Abstract
The genetic relationships between different behaviors used to index the rewarding or reinforcing effects of alcohol are poorly understood. To address this issue, ethanol-induced conditioned place preference (CPP) was tested in a genetically diverse panel of inbred mouse strains and strain means from this study and other inbred strain studies were used to examine the genetic correlation between CPP and several ethanol-related phenotypes, including activity measures recorded during CPP training and testing. Mice from each strain were exposed to a well-characterized unbiased place conditioning procedure using ethanol doses of 2 or 4 g/kg; an additional group from each strain was exposed to saline alone on all trials. Genotype had a significant effect on CPP, basal locomotor activity, ethanol-stimulated activity and the effect of repeated ethanol exposure on activity. Correlational analyses showed significant negative genetic correlations between CPP and sweetened ethanol intake and between CPP and test session activity, as well as a significant positive genetic correlation between CPP and chronic ethanol withdrawal severity. Moreover, there was a trend toward a positive genetic correlation between CPP and ethanol-induced conditioned taste aversion. These genetic correlations suggest overlap in the genetic mechanisms underlying CPP and each of these traits. The patterns of genetic relationships suggest a greater impact of ethanol’s aversive effects on drinking and a greater impact of ethanol’s rewarding effects on CPP. Overall, these data support the idea that genotype influences ethanol’s rewarding effect, a factor that may contribute importantly to addictive vulnerability.
Keywords: alcohol, reward, activity, learning, inbred strains
The idea that genotype contributes to the development of alcoholism is widely recognized, but the specific genes and the mechanisms whereby they influence alcohol (ethanol) seeking and taking remain largely unknown. Laboratory animals have been used to model various aspects of the addictive process and such studies have shown substantial genetic variability across many phenotypes hypothesized to be important (Crabbe, 2008; Crabbe, Kendler & Hitzemann, 2013; Crabbe & Phillips, 2004). One phenotype domain of particular interest comprises the reinforcing/rewarding and punishing/aversive effects of ethanol, reflecting the belief that vulnerability to addiction depends, in part, on genetic variation in sensitivity to these effects (Tabakoff & Hoffman, 1988). Various forms of drug self-administration (e.g., drinking, operant responding) as well as drug conditioning procedures (e.g., conditioned place preference (CPP), conditioned taste aversion (CTA)) have been used successfully to demonstrate genetic differences in drug reward/aversion in animals (Cunningham & Phillips, 2003), lending credibility to this point of view.
Although older papers sometimes gave the impression that CPP and self-administration procedures could be used interchangeably in the study of drug reinforcement (Katz & Gormezano, 1979), it is now clear that the relationship between these phenotypes is complicated (Stephens et al., 2010). For example, while many drugs are reinforcing or rewarding in both procedures, there are sometimes differences in the underlying mechanisms as well as discrepancies in the abilities of certain drugs to produce CPP and drug self-administration (Bardo & Bevins, 2000). The challenge to understanding how these behaviors are related is especially well illustrated by the inconsistent patterns of genetic differences when rat or mouse strains are compared (Green & Grahame, 2008). In some cases, the direction of the strain difference appears similar across both phenotypes. For example, mouse lines selectively bred to prefer drinking solutions that contain ethanol (Phillips et al., 2005) or methamphetamine (Shabani et al., 2011; Wheeler et al., 2009) have been shown to develop a stronger CPP when injected with those drugs than mouse lines bred to avoid such solutions. Such findings suggest commonality in the mechanisms underlying these phenotypes. In other cases, however, the direction of the strain difference is opposite. For example, inbred DBA/2 (D2) mice show strong ethanol-induced CPP but drink very little ethanol, whereas inbred C57BL/6 (B6) mice show the opposite pattern, readily drinking ethanol but developing weaker CPP (Belknap et al., 1993; Cunningham et al., 1992).
Attempts to understand genetic relationships between phenotypes using only two inbred strains are risky because, unlike selectively bred mouse lines, such strains are more likely to differ in many traits that are genetically (mechanistically) unrelated due to random fixation of genes as the result of inbreeding. Thus, it is difficult to know whether the negative relationship between CPP and ethanol drinking observed in D2 and B6 mice reflects chance or a true genetic correlation (i.e., pleiotropic influence, whereby one or more gene influences both traits). One solution to this problem is to examine the relationship in a larger number of inbred strains that show a range of responses to each of the target phenotypes and then estimate the genetic correlation by correlating the mean phenotypic values for each strain. Significant genetic correlations are potentially indicative of overlap in the genes influencing the two phenotypes, suggesting a mechanistic link (Crabbe et al. 1990). This approach was previously applied using data from a study of CPP in the B6 and D2 strains together with 20 recombinant inbred (RI) strains derived from crosses of the B6 and D2 strains (i.e., the BXD RI strains; Cunningham, 1995). Correlation of the strain means for CPP with strain means for ethanol intake or preference (Phillips et al., 1994) yielded a non-significant (though positive) genetic correlation (Phillips et al., 1998), suggesting there was little overlap in the underlying genetic mechanisms.
Generalization of correlations from BXD studies to the broader population are limited, however, because these strains only carry alleles from the two progenitor strains (i.e., B6, D2), which may not adequately reflect the full range of potential genetic influences on these behaviors (Crabbe et al., 1990). To address this limitation, the present study was designed to examine ethanol-induced CPP in a more genetically diverse panel of 15 standard inbred strains that included the B6 and D2 strains. The primary goal was to characterize genetic differences in ethanol-induced CPP, ethanol-induced changes in activity, as well as the development of tolerance or sensitization to such changes. Secondary goals were to examine the genetic correlations between: (a) CPP and the activity phenotypes, and (b) CPP and other commonly studied ethanol phenotypes like ethanol drinking (Belknap et al., 1993) and ethanol-induced CTA (Broadbent et al., 2002).
Method
Subjects
Adult male mice from 15 standard inbred strains were shipped from the Jackson Laboratory (Bar Harbor, ME). The specific strains were: 129P3/J (previously 129/J), A/HeJ, AKR/J, Balb/cJ, C3H/HeJ, C57BL/6J, C57L/J, C58/J, CBA/J, DBA/1J, DBA/2J, NZB/B1NJ1, PL/J, SJL/J, and SWR/J. These strains were selected to maximize the number of strains overlapping with those used in other multi-strain studies of ethanol-related phenotypes (Belknap et al., 1993; Broadbent et al., 2002; Metten & Crabbe, 2005). Mice were housed in same-strain groups of two to four in polycarbonate cages (27.9 × 9.5 × 12.7 cm) with corncob bedding on a normal 12:12 light-dark cycle. Water and rodent chow were available at all times in the home cage.
A total of 967 mice were used, ranging in age from 54 to 70 days old. Twenty-four subjects (2.5%) were excluded from the final data analysis due either to procedural errors (n = 3) or because the mice became unhealthy or died for unknown reasons (n = 21). Subject attrition was distributed across 8 of the 15 strains; the largest loss in a single strain was for the PL/J strain (n = 6). The final number of mice that completed testing in each strain ranged from 58 to 68. Mice were tested in 14 cohorts ranging in size from 47 to 120 subjects (with the exception of 3 cohorts which contained fewer mice due to limited vendor availability), with testing spanning a 5-month period. Each cohort contained mice from 1 to 15 different strains and each strain was represented in at least five different cohorts.
Apparatus
The apparatus has been described in detail elsewhere (Cunningham, Gremel & Groblewski, 2006). Briefly, it consisted of 12 identical acrylic and aluminum boxes (30 × 15 × 15 cm) enclosed in separate light- and sound-attenuating enclosures (Coulbourn Instruments Model E10-20). Six sets of infrared light sources and detectors were mounted opposite each other at 5-cm intervals on the long walls of each box, 2.2 cm above the floor. General activity and position (left vs. right) were recorded every minute by computer (10-ms resolution).
The floor of each box consisted of interchangeable halves made of one of two textures. The “grid” (G) floor was composed of 2.3 mm stainless-steel rods mounted 6.4 mm apart in plexiglas rails. The “hole” (H) floor was made from perforated stainless steel (16 GA) with 6.4-mm round holes in 9.5-mm staggered centers. These floor textures were selected on the basis of previous studies showing that drug-naive saline only groups of B6 and D2 mice spend about half their time on each floor type during preference tests (Cunningham, 1995; Cunningham et al., 1992). The apparatus and floors were wiped down with a damp sponge and the litter paper was changed after each animal.
Procedure
The experiment involved three phases: habituation (one session), conditioning (eight sessions), and testing (one session). Sessions were conducted 5 days a week with a 2-day break between the first four and second four conditioning sessions. Each mouse was weighed and injected (IP) just before being placed in the center of the apparatus for each session.
Habituation
The habituation session was intended to reduce the novelty and stress associated with handling, injection and exposure to the apparatus. All mice were injected with saline and immediately placed in the center of the conditioning box on a smooth paper floor. Mice were removed from the apparatus after 5 min and returned to the home cage.
Conditioning
During the conditioning phase, mice from each strain were randomly assigned to one of two ethanol dose groups (2 or 4 g/kg) or to a saline only group. Dose was manipulated by varying the volume (12.5 or 25.0 ml/kg) of a 20% v/v solution of ethanol in saline (Linakis & Cunningham, 1979). These doses were chosen on the basis of several previous studies indicating that they produce a reliable conditioned preference in both inbred and outbred mice under training conditions similar to those used here (e.g., Cunningham et al., 1992; Cunningham, 1995; Risinger & Oakes, 1996). Saline only groups (0 g/kg) received saline injections in the same volume (25 ml/kg) as the high dose groups. All injections were intraperitoneal.
Within each dose group, mice were randomly assigned to one of two conditioning subgroups and exposed to an unbiased place conditioning procedure (Cunningham, Ferree & Howard, 2003). Mice in the GRID+ conditioning subgroups received ethanol before placement on the grid floor (CS+ trial) and saline before placement on the hole floor (CS− trial). In contrast, mice in the GRID− subgroups received saline before placement on the grid floor (CS− trial) and ethanol before placement on the hole floor (CS+ trial). The saline only groups received a saline injection before all trials, regardless of the alternating floor type. The order of exposure to CS+ and CS− was counterbalanced within each conditioning subgroup. Mice had access to the whole apparatus and floor texture was identical on both sides on each trial (i.e., one-compartment procedure). Four 5-min conditioning trials of each type were given over an 8-day period. Because the conditioning subgroups within each strain were matched for overall exposure to each floor type, and differed only in the floor-drug contingency, any differences between subgroups during a preference test can be attributed to learning based on the Pavlovian relationship between the CS+ floor and ethanol (Cunningham, 1993).
Place preference test
A 30-min floor preference test was given 24 h after the last conditioning trial. All mice were weighed and received a saline injection just before placement in an apparatus configured with half grid floor and half hole floor. Relative position of the floors (i.e., left vs. right) was counterbalanced within each subgroup. The primary dependent variable was the amount of time spent on the grid floor.
Data Analyses
Data were evaluated using analysis of variance (ANOVA). Strain, dose and conditioning subgroup (GRID+ vs. GRID−) were treated as between-group factors; session was included as a repeated measure. The coefficient of genetic determination (i.e., the sum of squares between strains divided by the total sum of squares from a one-way ANOVA) was used to estimate heritability for each phenotype (Belknap et al., 1993). Genetic (Pearson) correlations were calculated using the phenotype means for each strain (Crabbe et al., 1990). Differences among individuals within-strain provides a measure of non-genetically determined variation in behavior.
Results
Due to equipment problems, activity data for four mice were lost from one or two sessions; preference test data were lost for one mouse. These mice were excluded only from analyses where data were lost.
Habituation session activity
Mean activity rates for the 0, 2 and 4 g/kg groups during the habituation session are shown in Tables 1 to 3, respectively. As can be seen, there were substantial genetic differences in habituation session activity, with strain mean (±SEM) activity counts/min (averaged across dose groups) ranging from 30.4 ± 1.3 (C3H/HeJ) to 121.9 ± 1.7 (C57L/J). Two-way ANOVA (Strain x Dose) indicated a significant difference among strains, F(14,896) = 119.1, p < .0001. There were no differences between the subgroups randomly assigned to each dose. Heritability of the habituation session activity phenotype was relatively high in each dose group (.67–.69).
