoniae lung infection and suggest that the survival disadvantage in DPPI +/+ mice is in part due to processing of surfactant protein D by DPPI.
Keywords: Bacterial killing, collectin, innate immunity, pulmonary inflammation
INTRODUCTION
Dipeptidyl peptidase I (DPPI) is a cysteine protease that removes NH2-terminal dipeptides from a variety of targets [1,2,3,4,5,6]. Studies of DPPI −/− mice show that DPPI is required for activation of the granule-associated serine proteases granzymes A and B, neutrophil elastase, cathepsin G and mast cell chymases [2,4,6]. Because these proteases are required for mounting an optimal response to bacterial infection, we previously studied DPPI −/− mice in a model of polymicrobial septic peritonitis [7], finding that the absence of DPPI is associated with significantly better survival and that this survival advantage depends on depletion of mast cell-DPPI and increased levels of IL-6.
Surfactant protein D (SPD) is a member of the collectin family of proteins, which act as pattern recognition molecules that bind polysaccharides and glycolipids expressed on the surface of a variety of microorganisms [8,9]. Structurally, SPD exists predominately as a dodecamer [10,11,12]. Multimerization of SPD is critical to its bioactivity [9,10,11,12]. Via direct binding to microorganisms, SPD causes aggregation and influences immune cell-mediated clearance of pathogens [8,9]. Microorganisms whose lung clearance depends on SPD include influenza A [13,14], respiratory syncytial virus [15], Pseudomonas aeruginosa [16] and Klebsiella pneumoniae [10].
In this report, experiments were conducted to examine whether DPPI regulates survival from bacterial lung infection. They reveal that DPPI-deficient mice have better survival following K. pneumoniae lung infection and that this survival advantage is associated with increased levels of SPD in the lungs of DPPI mice. These findings indicate that the absence of DPPI protects against severe bacterial lung infection and extends its importance as a mediator of the host response to severe bacterial infections.
MATERIAL AND METHODS
Experimental Animals
The experiments used DPPI +/+ and DPPI −/− mice [4] in a C57BL/6 background. All experimental procedures were performed in 8-12 week-old mice and were approved by the University of California, San Francisco Committee on Animal Research.
Induction of K. pneumoniae lung infection in mice
Mice were inoculated intranasally via a sterile pipet tip with 3000 CFU of K. pneumoniae (strain 43816, American Type Culture Collection, Manassas, VA) suspended in 50 μl of saline. Mice recovered from anesthesia and survival monitored three times daily. Moribund mice were euthanized by CO2 inhalation and cervical dislocation.
Quantification of cellular response to infection
Lungs of mice were lavaged 3x with 0.7 cc of sterile PBS. Lavage fluid (BAL) was pooled and centrifuged at 4°C and the supernatant saved for analysis. Cell pellets were suspended in PBS and cell numbers counted with a hemocytometer and differentials determined on cytospins of cells stained with Diff-Quik (American Scientific Products, McGaw Park, IL).
Quantification of bacterial colony forming units (CFU)
Immediately after recovery, 10 μl of lung lavage fluid or blood were diluted serially in sterile saline. 10 μl of each dilution were aseptically plated and cultured on nutrient agar for non-fastidious microorganism plates (Difco, Detroit, MI) at 37°C. After 24 h, the numbers of bacterial colonies were counted.
Type II cell Isolation
Alveolar type II cells were isolated by inflating mouse trachea with 1 ml of dispase (5 u/ml, Roche Indianoplis, IN) suspended in DMEM, the lungs harvested, and incubated at 18°C for 60 min. The lungs were then minced, filtered through 40 μM mesh filters. Cells were subsequently stained with rat anti-E-cadherin followed by APC-conjugated anti-rat IgG secondary antibody and FACS sorted for E-cadherin-positive cells using a MoFlo Cell Sorter. Sorted cells were analyzed by immunoblot for the presence of DPPI using a goat anti-mouse DPPI antibody (R&D systems, Minneapolis, MN).
Assay of bacterial aggregation
Surfactant aggregation of bacteria was quantified using a method described previously [17]. Briefly, 500 μl of E. coli K12 grown overnight in LB broth (Difco) were pelleted, and then resuspended in 1 ml of PBS containing calcium. 90 μl of this suspension and 10 μl of BAL fluid obtained from uninfected DPPI +/+ or DPPI −/− mice were incubated for varying lengths of time. The suspensions were shaken at 300 rpm, and bacterial density monitored at a wavelength of 400 nm in a spectrophotometer at 5-min intervals.
