Abstract
Background
Alcohol dependence is common in bipolar disorder (BPD) and associated with treatment non-adherence, violence, and hospitalization. Quetiapine is a standard treatment for BPD. We previously reported improvement in depressive symptoms, but not alcohol use, with quetiapine in BPD and alcohol dependence. However, mean alcohol use was low and a larger effect size on alcohol-related measures was observed in those with higher levels of alcohol consumption. In this study, efficacy of quetiapine in patients with BPD and alcohol dependence was examined in patients with higher mean baseline alcohol use than in the prior study.
Methods
Ninety outpatients with bipolar I or II disorders, depressed or mixed mood state, and current alcohol dependence were randomized to 12 weeks of sustained release quetiapine (to 600 mg/day) add-on therapy or placebo. Drinking was quantified using the Timeline Follow Back method. Additional assessment tools included the Hamilton Rating Scale for Depression (HRSD17), Inventory of Depressive Symptomatology–Self-Report (IDS-SR30), Young Mania Rating Scale (YMRS), Penn Alcohol Craving Scale (PACS), liver enzymes, and side effects. Alcohol use and mood were analyzed using a declining-effects random-regression model.
Results
Baseline and demographic characteristics in the two groups were similar. No significant between-group differences were observed on the primary outcome measure of drinks/day or other alcohol-related or mood measures (p>.05). Overall side effect burden, glucose and cholesterol were similar in the two groups. However, a significant weight increase was observed with quetiapine at week 6 (+2.9 lbs [SE 1.4] quetiapine vs. −2.0 lbs [SE 1.4], p=.03), but not at week 12. Scores on the Barnes Akathisia Scale increased significantly more (p=.04) with quetiapine (+0.40 (SE 0.3)) than placebo (−0.52 (SE 0.3)) at week 6 but not week 12. Retention (survival) in the study was similar in the groups.
Conclusions
Findings suggest that quetiapine does not reduce alcohol consumption in patients with BPD and alcohol dependence.
Keywords: quetiapine, bipolar disorder, depression, mania, alcohol dependence
Introduction
Bipolar disorder (BPD) is a debilitating illness that affects roughly 2.6 percent of the population (Kessler, Berglund et al., 2005). Approximately 28 percent of people with BPD have lifetime comorbid alcohol dependence, as compared to 14 percent without this illness (Regier, Farmer et al., 1990). Alcohol dependence significantly worsens symptoms and complications in BPD, increases inpatient hospitalization rates (Sonne, Brady et al., 1994), decreases treatment adherence (Aagaard and Vestergaard, 1990), decreases quality of life (Singh, Mattoo et al., 2005), and increases the risk for suicide attempt (Dalton, Cate-Carter et al., 2003).
Despite the high rates of co-occurrence of BPD and alcohol dependence, few randomized, controlled trials have examined potential pharmacologic treatments for this population (Brown, Carmody et al., 2009, Brown, Garza et al., 2008, Salloum, Cornelius et al., 2005, Stedman, Pettinati et al., 2010). Quetiapine, an atypical antipsychotic, is a standard treatment in BPD for both mania and depression (Geddes and Miklowitz, 2013). Quetiapine may also decrease alcohol use, but the data are mixed. Kampman et al. showed that quetiapine decreased alcohol consumption and number of heavy drinking days in Type B, but not Type A, alcohol-dependent participants (Kampman, Pettinati et al., 2007). Litten et al., however, observed “no efficacy for quetiapine compared with placebo at reducing alcohol consumption in heavy-drinking alcohol-dependent patients” (Litten, Fertig et al., 2012).
Three randomized, double-blind, placebo controlled trials (Stedman, Pettinati et al., 2010, Brown, Garza et al., 2008, Guardia, Roncero et al., 2011) found that quetiapine was not associated with reduced alcohol consumption in patients with BPD and alcohol dependence. Brown et al. however, observed significant improvement in depressive symptoms with quetiapine, as well as a larger effect sizes on alcohol consumption measures in a subgroup of patients with higher levels of baseline alcohol consumption. Two of these negative studies were published during the enrollment in the current study. These studies differed in design from the current study. The study by Guardia et al. used quetiapine as an adjunctive therapy to naltrexone (Guardia, Roncero et al., 2011), while the study by Stedman et al. included an extended washout phase with high attrition (Stedman, Pettinati et al., 2010). We conducted the current study in order to clarify whether quetiapine may be effective in reducing alcohol consumption in patients with BPD and alcohol dependence. The basic design of the current study was similar to our earlier study except that participants were required to have higher levels of baseline alcohol consumption because this clinical characteristic was associated with a lager effect size in the prior study.
