Abstract
Background
Few studies have investigated factors influencing participation rates for minority children with a chronic disease in clinical trials. The Silent Cerebral Infarct Multi-Center Clinical (SIT) Trial provides an opportunity to study the impact of demographic and socio-economic factors on randomization in a clinical trial among Black children. Our primary objective was to characterize the factors associated with successful randomization of children with sickle cell disease (SCD) and silent cerebral infarct (SCI) in the SIT Trial after initial consent.
Procedure
Differences in socio-economic and demographic variables, family history and disease-related variables were determined between eligible participants who were successfully randomized and those who were not randomized following initial consent. Head of household educational level and family income were examined separately for US versus non-US sites.
Results
Of 1,176 children enrolled in the SIT Trial, 1016 (86%) completed screening. Of 208 (20%) children with SCI on screening MRI, 196 (94%) were successfully randomized. There were no differences in socio-economic, demographic or disease-related variables between children who were or were not randomized. Participants from non-US sites were more likely to be randomized (22% vs. 12%, p = 0.011), although randomization by country was associated with neither head of household education nor family income.
Conclusion
In the SIT Trial, randomization after initial consent does not appear to be associated with socio-economic or demographic factors. Although these factors may represent barriers for some participants, they should not bias investigators caring for children with SCD in their approach to recruitment for clinical trial participation.
Keywords: Sickle cell disease, study recruitment, research participation, clinical trials, randomization
INTRODUCTION
Participation rates in clinical trials are lower for Black individuals (defined as African Americans and individuals of African descent) and other minority populations[1–4]. Non-participation of racial and ethnic minorities in clinical research, both observational studies and clinical trials, jeopardizes the generalizability of findings and limits the ability to do subgroup analysis. Non-participation also limits access to state-of-the-art treatment, a contributor to morbidity and mortality and fragmented equality within the health care system[5,6] for these individuals. Socio-economic factors such as limited ability to take time off work, lack of trust in the healthcare system, and an infrastructure that is culturally ineffective and insensitive to minorities, have been offered as explanations for why Black individuals do not participate in clinical research trials[7,8]. Further, some providers and research staff assume that Black individuals are unable to understand the details of clinical trials, value of participation, or adhere to study requirements[9]. Assumptions that Black individuals lack knowledge regarding current medical treatments and the availability of newer treatments through clinical trials may also hinder recruitment[9].
Little is known about the parental factors that influence research participation of Black children, although demographic and socio-economic factors are commonly cited barriers to participation in clinical trials among Black adults[7,10]. In their epidemiologic study of children with diabetes, Liese et al. found that non-Hispanic Whites and Hispanic youth had higher participation rates, when compared to Black children[11], without identifying the factors that contribute to the difference. Importantly, understanding the socio-economic and demographic factors that influence the willingness of Black parents and caregivers to allow their children to participate in clinical trials is essential in conditions disproportionately affecting the Black community, such as sickle cell disease (SCD). Currently, little literature exists to explain the factors that influence participation rates in clinical trials for children with SCD, one of the most common genetic diseases in the United States and worldwide[12,13].
The Silent Cerebral Infarct Multi-Center Clinical (SIT) Trial (5U01-NS042804-07) provides a rare opportunity to study the influence of demographic and socio-economic factors on parental willingness to allow screening and random allocation of a large population of Black children following enrollment in a clinical trial. The primary goal of the SIT Trial is to determine whether blood transfusion therapy will reduce further neurological morbidity in children with SCD and silent cerebral infarction (SCI), and if so, the magnitude of this benefit[14]. The overall purpose of this secondary analysis is to gain insight into the potential barriers to research participation, defined as parental willingness to undergo random allocation in the SIT Trial following initial consent. We sought to characterize the factors associated with successful randomization in this population and to test the primary hypothesis that parental acceptance of randomization in the SIT Trial after initial consent is associated with demographic and socio-economic factors, specifically family income and head of household educational level.
METHODS
SIT Trial Description
The SIT Trial is a multi-center clinical trial in which children with SCI were randomized to receive monthly blood transfusions or observation for 36 months. The SIT Trial included 29 clinical sites (7 international sites located in Canada, France and the United Kingdom) and 3 sub-sites, a Clinical Coordinating Center, and a Statistical and Data Coordinating Center. The institutional review board approved the study at all of the participating sites. Eligibility was limited to children between 5 and 14 years of age with hemoglobin SS or hemoglobin S-β0 thalassemia. Randomization required evidence of SCI on magnetic resonance imaging (MRI).
