Abstract
Epidermal growth factor-like domain 7 (Egfl7) expression in the developing embryo is largely restricted to sites of mesodermal progenitors of angioblasts/hemangioblasts and the vascular endothelium. We hypothesize that Egfl7 marks the endothelial lineage during embryonic development, and can be used to define the emergence of endothelial progenitor cells, as well as to visualize newly forming vasculature in the embryo and during the processes of physiological and pathological angiogenesis in the adult. We have generated a transgenic mouse strain that expresses enhanced green fluorescent protein (eGFP) under the control of a minimal Egfl7 regulatory sequence (Egfl7:eGFP). Expression of the transgene recapitulated that of endogenous Egfl7 at sites of vasculogenesis and angiogenesis in the allantois, yolk sac, and in the embryo proper. The transgene was not expressed in the quiescent endothelium of most adult organs. However, the uterus and ovary, which undergo vascular growth and remodeling throughout the estrus cycle, expressed high levels of Egfl7:eGFP. Importantly, expression of the Egfl7:eGFP transgene was induced in adult neovasculature. We also found that increased Egfl7 expression contributed to pathological revascularization in the mouse retina. To our knowledge, this is first mouse model that enables monitoring endothelial cells at sites of active vasculogenesis and angiogenesis. This model also facilitated the isolation and characterization of EGFL7+ endothelial cell populations by fluorescence activated cell sorting (FACS). Together, our results demonstrate that the Egfl7:eGFP reporter mouse is a valuable tool that can be used to elucidate the mechanisms by which blood vessels form during development and under pathologic circumstances.
Keywords: endothelial reporter, eGFP, transgenic mice, vascular development
Introduction
During development, the earliest blood vessels form by vasculogenesis, a process in which the large vessels and primitive vascular plexi are formed by the migration, coalescence, and de novo endothelial differentiation of mesoderm-derived angioblasts (Drake and Fleming, 2000; Risau and Flamme, 1995). In the mouse embryo, the first vascular structures appear in the blood islands of the yolk sac. The primitive plexus is then modified by angiogenesis, a group of coordinated events comprised of endothelial sprouting, branching, lumen formation, and remodeling (Carmeliet, 2000; Risau, 1997). During embryonic development, formation of a functional vascular system is essential during for the distribution of nutrients and gases during organ formation and growth, and also for the removal of waste products from the developing organism. New blood vessel growth, termed neoangiogenesis, is also imperative for physiological processes, such as wound healing and the successful establishment of pregnancy. Deregulation of vascular growth is implicated in pathophysiologic conditions, including cancer, tissue ischemia, and retinal diseases (Carmeliet, 2003; Chen and Smith, 2007; Gariano and Gardner, 2005; Herbert and Stainier, 2011).
Epidermal growth factor-like domain 7 (Egfl7) is an endothelial-specific gene that was identified in a retroviral gene entrapment screen during early in vitro differentiation of embryonic stem cells (ESC) and during early mouse embryonic development (Fitch et al., 2004). The gene was independently identified in zebrafish (Parker et al., 2004) and as a repressor of smooth muscle cell migration (Soncin et al., 2003). The EGFL7 protein is secreted from the cell, but is associated with the extracellular matrix (ECM) after secretion (Fitch et al., 2004; Schmidt et al., 2007; Soncin et al., 2003). Expression of Egfl7 during mouse development is restricted to sites of mesodermal precursors of angioblasts/hemangioblasts, and the vascular endothelium of the embryo proper and the yolk sac (Fitch et al., 2004). In the adult, Egfl7 expression is mostly down-regulated, with the exception of transient upregulation in sites of physiological and pathological angiogenesis (Campagnolo et al., 2005).
Functional studies in zebrafish and mice have shown that Eglf7 is important during vasculogenesis and angiogenesis. Modulation of Egfl7 expression levels results in vascular defects. Knockdown of Egfl7 in zebrafish embryos leads to defects in vascular tube formation (Parker et al., 2004). Endothelial overexpression of Egfl7 cDNA in a transgenic mouse model, Tie2-Egfl7, results in increased vessel diameter in the embryonic yolk sac and head, vascular cell stratification defects at vessel branch points, and increased vascular coverage in the developing retina (Nichol et al., 2010). Knockdown of Egfl7 in murine embryonic stem cells (mESC) causes defects in vascular structure formation during in vitro differentiation (Durrans and Stuhlmann, 2010). The presence of abnormal vascular sheets is due, in part, to increased proliferation of endothelial cells upon Egfl7 knockdown.
Based on its early developmental and endothelial-restricted expression, we hypothesized that Egfl7 would be a useful marker to study the emergence and formation of endothelial progenitors during early mammalian development. We also hypothesized that fluorescently marked Egfl7-expressing cells could be used to visualize and monitor newly forming vasculature under physiologic and pathologic conditions. To test this, we have generated a novel reporter model, the Egfl7:eGFP transgenic mouse. We show here that the transgene can be used as a reporter of active angiogenesis in embryos and adults. Our results suggest that Egfl7:eGFP transgenic mice can also be used to track endothelial progenitors during early embryonic development.
Results
Generation of Egfl7:eGFP transgenic mice
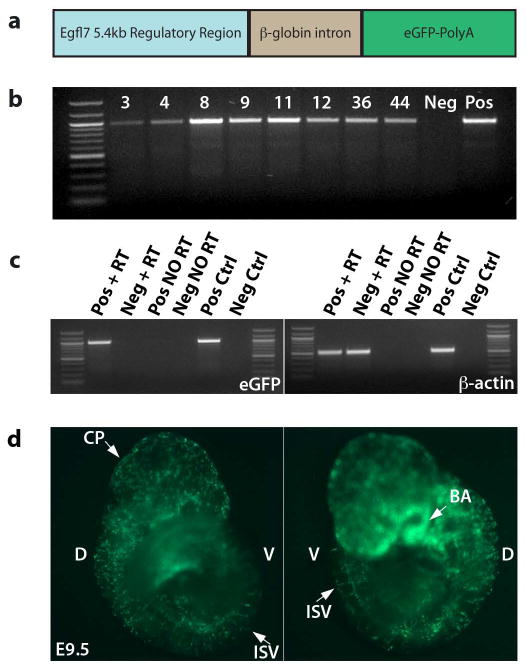
In order to track EGFL7-expressing cells in the endothelial lineage during mouse development and adult neoangiogenesis, we generated an Egfl7:eGFP transgenic mouse. Expression of endogenous Egfl7 and the microRNA-126, located in the intronic sequence between Egfl7 exons seven and eight, are controlled by 5.4kb of sequence located directly upstream of the Egfl7 transcriptional start site in exon 1b (Wang et al., 2008). This regulatory region contains two evolutionarily-conserved ETS regulatory elements (EREs) (Wang et al., 2008), in addition to a GATA-2 consensus binding site (Le Bras et al., 2010). Regulation by ETS and GATA transcription factors is a common feature of genes important for both endothelial and hematopoietic development (Donaldson et al., 2005; Nichol and Stuhlmann, 2011). Thus, we amplified the reported regulatory sequence from a bacterial artificial chromosome (BAC) containing the murine Egfl7 locus and surrounding genomic information, and cloned it directly upstream of the enhanced green fluorescent protein (eGFP) sequence (Figure 1a).
