Abstract
We aimed to determine whether frailty, a validated geriatric construct of increased vulnerability to physiologic stressors, predicts mortality in liver transplant (LT) candidates. Consecutive adult outpatients listed for LT with laboratory MELD≥12 at a single center (97% recruitment rate) underwent 4 frailty assessments: Fried Frailty, Short Physical Performance Battery (SPPB), Activities of Daily Living (ADL), and Instrumental ADL (IADL) scales. Competing risks models associated frailty with wait-list mortality (death/delisting for being too sick for LT). 294 listed LT patients with MELD≥12, median age 60y, and MELD 15 were followed for 12 months. By Fried Frailty score≥3, 17% were frail; 11/51 (22%) of the frail versus 25/243 (10%) of the not frail died/were delisted (p=0.03). Each 1-unit increase in the Fried Frailty score was associated with a 45% (95%CI, 4-202%) increased risk of wait-list mortality adjusted for MELD. Similarly, the adjusted risk of wait-list mortality associated with each 1-unit decrease (i.e., increasing frailty) in the SPPB (HR 1.19, 95%CI 1.07-1.32). Frailty is prevalent in LT candidates. It strongly predicts wait-list mortality, even after adjustment for liver disease severity demonstrating the applicability and importance of the frailty construct in this population.
Keywords: surgery, functional status, sarcopenia, age, disability
INTRODUCTION
Every year, one in five patients on the liver transplant wait-list in the United States will die or become too sick for transplantation (1). Although all candidates are prioritized for liver transplant by their risk of wait-list mortality as predicted by the laboratory-based Model for End-Stage Liver Disease (MELD) score (2), 84% of those who died or were delisted for being too sick for transplant received at least one liver offer – with a median of five liver offers (all of which were refused by the transplant clinician or patient) – prior to their death or delisting (3). This suggests that there are important unmeasured donor and/or recipient factors other than the MELD score that are being incorporated into the decision to proceed with transplantation.
One of the recipient factors of importance is the transplant clinician's assessment of the patient's global health status, which may be conceptualized as the patient's vulnerability to stresses while on the wait-list (i.e., wait-list mortality) combined with his or her vulnerability to poor outcomes after transplant surgery (i.e, perioperative risk). In patients with cirrhosis, this “state of health” is influenced not only by liver disease severity which is well-captured by the MELD score, but by factors such as age, muscle mass, nutritional status, and non-liver related co-morbidities that also contribute to wait-list outcomes but are not captured by the MELD score. There is robust rationale for the application of this clinical judgment, as several studies have shown that clinicians can predict outcomes in hospitalized or elderly patients (4-7). However, while inclusion of clinical assessment to liver transplant decisions may be justified, it is subjective and, as such, may differ from one clinician to another and lacks transparency. Moreover, components of this assessment are likely neither associated with nor validated to predict transplant-related outcomes. Less charitably, this assessment may incorporate inappropriate factors, including age, sex, race, and/or socio-economic status.
In the field of geriatrics, this concept of a patient's vulnerability to stress and decreased physiologic reserve has been widely recognized as a crucial determinant of health outcomes, and is generally operationalized as frailty. While there are several measures of frailty that are used in geriatrics, all definitions seek to identify patients who are at risk for adverse outcomes in the setting of stressors such as acute medical illness, hip fracture, or surgery. One of the most widely used definitions of frailty, the Fried Frailty phenotype (8), conceptualizes frailty as a distinct clinical syndrome characterized by five domains suggesting decreased physiologic reserve. Other definitions of frailty are more expansive, and often view frailty in the context of functional status deficits and/or disability. Importantly, these multiple definitions of frailty have been operationalized as objective, validated measures (8-11) that have important prognostic value and can predict morbidity and mortality in both geriatric and surgical populations. Moreover, these measures can substantially enhance the predictive ability of traditionally used risk indices such as the American Society of Anesthesiologists score or Lee's revised cardiac index (12,13).
We hypothesized that this concept of frailty could be applied to patients with cirrhosis awaiting liver transplantation, a population at increased risk for accelerated functional decline, to similarly predict clinically relevant outcomes independent of liver disease severity. Therefore, we designed the Frailty Assessment in Liver Transplant Candidates Study (FrAI-LT Study) to measure frailty in liver transplant candidates using measures of physiologic reserve and functional status. In this particular study, we aimed to assess the prevalence and degree of frailty in liver transplant candidates and evaluate the association between these measures and wait-list mortality.
METHODS
Study population
The FrAI-LT Study is an ongoing prospective cohort study of all adult (≥18 years) patients with cirrhosis who are actively listed for liver transplantation at the University of California, San Francisco (UCSF). Patients were enrolled during an outpatient visit to the UCSF Liver Transplant Clinic, independent of their wait-list date; therefore, patients who were never seen as an outpatient at UCSF were not included in this study. In order to ensure an adequate number of events during the follow up period, only candidates with a MELD score ≥12 were included. Patients were excluded if they had severe hepatic encephalopathy, as defined by the time to complete the Numbers Connection Test (14) of >120 seconds, as this may impair the patient's ability to provide signed informed consent and comply with study procedures. Patients who had undergone a prior transplant were also excluded. In total, 304 patients were asked to participate in this study; while the number of patients who are actively awaiting liver transplant at UCSF is dynamic, there are currently approximately 300 actively wait-listed patients (15). Of the 304 patients who were asked to participate, 294 (97%) consented and enrolled in the study.
