The TET2 (tet methylcytosine dioxygenase 2) gene encodes a methylcytosine dioxygenase that catalyses the hydrolysis of 5-methylcytosine (5mC) to 5-hydroxylmethylcytosine (5hmC) and promotes DNA demethylation through passive and active mechanisms (Shih, et al 2012). Loss-of-function mutations in TET2 are identified in patients with myeloid and lymphoid malignancies, and are particularly frequent in patients with chronic myelomonocytic leukaemia (CMML) (36–58%) (Shih, et al 2012). Consistent with the patient sequencing analysis, conditional knockout of Tet2 in mice dysregulates haematopoietic stem cell (HSC) function and promotes development of a myeloid malignancy closely resembling human CMML (Cimmino, et al 2011). Despite the high mutation frequency, the prognostic importance of TET2 mutations is unclear in many cases (Shih, et al 2012). We postulated that this could be due to the differential allelic strengths of distinct TET2 mutations (e.g. amorphic versus hypomorphic) and/or the influence of other concurrent genetic alterations.
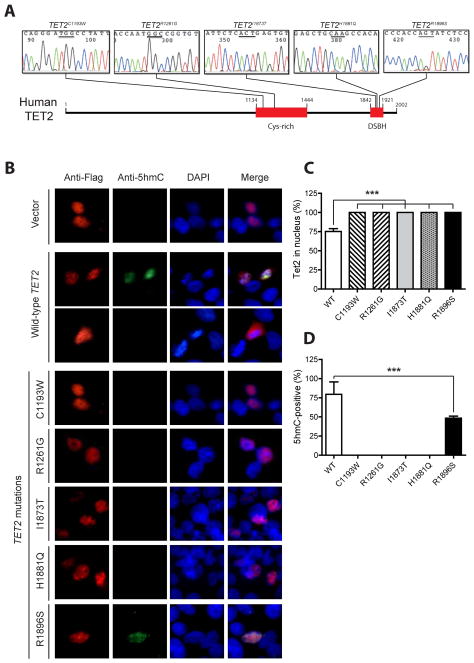
Although nonsense and frameshift mutations are found spread over the entire TET2 sequence, the majority of missense mutations occur in the two conserved regions of TET2 protein (Figure S1): a cysteine-rich region within 1134-1444 amino acids and a catalytic domain (double strand β helix, DSBH) in 1842-1921 amino acids (Fig. 1A). To determine the allelic strengths of distinct TET2 mutations, we characterized five missense mutations prevalent in the COSMIC database in the context of full length human TET2. Two of them (C1193W and R1261G) are located in the cysteine-rich domain and have not been examined before. The other three mutations (I1873T, H1881Q, and R1896S) are located in the DSBH domain and their equivalent mutations were previously evaluated in mouse Tet2 (Ko, et al 2010). Transient expression of full-length wild-type human TET2 in HEK293T cells showed a predominant nuclear localization (~75%) and concomitant detection of 5hmC in the nucleus (Fig. 1B–1D). We observed that in ~25% of TET2-expressing cells, TET2 protein was distributed in both cytoplasm and nucleus and 5hmC staining was diminished (Figure 1B and 1C). These results suggest that intracellular localization of TET2 influences production of 5hmC. In contrast, the mutant proteins containing C1193W, R1261G, I1873T or H1881Q mutations maintained their nuclear localization but 5hmC levels were not detectable, suggesting that these mutations are amorphic. TET2R1896S mutant only showed partial loss of function, suggesting that this mutation is hypomorphic (Fig. 1D). Importantly, our results of human TET2I1873T and TET2R1896S are not consistent with those obtained from equivalent mouse Tet2 mutants, which did not show diminished 5hmC staining (Ko, et al 2010). This could be due to the differences between human TET2 and mouse TET2, emphasizing the importance of validating discoveries from mouse genes in human genes. Nonetheless, our data indicate that leukaemia-associated TET2 mutations lead to complete or partial loss of TET2 function, providing a rational to further stratify leukaemia patients based on their specific TET2 mutations in future prognostic studies.
Fig 1. Missense mutations at the Cys-rich and DSBH regions of human TET2 attenuate its catalytic function.