Figure 1. Generation of Egfl7:eGFP transgenic mice.
a) Schematic of the Egfl7:eGFP transgene. 5.4kb upstream of exon 1b, containing two ETS response elements, was cloned upstream of the β-globin intron, eGFP cDNA, and poly-adenylation sequence. b) Genotyping of potential founders. Genomic DNA was isolated from tail snips of potential founder pups, and genotyped by PCR for the presence of the eGFP sequence. Eight potential founders were identified. c) Transcript expression in E9.5 line 12 embryos by semi-quantitative RT-PCR. Only those embryos that expressed eGFP by live fluorescence expressed the eGFP transcript by semi-quantitative RT-RCR. d) Live expression of eGFP in a representative transgenic E9.5 embryo. D = dorsal, V = ventral, CP = choroid plexus, ISV = intersomitic vessel(s), BA = branchial arches.
One of eight potential founders that contained the transgene, Founder #12 (Figure 1b), produced transgenic embryos at Mendelian ratios that displayed robust eGFP by live fluorescence in what appeared to be a vascular pattern (Figure 1d). Using live whole-mount imaging, uniformly strong punctuated fluorescence was detected in E9.5 transgenic embryos at sites of the choroid plexus in the head, the dorsal aorta, branchial arch vessels, and the intersomitic vessels (Figure 1d), similar to the localization of Egfl7 transcripts detected by RNA in situ hybridization (Fitch et al., 2004). Expression of eGFP-specific transcripts was detected only in those embryos that expressed the fluorescent reporter by eye and were positive for eGFP by genotyping PCR (Figure 1c). The remaining seven founders produced offspring that either expressed no eGFP or did not show endothelial-specific expression. All further studies were performed using transgenic line 12, which were bred as hemizygotes. Although the transgenic mice were healthy and fertile, intercrosses of hemizygous Egfl7:eGFP transgenic mice did not yield homozygous offspring, possibly due to positional effects of the transgene insertion.
Egfl7:eGFP transgene expression specifically marks endothelial cells and their progenitors
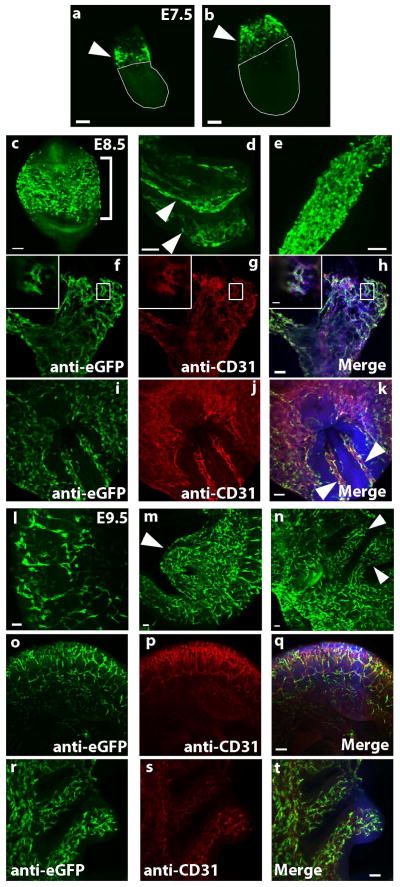
We examined expression of the transgene during the period of vascular development from embryonic day (E) 7.5 to E9.5, using whole-mount immunofluorescence staining for eGFP. Expression of the transgene was localized to sites of mesodermal progenitors, angioblasts and actively proliferating endothelium (Figure 2a–t). Specifically, at E7.5, expression of the Egfl7:eGFP reporter was detected in the extraembryonic compartment. Taken together with previous studies to localize endogenous Egfl7 expression (Fitch et al., 2004), our data are consistent with expression in the extraembryonic mesoderm (Figure 2a, b, arrow heads), the site of the first appearance of endothelial cells. At E8.5, strong expression of eGFP was detected at sites of vasculogenesis in the yolk sac (Figure 2c, bracket), the vasculature of the head folds (Figure 2d, arrow heads), and the dorsal aorta. In addition, the allantois, a site of de novo formation of vessels that later form the fetal vascular plexus of the placenta (Arora and Papaioannou, 2012; Downs et al., 1998; Drake and Fleming, 2000), contained a large number of eGFP+ cells (Figure 2e). eGFP+ cells in the allantois also stained for CD31 (Figure 2f–h), indicating that they were angioblasts or differentiating endothelial cells. Egfl7-eGFP expression also localized to CD31+ vasculature in the yolk sac and paired dorsal aortae of E8.5 embryos (Figure 2i–k). By E9.5, Egfl7:eGFP transgene expression was restricted to the developing vasculature, with expression detected in the intersomitic vessels (Figure 2l), the umbilical and vitelline artery remnants (Figure 2m, arrow head), the vessels of the branchial arches (Figure 2n, arrow heads), and the head vasculature (not shown). Co-staining for GFP and CD31 demonstrated that eGFP+ cells at E9.5 were also restricted to the vasculature in the trunk, including intersomitic vessels (o–q) and branchial arches (r–t).
Figure 2. Egfl7:eGFP is expressed at sites of mesodermal progenitors of endothelial cells and the developing vasculature.
Whole mount staining of Egfl7:eGFP transgenic embryos. Three litters were analyzed at each time point and for each staining condition. All images are compressed z-stacks unless otherwise noted. a–e) Anti-eGFP staining. (a–b) E7.5 embryos. Arrowheads point to extraembryonic mesoderm (EEM), (scale bars = 100 μm). (c–e) E8.5 embryos. Bracket in panel (c) indicates the yolk sac, arrowheads in panel (d) indicate head folds, and an allantois is pictured in panel (e). f–k) Anti-eGFP (green) and anti-CD31 (red) co-staining of E8.5 embryos. Allantois is pictured in (f–h) (scale bar = 50 μm) with high magnification image of a single optical section shown in inset (scale bar = 20 μm). Embryo within yolk sac is pictured in (i–k). Arrowheads point to paired dorsal aortae (scale bar = 50 μm). l–n) Anti-eGFP staining of E9.5 embryos. Intersomitic vessels are shown in panel (l), arrowhead in panel (m) indicates remnants of umbilical and vitelline arteries, and arrowheads in panel (n) point to branchial arches (scale bars = 50 μm). o–t) Anti-eGFP (green) and anti-CD31 (red) co-staining of E9.5 embryos. Trunk vasculature including intersomitic vessels is shown in panels (o–q) (scale bar = 100 μm). Branchial arches are shown in panels (r–t) (scale bar = 50 μm).
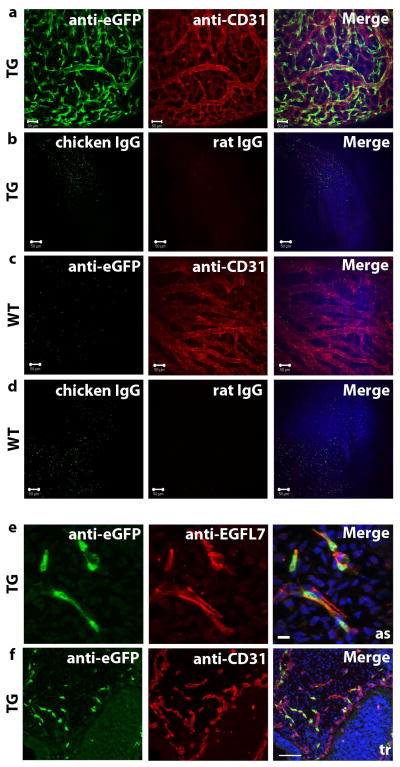
Next, we performed whole mount co-immunofluorescence staining of E10.5 Egfl7:eGFP transgenic (TG) and wild type (WT) littermate embryos using eGFP and CD31 antibodies to determine localization of Egfl7:eGFP expression (depicted in Figure 3a–d is the head vasculature). eGFP expression in transgenic embryos exclusively co-localized to CD31+ blood vessels (Figure 3a). In contrast, no eGFP staining was observed in the vasculature of WT embryos (Figure 3c). Specificity of antibody staining was demonstrated by comparing TG (Figure 3b) and WT (Figure 3d) embryos stained with species-specific IgG and secondary antibodies.