Study procedures and data collection
At enrollment, all patients underwent frailty testing using four measures listed in Table 1: Fried Frailty Instrument, Short Physical Performance Battery, Activities of Daily Living (ADL) Scale, and Instrumental Activities of Daily Living Scale (IADL) at a single time point in the outpatient clinic. The actual instruments and grading systems used are shown in the Appendix. Given the overlap of testing of gait speed between the Fried Frailty Instrument and the Short Physical Performance Battery, only one walk test (4 meters) was administered, but scored based on the number of meters per second for each instrument.
Table 1.
The four measures of frailty that were administered in this study.
| Measure | Type | Components | Range: Not frail→frail | Comments about use |
|---|---|---|---|---|
| Fried Frailty Instrument(8) | Performance-based/Self-report | 1) Gait speed 2) Exhaustion 3)Physical activity 4)Unintentional weight loss 5)Weakness |
0 – 5 | Identifies vulnerable elders at risk for death, long-term institutionalization, and post-surgical complications |
| Short Physical Performance Battery(9) | Performance-based | 1)Repeated chair stands 2)Balance testing 3)13 foot walk |
12 – 0 | Identifies vulnerable elders at risk of becoming disabled |
| Activities of Daily Living(10) | Self-report | Daily self-care activities | 6 – 0 | Assesses difficulty with performing the activity |
| Instrumental Activities of Daily Living(11) | Self-report | Activities that allow an individual to live independently | 8 – 0 | Assesses difficulty with performing the activity |
At the time of the frailty assessment, information regarding demographics, medical co-morbidities (e.g., hypertension, diabetes, coronary artery disease), degree of ascites, and laboratory tests were collected from the patient's electronic health record. Hepatic encephalopathy was classified as none/mild versus moderate based on the patient's performance on the Numbers Connection Test Score of < or >60 seconds, respectively.
Statistical analysis
The primary predictor was frailty, as assessed by the Fried Frailty instrument. This was selected as the primary predictor given its construct validity in general surgical and kidney transplant patients (12,16), whom we felt were most similar to the liver transplant population. Subjects were classified as “frail” if they scored ≥3 on the Fried Frailty instrument, using the same cut-offs that were used in the original study by Fried et al of 6,000 community-dwelling elderly men and women enrolled in the Cardiovascular Health Study (8). Differences in baseline characteristics by frail status were compared using chi-square or Wilcoxon ranksum tests for categorical and continuous variables, respectively. Secondary predictors included frailty as assessed by the Short Physical Performance Battery, ADL Scale, and IADL Scale.
The primary outcome in this study was wait-list mortality, defined as death prior to liver transplantation or delisting for being too sick for transplant. Patients who were delisted for reasons other than being too sick (e.g., substance abuse, non-adherence) were censored from the FrAI-LT Study at the time of wait-list removal.
In order to identify a subgroup of patients who might benefit most from frailty assessment in clinical practice, we evaluated rates of wait-list mortality by frail status (defined as a Fried Frailty score ≥3) and MELD categories, using the 75%ile MELD score for the cohort as the MELD cut-off. Competing risks analysis evaluated the association between frailty measures and wait-list mortality with liver transplant as the competing risk. To reduce the risk of overfitting models, only bi-variate and tri-variate analyses were performed using variables that may conceptually confound the association between frailty and wait-list mortality, following the general rule-of-thumb of allowing one predictor for every ten events. A cut-off p-value <0.05 was used to determine statistical significance.
The UCSF Institutional Review Board approved this study. STATA® v12 (College Station, Texas) was used for all statistical analyses.
RESULTS
Baseline characteristics of the cohort
A total of 294 liver transplant wait-list candidates with MELD ≥12 were included in the analyses. Baseline characteristics of the cohort are shown in Table 2, column A. Median (IQR) follow up time was 12 (5-15) months for the entire cohort. Notable characteristics include median (IQR) age of 60 years (53-64). Forty-nine percent had chronic HCV and only 22% had HCC, a percentage that was lower than expected due to the inclusion of patients with a laboratory MELD score ≥12. Median (IQR) MELD score at testing was 15 (13-18), sodium was 137 mEq/L (134-139), and albumin was 2.9 g/dL (2.6-3.3). The majority (62%) had no ascites and 19% had moderate hepatic encephalopathy defined as a Numbers Connection Test score between 60-120 seconds. The proportion of patients with Child-Pugh Class A, B, and C was 9%, 61%, and 30%, respectively.
Table 2.
Baseline characteristics of 267 liver transplant candidates with MELD ≥12 for the entire cohort and by frail status (defined as Fried Frailty Score ≥3).
