Abstract
Schizophrenia is a prevalent neurodevelopmental psychiatric disorder with poor prognosis and limited understanding of its etiology. This limited etiological understanding renders developing animal models of schizophrenia difficult. While attempts are made to recreate putative etiologies in models, these models may only enable the generation of treatments targeted at the mechanisms manipulated. Although the chakragati mouse was not created as a result of a specific gene target, reports to date suggest these mice exhibit behavioral abnormalities that are consistent with some observed in patients with schizophrenia.
As an initial screen on the relevance of these mice to schizophrenia, we tested the exploration and sensorimotor gating of male and female chakragati mice in the cross-species tests Behavioral Pattern Monitor (BPM) and prepulse inhibition (PPI), respectively. The chakragati mice exhibited hyperactive yet more meandering/circling movements of exploration compared with wildtype (WT) littermates. Moreover, chakragati mice exhibited impaired PPI compared with WT mice, primarily at high prepulse intensity levels. Thus, Chakragati mice share some of the abnormal exploratory and PPI behaviors that are observed in patients with schizophrenia. These behaviors can be used to screen for novel antipsychotics which may be based on novel mechanisms of action. The multivariate abnormal exploration of these mice may also yield further information for treatment effects. Further characterization of these mice in tasks with putative links to negative or cognitive symptoms may further advance the utility of these mice as a screen for novel treatments for schizophrenia.
Keywords: animal model, behavioral pattern monitor, prepulse inhibition
Introduction
Schizophrenia is a prevalent neuropsychiatric disorder affecting approximately 1% of the population (Cannon & Jones, 1996). The group of schizophrenias includes neurodevelopmental disorders with unclear etiologies. It is evident however, that numerous genes and environmental insults contribute to the development of the disorder (Klaning, 1999; van Os & McGuffin, 2003), which in adulthood results in abnormalities in more than 20 brain regions (Levitt, Bobrow, Lucia, & Srinivasan, 2010). This diversity in abnormalities likely contributes to the diverse symptoms of schizophrenia, which are grouped as positive (hallucinations, delusions etc.), negative (alogia, anhedonia, amotivation etc.), and cognitive (inattention, poor working memory, executive dysfunction etc).
Currently, treatments have only been approved for the positive symptoms of schizophrenia, referred to as antipsychotics (APs). APs are primarily dopamine D2-family antagonists, although atypical APs also act on numerous other receptors (Schotte, et al., 1996). The development of novel treatments for schizophrenia require the generation of animal models which recreate symptoms of the disorder (Wu, Hill, Gogos, & van den Buuse, 2013; Young, Zhou, & Geyer, 2010). Models are generated through pharmacological, genetic, or environmental insult to an animal species (Jones, Watson, & Fone, 2011; Young, Powell, & Geyer, 2012), the effects of which are assessed in specific behavioral tasks (Young, Powell, Risbrough, Marston, & Geyer, 2009). Targeted pharmacological or genetic insults may only recreate symptoms related to the target however, limiting the development of novel treatments (Dawe & Ratty, 2007). Chakragati Chakragati (ckr) mice are a transgenic insertional mutant line created following microinjection of a 24-kb genomic fragment containing the mouse Ren-2d rennin gene into BCF (C57BL/10Rospd x C3H/HeRos) fertilized oocytes (Ratty, et al., 1990). The transgene is not expressed but analyses of the insertion revealed that 2.5 copies of the transgene had integrated, with associated duplication and inversion events within chromosome 16 of the mouse genome, resulting in multiple genetic disruptions of a region of chromosome 16 that happens to contain a number of genes and trait loci that have been implicated in schizophrenia. This region of mouse chromosome 16 maps mostly to the 3p21-3q21 region of human chromosome 3 (Ratty, Matsuda, Elliott, Chapman, & Gross, 1992), which encompasses the 3p21 region that has been implicated in risk for five major psychiatric disorders including schizophrenia (GROUP, 2013). Abnormal dopaminergic neurotransmission supports the premise that these mice may be a useful screen for diseases with dopaminergic implications (Ratty, Glick, Mullins, Fitzgerald, & Gross, 1998). Ckr mice were homozygous for the transgene insertion and exhibit an asymmetrically higher D2-family receptor expression in the striatum (Ratty, et al., 1990), but normal D1-family receptor expression (Fitzgerald, et al., 1992). The enlarged ventricles of ckr mice (Torres, Meeder, Hallas, Spernyak, et al., 2005) are also consistent with patients with schizophrenia (Harrison, 1999). The hyperactivity (Torres, et al., 2004) and impaired sensorimotor gating as measured by prepulse inhibition (PPI; (Verma, et al., 2008)) observed in ckr mice are also consistent with patients with schizophrenia (D. Braff, et al., 1978; D. L. Braff, Swerdlow, & Geyer, 1999; Perry, et al., 2009).
