Abstract
Objective
Post-traumatic epilepsy (PTE) is a significant complication following traumatic brain injury (TBI), yet the role of genetic variation in modulating PTE onset is unclear. We hypothesized that TBI-induced inflammation likely contributes to seizure development. We assessed whether genetic variation in the IL-1β gene, Il-1β levels in cerebral spinal fluid (CSF) and serum, and CSF/serum IL-1β ratios would predict PTE development post-TBI.
Methods
We investigated PTE development in 256 Caucasian adults with moderate to severe TBI. IL-1β tagging and functional single nucleotide polymorphisms (SNPs) were genotyped. Genetic variance and PTE development were assessed. Serum and CSF IL-1β levels were collected from a subset of subjects (n=59) during first week post injury and evaluated for their associations with IL-1β gene variants and also PTE. Temporally matched CSF/serum IL-1β ratios were also generated to reflect the relative contribution of serum IL-1β to CSF IL-1β.
Results
Multivariate analysis showed that higher CSF/serum IL-1β ratios were associated with increased risk for PTE over time (p=0.008). Multivariate analysis for rs1143634 revealed an association between the CT genotype and increased PTE risk over time (p=0.005). The CT genotype group also had lower serum IL-1β levels (p=0.014) and higher IL-1β CSF/serum ratios (p=0.093).
Significance
This is the first report implicating IL-1β gene variability with PTE risk and linking 1) IL-1β gene variation with serum IL-1β levels observed after TBI and 2) IL-1β ratios with PTE risk. Given these findings, we propose that genetic and IL-1β ratio associations with PTE may be attributable to biological variability with blood brain barrier integrity during TBI recovery. These results provide a rationale for further studies 1) validating the impact of genetic variability on IL-1β production after TBI, 2) assessing genetically mediated signaling mechanisms that contribute to IL-1β CSF/serum associations with PTE, and 3) evaluating targeted IL-1β therapies that reduce PTE.
Keywords: Post-traumatic Epilepsy, Inflammation, TBI, genetic variation, IL-1β
Introduction
Post-traumatic epilepsy (PTE) accounts for 20% of symptomatic seizures and 5% of all seizures in the general population.1 For those with penetrating head injury, subdural hematoma (SDH), or depressed skull fracture, more than 20% develop PTE.2 Time to first seizure varies greatly, with clinical onset reported more than 10 years post injury.3 PTE is associated with increased mortality, and death at a younger age, compared to patients without PTE.4 Those with PTE also are at a significant disadvantage regarding physical, cognitive and psychosocial issues that adversely impact outcome.5 Despite evidence against effective PTE prevention treatments,6 people with TBI frequently receive long-term anticonvulsant therapy, often resulting in unwanted side effects and regular monitoring. Thus, identifying reliable biomarkers for epileptogenesis and PTE risk prognostication could have broad clinical implications on TBI treatment and recovery.
Increasing evidence implicates glial cell activation and subsequent cytokine production following acute seizures as an important contributor to epileptogenesis.7, 8 Interestingly, a similar glial cell and cytokine response is also observed following TBI. One of the most widely studied biomarkers for epileptogenesis is interleukin-1beta (IL-1β). IL-1β is a pro-inflammatory cytokine produced in the central nervous system (CNS) by activated microglia and astrocytes, as well as in the periphery by macrophages and other immune cells. Following TBI injured tissue increases extracellular adenosine triphosphate which mediates CNS microglia activation9 as well as IL-1β processing and release.10 Previous studies have reported increased IL-1β expression,11 microglial activation,12 and cell death up to a year following TBI,13 suggesting that IL-1β may be a useful marker of chronic inflammation that facilitates and perpetuates PTE risk.
Increased IL-1β production following TBI increases CNS hyperexcitability and excitotoxicity through Ca2+, glutamatergic, and GABAergic mechanisms potentially contributing to epileptogenesis.14 Interestingly, exogenous IL-1β administration increases seizure activity induced by various pro-convulsant drugs in rodent models.15 Furthermore, disruption of the IL-1β biosynthesis pathway, with IL-1β converting enzyme (ICE/Caspase-1) inhibitors, results in delayed onset time and frequency of chemically induced seizures.16 Studies investigating plasma and cerebrospinal fluid (CSF) IL-1β levels in populations with febrile seizures (FS) and TLE have shown mixed results17 but do implicate IL-1β with the pathology. However, no studies have assessed IL-1β levels in association with the evolution of epileptogenesis and PTE risk following TBI.
Genetic variant associations with epilepsy are another viable biomarker path for predicting PTE development. Previous studies have identified multiple single nucleotide polymorphisms (SNPs) associated with increased seizure risk following TBI.18,19 The gene coding for IL-1β (IL-1B) is located in the 2q12-13 region on the long arm of chromosome 2. One commonly studied IL-1B SNP, rs16944, located at position -511 within the promoter region, reportedly increases susceptibility to common types of TLE.20 Interestingly, variation within this same SNP increases lipopolysaccharide (LPS) induced IL-1β production 2–3 fold.21 Based on the known role of IL-1β in inflammation and risk for non-traumatic epilepsy, the goal of this study was to determine if genetic variability within the IL-1B gene and IL-1β biomarker profiles in CSF relative to serum in a TBI population were associated with PTE risk.
Methods
Study Design and Subjects
This study was approved by the Institutional Review Board at the University of Pittsburgh, using an IRB approved consenting process. There were 354 subjects screened for this study at a single academic medical center as a part of a larger study evaluating genetics and biomarkers on outcomes for individuals with TBI. Based on allelic frequency information obtained from the database of Single Nucleotide Polymorphisms (dbSNP: http://www.ncbi.nlm.nih.gov/snp), this longitudinal retrospective cohort study was limited to Caucasians, and 32 subjects were excluded from the analysis. Subjects were 18–70 years old, had moderate to severe TBI (Glasgow Coma Score (GCS) ≤ 12), positive computed tomography (CT) scan confirming intracranial injury (TBI), and no history of premorbid seizures. 13 subjects had GCS>12, but were included in the moderate TBI category based on positive CT findings. Six subjects screened had premorbid seizures and were excluded. The remaining (n=316) subjects who met the race, age, medical history, and injury criteria for inclusion were further screened based on our criteria for PTE analysis.
IL-1β Genetics Population
Time to first seizure was our primary variable of interest, and the cohort was further restricted to approximate the standard criteria for PTE.22 PTE was defined as the time to first seizure occurring beyond the first week post-injury. Therefore, individuals who had their first seizure during the first week post-injury (n=20) or died within the first week post-injury (n=40) were excluded. Three deceased individuals had documented history of PTE prior to death and were included in the analysis. This final cohort consisted of n=256 subjects. The time-course for PTE development can vary based on mortality status and affect genetic relationships with PTE status and time to first seizure. In addition to Kaplan-Meier (KM) approaches for assessing time to first seizure, mortality status was handled in bivariate categorical analysis by removing subjects who died after the first week post-injury but did not seize, leaving n=206 survivors. Time to mortality was handled in multivariate models using Cox Regression (see statistical approach).
IL-1β Level Population
This group included subjects (n=59) from the IL-1β genetics population that had ≥2 temporally matched CSF and serum samples available during the first week post-injury for IL-1β quantification (n=143 for serum and CSF samples). Similar to the IL-1β genetics cohort, subjects who died after the first week but did not seize were removed, leaving (n=46) survivors from which to analyze bivariate IL-1β associations with PTE. Time to mortality was adjusted in associated multivariate population analyses.