After informed consent was obtained, the screening process for the SIT Trial consisted of a comprehensive history and physical, a standardized neurological examination by a pediatric neurologist, a screening MRI of the brain, a transcranial Doppler ultrasound, a blood specimen for the genetic repository and completion of the relevant screening case report forms. Children who screened positive for SCI but had a normal neurological examination and transcranial Doppler ultrasound were subsequently eligible for randomization. These children had a second MRI (pre-randomization) prior to randomization to ensure there was no evidence for progression of disease.
Randomization in the SIT Trial consisted of assignment to either the transfusion or observation arm for a total of 36 months. Routine laboratory studies and an interval history and examination were required at least monthly for children randomized to receive blood transfusion therapy. Children on the observation arm of the study received monthly phone calls to review medical histories but returned to clinic every 3 months for physical examinations. Additional studies required for participants on either arm included: 1) Comprehensive neurocognitive testing at study entry and exit, 2) Annual neurological examinations by a pediatric neurologist, 3) Health-related quality of life questionnaires, and 4) repeat MRI and transcranial Doppler ultrasound at study exit. Other details of the clinical trial, including exclusion criteria and primary and secondary outcomes, have been described elsewhere[14].
Socio-economic and Demographic Variables Examined
For this analysis, we examined the following child and parent/caregiver demographic and socio-economic characteristics, family history, and clinical variables taken from standard case report forms completed as part of the SIT Trial: 1) participant sex, race, national origin, age and sickle cell diagnosis; 2) participant study site (US versus non-US); 3) number of siblings, siblings with SCD, number of persons 16 years old and younger, and 17 years and older, in the home; 4) mother's age, father's age, marital status of primary caregiver, relationship of primary caregiver to participant, head of household education level and family income; and 5) insurance type. We also examined a number of disease-related variables pertaining to the participants' medical history/disease severity or family history, including hospitalizations and emergency department visits for pain and acute chest syndrome.
Description of Comparison Groups
We evaluated the differences in these characteristics between multiple comparison groups based on participant randomization, screening and withdrawal (Figure 1). To test our primary hypothesis, we compared all eligible participants who signed consent but voluntarily did not proceed to randomization (groups 1, 2, and 6) to those participants who were successfully randomized (group 7). In this primary analysis, voluntary reasons for non-randomization would have included the following: missed screening or pre-randomization MRI, declined randomization either at the screening or randomization stages, or withdrawn by the Principal Investigator for participant non-adherence with the study protocol. Two other secondary comparisons were also performed, including: 1) Participants successfully randomized (group 7) versus those involuntarily withdrawn prior to randomization (group 3), most frequently due to evidence of SCI progression, abnormal transcranial Doppler ultrasound or MRI failures, defined as quality (e.g. motion artifact) and technical failures (e.g. incorrect image sequence), and 2) Participants successfully randomized (group 7) versus those involuntarily withdrawn prior to randomization (group 3) plus participants who underwent successful screening but whose MRIs were negative for SCI (group 4). The main purpose of these secondary comparisons was to assess for socio-economic, demographic and clinical differences that may account for participation rates at various points of the screening and randomization process.
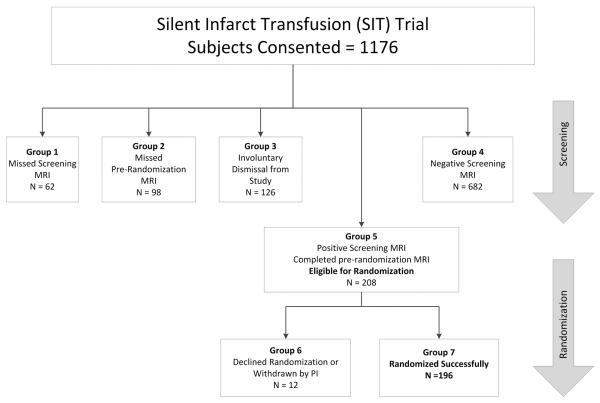
Figure 1. Study Participation and Withdrawal in the SIT Trial.