(A) Schematic illustration of TET2 protein structure and localization of constructed human TET2 point mutations. These mutations were confirmed by sequencing. (B–D) HEK293T cells were transiently transfected with pCMV6-TET2 or various mutant plasmids and simultaneously stained for Flag-tagged TET2 and 5hmC. (B) Representative images of transfected cells. (C, D) Quantification of nuclear TET2 (C) and 5hmC-positive cells (D). The results are presented as percentages of total TET2 expressing cells. The cytoplasm/nucleus distribution of TET2 proteins and 5hmC was quantified in at least 100 cells for each experiment under a fluorescent microscope. All experiments were performed at least three times, and values are presented as means ± standard deviation. *** P<0.001.
Recent work identified a significant synergy between loss-of-TET2 and loss-of other epigenetic regulators (Ezh2 (Muto, et al 2013) and Asxl1 (Abdel-Wahab, et al 2013) ) or NOTCH inactivation (loss of Ncstn) (Lobry, et al 2013) in mice. These results suggest that TET2 mutations might indicate a poor prognosis outcome in CMML patients with concurrent EZH2, ASXL1, or NCSTN mutations. However, the prognostic importance of TET2 mutations in patients with other concurrent mutations, for example, RAS signalling pathway mutations, has not been evaluated.
Given the high mutation rate of TET2 in CMML patients, we set out to find and characterize additional gene mutations concurrent with TET2 mutations. We performed whole exome sequence analysis of 5 CMML patients with a normal karyotype and at different stages of CMML development, including 2 collected from patients transformed to acute myeloid leukaemia (AML) with antecedent of CMML, 1 with Type II CMML, and 2 with Type I CMML (Fig. 2A). All of them contained TET2 mutations, including 4 frameshift and 11 missense mutations. Among the missense mutations, L1721W and I1762V were reported before (Kohlmann, et al 2011, Nibourel, et al 2010), L1340R was found in the COSMIC database (http://cancer.sanger.ac.uk/cancergenome/projects/cosmic/), while N7S, Q129K, and N196K have not been described but are absent from the SNP database (http://snp-nexus.org/index.html). Detection of more than one TET2 mutation in individual patients suggests the presence of multiple leukaemic clones. The mutation frequency of TET2 in our small cohort is much higher than previously reported (Shih, et al 2012). This could be due to the small sample size and the selection of myeloproliferative variant of CMML (indicated by high white blood cell and monocyte counts) and transformed AML in our study.
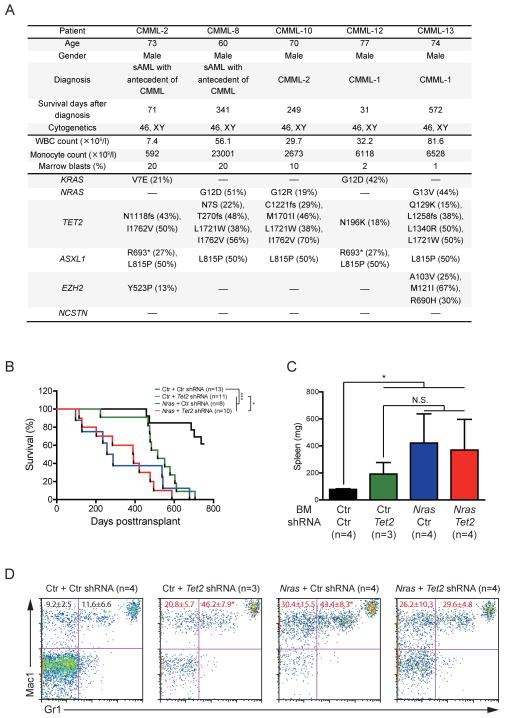
Fig 2. Tet2 knockdown does not promote NrasG12D/+-induced CMML.