Serum and CSF IL-1β levels for healthy adult control subjects were also evaluated. CSF was obtained via lumbar puncture (n=13), and serum was obtained by venipuncture (n=11) from healthy control subjects. Control subjects were between, 19–60 years old, with no past or current bleeding disorder, brain injury, or neurological disease. Women currently pregnant, or taking oral contraceptives or hormone replacement therapy, were excluded.
Critical Care Management of Severe TBI
Subjects evaluated were admitted to the neurotrauma intensive care unit over a 10 year period and treated in accordance with the Guidelines for the Management of Severe Head Injury.23 Standard treatment included initial extra-ventricular drain placement, central venous, and arterial catheters. Surgical intervention for mass lesion decompression was performed when clinically necessary. Intermittent electroencephalography (EEG) was used to evaluate patients with clinical suspicion of epileptic activity. In accordance with previously published studies, patients with severe TBI were typically prescribed PTE prophylaxis for the first week post-injury.24
Demographics and Injury Data
Patient demographic variables, including age and gender were recorded. Medical records and UPMC trauma registry data were reviewed to abstract clinical information regarding GCS scores, injury mechanism, depressed skull fracture, subdural hematoma (SDH), injury severity score (ISS), isolated head injury status (IHI), and length of hospital stay. The highest GCS score in the first 24 hours post-injury was recorded. Depressed skull fracture and/or SDH were identified based on radiographic reports. Information regarding anti-epileptic drugs (AEDs) use was recorded only if noted in the medical records as used for seizure prophylaxis or treatment.
ISS and IHI were determined based on the Abbreviated Injury Scale (AIS) scoring system, which determines severity of a specific injury based on the survivability of that injury.25 The AIS categorizes injury to a body region from 1–6, with 6 representing the highest chance of an injury being lethal. ISS, a measure of global anatomical injury severity, is defined as the sum of squares of scores from the three most severely affected body regions.26 IHI status was based on the head AIS score, with ≥3 being considered a severe TBI. All subjects had a head AIS ≥3. Isolated TBI (IHI) was defined as head AIS ≥3 in patients with AIS <3 for each of the three most injured extra-cranial body regions. Non-isolated TBI was defined as head AIS score ≥3, and an AIS score of ≥3 for at least one extra-cranial body region.27
Seizure Assessment
Electronic medical records were reviewed for information about PTE, and time to first seizure. PTE determinations were abstracted from patient history and physical, ambulance, emergency room, and EEG reports as well as discharge/transfer summaries and inpatient/outpatient progress notes. Any mention of seizures, convulsions, or status epilepticus occurring after the first week post-injury was considered as evidence of PTE. PTE follow-up was censored to three years post-injury since all subjects had complete ascertainment of PTE status during this period. There was no difference in EEG procurement between subjects included in this study and normal standard of care.
DNA Extraction, SNP Selection, and Genotyping
DNA was extracted from one of two sources for each subject, whole blood or CSF. Whole blood was collected into ethylenediaminetetraacetic acid (EDTA) vacutainer tubes, processed to retrieve the buffy coat, and DNA was extracted using a simple salting-out procedure.28 CSF was collected by passive drainage, and DNA was extracted from white blood cells using Qiamp DNA extraction protocol for extraction from body fluids (Qiagen Corporation). Tagging SNPs rs1143633, rs1143634, rs3136558 with a minor allele frequency of at least 20% were selected based on data from the HapMap database (International HapMap Project: http://hapmap.ncbi.nlm.nih.gov/) and SNP database (dbSNP: http://www.ncbi.nlm.nih.gov/projects/SNP). These tSNPs captured the variability of the gene, including 1000 bases 5’ upstream into the promoter region, based on data from build 36. Rs1143634 is also considered a functional SNP. Furthermore, functional SNPs rs1143627 and rs16944 were also selected for genotyping.
Genotyping was completed using TaqMan allele discrimination technology and commercially available 5’ exonuclease Assay-on-Demand TaqMan assays (Applied Biosystems Incorporated). Amplification and genotype assignments were conducted using the AB17000 and SDS 2.0 software (Applied Biosystems). Double-masked genotype assignments were made for each SNP, and each discrepancy was addressed using raw data or re-genotyping. Hardy-Weinberg equilibrium was verified for all SNPs, indicating genotype distributions were within the expected proportions. Call rates for each SNP were >95%.
CSF and Serum IL-1β Collection and Quantification
CSF samples were collected up to every 12 hours for the first six days post-injury, and serum was collected daily. CSF samples were taken from an extraventricular drain (EVD) collection bag and blood samples via peripheral venipuncture. Collected samples were then centrifuged, aliquoted, and stored at −80°C until assay. A Luminex™ bead array assay (Millipore; Milliplex High Sensitivity 9plex) was used to measure IL-1β levels in CSF and serum. IL-1β levels were measured in available CSF and serum samples. Coefficients of variance for IL-1β assessments were <10%.
Statistical Analysis
Statistical analyses were performed using SPSS version 20.0 (Chicago, IL) and SAS (Cary, NC). Summary statistics included means, medians, frequencies, and standard error of the mean (SEM). Independent t-test, or Mann-Whitney when appropriate, was used to assess differences between PTE groups and continuous variables. Chi-square analysis, using Fisher’s exact test when appropriate, was used to assess differences between PTE and categorical variables, including IL-1β SNP genotypes.
To analyze serum and CSF IL-1β levels in the context of PTE, weekly averages were calculated based on daily IL-1β levels for the first week post-injury. Daily values >3 standard deviations above/below the population mean were considered outliers and excluded from analysis (CSF n=8, serum n=7 samples). Given the induction of both peripheral monocytes and CNS microglia to activated macrophages, we hypothesized that CSF IL-1β levels represented a mixture of CNS derived and peripherally generated IL-1β transported into the CSF. To express the relative contribution of serum IL-1β to measured CSF IL-1β levels, IL-1β ratios were calculated by dividing temporally matched CSF/serum samples, and daily ratios were then averaged in order to obtain a weekly IL-1β ratio. Significant bivariate associations (p<0.05) were analyzed in multivariate models to determine genetic and other variable associations with cytokine ratios.
For those with PTE, Kaplan-Meier analysis was used to examine time to first seizure (eight days-three years) by candidate SNP variation while adjusting for time to mortality. IL-1β ratios, and SNPs with genotypes that differed significantly in terms PTE risk, were also examined using Cox Proportional Hazards models. Cox models were generated with time to first seizure (eight days-three years) as the dependent variable and PTE incidence as the event of interest. Multivariate Cox models examined either IL-1β ratios or significant SNPs while adjusting for covariates significantly associated with PTE status identified in bivariate analysis. Cox models were also adjusted for GCS and depressed skull fracture given their documented relevance to TBI severity and PTE.