| Characteristic* | A All n=294 | B Not frail n=243 (83%) | C Frail n=51 (17%) | D p-value | |
|---|---|---|---|---|---|
| Follow up time, mos | 12 (5-15) | 12 (6-15) | 10 (5-15) | 0.71 | |
| Age, yrs | 60 (53-64) | 59 (52-64) | 60 (56-63) | 0.50 | |
| % female | 34% | 32% | 43% | 0.13 | |
| Race/ethnicity | White | 57% | 58% | 53% | 0.18 |
| Black | 4% | 4% | 8% | ||
| Hispanic | 27% | 28% | 20% | ||
| Asian | 5% | 5% | 8% | ||
| Other | 7% | 5% | 12% | ||
| Etiology of liver disease | HCV | 49% | 51% | 40% | 0.61 |
| Alcohol | 17% | 17% | 18% | ||
| NASH | 15% | 15% | 18% | ||
| Cholestatic | 11% | 10% | 14% | ||
| Other | 9% | 8% | 12% | ||
| HCC | 22% | 23% | 18% | 0.40 | |
| Weight, kg | 85 (73-98) | 85 (74-98) | 83 (70-99) | 0.61 | |
| BMI | 29 (25-34) | 29 (25-33) | 30 (24-36) | 0.54 | |
| Medical co-morbidities | |||||
| Hypertension | 42% | 42% | 39% | 0.68 | |
| Diabetes | 29% | 28% | 31% | 0.67 | |
| Coronary artery Disease | 6% | 6% | 6% | 0.97 | |
| Laboratory tests | |||||
| Laboratory MELD | 15 (13-18) | 15 (13-18) | 18 (15-21) | <0.01 | |
| Total bilirubin, mg/dL | 2.5 (1.8-3.6) | 2.5 (1.9-3.6) | 3.0 (2.1-4.9) | 0.04 | |
| INR | 1.4 (1.3-1.6) | 1.4 (1.3-1.6) | 1.5 (1.2-1.8) | 0.24 | |
| Creatinine, mg/dL | 1.0 (0.8-1.3) | 0.9 (0.7-1.2) | 1.2 (0.9-1.8) | <0.01 | |
| Sodium, mEq/L | 137 (134-139) | 137 (135-139) | 135 (132-137) | <0.01 | |
| Albumin, g/dL | 2.9 (2.6-3.3) | 2.9 (2.6-3.4) | 2.8 (2.4-3.2) | 0.02 | |
| Ascites | Absent | 62% | 66% | 43% | 0.01 |
| Mild-moderate | 34% | 30% | 51% | ||
| Severe | 4% | 4% | 6% | ||
| % moderate hepatic encephalopathy† | 19% | 17% | 26% | 0.17 | |
| Child Pugh Score | A | 9% | 10% | 4% | 0.01 |
| B | 61% | 64% | 50% | ||
| C | 30% | 26% | 47% | ||
Median (interquartile range) or %
Defined as Numbers Connection Test score between 60 and 120 seconds. Patients with a Numbers Connection Test score >120 seconds were excluded from the study.
Baseline measurements of frailty
The median (IQR) Fried Frailty score was 1 (1-2). A total of 51 (17%) subjects were classified as “frail” as defined as a Fried Frailty score ≥3. As for the individual components of the Fried Frailty score, median (IQR) grip strength was 32 kg (23-39) and gait speed 1.4 meters/second (1.1-1.7), 53% reported exhaustion, 16% reported low physical activity, and 43% reported unintentional weight loss within the last 12 months.
On the Short Physical Performance Battery, the median (IQR) score was 11 (9-12), with 73% able to complete all three balance tests for 10 seconds each and 43% able to complete five chair stands within 11.1 seconds. With respect to the disability scales, 76% and 57% reported no difficulty with completing all six ADLs by the ADL Scale and all 8 IADLs by the IADL Scale.
Characteristics associated with frailty
The primary predictor in this study was frailty as assessed by the Fried Frailty instrument. Baseline characteristics among the not frail versus frail subjects are shown in Table 2, columns B and C, respectively. The two groups were similar with respect to age, gender, race/ethnicity, etiology of liver disease, body size, and proportion with HCC (p>0.05). Frail patients had higher MELD scores, sodium, rates of mild/moderate and severe ascites, and rates of moderate hepatic encephalopathy (p<0.05).
Frailty and outcome
By the end of follow-up, 36/294 (12%) died/were delisted for being too sick, 71 (24%) underwent liver transplant, and 14 (5%) were removed from the wait-list for other reasons; 173/294 (59%) were actively waiting on the list at the end of the study period. The proportion of those who died/were delisted differed significantly by frail status: 11/51 (22%) frail versus 25/243 (10%) not frail (p=0.03).
Among the entire cohort, 51/294 (17%) were classified as frail (Fried Frailty Score ≥3), 91/294 (31%) scored <9 on the Short Physical Performance Battery, 24% had difficulty with ≥1 ADL and 43% had difficulty with ≥1 IADL. Table 3 shows the scores on the four frailty measures by outcome (e.g., died/delisted, transplanted, waiting, other reason for removal). Patients who died/were delisted compared with those who were transplanted, still waiting, or removed for other reasons displayed higher rates of frailty as demonstrated by a higher Fried Frailty score (p<0.01), and higher degrees of physical inactivity (p=0.01). They also demonstrated higher rates of functional impairment by the Short Physical Performance Battery (p<0.01) [Table 3].
Table 3.
Functional status measures of the entire cohort, categorized by outcome.
| Functional status measure* | A Died/delisted n=36 (12%) | B Transplanted n=71 (24%) | C Waiting n=173 (59%) | D Other† n=14 (5%) | E p-value‡ |
|---|---|---|---|---|---|
| Fried Frailty Instrument (Instrument Range 0-5; 0=Not frail, 5=Frail) | |||||
| Summary score | 2 (1-3) | 2 (1-3) | 1 (1-2) | 1 (0-2) | <0.01 |
| Individual components | |||||
| Grip strength, kg | 25 (17-36) | 32 (22-39) | 33 (24-40) | 32 (23-38) | 0.10 |
| Exhaustion | 56% | 61% | 52% | 29% | 0.13 |
| Weight loss | 47% | 61% | 36% | 29% | <0.01 |
| Gait speed, m/sec | 1.2 (0.9-1.6) | 1.4 (1.2-1.8) | 1.4 (1.1-1.7) | 1.6 (1.0-2.0) | 0.19 |
| Physical inactivity | 28% | 21% | 10% | 29% | 0.01 |
| Short Physical Performance Battery (Instrument Range 0-12; 0=Impaired, 12=No Impairment) | |||||
| Summary Score | 9 (8-11) | 11 (9-12) | 11 (10-12) | 11 (8-12) | <0.01 |
| Individual Components§ | |||||
| Balance, seconds | 30 (25-30) | 30 (27-30) | 30 (30-30) | 30 (30-30) | 0.14 |
| Chair stands, seconds | 13 (10-17) | 13 (10-17) | 11 (10-14) | 12 (11-14) | 0.06 |
| Activities of Daily Living Scale¶ | 6 (5-6) | 6 (5-6) | 6 (6-6) | 6 (6-6) | 0.63 |
| Instrumental Activities of Daily Living Scale¶ | 7 (5-8) | 8 (5-8) | 8 (6-8) | 8 (7-8) | 0.08 |
Median (IQR) or %
Removed from the wait-list for reasons other than death, transplant, or being too sick for transplant (i.e., substance abuse, medical non-adherence)
By the Kruskal-Wallis test
The third component is gait speed in meters/second, which is listed above under the Fried Frailty Instrument.