Surfactant protein analysis and macrophage immunoblotting
Lungs were lavaged with 0.8 cc of saline and cells in lavage fluid separated by centrifugation. The supernatant was removed and separated by SDS-PAGE under reducing or non-reducing conditions, and analyzed by immunoblots probed with rabbit-anti mouse surfactant protein D (Chemicon) or rabbit-anti mouse surfactant protein A (Chemicon).
Surfactant protein D purification and sequencing
The lungs of 25 DPPI +/+ and 40 DPPI −/− mice were lavaged with 150 mM NaCl, 5 mM Tris, pH 7.4 and the recovered lavage fluids centrifuged at 3,000 × g for 45 min at 4°C. The recovered supernatants were incubated overnight with maltose-sepharose containing 5 mM CaCl2 as described [18,19]. Bound protein was eluted with 50 mM Tris, 150 mM NaCl, 10 mM EDTA, pH 7.8. Fractions containing SPD were pooled, concentrated using Microcon filters (MilliporeBillerica, MA) with a 3000 MW cutoff, separated by SDS-PAGE and transferred onto a PVDF membrane. The membrane was stained with Coomassie Blue and the band corresponding to surfactant protein D was sequenced by Edman degradation (performed by Midwest Analytical, Inc., Chesterfield, MO).
Statistics
Survival curves were analyzed using the two-tailed Fisher’s exact test. ANOVA followed by two-tailed t testing was used to compare markers of organ dysfunction, bacterial CFU, and mean cytokine concentrations. All calculations were performed using Statview 5.0.1 software (SAS Institute Inc., Cary, NC). Significance was assigned to P values < 0.05.
RESULTS
DPPI modulates survival from Klebsiella pneumoniae lung infections
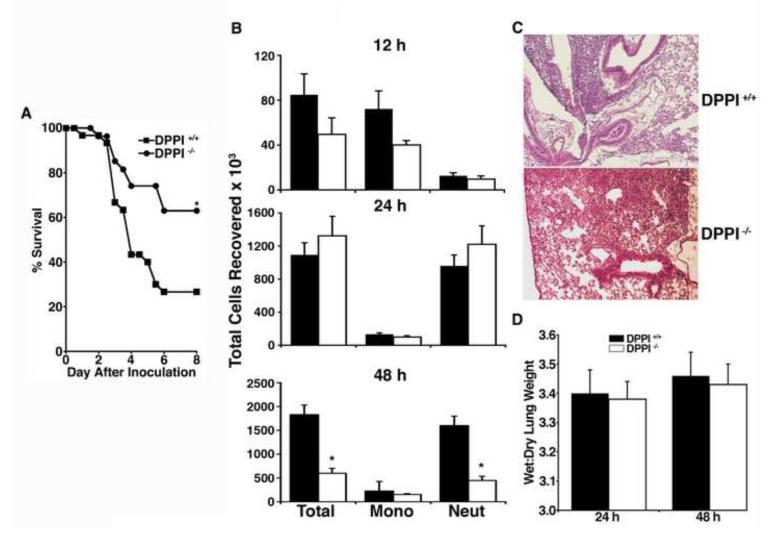
To test whether DPPI modulates survival from bacterial infections outside the peritoneum, the nares of DPPI +/+ and DPPI −/− mice were inoculated with 3000 CFU of K. pneumoniae. DPPI −/− mice have significantly improved survival compared to wild type controls (63% vs. 27%) 8 d after inoculation (Figure 1A).
Figure 1. DPPI −/− mice are protected from death from K. pneumonia lung infection.
(A) Survival of 30 DPPI +/+ and 27 DPPI −/− mice infected intranasally with 3000 CFU of K. pneumonia(*P = 0.008). Similar results were found in three separate experiments. (B) Total cell counts and cell differentials in BAL fluid obtained from DPPI +/+ and DPPI −/− mice 12 h, 24 h, and 48 h after inoculation with K. pneumonia (7-10 mice per group, *p < 0.01). (B) Representative sections of lung, stained with hematoxylin and eosin, obtained from DPPI +/+ and DPPI −/− mice 48 h after inoculation with K. pneumonia. (C) Wet: dry lung weights of DPPI +/+ and DPPI −/− mice 24 and 48 h after inoculation of K. pneumonia (7-10 mice per group).