Table 1.
Saline group strain means (± SEM) for activity (counts/min) during habituation, conditioning and testing and for time spent on the grid floor (s/min) during testing.
| Strain | n | Habituation | Saline: Trial 1* | Saline: Trial 4* | Preference Test | Test Activity |
|---|---|---|---|---|---|---|
| (activity/min) | (activity/min) | (activity/min) | (s/min) | (activity/min) | ||
| 129P3/J | 13# | 63.1±5.7 | 47.7±2.5b | 37.6±3.2b | 40.7±2.7 | 35.2±2.4 |
| A/HeJ | 12 | 50.6±5.8 | 46.8±3.2 | 30.2±3.4b | 30.6±2.8 | 36.8±3.6 |
| AKR/J | 12 | 80.0±5.3 | 57.7±6.4 | 37.4±3.0d | 30.2±1.9 | 44.6±1.5 |
| BALB/cJ | 12 | 83.1±4.9 | 66.6±3.6c | 71.2±4.7 | 33.3±2.2 | 60.0±6.8 |
| C3H/HeJ | 13 | 29.8±3.6 | 33.7±1.6 | 25.0±2.0 | 35.5±0.9 | 29.8±0.7 |
| C57BL/6J | 12 | 123.7±11.0 | 77.6±7.3c | 36.9±1.8d | 32.9±2.0 | 51.2±4.6 |
| C57L/J | 13 | 117.7±4.2 | 84.5±4.7d | 57.8±3.8d | 36.1±2.1 | 55.6±3.6 |
| C58/J | 12 | 112.7±7.3 | 88.0±5.5a | 59.2±7.4d | 30.5±1.5 | 80.9±7.6 |
| CBA/J | 12 | 58.1±5.4 | 52.6±2.2 | 37.2±2.4b | 33.7±1.9 | 34.8±1.0 |
| DBA/1J | 13 | 61.5±5.3 | 64.1±3.9 | 33.5±2.8b | 32.4±2.7 | 38.5±2.9 |
| DBA/2J | 13 | 55.4±4.4 | 56.7±3.2 | 44.4±3.6 | 28.5±1.6 | 42.0±1.9 |
| NZB/B1NJ | 12 | 46.7±3.6 | 34.8±2.3a | 25.7±4.6b | 26.6±2.9 | 23.7±2.3 |
| PL/J | 12 | 59.3±1.9 | 56.9±1.9 | 44.8±2.4c | 32.7±1.0 | 32.3±2.0 |
| SJL/J | 12 | 57.2±4.6 | 37.4±3.2c | 25.3±2.4d | 33.0±2.4 | 25.1±2.7 |
| SWR/J | 12 | 80.3±5.0 | 42.9±2.8d | 34.4±1.6d | 35.7±2.3 | 33.7±1.7 |
| Grand Mean | 71.8±2.4 | 56.6±1.6 | 40.1±1.3 | 32.9±0.6 | 41.6±1.4 | |
| Heritability | 0.67 | 0.61 | 0.54 | 0.18 | 0.60 | |
These scores are the mean activity rates on the first two saline trials (“Trial 1”) or the last two saline trials (“Trial 4”).
except for Habituation where n = 12
d= p<0.0001, c= p<0.001, b= p<0.01, a= p<0.05, significant change from habituation session
Table 3.
4 g/kg group strain means (± SEM) for activity (counts/min) during habituation and conditioning.
| Strain | n* | Habituation | Saline:Trial 1 | Saline: Trial 4 | Ethanol: Trial 1 | Ethanol: Trial 4 | Tol/Sens** |
|---|---|---|---|---|---|---|---|
| (activity/min) | (activity/min) | (activity/min) | (activity/min) | (activity/min) | (activity/min) | ||
| 129P3/J | 26 | 61.0±3.2 | 47.8±2.2c | 27.5±1.9d | 27.7±4.3y | 17.5±2.5ax | 10.1±4.6a |
| A/HeJ | 24 | 51.6±2.8 | 51.4±2.7 | 24.0±2.3d | 29.6±3.8z | 41.0±4.4ay | 38.8±5.6d |
| AKR/J | 26 | 86.6±3.7 | 64.4±3.2d | 40.0±3.0d | 56.1±4.1 | 49.8±5.3 | 18.1±8.7a |
| BALB/cJ | 26 | 84.0±2.4 | 68.9±2.7c | 56.5±3.5d | 50.8±7.7w | 67.5±6.7 | 29.1±10.8a |
| C3H/HeJ | 24# | 29.1±2.3 | 33.4±1.6 | 27.4±1.4 | 68.9±5.4z | 39.4±5.9bw | −23.1±8.9a |
| C57BL/6J | 25 | 114.2±4.7 | 78.7±4.6d | 38.7±2.6d | 58.5±6.9w | 42.4±5.3 | 23.9±9.5a |
| C57L/J | 25 | 119.9±2.0 | 97.0±3.3d | 49.7±4.0d | 74.6±5.8x | 51.2±6.1b | 23.8±9.5a |
| C58/J | 25 | 118.0±5.2 | 87.9±4.9d | 66.3±4.9d | 118.2±7.4y | 115.7±6.7z | 19.1±9.6 |
| CBA/J | 25 | 54.3±2.8 | 55.8±2.4 | 41.3±2.2c | 65.6±6.3 | 61.1±4.2z | 9.9±7.1 |
| DBA/1J | 27 | 79.2±5.6 | 74.5±3.9 | 37.4±2.1d | 51.0±7.1x | 70.1±9.0y | 56.1±11.2d |
| DBA/2J | 25 | 58.5±3.8 | 60.0±3.7 | 49.9±4.5a | 39.7±6.5w | 69.3±9.0bw | 39.8±11.0b |
| NZB/B1NJ | 26 | 47.9±2.3 | 34.0±2.4d | 20.2±2.3d | 21.1±4.9w | 23.0±3.7 | 15.7±6.5a |
| PL/J | 22 | 63.3±1.6 | 62.2±2.4 | 42.0±2.3d | 54.1±8.6 | 49.6±7.4 | 15.7±12.2 |
| SJL/J | 26 | 65.0±4.0 | 48.2±2.4b | 21.3±1.9d | 28.0±4.2y | 29.0±3.9 | 27.9±6.1d |
| SWR/J | 24 | 86.2±5.9 | 51.0±4.2d | 39.9±12.9d | 68.1±8.0w | 52.1±11.1y | −5.0±7.9 |
| Grand Mean | 74.9±1.6 | 61.1±1.2 | 38.8±1.3 | 53.9±2.0 | 51.9±2.0 | 20.4±2.4 | |
| Heritability | 0.67 | 0.55 | 0.27 | 0.38 | 0.35 | 0.15 |
Grid+ and Grid− groups combined
except for Habituation and Saline:Trial 1 where n = 23
d= p<.0001, c= p<.001, b= p<.01, a= p<.05, significant difference from habituation session (Saline) or Ethanol Trial 1 (Ethanol)
z= p<.0001, y= p<.001, x= p<.01, w= p<.05, significant difference from Saline on the same trial
p values based on within strain comparison between Ethanol-Saline differences on Trial 4 vs Trial 1
Conditioning trial activity
Saline-only (0 g/kg) groups
Data from 0-g/kg groups were initially analyzed separately because they provide an opportunity to examine activity changes over trials without the confounding influence of intermittent ethanol exposure. Table 1 lists mean activity for each strain averaged across the first two saline trials (“Trial 1”) and the last two saline trials (“Trial 4”) for the saline-only (0 g/kg) groups. These data were averaged over trial pairs to index saline activity at approximately the same stage of training examined in the 2- and 4-g/kg groups, which received saline as the first or second trial of each pair in a counterbalanced manner. Most strains showed a significant decrease in activity between the habituation session and either the first or last saline conditioning trial, suggesting habituation of the initial activity response. The two exceptions were the C3H/HeJ and DBA/2J strains, which showed non-significant decreases. No strain showed a significant activity increase over sessions. A two-way repeated measures ANOVA (Strain x Sessions) that included habituation and the first and last saline trials confirmed that the decrease in activity varied as a function of genotype, yielding a significant interaction, F(28,338) = 9.4, p < .0001, in addition to significant main effects of Strain, F(14,169) = 33.6, p < .0001, and Sessions, F(2,338) = 249.9, p < .0001. Heritabilities of the saline trial 1 (.61) and saline trial 4 (.54) activity phenotypes were lower than for the habituation activity phenotype (.67).
Ethanol groups: Habituation and CS− (saline) trials
Most strains also showed a significant decrease in activity across saline sessions when 2 or 4 g/kg ethanol was given on intervening CS+ trials (Tables 2 and 3). The exceptions were once again C3H/HeJ, which showed no change over sessions, and the DBA/2J 2-g/kg group, which actually showed a significant increase in activity between habituation and the first saline conditioning trial (Table 2). The effects of genotype and ethanol dose (2 vs. 4 g/kg) on the change in activity after saline treatment over trials were supported by a three-way ANOVA (Strain x Dose x Sessions) that yielded significant Strain x Sessions, F(28,1454) = 28.3, p < .0001, and Dose x Sessions, F(2,1454) = 21.4, p < .0001, interactions, in addition to main effects of Strain, F(14,727) = 87.9, p < .0001, Dose, F(1,727) = 8.9, p < .005, and Sessions, F(2,1454) = 873.1, p < .0001.
Table 2.
2 g/kg group strain means (± SEM) for activity (counts/min) during habituation and conditioning.