Materials and Methods
Outpatients (n=90) with BPD and alcohol dependence were enrolled. A UT Southwestern IRB-approved written informed consent process was completed. Possible participants were identified through physician referral and through flyers and brochures at clinics for this study. At baseline, a structured clinical interview for DSM-IV clinician version (SCID-CV) was used to establish diagnoses (First, Spitzer et al., 1995). Other assessments included the 17-item Hamilton Rating Scale for Depression (HRSD17) (Hamilton, 1960), 30-item Inventory of Depressive Symptomatology–Self-Report (IDS-SR30) (Rush, Carmody et al., 2000), Young Mania Rating Scale (YMRS) (Young, Biggs et al., 1978), Penn Alcohol Craving Scale (PACS) (Flannery, Volpicelli et al., 1999), Addiction Severity Index (ASI) (McLellan, Luborsky et al., 1980), Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised (CIWA-Ar) (Sullivan, Sykora et al., 1989), Psychobiology of Recovery in Depression III - Somatic Symptom Scale (PRD-III) (Thase, Fava et al., 1996), Abnormal Involuntary Movement Scale (AIMS) (Guy, 1976), Simpson-Angus Scale (SAS) (Simpson and Angus, 1970), and Barnes Akathisia Scale (BARS) (Barnes, 1989). Alcohol use was assessed with the Timeline Follow Back method (Sobell and Sobell, 1992). Blood was drawn for routine laboratory analyses including a complete blood count (CBC), liver tests, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyltransferase (GGT), glucose, and lipids. A physical examination was performed, and vital signs and weight were obtained. A urine drug screen (UDS) was obtained, and women of childbearing potential received a urine pregnancy test. At each weekly visit, the HRSD17, IDS-SR30, YMRS, PACS, and assessment of alcohol and drug use was completed. Changes in concomitant medications, when absolutely necessary, were managed using a treatment algorithm based on Bauer et al. (Bauer, Williford et al., 2001). The algorithm allowed for the consideration of concomitant medication changes if either the HRSD17 or YMRS increased by more than 10 points since the last assessment. The first option was to change the dose of a current concomitant medication. If this approach was not successful then an additional medication could be added. Adherence was assessed using pill counts. All participants received manual-driven cognitive behavioral therapy designed for persons with BPD and substance abuse (Schmitz, Averill et al., 2002). Participants were paid for their participation.
The study included men and women 18–65 years old with a diagnosis of bipolar I or II disorder, depressed or mixed phase, current alcohol dependence with alcohol use of at least 15 drinks in the 7 days prior to baseline, currently taking a mood stabilizer defined as lithium, divalproex/valproic acid, oxcarbazepine, or lamotrigine at a stable dose for ≥ 14 days. The study excluded persons with a baseline YMRS score ≥ 35 or HRSD17 score ≥ 35, current clinically significant psychotic features, CIWA-Ar score of > 8, history of hepatic cirrhosis or baseline liver enzymes > 3X upper limit of normal or other clinically significant findings on physical or laboratory examination, vulnerable persons (severe cognitive impairment, inmates, pregnant or nursing women), antipsychotic therapy within 14 days prior to randomization, current carbamazepine or benzodiazepine therapy, current treatment with medications shown to reduce alcohol consumption in large randomized, controlled trials (naltrexone, acamprosate, disulfiram, or topiramate), initiation of antidepressants or mood stabilizers or psychotherapy within past 14 days, high risk for suicide defined as any suicide attempts in the past 3 months or current suicidal ideation with plan and intent, intensive outpatient treatment for substance abuse (12-step programs or weekly psychotherapy that started at least 14 days prior to randomization were allowed), current treatment with ketoconazole, itraconazole, erythromycin, or nefazodone, severe or life-threatening medical condition or diabetes, or history of cataracts or suspected cataracts on ophthalmic exam.
Eligible participants were randomized to 12 weeks of sustained release quetiapine or placebo given in a double-blind fashion. Study drug was initiated at 50 mg/QHS at baseline, increased to 100 mg/QHS at week 1, 200 mg/day at week 2, 400 mg/QHS at week 3 and 600 mg/QHS at week 4. Slower titration or doses reductions were allowed, if needed, using clinician judgment, due to side effects.