Results
Population Description
IL-1β Genetics Population
There were 256 adults with moderate-severe TBI and genotype information that otherwise met the criteria for PTE analysis. Approximately 81.6 % of the population was men and 18.4% women. Mean age was 35.0±0.93yrs. Median GCS score was 6, and mean acute care length of stay (LOS) was 22.5±0.68d. The most common mechanisms of injury included automobile accidents (50.4%), motorcycle accidents (20.3%), and falls (11.3%). 249 individuals (97.3%) were given AEDs for seizure prophylaxis, and PTE developed in 42 individuals (16.4%). 51.2% of those with a diagnosis of PTE had at least one EEG obtained at the time of clinical presentation. 34.1% of PTE subjects had at least one EEG during acute care, while 70.7% of subjects had at least one EEG during the 1wk-3yr post-injury surveillance period.
A breakdown of the population based on PTE status is provided in Table 1. Individuals with a lower ISS score were more likely to develop PTE (p=0.026). IHI status (p=0.033) and SDH (p=0.002) were also associated with PTE. Mortality was significantly associated with PTE, with those who died having lower PTE incidence. Thus, a mortality adjusted PTE population was created (n=206). There were no differences in injury or demographic information between the unadjusted and mortality adjusted PTE populations (data not shown).
Table 1.
Demographics Table
| Variable | IL-1β Genetics Population (n=256) |
IL-1β Level Population (n=59) |
||||
|---|---|---|---|---|---|---|
| No PTE | PTE | p-value | No PTE | PTE | p-value | |
| Gender | ||||||
| Female | 37 (78.7%) | 10 (21.3%) | p=0.382 | 5 (71.4%) | 2 (28.6%) | p=0.589 |
| Male | 177 (84.7%) | 32 (15.3%) | 42 (80.8%) | 10 (19.2%) | ||
| AED treatment | ||||||
| Yes | 207 (83.1%) | 42 (16.9%) | p=0.603 | 45 (78.9%) | 12 (21.1%) | p=0.591 |
| No | 7 (100%) | 0 (0%) | 2 (100%) | 0 (0%) | ||
| GCS Score | ||||||
| 3–4 | 60 (87.0%) | 9 (13.0%) | p=0.643 | 6 (85.7%) | 1 (14.3%) | p=0.636 |
| 5–8 | 118 (82.5%) | 25 (17.5%) | 35 (81.4%) | 8 (18.6%) | ||
| 9–12 | 27 (87.1%) | 4 (12.9%) | 5 (100%) | 0 (0%) | ||
| 13–15 | 7 (77.8%) | 2 (22.2%) | 0 (0%) | 1 (100%) | ||
| Depressed Skull Fracture | ||||||
| Yes | 20 (71.4%) | 8 (28.6%) | p=0.106 | 5 (83.3%) | 1 (16.7%) | p=0.797 |
| No | 185 (84.5%) | 34 (15.5%) | 42 (79.2%) | 11 (20.8%) | ||
| Injury Mechanism | ||||||
| Motor Vehicle | 111 (86.0%) | 18 (14.0%) | p=0.363 | 20 (76.9%) | 6 (23.1%) | p=0.560 |
| Fall | 22 (75.9%) | 7 (24.1%) | 7 (77.8%) | 2 (22.2%) | ||
| Motorcycle | 43 (82.7%) | 9 (17.3%) | 13 (92.9%) | 1 (7.1%) | ||
| Other | 38 (82.6%) | 8 (17.4%) | 7 (70.0%) | 3 (30.0%) | ||
| Isolated Head Injury | ||||||
| Yes | 67 (76.1%) | 21 (23.9%) | p=0.033 | 18 (69.2%) | 8 (30.8%) | p=0.047 |
| No | 136 (87.2%) | 20 (12.8%) | 28 (90.3%) | 3 (9.7%) | ||
| Mortality | ||||||
| Alive | 166 (81.0%) | 39 (19.0%) | p=0.021 | 35 (76.1%) | 11 (23.9%) | p=0.846 |
| Dead | 48 (94.1%) | 3 (5.9%) | 12 (92.3%) | 1 (7.7%) | ||
| Age | 35.5 ± 1.04 | 32.5 ± 1.97 | p=0.374 | 37.2 ± 2.49 | 38.5 ± 4.80 | p=0.206 |
| ISS | 35.6 ± 0.69 | 32.6 ± 1.41 | p=0.026 | 34.7 ± 1.19 | 27.8 ± 1.93 | p=0.329 |
| Acute Care LOS | 22.4 ± 0.75 | 23.2 ± 1.56 | p=0.564 | 23.7 ± 1.65 | 28.0 ± 3.80 | p=0.437 |
| Subdural Hematoma | ||||||
| Yes | 122 (77.7%) | 35 (22.3%) | p=0.002 | 30 (75.0%) | 10 (25.0%) | p=0.037 |
| No | 90 (92.8%) | 7 (7.2%) | 17 (89.5%) | 2 (11.5%) | ||
IL-1β Level Population
There were 59 individuals with moderate to severe TBI and acute cytokine information to assess factors influencing IL-1β ratios and PTE (Table 1). IHI status was associated with higher IL-1β ratios compared to those with more severe extra-cerebral injuries (p=0.047). SDH was also associated with higher IL-1β ratios (p=0.037). Lower serum IL-1β levels were associated with IHI status (p=0.007) and SDH (p=0.022). Unlike IL-1β ratios, ISS was associated with higher serum IL-1β levels (p=0.006). IL-1β level and ratio (n=46) comparisons to PTE among survivors showed no significant differences in injury or demographic information (data not shown).
Healthy Control Population
Members of the healthy control population were all Caucasian and were 61.5% male (n=8). Mean age was 24.62 +/− 11.16 yrs. Sensitivity analyses showed significant differences between TBI and control cohorts in regards to age and gender distributions. Given some reports of possible sex differences in inflammatory response after TBI,29 we assessed whether or not there were differences by sex among controls and found no significant differences in serum (0.361+/−0.230 women vs. 0.072+/−0.036 men; p=0.257), CSF (0.030+/−0.005 women vs. 0.026+/−0.003 men; p=0.171), or ratios (0.400+/−0.667 women vs. 0.441+/−0.263 men; p=0.257) IL-1β levels. Age was not significantly correlated with IL-1β serum, CSF, or ratio levels among controls (p=0.686, 0.733, 0.788, respectively).
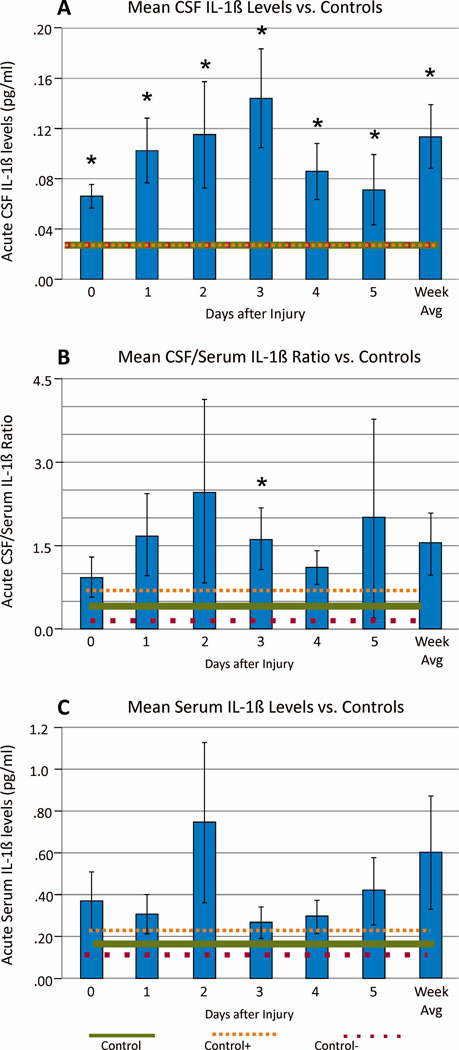
Acute IL-1β Levels
Daily and mean weekly CSF and serum IL-1β levels among survivors were compared to healthy controls. Daily and mean weekly CSF IL-1β levels for the TBI group were higher than controls (p=<0.001 all comparisons) (Figure 1a). CSF/serum IL-1β ratios were higher on d3 post-injury (p=0.025), and there was a trend towards higher weekly average levels (p=0.070) compared to controls (Figure 1b). Serum IL-1β levels did not differ from controls (Figure 1c). There were no significant sex differences in serum (0.377+/−0.543 women vs. 0.550+/−1.719 men; p=0.722), CSF (0.088+/−0.109 women vs. 0.123+/−0.167 men; p=0.454), or ratio (0.766+/−0.565 women vs. 1.699+/−3.771 men; p=0.589) IL-1β weekly average values.