Figure 3. Egfl7:eGFP marks the developing vasculature and faithfully recapitulates endogenous EGFL7 expression.
a–b) Whole mount staining of E10.5 transgenic (TG) embryos (scale bar = 50 μm), head vasculature shown. a) Anti-eGFP and anti-CD31. b) IgG controls. c–d) Whole mount staining of E10.5 wild type (WT) embryos (scale bar = 50 μm), head vasculature shown. c) Anti-eGFP and anti-CD31. d) IgG controls. e) Anti-EGFL7 (red) and anti-eGFP (green) immunofluorescence staining of sections of an E9.5 transgenic embryo (scale bar = 10 μm). eGFP expression is restricted to EGFL7-expressing cells. as = aortic sac. f) Anti-CD31 (red) and anti-eGFP (green) immunofluorescence staining of sections of and E9.5 transgenic embryo (scale bar = 100 μm). tr = trunk.
To determine if expression of this fluorescent reporter faithfully and reliably marks EGFL7-expressing cells, we analyzed sections of E9.5 and E10.5 transgenic embryos by co-immunofluorescence staining for eGFP and EGFL7. eGFP staining specifically localized to EGFL7-expressing cells (Figure 3e). Furthermore, staining of sections confirmed that Egfl7:eGFP expressing cells are restricted to CD31+ vascular structures, as exemplified in the aortic sac and trunk of E9.5 transgenic embryos (Figure 3f). Egfl7:eGFP+ cells also localized strictly to vessels marked by VE-Cadherin at E9.5 (data not shown). Together, these data indicate that the Egfl7:eGFP transgenic mouse is a reliable reporter model, and that it can be used to specifically visualize EGFL7-positive endothelial progenitors and endothelial cells in the developing vasculature.
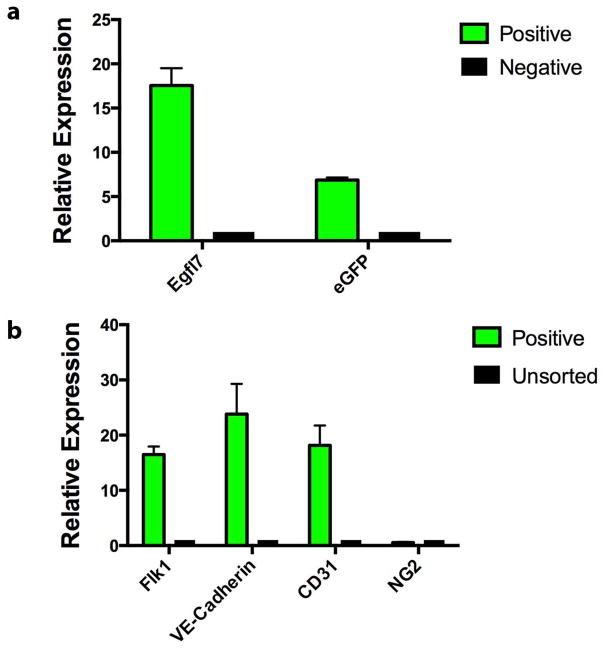
To quantify endothelial gene expression in cells marked by the Egfl7:eGFP transgene, we isolated eGFP+ cells from E9.5 embryos by fluorescence activated cell sorting (FACS) and performed Real Time (RT)-PCR on total RNA for endothelial lineage genes. eGFP+, eGFP− and unsorted cells were collected from E9.5 Egfl7:eGFP transgenic embryos. Egfl7 and eGFP transcripts were highly enriched in the eGFP+ population, with expression levels 17.5- and 6.6-fold higher than in GFP− cells, respectively (Figure 4a). In addition, eGFP+ cells expressed high levels of endothelial gene-specific transcripts compared to unsorted cells. Specifically, we found a 16.5-fold, 23.8-fold and 18.2-fold enrichment in expression of early endothelial genes Flk1, VE-Cadherin, and CD31, respectively (Figure 4b). In contrast, we did not observe enrichment in expression of NG2, a marker of pericytes (Figure 4b). The results indicate that the Egfl7:eGFP transgenic mouse model can be used to isolate purified populations of Egfl7-expressing endothelial cells.
Figure 4. Embryonic Egfl7:eGFP+ cells express endothelial genes.
Gene expression analysis of eGFP+ cells sorted from E9.5 embryos. eGFP+ and eGFP−, as well as unsorted control cells were isolated and analyzed for expression of Egfl7, eGFP (a), Flk1, VE-Cadherin, CD31, and NG2 transcripts (b). Graph contains data from three independent sorting experiments. Error bars represent SEM.
Egfl7:eGFP is expressed in sites of physiologic angiogenesis
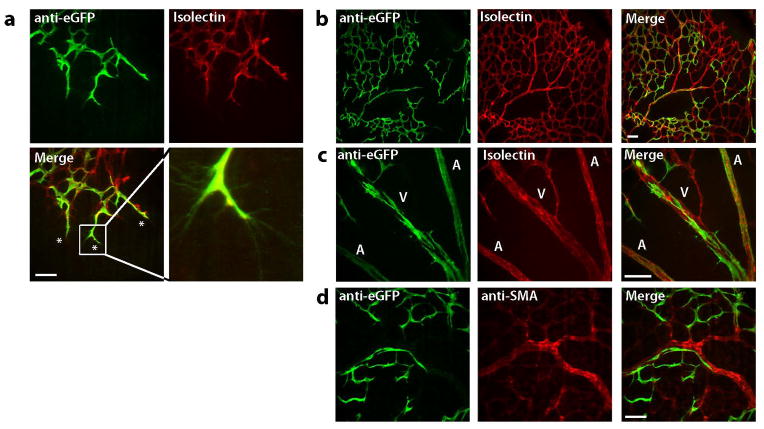
In order to determine the contribution of Egfl7-expressing cells to the nascent vasculature, we examined expression of the Egfl7:eGFP transgene in the developing vasculature of the retina. Retinas of pups on postnatal day 0 (P0) are completely avascular (Fruttiger, 2007). The vascular plexus of the retina develops via angiogenesis over the first 21 days of postnatal life, by a process in which endothelial tip cells at the vascular front project filopodia and guide the growing plexus towards the periphery of the retina. The newly formed plexus subsequently undergoes pruning and remodeling (Fruttiger, 2007; Gerhardt et al., 2003). Retinas were isolated from P6-P7 pups and stained with anti-GFP antibody and DyLight-594-labeled isolectin B4 (Figure 5a–c) or anti-GFP and anti-alpha smooth muscle actin (αSMA) antibodies (Figure 5d). eGFP was restricted to the developing vasculature throughout the retina, as demonstrated by co-localization with isolectin B4 signal (Figure 5a–c). The majority of tip cells with filopodia at the vascular front expressed high levels of the Egfl7:eGFP transgene (Figure 5a). The remodeling plexus toward the center of the retina showed overall reduced and patchy levels of expression (Figure 5b). Arteries and veins displayed somewhat distinct patterns of Egfl7:eGFP expression, with lower levels of eGFP immunofluorescence detected in the arteries at P7 (Figure 5c). Egfl7:eGFP transgene expression was restricted to endothelial cells, and was not detected in smooth muscle cells (Figure 5c). Together, these data demonstrate that Egfl7:eGFP transgene expression marks sites of active angiogenic growth and remodeling.
Figure 5. Egfl7:eGFP is expressed in sites of physiologic angiogenesis.