To understand how DPPI modulates survival following Klebsiella pneumonia, endpoints commonly explaining differences in survival in this model were examined at various intervals after inoculation with K. pneumoniae. No differences were found in either wet : dry lung weights or histopathology. The DPPI −/− mice had fewer neutrophils in their BAL fluid 48 hours after inoculation (Figures 1B, 1C, and 1D). There were no differences in levels of TNF-α, IL1β, or IL-6 in BAL fluid obtained 12, 24, or 48 hours after inoculation (data not shown).
DPPI −/− mice have enhanced bacterial clearance following K. pneumonia lung infection
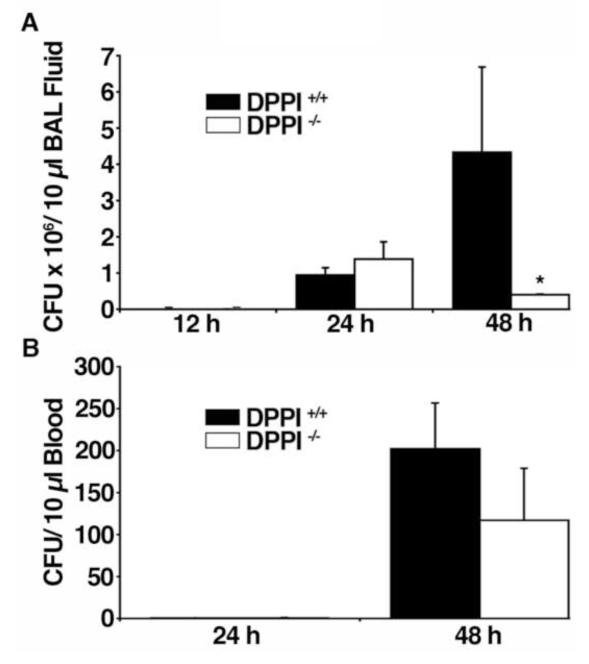
Next, bacterial loads in the lung and blood of DPPI −/− mice to DPPI +/+ controls were compared at various times after inoculation. Bacterial loads were similar in BAL fluid and blood of DPPI −/− mice and DPPI +/+ controls 12 and 24 h after inoculation (Figure 2A). By 48 h, DPPI −/− mice have fewer bacteria in BAL fluid (Figure 2A) and a trend toward fewer bacteria in the blood (Figure 2B) than DPPI +/+ mice, demonstrating that DPPI −/− mice have enhanced clearance of Klebsiella from the lung.
Figure 2. Bacterial clearance is enhanced in DPPI −/− mice.
Quantification of K. pneumonia in BAL fluid (A) or blood (B) from DPPI +/+ and DPPI −/− mice 12 h, 24 h, and 48 h after intranasal inoculation with K. pneumonia (n = 6-9 mice/group; *P < 0.001).
DPPI-deficient mice have increased levels of surfactant protein D
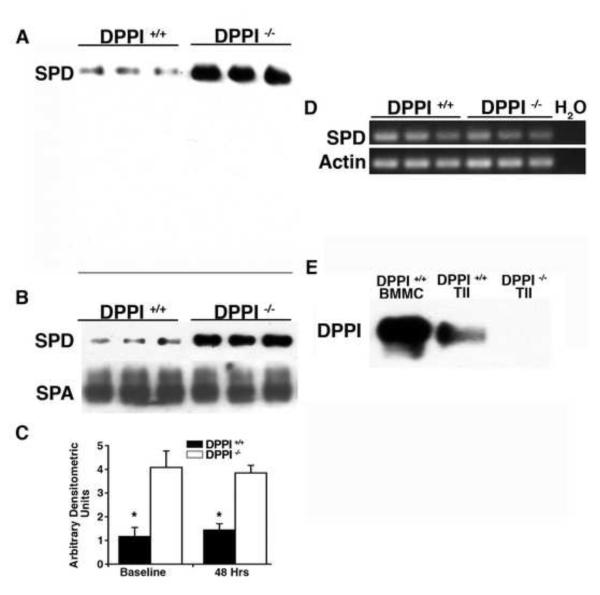
The collectins surfactant protein A (SPA) and surfactant protein D (SPD) are major mediators of clearance of bacteria from the lung [8,9,17]. Prior work suggested that the neutrophil serine proteases regulate SPD levels by proteolytic destruction during lung infection [20]. Because these enzymes are inactive in DPPI −/− mice [6], we used immunoblots to examine whether SPA or SPD are differentially processed in infected DPPI −/− mice. Immunoblots of BAL fluid obtained from DPPI −/− and DPPI +/+ mice 48 h after Klebsiella infection revealed that BAL fluid from DPPI −/− mice had increased levels of SPD, but not SPA (not shown). Immunoblots did not detect fragments of SPD (Figure 3A), suggesting the increased level of SPD in DPPI −/− mice was not due to limited degradation by the absence of active neutrophil proteases in DPPI −/− mice.