Figure 1.
Daily and week 1 mean IL-1β levels (+/−SEM) among survivors (n=46), (A) CSF IL-1β levels compared to healthy controls (n=13 controls) (p<0.001 for each time point), (B) CSF/Serum IL-1β ratios compared to healthy controls (n=11 controls) (p=0.025 day 3, and p=0.070 weekly average), (C) Serum IL-1β levels compared to healthy controls (n=11 controls) (p>0.05 all comparisons).
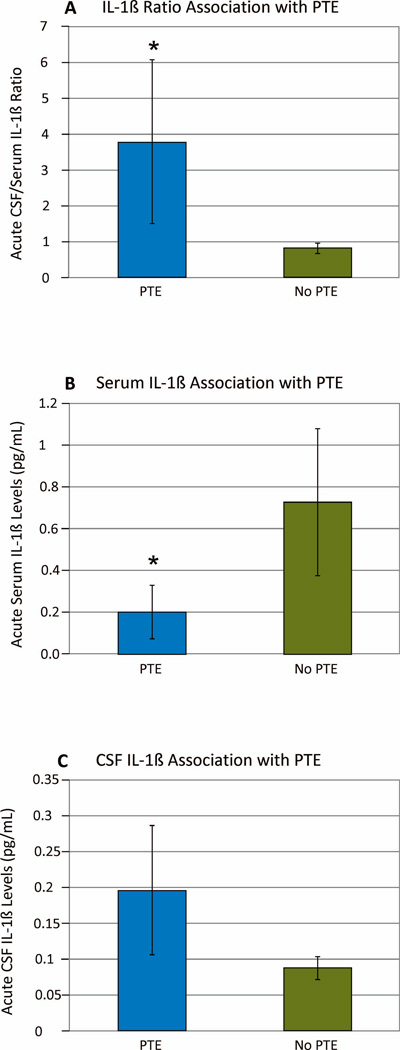
Acute IL-1β Levels and PTE Development
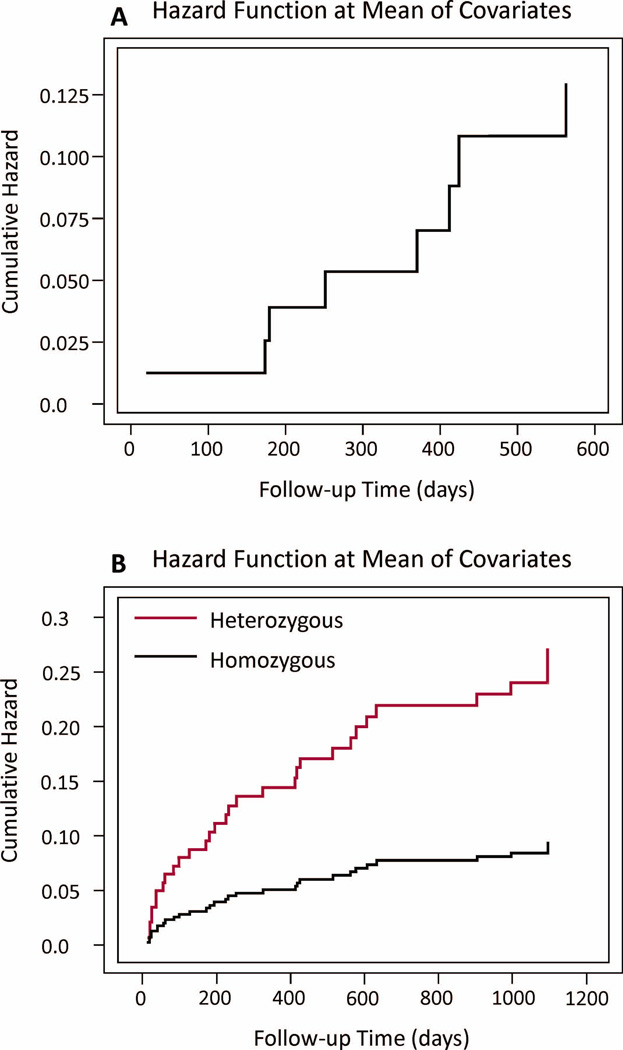
Among survivors, weekly IL-1β ratios were higher in those with PTE vs. No-PTE (PTE=3.794±2.274; No-PTE=0.8207±0.1495; p=0.020) (Figure 2a). Serum IL-1β was lower in the PTE group (PTE=0.2007±0.1290pg/mL; no-PTE=0.7239±0.3501pg/mL; p=0.016) (Figure 2b). CSF IL-1β levels were not different between PTE groups (PTE=0.1964±0.0901; no-PTE=0.0876±0.0159; p=0.864) (Figure 2c). A Cox model, for the IL-1β level population described in table 1 and adjusted for time to mortality, ISS, GCS, SDH, and depressed skull fracture, showed that higher IL-1β ratios were also associated with increased PTE risk over time (Hazard ratio=1.341; CI 1.081–1.665; p=0.008) (Table 3, Figure 3a). A similar Cox model showed no association between serum IL-1β levels and PTE risk over time (data not shown).
Figure 2.
Mean IL-1β levels (+/−SEM) over the first week post-injury by PTE status among survivors (n=46), (A) CSF/Serum IL-1β ratio by PTE (p=0.020), (B) Serum IL-1β levels by PTE (p=0.016), (C) CSF IL-1β levels by PTE (p>0.05).
Table 3.
Cox Proportional Hazard
| IL-1β Ratio Associations with PTE (n=52) | ||||||
| IL-1β CSF/Serum Ratio | Hazard Ratio | Confidence Interval | p-value | |||
| GCS | 0.758 | 0.489–1.175 | 0.215 | |||
| Subdural Hematoma | 0.873 | 0.109–6.989 | 0.898 | |||
| ISS | 0.849 | 0.749–0.963 | 0.011 | |||
| Depressed Skull Fracture | 0.910 | 0.104–7.944 | 0.932 | |||
| IL-1β Ratio | 1.341 | 1.081–1.665 | 0.008 | |||
| rs1143634 Associations with PTE (n=199) | ||||||
| Age | 0.976 | 0.941–1.006 | 0.115 | |||
| Gender | 2.335 | 0.941–5.791 | 0.067 | |||
| GCS | 1.136 | 0.981–1.316 | 0.089 | |||
| Subdural Hematoma | 0.286 | 0.118–0.691 | 0.005 | |||
| ISS | 0.988 | 0.948–1.029 | 0.550 | |||
| Depressed Skull Fracture | 0.436 | 0.187–1.013 | 0.054 | |||
| Genotype (CT vs.CC+TT) | 2.845 | 1.372–5.900 | 0.005 | |||
Shaded cells denote a significant statistic (p<0.05)
Figure 3.