Expression of Egfl7:eGFP in the neonatal retina. P6 and P7 retinas were isolated and whole-mount stained for eGFP (green) and Isolectin B4 orα-SMA (red). a) eGFP is expressed in tip cells at the sprouting vascular front (scale bar = 50 μm) and in the endothelium (b–c, scale bar = 100 μm), but not in smooth muscle cells of the remodeling vascular plexus (d, scale bar = 50 μm). A = artery, V = vein.
Egfl7:eGFP is downregulated in the quiescent adult vasculature
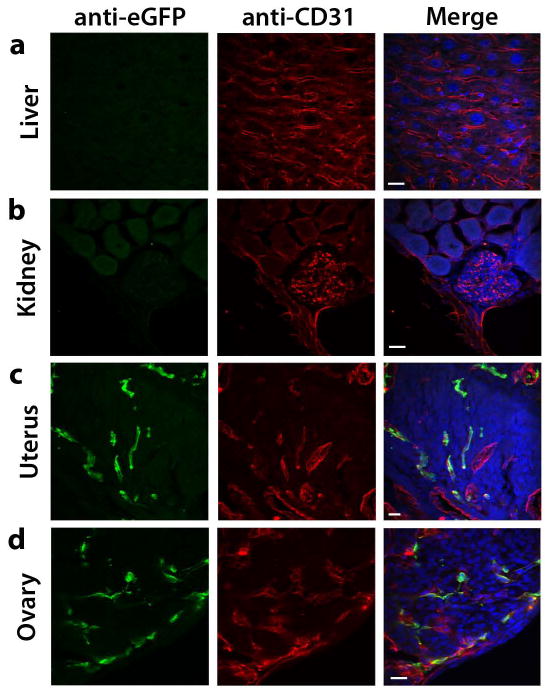
The vascular endothelium of most adult organs is quiescent with active angiogenesis occurring only rarely under physiological conditions, such as during pregnancy and tissue repair. However, in response to appropriate stimuli such as hypoxia, the quiescent vasculature becomes activated to undergo neoangiogenesis (Carmeliet, 2005; Hanahan and Folkman, 1996). To determine expression of the Egfl7:eGFP transgene in the adult vasculature, we harvested organs from 10- to 12-week-old mice and performed co-immunofluorescence staining on sections, using GFP and CD31 antibodies. Very low or no Egfl7:eGFP expression was detected in the vasculature of the adult liver, kidney, heart, testes, and lung (Figure 6a, b and data not shown). In contrast, a substantial subpopulation of CD31+ cells in the uterus and in ovaries expressed the Egfl7:eGFP transgene (Figure 6c, d). Both of these organs undergo active angiogenesis throughout the estrus cycle (Reynolds et al., 1992).
Figure 6. Egfl7:eGFP is downregulated in the quiescent adult vasculature.
Anti-CD31 (red) and anti-eGFP (green) immunofluorescence staining on sections of organs harvested from 10–12 week-old Egfl7:eGFP transgenic mice (scale bar = 20 μm). a) Liver. b) Kidney. c) Uterus. d) Ovary.
Egfl7:eGFP transgene expression is induced during neoangiogenesis in the adult
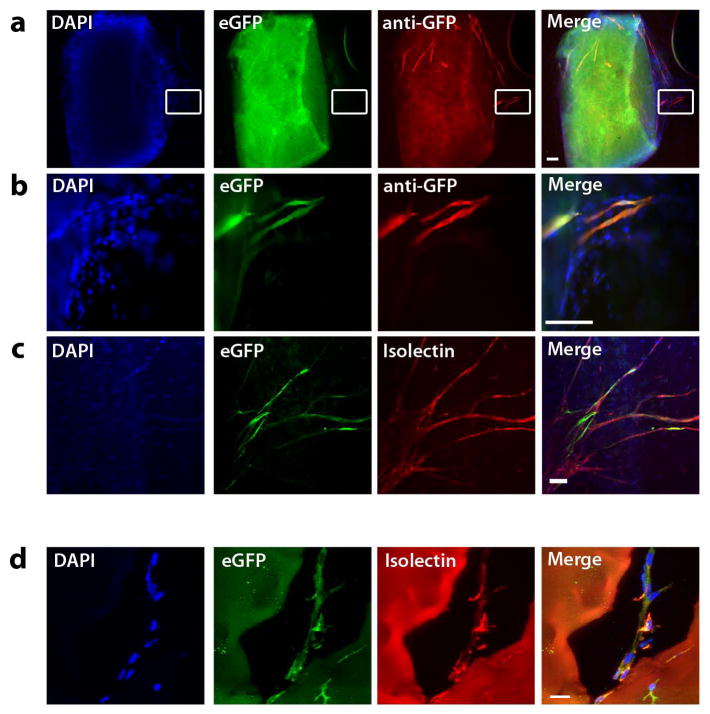
Next, we examined expression of the Egfl7:eGFP transgene in adult mice using two models of neoangiogenesis. First, we used the ex vivo aortic ring assay to investigate expression of Egfl7:eGFP during sprouting angiogenesis (Baker et al., 2012; Nicosia and Ottinetti, 1990). In this assay, aortic explants are cultured in a type I collagen matrix that provides a physiologically relevant matrix to study endothelial sprouting. Due to its high content of connective tissue and extracellular matrix components, the aortic ring typically shows significant levels of background autofluorescence. Nevertheless, eGFP+ sprouts were readily observed emerging from the quiescent aorta when cultured in the presence of VEGF (Figure 7a, b). Expression of eGFP was detectable by both live fluorescence and after staining with an anti-GFP antibody. Importantly, eGFP expression was restricted to isolectin B4-labeled endothelial cells (Figure 7c).
Figure 7. Egfl7:eGFP+ cells contribute to neovasculature.
a) Aortic Ring assay. Aortae from Egfl7:eGFP transgenic adult mice embedded in collagen I gels for eight days develop eGFP+ endothelial sprouts from the quiescent aorta. Endogenous eGFP (green), anti-eGFP immunofluorescence (red) (scale bar = 100 μm). b) Enlargement of boxed area in panels in A (scale bar = 100 μm). c) Anti-eGFP immunofluorescence (green) and DyLight-594-conjugated isolectin B4 labeling (red) (scale bar = 100 μm). d) Matrigel implants. Day 10 Matrigel implants harvested from Egfl7:eGFP transgenic mice. Anti-eGFP immunofluorescence (green) and DyLight-594-conjugated isolectin B4 labeling (red) (scale bar = 20 μm).
Second, we used the Matrigel plug assay to examine the capability of Egfl7:eGFP+ cells to contribute to neovasculature in the adult mouse in vivo (Passaniti et al., 1992). Matrigel plugs (0.5mL) containing 200ng/mL of VEGF were injected intradermally into 8- to 12-week-old Egfl7:eGFP transgenic mice and harvested four and ten days later. Immunohistochemical analysis of stained sections showed that the plugs contained isolectin B4+, eGFP+ (Figure 7d) vessels that had sprouted from the dermis into the avascular Matrigel. In summary, these data indicate that while Egfl7:eGFP is downregulated in the quiescent adult vasculature, adult endothelial cells are competent to re-express Egfl7:eGFP under conditions that initiate angiogenesis.