Participants in the SIT Trial underwent both an initial screening and pre-randomization MRI. Those with SCI on screening MRI but no progression of disease on pre-randomization MRI or transcranial Doppler were subsequently randomized to transfusions or observation. Groups were defined as: 1) consented participants who missed initial screening MRI, 2) consented participants who had a positive screening MRI but missed pre-randomization MRI, 3) consented participants who were involuntarily dismissed from the study due to abnormal transcranial Doppler ultrasound or progression of disease on pre-randomization MRI and those with MRI failures, 4) consented participants who screened negative on initial MRI, 5) all participants who successfully completed screening MRI, pre-randomization MRI and were eligible for randomization; 6) participants who were eligible for randomization but either declined randomization or were dismissed from the study due to non-adherence, lost to follow-up, etc., and 7) consented participants who were successfully randomized.
Statistical Analysis
Categorical data were analyzed using chi-square tests, including Fisher's exact test where appropriate. Continuous data were analyzed using Student t-tests with the Mann-Whitney-Wilcoxon test used for variables with unequal variance or a non-normal distribution. Analyses were conducted using IBM SPSS Statistics (Version 20, Chicago, IL, IBM). The primary analysis compared all participants who voluntarily withdrew from study pre-randomization to participants who proceeded to randomization. Head of household education level and household income were also compared separately in US versus non-US participants given the potential cross-national differences in these measures depending on country of origin. For US participants only, we also performed logistic regression modeling with successful randomization as the dependent variable, including all covariates from Table 1. For measures of clinical severity, we included in the model only percentages of participants reporting hospitalizations or emergency department visits for pain or acute chest syndrome during the past 3 years, rather than also including absolute numbers of events, due to multicollinearity of these variables. All variables were entered in one step.
Table 1.
Univariate analysis of demographic and socio-economic factors associated with successful randomization
| Primary Comparison | Secondary Comparisons | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Groups | Group 7 aSuccessful Randomization |
Groups 1, 2, and 6 blncomplete MRI or Randomization Declined |
Group 3 cinvoluntary Withdrawal from Study |
Groups 3 and 4 dlnvoluntary Withdrawal or Negative Screening MRI |
|||||||||
| Variable | N | Mean ±SD or Percent | N | Mean ±SD or Percent | N | Mean ±SD or Percent | P value | N | Mean ±SD or Percent | P value | N | Mean ±SD or Percent | P value |
| Sex (% female) | 1176 | 48.5 | 196 | 43.4 | 172 | 40.7 | 0.605 | 126 | 53.2 | 0.085 | 808 | 51.4 | 0.045 |
| Age (years) | 1176 | 9.0 ±2.5 | 196 | 9.4 ±2.5 | 172 | 9.1 ±2.5 | 0.234 | 126 | 8.1 ±2.3 | 0.000 | 808 | 8.8 ±2.4 | 0.002 |
| From non-US site (%) | 1176 | 19.2 | 196 | 22.4 | 172 | 12.8 | 0.016 | 126 | 8.7 | 0.001 | 808 | 19.8 | 0.409 |