Statistical Analysis
Randomization was conducted through a computerized randomization process which was downloaded to a spread sheet used by an unblinded clinic staff member to allocate medication. The randomization was stratified based on ≥ or < 4 drinking days in the past 7 days and use of divalproex/valproic acid (a medication that decreases alcohol use in patients with BPD) at baseline (Salloum, Cornelius et al., 2005). All direct care staff (i.e. study physicians and raters) were blinded. Demographic and baseline clinical characteristics were compared between treatment groups using t-tests for continuous measures and chi squared tests for categorical measures. The primary outcome measure was number of drinks per day, with number of heavy drinking days (defined as a day with ≥ 5 drinks for man and ≥ 4 drinks for women)/week, days of alcohol use/week, and GGT levels as secondary outcomes. All participants completing baseline and at least one post-baseline assessment were used in the analysis (intent-to-treat [ITT] sample). Data on non-completers were analyzed up to the point of study discontinuation.
Mean drinks/day and other continuous outcome measures assessed weekly were analyzed using declining-effects random-regression models.
These models included terms for time, treatment group, and treatment group by time interaction. The baseline value of the outcome measure and type of bipolar disorder were always used as covariates. Additional covariates were included if they improved the Bayesian information criterion (BIC; a measure of goodness of fit). Covariates were selected without regard to whether they enhanced or diminished the significance of the group effect. Models were checked for the presence of outliers and influential points. AST, ALT, GGT, and PRD-III (side effects) values, were measured only at baseline and weeks 6 and 12. Therefore, these values were assessed using analysis of covariance (ANCOVA) with the baseline level of the outcome measure as the covariate. The above analyses were repeated using only participants with study drug adherence greater than or equal to 90% where adherence was computed as the percent of pills taken per week (pills taken between visits/pills that should have been taken between visits). Retention was assessed between groups using a Kaplan-Meier Survival Curve. Baseline to exit changes in alcohol and mood measures were explored in each treatment group using Pearson’s correlation coefficient.
Two post hoc analyses were conducted. The first analysis was of PRD-III scores in those with a ≥ vs. < 90% medication adherence, using the ANCOVA methods described above. This analysis was conducted to explore whether side effect burden was different in those with greater adherence. The second analysis examined whether baseline use of an anticonvulsant was related to quetiapine response by adding baseline anticonvulsant use (yes/no) to the model for the primary outcome measure of drinks per day.
Results
Of 90 participants randomized, 88 participants returned for at least one post-baseline assessment and were used in the data analysis (ITT sample). Demographic information about the two treatment groups is provided in Table 1. The two groups were similar at baseline except for higher GGT levels and more frequent sedative/hypnotic/anxiolytic use in the quetiapine group.
Table 1.
Baseline Demographic and Clinical Characteristics of Quetiapine and Placebo Groups (Intent-to-Treat Sample, N=88), *p<0.05.
| Baseline Characteristic | Quetiapine (N=44) | Placebo (N=44) |
|---|---|---|
|
| ||
| Age, mean in years (SD) | 43.3 (8.2) | 39.7 (10.1) |
| Gender, N (%) | ||
| Female | 17 (38.6%) | 19 (43.2%) |
| Male | 27 (61.4%) | 25 (56.8%) |
| Married, N (%) | 10 (23.3%) | 8 (19.1%) |