While hyperactivity in animals have consistently been described as modeling aspects of schizophrenia, it is only recently that hyperactivity in an exploratory environment is observed in patients with schizophrenia, measured using the human behavioral pattern monitor (BPM) (Perry, et al., 2009). This abnormal exploratory profile of patients with schizophrenia also included more linear movements through space, as measured by spatial d, an aspect of behavior rarely described in animal models. Examining the exploratory behavior of ckr mice in the mouse BPM may provide further evidence of the similarity of behavioral abnormalities between these mice and patients with schizophrenia. Moreover, while ckr mice exhibit reduced PPI when measured using varying prepulse intensities (Verma, et al., 2008), it is unclear whether these mice exhibit altered PPI when measured across inter-stimulus intervals, or altered startle reactivity of varying intensities, which can impact sensorimotor gating (Geyer & Swerdlow, 2001).
Thus, in the present studies we examined the exploratory and sensorimotor gating behaviors of ckr and heterozygous mice and their wildtype littermates, using the BPM and PPI. We hypothesized that consistent with patients with schizophrenia ckr mice would exhibit hyperactivity and reduced spatial d. Moreover, we hypothesized that the ckr mice would exhibit a consistent deficit in PPI that was not driven by altered startle reactivity to varying pulse intensities.
Methods
Animals
Chakragati (ckr) mice and their heterozygote (HT) and wildtype (WT) littermates (20–30 g) were provided by Cerca Insights Sdn Bhd, Malaysia (see table 1 for sample sizes). Mice were housed in groups of 4/cage, with water available ad libitum, and housed in a vivarium on a reversed day-night cycle (lights off at 0800, on at 2000 h). Mice were brought to the laboratory 60 min before testing between 0900 and 1800 h. All behavioral testing procedures were approved by the UCSD Institutional Animal Care and Use Committee. All mice were maintained in an animal facility that meets all federal and state requirements for animal care and was approved by the American Association for Accreditation of Laboratory Animal Care.
Table 1.
Sample sizes of the male and female chakragati wildtype (WT), heterozygous (HT), and knockout (KO) mice used in the current studies.
| WT | HT | KO | |
|---|---|---|---|
| Male | 12 | 13 | 12 |
| Female | 10 | 15 | 4 |
Behavioral Pattern Monitor
Nine mouse BPM chambers (San Diego Instruments, CA) were used to assess the spontaneous exploratory behavior as described previously (Halberstadt, et al., 2009; Risbrough, et al., 2006). Each chamber is illuminated from a single light source above the arena (350 lux in the center and 92 lux in the four corners) which was 30.5 × 61 × 38 cm area with a Plexiglas hole board floor equipped with 3 floor holes and 8 wall holes (J. W. Young, A. K. Goey, et al., 2010b). An outer chamber minimized external light and noise. Nose-poking behavior was detected using an infrared photobeam in each hole. The location of the mouse was recorded every 0.1 s using a grid of 12 × 24 infrared photobeams located 1 cm above the floor recorded. The position of the mouse was defined across nine unequal regions (Geyer, Russo, & Masten, 1986; Risbrough, et al., 2006; J. W. Young, A. K. Goey, et al., 2010b). Rearing behavior was recorded using an array of 16 infrared photobeams 2.5 cm above the floor aligned with the long axis of the chamber. At the start of each test session, mice were placed in the bottom left hand corner of the chamber, facing the corner and the test session started immediately. The exploratory profile of these mice was assessed for 60 min.
Three main factors were investigated based on previous analysis of multivariate factor loading (Paulus & Geyer, 1993): locomotor activity as measured by transitions (calculated as a movement across a defined region); exploratory behavior as measured by holepoking, varied holepoking (total holepokes minus repetitious poking), and rearing; and locomotor pattern as assessed by the spatial d measure of dimensionality. Spatial d uses analyses based on fractal geometry to quantify the geometrical structure or dimensionality of the locomotor path, where a value of 2 represents highly localized 2-dimensional movements and 1 represents 1-dimensional straight distance-covering movements (for calculations of the spatial d value, please see (Paulus & Geyer, 1991). Detailed description of the BPM, its measures, and use in psychiatric research can be found (Young & Geyer, 2011).
Prepulse Inhibition
Startle chambers (SR-LAB, San Diego Instruments, San Diego, CA) consisted of nonrestrictive Plexiglas cylinders 5 cm in diameter resting on a Plexiglas platform in a ventilated chamber. High-frequency speakers mounted 33 cm above the cylinders produced all acoustic stimuli. Piezoelectric accelerometers mounted under the cylinders transduced movements of the animal, which were digitized and stored by an interface and computer assembly. Beginning at the stimulus onset, 65 consecutive 1 ms readings were recorded to obtain the peak amplitude of the animals’ startle response to acoustic (40 ms) startle stimuli. Peak responses to these stimuli are presented in arbitrary units. A dynamic calibration system was used to ensure comparable sensitivities across chambers. Sound levels were measured as described elsewhere (Mansbach & Geyer, 1988) using the A weighting scale in units of dBA SPL. The light was delivered via a bare 15 W incandescent bulb located on the ceiling of the testing chamber. A 65 dB background noise was presented continuously throughout the session.