Figure 3. Lung SPD levels are increased in DPPI −/− mice.
(A) Immunoblot of 5 μl of BAL fluid obtained 48 h after intranasal inoculation with 3000 CFU of K. pneumonia probed for SPD. Note: line indicates the bottom of the gel. (B) Immunoblot of 5 μl of BAL fluid from uninfected mice probed for SPD or SPA. (C) Average densitometric units of immunoreactive SPD in DPPI +/+ and DPPI −/− BAL fluid (n = 6-9 mice/group). (D) PCR for SPD and β-actin of mRNA from lung lysates. (E) Immunoblots of bone marrow mast cells (BMMC, positive control) and alveolar type II cells (TII) from DPPI +/+ and DPPI −/− mice probed for DPPI demonstrate immunoreactive DPPI in TII cells isolated from DPPI +/+ but not DPPI −/− mice. (*P <0.01 compared to DPPI −/− mice).
Next, immunoblots of BAL fluid were used to examine whether SPA or SPD levels are altered in uninfected DPPI −/− mice. Levels of immunoreactive SPD but not SPA were markedly higher in BAL fluid from DPPI −/− mice (Figures 3B and 3C), indicating that the absence of DPPI alters the basal levels of SPD. To begin to understand why DPPI −/− mice have increased levels of SPD, levels of SPD mRNA were measured and found to be similar in lung extracts from DPPI −/− and DPPI +/+ mice (Figure 3D), indicating that the biochemical change causing increased SPD levels in DPPI −/− mice occurs posttranslationally. Finally, alveolar type II cells were shown to be immunoreactive for DPPI, indicating they manufacture the protease (Figure 3E). These findings suggest that type II cell DPPI could regulate lung levels of SPD posttranslationally, and that higher levels of SPD may explain the survival advantage of DPPI −/− mice to Klebsiella lung infections.
BAL fluid from DPPI-deficient mice has enhanced clumping of bacteria
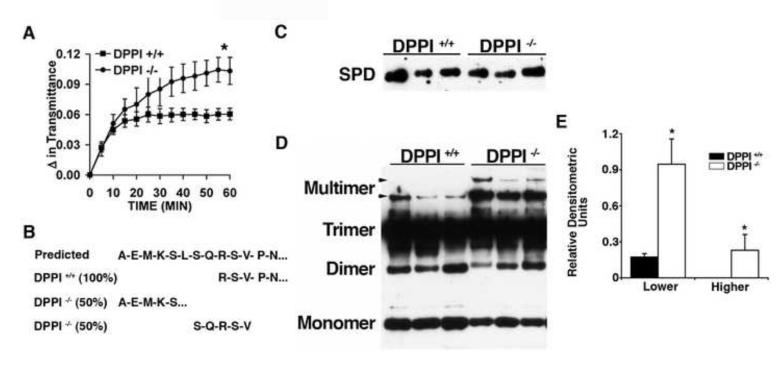
To examine whether the increased SPD levels in DPPI −/− mice is potentially biologically relevant, a bioassay for SPD surfactant activity, was used to examine whether BAL fluid from DPPI −/− mice aggregates bacteria differently than that from DPPI +/+ mice. Consistent with the higher SPD levels in DPPI −/− mice, the BAL fluid from DPPI −/− mice aggregates bacteria with significantly greater efficiency than that from DPPI +/+ controls (Figure 4A).
Figure 4. Characterization of BAL fluid from DPPI −/− mice.