Egfl7:eGFP is induced during retinal revascularization
To determine whether expression of the Egfl7:eGFP transgene is induced during revascularization, we used the murine model of oxygen-induced retinopathy (OIR) (Connor et al., 2009; Smith et al., 1994). Retinas were harvested from Egfl7:eGFP transgenic mice exposed from P7-P12 to high oxygen (75% O2) and then transferred to normoxia for 5 days (OIR). Control retinas were harvested from mice that were kept in room air (RA) from P7-P17. Whole-mount immunofluorescence staining for GFP and isolectin B4 revealed an overall increased eGFP expression in OIR retinas when compared to those of RA controls, an example of which is shown (Figure 8a, b). This suggests that Egfl7 is upregulated during the hypoxia-induced revascularization process. Quantification showed an increase of GFP+/isolectin+ pixels in the OIR retinas when compared to room air retinas. However, the extent of new eGFP+ vessels was highly variable within the cohorts of OIR and room air retinas, and therefore the result was not statistically significant (Figure 8c). At the RNA transcript level, slight increases in Egfl7 and CD31 expression were observed in the OIR cohort compared to RA cohort without reaching significance (Figure 8d). We surmise that these results reflect the relatively mild angiogenic response of the CD1 mouse strain in this assay (Chan et al., 2005).
Figure 8. Egfl7:eGFP is induced during retinal revascularization.
a) Retinas were isolated from P17 pups and stained using anti-eGFP antibody (green) and DyLight-594-labeled isolectin B4 (red) (n = 11) (scale bar = 100 μm). b) After exposure to 75% oxygen, mice were returned to room air and retinas were isolated at P17, and stained and imaged as in panel A (n = 9). c) Quantification of eGFP+/isolectin+ pixels. Values are shown as mean +/− SD. d) Gene expression analysis of P17 retinas. Egfl7 and CD31 transcripts are slightly induced in OIR retinas compared to room air controls. Values are represented as mean +/− SEM (RA n = 11, OIR n = 9).
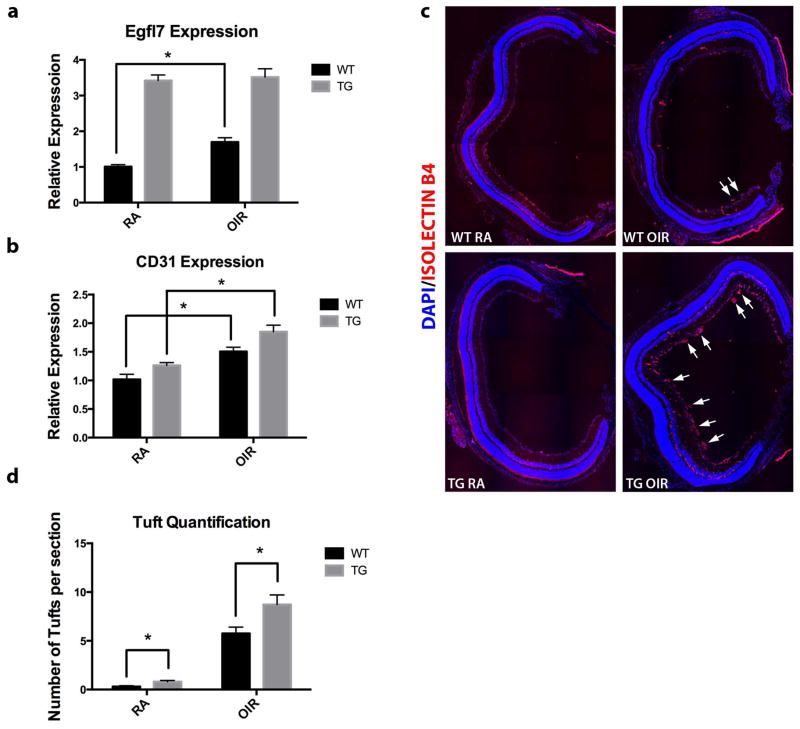
Egfl7 regulates pathologic revascularization in the mouse model of retinopathy of prematurity
To further investigate whether Egfl7 plays a causative role in the pathologic angiogenic process in the OIR model, we analyzed the response of wild type and Tie2-Egfl7 transgenic mice to OIR. Tie2-Egfl7 transgenic (TG) mice express the Egfl7 cDNA under the control of the Tie2 promoter and enhancer (Nichol et al., 2010). TG control retinas from mice maintained in RA expressed the Egfl7 transcript at a 3.4-fold higher level that WT room air controls (Figure 9a). Both WT and TG mice showed induction of both Egfl7 and CD31 transcripts in response to hypoxic conditions (Figure 9a, b). Notably, induction of the Egfl7 transcript in the TG OIR retinas was very slight compared to TG RA controls. This could be due to the fact that the induction of endogenous Egfl7 is masked by the already elevated expression of the transgene driven by the Tie2 promoter. In contrast, CD31 transcript expression was significantly induced in the TG OIR mice. This correlates with the finding that retinas of TG OIR pups contained significantly more pathological tufts per section than the WT OIR counterparts (Figure 9c, d). Together, these data suggest that not only is Egfl7 induced during pathologic revascularization in response to OIR, but importantly, that Egfl7 contributes to the pathology of this disease model.
Figure 9. Increased Egfl7 expression contributes to pathological neovascularization in mouse model of ROP.
a) Expression of Egfl7 transcript in WT and Tie2-Egfl7 TG room air and OIR pups at P17. b) Expression of CD31 transcript in WT and Tie2-Egfl7 TG room air and OIR pups at P17. c) Retinas were isolated from P17 pups, sectioned, and stained using DyLight-594-labeled isolectin B4 (red). Representative figures contain composites of 20x tiled images of the entire retina. Arrows indicate pathological vascular tufts. d) Quantification of pathological vascular tufts in P17 retinas (five sections per retina were quantified). Values are represented as mean +/− SEM; *p<0.05. n = 6 for each genotype and each condition.
Discussion
We have established a novel transgenic mouse model, Egfl7:eGFP, in which a green fluorescent protein is expressed under the control of a minimal Egfl7 promoter. Expression of the reporter marks endothelial progenitors and developing vasculature throughout embryonic development and during physiologic and pathologic angiogenesis. Importantly, the strong fluorescent signal facilitates the isolation of live cells by fluorescence activated cell sorting.
The Egfl7:eGFP transgenic mice provide an attractive alternative to the commonly used Flk-1 reporter mice (Fraser et al., 2005; Larina et al., 2009). Both of these models can be used to follow early morphogenesis of the vascular system by real-time imaging, for cell lineage tracing, determining developmental potential, and for studying vascular phenotypes in mutant mouse strains. However, an important difference between these two reporter models is that while Flk1::H2B-EYFP transgenic mice retain fluorescence transgene expression in smaller vessels of most adult organs (Fraser et al., 2005), expression of the Egfl7:eGFP transgene in the adult vasculature exclusively marks activated endothelial cells that undergo neoangiogenesis.
Our findings show that Egfl7:eGFP expression faithfully recapitulates endogenous Egfl7 expression in the developing embryo and thus serves as a useful marker for the characterization of cells that express the gene. Interestingly, while every eGFP+ cell localized to the vasculature, not all endothelial cells co-stained for eGFP. This is in keeping with findings from previous transgenic studies, in which the majority of endothelial cell promoters direct transgene expression in subsets of endothelium (Minami and Aird, 2005). Patchy expression of the Egfl7:eGFP transgene was also observed in the retinal vasculature. This result is reminiscent of the expression pattern of several genes in the postnatal retina, including vasculature-specific Notch signaling pathway receptors and ligands (Hofmann and Luisa Iruela-Arispe, 2007), and may reflect endothelial cell heterogeneity. In this context, it is of interest to note that EGFL7 is co-expressed with Notch4 in a subset of retinal endothelial cells and functions as a Notch signaling modulator (Nichol et al., 2010). Similar to Egfl7 in situ hybridization results described in a recent study, we found Egfl7:eGFP reporter expression to be higher in veins than in arteries (Poissonnier et al., 2014). However, in contrast to the results by Poissonnier et al., we detected Egfl7:eGFP reporter expression in most of the tip cells at the vascular front.