(A) Summary of KRAS, NRAS, TET2, ASXL1, EZH2 and NCSTN mutation status in 5 human CMML patients. The percentages of mutant alleles are shown in parentheses. (B–D) Bone marrow cells from control or NrasG12D/+ mice were infected with retrovirus encoding control or Tet2 shRNA and transplanted into lethally irradiated recipient mice. (B) Kaplan-Meier survival curves of different groups of recipient mice were plotted against days post-transplant. P values were determined by the Log-rank test. (C) Splenomegaly in different groups of recipient mice.. (D) Quantification of donor-derived myeloid cells in peripheral blood of moribund mice. The percentages of monocytes (Mac1+ Gr1−) and neutrophils (Mac1+ Gr1+) are indicated in their corresponding quadrants. The results are presented as mean ± standard deviation. Red font indicates significant changes compared with recipients transplanted with control cells infected with control (Ctr) shRNA. Asterisks indicate significant changes compared with recipients transplanted with NrasG12D/+ cells infected with Tet2 shRNA. * P<0.05; *** P<0.001. BM, bone marrow; N.S., not significant; CMML, chronic monomyelocytic leukaemia; WBC, white blood cell
Consistent with the mouse studies, our sequencing results revealed that all patients carried ASXL1 mutations and two patients carried EZH2 mutations. However, NCSTN mutations were not detected in any of the patients (Fig. 2A). In addition, four patients carried canonical oncogenic mutations in NRAS or KRAS, and one patient carried the KRASV7E mutation, which has not been reported in human cancers. However, V7 codon was recently suggested as a key residual in regulating oncogenic Kras activity (Maurer, et al 2012). Our finding of concurrent TET2 mutations with oncogenic RAS mutations in CMML patients is consistent with our data-mining result of the COSMIC database (Table S1) and other reports (Table S2).
To determine whether loss-of-Tet2 co-operates with oncogenic Ras to promote CMML development, we knocked down Tet2 expression in NrasG12D/+ bone marrow cells (Fig. 2B–2D). Compared with recipients transplanted with control cells expressing a scrambled shRNA, recipients transplanted with control cells expressing Tet2 shRNA (Ko, et al 2010) developed CMML-like phenotypes after a prolonged latency, consistent with previous reports of Tet2 knockout mice (Cimmino, et al 2011). To our surprise, knockdown of Tet2 did not accelerate NrasG12D/+ induced CMML (Fig. 2B) or further promote CMML phenotypes (Fig. 2C and 2D). All CMML mice displayed comparably enlarged spleen and significantly higher percentage of monocytes (Mac1+ Gr1−) and neutrophils (Mac1+ Gr1+) in peripheral blood compared to controls.
It is likely that Tet2 knockdown does not result in long-term abrogation of Tet2 expression. Alternatively, oncogenic Ras signalling might alter the subcellular localization of TET2 protein to promote Tet2 loss-of-function during CMML development as shown in BCR-ABL1-driven chronic myeloid leukaemia (Mancini, et al 2012). Thus, further downregulation of Tet2 expression in NrasG12D/+ bone marrow cells does not significantly accelerate CMML progression. It is also possible that the order of mutational acquisition is important. However, current technologies do not allow us to assess this possibility under physiological conditions.
In summary, our results provide a rationale to further stratify leukaemia patients based on their specific TET2 mutations and presence of specific additional genetic mutations in future prognostic studies.
Supplementary Material
Acknowledgments
We are grateful to Coral Wille for sharing reagents and protocols with us and Dr. Anjana Rao for providing retroviral constructs encoding Tet2 shRNA and scrambled shRNA. We would like to thank the University of Wisconsin Carbone Comprehensive Cancer Center (UWCCC) for use of its Shared Services to complete this research. This work was supported by R01 grants CA152108 and HL113066, a Shaw Scientist Award from the Greater Milwaukee Foundation, a Scholar Award from the Leukemia & Lymphoma Society, and an Investigator Initiated Grant from UWCCC to J.Z. This work was also supported in part by NIH/NCI P30 CA014520--UW Comprehensive Cancer Center Support.
Footnotes
Author contributions
Yuan-I Chang and Alisa Damnernsawad for experimental design & execution as well as writing manuscript; Laura K. Allen, Guangyao Kong, Jinyong Wang, and Yangang Liu for experimental execution; Jingfang Zhang, Hsu-Yuan Fu, and Chii-Shen Yang for exome sequencing analysis; David Yang, Erik A. Ranheim, and Ken H. Young for obtaining CMML patient samples; Junjie Guo and Hongjun Song for providing human TET2 construct; Jing Zhang for experimental design and writing manuscript.
Conflicts of interest
We declare no competing financial interests.