| Number of siblings | 1161 | 2.4 ±1.8 | 195 | 2.4 ±1.9 | 169 | 2.4 ±1.6 | 0.949 | 125 | 2.4 ±1.8 | 0.995 | 797 | 2.4 ±1.8 | 0.991 |
| Sibling with sickle cell disease (%) | 1176 | 24.8 | 196 | 23.0 | 172 | 19.2 | 0.377 | 126 | 20.6 | 0.623 | 808 | 26.5 | 0.311 |
| Number in household ≤ 16 years old | 1169 | 2.6 ±1.3 | 196 | 2.7 ±1.4 | 168 | 2.5 ±1.2 | 0.238 | 125 | 2.8 ±1.5 | 0.373 | 805 | 2.6 ±1.4 | 0.542 |
| Number in household ≥ 17 years old | 1166 | 1.9 ±1.0 | 195 | 1.8 ±0.9 | 167 | 1.9 ±1.0 | 0.352 | 125 | 2.0 ±0.9 | 0.086 | 804 | 1.9 ±1.0 | 0.085 |
| Mother's age (years) | 1103 | 35.1 ±6.9 | 183 | 35.5 ±6.9 | 160 | 34.9 ±6.9 | 0.454 | 118 | 33.7 ±6.9 | 0.036 | 760 | 35.1 ±6.8 | 0.484 |
| Father's age (years) | 946 | 38.4 ±8.1 | 153 | 38.5 ±8.0 | 132 | 38.7 ±8.4 | 0.786 | 106 | 37.7 ±8.0 | 0.455 | 651 | 38.4 ±8.0 | 0.869 |
| Primary caregiver (% mother) | 1172 | 82.8 | 196 | 82.7 | 172 | 84.3 | 0.671 | 125 | 84.8 | 0.613 | 804 | 82.5 | 0.950 |
| Marital status primary caregiver (% married/living with partner) | 1120 | 46.1 | 185 | 45.4 | 159 | 45.3 | 0.721 | 121 | 41.3 | 0.394 | 776 | 46.4 | 0.952 |
| Education head of household (% college) | 1128 | 54.4 | 191 | 58.6 | 158 | 53.2 | 0.305 | 124 | 56.5 | 0.701 | 779 | 53.7 | 0.215 |
| Family income (% $50K and above) | 903 | 19.5 | 146 | 21.9 | 122 | 21.3 | 0.904 | 99 | 13.1 | 0.081 | 635 | 18.6 | 0.356 |
| Hospitalized for sickle cell pain (%) | 1166 | 61.8 | 196 | 59.2 | 172 | 58.1 | 0.839 | 126 | 60.3 | 0.840 | 805 | 63.6 | 0.251 |
| Hospitalized for acute chest syndrome (%) | 1169 | 29.0 | 196 | 32.7 | 172 | 29.7 | 0.535 | 126 | 27.8 | 0.355 | 805 | 28.3 | 0.232 |
| # Hosp for sickle cell pain | 1166 | 1.9 ±2.5 | 195 | 2.0 ±2.9 | 172 | 1.8 ±2.4 | 0.750 | 125 | 1.6 ±2.0 | 0.596 | 799 | 1.9 ±2.5 | 0.619 |
| # Hosp for acute chest syndrome | 1169 | 0.4 ±0.9 | 196 | 0.5 ±1.1 | 172 | 0.4 ±0.8 | 0.657 | 126 | 0.4 ±0.9 | 0.361 | 801 | 0.4 ±0.8 | 0.230 |
| ED visit for sickle cell pain (%) | 1162 | 59.3 | 196 | 55.1 | 172 | 55.2 | 0.980 | 126 | 60.3 | 0.356 | 803 | 61.6 | 0.093 |
| ED visit for acute chest (%) | 1170 | 12.0 | 195 | 13.8 | 172 | 8.7 | 0.124 | 126 | 11.1 | 0.473 | 804 | 12.3 | 0.563 |
| # ED visits for sickle cell pain | 1162 | 2.2 ±3.3 | 195 | 2.3 ±3.8 | 171 | 1.8 ±2.9 | 0.498 | 124 | 2.7 ±4.8 | 0.547 | 796 | 2.2 ±3.3 | 0.456 |
| # ED visits for acute chest | 1170 | 0.2 ±0.6 | 195 | 0.2 ±0.5 | 172 | 0.1 ±0.5 | 0.128 | 126 | 0.2 ±0.7 | 0.479 | 803 | 0.2 ±0.6 | 0.549 |
| Medicaid use (%) – US only | 945 | 71.3 | 151 | 70.2 | 149 | 72.5 | 0.662 | 115 | 72.2 | 0.725 | 645 | 71.3 | 0.785 |
| Private insurance (%) – US only | 945 | 32.0 | 151 | 33.8 | 149 | 30.2 | 0.507 | 115 | 24.3 | 0.096 | 645 | 31.9 | 0.664 |
| Private insurance (%) – non-US only | 226 | 16.8 | 44 | 15.9 | 22 | 13.6 | 0.808 | 11 | 9.1 | 0.924 | 160 | 17.5 | 0.804 |
Includes participants successfully randomized.
Includes participants who did not complete MRIs (screening or pre-randomization) or who declined randomization at screening or randomization stages.
Includes participants who were involuntarily withdrawn from study, usually due to disease progression or MRI failure.
Includes participants who were involuntarily withdrawn from study, usually due to disease progression or MRI failure, or who had a negative screening MRI.