The PPI test session consisted of 5 testing blocks (Young, Meves, Tarantino, Caldwell, & Geyer, 2011; Young, Wallace, Geyer, & Risbrough, 2010). Block 1 (Habituation 1) acclimated the mice to 120-dB startle pulse-alone intensities by presenting 5 sequentially. Block 2 (prepulse intensities) consisted of 12 120-dB startle pulse-alone intensities interspersed with 10 each of 3 different prepulse trials: 69, 73, and 81 dB prepulses preceding a 120 dB pulse. Prepulses preceded the pulse by 100 ms (i.e. interstimulus interval [ISI], onset to onset). Block 3 (ISI variation) varied the ISI. The block consisted of 7 startle pulses at 120 dB and 4 each 73 dB prepulses preceding a 120 dB pulse by 20, 50, 100, 200, 500 and 1000 ms (onset to onset). Block 4 (pulse intensities) assessed acoustic startle responding across stimulus intensities with 4 presentations of 80, 90, 100, 110, and 120-dB pulse-alone trials. Block 5 (Habituation 2) tested the overall habituation of the mice to the 120-dB startle pulse-alone intensities by presenting 5 sequentially. There was on average a 15 s inter-trial interval (ITI) between each trial. Within each ITI, a nostim trial was interspersed whereby unstimulated behavior was recorded. Habituation to the 120-dB pulse alone trials was examined over the session by measuring the startle amplitude to the 120 dB pulse across the 5-block testing session.
Statistical Analyses
For every study, an analysis of variance was used to examine performance by genotype and sex as between-subjects factors. For the BPM, time was analyzed as a within-subject factor. For PPI, varying prepulses, ISIs, pulse intensities, and habituation were analyzed as within-subjects factors. Tukey post hoc analyses were performed on any significant main effect or interaction. Alpha level was set to 0.05. Data were analyzed using Biomedical Data Programs (BMDP) statistical software (Statistical Solutions Inc., Saugus, MA).
Results
Behavioral Pattern Monitor
Transitions
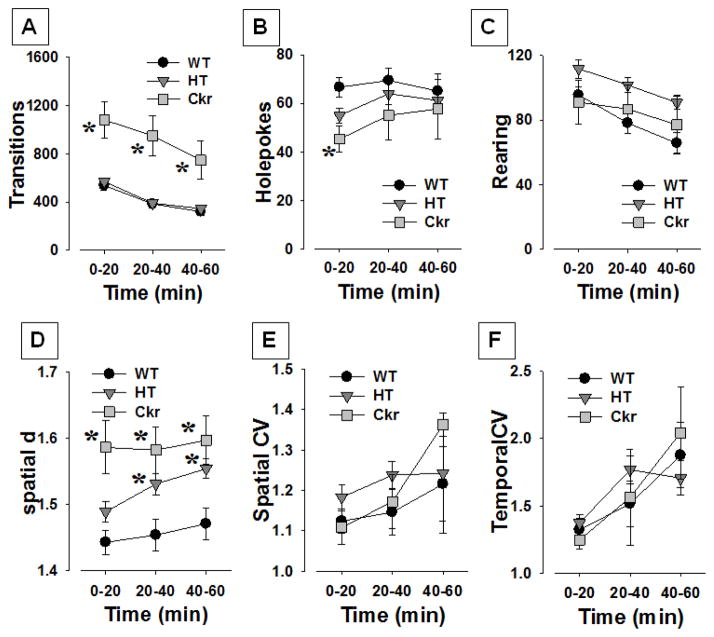
Ckr mice were more active when compared with WT and HT mice as measured transitions (F(2,60)=10.4, p<0.0001; Fig. 1) and confirmed with post hoc analyses (p<0.001). No effect of sex or a genotype by sex interaction was observed for transitions (F<1,1, ns). No time by genotype (F<1, ns), or time by sex by genotype (F(4,120)=2.0, p=0.082) interaction were observed, while an interaction between time and sex (F(2,120)=4.6, p<0.05), was observed.
Figure 1. Altered exploration of ckr mice in the Behavioral Pattern Monitor.
The exploratory behavior of ckr (n=16) mice was compared with heterozygous (HT; n=28) and wildtype (WT; n=22) littermate mice in the cross-species behavioral pattern monitor. Ckr mice exhibited increased activity as measured by transitions (A), a modest reduction in holepoking (B), no difference in rearing (C), and a more meandering localized movement pattern of exploration (increased spatial d, D). HT mice also exhibited higher spatial d values over time, while no effect of genotype was observed for spatial (E) or temporal CV (F). Data expressed as mean ± SEM, * denotes p<0.05 when compared with WT mice.
Holepoking
No effect of genotype or a genotype by sex interaction was observed for holepoking (F<1, ns; Fig. 1), but a main effect of sex was observed (F(1,60)=4.9, p<0.05). No time by genotype or time by sex by genotype (F<1.6, ns) interactions were observed, while an interaction between time and sex (F(2,120)=7.2, p<0.005), was observed.
Rearing
No effect of genotype (F(2,60)=2.3, p>0.1; Fig. 1) or sex, or a sex by genotype interaction (F<1, ns) was observed. No time by genotype or time by sex interaction (F<1, ns) were observed for rearing. A time by genotype by sex interaction (F(4,120)=2.7, p<0.05) was observed for rearing. Post hoc analyses did not reveal any significant differences of genotype within time or sex.