Cox Proportional Hazards cumulative PTE risk based on (A) mean CSF/Serum IL-1β ratio and adjusted for time to mortality (in days), ISS, GCS, SDH, and depressed skull fracture (p=0.008), (B) rs1143634 genotype and adjusted for time to mortality (in days), age, gender, ISS, GCS, SDH, and depressed skull fracture (p=0.005).
rs1143634 Association with PTE Development
Table 2 depicts the overall allelic frequencies for each SNP as well as bivariate associations within a mortality adjusted subset of the overall population. There were no significant bivariate associations with PTE for rs1143633, rs3136558, rs1143627, and rs16944, regardless of mortality adjustment. However, bivariate evaluation showed that rs1143634 was related to PTE risk. PTE occurred in 17.6% of individuals with a CC genotype, 47.7% of individuals with a CT genotype, and no individuals with a TT genotype (p=0.008). Consistent with previous studies,30 individuals with the TT genotype (n=13) at rs1143634 comprised only a small percentage of the overall population. Grouped genotype analysis revealed that heterozygotes (CT) were at greater risk (p=0.005) of developing PTE compared to homozygotes. Kaplan-Meier analysis, adjusting for time to mortality, showed that heterozygotes had a shorter time to first seizure compared to homozygotes (CT mean=854.37 days, CI 759.28–949.46; CC+TT mean=1010.51days, CI 959.40–1061.62; p=0.006). A Cox Proportional Hazards model adjusted for time to mortality, age, gender, GCS, ISS, SDH, and depressed skull fracture, showed the CT genotype was associated with increased PTE risk over time compared to homozygotes (Hazard ratio=2.845; CI 1.372–5.900; p=0.005) (Table 3, Figure 3b).
Table 2.
Bivariate IL-1β Tagging SNP Results
| SNP | Allele frequencies overall |
Genetics Population (PTE) | Level Population (IL-1β Ratio) | Level Population (Serum IL-1β) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wta | Varb | Heteroc | Genod | Wta | Varb | Heteroc | Genod | Wta | Varb | Heteroc | Genod | ||
| IL-1β GENE | |||||||||||||
| rs1143634 | C=.75 (wt) | .123 | .086 | .005 | .008 | .272 | .111 | .062 | .093 | .101 | .037 | .009 | .014 |
| T=.25 (var) | |||||||||||||
| rs16944 | G=.64 (wt) | .354 | .700 | .441 | .463 | .199 | .461 | .088 | .215 | .692 | .562 | .473 | .696 |
| A=.36 (var) | |||||||||||||
| rs1143627 | T=.63 (wt) | .559 | .847 | .571 | .711 | .279 | .916 | .248 | .486 | .800 | .692 | .618 | .846 |
| C=.37 (var) | |||||||||||||
| rs3136558 | T=.77 (wt) | .204 | .337 | .081 | .105 | .610 | .159 | .143 | .172 | .551 | .060 | .065 | .058 |
| C=.23 (var) | |||||||||||||
| rs1143633 | C=.69 (wt) | .494 | 1.00 | .701 | .707 | .554 | .471 | .950 | .703 | .602 | .992 | .593 | .846 |
| T =.31 (var) | |||||||||||||
presence of wild type (wt) versus homozygous variant trait
presence of variant (var) versus homozygous wild type trait
presence of heterozygous (hetero) versus homozygous trait
genotype (3 group) comparisons
Shaded cells denote a significant statistic (p < 0.05)
rs1143634 Associations with IL-1β Levels
There were no significant bivariate associations with IL-1β ratios, serum, or CSF levels for rs1143633, rs1143627, rs3136558, and rs16944 among subjects in the mortality adjusted IL-1β level population. Serum IL-1β levels were significantly different (p=0.014) and IL-1β ratios showed a trend (p=0.093) based on rs1143634 genotype (Table 2). Grouped genotype analysis revealed that CT heterozygotes had lower serum IL-1β levels compared homozygotes (CT mean=0.1571±0.0552 pg/mL; CC+TT mean=0.9661±0.5048 pg/mL; p=0.009). Also, CT heterozygotes also tended to have higher IL-1β ratios compared to homozygotes (CT mean=2.615±1.583; CC+TT mean=0.8251±0.2094; p=0.062). Rs3136558 also tended to be associated (p=0.058) with serum IL-1β levels but further analysis showed a high degree of linkage disequilibrium (LD) between rs3136558 and rs1143634 within our population (p=<0.001).
Discussion
PTE is a significant complication following TBI that leads to increased disability and long-term AED treatment. Mechanisms underlying epileptogenesis and PTE are not well understood and despite characterized clinical risk factors for PTE, not all patients possessing these risk factors go on to develop PTE. Genetic variability, and associated variation in biological responses to TBI, may account for increased PTE incidence in certain individuals. The most recent Epilepsy Benchmark Research Progress Report lists IL-1β as one potential biomarker for epilepsy based on recently published findings.31 Previous work in our lab has shown that daily levitiracetam, a widely prescribed AED reduces regional IL-1β expression, potentially contributing to its anti-seizure effects in TBI.32 This observation, along with other data showing a link between IL-1β and non-traumatic epilepsy,33 provide a rationale for studying IL-1β and PTE development.
IL-1β is produced in the CNS and periphery. In healthy individuals, CNS IL-1β levels are very low and beneficial to physiological processes like memory formation and sleep.34 However, TBI is associated with increased IL-1β expression,11 which can persist for months post-injury.35,36 Elevated IL-1β levels are associated with early neuronal death and excitotoxicity,13,14 suggesting the importance of IL-1β regulation during TBI recovery. Based on these findings, and the known pro-convulsant effects of IL-1β, we hypothesized that early TBI induced changes in IL-1β levels would be associated with PTE. Given that both blood brain barrier (BBB) disruption and peripheral inflammation occurs with TBI,37 and that CSF IL-1β levels are likely to be a product of both CNS and peripheral production, we also explored serum/CSF IL-1β ratios as a reflection of the relative contribution of serum IL-1β to CSF IL-1β levels. While our data showed CSF IL-1β levels were elevated in our PTE subpopulation, compared to controls, no difference in average CSF IL-1β was observed between the PTE and no-PTE groups. Serum IL-1β levels did not differ from controls. However, serum IL-1β levels were significantly lower in the PTE vs. no-PTE group, suggesting relative differences in serum IL-1β transit into the CNS between these two groups. Interestingly, individuals with high CSF/serum IL-1β ratios during the first week post-injury had significantly higher PTE risk, suggesting that while absolute CSF IL-1β are elevated after TBI, the relative contribution of IL-1β from the periphery is the measurement most sensitive to eventual PTE development. This association is interesting and has implications for early screening, yet further work is needed to determine if/how the serum and ratio associations also reflect something about how a persistent peripheral inflammatory state, recently characterized over the first year in a clinical population with moderate to severe injury,36 may contribute to epileptogenesis and PTE development over the long-term. Multivariate Cox models showed that only IL-1β ratios were significantly associated with PTE risk overtime, indicating CSF/serum ratios appear to increase the specificity of the contribution of peripheral inflammation to CNS pathology.