Bold P values < 0.05 calculated by T-Test, Mann-Whitney U Test or Chi-Square Test where appropriate
RESULTS
A total of 1,176 children were consented and enrolled in the SIT Trial (Figure 1), 1,016 (86%) of whom successfully underwent all required elements of screening, including a pre-randomization MRI following a positive screening MRI. Conversely, 160 (14%) children were not screened successfully, 62 (39%) missed the initial screening MRI and 98 (61%) either missed or declined the pre-randomization MRI. Of those who were screened successfully, 126 (12%) children were subsequently withdrawn involuntarily from study, most frequently due to medical (e.g., progressive disease on MRI or abnormal transcranial Doppler ultrasound) or technical (e.g., MRI failure) reasons. A total of 208 (20%) children upon screening demonstrated evidence of SCI on MRI, and 196 (94%) of these children were successfully randomized. The remaining 12 children were not randomized either due to parent/guardian declining randomization or voluntary withdrawal by the study principal investigator because of concerns of non-adherence to study procedures.
Demographics of Consented Participants
Among the entire group of participants who consented for participation in the SIT Trial and were eligible for screening and random allocation, 1,111 (95%) were Black, 576 (49%) were female and the average age was 9.0 years. The average number of siblings per household was 2.4 with 294 (25%) of siblings also living with SCD. The average mother's age was 35.1 years and the average father's was 38.4 years. The primary caregiver for each participant was the mother (83%) and approximately half (46%) of the primary caregivers were married or living with their partner. A total of 609 (54%) of the heads of household attended college, with 181 (20%) of the family income above $50,000. The majority of US participants (71%) were on Medicaid.
Primary Comparison
In our primary comparison, we examined demographic, socio-economic and disease-related variables in participants who were randomized successfully after initial consent (group 7, n=196) versus participants who voluntarily did not successfully complete the required screening stages of the trial or declined randomization either at the screening or randomization stage, collectively (groups 1, 2 and 6, n=172). We found no differences between groups in almost all socio-economic and demographic variables (Table 1), including the number of children in the family, head of household education level, family income or insurance status. Additionally, participants who were successfully randomized did not differ in hospitalizations or emergency department visits for pain or acute chest syndrome from those who voluntarily did not complete screening or declined randomization. We confirmed that no socio-economic and demographic variables, including family income or head of household education level, independently predicted successful randomization in a logistic regression model for US participants (n=223) (Table 2). Excluding family income, which had 26% missing data, from the model did not change the results of the other variables.
Table 2.
Multivariate logistic regression of demographic and socio-economic factors and their association with successful randomizationa (N = 223)
| Variable | Odds Ratio [95% CI] | P Value |
|---|---|---|
| Age (years) | 1.05 [0.93, 1.17] | 0.46 |
| Sex (% female) | 1.06 [0.6, 1.89] | 0.83 |
| Number of siblings | 1.0 [0.83, 1.20] | 0.98 |
| Marital status primary caregiver (% married/living with partner) | 1.07 [0.74, 1.56] | 0.72 |
| Primary caregiver (% mother) | 1.05 [0.48, 2.29] | 0.90 |
| Education head of household – US only (% college) | 1.12 [0.6, 2.08] | 0.73 |
| Number in household ≤ 16 years old | 1.23 [0.95, 1.58] | 0.12 |
| Number in household ≥ 17 years old | 1.01 [0.71, 1.43] | 0.95 |
| Sibling with sickle cell disease (%) | 1.32 [0.66, 2.62] | 0.43 |
| Private insurance (%) – US only | 0.94 [0.33, 2.66] | 0.91 |
| Medicaid use (%) | 1.08 [0.39, 3.0] | 0.89 |
| Hospitalized for sickle cell pain (%) | 0.82 [0.43, 1.57] | 0.54 |
| Hospitalized for acute chest syndrome (%) | 0.95 [0.51, 1.79] | 0.88 |
| ED visit for sickle cell pain (%) | 0.84 [0.44, 1.58] | 0.58 |
| ED visit for acute chest (%) | 0.66 [0.27, 1.64] | 0.37 |
| Family income – US only (% $50K and above) | 1.3 [0.51, 3.34] | 0.58 |
US participants only
Secondary Comparisons
We performed secondary analyses comparing the same demographic, socio-economic and disease-related variables in successfully randomized participants with the following other subgroups: 1) participants who were involuntarily withdrawn from the study prior to randomization, usually due to disease progression or MRI failure during screening (group 3, n=126), and 2) all participants involuntarily withdrawn plus those who completed successful screening but had a negative screening MRI (groups 3 and 4, n=808). As with our primary comparison, we found no differences in the majority of variables for these secondary comparisons. However, participants who were successfully randomized were significantly older than participants who were involuntarily withdrawn from study (9.4 vs. 8.1 years, p < 0.001). They were also older than the group of participants who were involuntarily withdrawn from study or had a negative screening MRI (9.4 vs. 8.8 years, p = 0.002). We found no difference in hospitalizations or emergency department visits for pain or acute chest syndrome between groups in these secondary comparisons.