(A) Enhanced aggregation of bacteria by BAL fluid obtained from DPPI −/− mice. E. coli K12 suspended in media were mixed with BAL fluid from DPPI +/+ (squares) or DPPI −/− (circles) mice and incubated at 37°C. Bacterial density was monitored by absorbance at 400 nm (n= 6/group, *p= 0.006 at 60 min). (B) Amino terminal sequence of SPD purified from the BAL fluid of uninfected DPPI +/+ and DPPI −/− mice. (C) Immunoblot for SPD of BAL fluid obtained from DPPI +/+ and DPPI −/− mice separated under reducing conditions with the relative amounts loaded normalized for levels of SPD. (D) Immunoblot for SPD of the same quantities of BAL fluid loaded in panel C separated by SDS-PAGE under non-reducing conditions. (E) Relative densitometric units of the multimeric forms of SPD compared to total SPD (panel C). (*P <0.02)
Surfactant Protein D Isolated from DPPI −/− mice has a unique amino-terminus
To examine whether SPD was differentially processed in the absence of DPPI, SPD was purified from BAL fluid from DPPI −/− and DPPI +/+ mice. The amino terminus was sequenced by Edman degradation. SPD purified from DPPI +/+ mice consisted of a single amino terminus (RSVPN) corresponding to residues 28-32 of mouse SPD (Figure 4B). In contrast, the amino terminus of SPD from DPPI −/− mice consisted of approximately equal proportions of two different sequences (AEMKS and SQRSV) corresponding to amino acids 20-24 and 26-30 of mouse SPD (Figure 4B). These results show that the amino terminus of SPD in DPPI −/− mice contains either two or eight additional amino acids.
DPPI deficient mice have increased levels of the multimeric form of surfactant protein D
SPD is recovered from BAL fluid in multiple forms including trimers, oligomers, and higher-order structures [9,21], which account for its anti-bacterial activity [10,12]. To examine whether the multimeric forms of SPD are proportionately greater in DPPI −/− mice, the total levels of SPD were first normalized by immunoblotting for SPD under reducing conditions (Figure 4C). Next, the levels of the multimeric forms of SPD were compared by immunoblotting the normalized samples separated under non-reducing conditions (Figures 4D and 4E). BAL fluid from DPPI −/− mice had higher levels of the multimeric and higher order forms of SPD and lower levels of the monomer. These data indicate that DPPI −/− mouse lungs contain proportionately greater amounts of SPD multimers.
DISCUSSION
This study shows that DPPI regulates survival from Klebsiella lung infections, possibly by regulating lung levels of surfactant protein D. The findings are significant because they identify a previously unrecognized role for DPPI in SPD processing, which influences both the level of SPD and its ability to assemble into multimeric forms. Furthermore, they show that in addition to protecting mice from death from septic peritonitis [7], DPPI deficiency protects mice from death from severe lung infection.
A prior investigation showed that genetic deletion of the cysteine protease DPPI improves survival following polymicrobial septic peritonitis [7]. In that study, the survival advantage was attributed to regulation of peritoneal IL-6 levels by mast cell DPPI. Because of those observations, similar experiments were considered to investigate this mechanism explains the improved survival of DPPI −/− mice in the Klebsiella lung infection model. Cytokine analysis did not confirm differences in IL-6 levels at various time-points after infection. Furthermore, because reconstitution of mast cell-deficient mice do not permit investigation of mast cell roles in this Klebsiella lung infection model [22] we were unable to adequately examine whether mast cell DPPI similarly influences survival in Klebsiella lung infections. In fact, survival of mast cell deficient Wsh mice reconstituted with DPPI +/+ and DPPI −/− mast cells [23] had similar survival in the Klebsiella lung infection model (data not shown). This is likely because the location and number of mast cells in lungs of reconstituted mice differ from those in wild type controls, making it technically unfeasible to definitively examine the role of lung mast cell DPPI or its regulation of IL-6 in Klebsiella lung infection [22].
Neutrophils play a central role in the killing and clearing of Klebsiella infections. The killing of bacteria by neutrophils is dependent on the proteases neutrophil elastase and cathepsin G [24,25]. Neutrophils are abnormal in DPPI −/− mice since they lack active neutrophil elastase, cathepsin G, and have reduced quantities of active proteinase-3 (54). Because neutrophil elastase −/− or neutrophil elastase −/− and cathepsin G −/− double-null mice have increased mortality following Klebsiella infection, it is unlikely that the absence of active neutrophil elastase or cathepsin G explains the survival advantage of DPPI −/− mice. Furthermore, neutrophils and their proteases may contribute to lung injury [25,26]. However, differences in lung injury due to the lower numbers of neutrophils at the 48 hour time-point or lack of active neutrophil elastase and cathepsin G in DPPI −/− mice do not explain the survival advantage of DPPI −/− mice.