Spatial d
A main effect of genotype (F(2,60)=8.5, p<0.001; Fig. 1) was observed for spatial d, with no sex (F<1, ns), or sex by genotype interaction (F(2,60)=2.5, p=0.094) observed. Post hoc analyses revealed that ckr mice exhibited higher spatial d compared with WT mice (p<0.01), while HT mice exhibited a trend toward higher spatial d compared with WT mice (p<0.1). No time by genotype, time by sex, or time by sex by genotype interactions were observed (F<1.7, ns).
Spatial CV
No genotype, sex, genotype by sex, time by genotype, time by sex, or time by sex by genotype interactions (F<1.8, ns; Fig. 1) were observed for spatial CV.
Temporal CV
No genotype, sex, genotype by sex, time by genotype, time by sex, or time by sex by genotype interactions (F<1.7, ns; Fig. 1) were observed for temporal CV.
Prepulse Inhibition
Varying the prepulse intensity
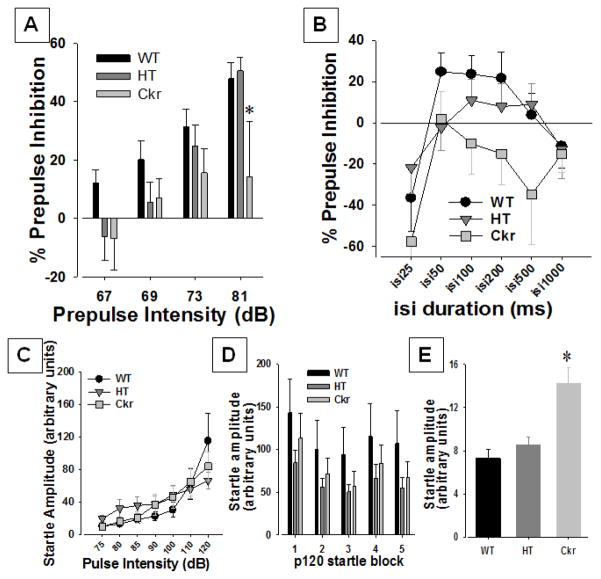
A trend toward a genotype effect was observed on prepulse intensity (F(2,62)=2.7, p=0.07), driven by lower PPI of ckr mice across intensities. This lower PPI of ckr mice was only significant at the highest prepulse however, as confirmed by a genotype by prepulse intensity interaction (F(6,186)=2.6, p<0.05; Fig. 2) and post hoc analyses when compared with both WT and HT mice (p<0.05). Removal of HT mice from the analysis resulted in significantly lower PPI of ckr mice across prepulse intensities (F(1,35)=5.8, p<0.05) with no interactions between other factors (F<1.5, ns). No effect of sex (F(1,62)=2.1, p=0.15), genotype by sex, prepulse intensity by sex, or genotype by prepulse intensity by sex effects were observed (F<1, ns). No other differences were observed.
Figure 2. Prepulse Inhibition of chakragati wildtype (WT), heterozygous (HT), and ckr litter mate mice.
The prepulse inhibition (PPI) ckr (n=16) mice was compared with heterozygous (HT; n=28) and wildtype (WT; n=22) littermate mice. Ckr mice exhibited lower PPI when measured at the highest dB prepulse (81 dB) only (A). No effect of genotype was observed when the interstimulus intervals (isi) between the prepulse (73 dB) and pulse (120 dB) was varied (B). When the pulse intensity was varied, the startle amplitude of the mice did not differ (C). No difference in the habituation of the startle amplitude of these mice to 120 dB pulse was observed across the 5 testing blocks (D). The ckr mice did exhibit higher activity levels during periods when no stimuli were presented (E). Data presented as mean ± SEM, * denotes p<0.05 compared with WT mice.
Varying the inter-stimulus interval
No effect of genotype, sex, sex by genotype, ISI by genotype, ISI by sex, ISI by genotype by sex effects were observed (F<1.4, ns; Fig. 2).
Varying the pulse intensity level
No effect of genotype or genotype by sex interaction was observed (F<1, ns). No effect of sex was observed (F(1,62)=2.2, p=0.15). A trend toward a pulse intensity by genotype (F(12,372)=1.7, p=0.07; 1C) was observed. A pulse intensity by sex interaction was observed (F(6,372)=3.2, p<0.005), while no pulse intensity by genotype by sex interaction was observed (F<1, ns). Post hoc analyses revealed that HT mice exhibited a trend toward higher startle at the 75 dB pulse intensity compared with WT mice (p<0.1). No effect of genotype was observed for any other pulse intensity. When HT mice were removed from the analysis, a significant pulse by genotype interaction was observed (F(6,210)=2.8, p<0.05), with no main genotype effect or interaction with other factors (F<1.8, ns). Post hoc analyses revealed no clear startle differences between genotypes at any pulse intensity however.