Comparison of US versus Non-US Sites
We further sought to determine if successful randomization, involuntary withdrawal or successful screening was affected by participant study site and country. When compared to participants from US sites, participants from non-US sites were more likely to be successfully randomized than to miss critical elements of screening or decline randomization (22% vs. 12%, p = 0.011) in our primary comparison (Table 1). Participants from non-US sites, compared to participants from US sites, also were more likely to be randomized than to be involuntarily withdrawn from study because of MRI failure or disease progression (22% vs. 9%, p = 0.001) (Table 1).
Successful randomization was associated with neither family income nor head of household education level for both US and non-US sites. The proportion of randomized versus non-randomized participants with heads of household completing college or the equivalent was similar for US (55% vs. 51%, p = 0.579) and non-US sites (72% vs. 63%, p = 0.482). The proportion of randomized versus non-randomized participants with household incomes the equivalent of $50,000 or greater was also similar for US sites (23 vs. 19%, p = 0.484) and non-US sites (15 vs. 39%, p = 0.213).
DISCUSSION
Clinical trial recruitment and retention for Black individuals, including both adults and children, have historically been challenging[15–18]. Few studies, however, have examined the factors that influence research participation rates in conditions that primarily affect this population, such as SCD. The results of this analysis did not support our primary hypothesis that parental acceptance of randomization in the SIT Trial is associated with demographic and socio-economic factors. We found that parental willingness to allow eligible children to be randomized in the SIT Trial after initial consent was not associated with standard demographic and socio-economic variables, including family income and head of household education level. However, successful randomization was more frequent among participants enrolled across non-US sites, including Canada, France and the United Kingdom. We also found that, in general, randomized participants in the SIT Trial were no different either from participants who were removed involuntarily at the screening stage or from all participants who were negative for SCI on initial screening by MRI.
Our results may challenge assumptions that lower socio-economic status and education represent major barriers to study randomization following initial consent, even among Black children enrolled in a high burden clinical trial such as the SIT Trial. Several studies have demonstrated that non-white races, lower education and low socio-economic status, defined by personal or family income, are associated with low participation rates and poor adherence in research studies involving children and adults[19–22]. Studies like these may lead to biases, both conscious and unconscious, by investigators in their recruitment practices of racial and ethnic minorities. In contrast, our results suggest children with SCD and their parents, regardless of family income, education level or perceived ability, can handle the burden of screening procedures required of successful randomization following initial consent and study entry and thus, should be approached for participation in studies like the SIT Trial.
Importantly, our results contribute to the existing SCD literature because few data exist regarding the factors that impact parental acceptance of research participation in the SCD population[23]. Investigators for the BABY HUG Trial, a study that assessed hydroxyurea in very young children with SCD, found that families declined participation primarily due to the lack of transportation, demanding nature of the study and general fear of research[24]. Through their development of a survey tool to examine barriers to clinical trials participation, Barakat et al. also demonstrated that parental perceptions of potential harm and mistrust of medical researchers might greatly influence research participation decisions in SCD[25]. In contrast to our analysis, neither of these studies specifically examined the impact of parental socio-economic status, education and other demographic characteristics on actual randomization rates in a multi-center clinical trial that includes children with SCD.
Other unique aspects of our analysis lend additional insight into the factors associated with research participation decision-making among parents and guardians of children with SCD. Overall, randomization rates after initial consent in the SIT Trial were higher at non-US sites, although the reasons for this are not clear and no prior studies have directly compared clinical trial participation in different countries. However, we found that family income and head of household educational level did not affect randomization rates at US sites versus sites in the United Kingdom, France and Canada. To the extent that family income and head of household educational level represent key demographic variables for research participation, our findings not only are important in the US but also may be generalizable across other developed countries.