| Education, mean in years (SD) | 13.6 (2.5) | 13.3 (2.4) |
| Race, N (%) | ||
| Caucasian | 21 (48.8%) | 21 (47.7%) |
| African American | 13 (30.2%) | 12 (27.3%) |
| Hispanic | 7 (16.3%) | 10 (22.7%) |
| Other | 2 (4.6%) | 1 (2.3%) |
| Mood States, N (%) | ||
| Depressed | 38 (86.4%) | 40 (90.9%) |
| Mixed | 6 (13.6%) | 4 (9.1%) |
| History of Treatment for Drug/Alcohol Use, N (%) | 30 (68.2%) | 25 (56.8%) |
| % Days Alcohol Use, mean (SD) | 74.2 (27.3) | 74.6 (26.1) |
| % Days Heavy Alcohol Use, mean (SD) | 53.0 (30.9) | 60.0 (30.1) |
| Drinks Per Day, mean (SD) | 6.0 (3.4) | 6.5 (3.4) |
| Mood and Craving Scales, mean (SD) | ||
| HRSD17 Total Score | 18.6 (7.0) | 18.3 (6.7) |
| IDS-SR Total Score | 33.9 (14.6) | 28.6 (10.7) |
| YMRS Total Score | 13.9 (6.7) | 13.6 (8.2) |
| PACS Total Score | 20.6 (6.3) | 19.2 (6.6) |
| Liver Enzymes, mean (SD) | ||
| GGT (IU/L)* | 74.3 (69.3) | 47.8 (37.6) |
| AST (IU/L) | 29.7 (13.5) | 27.4 (14.8) |
| ALT (IU/L) | 28.9 (17.3) | 29.0 (24.5) |
| Concomitant Medications, N (%) | ||
| Lithium | 27 (67.5%) | 28 (68.3%) |
| Anticonvulsants | 13 (32.5%) | 13 (31.7%) |
| Antidepressants | 11 (27.5%) | 6 (14.6%) |
| Sedatives/Hypnotics* | 8 (20.0%) | 2 (4.9%) |
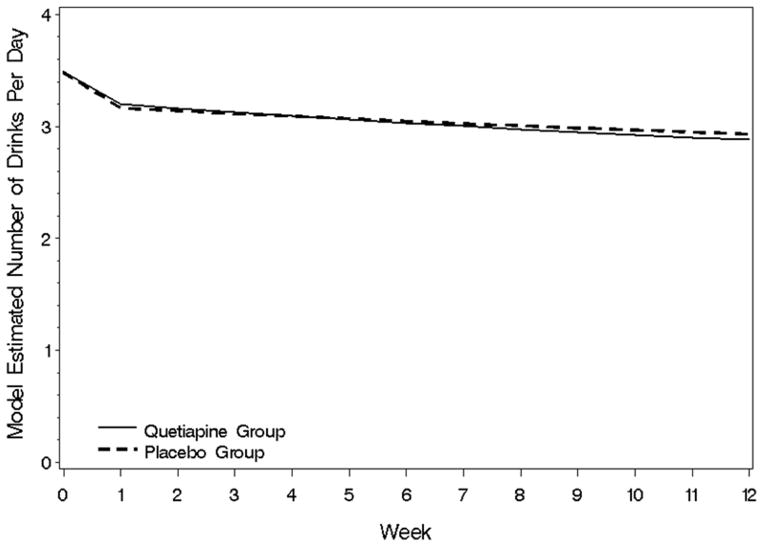
Results of the random regression analysis are given in Table 2. Drinks per day (covariates: baseline drinks per day, bipolar type, race-African American vs. non-African American) did not demonstrate a significant treatment group (F(1,78)= 0.1, p =.75) or week by treatment (F(1.61)=0.5, p=.47) group effect (Table 2, Figure 1). Similar non-significant results were obtained for percent days of alcohol use, drinks per drinking day, percent of heavy drinking days, drinks per heavy drinking day, and alcohol craving as assessed by the PACS. Results of ANCOVAs examining GGT (F(1,42)=0.0, p=.96 week 6, F(1,41)=0.6, p=.45 week 12), AST (F(1,42)=0.2, p=.63 week 6, F(1,41)=0.0, p=.86 week 12) and ALT(F(1,45)=0.0, p=.91 week 6, F(1,44)=0.0, p=.90 week 12) levels also did not reveal significant treatment effects. Because anticonvulsants, particularly valproate (Salloum, Cornelius et al., 2005), have shown promise in reducing alcohol use, an exploratory analysis of drinks per day (the primary outcome measure) was conducted with anticonvulsant use (yes. vs. no) included in the random-regression model. In this analysis, both anticonvulsant use (F-0.2, p=.68) and anticonvulsant by treatment group interaction (F=0.2, p=.70) were non-significant.
Table 2.
Results of Between-Groups Analysis, (N=88).
| Outcome Measure | F-value | Significance (p-value) |
|---|---|---|
|
| ||
| Drinks per day | ||
| Treatment group | F(1,78) = 0.1 | 0.75 |
| Week by Treatment group | F(1,61) = 0.5 | 0.47 |
| Percent Days of Alcohol Use | ||
| Treatment group | F(1,81) = 1.3 | 0.27 |
| Log week by Treatment group | F(1,75) = 0.5 | 0.47 |
| Mean drinks per drinking day | ||
| Treatment group | F(1,152) = 0.2 | 0.63 |
| Week by Treatment group | F(1,181) = 0.8 | 0.36 |
| Percent heavy drinking days per week | ||
| Treatment group | F(1,72) = 0.3 | 0.60 |
| Week by Treatment group | F(1,173) = 1.8 | 0.18 |
| Drinks per heavy drinking day | ||
| Treatment group | F(1,159) = 0.1 | 0.73 |
| Week by Treatment group | F(1,156) = 0.1 | 0.79 |
| PACS (alcohol craving scale) | ||
| Treatment group | F(1,76) = 2.3 | 0.14 |
| Week by Treatment group | F(1,64) = 1.6 | 0.22 |
| HRSD (depression scale rating) | ||
| Treatment group | F(1,69) = 2.5 | 0.12 |
| Week by Treatment group | F(1,59) = 2.0 | 0.16 |
| IDS-SR(self-rated depression) | ||
| Treatment group | F(1,70) = 3.3 | 0.07 |
| Week by Treatment group | F(1,54) = 1.9 | 0.17 |
| YMRS (mania scale rating) | ||
| Treatment group | F(1,73) = 0.0 | 0.88 |
| Week by Treatment group | F(1,58) = 0.0 | 0.97 |
Figure 1.