Habituation to the 120 dB pulse across the session
No effect of genotype (F(2,62)=2.2, p=0.123; Fig. 2), sex by genotype, block by genotype, or block by genotype by sex effects were observed (F<1, ns).
Baseline activity
Interestingly, ckr mice exhibited increased movements during periods where no stimuli were presented (F(2,62)=12.9, p<0.0001: Fig 2), compared to both WT and HT mice (p<0.01). This increase could reflect the increased general activity of these mice. When the nostim values were used as a covariate to each of the analyses described above, no change in significant differences between genotype were observed.
Discussion
Ckr mice exhibited abnormal exploratory behaviors and impaired sensorimotor gating as compared to their WT littermates. The ckr mice were hyperactive and exhibited a more localized, circumscribed pattern of movement through space compared with WT mice. The ckr mice also exhibited lower specific exploration compared with WT mice, an effect limited to the first 20 min in the novel environment. We also observed reduced PPI of ckr compared with WT mice at the 81 dB prepulse intensity and increased baseline activity of ckr mice during no stimulation. Several of these abnormalities are consistent with patients with schizophrenia, supporting the use of these mice as a model platform for drug development for schizophrenia (Dawe & Ratty, 2007).
The multivariate mouse BPM was utilized to examine the exploratory profile of ckr mice because a specific abnormal exploratory pattern of exploration has been described for patients with schizophrenia using the human BPM. Patients with schizophrenia exhibit increased activity in the BPM, reflected primarily as greater activity towards the end of the testing session (Perry et al, 2009). This pattern contrasts with patients with bipolar disorder (BD) whom are more active at the start of a session and rapidly habituate to the environment In terms of specific exploration (Henry, et al., 2010). Moreover, patients with BD interact with objects more compared with healthy subjects, while patients with schizophrenia do not (Perry, et al., 2010). Both patients with schizophrenia and BD exhibit more linear movement through space as measured by reduced spatial d (Perry et al, 2009). Thus, while the hyperactivity of ckr mice is consistent with patients with schizophrenia (albeit over 60 min in mice vs. 15 min in patients), the increased spatial d and reduced specific exploration of ckr mice is in contrast with schizophrenia patients. This increased spatial d quantifies the ‘circling’ behavior first described in these mice in 1990 (Ratty, et al., 1990) and thus is likely a reliable behavior. Interestingly, while many studies do not report altered behavior of the heterozygous ckr mice, here we observed increased spatial d of these mice, consistent with ckr mice, but only in the latter 2 time blocks of exploration. The hyperactive behavior of the ckr mice can be blocked by doses of antipsychotics (APs) such as haloperidol, clozapine, olanzapine, and pimozide, at clinically prescribed doses (Dawe, et al., 2010). These doses coincide with approximately 70% dopamine D2-family receptor occupancy (Peroutka & Synder, 1980; Seeman, Lee, Chau-Wong, & Wong, 1976) and are consistent with the doses required to block apomorphine-induced disruption in PPI in rats (Swerdlow, Braff, Taaid, & Geyer, 1994). D2-blockade-induced remediation of the hyperactivity of ckr mice may be due to their higher D2-family receptor expression in the striatum (Ratty, et al., 1990). That the increased D2 expression is asymmetric may explain why these mice circle similarly to mice with unilateral striatal 6-hydroxydopamine lesions (Watanabe, Ikeda, & Watanabe, 1981).
Dopaminergic stimulant treatment, such as amphetamine, modafinil, or GBR 12909, typically reduces spatial d in intact mice (Perry, et al., 2009; Risbrough, et al., 2006; J. W. Young, A. K. Goey, et al., 2010a; Young, Kooistra, & Geyer, 2011), indicative of more linear movement through space. This behavior contrasts with the higher spatial d yet increased activity observed in ckr mice. Interestingly, the hallucinogen psilocin, which primarily acts via serotonergic mechanisms, increases spatial d in mice (Halberstadt, Koedood, Powell, & Geyer, 2010), although it reduces activity levels, supporting the asymmetric increase in dopamine D2-family receptor expression may underlie the higher spatial d of ckr mice Thus, the abnormal exploratory behavior of these mice could be as a result of combinations of alterations in dopaminergic and serotonergic mechanisms (van den Buuse, Ruimschotel, Martin, Risbrough, & Halberstadt, 2011). It would be interesting to determine whether the abnormal circling behavior quantified here would be remediated by treatment with APs. Specifically one could hypothesize that typical APs - which block dopamine D2-family receptors - would only remediate the hyperactivity of these mice, while atypical APs - which block dopamine D2-family receptors and serotonergic receptors - would remediate the hyperactivity and circling behavior of these mice (Richelson & Souder, 2000; Schotte, et al., 1996).