Interestingly, type II IL-1 transporters have been implicated in one report with blood to brain IL-1β transport across the BBB using an in vitro approach using cerebromicrovascular endothelial cells.38 Although clinical TBI studies suggest that some cytokines follow a brain-to-blood transport gradient,39 similar studies do not identify such a gradient for IL-1β transport.40 Our results show high serum IL-1β concentrations, relative to CSF, in our cohort with IL-1β levels that are suggestive of a blood-to-brain gradient for IL-1β (figure 1). Additionally, higher CSF to serum IL-1β ratios among those with PTE also supports this hypothesis of blood-to-brain IL-1β transport (figure 2). This finding, taken with rs11436234 associations with serum levels and the ratio, also suggests genetic variation influencing IL-1β transport capacity into the CNS over time as a plausible biological mechanism to explore further for how IL-1β pathology influences PTE risk.
Further work is needed to establish why higher IL-1β ratios acutely post-TBI can predict PTE onset months to years post-injury. An increased acute inflammatory response might predict a prolonged period of chronic (CNS and peripheral) inflammation, and BBB dysfunction that contributes to epileptogenesis and PTE. Notably, serum cytokine levels are elevated for at least a year after moderate to severe TBI,36 which may contribute to BBB dysfunction and ongoing CNS inflammation. IL-1β induces IL-1β mRNA production in several cell types, including activated microglia, resulting in a positive feedback system that may perpetuate CNS inflammation over time.41 Interestingly, clinical imaging studies suggest that microglial activation can persist for years after TBI and is associated with ongoing white matter degeneration.12 Furthermore, studies showing progressive cell death up to a year post-TBI,13 suggest increases in extracellular ATP associated with apoptosis may chronically activate inflammasomes necessary for IL-1β production.10
Higher acute IL-1β CSF/serum ratios within the PTE group may indicate various long-term physiological changes, associated with chronic inflammation, which promote epileptogenesis. Intraventricular IL-1β infusion can activate both the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system, decreasing peripheral cellular immune responses and resulting in suppressed macrophage IL-1β secretion.42 Thus, individuals with PTE may have acute hyperactivation of this neuroimmunomodulation system resulting in the lower serum IL-1β levels as we report in this study. Studies have also shown increases in IL-1β and/or decreases in IL-1 receptor antagonist (IL-1Ra) significantly alters the frequency, duration, and threshold of chemical induced seizures.15 Additionally, delayed seizure progression has been reported in kindling studies that block nerve growth factor (NGF).43 Interestingly, IL-1β up-regulates NGF both in vitro and in vivo,44,45 and application of IL-1Ra in a TBI rat model suppresses NGF-mediated plasticity.46 Further work is needed to discern which, if any, of these altered physiological states are reflected by the IL-1β CSF/serum ratio.
IL-1β gene variant analysis showed a relationship between rs1143634 genotype, PTE, and serum IL-1β levels as well as the CSF/serum IL-1β ratio. Individuals with a CT genotype at rs1143634 had a significantly increased risk of PTE and those with the CT genotype had a significantly shorter time to first seizure (~5 months shorter on average), implicating variability at this locus in accelerating epileptogenesis. Although the minor allele frequency (MAF) for this SNP is 0.25, only 13 subjects were TT homozygotes. Interestingly none of these subjects developed PTE. So while this TT subgroup is small and potentially subject to sample bias, the data suggests that TT homozygotes are relatively protected against PTE and the CT heterozygotes are uniquely the “at risk” group. Further, future validation studies would be unlikely to yield a dose response association with PTE risk.
Heterozygote associations for genetic variation for genes coding membrane receptors can occur and may result in multiple actions that affect the phenotype of interest (e.g. differential effects of membrane trafficking for receptor protein hetero-dimers vs. homo-dimers). We have previously reported heterozygote associations with PTE for rs10920573 located on the ADORA1 gene.19 While not a membrane receptor, one might speculate an extracellular milieu with a mixture of different IL-1β isoforms theoretically may have a differential impact on target receptor binding, activation, or downstream signaling within inflammation pathways that leads to increased PTE risk. As heterozygosity at rs1143634 is also associated with decreased serum IL-1β, and given that IL-1β can influence BBB permeability, it also stands to reason that IL-1β physiology associated heterozygosity at rs1143634 may differentially facilitate serum IL-1β transport into the CSF compartment. Notably, IL-1β modulates expression and efflux functionality for BBB carrier transporters like ATP-binding cassette (ABC) transporters,47 for which genetic variation may have differential effects on IL-1β levels48 as well as other molecules like cortisol,49 in the setting of TBI. Interestingly, ABC transporters also facilitate IL-1β efflux from stim ulated macrophages,50,51 a function which may be particularly relevant in the context of IL-1β contributions from chronically activated microglia12 to PTE development overtime. Finally, significant widespread epigenetic modification is known to occur after TBI,52 and given previous reports on how epigenetic changes within the IL-1β promoter can occur,53 this may be another area for further study regarding mechanisms associated with increased PTE risk with rs1143634.
The rs1143634 SNP is located in a coding region on exon 5 of the IL1β gene, however its functionality is still controversial.54 Studies suggest that the T allele is associated with increased IL-1β production,48 while others found no difference in IL-1β secretion.55 Rs1143634 tags approximately a 1Kb region of the IL-1β gene, located on chromosome 2, that also contains missense SNPs rs141525736, rs139843362, and rs114640380 (HapMap phase 3; hapmap.org). However, these SNPs are not highly variable, and no literature exists regarding their functionality. Also, it is unclear where LD decays for the region tagged by rs1143634, thus additional genotyping around the DNA block representing rs1143634 may be helpful to better understand the functional implications of variation, including heterozygosity, at this locus.
ISS, IHI, and SDH were all linked to PTE. Individuals with lower ISS, an IHI, or a subdural hematoma were more likely to develop PTE. As ISS is a measure of global, anatomical injury severity, it is not surprising that individuals with an IHI also have lower ISS. However, ISS/IHI were not significant predictors of PTE in multivariate analysis. Our findings do support previous studies showing SDH can increase PTE risk.2
Despite the promising findings linking IL-1β and PTE, some limitations should be considered. Medical record data abstraction makes it difficult to accurately assess recurrent seizures and PTE severity. As such, recurrent seizures were not tracked. Also, subclinical or non-convulsive seizures occurring in subjects still intubated and sedated beyond the first week post-injury may have been missed. The sample was limited to Caucasians with moderate to severe injury, limiting the generalizability to those with different racial backgrounds. Also, the overall sample size is modest for a genetic association study. However, the cohort available for biomarker and genetic analysis used is one of the largest moderate to severe TBI cohorts currently available. Nonetheless, to our knowledge, this is the first research report implicating serum IL-1β contributions to CSF IL-1β levels and also genetic variability in the IL-1B gene with PTE. Further work is needed to replicate these findings in independent populations and better understand the mechanistic implications associated with these relationships. If validated, the findings might stimulate further research testing agents that target IL-1β to prevent and treat PTE. If validated, the findings might also support future work assessing the utility of genetic screening paradigms, paired with EEG screening and clinical care pathways, which result in risk stratified treatment and prevention protocols for PTE. Additionally, future work exploring genetic variation and levels for other biomarkers relevant to TBI induced inflammation and PTE development is warranted.