| Strain | n* | Habituation | Saline: Trial 1 | Saline: Trial 4 | Ethanol: Trial 1 | Ethanol: Trial 4 | Tol/Sens** |
|---|---|---|---|---|---|---|---|
| (activity/min) | (activity/min) | (activity/min) | (activity/min) | (activity/min) | (activity/min) | ||
| 129P3/J | 25 | 59.4±3.6 | 52.4±2.8 | 36.4±2.0d | 92.7±5.9z | 45.6±3.4dw | −31.2±6.3d |
| A/HeJ | 25 | 58.9±2.9 | 56.8±2.8 | 29.6±2.9d | 90.4±6.2z | 64.7±4.7cz | 1.4±7.5 |
| AKR/J | 25 | 85.9±4.3 | 63.3±3.9d | 51.7±3.9d | 88.8±5.2y | 84.2±4.6z | 7.0±7.3 |
| BALB/cJ | 26 | 81.3±2.3 | 66.6±2.6d | 70.0±3.2b | 116.8±4.2z | 138.3±5.8az | 18.0±6.6a |
| C3H/HeJ | 24 | 32.0±1.6 | 34.1±1.3 | 30.2±1.6 | 93.5±5.6z | 53.0±3.3dz | −36.7±4.6d |
| C57BL/6J | 26 | 112.0±4.8 | 77.8±3.5d | 51.3±3.7d | 132.8±6.7z | 69.1±5.3dx | −37.3±7.7d |
| C57L/J | 26 | 125.9±3.0 | 88.2±5.5d | 59.0±3.3d | 153.5±8.4z | 91.8±9.0dy | −32.4±9.6b |
| C58/J | 25 | 116.2±5.8 | 91.7±4.6b | 69.3±4.5d | 151.7±8.9z | 118.2±11.5cz | −11.0±7.5 |
| CBA/J | 26 | 56.6±2.6 | 55.1±1.4 | 48.8±2.8 | 157.4±6.4z | 137.7±7.2az | −13.3±8.7 |
| DBA/1J | 28 | 77.4±4.1 | 75.5±4.9 | 55.7±4.6d | 126.3±6.2z | 137.9±6.5z | 31.5±10.9b |
| DBA/2J | 26 | 57.2±2.6 | 66.2±3.0b | 62.1±3.9 | 132.6±6.7z | 169.4±8.6cz | 41.0±9.8c |
| NZB/B1NJ | 26# | 48.7±2.4 | 35.5±2.7d | 25.7±2.5d | 30.8±3.3 | 25.0±3.3 | 5.9±4.4 |
| PL/J | 24 | 63.1±2.9 | 64.6±2.9 | 52.8±2.8c | 105.4±5.7z | 96.6±6.3z | 3.0±7.6 |
| SJL/J | 26 | 60.6±3.6 | 52.3±4.1 | 30.1±2.8d | 87.7±6.0z | 43.5±3.9dy | −22.1±6.4b |
| SWR/J | 24 | 77.1±3.2 | 45.1±1.6d | 38.1±2.1d | 108.1±7.4z | 69.3±3.5dz | −31.8±5.9d |
| Grand Mean | 74.4±1.6 | 61.9±1.2 | 47.5±1.1 | 111.9±2.3 | 90.2±2.7 | −6.8±2.3 | |
| Heritability | 0.69 | 0.48 | 0.44 | 0.51 | 0.64 | 0.29 |
Grid+ and Grid− groups combined
Except for Ethanol:Trial 1 where n = 24
d= p<.0001, c= p<.001, b= p<.01, a= p<.05, significant change from habituation session (Saline) or Ethanol Trial 1 (Ethanol)
z= p<.0001, y= p<.001, x= p<.01, w= p<.05, significant difference from Saline on the same trial
p values based on within-strain comparison between Ethanol-Saline differences on Trial 4 vs Trial 1
The Strain x Sessions and Dose x Sessions interactions, in the absence of a significant 3-way interaction, suggest that strain and ethanol dose independently influenced activity habituation on saline trials. For example, although all three dose groups showed similar activity rates during the habituation session before ethanol exposure, activity rates on the first and last saline conditioning trials differed as a function of ethanol dose (Tables 1–3). This observation was supported by significant main effects of Dose in two-way (Strain x Dose) ANOVAs applied separately to the data from the first, F(2,898) = 6.6, p < .01, and last, F(2,898) = 23.5, p < .0001, saline trials. Post-hoc pairwise comparisons showed that mean activity rates in the 2 (61.9 ± 1.2) and 4 (61.1 ± 1.2) g/kg groups were significantly higher than in the 0-g/kg group (56.6 ± 1.6) on the first saline trial (p’s < .01). On the fourth saline trial, mean activity rate in the 2-g/kg group (47.5 ± 1.1) was significantly higher than in either the 0- (40.0 ± 1.3) or 4- (38.8 ± 1.3) g/kg groups (p’s < .0001), which did not differ. This difference might reflect a generalized conditioned activity response induced by 2 g/kg ethanol (Cunningham & Noble, 1992). The two-way ANOVAs also yielded significant main effects of Strain on Trials 1, F(14,898) = 62.9, p < .0001 and 4, F(14,898) = 30.7, p < .0001, but no Strain x Dose interactions. As in the saline-only group, heritabilities for saline trial activity in the ethanol groups were lower than those for habituation trial activity (see Tables 2 and 3).
Ethanol groups: CS+ (ethanol) trials
Mean activity rates on the first and last ethanol conditioning trials are shown for the 2- and 4-g/kg groups (averaged across conditioning subgroups) in Tables 2 and 3. The lower ethanol dose initially had an activating effect in most strains. The overall mean activity rate for the 2-g/kg groups was 111.9 ± 2.3 counts per min on the first ethanol trial compared to only 61.9 ± 1.2 counts per min on their first saline trial. In contrast, the 4-g/kg dose initially tended to suppress activity in most strains as indicated by lower average activity rates on the first ethanol trial (53.9 ± 2.0) compared to the first saline trial (61.1 ± 1.2).
Interpretation of strain differences in absolute levels of activity after ethanol injection is complicated by the fact that strain means on the first ethanol trial were significantly correlated with strain means on the first saline trial for both the 2-, r = +.73, p < .01, and 4-, r = +.59, p < .05, g/kg groups. To correct for strain differences in basal activity and to simplify strain comparisons, ethanol’s acute activating effect was indexed by subtracting activity rate on the first saline trial from activity rate on the first ethanol trial (E1-S1) for all mice (Cunningham, 1995). This measure, depicted by the solid bars in Figure 1, was not genetically correlated with saline activity on the first trial in either dose group, p’s > .26. Heritabilities for this measure were .35 and .25 for the 2- and 4-g/kg groups, respectively.
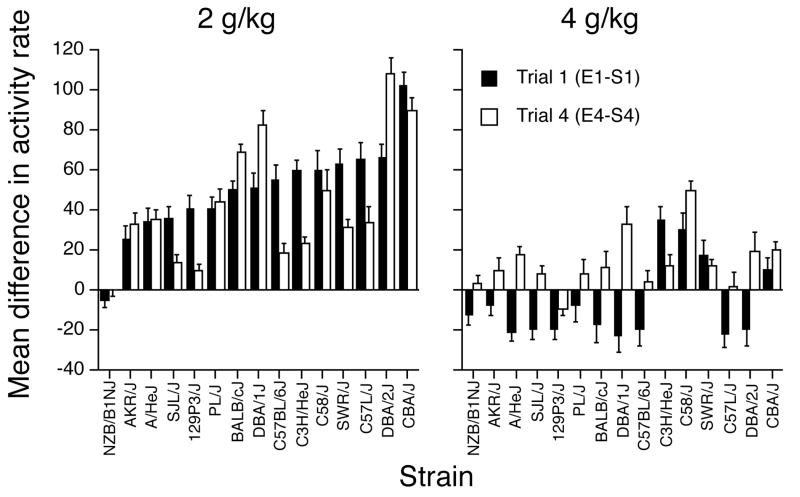
Figure 1.
Mean difference (+ SEM) in activity rate (counts per minute) between the ethanol and saline trials in the 2- (left panel) and 4- (right panel) g/kg ethanol groups on the first (solid bars) and last (open bars) pairs of conditioning trials. Activity is plotted as the difference between the ethanol and saline trials for each pair of trials (E1-S1, E4-S4). The strains are ordered according to magnitude of the trial 1 difference in the 2-g/kg groups.
As can be seen in the figure, there was substantial strain variation in the magnitude and direction of ethanol’s initial effect on activity at the 2 and 4 g/kg doses, with some strains showing very small differences between saline and ethanol and other strains showing relatively large differences. An overall two-way ANOVA (Strain x Dose) of trial 1 difference scores yielded significant main effects of Strain, F(14,726) = 15.1, p < .0001, and Dose, F(1,726) = 553.3, p < .0001, as well as a significant Strain x Dose interaction, F(14,726) = 7.4, p < .0001. Separate one-way follow-up ANOVAs confirmed that the Strain effect was significant at both the low, F(14,365) = 14.0, p < .0001, and high, F(14,361) = 8.7, p < .0001, dose. The interaction reflected the substantial difference in the pattern of strain differences at each dose. At 2 g/kg, all but one strain (NZB/B1NJ) showed an increase in activity, with mean activity changes ranging between 25 and 102 counts/min. In contrast, at 4 g/kg, most strains showed decreases in activity ranging from −8 to −23 counts/min. However, four strains (C3H/HeJ, C58/J, SWR/J, CBA/J) showed mean activity increases at the high dose ranging from 9 to 35 counts/min. The difference in the pattern of strain effects at each dose was also supported by the absence of a significant genetic correlation between the strain mean difference scores for the 2- and 4-g/kg dose groups on trial 1 [r = .35, p = .20].
Sensitization/Tolerance (ethanol trials)
The place conditioning procedure provides an opportunity to examine within-group tolerance or sensitization to ethanol’s effects on activity based on the comparison between responses to the first and to fourth ethanol injections. As noted previously (Cunningham, 1995), there are two alternative approaches for indexing this change in the response to ethanol. The first approach is simply to examine changes across trials in the absolute levels of activity elicited by ethanol injection (E4-E1). Heritability of this measure was .40 and .12 for the 2- and 4-g/kg groups, respectively. One-way ANOVAs revealed significant strain differences in the size and direction of the change in activity between trials 1 and 4 for both the 2-, F(14, 365) = 17.1, p < .0001, and 4-, F(14,361) = 3.6, p < .0001, g/kg groups. Within-strain post-hoc comparisons are shown in Tables 2 and 3.
At 2 g/kg, most strains (60%) showed a significant decrease in ethanol activity across trials, suggesting development of tolerance to ethanol-induced activation. However, two strains (BALB/cJ and DBA/2J) showed significant increases over trials in their response to 2 g/kg, consistent with development of sensitization to ethanol’s activating effect. At 4 g/kg, however, most strains (67%) showed no significant change in their ethanol response over trials. For the five strains that showed significant increases or decreases over trials in their activity response to 4-g/kg ethanol, interpretation is complicated by the direction of the acute response to ethanol (relative to saline) on trial 1. For example, because C3H/HeJ mice were initially activated by 4 g/kg, the significant activity decrease over trials suggests development of tolerance to ethanol-induced activation. In contrast, because activity in 129P3/J and C57L/J mice was initially suppressed by 4 g/kg, the significant decrease in their activity response over trials could reflect sensitization to ethanol’s depressant effect. The activity levels of two strains that showed a significant increase over trials in their response to 4 g/kg (A/HeJ and DBA/2J) were initially suppressed by that dose, suggesting development of tolerance to ethanol’s depressant effect.
One problem in using changes in the absolute ethanol response to index drug tolerance and sensitization is that this measure is potentially confounded by changes in activity that are unrelated to drug exposure (e.g., habituation to the test apparatus or handling/injection stress). Thus, in order to correct for such potential changes, the change over trials in the ethanol-saline difference score was also analyzed (Cunningham, 1995). The mean change across trials ([E4-S4] – [E1-S1]), which is represented graphically by the difference between adjacent bars in Figure 1, is listed for each strain in Tables 2 and 3 (Tol/Sens). Heritabilities for this measure were .29 and .15 for the 2- and 4-g/kg groups, respectively. One-way ANOVAs on the difference between Trials 4 and 1 revealed a significant effect of strain on the size and direction of activity change for both the 2-, F(14, 365) = 10.8, p < .0001, and 4-, F(14,361) = 4.5, p < .0001, g/kg groups.
At 2 g/kg, six strains showed no change across trials (NZB/B1NJ, AKR/J, A/HeJ, PL/J, C58/J, and CBA/J), suggesting no development of drug tolerance or sensitization (Table 2). Six strains showed a significant decrease in the ethanol-saline difference across trials (SJL/J, 129P3/J, C57BL/6J, C3H/HeJ, SWR/J, and C57L/J), whereas three strains showed significant increase in that difference across trials (BALB/cJ, DBA/1J, and DBA/2J). Because ethanol initially produced a stimulant effect in all of these strains, these decreases and increases over trials are consistent with the development of tolerance and sensitization to ethanol’s activating effect, respectively. At 4 g/kg, in contrast to findings based on changes in the absolute ethanol response, most strains (67%) showed a significant increase in the ethanol-saline difference over trials (Table 3). Because activity was reduced by ethanol on the first trial for all of these strains, this increase over trials is probably best interpreted as evidence of tolerance to ethanol’s depressant effect. However, given the findings at the lower dose, one cannot rule out the possible contribution of sensitization to ethanol’s activating effect in at least some of these strains (e.g., BALB/cJ, DBA/1J, and DBA/2J). Only one strain (C3H/HeJ) showed a significant decrease in the ethanol-saline difference over trials at 4 g/kg. Because this strain showed activation on the first ethanol trial at that dose, the decrease appears to reflect tolerance to ethanol-induced activation. This conclusion is consistent with that based on the analysis of changes across trials in the absolute ethanol response for the C3H/HeJ strain (see Table 4).
Table 4.