Importantly, Egfl7:eGFP expression in the yolk sac and allantois of late primitive streak stage embryos (E7.5) is consistent with EGFL7 expression marking early, mesoderm-derived progenitors of not only the endothelial lineage and possibly the primitive hematopoietic lineage. Therefore, this model will prove to be a useful tool for studying the origin of vascular progenitor populations. It is important to note that in primitive streak stage mouse embryos, EGFL7 and FLK1 mark endothelial progenitor populations that only partially overlap in their expression domains (K. Bambino and H. Stuhlmann, unpublished). As FLK1 has been the earliest known marker of the mesodermal progenitors of endothelial and hematopoietic cells, determining similarities and differences in the cell populations that express Egfl7:eGFP and the Flk1 reporter(s) will facilitate our understanding of the origin of vascular cell lineages.
The Egfl7:eGFP transgene was not expressed in the quiescent endothelium of adult mice, consistent with previous studies using in situ hybridization (Campagnolo et al., 2005). However, expression was detected during physiologic angiogenesis in the female reproductive organs of non-pregnant mice. Together with our previous studies showing high levels of Egfl7 transcripts in pregnant uteri of mice, these results suggest that Egfl7 expression is regulated during the estrus cycle (Campagnolo et al., 2005). Importantly, we have demonstrated that the Egfl7:eGFP transgene is re-expressed in two models of adult neoangiogenesis. This is in keeping with previous findings that Egfl7 expression is induced during pathologic angiogenesis, following arterial injury, during regeneration of the endothelial lining (Campagnolo et al., 2005), and in tumor vasculature (Huang et al., 2010).
Our studies also provide evidence that EGFL7 may be a druggable target in diseases of the ischemic retina and other possibly pathological angiogenic disease states. The OIR model mimics the two phases of the human disease ROP. In the first phase, exposure of mouse pups to hyperoxia results in vessel regression in the central retina and dilation and abnormal growth of the large radial vessels. During the second, proliferative phase, pups returned to room air experience relative hypoxia in the avascular central retina, resulting in the induction of angiogenic factors - including VEGF (Pierce et al., 1995) and EGFL7 (Sato et al., 2009) - and abnormal retinal neovascularization. We show here that Egfl7 expression is induced in OIR retinas, and, importantly, that Egfl7 overexpression in the retina results in a more pronounced pathological OIR phenotype. Interestingly, Egfl7 expression has previously been shown to be induced in response to hypoxic preconditioning in the premature rat brain (Gustavsson et al., 2007), supporting a role for Egfl7 in revascularization in response to hypoxic injury. In addition, Egfl7 was also shown to protect endothelial cells against hyperoxia-induced cell death (Xu et al., 2008). Based on these studies, it would be interesting to try to separate the distinct functions of Egfl7 during the two phases of ROP using the OIR mouse model. However, the Egfl7:eGFP transgenic mice are presently maintained in an outbred CD1 genetic background, which responds weakly in angiogenesis assays including OIR (Chan et al., 2005). To facilitate future studies that will utilize the Egfl7:eGFP mice as a reporter for pathologic angiogenesis, we are currently backcrossing the mice into the 129Sv genetic background.
Investigation of the role of Egfl7 in vascular development and angiogenesis have been complicated by the function of miR-126, which is located in and co-transcribed with the Egfl7 locus (Fish et al., 2008; Kuhnert et al., 2008). Recently, it was shown that mice that lack miR-126 but retain Egfl7 expression show partial embryonic lethality, attributed to a loss of vascular integrity and defects in endothelial cell migration, proliferation, and angiogenesis. These phenotypes can be attributed to promotion of VEGF signaling through miR-126-mediated repression of SPRED1 and PIK3R2, two downstream repressors of the VEGF signaling pathway (Fish et al., 2008; Wang et al., 2008). Recent studies from our laboratory demonstrated that alteration of Egfl7 expression levels, without affecting miR-126, leads to vascular defects both in vitro (Durrans and Stuhlmann, 2010) and in vivo (Nichol et al., 2010). Two recent publications from other groups have also explored the role of Egfl7 in multiple in vitro and in vivo vertebrate systems, independent of miR-126 activity (Charpentier et al., 2013; Nikolic et al., 2013). In conclusion, we propose that the Egfl7:eGFP transgenic mouse model is an important and novel tool for studying angiogenesis during development and in the adult. This model will facilitate the understanding of the role of Egfl7-expressing cells in the formation of a functional vasculature in development and disease.
Methods
Ethics Statement
All mouse procedures were performed under a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at the Research Animal Resource Center (RARC) at Weill Cornell Medical College.
Generation of Egfl7:eGFP Transgenic Mice
5.4kb of Egfl7 genomic sequence, directly upstream of the transcriptional start site in exon 1b, (Wang et al., 2008) was PCR amplified from BAC clone RP24-99G313 (CHORI), using Hi-fidelity Platinum Taq Polymerase (Invitrogen, Life Technologies) and the following primers: forward: 5′-AATTGGCCATCGATGGAGCCTTTGAGCTTTTTC and reverse: 5′-AATTGGCCACGCGTCTTCCAGGGAATGTCTGCTG. The amplified sequence was cloned directly upstream of the β-Globin intron (containing the splice donor and splice acceptor) and the eGFP- SV40-PolyA sequence from pEGFP-C1 (Clontech). The resulting 7-kb transgene was excised from the vector by digestion with ClaI and NotI restriction enzymes, and injected into fertilized oocytes by the Transgenic Core Facility at Memorial Sloan Kettering Cancer Center (MSKCC). Oocytes were subsequently transferred to pseudopregnant females. Genomic DNA from tail biopsies of the resulting pups was analyzed by PCR for the presence of the eGFP transgene using the following primers: Forward: 5′-ATGGTGAGCAAGGGCGAGGAGC and Reverse: 5′-GCAGTGAAAAAAATGCTTTATT. Egfl7:eGFP transgenic mice were maintained as hemizygotes in the CD1 background (Charles River Laboratories). Upon publication, the Egfl7:eGFP transgenic mice will be made available to the research community.
Immunofluorescence staining of embryos
For timed pregnancies, the morning of the vaginal plug was considered embryonic day 0.5 (E0.5). Embryos were dissected from pregnant females in cold phosphate buffered saline with calcium and magnesium (PBS + C/M). For tissue sections, embryos were fixed for thirty minutes in 4% paraformaldehyde (PFA) at room temperature. Embryos were washed and cryopreserved in 30% sucrose, and then embedded in 2:1 Tissue-Tek O.C.T. compound (Electron Microscopy Sciences):30% sucrose and cryosectioned at 10–20μm. Sections were permeabilized in ice-cold acetone, blocked in PBS + 10% donkey serum and stained sequentially overnight at 4°C using rat anti-mouse CD31 (Mec13.3 1:100, BD Pharmingen) and chicken anti-GFP (ab13970 1:1000, Abcam). Alternatively, sections from embryos fixed in 4% PFA on ice for 10 minutes were permeabilized in 0.5% Triton X-100 + 0.1% Saponin in 1x PBS (TSP) and stained in PBS + 0.1% TSP for EGFL7 (clone R-12, sc-34416 1:100, Santa Cruz) and eGFP for 3 hours at 37°C. Secondary antibodies donkey anti-rat Cy3, donkey anti-chicken AlexaFluor 488, and donkey anti-goat DyLight-594 (Jackson Immunoresearch) were incubated for one hour at room temperature. Slides were mounted in ProLong Gold Antifade Reagent with DAPI (Invitrogen). IgG controls were performed for each antibody and showed no staining above background. Slides were imaged using the Zeiss Axioplan2 upright microscope using Open Lab software (PerkinElmer) or the Zeiss LSM 5 Live confocal microscope using Zeiss Axiovision software (Zeiss).