Number of items in which the patient was independent.
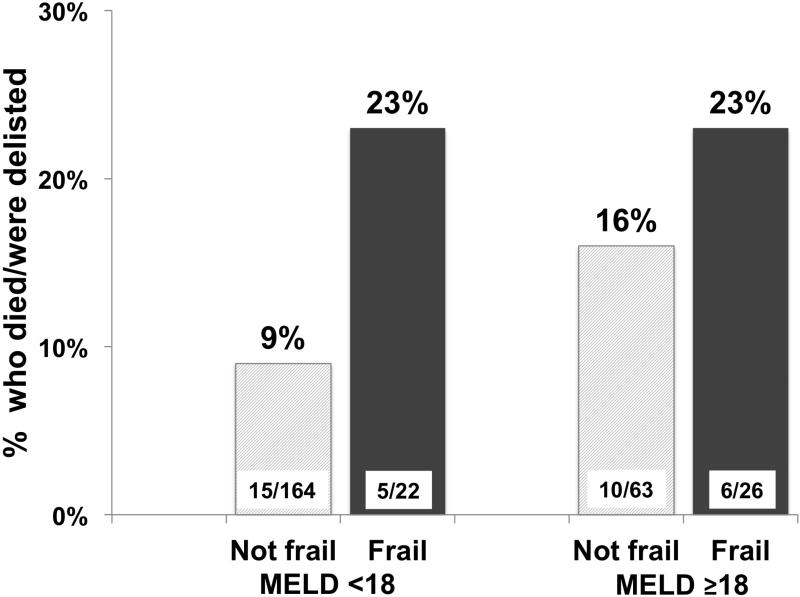
Given the conceptual association between frailty and liver disease severity, we evaluated the proportion of patients that achieved a wait-list event by MELD score using MELD 18, the 75%ile cut-point for the cohort, and frailty status. Among those with MELD<18, 5/22 (23%) who were classified as frail died/were delisted compared to 15/164 (9%) of the non-frail, and among those with MELD ≥18, 5/22 (23%) frail patients and 10/63 (16%) non-frail patients died/were delisted (p=0.07 for the comparison of all four groups) [Figure 1].
Figure 1.
Proportion of candidates who died or were delisted, by frail status (Fried Frailty Score ≥ 3) and MELD score category (<18 or ≥18).
With respect to the primary predictor (Fried Frailty score), univariable competing risks analysis revealed that a one unit increase in Fried Frailty score was associated with a 50% increased risk of wait-list mortality (p=0.01; Table 4). The hazard ratio (HR) associated with the Fried Frailty score did not change substantially after adjustment for MELD score, MELD and age, MELD and sodium, or MELD and hepatic encephalopathy as characterized by the Numbers Connection Test score in seconds (Table 4). Competing risks models evaluating the association between the Short Physical Performance Battery, a secondary predictor, and wait-list mortality produced similar results (HR 1.20, p<0.01) [Table 4]. Although every unit increase in the IADL scale was significantly associated with wait-list mortality in unadjusted analysis (HR 1.17, p=0.03), adjustments for confounders in bi- and tri-variate models attenuated these differences (Table 4).
Table 4.
Unadjusted and adjusted risk of wait-list mortality associated with each frailty measure.
| Hazard Ratio (95% CI) for each Frailty Measure p-value | ||||
|---|---|---|---|---|
| Adjustment | Fried Frailty* (per point increase) | Short Physical Performance Battery† (per point decrease) | ADL scale‡ (per point decrease) | IADL scale§ (per point decrease) |
| No adjustment | 1.50 (1.09-2.05) 0.01 |
1.20 (1.08-1.33) <0.01 |
1.21 (0.90-1.62) 0.20 |
1.17 (1.02-1.36) 0.03 |
| MELD | 1.45 (1.04-2.02) 0.03 |
1.19 (1.07-1.32) <0.01 |
1.18 (0.87-1.58) 0.28 |
1.14 (0.97-1.35) 0.11 |
| MELD and age | 1.43 (1.04-1.96) 0.03 |
1.16 (1.04-1.30) <0.01 |
1.17 (0.87-1.59) 0.31 |
1.16 (0.99-1.37) 0.07 |
| MELD and albumin | 1.36 (0.95-1.96) 0.10 |
1.18 (1.06-1.31) <0.01 |
1.09 (0.80-1.50) 0.60 |
1.12 (0.96-1.32) 0.15 |
| MELD and sodium | 1.42 (1.01-2.00) 0.046 |
1.19 (1.07-1.33) <0.01 |
1.14 (0.85-1.53) 0.38 |
1.13 (0.96-1.434) 0.14 |
| MELD and hepatic encephalopathy | 1.39 (1.00-1.97) 0.06 |
1.18 (1.06-1.30) <0.01 |
1.23 (0.91-1.66) 0.17 |
1.11 (0.93-1.33) 0.25 |
Performance-based and self-report instrument that includes five domains (gait speed, exhaustion, physical activity, unintentional weight loss, and weakness). Range 0 (not frail) to 5 (frail).