In the present studies we investigated numerous aspects of sensorimotor gating in these mice including: 1) Prepulse intensity variation; 2) Inter-stimulus interval variation; 3) Variations in startle-pulse intensities; 4) Habituation to the 120 dB startle pulse; and 5) The baseline activity of the mice during no stimulation. The breadth of examination provided in the present study support that these mice exhibit poor sensorimotor gating as measured by lower PPI, as described previously (Verma, et al., 2008). Moreover, the prepulse intensity effect on PPI observed in WT, HT, and ckr mice indicate that while the ckr mice exhibit normal prepulse intensity-induced variation in PPI, the levels of PPI exhibited by these mice at higher prepulses are lower than their WT littermates. Previously, PPI deficits were reported for every prepulse level (Verma, et al., 2008), where the PPI of WT mice varied from 65–75% (68–77 dB prepulses on a 65 dB background). In the present studies, we observed PPI deficits of ckr mice only at 81 dB prepulse, and observed the PPI of WT mice to vary from 15–50% (67–81 dB prepulses on a 65 dB background). Thus, the discrepancy between these studies could be because of higher baseline PPI in the earlier study. Removal of HT mice from the analyses, revealed significantly lower PPI levels of ckr mice compared with WT mice across prepulse intensities, supporting the previous findings. Consistent with the earlier report on startle reactivity (Verma, et al., 2008), ckr mice did not differ from HT or WT mice in acoustic startle reactivity at the 120 dB, nor at other startle levels in the present study (80, 90, 100, 110 dB). Thus, the reduced PPI of ckr mice were unlikely to be as a result of abnormal startle reactivity. The lack of interaction with sex supports this PPI deficit of ckr mice is sex-independent. Ckr mice exhibited prepulse facilitation when inter-stimulus intervals (isi) between the prepulse and pulses were 25 or 1000 ms, consistent with WT mice. Hence, the temporal processing of incoming stimuli appear to be intact in these mice. Interestingly, the ckr mice exhibited higher ‘startle’ values during periods when no startling stimuli were present. This higher level of activity was likely as a result of the hyperactive phenotype of these mice described above, exacerbated under stressful conditions (Ratty, et al., 1990). Crucially, PPI deficits were present in these mice even when higher baseline activity was controlled for. The independent confirmation of PPI deficits in these mice support their use as a model of impaired PPI for treatment development in diseases such as schizophrenia as such patients exhibit robust PPI deficits (D. Braff, et al., 1978; Swerdlow, Weber, Qu, Light, & Braff, 2008).
The abnormal exploration and sensorimotor gating of ckr mice resemble some aspects of abnormal behaviors ascribed to patients with schizophrenia (D. Braff, et al., 1978; Perry, et al., 2009). Hence, ckr mice may prove a viable model for developing novel treatments of behavioral deficits in schizophrenia that are not limited by a targeted insult to generate the model (Dawe & Ratty, 2007). The utility of PPI and or exploratory measurements alone for developing treatments for schizophrenia is limited however, given the wide range of symptoms experienced by patients that require modeling (Jones, et al., 2011; Swerdlow, et al., 2008; Young, et al., 2009; J. W. Young, et al., 2010). For example, patients with schizophrenia exhibit abnormal social behaviors, which could reflect cognitive deficits and/or negative symptoms (Gard, Kring, Gard, Horan, & Green, 2007; Horan, Subotnik, Snyder, & Nuechterlein, 2006; Mancuso, Horan, Kern, & Green, 2011). When compared with WT littermates, ckr and HT mice exhibit reduced time in social interaction (Torres, Meeder, Hallas, Gross, & Horowitz, 2005). While the deficient sociability mice of ckr observed was not controlled for their abnormal activity levels or whether the mice exhibited reduced approach to non-social situations, these data support the further investigation of other aspects of abnormal behavior of ckr mice as pertains to schizophrenia. Examining the cognitive performance of these mice in tests with cross-species translational validity for human cognitive test batteries (Young, et al., 2009) would also be useful. Basic cognitive profiling of these mice would also be useful (Arguello & Gogos, 2010). The potential for these mice to be used to develop treatments beyond that of positive symptomatology for schizophrenia – as AP currently target – should be examined.
These data provide independent verification that ckr mice exhibit hyperactive behavior and reduced sensorimotor gating. Moreover, the circling behavior of these mice has been confirmed and quantified, while the selectivity of the sensorimotor gating deficits have been supported. While these mice do not recreate every exploratory abnormality seen in patients with schizophrenia, the hyperactivity and impaired PPI of these mice support the use of ckr mice as a platform for identifying novel APs. Future studies should examine whether ckr mice reproduce other symptoms of schizophrenia that remain untreated.
Acknowledgments
We thank Mahalah Buell, Michael Schuchbauer, and Virginia Masten for their advice and support. This study was supported by NIH grants R01-MH071916, the Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center, and by grant support from Cerca Insights.
Footnotes
Conflict of Interest
These studies were supported by a grant from Cerca Insights for which Anil Ratty is employed. The design, conductance, and interpretation of these findings were not influenced by any member however. Mark Geyer holds equity interest in San Diego Instruments. There are no other perceived conflicts of interest.