Additional Supporting Information may be found in the online version of this article: Supplemental Information (including Supplemental Methods and Supplemental Figure Legends)
References
- Abdel-Wahab O, Gao J, Adli M, Dey A, Trimarchi T, Chung YR, Kuscu C, Hricik T, Ndiaye-Lobry D, Lafave LM, Koche R, Shih AH, Guryanova OA, Kim E, Li S, Pandey S, Shin JY, Telis L, Liu J, Bhatt PK, Monette S, Zhao X, Mason CE, Park CY, Bernstein BE, Aifantis I, Levine RL. Deletion of Asxl1 results in myelodysplasia and severe developmental defects in vivo. Journal of Experimental Medicine. 2013;210:2641–2659. doi: 10.1084/jem.20131141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimmino L, Abdel-Wahab O, Levine RL, Aifantis I. TET family proteins and their role in stem cell differentiation and transformation. Cell Stem Cell. 2011;9:193–204. doi: 10.1016/j.stem.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko M, Huang Y, Jankowska AM, Pape UJ, Tahiliani M, Bandukwala HS, An J, Lamperti ED, Koh KP, Ganetzky R, Liu XS, Aravind L, Agarwal S, Maciejewski JP, Rao A. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature. 2010;468:839–843. doi: 10.1038/nature09586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmann A, Klein HU, Weissmann S, Bresolin S, Chaplin T, Cuppens H, Haschke-Becher E, Garicochea B, Grossmann V, Hanczaruk B, Hebestreit K, Gabriel C, Iacobucci I, Jansen JH, te Kronnie G, van de Locht L, Martinelli G, McGowan K, Schweiger MR, Timmermann B, Vandenberghe P, Young BD, Dugas M, Haferlach T. The Interlaboratory RObustness of Next-generation sequencing (IRON) study: a deep sequencing investigation of TET2, CBL and KRAS mutations by an international consortium involving 10 laboratories. Leukemia. 2011;25:1840–1848. doi: 10.1038/leu.2011.155. [DOI] [PubMed] [Google Scholar]
- Lobry C, Ntziachristos P, Ndiaye-Lobry D, Oh P, Cimmino L, Zhu N, Araldi E, Hu W, Freund J, Abdel-Wahab O, Ibrahim S, Skokos D, Armstrong SA, Levine RL, Park CY, Aifantis I. Notch pathway activation targets AML-initiating cell homeostasis and differentiation. Journal of Experimental Medicine. 2013;210:301–319. doi: 10.1084/jem.20121484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini M, Veljkovic N, Leo E, Aluigi M, Borsi E, Galloni C, Iacobucci I, Barbieri E, Santucci MA. Cytoplasmatic compartmentalization by Bcr-Abl promotes TET2 loss-of-function in chronic myeloid leukemia. Journal of Cellular Biochemistry. 2012;113:2765–2774. doi: 10.1002/jcb.24154. [DOI] [PubMed] [Google Scholar]
- Maurer T, Garrenton LS, Oh A, Pitts K, Anderson DJ, Skelton NJ, Fauber BP, Pan B, Malek S, Stokoe D, Ludlam MJ, Bowman KK, Wu J, Giannetti AM, Starovasnik MA, Mellman I, Jackson PK, Rudolph J, Wang W, Fang G. Small-molecule ligands bind to a distinct pocket in Ras and inhibit SOS-mediated nucleotide exchange activity. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5299–5304. doi: 10.1073/pnas.1116510109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto T, Sashida G, Oshima M, Wendt GR, Mochizuki-Kashio M, Nagata Y, Sanada M, Miyagi S, Saraya A, Kamio A, Nagae G, Nakaseko C, Yokote K, Shimoda K, Koseki H, Suzuki Y, Sugano S, Aburatani H, Ogawa S, Iwama A. Concurrent loss of Ezh2 and Tet2 cooperates in the pathogenesis of myelodysplastic disorders. Journal of Experimental Medicine. 2013;210:2627–2639. doi: 10.1084/jem.20131144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibourel O, Kosmider O, Cheok M, Boissel N, Renneville A, Philippe N, Dombret H, Dreyfus F, Quesnel B, Geffroy S, Quentin S, Roche-Lestienne C, Cayuela JM, Roumier C, Fenaux P, Vainchenker W, Bernard OA, Soulier J, Fontenay M, Preudhomme C. Incidence and prognostic value of TET2 alterations in de novo acute myeloid leukemia achieving complete remission. Blood. 2010;116:1132–1135. doi: 10.1182/blood-2009-07-234484. [DOI] [PubMed] [Google Scholar]
- Shih AH, Abdel-Wahab O, Patel JP, Levine RL. The role of mutations in epigenetic regulators in myeloid malignancies. Nature Reviews Cancer. 2012;12:599–612. doi: 10.1038/nrc3343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.