Performance-based instrument that consists of three tests: repeated chair stands, balance testing, and gait speed. Range 0 (frail) to 12 (robust).
Self-report instrument that asks about of daily self-care activities (e.g., bathing, feeding). Range 0 (dependent) to 6 (independent).
Self-report instrument that asks about activities necessary for an individual to live independently (e.g., preparing meals, doing laundry). Range 0 (dependent) to 12 (independent).
DISCUSSION
The implementation of the MELD-based liver allocation system in the United States in 2002 has been heralded for its objectivity, fairness, and success in reducing wait-list mortality (17). Although the MELD score can accurately predict 90-day wait-list mortality for the vast majority of cirrhotics (18), there are clearly certain wait-list candidates whose mortality risk is underestimated by MELD. We hypothesize that progressive under-nutrition, sarcopenia, and functional decline – three components that are arguably the sine qua non of advanced cirrhosis – impact mortality in ways that are not fully captured by total bilirubin, INR, and creatinine.
In this study, we introduce tools to identify a subgroup of candidates that might be particularly vulnerable to adverse outcomes on the liver transplant wait-list. Borrowing the concept of frailty from geriatrics, we found that four common measures of physical frailty can be utilized to predict waitlist mortality in cirrhotics with a minimum MELD score of 12. While patients displaying the frail phenotype, as defined by a Fried Frailty Score ≥3, had statistically significantly higher MELD scores and rates of ascites and hepatic encephalopathy, our analyses demonstrated a robust, independent association between frailty and mortality even after adjustment for liver disease severity. The construct validity of frailty in liver transplant candidates was further strengthened by the qualitatively similar associations we observed between the Short Physical Performance Battery, a well-established metric of physical function, and wait-list mortality. Importantly, the measures of physical frailty that we tested in this study offer distinct advantages over other measures that must be assessed in an exercise physiology laboratory (e.g., cardiopulmonary exercise testing, isokinetic muscle testing) or by an imaging study (e.g., psoas muscle area) in that they can be performed quickly, repeatedly, and in a clinic setting without costly equipment or specialized technicians.
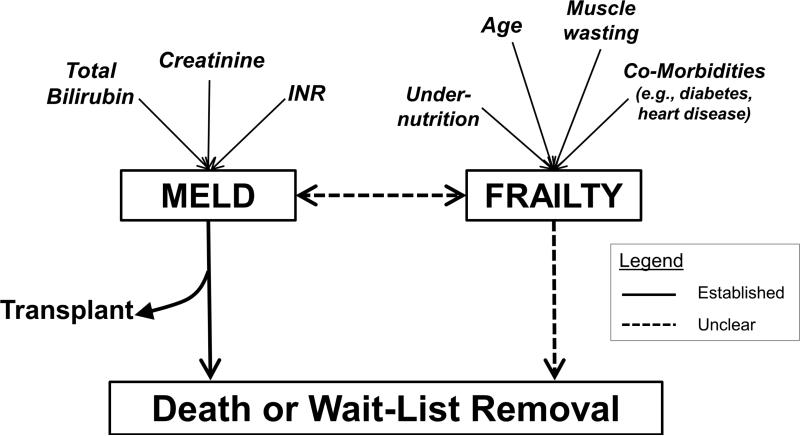
What exactly do these frailty measures capture? As described in the geriatrics literature, frailty is a distinct clinical state of increased vulnerability to health stressors and decreased physiologic reserve that leads to adverse health outcomes, including disability, short- and long-term institutionalization, and ultimately mortality (8,19). Although the underlying pathophysiology remains elusive, it is believed to result from the aggregate decline of numerous systems including, but not limited to, neuromuscular, inflammatory, skeletal, and endocrine systems (20). For patients with cirrhosis, the liver failure itself inevitably drives this process; there is obvious overlap between the manifestations of synthetic liver dysfunction and those of frailty. But, as anyone who has cared for these patients can attest to, there are individuals whose co-morbidities, under-nutrition, sarcopenia, and functional decline appear to exceed the severity of their liver disease. We have conceptualized that the combination of these factors – i.e., frailty – not only worsens with liver disease severity but contributes to wait-list mortality independent of disease severity (Figure 2).
Figure 2.
Conceptual model of the relationship between MELD, frailty, and waitlist outcomes.
To an extent, certain components of the Fried Frailty Instrument are subjective in that they are patient-reported (exhaustion, physical activity, unintentional weight loss). Rather than confound the association between frailty and wait-list mortality, however, we believe that this “subjectivity” directly contributes to the association. For example, two patients with refractory ascites can perceive the burden of their ascites in different ways: one patient will spend most of his day in bed and report feeling “exhausted” (by the Fried Frailty Instrument), the other patient will pace the hospital hallways and not meet the same Fried Frailty criteria for “exhausted”. We hypothesize that the patient who perceives himself to be exhausted will restrict his physical activity leading to worsening sarcopenia, risk of infection, and ultimately, increased vulnerability to wait-list mortality that exceeds his liver disease severity. Similarly, complications of cirrhosis such as massive ascites and poorly controlled encephalopathy will lead patients to reduce their physical activity and increase vulnerability to poor outcomes.