Summary of strain differences in development of tolerance and sensitization to ethanol’s effect on activity.
| Change over trials in Ethanol response [E4 – E1] | Change over trials in Ethanol-Saline difference [Tol/Sens] | |||
|---|---|---|---|---|
| Strain | 2 g/kg | 4 g/kg | 2 g/kg | 4 g/kg |
| 129P3/J | TA | SD | TA | TD |
| A/HeJ | TA | TD | - | TD |
| AKR/J | - | - | - | TD |
| BALB/cJ | SA | - | SA | TD |
| C3H/HeJ | TA | TA | TA | TA |
| C57BL/6J | TA | - | TA | TD |
| C57L/J | TA | SD | TA | TD |
| C58/J | TA | - | - | - |
| CBA/J | TA | - | - | - |
| DBA/1J | - | - | SA | TD |
| DBA/2J | SA | TD | SA | TD |
| NZB/B1NJ | - | - | - | TD |
| PL/J | - | - | - | - |
| SJL/J | TA | - | TA | TD |
| SWR/J | TA | - | TA | - |
T = Tolerance; S = Sensitization; - = no change over trials
A = Activating effect; D = Depressant effect
Conclusions about the development of ethanol tolerance and sensitization based on these two different phenotypes are summarized for each strain in Table 4. In general, conclusions about development of tolerance or sensitization in each strain were similar across these phenotypes at 2 g/kg. At 4 g/kg, however, conclusions about the significance and direction of the changes varied with phenotype. In only three cases (A/HeJ, C3H/HeJ, and DBA/2J) were conclusions derived from the analysis of these two phenotypes identical at the higher dose. Nevertheless, analysis showed a strong genetic correlation between these phenotypes for both the 2-, r = .95, p < .0001, and 4-, r = .78, p < .001, g/kg groups.
Place preference test
Ethanol groups
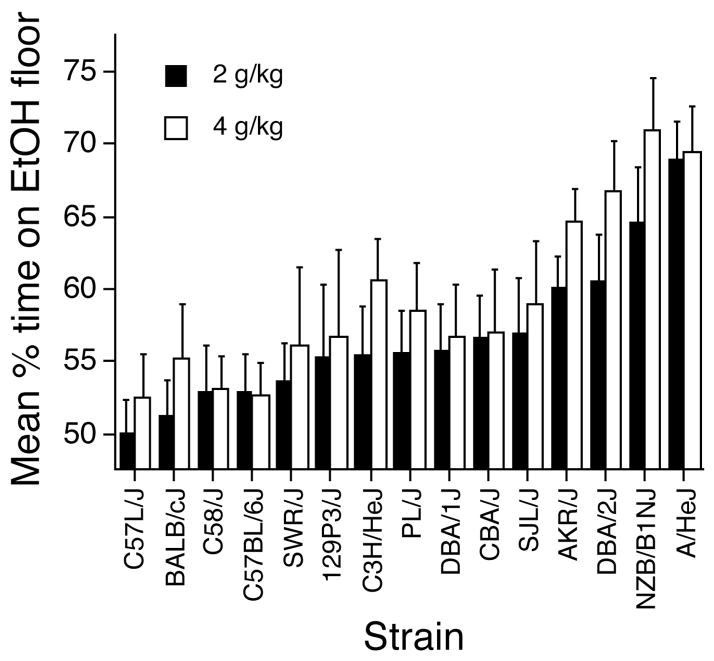
Mean times spent on the grid floor by both conditioning subgroups within each strain are listed in Tables 5 (2 g/kg) and 6 (4 g/kg). Overall, mice in the GRID+ subgroups spent more time on the grid floor than mice in the GRID- subgroups, providing evidence that both ethanol doses induced a conditioned floor preference (Cunningham et al., 2003). However, the size of the difference between conditioning subgroups varied widely across strains, indicating genetic differences in sensitivity to ethanol-induced place preference. To simplify strain and dose comparisons, the raw time data for both subgroups were converted to percent time on the ethanol-paired floor and averaged to create a single score for each strain at each dose (Figure 2). Heritability for this measure was .09 for both doses. As can be seen, some strains (e.g., C57L/J, BALB/cJ) spent about 50% of the session on the ethanol-paired floor (i.e., no preference), while others approached 70% (DBA/2J, NZB/B1NJ, A/HeJ). No strain in either dose group showed a conditioned place aversion (i.e., less than 50% on ethanol paired floor). Moreover, all strains showed a monotonic dose effect function, with preference at the higher (4 g/kg) dose either the same or slightly greater than preference at the lower (2 g/kg) dose. In no case did 4 g/kg produce a weaker preference than that seen at 2 g/kg in the same strain.
Table 5.
2 g/kg group strain means (± SEM) for time spent on the grid floor (s/min), percent time on the ethanol floor and activity (counts/min) during testing.
| Strain | (n) | GRID+ (s/min) | (n) | GRID− (s/min) | Percent EtOH Time* | Test Activity* (activity/min) |
|---|---|---|---|---|---|---|
| 129P3/J | 12 | 44.4±2.9 | 13 | 37.2±3.2 | 55.3±5.1 | 29.8±1.2 |
| A/HeJ | 13 | 46.3±1.4 | 12 | 24.0±1.8d | 69.0±2.5 | 33.7±1.8 |
| AKR/J | 12 | 34.8±2.2 | 13 | 22.7±1.3d | 60.2±2.1 | 47.4±2.0 |
| BALB/cJ | 13 | 34.1±1.5 | 13 | 32.6±2.0 | 51.3±2.4 | 59.9±2.7 |
| C3H/HeJ | 12 | 40.0±2.0 | 12 | 33.4±2.0a | 55.5±3.3 | 29.8±1.1 |
| C57BL/6J | 13 | 35.9±1.4 | 13 | 32.4±2.1 | 53.0±2.5 | 50.7±3.5 |
| C57L/J | 13 | 32.9±1.8 | 13 | 32.8±1.8 | 50.1±2.3 | 59.3±3.6 |
| C58/J | 13 | 33.6±2.9 | 12 | 30.3±2.3 | 52.9±3.1 | 75.5±3.1 |
| CBA/J | 12 | 40.2±1.1 | 14 | 31.2±2.2b | 56.7±2.9 | 35.1±0.9 |
| DBA/1J | 14 | 33.0±3.1 | 14 | 26.0±2.2 | 55.8±3.1 | 41.6±1.7 |
| DBA/2J | 13 | 38.4±2.1 | 13 | 25.8±3.1b | 60.5±3.2 | 46.0±2.1 |
| NZB/B1NJ | 13 | 42.7±2.5 | 13 | 25.1±3.4c | 64.7±3.7 | 23.8±1.6 |
| PL/J | 12 | 38.2±1.3 | 12 | 31.4±2.3a | 55.7±2.7 | 39.5±3.5 |
| SJL/J | 12 | 40.3±3.0 | 14 | 31.0±2.6a | 57.0±3.7 | 27.4±1.6 |
| SWR/J | 12 | 37.1±1.7 | 12 | 32.7±1.7 | 53.7±2.6 | 35.6±1.0 |
| Grand Mean | 38.1±0.6 | 29.9±0.7d | 56.8±0.8 | 42.4±0.9 | ||
| Heritability | 0.23 | 0.19 | 0.09 | 0.60 |
GRID+ and GRID− groups combined
d= p < .0001, c= p < .001, b= p < .01, a= p < .05, significant difference from GRID+
Figure 2.
Mean percent (+ SEM) time spent on the ethanol-paired floor by the 2- (solid bars) and 4- (open bars) g/kg ethanol groups during the 30-min preference test. Data are collapsed over conditioning subgroups (i.e., GRID+ vs. GRID−) within each strain. Strains are ordered according to magnitude of effect in the 2-g/kg ethanol groups.
Two different statistical approaches were used to analyze the preference test data (Cunningham et al., 2003). The first analysis examined raw time scores (i.e., sec/min on grid floor) using Strain, Dose and Conditioning Subgroup as factors. The second analysis examined preference scores (i.e., percent time on ethanol-paired floor) using Strain and Dose as factors. The three-way ANOVA on time scores yielded significant main effects of Strain, F(14,697) = 7.6, p < .0001, and Conditioning Subgroup, F(1,697) = 226.1, p < .0001. There was also a significant Dose x Conditioning Subgroup interaction, F(1,697) = 5.4, p < .03, reflecting slightly greater place preference at 4 g/kg than 2 g/kg. Strain differences in strength of place conditioning were supported by a significant Strain x Conditioning Subgroup interaction, F(14,697) = 6.6, p < .0001. Statistical comparisons between the conditioning subgroups (GRID+ vs. GRID−) within each strain at each dose are shown in Tables 5–6 and are summarized in Table 7. A two-way ANOVA (Strain x Dose) applied to preference scores supported conclusions from the analysis of raw time scores, yielding significant main effects of Strain, F(14,727) = 5.0, p < .0001, and Dose, F(1,727) = 4.2, p < .05, but no interaction. Thus, Strain did not interact with Dose in either analysis, supporting similar effects of these two ethanol doses in these strains.
Table 6.
4 g/kg group strain means (± SEM) for time spent on the grid floor (s/min), percent time on the ethanol floor and activity (counts/min) during testing.
| Strain | (n) | GRID+ (s/min) | (n) | GRID− (s/min) | Percent Drug Time* | Test Activity* (activity/min) |
|---|---|---|---|---|---|---|
| 129/J | 12 | 50.1±2.1 | 14 | 39.8±3.3a | 56.7±6.0 | 24.3±1.3 |
| A/HeJ | 13 | 45.0±2.3 | 11 | 22.2±2.7d | 69.5±3.1 | 28.5±2.8 |
| AKR/J | 13 | 38.6±1.6 | 13 | 21.1±2.3d | 64.6±2.3 | 38.5±2.3 |
| BALB/cJ | 14 | 35.3±3.3 | 12 | 29.5±2.9 | 55.1±3.7 | 47.7±3.1 |
| C3H/HeJ | 12 | 43.0±1.5 | 12 | 30.3±1.6d | 60.5±2.9 | 30.6±1.1 |
| C57BL/6J | 12 | 32.3±2.0 | 13 | 29.1±1.8 | 52.7±2.2 | 38.1±2.6 |
| C57L/J | 13 | 36.7±1.8 | 12 | 34.2±2.4 | 52.4±3.1 | 51.6±2.6 |
| C58/J | 12 | 33.8±1.9 | 13 | 29.9±1.8 | 53.2±2.2 | 74.3±4.5 |
| CBA/J | 13 | 42.4±1.8 | 12 | 34.6±3.7 | 57.0±4.4 | 31.6±1.1 |
| DBA/1J | 13 | 33.4±3.5 | 14 | 25.5±2.8 | 56.6±3.6 | 33.3±1.4 |
| DBA/2J | 12 | 41.3±3.0 | 13 | 21.1±2.8d | 66.7±3.4 | 38.4±2.8 |
| NZB/B1NJ | 13 | 43.0±3.0 | 13 | 17.8±3.1d | 71.0±3.5 | 20.2±1.5 |
| PL/J | 11 | 40.7±1.5 | 11 | 30.4±2.8b | 58.5±3.3 | 37.0±4.1 |
| SJL/J | 12 | 43.1±3.4 | 13 | 31.7±2.8a | 59.0±4.4 | 20.5±1.3 |
| SWR/J | 12 | 39.5±3.7 | 12 | 32.2±4.8 | 56.1±5.3 | 37.1±10.3 |
| Grand Mean | 39.8±0.7 | 28.6±0.8d | 59.3±1.0 | 36.7±1.1 | ||
| Heritability | 0.23 | 0.27 | 0.09 | 0.37 |
GRID+ and GRID− groups combined
d= p < .0001, c= p < .001, b= p < .01, a= p < .05, significant difference from GRID+
Table 7.