Drinks per day during the trial for the quetiapine and placebo groups.
Because treatment non-adherence is a concern in dual diagnosis patients, we conducted an exploratory analysis of participants with high medication adherence (defined as ≥ 90% of doses taken based on pill counts). The findings in the high adherence subgroup (n=63, ITT sample) also did not demonstrate significant between-group differences except on drinks per heavy drinking day (the number of drinks in a day with heavy drinking defined as ≥ 5 drinks for men and ≥ 4 for women), which had a significant treatment group effect favoring quetiapine (F(1,126)=5.1, p=.03). To assess whether differences in side effect burden or medication tolerability might have led to differences in response on this outcome measure in those with higher medication adherence, medication adherence was added to an ANCOVA of PRD-III scores. Both adherence (F=2.9, p=.098) and adherence by treatment group interaction (F=0.3, p=.60) were non-significant. The relationship change in PRD scores and drinks per heavy drinking day from baseline to Week 12 was explored using Pearson’s correlation coefficient (n=34). In the quetiapine group (n=17) the correlation was r = 0.55 (p=.023) while in the placebo group (n=17) it was r = −0.14 (p=.59).
Assessments of depressive and manic symptoms did not reveal significant between-group differences (Table 2). However, a statistical trend favoring quetiapine was noted on the IDS-SR (F(1,70)=3.3, p=.07). In the quetiapine group, baseline to exit change HRSD17 correlated significantly with change in drinking days (r=0.314, p=.045). In the placebo group changes in the HRSD17 correlated with changes in the PACS (r=0.545, p=.003), drinking days (r=0.419, p=.006), and heavy drinking days (r=0.343, p=.026), changes in the YMRS with the PACS (r=0.350, p=.023), and changes in the IDS-SR with the PACS (r=0.456, p=.003), drinking days (r=0.367, p=.018), and heavy drinking days (r=0.351, p=.025).
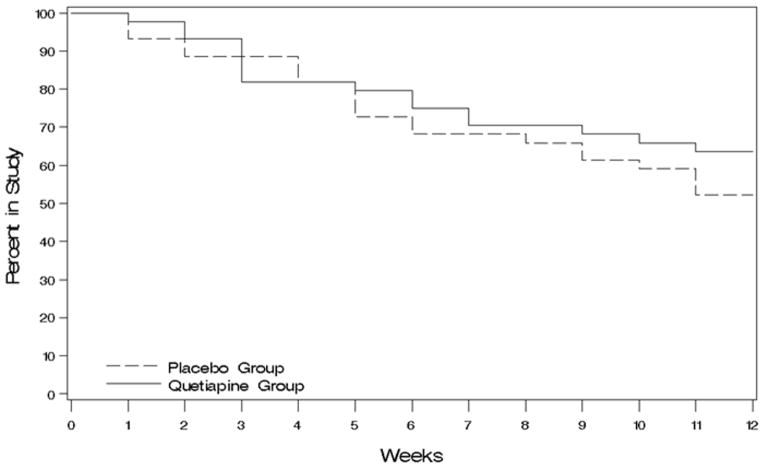
Quetiapine appeared to be reasonably safe and well tolerated in this population (Table 3). Overall side effect burden (PRD-III Somatic Symptom Scale total score), glucose, cholesterol, AIMS, SAS did not differ significantly between groups. Weight showed a significant difference at week 6 (F(1.14)=6.2, p=.03) due to a mean 2.9 (SE 1.4) lb. increase and 2.0 (SE 1.4) lb. decrease in the quetiapine and placebo groups, respectively. The BARS (akathisia) also demonstrated a significant difference (F(1,48)=4.3, p=.04) at week 6 due to a mean 0.40 (SE 0.3) point increase in the quetiapine group and 0.52 (SE 0.3) point decrease with placebo. A total of eight serious adverse events (5 in the quetiapine group and 3 in the placebo group) were reported including two falls (placebo), two arrests for public intoxication (1 in each group), one panic attack (quetiapine), one asthma exacerbation (quetiapine), one victim of a sexual assault (quetiapine) and a myocardial infarction (quetiapine). The participant with the myocardial infraction had cardiac risk factors and was discharged from the hospital following placement of a stent. All of these adverse events were deemed unrelated to the study. Treatment retention was similar in the two treatment groups (logrank test p=.33) (Figure 2).
Table 3.