Embryo whole-mount preparation
Embryos were fixed in 4% PFA for 15–30 minutes at room temperature, washed in PBS + 0.1% Triton X-100, and permeabilized in PBS + 0.25% Triton X-100. Embryos were then blocked in PBS + 0.1% Triton X-100 + 10% donkey serum, and stained for GFP or GFP and CD31 overnight at 4°C. Embryos were incubated with secondary antibody for one hour at room temperature and mounted in 100% glycerol or ProLong Gold antifade reagent with DAPI using FastWells (Grace Bio-Labs). Slides were imaged as above.
FACS analysis
Egfl7:eGFP transgenic and wild type littermates were isolated from timed pregnancies and separated into pools. After digestion in Collagenase/Dispase (Roche) and DNase I (Roche) at 37°C for 15–30 minutes, tissues were passed through a 40μm filter and resuspended in PBS + 2% FBS. eGFP+ and eGFP− populations, as well as unsorted cells were collected using the BD FACSAriaII SORP high-speed cell sorter and analyzed using BD FACSDiva Software (BD Biosciences).
Semi-Quantitative and Quantitative Real Time-PCR (qRT-PCR)
Total RNA was isolated from whole embryos or FACS sorted populations by extraction in Trizol Reagent (Ambion). RNA (1μg from embryos or 100ng from sorted cells) was treated with DNase I (Invitrogen) and reverse transcribed using the qScript cDNA synthesis kit (Quanta) in accordance with the manufacturer’s instructions. Semi-quantitative RT-PCR was performed using Platinum Taq (Invitrogen/Life Technologies) and run using an Eppendorf Mastercycler EP (Eppendorf). qRT-PCR was run using PerfeCTa SYBR Green Fast Mix for iQ (Quanta). Triplicate samples were run on the iQ5 cycler (Bio-Rad), in a reaction volume of 20μL. Cycle conditions were 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds, 60°C for 1 minute. Gene expression levels were normalized to β-actin, and were determined using the comparative threshold cycle (ΔΔCt) method. Primer sequences are listed below:
| Primer | Sequence (5′→3′) |
|---|---|
| eGFP Semi-Q Fwd | ATGGTGAGCAAGGGCGAGGAGC |
| eGFP Semi-Q Rev | GCAGTGAAAAAAATGCTTTATT |
| β-actin Semi-Q Fwd | GTGGGCCGCTCTAGGCACCAA |
| β-actin Semi-Q Rev | CTCTTTGATGTCACGCACGATTTC |
| eGFP Fwd | ATGGTGAGCAAGGGCGAGGA |
| eGFP Rev | AGGGTGGTCACGAGGGTGGG |
| Egfl7 Fwd | AGAGGAGGTGTACAGGCTGCA |
| Egfl7 Rev | TTCGGTCCAGCTGCTGGAAGGAAT |
| Flk1 Fwd | CCAGAACAGTAAGCGAAAGAGC |
| Flk1 Rev | CCTGTCTTCCAGAGTTTTCAGC |
| VE-Cadherin Fwd | ACTGGAACCAGCACGCTAAC |
| VE-Cadherin Rev | CAACTGCTCGTGAATCTCCA |
| CD31 Fwd | ACTTCTGAACTCCAACAGCGA |
| CD31 Rev | CCATGTTCTGGGGGTCTTTAT |
| NG2 Fwd | ATACACTGGCCTTCCACCAG |
| NG2 Rev | GGTCAGGTCCTCCACTGTGT |
| β-actin Fwd | CCATCATGAAGTGTGACGTTG |
| β-actin Rev | CAATGATCTTGATCTTCATGGTG |
Aortic Ring Assay
Aortic Ring assay was performed as previously described (Baker et al., 2012). Briefly, aortae from 12-week-old-mice were dissected, cut into 0.5–1.0mm rings, and embedded in Collagen Type I (Millipore) gels in 96-well dishes. Gels were cultured with OptiMEM + Gluta Max (Invitrogen) + 2.5% FBS + Penicillin/Streptomycin + 30ng/mL VEGF (R&D Systems). Cultures were fed for the first time on day 3 and every other day thereafter. Gels were stained in well and transferred to slides for mounting and imaging.
Matrigel Plug Assay
Growth factor reduced, phenol red-free Matrigel (BD Biosciences) was mixed with heparin (30U/mL, Sigma) and VEGF (200ng/mL, R&D Systems). The mixture (0.5mL per plug) was injected intradermally into the lower abdomen of 8- to 12-week-old Egfl7:eGFP transgenic mice. Four or ten days after injection, mice were euthanized and the Matrigel plugs were removed. Plugs were fixed, cryopreserved in 30% sucrose, and embedded in O.C.T. compound. Sections were stained for GFP and CD31 or DyLight-594-labeled Isolectin B4 (Vector Labs).
Retina angiogenesis model
For whole-mount staining of the retina, eyes were harvested from Egfl7:eGFP pups at postnatal day (P) 6–7 and fixed in 4% PFA. Following fixation, retinal cups were dissected from the eyes, blocked, and stained for GFP and/or α-SMA (A2547 1:500, Sigma) overnight at 4°C. Secondary antibody (donkey anti-chicken-DyLight 488 and donkey anti-mouse Cy3) and/or DyLight 594-labeled Isolectin B4 were added and incubated for one hour at room temperature. After washing, retinas were flat mounted onto slides in ProLong Gold Antifade Reagent.
Oxygen-induced retinopathy model
Oxygen-induced retinopathy (OIR) was induced as described in Smith et al. (Smith et al., 1994) P7 Egfl7:eGFP, Tie2-Egfl7 transgenic (Nichol et al., 2010), and wild type C57Bl/6J pups their with mothers were exposed to 75% oxygen for five days and returned to room air at P12. Control litters of the same age were kept at room air for the duration of the experiment. Pups were sacrificed at P17 and eyes were processed as above.
Statistical Analysis
Data were analyzed using GraphPad Prism 6 software. Differences between experimental groups were analyzed by unpaired Student t test, and P values <0.05 were considered statistically significant. For immunofluorescence staining, representative images from one experiment are shown.
Acknowledgments
Grant Support: This work was supported by NIH RO1HL082098 to HS and NIH T32GM008539-16 to KB.
We thank the Memorial Sloan-Kettering Cancer Center Transgenic Core Facility for injection of the transgenic construct and Steven Merlin and Jason McCormack at the Weill Cornell Medical College Department of Pathology and Laboratory Medicine Flow Cytometry Core for FACS assistance. We also thank Drs. Daniel J. Nolan and Romulo Hurtado for technical advice, Dr. Donna Nichol for scientific advice, and Samantha Hinds and Dena Almeida for technical support.
Footnotes
Disclosure of Conflicts of Interest
The authors declare no competing financial interests.