Summary of analyses to determine whether significant CPP was obtained in each strain (see text).
| Strain | GRID+ vs. GRID− | PDT vs. 50% | GRID± vs. Saline-Only Group | |||||
|---|---|---|---|---|---|---|---|---|
| 2 g/kg | 4 g/kg | 2 g/kg | 4 g/kg | 2:GRID+ | 2:GRID− | 4:GRID+ | 4:GRID− | |
| 129P3/J | -- | a | -- | -- | -- | -- | -- | -- |
| A/HeJ | d | d | d | d | c | -- | a | -- |
| AKR/J | d | d | d | d | -- | a | a | a |
| BALB/cJ | -- | -- | -- | -- | -- | -- | -- | -- |
| C3H/HeJ | a | d | -- | b | -- | -- | b | a |
| C57BL/6J | -- | -- | -- | -- | -- | -- | -- | -- |
| C57L/J | -- | -- | -- | -- | -- | -- | -- | -- |
| C58/J | -- | -- | -- | -- | -- | -- | -- | -- |
| CBA/J | b | -- | a | -- | b | -- | b | -- |
| DBA/1J | -- | -- | -- | -- | -- | a | -- | -- |
| DBA/2J | b | d | b | d | b | -- | b | -- |
| NZB/B1NJ | b | d | c | d | -- | -- | -- | -- |
| PL/J | a | b | a | a | b | -- | b | -- |
| SJL/J | a | a | -- | a | a | -- | a | -- |
| SWR/J | -- | -- | -- | -- | -- | a | -- | -- |
d= p < .0001, c= p < .001, b= p < .01, a= p < .05; Note: In all cases, the difference showed place preference, not aversion.
Also listed in Tables 5–6 are the mean activity rates during the 30-min preference test (GRID+ and GRID− subgroups collapsed). There were strain differences in test activity in both the 2-, F(14,367) = 39.0, p < .0001, and 4-, F(14,360) = 15.0, p < .0001, g/kg groups.
Saline-only groups
Table 1 shows the strain means for time spent on the grid floor by the saline-only groups. Overall, the saline-only groups spent an average of 32.9 ± 0.6 s/min on the grid floor during the preference test. Percentage time on drug-paired floor was not used for the saline only groups because neither floor was paired with ethanol. Since saline-only animals were exposed to each floor type as often as experimental animals, their performance can be viewed as a measure of unconditioned preference for the two floor types. As can be seen, most strains (11/15) had preference scores in the middle of the response range (30 ± 3.7 s/min on grid), although a few showed a stronger bias in favor of the grid floor (C3H/HeJ, SWR/J, C57L/J, NZB/B1NJ). One-way ANOVA indicated a significant difference in unconditioned floor preference among strains, F(14,170) = 2.6, p < .002. Table 7 summarizes separate comparisons between the saline-only group and each conditioning subgroup using grid time scores. Finally, the saline-only groups showed a significant strain difference in test session activity levels, F(14,170) = 18.0, p < .0001, which are listed in Table 1.
Genetic correlations
Table 8 lists genetic correlations among the various activity phenotypes measured during the habituation and conditioning sessions, while Table 9 lists genetic correlations between various activity phenotypes and phenotypes measured during the final preference test. Table 10 lists genetic correlations between CPP (indexed as percent time on the ethanol-paired floor) with several ethanol-related phenotypes measured in previous multi-strain studies, including ethanol blood concentration (BEC)(Crabbe et al., 1994), ethanol-induced CTA (Broadbent et al., 2002), ethanol drinking (Belknap et al., 1993) and chronic ethanol withdrawal severity (Metten & Crabbe, 2005).
Table 8.
Genetic correlations (Pearson r) between activity phenotypes during habituation and conditioning.
| Phenotype* | HAB -0 |
HAB- 2 |
HAB- 4 |
S1-0 | S1-2 | S1-4 | S4-0 | S4-2 | S4-4 | (S4- HAB)- 0 |
(S4- HAB)- 2 |
(S4- HAB)- 4 |
E1-2 | E1-4 | (E1- S1)-2 |
(E1- S1)-4 |
E4-2 | E4-4 | (E4- S4)-2 |
(E4- S4)-4 |
Tol/Se ns-2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HAB-0 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| HAB-2 | .97d | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| HAB-4 | .98d | .99d | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| S1-0 | .87d | .90d | .87d | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| S1-2 | .79c | .86d | .83d | .96d | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| S1-4 | .85d | .92d | .89d | .97d | .97d | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| S4-0 | .60a | .61a | .60a | .75b | .68b | .71b | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| S4-2 | .60a | .64a | .64a | .84d | .82c | .81c | .87d | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| S4-4 | .63a | .64a | .65a | .80c | .74b | .76b | .88d | .94d | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| (S4-HAB)-0 | −.87d | −.83d | −.84d | −.61a | −.56a | −.63a | −.14 | −.22 | −.25 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| (S4-HAB)-2 | −.82c | −.83d | −.82c | −.56a | −.53a | −.61a | −.17 | −.10 | −.15 | .92d | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| (S4-HAB)-4 | −.86d | −.87d | −.88d | −.62a | −.61a | −.67b | −.22 | −.23 | −.21 | .93d | .96d | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| E1-2 | .56a | .58a | .56a | .74b | .73b | .75b | .57a | .73b | .74b | −.35 | −.22 | −.25 | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| E1-4 | .57a | .59a | .58a | .64a | .54a | .59a | .49 | .57a | .74b | −.41 | −.35 | -.28 | .67b | -- | -- | -- | -- | -- | -- | -- | -- |
| (E1-S1)-2 | .22 | .19 | .19 | .34 | .31 | .35 | .31 | .44 | .51 | −.09 | .07 | .08 | .87d | .55a | -- | -- | -- | -- | -- | -- | -- |
| (E1-S1)-4 | −.08 | −.12 | −.10 | −.10 | −.22 | −.18 | −.05 | −.04 | .22 | .07 | .13 | .27 | .14 | .68b | .35 | -- | -- | -- | -- | -- | -- |
| E4-2 | .17 | .22 | .22 | .51 | .55a | .51 | .59a | .83c | .75b | .15 | .31 | .19 | .72b | .35 | .61a | -.03 | -- | -- | -- | -- | -- |
| E4-4 | .41 | .48 | .49 | .67b | .66b | .62a | .62a | .79c | .87d | −.14 | −.05 | −.08 | .68b | .77c | .47 | .38 | .75b | -- | -- | -- | -- |
| (E4-S4)-2 | −.06 | .00 | .00 | .30 | .36 | .31 | .39 | .65b | .57a | .31 | .46 | .36 | .62a | .21 | .62a | −.02 | .96d | .64b | -- | -- | -- |
| (E4-S4)-4 | .11 | .21 | .21 | .37 | .42 | .34 | .22 | .46 | .53a | .00 | .06 | .06 | .45 | .61a | .33 | .43 | .57a | .88d | .56a | -- | -- |
| Tol/Sens-2 | −.29 | −.18 | −.18 | .05 | .15 | .06 | .20 | .39 | .23 | .47 | .51 | .38 | −.05 | −.26 | −.18 | −.37 | .62 | .35 | .66b | .38 | -- |
| Tol/Sens-4 | .16 | .28 | .26 | .38 | .55a | .45 | .21 | .38 | .16 | −.08 | −.09 | −.24 | .19 | −.27 | −.13 | −.74b | .46 | .25 | .44 | .28 | .67b |
d= p < 0.0001, c= p < 0.001, b= p < 0.01, a= p < 0.05
The suffix (−0, −2, −4) indicates the ethanol dose group; HAB = habituation day activity rate; S1 = saline trial 1 activity rate; S4 = saline trial 4 activity; E1 = ethanol trial 1 activity rate; E4 = ethanol trial 4 activity rate; E1-S1 = difference in activity rates between ethanol trial 1 and saline trial 1; E4-S4 = difference in activity rates between ethanol trial 4 and saline trial 4; Tol/Sens = difference between E4-S4 and E1-S1
Table 9.
Genetic correlations (Pearson r) between CPP and activity phenotypes.
| Phenotype | GT-0 | PDT-2 | PDT-4 | TACT-0 | TACT-2 | TACT-4 |
|---|---|---|---|---|---|---|
| GT-0 | -- | -- | -- | -- | -- | -- |
| PDT-2 | −.58a | -- | -- | -- | -- | -- |
| PDT-4 | −.63a | .94d | -- | -- | -- | -- |
| TACT-0 | −.04 | −.48 | −.52a | -- | -- | -- |
| TACT-2 | −.09 | −.53a | −.53a | .97d | -- | -- |
| TACT-4 | −.06 | −.52a | −.52a | .94d | .96d | -- |
| HAB-0 | .12 | −.59a | −.66a | .77c | .79c | .71b |
| HAB-2 | .06 | −.55a | −.62a | .79c | .82c | .74b |
| HAB-4 | .08 | −.59a | −.66a | .77c | .81c | .73b |
| (E1-S1)-2 | .38 | −.51 | −.57a | .30 | .32 | .37 |
| (E1-S1)-4 | .09 | −.22 | −.18 | .15 | .10 | .35 |
| (E4-S4)-2 | −.22 | −.08 | −.07 | .26 | .34 | .31 |
| (E4-S4)-4 | −.41 | −.01 | −.10 | .51 | .50 | .59a |
| Tol/Sens-2 | −.64b | .39 | .46 | .04 | .12 | .03 |
| Tol/Sens-4 | −.40 | .22 | .11 | .21 | .26 | .07 |
d= p< .0001, c= p< .001, b= p< .01, a= p< .05
Table 10.
Genetic correlations (Pearson r) between CPP and other ethanol phenotypes.
| Reference | Phenotype | n | CPP PDT-2 | CPP PDT-4 |
|---|---|---|---|---|
| Belknap et al. (1993) | ||||
| 3% ethanol intake | 13 | −.28 | −.47 | |
| 6% ethanol intake | 13 | −.10 | −.17 | |
| 10% ethanol intake | 13 | −.40 | −.49 | |
| 3% ethanol + saccharin intake | 13 | −.49 | −.57a | |
| 6% ethanol + saccharin intake | 13 | −.54 | −.62a | |
| 10% ethanol + saccharin intake | 13 | −.63a | −.69b | |
| Saccharin alone intake | 13 | −.37 | −.46 | |
| Broadbent et al. (2002) | ||||
| Conditioned Taste Aversion (2 g/kg) | 15 | −.32 | −.29 | |
| Conditioned Taste Aversion (4 g/kg) | 15 | −.45 | −.50 | |
| Crabbe et al. (1994) | ||||
| Blood Ethanol Concentration (1 g/kg) | 13 | .03 | .03 | |
| Blood Ethanol Concentration (2 g/kg) | 13 | −.15 | −.26 | |
| Blood Ethanol Concentration (3 g/kg) | 13 | −.28 | −.26 | |
| Blood Ethanol Concentration (4 g/kg) | 13 | −.23 | −.22 | |
| Metten & Crabbe (2005) | ||||
| Chronic ethanol withdrawal (Δ AREA25) | 11 | .53 | .67a | |
p < .05
p < .01
Habituation and conditioning activity (Table 8)
The genetic correlations among the various saline session phenotypes were quite strong (HAB, S1, S4). In general, the correlations between dose groups were greatest when compared at the same point in training (Habituation: .97 ≤ r ≤ .99; Trial 1: .96 ≤ r ≤ .97; Trial 4: .87 ≤ r ≤ .94). The decrease in magnitude of correlations across phases of training within each dose group most likely reflects the genetic differences in rates of habituation described earlier. The somewhat lower genetic correlations between dose groups on the final saline trial compared to the earlier trials possibly reflects an effect of the ethanol dose experienced on intervening CS+ conditioning trials. The strain mean change in activity between the habituation session and last saline trial (S4-HAB) was negatively correlated with the strain mean activity during the habituation session, −.88 ≤ r ≤ −.83, p’s < .0001. That is, strains showing higher levels of activity during the habituation session tended to show larger decreases in activity across sessions.
As noted earlier, absolute activity levels on ethanol trials (E1, E4) were correlated with activity levels on saline trials (S1, S4). However, those correlations generally became non-significant when ethanol-induced activation was defined by the difference between activity on the ethanol and saline trials (E1-S1, E4-S4). Of particular interest, there was no significant genetic correlation between the strain mean difference scores at 2 and 4 g/kg on trial 1, r = .35, p = .20, suggesting little overlap in the genes that control the initial activity responses to these two ethanol doses. Finally, although there was a significant genetic correlation between the Tol/Sens phenotypes [(E4-S4)-(E1-S1)] in the 2- and 4-g/kg groups, r = .67, p < .01, most of the activity phenotypes were not predictive of this index of change in ethanol-induced activation.