Treatment Group Effects of Safety Measures from ANCOVA Analysis.
| Week 6 | Week 12 | |||
|---|---|---|---|---|
|
| ||||
| Safety Measure | F value | Significance (p-value) | F-value | Significance (p-value) |
|
| ||||
| SAS | F(1,46) = 2.3 | 0.13 | F(1,38) = 2.4 | 0.13 |
| AIMS | F(1,46) = 0.8 | 0.38 | F(1,41) = 2.2 | 0.15 |
| BARS | F(1,48) = 4.3 | 0.04 | F(1,39) = 1.0 | 0.31 |
| Glucose | F(1,46) = 0.0 | 0.92 | F(1,45) = 0.5 | 0.49 |
| Cholesterol | F(1,41) = 0.8 | 0.37 | F(1,43) = 0.4 | 0.51 |
| Weight | F(1,14) = 6.2 | 0.03 | F(1,34) = 0.4 | 0.51 |
| PRD-III | F(1,46) = 2.5 | 0.12 | F(1,44) = 0.6 | 0.43 |
Figure 2.
Kaplan–Meier survival curve for quetiapine and placebo groups.
Discussion
This study did not find significant between-group differences in alcohol consumption or craving. We observed greater improvement in drinks per heavy drinking day in a subgroup with greater than 90% study medication adherence. This difference in response did not appear to be related to a reduction in side effect burden in those with higher adherence, as a reduction is side effect burden appeared to be associated with greater reduction in this alcohol use outcome. This could suggest some value for quetiapine in reducing drinking on days with relatively heavy alcohol consumption if medication adherence can be maintained. However, this finding should be interpreted with great caution because it was from a subgroup analysis, the between-group differences while significant were modest, and similar findings were not observed on other alcohol use measures in the more adherent subgroup. The observation that quetiapine was not superior to placebo in reducing craving is consistent with prior quetiapine studies in alcohol dependence (Brown, Garza et al., 2008, Guardia, Roncero et al., 2011, Litten, Fertig et al., 2012, Stedman, Pettinati et al., 2010), with the exception of the study by Kampman et al. that observed a reduction in PACS scores in Type B but not Type A alcoholics (Kampman, Pettinati et al., 2007, Salloum, Cornelius et al., 2005). Because Salloum et al (Salloum, Cornelius et al., 2005). reported reduction in alcohol use with valproate in patients with BPD and alcohol dependence, we explored difference in quetiapine response based on the use of concomitant anticonvulsants. However, anticonvulsant use did not appear to influence response to quetiapine.
Although quetiapine is FDA-approved for the treatment of bipolar depression, we did not observe between-group differences in depressive symptoms during the trial other than a trend on the IDS-SR. In our previous study of quetiapine in patients with BPD and alcohol dependence, we observed a significant improvement in HRSD scores with quetiapine as compared to placebo (Brown, Garza et al., 2008). However, a multisite study of quetiapine in this population did not find a significant difference between treatment groups on the Montgomery-Asberg Depression Rating Scale (Stedman, Pettinati et al., 2010). Differences in the study populations may explain these dissimilar findings. Our earlier quetiapine study had lower mean levels of alcohol use (mean approximately 2 drinks per day, 1/3 heavy drinking days) than the multisite study (mean approximately 7 drinks per day, 2/3 heavy drinking days) or current report (mean approximately 6 drinks per day, 57% heavy drinking days). Therefore, effects of quetiapine on depressive symptoms may diminish in patients with higher levels of alcohol use and more severe level of alcohol dependence. Our prior quetiapine study also had slightly higher (approximately 1.5 points) baseline HRSD scores than the current study. Although this clinical feature has not been investigated in quetiapine, data suggest that the mood stabilizer lamotrigine is associated with greater improvement in depressive symptoms as compared to placebo in patients with relatively high baseline HRSD scores (Geddes, Calabrese et al., 2009). Thus, we might have observed a more robust effect of quetiapine on depression in a sample with more severe baseline depressive symptom severity. Similarly, the lack of between-groups difference in YMRS score during the study may have been due to the enrollment of almost exclusively depressed, not mixed, mood state patients at baseline. Consequently, baseline mean YMRS scores were low.