Additionally, we performed secondary comparisons to ensure that randomized participants also were not different in demographic or socio-economic characteristics when compared to participants involuntarily removed before randomization and to participants who had a negative MRI on initial screening. Finally, we examined disease severity markers and their association with randomization rates following initial consent. We found that neither hospitalizations nor emergency department visits for either pain or acute chest syndrome was associated with greater likelihood of randomization in the SIT Trial. This is in contrast to results from a survey, which suggested an association between perceived disease severity and greater parental willingness to allow their child to participate in research[23].
Some limitations of this study should be noted. This analysis was performed using secondary demographic data collected as part of the SIT Trial and as such, our results were not derived from direct questions designed to ascertain parental willingness to participate in screening and/or randomization. Because of this, we were also not able to examine all potential contributors to participation. These include variables cited as barriers in other studies, such as distance away from study site and transportation methods. However, our analysis of demographic and socio-economic variables still provides valuable insight, albeit indirectly, into the factors determining successful randomization in a large pediatric clinical trial. Lastly, the SIT Trial was not set up to reliably collect detailed demographic data from individuals who were approached for study but did not consent to screening and randomization. It may be that the patients/parents approached for this trial who were unwilling to even undergo screening have different characteristics and barriers to participation. Thus, our findings cannot be generalized to decision making at study entry and apply only to the randomization stage after initial consent, nor can they be generalized to all Black children since the impact of demographic variables on research participation may vary by underlying medical condition within the same race or ethnic group. Nonetheless, our present findings are important in the setting of trials such as the SIT Trial and others that are associated with a high participant burden due to required screening evaluations after consent but before randomization. Drop out prior to randomization represents a real world logistical challenge for investigators. As such, our findings provide some insight into factors that should or should not be considered during the development phase of a clinical trial. They underscore a need to develop better methods to prospectively evaluate research participation decision making as part of the development of future trials.
In summary, parental and caregiver decisions about post-consent randomization of children with SCD, a condition that affects primarily Black individuals, do not appear to be significantly associated with socio-economic or demographic factors, specifically family income and head of household education level, in the SIT Trial. Although these factors may be important in addressing logistical barriers for specific families, they should not bias investigators caring for children with SCD in their approach to recruitment for clinical trial participation. In accordance with common themes that have emerged in the general literature on minority research recruitment, future efforts instead should focus on improving education about research participation and allaying concerns stemming from general misunderstanding, mistrust and fear of research among racial and ethnic minority populations.
ACKNOWLEDGMENTS
The SIT Trial was funded by 5U01-NS042804-07, MRD
Footnotes
DISCLOSURE STATEMENT Conflict of Interest Statement: To the best of our knowledge, no potential conflicts of interest exist for the specified authors.
REFERENCES
- 1.Katz RV, Green BL, Kressin NR, et al. Exploring the “legacy” of the Tuskegee Syphilis Study: a follow-up study from the Tuskegee Legacy Project. J Natl Med Assoc. 2009;101(2):179–183. doi: 10.1016/s0027-9684(15)30833-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durant RW, Davis RB, St George DM, et al. Participation in research studies: factors associated with failing to meet minority recruitment goals. Annals of epidemiology. 2007;17(8):634–642. doi: 10.1016/j.annepidem.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Penberthy L, Brown R, Wilson-Genderson M, et al. Barriers to therapeutic clinical trials enrollment: differences between African-American and white cancer patients identified at the time of eligibility assessment. Clinical trials. 2012;9(6):788–797. doi: 10.1177/1740774512458992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Archives of internal medicine. 2002;162(15):1682–1688. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- 5.BeLue R, Taylor-Richardson KD, Lin J, et al. African Americans and participation in clinical trials: differences in beliefs and attitudes by gender. Contemp Clin Trials. 