Supplementary Material
Acknowledgments
Sources of support and acknowledgements: This work was supported by DODW81XWH-071-0701, NIH R01 HD048162-02, NIH R01NR008424, NIH 5P01NS030318. Thanks to Sandra Deslouche for support with genotyping, the subjects and their families for their generous participation. Thanks to the UPMC Trauma Registry for providing some elements of data collection. Thanks to the University of Pittsburgh Cancer Institute for Luminex services.
Biography

Matthew Diamond is completing a BPhil in neuroscience at the University of Pittsburgh.
Footnotes
Disclosures: None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Englander J, Bushnik T, Duong TT, et al. Analyzing risk factors for late posttraumatic seizures: a prospective, multicenter investigation. Arch Phys Med Rehabil. 2003;84:365–373. doi: 10.1053/apmr.2003.50022. [DOI] [PubMed] [Google Scholar]
- 2.Temkin NR. Risk factors for posttraumatic seizures in adults. Epilepsia. 2003;44:18–20. doi: 10.1046/j.1528-1157.44.s10.6.x. [DOI] [PubMed] [Google Scholar]
- 3.Annegers JF, Hauser WA, Coan SP, et al. A population-based study of seizures after traumatic brain injuries. N Engl J Med. 1998;338:20–24. doi: 10.1056/NEJM199801013380104. [DOI] [PubMed] [Google Scholar]
- 4.Englander J, Bushnik T, Wright JM, et al. Mortality in late post-traumatic seizures. J Neurotrauma. 2009;26:1471–1477. doi: 10.1089/neu.2008.0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolakowsky-Hayner SA, Wright J, Englander J, et al. Impact of late post-traumatic seizures on physical health and functioning for individuals with brain injury within the community. Brain Inj. 2013;27:578–586. doi: 10.3109/02699052.2013.765595. [DOI] [PubMed] [Google Scholar]
- 6.Formisano R, Barba C, Buzzi MG, et al. The impact of prophylactic treatment on post-traumatic epilepsy after severe traumatic brain injury. Brain Inj. 2007;21:499–504. doi: 10.1080/02699050701310994. [DOI] [PubMed] [Google Scholar]
- 7.Vezzani A, Ravizza T, Balosso S, et al. Glia as a source of cytokines: implications for neuronal excitability and survival. Epilepsia. 2008;49(Suppl 2):24–32. doi: 10.1111/j.1528-1167.2008.01490.x. [DOI] [PubMed] [Google Scholar]
- 8.Holtman L, van Vliet EA, Aronica E, et al. Blood plasma inflammation markers during epileptogenesis in post-status epilepticus rat model for temporal lobe epilepsy. Epilepsia. 2013;54:589–595. doi: 10.1111/epi.12112. [DOI] [PubMed] [Google Scholar]
- 9.Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari D, Villalba M, Chiozzi P, et al. Mouse microglial cells express a plasma membrane pore gated by extracellular ATP. J Immunol. 1996;156:1531–1539. [PubMed] [Google Scholar]
- 11.Lu KT, Wang YW, Yang JT, et al. Effect of interleukin-1 on traumatic brain injury-induced damage to hippocampal neurons. J Neurotrauma. 2005;22:885–895. doi: 10.1089/neu.2005.22.885. [DOI] [PubMed] [Google Scholar]
- 12.Johnson VE, Stewart JE, Begbie FD, et al. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain J Neuro. 2013;135:28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith DH, Chen XH, Pierce JE, et al. Progressive atrophy and neuron death for one year following brain trauma in the rat. J Neurotrauma. 1997;14:715–727. doi: 10.1089/neu.1997.14.715. [DOI] [PubMed] [Google Scholar]
- 14.Zhu G, Okada M, Yoshida S, et al. Effects of interleukin-1beta on hippocampal glutamate and GABA releases associated with Ca2+-induced Ca2+ releasing systems. Epilepsy Res. 2006;71:107–116. doi: 10.1016/j.eplepsyres.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Vezzani A, Moneta D, Conti M, et al. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc Natl Acad Sci U S A. 2000;97:11534–11539. doi: 10.1073/pnas.190206797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravizza T, Lucas SM, Balosso S, et al. Inactivation of caspase-1 in rodent brain: a novel anticonvulsive strategy. Epilepsia. 2006;47:1160–1168. doi: 10.1111/j.1528-1167.2006.00590.x. [DOI] [PubMed] [Google Scholar]
- 17.Rijkers H, Majoie HJ, Hoogland G, et al. The role of interleukin-1 in seizures and epilepsy: A critical review. Exp Neurol. 2009;216:258–271. doi: 10.1016/j.expneurol.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Darrah SD, Miller MA, Ren D, et al. Genetic variability in glutamic acid decarboxylase genes: associations with post-traumatic seizures after severe TBI. Epilepsy Res. 2013;103:180–194. doi: 10.1016/j.eplepsyres.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner AK, Miller MA, Scanlon J, et al. Adenosine A1 receptor gene variants associated with post-traumatic seizures after severe TBI. Epilepsy Res. 2010;90:259–272. doi: 10.1016/j.eplepsyres.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kauffman MA, Moron DG, Consalvo D, et al. Association study between interleukin 1 beta gene and epileptic disorders: a HuGe review and meta-analysis. Genet Med. 2008;10:83–88. doi: 10.1097/GIM.0b013e318161317c. [DOI] [PubMed] [Google Scholar]
- 21.Hall SK, Perregaux DG, Gabel CA, et al. Correlation of polymorphic variation in the promoter region of the interleukin-1 beta gene with secretion of interleukin-1 beta protein. Arthritis Rheum. 2004;50:1976–1983. doi: 10.1002/art.20310. [DOI] [PubMed] [Google Scholar]
- 22.Fisher RS, van Emde Boas W, Blume W, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46:470–472. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 23.Bratton SL, Bullock RM, Carney N, et al. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24:s37–s44. doi: 10.1089/neu.2007.9990. [DOI] [PubMed] [Google Scholar]
- 24.Temkin NR, Dikmen SS, Wilensky AJ, et al. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323:497–502. doi: 10.1056/NEJM199008233230801. [DOI] [PubMed] [Google Scholar]
- 25.Gennarelli TA, Wodzin E. AIS 2005: a contemporary injury scale. Injury. 2006;37:1083–1091. doi: 10.1016/j.injury.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Baker SP, O’Neill B, Haddon W, Jr, et al. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–196. [PubMed] [Google Scholar]
- 27.Berry C, Ley EJ, Tillou A, et al. The effect of gender on patients with moderate to severe head injuries. J Trauma. 2009;67:950–953. doi: 10.1097/TA.0b013e3181ba3354. [DOI] [PubMed] [Google Scholar]
- 28.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mellergard P, Aneman O, Sjogren F, et al. Differences in cerebral extracellular response of interleukin-1β, interleukin-6, and interleukin- 10 after subarachnoid hemorrhage or severe trauma in humans. J Neurosurgery. 2011;68:12–19. doi: 10.1227/NEU.0b013e3181ef2a40. [DOI] [PubMed] [Google Scholar]
- 30.Camargo MC, Mera R, Correa P, et al. Interleukin-1beta and interleukin-1 receptor antagonist gene polymorphisms and gastric cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1674–1687. doi: 10.1158/1055-9965.EPI-06-0189. [DOI] [PubMed] [Google Scholar]
- 31.National Institute of Neurological Disorders and Stroke. Epilepsy Research Benchmarks Progress Report 2007–2012 [document on the internet]. NINDS online. [cited 2013 Sept 4];2013 Available from: http://www.ninds.nih.gov/research/epilepsyweb/benchmarks_2007-2012progress.pdf.