Quetiapine appeared to be reasonably safe and well tolerated in patients with BPD and active alcohol use. Overall side effect burden, glucose, cholesterol, SAS, and AIMS scores did not differ significantly between the two groups. However, weight gain was noted in the quetiapine group at week 6 but, perhaps due to attrition, not at week 12. Weight gain is a common side effect with quetiapine (Sanford and Keating, 2012) and other atypical antipsychotics (Spielmans, Berman et al., 2013). Interestingly, scores on the BARS (a measure of akathisia) increased with quetiapine as compared to placebo at week 6 but not week 12. Other clinical trials in patients with BPD have not found increases in akathisia with quetiapine (Nasrallah, Brecher et al., 2006, Sanford and Keating, 2012), and no differences in BARS scores were observed in prior clinical trials of quetiapine in patients with alcohol dependence. The mean increase in BARS scores of 0.4 observed in the current study is quite modest on a 0–14 point scale and may not be of clinical significance. Consistent with good tolerability for quetiapine, retention was similar in the two treatment groups as can be seen in the Kaplan-Meier survival curve (Figure 1).
The study has several limitations. Alcohol use, while higher than in our earlier quetiapine study and comparable to use in Stedman et al. (Stedman, Pettinati et al., 2010), at a mean of about 6 drinks per day was relatively modest for a study of alcohol-dependent participants. For comparison, the participants who were alcohol dependent without BPD in the quetiapine study by Litten et. al., had a mean of about 13 drinks per day at baseline (Litten, Fertig et al., 2012). Thus, a remaining limitation of the quetiapine studies in BPD and alcohol dependence is the modest alcohol use as compared to most alcohol dependence clinical trials. The modest alcohol use in these trials may be due to the challenges of enrolling dual diagnosis patients with current very heavy alcohol in outpatient clinical trials. Such patients may be unwilling or unable to participate in research studies. Alternatively, some data suggest that dual diagnosis patients may actually use less alcohol than patients with alcohol dependence alone but, nonetheless, have significant disability (Lehman, Myers et al., 1994). Although we limited the range of mood stabilizers, some heterogeneity in concomitant medications was present. While this is the second largest clinical trial reported to date in patients with BPD and alcohol dependence a larger sample size may have allowed for detection of between-groups differences in outcome measures. Finally, pill counts are not an optimum measure of medication adherence.
In summary, statistically significant improvement in alcohol use and craving was not observed with quetiapine as compared to placebo in patients with BPD and alcohol dependence. In addition, an increase in weight and akathisia was also observed early in treatment. Quetiapine is an effective treatment for both manic and depressive symptoms in patients with BPD. However, it does not appear to reduce alcohol use in either bipolar disorder or non-comorbid alcohol dependence.
Acknowledgments
Supported by NIH grant AA016379.
References
- Aagaard J, Vestergaard P. Predictors of outcome in prophylactic lithium treatment: a 2-year prospective study. J Affect Disord. 1990;18(4):259–266. doi: 10.1016/0165-0327(90)90077-l. [DOI] [PubMed] [Google Scholar]
- Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- Bauer MS, Williford WO, Dawson EE, Akiskal HS, Altshuler L, Fye C, Gelenberg A, Glick H, Kinosian B, Sajatovic M. Principles of effectiveness trials and their implementation in VA Cooperative Study #430: ‘Reducing the efficacy-effectiveness gap in bipolar disorder’. J Affect Disord. 2001;67(1–3):61–78. doi: 10.1016/s0165-0327(01)00440-2. [DOI] [PubMed] [Google Scholar]
- Brown ES, Carmody TJ, Schmitz JM, Caetano R, Adinoff B, Swann AC, John Rush A. A randomized, double-blind, placebo-controlled pilot study of naltrexone in outpatients with bipolar disorder and alcohol dependence. Alcoholism, clinical and experimental research. 2009;33(11):1863–1869. doi: 10.1111/j.1530-0277.2009.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ES, Garza M, Carmody TJ. A randomized, double-blind, placebo-controlled add-on trial of quetiapine in outpatients with bipolar disorder and alcohol use disorders. J Clin Psychiatry. 2008;69(5):701–705. doi: 10.4088/jcp.v69n0502. [DOI] [PubMed] [Google Scholar]
- Dalton EJ, Cate-Carter TD, Mundo E, Parikh SV, Kennedy JL. Suicide risk in bipolar patients: the role of co-morbid substance use disorders. Bipolar Disord. 2003;5(1):58–61. doi: 10.1034/j.1399-5618.2003.00017.x. [DOI] [PubMed] [Google Scholar]
- First M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders. New York: Biometrics Research Department, New York State Psychiatric Institute, Department of Psychiatry, Columbia University; 1995. [Google Scholar]