There is strong rationale for the application of frailty measures to a cirrhotic population who are typically younger than the geriatric population. First, cirrhosis is a state of accelerated physiologic aging. This is evidenced by the fact that 17% of liver transplant candidates in our study was categorized as frail by the Fried Frailty instrument, a proportion that is commensurate to community-dwelling elderly individuals 80 years and older (8). Second, the concept of frailty has already been applied to other similar conditions that can affect individuals in non-geriatric populations, such as end-stage renal disease (including renal transplant) and human immunodeficiency virus infection, and shown to predict clinically important outcomes such as long-term institutionalization, disability, and mortality (21-25). Lastly, two key pathophysiologic components of frailty – sarcopenia and decreased cardiopulmonary reserve – have been measured in liver transplant candidates and shown to be associated with transplant-related outcomes (26,27).
While we enrolled a large cohort that represents nearly the entire wait-list at UCSF with MELD ≥12, we acknowledge that there were relatively few deaths and delistings during our study period. This led to wide confidence intervals and uncertainty about the exact point estimate of the association and limited the ability to adjust for multiple confounders in our analyses. However, in our bi- and tri-variate models, the hazard ratios associated with each of the frailty measures remained remarkably similar to the univariate results, supporting our hypothesis that these frailty tools capture something other than the effects of the liver disease itself. In addition, all four measures of frailty revealed similar qualitative associations between increasing frailty and increased risk of wait-list mortality. Another potential limitation is that we did not include patients with MELD scores <12, a group for whom the frail phenotype may more accurately predict mortality compared with MELD score alone. This was intentional – to develop a cohort of patients who were theoretically just as likely to receive a transplant as experience death within the study follow-up period as well as achieve a sufficient number of wait-list events for our analyses.
Despite these limitations, our analyses have important conceptual and practical clinical implications. This is the first study to apply clinic-based measures of frailty to a population of liver transplant candidates. Frailty has already been applied and found to be highly prevalent in other solid organ transplant candidates including kidney (16,22) and lung (28,29) and predictive of transplant-related outcomes, supporting the utility of this geriatric concept in transplant hepatology. Although prior studies have shown that specific components of frailty, such as muscle mass (measured by quantitative morphomics (26)) and cardiopulmonary reserve (measured by cardiopulmonary exercise testing (27)), are predictive of transplant-related outcomes, all four of these frailty measures used in this study can be administered in a clinic setting within fewer than ten minutes. Perhaps of greater clinical utility, patients with a MELD score (<18) and frail phenotype (by the Fried Frailty instrument) experienced rates of wait-list mortality that were higher than those with MELD <18 and not frail or those with MELD ≥18. It is this subgroup (low MELD, high frailty) of patients who may derive the greatest benefit from implementation of these measures. Objective measurement of frailty can facilitate difficult conversations between transplant clinicians and the most debilitated candidates about their increased risk of wait-list mortality. Understanding this can motivate these patients to seek live donor transplantation or accept livers from a broadened spectrum of deceased donors. Lastly, we believe that the primary factor underlying the frail phenotype in cirrhotics – sarcopenia – may be reversible with physical therapy and nutritional support, and objective measures such as the Fried Frailty Instrument and Short Physical Performance Battery can be used to assess response to these interventions.
In summary, we have demonstrated in this study that the concept of frailty can be applied to liver transplant candidates and predict wait-list mortality even after adjustment for liver disease severity. Our next steps are to: 1) confirm these findings in a larger cohort with longer follow-up to better understand the complex relationship between frailty and liver disease severity and 2) to determine whether these same measures before transplant can predict outcomes after transplant. Our data provide the justification to initiate a multi-center collaboration to evaluate the feasibility of applying these measures on a larger scale and evaluate their association with transplant outcomes. Ultimately, objective measurement of frailty in liver transplant candidates and a better understanding of the impact of frailty on their outcomes before and after transplant are critical to ensuring the timely, equitable, and effective application of liver transplantation.
Acknowledgments
Financial support: This study was funded by an American College of Gastroenterology Junior Faculty Development Award, P30AG044281 (UCSF Older Americans Independence Center), and P30 DK026743 (UCSF Liver Center).
List of abbreviations
- ADL
activities of daily living
- HR
hazard ratio
- IADL
instrumental activities of daily living
- IQR
interquartile range
- MELD
model for end-stage liver disease
Appendix
Appendix.