Preference and activity (Table 9)
There was a strong genetic correlation between CPP (percent time on the ethanol-paired floor, PDT) induced by the 2- and 4-g/kg dose groups, r = .94, p < .0001. Furthermore, consistent with a previous multi-strain study (Cunningham 1995), there were several significant negative genetic correlations between CPP and test activity (TACT), r = −.53 and −.52, p < .05. These correlations suggest that test activity levels influenced CPP expression (Gremel & Cunningham, 2007), although the reverse could also be argued, i.e., strain differences in CPP expression might have caused the differences in test activity. However, there were also significant negative genetic correlations between CPP and activity measured during the habituation session before exposure to any conditioning trials, −.66 ≤ r ≤ −.55, p < .05, contradicting the idea that CPP strain differences caused activity strain differences.
There were also significant negative genetic correlations between unconditioned preference in saline-only mice (GT-0) and percent time spent on the ethanol-paired floor (PDT) by ethanol-treated mice, r = −.58 and −.63, p < .05, indicating that strain differences in CPP were influenced by strain differences in unconditioned preference (i.e., CPP in ethanol-treated mice was generally higher in strains where saline-only mice showed lower times on the grid floor).
Other ethanol phenotypes (Table 10)
Genetic correlations were calculated between CPP and BECs taken 30 min after an acute injection of 1, 2, 3 or 4 g/kg ethanol in 13 of the 15 strains tested here (Crabbe et al., 1994). None of those correlations was significant for either of the CPP dose groups (smallest p > .35), suggesting that strain differences in CPP magnitude are not explained by differences in ethanol pharmacokinetics. Genetic correlations between CPP at each dose and CTA induced by 2 or 4 g/kg (Broadbent et al., 2002) were also not significant, although there was a statistical trend suggesting that strains showing stronger CTA at 4 g/kg also tended to show stronger CPP at 4 g/kg (p = .056).
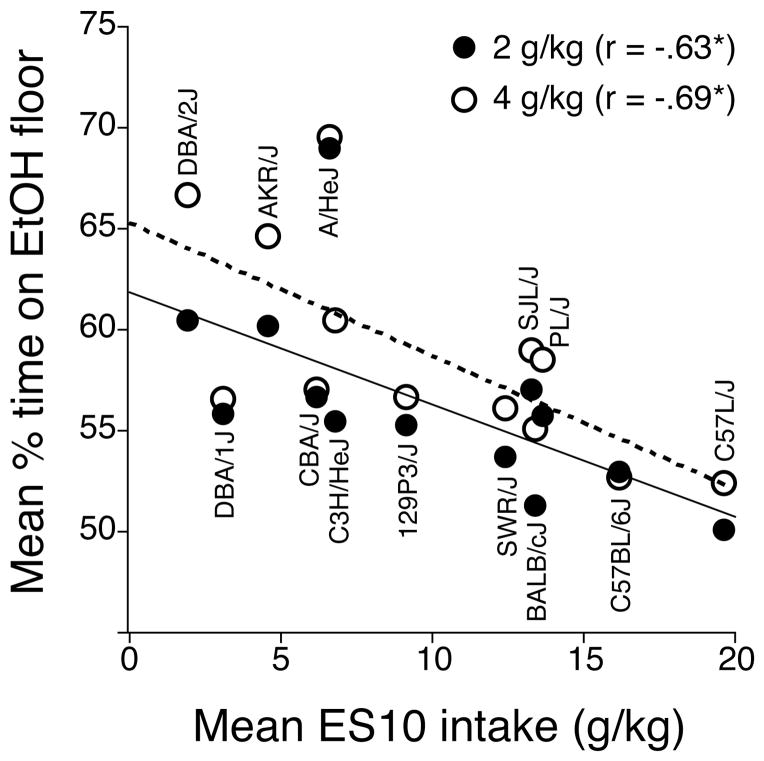
Strain means for CPP at each ethanol dose were also examined for correlation with strain mean 24-h ethanol intakes (g/kg) for increasing concentrations of ethanol (3, 6 or 10% v/v) either alone (E3CON, E6CON, E10CON) or mixed with 0.2% saccharin (ES3CON, ES6CON, ES10CON) in a two-bottle choice procedure (Belknap et al., 1993). These genetic correlations were consistently negative (i.e., higher ethanol intake predicted weaker CPP), reaching statistical significance for several of the correlations with sweetened ethanol intake (Table 10). There were non-significant genetic correlations (smallest p > .11) between CPP and intake of 0.2% saccharin alone (ASACCON). A scatterplot of the genetic correlation between sweetened 10% ethanol intake and CPP at each dose is shown in Figure 3.
Figure 3.
Scatterplot showing the genetic correlations between mean percent time on the ethanol-paired floor (y-axis) and mean oral intake (g/kg) of 10% ethanol and saccharin (ES10, x-axis) for the 2- (solid symbols, solid line) and 4- (open symbols, dashed line) g/kg groups. Each point represents the strain mean scores for both phenotypes. The points for each strain are arranged vertically. Means for ES10 intake are from Belknap et al. (1993).
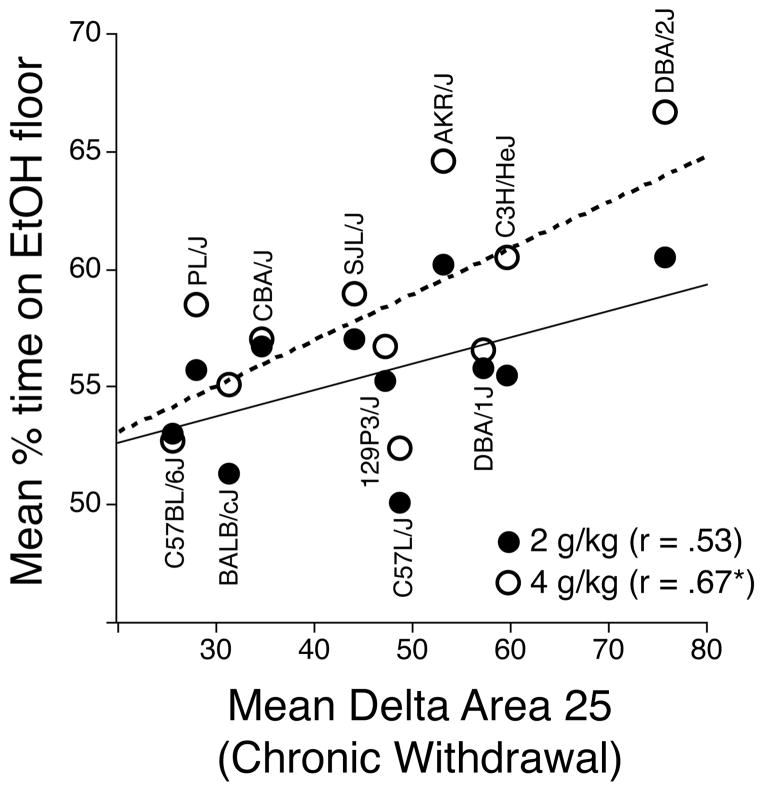
Finally, genetic correlations were calculated between CPP at each ethanol dose and chronic ethanol withdrawal severity after 72 h ethanol vapor exposure in 11 of the 15 inbred strains (Metten & Crabbe, 2005). As shown in the scatterplot in Figure 4, the correlations were positive for both CPP doses, with strains showing greater withdrawal severity also tending to show stronger CPP. The genetic correlation was significant for the 4-g/kg CPP groups, but fell short of the criterion for significance in the 2-g/kg groups (p = .09).
Figure 4.
Scatterplot showing the genetic correlations between mean percent time on the ethanol-paired floor (y-axis) and chronic ethanol withdrawal for the 2- (solid symbols, solid line) and 4- (open symbols, dashed line) g/kg groups. Each point represents the strain mean scores for both phenotypes. The points for each strain are arranged vertically. Means for Chronic Withdrawal (mean delta area 25) are from Metten & Crabbe (2005).
Discussion
This study is the first to assess ethanol-induced CPP in a large number (n = 15) of genetically diverse inbred mouse strains. The results show a significant effect of genotype at both doses, generally confirming the results of many previous studies showing a genetic influence on ethanol-induced CPP in mice (e.g., Chester et al., 1998; Crabbe et al., 1992; Cunningham, 1995; Cunningham et al., 1992, 2000). Consistent with previous dose-effect studies (Cunningham et al., 1992; Groblewski et al., 2008), CPP magnitude was positively related to ethanol dose, although the increment produced by the higher dose varied significantly across strains. Also consistent with many previous studies, there were significant genetic differences in basal locomotor activity, ethanol-stimulated activity and the effect of repeated ethanol exposure on ethanol stimulation (e.g., Crabbe et al., 1994; Phillips et al., 1995).
Heritabilities for basal activity (habituation) were relatively high in all groups, indicating that genetic differences explained about two thirds of the variation. However, genotype had a smaller influence on ethanol-stimulated activity (corrected for baseline, E1-S1), determining only 25–35% of phenotypic variation. Furthermore, genetic differences accounted for even less of the variation (15–29%) in the change in activity after repeated ethanol exposure (Tol/Sens). CPP (indexed as percent time on the ethanol-paired floor) had the lowest heritability, with genotype explaining only 9% of phenotypic variation at either dose. Thus, the genetic influence on CPP is substantially smaller than that for other commonly studied measures of ethanol’s motivational effects such as ethanol drinking (23% of variation for 10% ethanol intake, Belknap et al., 1993) or ethanol-induced conditioned taste aversion (24–43% of variation, Broadbent et al., 2002; Risinger & Cunningham, 1998). The implication is that most of the variation in CPP is attributable to non-genetic (environmental) influences or gene x environment interactions.
Despite the low heritability of CPP, analysis revealed significant negative genetic correlations between CPP and sweetened ethanol intake (Belknap et al., 1993) and a significant positive genetic correlation between CPP and chronic ethanol withdrawal severity (Metten & Crabbe, 2005). Furthermore, there was a strong trend toward a genetic correlation between CPP and ethanol-induced CTA (Broadbent et al., 2002). These genetic correlations suggest overlap in the genetic mechanisms underlying CPP and each of these traits (Crabbe et al., 1990). The absence of a significant genetic correlation between CPP and BEC suggests that these links are not simply a byproduct of strain differences in ethanol pharmacokinetics.
These data show that the negative genetic relationship between CPP and ethanol drinking originally observed in the two-strain comparison of B6 and D2 mice (Cunningham et al., 1992) is maintained when a more diverse set of inbred strains is considered. Further, these data suggest that the absence of a significant genetic correlation in the BXD RI strains (Phillips et al., 1998) was likely due to limited genetic diversity in that genetic model, which involves alleles from only two inbred strains. The direction of the genetic correlation in the current study is opposite that reported in a recent study of ethanol-induced CPP in mouse lines selectively bred for high (STDRHI) or low (STDRLO) alcohol intake (Phillips et al., 2005), but the direction is the same as that reported in the HAP and LAP selectively bred mouse lines (Grahame et al., 2001). The latter study showed that low drinking LAP mice developed stronger ethanol-induced CPP at 4 g/kg than high drinking HAP mice, although both lines acquired similar CPP at lower doses (1.5 and 3 g/kg). One possible reason for the discrepancy between these studies is the genetic characteristics of the parental population. The STDRHI/STDRLO lines were bred from the second generation cross of mice from the B6 and D2 strains (B6D2F2) whereas HAP/LAP mice were bred from the HS/Ibg strain (Grahame et al., 1999), created from a cross of eight inbred mouse strains that included six of the strains tested here (A, AKR, BALB/c, C3H, B6 and D2). Thus, like the current panel of inbred strains, the HAP/LAP lines reflect a wider range of genetic diversity than is represented in the STDRHI/STDRLO lines or BXD RI strains.