- 32.Zou H, Brayer SW, Hurwitz M, et al. Neuroprotective, Neuroplastic, and Neurobehavioral Effects of Daily Treatment With Levetiracetam in Experimental Traumatic Brain Injury. Neurorehabil Neural Repair. 2013;27:878–888. doi: 10.1177/1545968313491007. [DOI] [PubMed] [Google Scholar]
- 33.Omran A, Peng J, Zhang C, et al. Interleukin-1β and microRNA-146a in an immature rat model and children with mesial temporal lobe epilepsy. Epilepsia. 2012;53:1215–1224. doi: 10.1111/j.1528-1167.2012.03540.x. [DOI] [PubMed] [Google Scholar]
- 34.Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- 35.Acosta SA, Tajiri N, Shinozuka K, et al. Long-term upregulation of inflammation and suppression of cell proliferation in the brain of adult rats exposed to traumatic brain injury using the controlled cortical impact model. PloS One. 2013;8:e53376. doi: 10.1371/journal.pone.0053376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boles J, Goyal A, Kumar R, et al. Chronic Inflammation after Severe Traumatic Brain Injury: Characterization and Associations with Outcome. Presented at Annual Symposium of the National Neurotrauma Society; 2012 Jul 22–25; Phoenix, Arizona. 2013. [Google Scholar]
- 37.Das M, Mohapatra S, Mohapatra SS. New perspectives on central and peripheral immune responses to acute traumatic brain injury. J Neuroinflammation. 2012;9:236. doi: 10.1186/1742-2094-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skinner RA, Gibson RM, Rothwell NJ, et al. Transport of interleukin-1 across cerebromicrovascular endothelial cells. Br J Pharmacol. 2009;156:1115–1123. doi: 10.1111/j.1476-5381.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aibiki M, Maekawa S, Ogura S, et al. Effect of moderate hypothermia on systemic and internal jugular plasma IL-6 levels after traumatic brain injury in humans. J Neurotrauma. 1999;16:225–232. doi: 10.1089/neu.1999.16.225. [DOI] [PubMed] [Google Scholar]
- 40.Helmy A, Carpenter KLH, Menon DK, et al. The cytokine response to human traumatic brain injury: temporal profiles and evidence for cerebral parenchymal production. J Cereb Blood Flow Metab. 2011;31:658–670. doi: 10.1038/jcbfm.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee SC, Liu W, Dickson DW, et al. Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J. Immunol. 1993;150:2659–2667. [PubMed] [Google Scholar]
- 42.Brown R, Li Z, Vriend CY, et al. Suppression of splenic macrophage interleukin-1 secretion following intracerebroventricular injection of interleukin-1 beta: evidence for pituitary-adrenal and sympathetic control. Cell Immunol. 1991;132:84–93. doi: 10.1016/0008-8749(91)90008-y. [DOI] [PubMed] [Google Scholar]
- 43.Van der Zee CE, Rashid K, Le K, et al. Intraventricular administration of antibodies to nerve growth factor retards kindling and blocks mossy fiber sprouting in adult rats. J Neurosci. 1995;15:5316–5323. doi: 10.1523/JNEUROSCI.15-07-05316.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spranger M, Lindholm D, Bandtlow C, et al. Regulation of Nerve Growth Factor (NGF) Synthesis in the Rat Central Nervous System: Comparison between the Effects of Interleukin-1 and Various Growth Factors in Astrocyte Cultures and in vivo. Eur J Neurosci. 1990;2:69–76. doi: 10.1111/j.1460-9568.1990.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 45.DeKosky ST, Goss JR, Miller PD, et al. Upregulation of nerve growth factor following cortical trauma. Exp Neurol. 1994;130:173–177. doi: 10.1006/exnr.1994.1196. [DOI] [PubMed] [Google Scholar]
- 46.DeKosky ST, Styren SD, O’Malley ME, et al. Interleukin-1 receptor antagonist suppresses neurotrophin response in injured rat brain. Ann Neurol. 1996;39:123–127. doi: 10.1002/ana.410390118. [DOI] [PubMed] [Google Scholar]
- 47.Von Wedel-Parlow M, Wolte P, Galla HJ, et al. Regulation of major efflux transporters under inflammatory conditions at the blood-brain barrier in vitro. J Neurochem. 2009;111:111–118. doi: 10.1111/j.1471-4159.2009.06305.x. [DOI] [PubMed] [Google Scholar]
- 48.Pociot F, Mølvig J, Wogensen L, et al. A TaqI polymorphism in the human interleukin-1 beta (IL-1 beta) gene correlates with IL-1 beta secretion in vitro. Eur J Clin Invest. 1992;22:396–402. doi: 10.1111/j.1365-2362.1992.tb01480.x. [DOI] [PubMed] [Google Scholar]
- 49.Santarsieri M, Niyonkuru C, McCullough EH, et al. CNS Cortisol and Progesterone Profiles and Outcomes after Severe TBI. J. Neurotrauma. doi: 10.1089/neu.2013.3177. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmitz G, Kaminski WE, Porsch-Ozcurumez M, et al. ATP-binding cassette transporter A1 (ABCA1) in macrophages: a dual function in inflammation and lipid metabolism. Pathobiology. 1999;67:236–240. doi: 10.1159/000028100. [DOI] [PubMed] [Google Scholar]
- 51.Hamon Y, Luciani MF, Becq F, et al. Interleukin-1beta secretion is impaired by inhibitors of the ATP bind cassette transport, ABC1. Blood. 1997;90:2911–2915. [PubMed] [Google Scholar]
- 52.Gao WM, Chadha MS, Kline AE, et al. Immunohistochemical analysis of histone H3 acetylation and methylation--evidence for altered epigenetic signaling following traumatic brain injury in immature rats. Brain Res. 2006;1070:31–34. doi: 10.1016/j.brainres.2005.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen H, Wilkins LM, Aziz N, et al. Single nucleotide polymorphisms in the human interleukin-1B gene affect transcription according to haplotype context. Hum Mol Genet. 2006;15:519–529. doi: 10.1093/hmg/ddi469. [DOI] [PubMed] [Google Scholar]
- 54.Chen X, El Gazzar M, Yoza BK, et al. The NF-kappaB factor RelB and histone H3 lysine methyltransferase G9a directly interact to generate epigenetic silencing in endotoxin tolerance. J Biol Chem. 2009;284:27857–27865. doi: 10.1074/jbc.M109.000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dominici R, Malferrari G, Mariani C, et al. The Interleukin 1-beta exonic (+3953) polymorphism does not alter in vitro protein secretion. Exp Mol Pathol. 2002;73:139–141. doi: 10.1006/exmp.2002.2435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.