References
- Arguello PA, Gogos JA. Cognition in mouse models of schizophrenia susceptibility genes. Schizophr Bull. 2010;36(2):289–300. doi: 10.1093/schbul/sbp153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15(4):339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Braff DL, Swerdlow NR, Geyer MA. Symptom correlates of prepulse inhibition deficits in male schizophrenic patients. Am J Psychiatry. 1999;156(4):596–602. doi: 10.1176/ajp.156.4.596. [DOI] [PubMed] [Google Scholar]
- Cannon M, Jones P. Schizophrenia. J Neurol Neurosurg Psychiatry. 1996;60(6):604–613. doi: 10.1136/jnnp.60.6.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe GS, Nagarajah R, Albert R, Casey DE, Gross KW, Ratty AK. Antipsychotic drugs dose-dependently suppress the spontaneous hyperactivity of the chakragati mouse. Neuroscience. 2010;171(1):162–172. doi: 10.1016/j.neuroscience.2010.08.061. [DOI] [PubMed] [Google Scholar]
- Dawe GS, Ratty AK. The chakragati mouse: a mouse model for rapid in vivo screening of antipsychotic drug candidates. Biotechnol J. 2007;2(11):1344–1352. doi: 10.1002/biot.200700145. [DOI] [PubMed] [Google Scholar]
- Fitzgerald LW, Miller KJ, Ratty AK, Glick SD, Teitler M, Gross KW. Asymmetric elevation of striatal dopamine D2 receptors in the chakragati mouse: neurobehavioral dysfunction in a transgenic insertional mutant. Brain Res. 1992;580(1–2):18–26. doi: 10.1016/0006-8993(92)90922-v. [DOI] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93(1–3):253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacol Biochem Behav. 1986;25(1):277–288. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Swerdlow NR. Measurement of startle response, prepulse inhibition, and habituation. Curr Protoc Neurosci. 2001;Chapter 8(Unit 8):7. doi: 10.1002/0471142301.ns0807s03. [DOI] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium; Genetic Risk Outcome of Psychosis (GROUP) Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381(9875):1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Koedood L, Powell SB, Geyer MA. Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J Psychopharmacol. 2010 doi: 10.1177/0269881110388326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, et al. 5-HT(2A) and 5-HT(2C) receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology. 2009;34(8):1958–1967. doi: 10.1038/npp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(Pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Young JW, Paulus MP, Geyer MA, Perry W. Cross-species assessments of motor and exploratory behavior related to bipolar disorder. Neurosci Biobehav Rev. 2010;34(8):1296–1306. doi: 10.1016/j.neubiorev.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Subotnik KL, Snyder KS, Nuechterlein KH. Do recent-onset schizophrenia patients experience a “social network crisis”? Psychiatry. 2006;69(2):115–129. doi: 10.1521/psyc.2006.69.2.115. [DOI] [PubMed] [Google Scholar]
- Jones CA, Watson DJ, Fone KC. Animal models of schizophrenia. Br J Pharmacol. 2011;164(4):1162–1194. doi: 10.1111/j.1476-5381.2011.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaning U. Greater occurrence of schizophrenia in dizygotic but not monozygotic twins. Register-based study. Br J Psychiatry. 1999;175:407–409. doi: 10.1192/bjp.175.5.407. [DOI] [PubMed] [Google Scholar]
- Levitt JJ, Bobrow L, Lucia D, Srinivasan P. A selective review of volumetric and morphometric imaging in schizophrenia. Curr Top Behav Neurosci. 2010;4:243–281. doi: 10.1007/7854_2010_53. [DOI] [PubMed] [Google Scholar]
- Mancuso F, Horan WP, Kern RS, Green MF. Social cognition in psychosis: multidimensional structure, clinical correlates, and relationship with functional outcome. Schizophr Res. 2011;125(2–3):143–151. doi: 10.1016/j.schres.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA. Blockade of potentiated startle responding in rats by 5-hydroxytryptamine1A receptor ligands. Eur J Pharmacol. 1988;156(3):375–383. doi: 10.1016/0014-2999(88)90283-x. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA. A temporal and spatial scaling hypothesis for the behavioral effects of psychostimulants. Psychopharmacology (Berl) 1991;104(1):6–16. doi: 10.1007/BF02244547. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA. Three independent factors characterize spontaneous rat motor activity. Behav Brain Res. 1993;53(1–2):11–20. doi: 10.1016/s0166-4328(05)80262-1. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ, Synder SH. Relationship of neuroleptic drug effects at brain dopamine, serotonin, alpha-adrenergic, and histamine receptors to clinical potency. Am J Psychiatry. 1980;137(12):1518–1522. doi: 10.1176/ajp.137.12.1518. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Henry B, Kincaid M, Young JW, Geyer MA. Quantifying over-activity in bipolar and schizophrenia patients in a human open field paradigm. Psychiatry Res. 2010;178(1):84–91. doi: 10.1016/j.psychres.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, et al. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Arch Gen Psychiatry. 2009;66(10):1072–1080. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratty AK, Fitzgerald LW, Titeler M, Glick SD, Mullins JJ, Gross KW. Circling behavior exhibited by a transgenic insertional mutant. Brain Res Mol Brain Res. 1990;8(4):355–358. doi: 10.1016/0169-328x(90)90050-n. [DOI] [PubMed] [Google Scholar]