One simple interpretation of the negative correlation between CPP and drinking is that low-drinking strains experience greater ethanol-induced reward than high-drinking strains. In other words, low drinking strains might drink less ethanol because they need less to experience ethanol reward. Conversely, high drinking strains might drink more because they need more to experience ethanol reward. Although that analysis might apply to some of the strains tested here, it cannot be applied to all strains. For example, D2 mice are known to reject ethanol given orally well before they experience any significant pharmacological effect (Belknap et al., 1977), arguing against the idea that their intake is low because they quickly achieve a desired rewarding effect.
An alternative interpretation of this relationship might be that low-drinking, high-CPP strains are more sensitive to ethanol’s aversive effects than high-drinking, low-CPP strains. This possibility is suggested by the well-documented negative genetic correlation between drinking and CTA (see review by Cunningham et al., 2009) as well as by the near-significant positive genetic correlation between CPP and CTA seen here (Table 10). Although the idea that low drinking reflects high sensitivity to ethanol’s aversive pharmacological effects is plausible, it is more difficult to understand why greater sensitivity to such aversive effects would lead to greater preference for a paired contextual cue in the CPP procedure.
A partial solution to this problem is suggested by previous studies showing that ethanol has temporally distinct aversive and rewarding effects in the place conditioning procedure. These studies show that the direction of place conditioning depends critically on when ethanol is injected, with pre-trial injections producing CPP and post-trial injections producing conditioned place aversion (CPA; Cunningham et al., 1997; Cunningham & Henderson, 2000; Cunningham et al., 2002). This finding has been explained in terms of a temporally biphasic hedonic effect, initially aversive but subsequently rewarding. Mice presumably associate context with ethanol’s initial aversive effect after post-trial injections, but they associate context with the delayed rewarding effect after pre-trial injections. Thus, while there might be a mechanistic link between ethanol’s initial aversive and delayed rewarding effects, the pre-trial injection procedure used here is more sensitive to ethanol reward. Future studies could examine genetic correlations between CPA induced by post-trial ethanol injections and other ethanol phenotypes to further examine the relationship between ethanol reward and aversion.
The foregoing analysis, however, does not explain why sensitivity to the rewarding effect of ethanol indexed by CPP is not better reflected in ethanol drinking. The answer may be that drinking is simply more sensitive to post-absorptive aversive drug effects than to rewarding drug effects. Although the literature offers examples of flavor preferences induced by pairing with self-administered ethanol (e.g., Ackroff & Sclafani, 2001; Cunningham & Niehus, 1997; Mehiel & Bolles, 1984), the doses of ethanol consumed have typically been low and questions remain about whether BECs were sufficient to produce rewarding effects. In contrast, the literature yields many more examples where flavor aversions have resulted from pairing flavors with ethanol doses similar to those used here (e.g., Broadbent et al., 2002). The suggestion that drinking might be more sensitive to post-absorptive aversive effects is also generally consistent with theories and data suggesting that taste cues are more readily (selectively) associated with aversive interoceptive drug effects (Reicher & Holman, 1977; Verendeev & Riley, 2012).
The positive genetic correlation between CPP and CTA raises the possibility that strain differences in associative learning underlie this relationship. That is, since both procedures require learning, strains that show strong CPP and strong CTA might generally excel in their learning ability while those showing weak CPP and CTA do not. To address this possibility, strain means for ethanol-induced CPP were correlated with strain means from recent studies in which at least five of the strains used here were tested in a learning procedure (Table 11). Three of these correlations were statistically significant (p < .05) and several others approached significance (.05 < p < .10) despite the relatively low number of strains (n = 5–7). However, while some of these correlations supported the learning ability hypothesis, others did not. For example, there was a significant positive genetic correlation between ethanol-induced CPP (both doses) and learning in a lithium-chloride taste aversion procedure (Ishiwatari & Bachmanov, 2012), but a significant negative genetic correlation between ethanol-induced CPP and conditioned preference for an odor previously paired with sugar (Brown & Wong, 2007). Similarly, there were trends toward positive genetic correlations between CPP and performance in visual learning tasks (Wong & Brown, 2006) and between CPP and conditioned preference for a flavor previously paired with fructose (Pinhas et al., 2012), but trends also suggesting that strains expressing strong CPP made more errors during acquisition of escape learning in the Barnes maze (O’Leary et al., 2011). Other studies showed no significant genetic correlations between CPP and expression of contextual or cued fear conditioning (Portugal et al., 2012) or between CPP and learning on the accelerating rotarod (Brown & Wong, 2007). Although the low number of strains urges caution in the interpretation of these correlations, the overall pattern of findings fails to support the idea that the genetic correlation between ethanol-induced CPP and drinking or CTA can be explained simply in terms of strain differences in learning ability.
Table 11.
Genetic correlations (Pearson r) between CPP and learning phenotypes.
| Reference | Phenotype | n | CPP PDT-2 | CPP PDT-4 |
|---|---|---|---|---|
| Wong & Brown (2006) | ||||
| Visual detection task (D8 % correct) | 6 | .65 | .54 | |
| Visual detection task (D8 latency) | 6 | −.67 | −.49 | |
| Pattern discrimination task (D8 % correct) | 6 | .76 | .66 | |
| Pattern discrimination task (D8 latency) | 6 | −.72 | −.49 | |
| Swim speed (cm/sec) | 6 | .73 | .72 | |
| Brown & Wong (2007) | ||||
| Visual detection task (D8 % correct) | 5 | .50 | .42 | |
| Pattern discrimination task (D8 % correct) | 5 | .67 | .58 | |
| Reversal latency in MWM (sec) | 5 | −.15 | −.04 | |
| Reversal swim distance in MWM (cm) | 5 | −.08 | .11 | |
| Swim speed (cm/sec) | 5 | .48 | .53 | |
| Probe trial in MWM (% time in correct quadrant) | 5 | −.08 | −.16 | |
| Probe trial in MWM (annulus crossings) | 5 | −.39 | −.51 | |
| Latency to visible platform in MWM (sec) | 5 | −.15 | −.04 | |
| Conditioned odor preference task (% CS+ digging) | 5 | −.93a | −.85 | |
| Latency to fall on the Rotarod-males (sec) | 5 | −.05 | −.16 | |
| O’Leary et al. (2011) | ||||
| Acquisition latency (s) | 6 | .63 | .78 | |
| Reversal latency (s) | 6 | .68 | .73 | |
| Reversal difference latency | 6 | .44 | .16 | |
| Number of acquisition errors | 6 | .73 | .76 | |
| Number of reversal errors | 6 | .59 | .64 | |
| Reversal difference errors | 6 | .32 | −.01 | |
| % time correct quadrant | 6 | −.10 | −.29 | |
| Portugal et al. (2012) | ||||
| Contextual Fear Conditioning (% immobility) | 5 | −.37 | −.51 | |
| Cued Fear Conditioning (% immobility) | 5 | −.44 | −.27 | |
| Pinhas et al. (2012) | ||||
| CS+Sucrose (% CS+ intake) | 7 | .22 | .45 | |
| CS+Fructose (% CS+ intake) | 7 | .36 | .68 | |
| CS+Saccharin (% CS+ intake) | 7 | −.14 | .19 | |
| Ishiwatari & Bachmanov (2012) | ||||
| CS intake (mL) Difference (Trial 1-Trial 2) | 6 | .96b | .91a | |
p < .05
p < .01
The significant positive genetic correlation between CPP and sensitivity to ethanol withdrawal is of interest because it replicates, in a genetically diverse panel of inbred strains, a genetic correlation previously observed in two independent studies of mouse lines selectively bred for high or low ethanol withdrawal severity. In the first case, Withdrawal Seizure Prone (WSP) and Withdrawal Seizure Resistant (WSR) mice were selectively bred from genetically heterogeneous mice (HS/Ibg) for high or low ethanol withdrawal convulsions, respectively, after chronic (72 h) ethanol vapor inhalation (Crabbe et al., 1985). In the second case, High Alcohol Withdrawal (HAW) and Low Alcohol Withdrawal (LAW) mice were bred from B6D2F2 mice for differential sensitivity to withdrawal severity measured 2–12 h after a single 4-g/kg injection of ethanol (Metten et al., 1998). In both instances, the line bred for high withdrawal sensitivity showed stronger CPP than the line bred for low withdrawal sensitivity (Chester et al., 1998; Crabbe et al., 1992), mirroring the positive genetic correlation observed in the present study. The consistency of this genetic correlation across three different genetic models offers strong support for the conclusion that common genes influence sensitivity to ethanol withdrawal and ethanol reward as indexed by CPP. However, the neurobiological basis for this relationship remains unknown.
Given the near significant genetic correlation between CPP and CTA, it is interesting to note that a previous study showed a significant positive genetic correlation between CTA and chronic ethanol withdrawal severity in the same 15 inbred strains used here (Broadbent et al., 2002). However, CTA studies in the lines selectively bred for ethanol withdrawal sensitivity failed to support this genetic correlation (Chester et al., 1998). Nevertheless, although the positive genetic relationship between CTA and withdrawal has been less consistently supported across genetic models than the relationship between CPP and withdrawal, the overall pattern of findings suggests partial overlap in the mechanisms underlying all three of these phenotypes.
Consistent with previous findings in the BXD RI strains (Cunningham, 1995), genetic correlations in this study provided no support for theories proposing a positive relationship between sensitivity to ethanol-induced locomotor activation and ethanol reward as indexed by CPP. Correlations also failed to support the idea of a predictive relationship between sensitization (or tolerance) to ethanol-induced activation and CPP. In contrast, but also consistent with the BXD study, there was strong evidence for a negative genetic correlation between CPP and test session activity in the absence of ethanol, with less active strains showing stronger CPP than more active strains. This observation is generally consistent with previous data indicating that experimental manipulations (e.g., drug treatments or apparatus configurations) that alter test activity can have a similar impact on CPP expression (Gremel & Cunningham, 2007; Neisewander et al., 1990; Vezina & Stewart, 1987). The simplest interpretation of such findings is that CPP and elevated activity are competing responses, and that test activity is affected by both genotype and environmental manipulations.
In conclusion, these data offer support for the idea that genotype influences ethanol’s rewarding effect, a factor that may contribute to addictive vulnerability. Moreover, this study provides important new information on the genetic relationship between two behavioral phenotypes commonly used to draw inferences about ethanol’s reinforcing or rewarding effects, showing that CPP-sensitive strains (e.g., D2) generally tend to drink less than CPP-insensitive strains (e.g., B6). Although there are several possible explanations for this relationship, genetic correlations between each of these phenotypes and CTA reinforce the suggestion that low-drinking, high-CPP strains are more sensitive to ethanol’s aversive effects than high-drinking, low-CPP strains. By this account, the observed patterns of genetic relationships reflect the greater impact of ethanol’s aversive effects on drinking as well as the greater impact of ethanol’s rewarding effects on CPP. Finally, these data encourage additional research directed toward better understanding the relationship between ethanol’s rewarding and aversive effects, including the possibility of overlap in the mechanisms underlying sensitivity to ethanol’s aversive effects and ethanol withdrawal.
Acknowledgments
Research reported in this paper was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under award number R01AA007702. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health. Dobrina M. Okorn, Christine E. Howard and Carly Henderson are gratefully acknowledged for their assistance in data collection, analysis and manuscript preparation. I also thank John Belknap, John Crabbe and Pamela Metten for help in gathering strain means for several of the genetic correlational analyses, and Tamara Phillips for helpful comments on an initial draft of this manuscript.
Footnotes
After these experiments were completed, the Jackson Laboratory informed us of a possible breeding error in their NZB/B1NJ colony around the time our mice had been bred. Based on their information, as many as 56 of the 64 NZB/B1NJ mice we used may have been the progeny of a cross with a C57BL/6J. The other 8 mice were confirmed to be pure NZB/B1NJ. Because we had no way to know whether any of the 56 suspect NZB/B1NJ mice in the current study were affected by this breeding error, all mice in this strain were included in the final data analyses reported here. CPP measured in the NZB 2 g/kg group tested here was very similar to that recorded in NZB mice in a separate CPP study using an identical procedure (Gremel & Cunningham, 2007), increasing confidence that the data reported here accurately represent the NZB genotype.