The lack of confirmation of the mechanism defined in the peritonitis model prompted investigation of an alternative explanation for the improved survival of DPPI −/− mice following Klebsiella lung infection. Because neutrophil elastase and cathepsin G may regulate levels of SPD in some contexts [20], we investigated whether SPD was differentially expressed in DPPI −/− mice. Remarkably, SPD levels under physiologic and inflamed conditions were increased in the absence of active DPPI, indicating that DPPI regulates SPD levels in mice. Furthermore, the SPD purified from DPPI −/− mice has additional amino acids, suggesting these amino acids contribute to the increased SPD levels in DPPI −/− mice. Because neutrophils are rarely found in non-inflamed lungs, it is unlikely the lack of active neutrophil elastase or cathepsin G explain the elongated amino-terminus of SPD in DPPI −/− mice. Further, the finding that the amino-terminus of SPD isolated from DPPI −/− mice has two or eight additional amino acids, and that DPPI is made by type II cells suggest that DPPI processes the amino-terminus of SPD prior to secretion and that SPD processing by DPPI regulates both the level of SPD and the proportion of SPD that assembles into its multimeric form.
Surfactant proteins B and C undergo proteolysis prior to secretion and this processing is essential for biological activity [27,28]. The present study is novel because it reports the proteolytic processing of SPD under physiologic conditions and demonstrates the importance of this processing with respect to overall levels of SPD. The SPD amino-terminus has been sequenced for multiple species, including pigs, rats and humans [29,30,31]. These reported sequences of purified SPD correspond to the amino-termini of SPD identified in DPPI −/− mice rather than the truncated form identified in DPPI +/+ controls. The exception is a report of a minor amino-terminus in rats that is two amino acids shorter than the major sequence [30]. Truncation by two amino acids is consistent with processing by DPPI. One explanation for why the amino terminus of SPD isolated in prior studies corresponds to the sequence identified in DPPI −/− mice is that SPD in those studies was isolated either from patients with alveolar proteinosis [29,31,32] or from animals provoked to manufacture increased amounts of SPD [30]. This gives rise to the possibility that during circumstances of pathologic stress the unprocessed form of SPD predominates, contributing to increased levels of SPD in these diseased lungs.
The biological activity of SPD, including its ability to clear bacterial infections, depends on formation of oligomeric structures [11,12,33]. Another noteworthy finding in these studies is that the lungs of DPPI −/− mice have relatively higher amounts of the multimeric forms of SPD, suggesting that the relative increase in multimeric forms of SPD-- in addition to the increase in total SPD-- explain the enhanced bacterial clearance in DPPI −/− mice. Because SPD isolated from DPPI −/− mice has two or eight additional amino acids at its amino terminus, the data indicate that these eight amino acids play a key role either in the formation or stabilization of SPD in its oligomeric form.
In summary, the data presented here establish that DPPI is a major mediator of survival following K. pneumoniae lung infection and that absence of its activity is associated with improved survival. It identifies SPD as a novel DPPI substrate and establishes that amino terminal processing of SPD by DPPI influences SPD levels and multimer formation. Targeting DPPI with protease inhibitors could be a novel approach for manipulating levels of SPD and treating severe bacterial pneumonia.
DPPI −/− mice are protected from death following intranasal infection with Klebsiella pneumonia.
The survival advantage is due to enhanced bacterial clearance by DPPI −/− mice.
DPPI −/− mice have significantly higher levels of surfactant protein D in their lungs.
BAL fluid from DPPI −/− mice aggregate bacteria more effectively than control BAL fluid.
The amino terminus of Surfactant protein D is alternatively processed in DPPI −/− mice.
Acknowledgements
The authors thank Frank McCormack for helpful discussions and reagents for the bacterial aggregation assay.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was supported by NIH grants P01 HL024136 (GHC) and HL075026 (PJW).