The four instruments to measure frailty that were used in this study
| A) Fried Frailty Instrument(8) | |||
|---|---|---|---|
| Component | Definition/Criterion (1 point each) | ||
| Slowness | Time in seconds needed to complete a 4-meter walk test. “Short” for males is defined as ≤173 cm for males and ≤159 cm for females. | ||
| Height | Criterion | ||
| Short | ≥7 seconds | ||
| Not short | ≥6 seconds | ||
| Exhaustion | “How many days in the last week did you feel this way?” 1.I felt that everything I did was an effort 2.I could not get going Criterion: ≥3 days for either question |
||
| Physical Activity | Based on the Minnesota Leisure Time Activity questionnaire (30), which asks about activities such as walking, gardening, aerobics over the last 4 weeks. Inpatients will be asked about their activity in the 4 weeks prior to their hospitalization. | ||
| Sex | Criterion | ||
| Male | <383 kilocalories per week | ||
| Female | <270 kilocalories per week | ||
| Unintentional weight loss | Self-reported weight loss >10 pounds or measured weight loss of >5% of total body weight in the previous year | ||
| Weakness | Grip strength quantified by a hand-held dynamometer. The mean of the values of three serial tests of maximum grip strength with the dominant hand will be calculated. Categories for body mass index (BMI) vary by sex. | ||
| Criterion | |||
| BMI* | Male | Female | |
| Low | ≤29 kg | ≤17 kg | |
| Average | ≤30 kg | ≤17.3 kg | |
| High | ≤32 kg | ≤18 kg | |
| Very high | ≤32 kg | ≤21 kg | |
| B) Short Physical Performance Battery(9) | ||
|---|---|---|
| Component | Instructions | Grading |
| Timed repeated chair stands | Ask the subject to fold his arms over his chest while sitting in a chair, then stand up and sit down five times. Time begins when the subject begins to stand up and ends when he has sat down completely for the 5th time. |
4 = < 11.1 sec 3 = 13.6-11.2 sec 2 = 16.6-13.7 sec 1 = > 16.7 sec 0 = unable |
| Balance testing | Ask subject to stand in 3 positions for up to 10 seconds each: 1)Semitandem (side of the heel of one foot touching the big toe of the other foot) 2)Side-by-side (feet together, side-by-side) 3)Tandem (heel of one foot in front and touching the toes of the other foot) |
4 = tandem 10 sec 3 = semitandem 10 sec, tandem 3-9.9 sec 2 = semitandem 10 sec, tandem 0-2.9 sec 1 = side by side 10 sec, semitandem <10 sec 0 = side by side 0-9.9 sec or unable |
| 8 foot walk (2.44 meters) | Subject walks at his usual pace from the start to the end of a walking course (flat 8 foot walking surface) | 4 = <3.1 sec (>0.78 m/sec) 3 = 3.2-4.0 (0.61-0.77 m/sec) 2 = 4.1-6.5 sec (0.44-0.60 m/sec) 1 > 5.7 sec (<0.43 m/sec) 0 = could not do |
| C) Activities of Daily Living Scale(10) | ||
|---|---|---|
| KATZ BASIC ACTIVITIES OF DAILY LIVING (ADL) SCALE | ||
| Independent | ||
| YES | NO | |
| 1. Bathing (sponge bath, tub bath, or shower) Receives either no assistance or assistance in bathing only one part of body. |
||
| 2. Dressing – Gets clothes and dresses without any assistance except for tying shoes. | ||
| 3. Toileting – Goes to toilet room, uses toilet, arranges clothes, and returns without any assistance (may use cane or walker for support and may use bedpan/urinal at night). | ||
| 4. Transferring – Moves in and out of bed and chair without assistance (may use cane or walker). | ||
| 5. Continence – Controls bowel and bladder completely by self (without occasional “accidents”). | ||
| 6. Feeding – Feeds self without assistance (except for help with cutting meat or buttering bread). | ||
| D) Instrumental Activities of Daily Living Scale(11) | |||
|---|---|---|---|
| LAWTON – BRODY INSTRUMENTAL ACTIVITIES OF DAILING LIVING SCALE (IADL) | |||
| A. Ability to Use Telephone | E. Laundry | ||
| 1.Operates telephone on own initiative-looks up and dials numbers, etc. 2.Dials a few well-known numbers 3.Answers telephone but does not dial 4.Does not use telephone at all |
1 1 1 0 |
1.Does personal laundry completely 2.Launders small items-rinses stockings, etc. 3.All laundry must be done by others |
1 1 0 |
| B. Shopping | F. Mode of Transportation | ||
| 1.Takes care of all shopping needs independently 2.Shops independently for small purchases 3.Needs to be accompanied on any shopping trip 4.Completely unable to shop |
1 0 0 0 |
1.Travels independently on public transportation or drives own car 2.Arranges own travel via taxi, but does not otherwise use public transportation 3.Travels on public transportation when accompanied by another 4.Travel limited to taxi or automobile with assistance of another 5.Does not travel at all |
1 1 1 0 0 |
| C. Food Preparation | G. Responsibility for Own Medications | ||
| 1.Plans, prepares and serves adequate meals independently 2.Prepares adequate meals if supplied with ingredients 3.Heats, serves and prepares meals, or prepares meals, or prepares meals but does not maintain adequate diet 4.Needs to have meals prepared and served |
1 0 0 0 |
1.Is responsible for taking medication in correct dosages at correct time 2.Takes responsibility if medication is prepared in advance in separate dosage 3.Is not capable of dispensing own medication |
1 0 0 |
| D. Housekeeping | H. Ability to Handle Finances | ||
| 1.Maintains house alone or with occasional assistance (e.g. “heavy work domestic help”) 2.Performs light daily tasks such as dish washing, bed making 3.Performs light daily tasks but cannot maintain acceptable level of cleanliness 4.Needs help with all home maintenance tasks 5.Does not participate in any housekeeping tasks |
1 1 1 1 0 |
1.Manages financial matters independently (budgets, writes checks, pays rent, bills, goes to bank), collects and keeps track of income 2.Manages day-to-day purchases, but needs help with banking, major purchases, etc. 3.Incapable of handling money |
1 1 0 |
For males, “low” = <24, “average” = 24.1 – 28, “high/very high” = >28. For females, “low” = <23, “average” = 23.1-26, “high” = 26-29, “very high” = >29.
Footnotes
Disclosures: The authors of this manuscript have no conflicts of interest to disclose as described by American Journal of Transplantation.