- Ratty AK, Glick SD, Mullins JJ, Fitzgerald LW, Gross KW. US Patent No 1998
- Ratty AK, Matsuda Y, Elliott RW, Chapman VM, Gross KW. Genetic mapping of two DNA markers, D16Ros1 and D16Ros2, flanking the mutation site in the chakragati mouse, a transgenic insertional mutant. Mamm Genome. 1992;3(1):5–10. doi: 10.1007/BF00355834. [DOI] [PubMed] [Google Scholar]
- Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000;68(1):29–39. doi: 10.1016/s0024-3205(00)00911-5. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA. Differential contributions of dopamine D(1), D(2), and D(3) receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31(11):2349–2358. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, et al. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl) 1996;124(1–2):57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261(5562):717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Taaid N, Geyer MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiatry. 1994;51(2):139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl) 2008;199(3):331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres G, Hallas BH, Vernace VA, Jones C, Gross KW, Horowitz JM. A neurobehavioral screening of the ckr mouse mutant: implications for an animal model of schizophrenia. Brain Res Bull. 2004;62(4):315–326. doi: 10.1016/j.brainresbull.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Torres G, Meeder BA, Hallas BH, Gross KW, Horowitz JM. Preliminary evidence for reduced social interactions in Chakragati mutants modeling certain symptoms of schizophrenia. Brain Res. 2005;1046(1–2):180–186. doi: 10.1016/j.brainres.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Torres G, Meeder BA, Hallas BH, Spernyak JA, Mazurchuk R, Jones C, et al. Ventricular size mapping in a transgenic model of schizophrenia. Brain Res Dev Brain Res. 2005;154(1):35–44. doi: 10.1016/j.devbrainres.2004.08.011. [DOI] [PubMed] [Google Scholar]
- van den Buuse M, Ruimschotel E, Martin S, Risbrough VB, Halberstadt AL. Enhanced effects of amphetamine but reduced effects of the hallucinogen, 5-MeO-DMT, on locomotor activity in 5-HT(1A) receptor knockout mice: implications for schizophrenia. Neuropharmacology. 2011;61(1–2):209–216. doi: 10.1016/j.neuropharm.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, McGuffin P. Can the social environment cause schizophrenia? Br J Psychiatry. 2003;182:291–292. doi: 10.1192/bjp.182.4.291. [DOI] [PubMed] [Google Scholar]
- Verma V, Tan CH, Ong WY, Grigoryan GA, Jones CA, Stolzberg D, et al. The chakragati mouse shows deficits in prepulse inhibition of acoustic startle and latent inhibition. Neurosci Res. 2008;60(3):281–288. doi: 10.1016/j.neures.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Ikeda M, Watanabe K. Properties of rotational behaviour produced by methylxanthine derivatives in mice with unilateral striatal 6-hydroxydopamine-induced lesions. J Pharmacobiodyn. 1981;4(4):301–307. doi: 10.1248/bpb1978.4.301. [DOI] [PubMed] [Google Scholar]
- Wu YC, Hill RA, Gogos A, van den Buuse M. Sex differences and the role of estrogen in animal models of schizophrenia: interaction with BDNF. Neuroscience. 2013;239:67–83. doi: 10.1016/j.neuroscience.2012.10.024. [DOI] [PubMed] [Google Scholar]
- Young JW, Geyer MA. Using behavioral patterns across species in mood disorder research. In: Gould TD, editor. Mood and anxiety releated phenotypes in mice. Vol. 63. New York: Humana Press; 2011. pp. 21–41. [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. GBR 12909 administration as a mouse model of bipolar disorder mania: mimicking quantitative assessment of manic behavior. Psychopharmacology (Berl) 2010a;208(3):443–454. doi: 10.1007/s00213-009-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. The mania-like exploratory profile in genetic dopamine transporter mouse models is diminished in a familiar environment and reinstated by subthreshold psychostimulant administration. Pharmacology Biochemistry and Behavior. 2010b;96(1):7–15. doi: 10.1016/j.pbb.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Kooistra K, Geyer MA. Dopamine receptor mediation of the exploratory/hyperactivity effects of modafinil. Neuropsychopharmacology. 2011;36(7):1385–1396. doi: 10.1038/npp.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Meves JM, Tarantino IS, Caldwell S, Geyer MA. Delayed procedural learning in alpha7-nicotinic acetylcholine receptor knockout mice. Genes Brain Behav. 2011;10(7):720–733. doi: 10.1111/j.1601-183X.2011.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Geyer MA. Mouse pharmacological models of cognitive disruption relevant to schizophrenia. Neuropharmacology. 2012;62(3):1381–1390. doi: 10.1016/j.neuropharm.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol Ther. 2009;122(2):150–202. doi: 10.1016/j.pharmthera.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Wallace CK, Geyer MA, Risbrough VB. Age-associated improvements in cross-modal prepulse inhibition in mice. Behav Neurosci. 2010;124(1):133–140. doi: 10.1037/a0018462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Zhou X, Geyer MA. Animal models of schizophrenia. In: Swerdlow NR, editor. Behavioral Neurobiology of Schiozphrenia and tis Treatment. Berlin: Springer; 2010. pp. 391–433. [DOI] [PubMed] [Google Scholar]