References
- [1].Wolters PJ, Raymond WW, Blount JL, Caughey GH. Regulated expression, processing, and secretion of dog mast cell dipeptidyl peptidase I. J Biol Chem. 1998;273:15514–15520. doi: 10.1074/jbc.273.25.15514. [DOI] [PubMed] [Google Scholar]
- [2].Wolters PJ, Pham CT, Muilenburg DJ, Ley TJ, Caughey GH. Dipeptidyl peptidase I is essential for activation of mast cell chymases, but not tryptases, in mice. J Biol Chem. 2001;276:18551–18556. doi: 10.1074/jbc.M100223200. [DOI] [PubMed] [Google Scholar]
- [3].Wolters PJ, Laig-Webster M, Caughey GH. Dipeptidyl peptidase I cleaves matrix-associated proteins and is expressed mainly by mast cells in normal dog airways. Am J Respir Cell Mol Biol. 2000;22:183–190. doi: 10.1165/ajrcmb.22.2.3767. [DOI] [PubMed] [Google Scholar]
- [4].Pham CT, Ley TJ. Dipeptidyl peptidase I is required for the processing and activation of granzymes A and B in vivo. Proc Natl Acad Sci U S A. 1999;96:8627–8632. doi: 10.1073/pnas.96.15.8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McGuire MJ, Lipsky PE, Thiele DL. Purification and characterization of dipeptidyl peptidase I from human spleen. Arch Biochem Biophys. 1992;295:280–288. doi: 10.1016/0003-9861(92)90519-3. [DOI] [PubMed] [Google Scholar]
- [6].Adkison AM, Raptis SZ, Kelley DG, Pham CT. Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J Clin Invest. 2002;109:363–371. doi: 10.1172/JCI13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mallen-St. Clair J, Pham CTN, Villalta SA, Caughey GH, Wolters PJ. Mast Cell Dipeptidyl Peptidase I Mediates Survival from Sepsis. J Clin Invest. 2004;113:628–634. doi: 10.1172/JCI19062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McCormack FX, Whitsett JA. The pulmonary collectins, SP-A and SP-D, orchestrate innate immunity in the lung. J Clin Invest. 2002;109:707–712. doi: 10.1172/JCI15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- [10].Sahly H, Ofek I, Podschun R, Brade H, He Y, Ullmann U, Crouch E. Surfactant protein D binds selectively to Klebsiella pneumoniae lipopolysaccharides containing mannose-rich O-antigens. J Immunol. 2002;169:3267–3274. doi: 10.4049/jimmunol.169.6.3267. [DOI] [PubMed] [Google Scholar]
- [11].White M, Kingma P, Tecle T, Kacak N, Linders B, Heuser J, Crouch E, Hartshorn K. Multimerization of surfactant protein D, but not its collagen domain, is required for antiviral and opsonic activities related to influenza virus. J Immunol. 2008;181:7936–7943. doi: 10.4049/jimmunol.181.11.7936. [DOI] [PubMed] [Google Scholar]
- [12].Zhang L, Ikegami M, Crouch EC, Korfhagen TR, Whitsett JA. Activity of pulmonary surfactant protein-D (SP-D) in vivo is dependent on oligomeric structure. J Biol Chem. 2001;276:19214–19219. doi: 10.1074/jbc.M010191200. [DOI] [PubMed] [Google Scholar]
- [13].Hartshorn KL, Crouch EC, White MR, Eggleton P, Tauber AI, Chang D, Sastry K. Evidence for a protective role of pulmonary surfactant protein D (SP-D) against influenza A viruses. J Clin Invest. 1994;94:311–319. doi: 10.1172/JCI117323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].LeVine AM, Whitsett JA, Hartshorn KL, Crouch EC, Korfhagen TR. Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. J Immunol. 2001;167:5868–5873. doi: 10.4049/jimmunol.167.10.5868. [DOI] [PubMed] [Google Scholar]
- [15].LeVine AM, Elliott J, Whitsett JA, Srikiatkhachorn A, Crouch E, DeSilva N, Korfhagen T. Surfactant protein-d enhances phagocytosis and pulmonary clearance of respiratory syncytial virus. Am J Respir Cell Mol Biol. 2004;31:193–199. doi: 10.1165/rcmb.2003-0107OC. [DOI] [PubMed] [Google Scholar]
- [16].Restrepo CI, Dong Q, Savov J, Mariencheck WI, Wright JR. Surfactant protein D stimulates phagocytosis of Pseudomonas aeruginosa by alveolar macrophages. Am J Respir Cell Mol Biol. 1999;21:576–585. doi: 10.1165/ajrcmb.21.5.3334. [DOI] [PubMed] [Google Scholar]