REFERENCES
- 1.Based on OPTN data as of. 2012 Jul 20; [Google Scholar]
- 2.Kamath P. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001 Feb 1;33(2):464–70. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 3.Lai JC, Feng S, Roberts JP. An Examination of Liver Offers to Candidates on the Liver Transplant Wait-List. Gastroenterology. 2012 Jul;143(5):1261–5. doi: 10.1053/j.gastro.2012.07.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kruse JA, Thill-Baharozian MC, Carlson RW. Comparison of clinical assessment with APACHE II for predicting mortality risk in patients admitted to a medical intensive care unit. JAMA: The Journal of the American Medical Association. 1988 Sep;260(12):1739–42. [PubMed] [Google Scholar]
- 5.Charlson ME, Hollenberg JP, Hou J, Cooper M, Pochapin M, Pecker M. Realizing the potential of clinical judgment: a real-time strategy for predicting outcomes and cost for medical inpatients. The American Journal of Medicine. 2000 Aug 15;109(3):189–95. doi: 10.1016/s0002-9343(00)00477-0. [DOI] [PubMed] [Google Scholar]
- 6.Rockwood K. A global clinical measure of fitness and frailty in elderly people. Canadian Medical Association Journal. 2005 Aug 30;173(5):489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knaus WA, Harrell FE, Lynn J, Goldman L, Phillips RS, Connors AF, et al. The SUPPORT prognostic model. Objective estimates of survival for seriously ill hospitalized adults. Study to understand prognoses and preferences for outcomes and risks of treatments. Ann. Intern. Med. 1995 Feb 1;122(3):191–203. doi: 10.7326/0003-4819-122-3-199502010-00007. [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar 1;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 9.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994 Mar;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 10.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA: The Journal of the American Medical Association. 1963 Sep 21;185:914–9. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 11.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–86. [PubMed] [Google Scholar]
- 12.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, et al. Frailty as a predictor of surgical outcomes in older patients. J. Am. Coll. Surg. 2010 Jun;210(6):901–8. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 13.Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of Frailty in Patients With Cardiovascular Disease. AJC. Elsevier Inc. 2009 Jun 1;103(11):1616–21. doi: 10.1016/j.amjcard.2009.01.375. [DOI] [PubMed] [Google Scholar]
- 14.Weissenborn K, Rückert N, Hecker H, Manns MP. The number connection tests A and B: interindividual variability and use for the assessment of early hepatic encephalopathy. J. Hepatol. 1998 Apr;28(4):646–53. doi: 10.1016/s0168-8278(98)80289-4. [DOI] [PubMed] [Google Scholar]
- 15.Based on OPTN data as of October. 9:2013. [Google Scholar]
- 16.McAdams-DeMarco MA, Law A, Salter ML, Chow E, Grams M, Walston J, et al. Frailty and early hospital readmission after kidney transplantation. American journal of transplantation. 13(8):2091–5. doi: 10.1111/ajt.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman RB, Wiesner RH, Edwards E, Harper A, Merion R, Wolfe R. Results of the first year of the new liver allocation plan. Liver Transpl. 2004;10(1):7–15. doi: 10.1002/lt.20024. [DOI] [PubMed] [Google Scholar]
- 18.Wiesner R. MELD and PELD: Application of survival models to liver allocation. Liver Transpl. 2001 Jul 1;7(7):567–80. doi: 10.1053/jlts.2001.25879. [DOI] [PubMed] [Google Scholar]
- 19.Lang P- O, Michel J- P, Zekry D. Frailty Syndrome: A Transitional State in a Dynamic Process. Gerontology. 2009;55(5):539–49. doi: 10.1159/000211949. [DOI] [PubMed] [Google Scholar]
- 20.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004 Mar;59(3):255–63. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 21.Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL. Frailty, Dialysis Initiation, and Mortality in End-Stage Renal DiseaseFrailty, Dialysis Initiation, & Mortality in ESRD. Arch Intern Med. 2012 Jul 23;172(14):1071. doi: 10.1001/archinternmed.2012.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garonzik-Wang JM, Govindan P, Grinnan JW, Liu M, Ali HM, Chakraborty A, et al. Frailty and delayed graft function in kidney transplant recipients. Arch Surg. 2012 Feb;147(2):190–3. doi: 10.1001/archsurg.2011.1229. [DOI] [PubMed] [Google Scholar]
- 23.Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of Frailty among Dialysis Patients. Journal of the American Society of Nephrology. 2007 Oct 17;18(11):2960–7. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 24.Onen NF, Agbebi A, Shacham E, Stamm KE, Onen AR, Overton ET. Frailty among HIV-infected persons in an urban outpatient care setting. J. Infect. 2009 Nov;59(5):346–52. doi: 10.1016/j.jinf.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Piggott DA, Muzaale AD, Mehta SH, Brown TT, Patel KV, Leng SX, et al. Landay A, editor. Frailty, HIV Infection, and Mortality in an Aging Cohort of Injection Drug Users. PLoS ONE. 2013 Jan 31;8(1):e54910. doi: 10.1371/journal.pone.0054910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, et al. Sarcopenia and Mortality after Liver Transplantation. J. Am. Coll. Surg. 2010 Aug;211(2):271–8. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prentis JM, Manas DMD, Trenell MI, Hudson M, Jones DJ, Snowden CP. Submaximal cardiopulmonary exercise testing predicts 90-day survival after liver transplantation. Liver Transpl. 2012 Jan 25;18(2):152–9. doi: 10.1002/lt.22426. [DOI] [PubMed] [Google Scholar]
- 28.Singer JP, Katz PP, Dean MY, Chen J, Su B, Kern R, et al. Journal of Heart and Lung Transplantation. Vol. 32. Elsevier; Supplement: Apr 1, 2013. Frailty Is Common in Lung Transplant Candidates and Associated with Poorer Health-Related Quality of Life. p. S43. [Google Scholar]
- 29.Lederer DJ, Sonett JR, Philip NA, Larkin M, Peterson ER, Desai A, et al. Journal of Heart and Lung Transplantation. Supplement. Vol. 32. Elsevier; Apr 1, 2013. Frailty and Early Mortality after Lung Transplantation Preliminary Results. pp. S119–20. [Google Scholar]
- 30.Taylor HL, Jacobs DR, Schucker B, Knudsen J, S LA, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978 Jan 1;31(12):741–55. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]