- Flannery BA, Volpicelli JR, Pettinati HM. Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res. 1999;23(8):1289–1295. [PubMed] [Google Scholar]
- Geddes JR, Calabrese JR, Goodwin GM. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br J Psychiatry. 2009;194(1):4–9. doi: 10.1192/bjp.bp.107.048504. [DOI] [PubMed] [Google Scholar]
- Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672–1682. doi: 10.1016/S0140-6736(13)60857-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia J, Roncero C, Galan J, Gonzalvo B, Burguete T, Casas M. A double-blind, placebo-controlled, randomized pilot study comparing quetiapine with placebo, associated to naltrexone, in the treatment of alcohol-dependent patients. Addict Behav. 2011;36(3):265–269. doi: 10.1016/j.addbeh.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Guy W. NIoM Health, editor. Early Clinical Drug Evaluation Unit (ECDEU) Assessment Manual for Psychopharmacology, Revised. Bethesda MD: NIMH Publication; 1976. pp. 217–222. [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman KM, Pettinati HM, Lynch KG, Whittingham T, Macfadden W, Dackis C, Tirado C, Oslin DW, Sparkman T, O’Brien CP. A double-blind, placebo-controlled pilot trial of quetiapine for the treatment of Type A and Type B alcoholism. J Clin Psychopharmacol. 2007;27(4):344–351. doi: 10.1097/JCP.0b013e3180ca86e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Lehman AF, Myers CP, Dixon LB, Johnson JL. Defining subgroups of dual diagnosis patients for service planning. Hosp Community Psychiatry. 1994;45(6):556–561. doi: 10.1176/ps.45.6.556. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Fertig JB, Falk DE, Ryan ML, Mattson ME, Collins JF, Murtaugh C, Ciraulo D, Green AI, Johnson B, Pettinati H, Swift R, Afshar M, Brunette MF, Tiouririne NA, Kampman K, Stout RNCIG 001 Study Group. A double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate XR in very heavy-drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36(3):406–416. doi: 10.1111/j.1530-0277.2011.01649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168(1):26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Nasrallah HA, Brecher M, Paulsson B. Placebo-level incidence of extrapyramidal symptoms (EPS) with quetiapine in controlled studies of patients with bipolar mania. Bipolar Disord. 2006;8(5 Pt 1):467–474. doi: 10.1111/j.1399-5618.2006.00350.x. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511–2518. [PubMed] [Google Scholar]
- Rush A, Carmody T, RPE The Inventory of Depressive Symptomatology (IDS): Clinician (IDS-C) and self-report (IDS-SR) ratings of depressive symptoms. Int J Meth Psychiatr Res. 2000;9:45–59. [Google Scholar]
- Salloum IM, Cornelius JR, Daley DC, Kirisci L, Himmelhoch JM, Thase ME. Efficacy of valproate maintenance in patients with bipolar disorder and alcoholism: a double-blind placebo-controlled study. Arch Gen Psychiatry. 2005;62(1):37–45. doi: 10.1001/archpsyc.62.1.37. [DOI] [PubMed] [Google Scholar]
- Sanford M, Keating GM. Quetiapine: a review of its use in the management of bipolar depression. CNS Drugs. 2012;26(5):435–460. doi: 10.2165/11203840-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Averill P, Sayre S, McCleary P. Cognitive-Behavioral treatment of bipolar disorder and substance abuse: a preliminary randomized study. Addict Disord Their Treat. 2002;1(1):17–24. [Google Scholar]
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Singh J, Mattoo SK, Sharan P, Basu D. Quality of life and its correlates in patients with dual diagnosis of bipolar affective disorder and substance dependence. Bipolar Disord. 2005;7(2):187–191. doi: 10.1111/j.1399-5618.2004.00173.x. [DOI] [PubMed] [Google Scholar]
- Sobell L, Sobell M. Timeline followback: A technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. New Jersey: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Sonne SC, Brady KT, Morton WA. Substance abuse and bipolar affective disorder. J Nerv Ment Dis. 1994;182(6):349–352. doi: 10.1097/00005053-199406000-00007. [DOI] [PubMed] [Google Scholar]
- Spielmans GI, Berman MI, Linardatos E, Rosenlicht NZ, Perry A, Tsai AC. Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. PLoS Med. 2013;10(3):e1001403. doi: 10.1371/journal.pmed.1001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman M, Pettinati HM, Brown ES, Kotz M, Calabrese JR, Raines S. A double-blind, placebo-controlled study with quetiapine as adjunct therapy with lithium or divalproex in bipolar I patients with coexisting alcohol dependence. Alcohol Clin Exp Res. 2010;34(10):1822–1831. doi: 10.1111/j.1530-0277.2010.01270.x. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84(11):1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Thase ME, Fava M, Halbreich U, Kocsis JH, Koran L, Davidson J, Rosenbaum J, Harrison W. A placebo-controlled, randomized clinical trial comparing sertraline and imipramine for the treatment of dysthymia. Arch Gen Psychiatry. 1996;53(9):777–784. doi: 10.1001/archpsyc.1996.01830090023004. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]