References
- Arora R, Papaioannou VE. The murine allantois: a model system for the study of blood vessel formation. Blood. 2012;120:2562–2572. doi: 10.1182/blood-2012-03-390070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, Robinson SD, Lechertier T, Barber PR, Tavora B, D’Amico G, Jones DT, Vojnovic B, Hodivala-Dilke K. Use of the mouse aortic ring assay to study angiogenesis. Nat Protoc. 2012;7:89–104. doi: 10.1038/nprot.2011.435. [DOI] [PubMed] [Google Scholar]
- Campagnolo L, Leahy A, Chitnis S, Koschnick S, Fitch MJ, Fallon JT, Loskutoff D, Taubman MB, Stuhlmann H. EGFL7 is a chemoattractant for endothelial cells and is up-regulated in angiogenesis and arterial injury. Am J Pathol. 2005;167:275–284. doi: 10.1016/S0002-9440(10)62972-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Chan CK, Pham LN, Zhou J, Spee C, Ryan SJ, Hinton DR. Differential expression of pro- and antiangiogenic factors in mouse strain-dependent hypoxia-induced retinal neovascularization. Lab Invest. 2005;85:721–733. doi: 10.1038/labinvest.3700277. [DOI] [PubMed] [Google Scholar]
- Charpentier MS, Christine KS, Amin NM, Dorr KM, Kushner EJ, Bautch VL, Taylor JM, Conlon FL. CASZ1 Promotes Vascular Assembly and Morphogenesis through the Direct Regulation of an EGFL7/RhoA-Mediated Pathway. Dev Cell. 2013;25:132–143. doi: 10.1016/j.devcel.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007;10:133–140. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, Sapieha P, Stahl A, Willett KL, Smith LE. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009;4:1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson IJ, Chapman M, Kinston S, Landry JR, Knezevic K, Piltz S, Buckley N, Green AR, Göttgens B. Genome-wide identification of cis-regulatory sequences controlling blood and endothelial development. Hum Mol Genet. 2005;14:595–601. doi: 10.1093/hmg/ddi056. [DOI] [PubMed] [Google Scholar]
- Downs KM, Gifford S, Blahnik M, Gardner RL. Vascularization in the murine allantois occurs by vasculogenesis without accompanying erythropoiesis. Development. 1998;125:4507–4520. doi: 10.1242/dev.125.22.4507. [DOI] [PubMed] [Google Scholar]
- Drake CJ, Fleming PA. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood. 2000;95:1671–1679. [PubMed] [Google Scholar]
- Durrans A, Stuhlmann H. A role for Egfl7 during endothelial organization in the embryoid body model system. J Angiogenes Res. 2010;2:4. doi: 10.1186/2040-2384-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MJ, Campagnolo L, Kuhnert F, Stuhlmann H. Egfl7, a novel epidermal growth factor-domain gene expressed in endothelial cells. Dev Dyn. 2004;230:316–324. doi: 10.1002/dvdy.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser ST, Hadjantonakis AK, Sahr KE, Willey S, Kelly OG, Jones EA, Dickinson ME, Baron MH. Using a histone yellow fluorescent protein fusion for tagging and tracking endothelial cells in ES cells and mice. Genesis. 2005;42:162–171. doi: 10.1002/gene.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruttiger M. Development of the retinal vasculature. Angiogenesis. 2007;10:77–88. doi: 10.1007/s10456-007-9065-1. [DOI] [PubMed] [Google Scholar]
- Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438:960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson M, Mallard C, Vannucci SJ, Wilson MA, Johnston MV, Hagberg H. Vascular response to hypoxic preconditioning in the immature brain. J Cereb Blood Flow Metab. 2007;27:928–938. doi: 10.1038/sj.jcbfm.9600408. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12:551–564. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann JJ, Luisa Iruela-Arispe M. Notch expression patterns in the retina: An eye on receptor-ligand distribution during angiogenesis. Gene Expr Patterns. 2007;7:461–470. doi: 10.1016/j.modgep.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CH, Li XJ, Zhou YZ, Luo Y, Li C, Yuan XR. Expression and clinical significance of EGFL7 in malignant glioma. J Cancer Res Clin Oncol. 2010;136:1737–1743. doi: 10.1007/s00432-010-0832-9. [DOI] [PubMed] [Google Scholar]
- Kuhnert F, Mancuso MR, Hampton J, Stankunas K, Asano T, Chen CZ, Kuo CJ. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development. 2008;135:3989–3993. doi: 10.1242/dev.029736. [DOI] [PubMed] [Google Scholar]
- Larina IV, Shen W, Kelly OG, Hadjantonakis AK, Baron MH, Dickinson ME. A membrane associated mCherry fluorescent reporter line for studying vascular remodeling and cardiac function during murine embryonic development. Anat Rec (Hoboken) 2009;292:333–341. doi: 10.1002/ar.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bras A, Samson C, Trentini M, Caetano B, Lelievre E, Mattot V, Beermann F, Soncin F. VE-statin/egfl7 expression in endothelial cells is regulated by a distal enhancer and a proximal promoter under the direct control of Erg and GATA-2. PLoS One. 2010;5:e12156. doi: 10.1371/journal.pone.0012156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami T, Aird WC. Endothelial cell gene regulation. Trends Cardiovasc Med. 2005;15:174–184. doi: 10.1016/j.tcm.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Nichol D, Shawber C, Fitch MJ, Bambino K, Sharma A, Kitajewski J, Stuhlmann H. Impaired angiogenesis and altered Notch signaling in mice overexpressing endothelial Egfl7. Blood. 2010;116:6133–6143. doi: 10.1182/blood-2010-03-274860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol D, Stuhlmann H. EGFL7: a unique angiogenic signaling factor in vascular development and disease. Blood. 2011 doi: 10.1182/blood-2011-10-322446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia RF, Ottinetti A. Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Lab Invest. 1990;63:115–122. [PubMed] [Google Scholar]
- Nikolic I, Stankovic ND, Bicker F, Meister J, Braun H, Awwad K, Baumgart J, Simon K, Thal SC, Patra C, Harter PN, Plate KH, Engel FB, Dimmeler S, Eble JA, Mittelbronn M, Schäfer MK, Jungblut B, Chavakis E, Fleming I, Schmidt MH. EGFL7 ligates αvβ3 integrin to enhance vessel formation. Blood. 2013;121:3041–3050. doi: 10.1182/blood-2011-11-394882. [DOI] [PubMed] [Google Scholar]
- Parker LH, Schmidt M, Jin SW, Gray AM, Beis D, Pham T, Frantz G, Palmieri S, Hillan K, Stainier DY, De Sauvage FJ, Ye W. The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature. 2004;428:754–758. doi: 10.1038/nature02416. [DOI] [PubMed] [Google Scholar]
- Passaniti A, Taylor RM, Pili R, Guo Y, Long PV, Haney JA, Pauly RR, Grant DS, Martin GR. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest. 1992;67:519–528. [PubMed] [Google Scholar]
- Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poissonnier L, Villain G, Soncin F, Mattot V. Egfl7 Is Differentially Expressed in Arteries and Veins during Retinal Vascular Development. PLoS One. 2014;9:e90455. doi: 10.1371/journal.pone.0090455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LP, Killilea SD, Redmer DA. Angiogenesis in the female reproductive system. FASEB J. 1992;6:886–892. [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- Sato T, Kusaka S, Hashida N, Saishin Y, Fujikado T, Tano Y. Comprehensive gene-expression profile in murine oxygen-induced retinopathy. Br J Ophthalmol. 2009;93:96–103. doi: 10.1136/bjo.2008.142646. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Paes K, De Mazière A, Smyczek T, Yang S, Gray A, French D, Kasman I, Klumperman J, Rice DS, Ye W. EGFL7 regulates the collective migration of endothelial cells by restricting their spatial distribution. Development. 2007;134:2913–2923. doi: 10.1242/dev.002576. [DOI] [PubMed] [Google Scholar]
- Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- Soncin F, Mattot V, Lionneton F, Spruyt N, Lepretre F, Begue A, Stehelin D. VE-statin, an endothelial repressor of smooth muscle cell migration. EMBO J. 2003;22:5700–5711. doi: 10.1093/emboj/cdg549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Perez RE, Ekekezie II, Navarro A, Truog WE. Epidermal growth factor-like domain 7 protects endothelial cells from hyperoxia-induced cell death. Am J Physiol Lung Cell Mol Physiol. 2008;294:L17–23. doi: 10.1152/ajplung.00178.2007. [DOI] [PubMed] [Google Scholar]