References
- Ackroff K, Sclafani A. Flavor preferences conditioned by intragastric infusion of ethanol in rats. Pharmacol Biochem Behav. 2001;68(2):327–338. doi: 10.1016/s0091-3057(00)00467-6. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Belknap ND, Berg JH, Coleman R. Preabsorptive vs postabsorptive control of ethanol intake in C57BL/6J and DBA/2J mice. Behavior Genetics. 1977;7:413–425. doi: 10.1007/BF01066776. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Broadbent J, Muccino KJ, Cunningham CL. Ethanol-induced conditioned taste aversion in 15 inbred mouse strains. Behavioral Neuroscience. 2002;116:138–148. [PubMed] [Google Scholar]
- Brown RE, Wong AA. The influence of visual ability on learning and memory performance in 13 strains of mice. Learn Mem. 2007;14(3):134–144. doi: 10.1101/lm.473907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester J, Risinger F, Cunningham C. Ethanol reward and aversion in mice bred for sensitivity to ethanol withdrawal. Alcohol Clin Exp Res. 1998;22(2):468–473. [PubMed] [Google Scholar]
- Crabbe J, Phillips T, Cunningham C, Belknap J. Genetic determinants of ethanol reinforcement. Ann N Y Acad Sci. 1992;654:302–310. doi: 10.1111/j.1749-6632.1992.tb25976.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. Review. Neurogenetic studies of alcohol addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3201–3211. doi: 10.1098/rstb.2008.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Gallaher ES, Phillips TJ, Belknap JK. Genetic determinants of sensitivity to ethanol in inbred mice. Behavioral Neuroscience. 1994;108:186–195. doi: 10.1037//0735-7044.108.1.186. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Kendler KS, Hitzemann RJ. Modeling the diagnostic criteria for alcohol dependence with genetic animal models. Curr Top Behav Neurosci. 2013;13:187–221. doi: 10.1007/7854_2011_162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Kosobud A, Young ER, Tam BR, McSwigan JD. Bidirectional selection for susceptibility to ethanol withdrawal seizures in Mus musculus. Behav Genet. 1985;15(6):521–536. doi: 10.1007/BF01065448. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ. Pharmacogenetic studies of alcohol self-administration and withdrawal. Psychopharmacology. 2004;174(4):539–560. doi: 10.1007/s00213-003-1608-6. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. Estimation of genetic correlation: Interpretation of experiments using selectively bred and inbred animals. Alcoholism: Clinical and Experimental Research. 1990;14:141–151. doi: 10.1111/j.1530-0277.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Niehus J. Flavor preference conditioning by oral self-administration of ethanol. Psychopharmacology (Berl) 1997;134(3):293–302. doi: 10.1007/s002130050452. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Okorn D, Howard C. Interstimulus interval determines whether ethanol produces conditioned place preference or aversion in mice. Animal Learning & Behavior. 1997;25:31–42. [Google Scholar]
- Cunningham CL. Pavlovian drug conditioning. In: van Haaren F, editor. Methods in behavioral pharmacology. Amsterdam: Elsevier; 1993. pp. 349–381. [Google Scholar]
- Cunningham CL. Localization of genes influencing ethanol-induced conditioned place preference and locomotor activity in BXD recombinant inbred mice. Psychopharmacology. 1995;120:28–41. doi: 10.1007/BF02246142. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 2003;170(4):409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nature Protocols. 2006;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Genetic influences on conditioned taste aversion. In: Reilly S, Schachtman TR, editors. Conditioned taste aversion: Behavioral and neural processes. New York: Oxford University Press; 2009. pp. 387–421. [Google Scholar]
- Cunningham CL, Henderson CM. Ethanol-induced conditioned place aversion in mice. Behav Pharmacol. 2000;11(7–8):591–602. doi: 10.1097/00008877-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Howard MA, Gill SJ, Rubinstein M, Low MJ, Grandy DK. Ethanol-conditioned place preference is reduced in dopamine D2 receptor- deficient mice. Pharmacol Biochem Behav. 2000;67(4):693–699. doi: 10.1016/s0091-3057(00)00414-7. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus DR, Malott DH, Prather LK. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology. 1992;107:385–393. doi: 10.1007/BF02245166. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Noble D. Conditioned activation induced by ethanol: role in sensitization and conditioned place preference. Pharmacol Biochem Behav. 1992;43(1):307–313. doi: 10.1016/0091-3057(92)90673-4. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Phillips TJ. Genetic basis of ethanol reward. In: Maldonado R, editor. Molecular biology of drug addiction. Totowa, NJ: Humana Press, Inc; 2003. pp. 263–294. [Google Scholar]
- Cunningham CL, Tull LE, Rindal KE, Meyer PJ. Distal and proximal pre-exposure to ethanol in the place conditioning task: tolerance to aversive effect, sensitization to activating effect, but no change in rewarding effect. Psychopharmacology (Berl) 2002;160(4):414–424. doi: 10.1007/s00213-001-0990-1. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Chester JA, Rodd-Henricks K, Li TK, Lumeng L. Ethanol place preference conditioning in High- and Low- Alcohol Preferring selected lines of mice. Pharmacology Biochemistry & Behavior. 2001;68:805–814. doi: 10.1016/s0091-3057(01)00476-2. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Li TK, Lumeng L. Selective breeding for high and low alcohol preference in mice. Behav Genet. 1999;29(1):47–57. doi: 10.1023/a:1021489922751. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42(1):1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL. Role of test activity in ethanol-induced disruption of place preference expression in mice. Psychopharmacology (Berl) 2007;191(2):195–202. doi: 10.1007/s00213-006-0651-5. [DOI] [PubMed] [Google Scholar]
- Groblewski PA, Bax LS, Cunningham CL. Reference-dose place conditioning with ethanol in mice: empirical and theoretical analysis. Psychopharmacology (Berl) 2008;201(1):97–106. doi: 10.1007/s00213-008-1251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwatari Y, Bachmanov AA. NaCl taste thresholds in 13 inbred mouse strains. Chem Senses. 2012;37(6):497–508. doi: 10.1093/chemse/bjr135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz RJ, Gormezano G. A rapid and inexpensive technique for assessing the reinforcing effects of opiate drugs. Pharmacology, Biochemistry and Behavior. 1979;11:231–233. doi: 10.1016/0091-3057(79)90019-4. [DOI] [PubMed] [Google Scholar]
- Linakis JG, Cunningham CL. Effects of concentration of ethanol injected intraperitoneally on taste aversion, body temperature, and activity. Psychopharmacology. 1979;64:61–65. doi: 10.1007/BF00427346. [DOI] [PubMed] [Google Scholar]
- Mehiel R, Bolles RC. Learned flavor preferences based on caloric outcome. Animal Learning & Behavior. 1984;12:421–427. [Google Scholar]
- Metten P, Belknap JK, Crabbe JC. Drug withdrawal convulsions and susceptibility to convulsants after short-term selective breeding for acute ethanol withdrawal. Behav Brain Res. 1998;95(1):113–122. doi: 10.1016/s0166-4328(97)00216-7. [DOI] [PubMed] [Google Scholar]
- Metten P, Crabbe JC. Alcohol withdrawal severity in inbred mouse (Mus musculus) strains. Behav Neurosci. 2005;119(4):911–925. doi: 10.1037/0735-7044.119.4.911. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Pierce RC, Bardo MT. Naloxone enhances the expression of morphine-induced conditioned place preference. Psychopharmacology. 1990;100:201–205. doi: 10.1007/BF02244406. [DOI] [PubMed] [Google Scholar]
- O’Leary TP, Savoie V, Brown RE. Learning, memory and search strategies of inbred mouse strains with different visual abilities in the Barnes maze. Behav Brain Res. 2011;216(2):531–542. doi: 10.1016/j.bbr.2010.08.030. [DOI] [PubMed] [Google Scholar]
- Phillips T, Broadbent J, Burkhart-Kasch S, Henderson C, Wenger C, McMullin C, et al. Genetic correlational analyses of ethanol reward and aversion phenotypes in short-term selected mouse lines bred for ethanol drinking or ethanol-induced conditioned taste aversion. Behav Neurosci. 2005;119(4):892–910. doi: 10.1037/0735-7044.119.4.892. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Belknap JK, Buck KJ, Cunningham CL. Genes on mouse chromosomes 2 and 9 determine variation in ethanol consumption. Mammalian Genome. 1998;9:936–941. doi: 10.1007/s003359900903. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Crabbe JC, Metten P, Belknap JK. Localization of genes affecting alcohol drinking in mice. Alcoholism: Clinical and Experimental Research. 1994;18:931–941. doi: 10.1111/j.1530-0277.1994.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Huson M, Gwiazdon C, Burkhart-Kasch S, Shen EH. Effects of acute and repeated ethanol exposures on the locomotor activity of BXD recombinant inbred mice. Alcoholism: Clinical and Experimental Research. 1995;19:269–278. doi: 10.1111/j.1530-0277.1995.tb01502.x. [DOI] [PubMed] [Google Scholar]
- Pinhas A, Aviel M, Koen M, Gurgov S, Acosta V, Israel M, et al. Strain differences in sucrose- and fructose-conditioned flavor preferences in mice. Physiol Behav. 2012;105(2):451–459. doi: 10.1016/j.physbeh.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Wilkinson DS, Kenney JW, Sullivan C, Gould TJ. Strain-dependent Effects of Acute, Chronic, and Withdrawal from Chronic Nicotine on Fear Conditioning. Behav Genet. 2012;42(1):133–150. doi: 10.1007/s10519-011-9489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reicher MA, Holman EW. Location preference and flavor aversion reinforced by amphetamine in rats. Animal Learning & Behavior. 1977;5:343–346. [Google Scholar]
- Risinger F, Cunningham C. Ethanol-induced conditioned taste aversion in BXD recombinant inbred mice. Alcohol Clin Exp Res. 1998;22(6):1234–1244. [PubMed] [Google Scholar]
- Risinger FO, Oakes RA. Dose- and conditioning-trial dependent ethanol-induced conditioned place preference in Swiss-Webster mice. Pharmacology Biochemistry & Behavior. 1996;55:117–123. doi: 10.1016/0091-3057(96)00069-x. [DOI] [PubMed] [Google Scholar]
- Shabani S, McKinnon CS, Reed C, Cunningham CL, Phillips TJ. Sensitivity to rewarding or aversive effects of methamphetamine determines methamphetamine intake. Genes Brain Behav. 2011;10(6):625–636. doi: 10.1111/j.1601-183X.2011.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DN, Duka T, Crombag HS, Cunningham CL, Heilig M, Crabbe JC. Reward sensitivity: issues of measurement, and achieving consilience between human and animal phenotypes. Addict Biol. 15(2):145–168. doi: 10.1111/j.1369-1600.2009.00193.x. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Hoffman P. Tolerance and the etiology of alcoholism: hypothesis and mechanism. Alcohol Clin Exp Res. 1988;12(1):184–186. doi: 10.1111/j.1530-0277.1988.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Verendeev A, Riley AL. Conditioned taste aversion and drugs of abuse: history and interpretation. Neurosci Biobehav Rev. 2012;36(10):2193–2205. doi: 10.1016/j.neubiorev.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Vezina P, Stewart J. Morphine conditioned place preference and locomotion: The effect of confinement during training. Psychopharmacology. 1987;93:257–260. doi: 10.1007/BF00179944. [DOI] [PubMed] [Google Scholar]
- Wheeler JM, Reed C, Burkhart-Kasch S, Li N, Cunningham CL, Janowsky A, et al. Genetically correlated effects of selective breeding for high and low methamphetamine consumption. Genes Brain Behav. 2009;8(8):758–771. doi: 10.1111/j.1601-183X.2009.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AA, Brown RE. Visual detection, pattern discrimination and visual acuity in 14 strains of mice. Genes Brain Behav. 2006;5(5):389–403. doi: 10.1111/j.1601-183X.2005.00173.x. [DOI] [PubMed] [Google Scholar]