2006;27(6):498–505. doi: 10.1016/j.cct.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Shaya FT, Gbarayor CM, Huiwen Keri Y, et al. A perspective on African American participation in clinical trials. Contemp Clin Trials. 2007;28(2):213–217. doi: 10.1016/j.cct.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Sweet S, Legro RS, Coney P. A comparison of methods and results in recruiting white and black women into reproductive studies: the MMC-PSU cooperative center on reproduction experience. Contemp Clin Trials. 2008;29(4):478–481. doi: 10.1016/j.cct.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawley LM. African-American participation in clinical trials: situating trust and trustworthiness. J Natl Med Assoc. 2001;93(12 Suppl):14S–17S. [PMC free article] [PubMed] [Google Scholar]
- 9.DeBaun MR, Gorelick PB, Som S. Strategies for recruiting and retaining minorities. Front Neurol Neurosci. 2009;25:118–120. doi: 10.1159/000209485. [DOI] [PubMed] [Google Scholar]
- 10.Arega A, Birkmeyer NJ, Lurie JD, et al. Racial variation in treatment preferences and willingness to randomize in the Spine Patient Outcomes Research Trial (SPORT) Spine (Phila Pa 1976) 2006;31(19):2263–2269. doi: 10.1097/01.brs.0000232708.66608.ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liese AD, Liu L, Davis C, et al. Participation in pediatric epidemiologic research: the SEARCH for Diabetes in Youth Study experience. Contemporary clinical trials. 2008;29(6):829–836. doi: 10.1016/j.cct.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Field JJ, DeBaun MR. Asthma and sickle cell disease: two distinct diseases or part of the same process? Hematology Am Soc Hematol Educ Program. 2009:45–53. doi: 10.1182/asheducation-2009.1.45. [DOI] [PubMed] [Google Scholar]
- 13.Montanaro M, Colombatti R, Pugliese M, et al. Intellectual function evaluation of first generation immigrant children with sickle cell disease: the role of language and sociodemographic factors. Italian journal of pediatrics. 2013;39:36. doi: 10.1186/1824-7288-39-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casella JF, King AA, Barton B, et al. Design of the silent cerebral infarct transfusion (SIT) trial. Pediatr Hematol Oncol. 2010;27(2):69–89. doi: 10.3109/08880010903360367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivers D, August EM, Sehovic I, et al. A systematic review of the factors influencing African Americans' participation in cancer clinical trials. Contemp Clin Trials. 2013;35(2):13–32. doi: 10.1016/j.cct.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Gadegbeku CA, Stillman PK, Huffman MD, et al. Factors associated with enrollment of African Americans into a clinical trial: results from the African American study of kidney disease and hypertension. Contemp Clin Trials. 2008;29(6):837–842. doi: 10.1016/j.cct.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 18.Lund MJ, Eliason MT, Haight AE, et al. Racial/ethnic diversity in children's oncology clinical trials: ten years later. Cancer. 2009;115(16):3808–3816. doi: 10.1002/cncr.24437. [DOI] [PubMed] [Google Scholar]
- 19.DeVita DA, White MC, Zhao X, et al. Determinants of subject visit participation in a prospective cohort study of HTLV infection. BMC medical research methodology. 2009;9:19. doi: 10.1186/1471-2288-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drotar D, Miller V, Willard V, et al. Correlates of parental participation during informed consent for randomized clinical trials in the treatment of childhood leukemia. Ethics & behavior. 2004;14(1):1–15. doi: 10.1207/s15327019eb1401_1. [DOI] [PubMed] [Google Scholar]
- 21.Heinrichs N, Bertram H, Kuschel A, et al. Parent recruitment and retention in a universal prevention program for child behavior and emotional problems: barriers to research and program participation. Prevention science : the official journal of the Society for Prevention Research. 2005;6(4):275–286. doi: 10.1007/s11121-005-0006-1. [DOI] [PubMed] [Google Scholar]
- 22.Read K, Fernandez CV, Gao J, et al. Decision-making by adolescents and parents of children with cancer regarding health research participation. Pediatrics. 2009;124(3):959–965. doi: 10.1542/peds.2008-2878. [DOI] [PubMed] [Google Scholar]
- 23.Liem RI, Cole AH, Pelligra SA, et al. Parental attitudes toward research participation in pediatric sickle cell disease. Pediatr Blood Cancer. 2010;55(1):129–133. doi: 10.1002/pbc.22450. [DOI] [PubMed] [Google Scholar]
- 24.Wynn L, Miller S, Faughnan L, et al. Recruitment of infants with sickle cell anemia to a Phase III trial: data from the BABY HUG study. Contemporary clinical trials. 2010;31(6):558–563. doi: 10.1016/j.cct.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barakat LP, Patterson CA, Mondestin V, et al. Initial development of a questionnaire evaluating perceived benefits and barriers to pediatric clinical trials participation. Contemporary clinical trials. 2013;34(2):218–226. doi: 10.1016/j.cct.2012.11.001. [DOI] [PubMed] [Google Scholar]