- [17].Wu H, Kuzmenko A, Wan S, Schaffer L, Weiss A, Fisher JH, Kim KS, McCormack FX. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J Clin Invest. 2003;111:1589–1602. doi: 10.1172/JCI16889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fornstedt N, Porath J. Characterization studies on a new lectin found in seeds of Vicia ervilia. FEBS Lett. 1975;57:187–191. doi: 10.1016/0014-5793(75)80713-7. [DOI] [PubMed] [Google Scholar]
- [19].Stamme C, Walsh E, Wright JR. Surfactant protein A differentially regulates IFN-gamma- and LPS-induced nitrite production by rat alveolar macrophages. Am J Respir Cell Mol Biol. 2000;23:772–779. doi: 10.1165/ajrcmb.23.6.4083. [DOI] [PubMed] [Google Scholar]
- [20].Hirche TO, Crouch EC, Espinola M, Brokelman TJ, Mecham RP, DeSilva N, Cooley J, Remold-O’Donnell E, Belaaouaj A. Neutrophil serine proteinases inactivate surfactant protein D by cleaving within a conserved subregion of the carbohydrate recognition domain. J Biol Chem. 2004;279:27688–27698. doi: 10.1074/jbc.M402936200. [DOI] [PubMed] [Google Scholar]
- [21].Crouch E, Persson A, Chang D, Heuser J. Molecular structure of pulmonary surfactant protein D (SP-D) J Biol Chem. 1994;269:17311–17319. [PubMed] [Google Scholar]
- [22].Sutherland RE, Olsen JS, McKinstry A, Villalta SA, Wolters PJ. Mast cell IL-6 improves survival from Klebsiella pneumonia and sepsis by enhancing neutrophil killing. J Immunol. 2008;181:5598–5605. doi: 10.4049/jimmunol.181.8.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wolters PJ, Mallen-St Clair J, Lewis CC, Villalta SA, Baluk P, Erle DJ, Caughey GH. Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient Kit(W-sh)/Kit(W-sh) sash mice. Clin Exp Allergy. 2005;35:82–88. doi: 10.1111/j.1365-2222.2005.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Belaaouaj A, McCarthy R, Baumann M, Gao Z, Ley TJ, Abraham SN, Shapiro SD. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat Med. 1998;4:615–618. doi: 10.1038/nm0598-615. [DOI] [PubMed] [Google Scholar]
- [25].Tkalcevic J, Novelli M, Phylactides M, Iredale JP, Segal AW, Roes J. Impaired immunity and enhanced resistance to endotoxin in the absence of neutrophil elastase and cathepsin G. Immunity. 2000;12:201–210. doi: 10.1016/s1074-7613(00)80173-9. [DOI] [PubMed] [Google Scholar]
- [26].Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- [27].Beers MF, Mulugeta S. Surfactant protein C biosynthesis and its emerging role in conformational lung disease. Annu Rev Physiol. 2005;67:663–696. doi: 10.1146/annurev.physiol.67.040403.101937. [DOI] [PubMed] [Google Scholar]
- [28].Hawgood S, Derrick M, Poulain F. Structure and properties of surfactant protein B. Biochim Biophys Acta. 1998;1408:150–160. doi: 10.1016/s0925-4439(98)00064-7. [DOI] [PubMed] [Google Scholar]
- [29].Mason RJ, Nielsen LD, Kuroki Y, Matsuura E, Freed JH, Shannon JM. A 50-kDa variant form of human surfactant protein D. Eur Respir J. 1998;12:1147–1155. doi: 10.1183/09031936.98.12051147. [DOI] [PubMed] [Google Scholar]
- [30].Shimizu H, Fisher JH, Papst P, Benson B, Lau K, Mason RJ, Voelker DR. Primary structure of rat pulmonary surfactant protein D. cDNA and deduced amino acid sequence. J Biol Chem. 1992;267:1853–1857. [PubMed] [Google Scholar]
- [31].van Eijk M, van de Lest CH, Batenburg JJ, Vaandrager AB, Meschi J, Hartshorn KL, van Golde LM, Haagsman HP. Porcine surfactant protein D is N-glycosylated in its carbohydrate recognition domain and is assembled into differently charged oligomers. Am J Respir Cell Mol Biol. 2002;26:739–747. doi: 10.1165/ajrcmb.26.6.4520. [DOI] [PubMed] [Google Scholar]
- [32].Crouch E, Persson A, Chang D. Accumulation of surfactant protein D in human pulmonary alveolar proteinosis. Am J Pathol. 1993;142:241–248. [PMC free article] [PubMed] [Google Scholar]
- [33].Kingma PS, Whitsett JA. In defense of the lung: surfactant protein A and surfactant protein D. Curr Opin Pharmacol. 2006;6:277–283. doi: 10.1016/j.coph.2006.02.003. [DOI] [PubMed] [Google Scholar]