Abstract
Objectives/Hypothesis
Recurrent respiratory papillomatosis (RRP) is a devastating disease, caused by infection of the upper aerodigestive tract with human papillomavirus types 6 and 11. There is no cure for RRP, and surgical removal is the mainstay of treatment. The purpose of this project was to compare genes of cell cycle, apoptosis, and inflammatory cytokines in laryngeal papilloma versus normal tissue for a better understanding of the molecular mechanisms of the disease to discover novel therapies.
Study Design
Basic science research study.
Methods
Papilloma tissue was obtained from patients requiring surgical debridement. For comparison, normal mucosa was obtained from the excised uvula of patients undergoing uvulopalatopharyngoplasty. Total RNA was extracted from both groups and then probed using customized reverse transcriptase real time polymerase chain reaction gene arrays.
Results
The custom arrays examine expression of 84 separate genes within the cell cycle, apoptosis, and inflammatory cytokine pathways. Our findings based on 11 papilloma samples run in comparison to normal mucosa shows that the MCL–1 gene of the apoptosis pathway is significantly downregulated. cytokine genes IL1-A, IL-8, IL-18, and IL-31 are also significantly dysregulated.
Conclusions
Genes of cell cycle and apoptosis are generally upregulated and downregulated, respectively, as expected in papilloma tissue, with MCL-1 achieving significance when compared to normal tissue. The finding of particular interest is that inflammatory cytokine genes were significantly downregulated, including IL1-A, IL-18, and IL-31. This finding may explain why patients infected with the virus are unable to mediate a T-cell immune clearance of their disease.
Keywords: Recurrent respiratory papillomatosis, papillomavirus infections, cell cycle, apoptosis, cytokines respiratory papillomatosis, laryngeal papilloma, recurrent juvenile laryngeal papilloma, upper aerodigestive tract infection
INTRODUCTION
Recurrent respiratory papillomatosis (RRP) is a rare but devastating disease affecting millions of people worldwide.1 RRP is caused by infection of the upper aerodigestive tract with human papillomavirus (HPV) types 6 and 11 (Fig. 1).2 The disease incidence has a bimodal distribution, presenting during childhood or adulthood. The disease prevalence in children <14 years of age is estimated to be 4.3/105 and 1.8/105 in adults.3 Consequences of the disease on vocal quality and overall quality of life can be quite devastating. Currently, there is no cure for RRP, and surgical removal remains the mainstay of treatment.
Fig. 1.
A laryngeal papilloma before surgical excision. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Despite a wealth of knowledge on the high-risk HPV types associated with cancer, such as HPV 16 and 18, less is known at the molecular level about RRP-related HPVs. The high-risk viral oncoproteins, E6 and E7, are the most studied. The E6 protein binds to p53, targeting it for degradation, which results in the decreased ability for cells to stimulate DNA repair or trigger apoptosis.4 The E7 protein targets the tumor suppressor protein Rb for degradation, which leads to cell immortalization and the ability to overcome DNA damage arrest signals.5
It is unclear why different patients have such variable courses of the disease.6 Infected patients mount an immune response that is initially characterized by the production of measurable serum antibodies.7–9 However, patients who develop symptoms of the disease develop a tolerance to the virus, rather than a cell-mediated immune clearance of the virus.10 One explanation theorizes that T cells are misdirected from a Th1 response to a Th2-like response, illustrated by an altered CD8+ T-cell subset and Th1/Th2 cytokine imbalance in RRP patients.11 Altered chemokine repertoire expression,12 altered frequency of killer cell immunoglobulin-like receptor gene haplotypes, and defective natural killer (NK) cells may also play a role.10
Our objective was to compare the gene expression profiles in papillomas to normal tissue for genes involved in cell cycle, apoptosis, and inflammatory cytokine pathways. To date, no one has yet performed a comprehensive review of these gene expression profiles in low-risk HPVs. In addition to p53 and Rb, we selected various genes of the cell cycle and apoptosis pathways, including some that have been examined in HPV-associated cervical, lung, and oropharyngeal cancer. We also selected genes for inflammatory cytokines involved in the viral-mediated immune response (Fig. 2). A clearer understanding of the molecular basis for transformation into RRP will aid in developing novel and more effective treatment modalities.
Fig. 2.
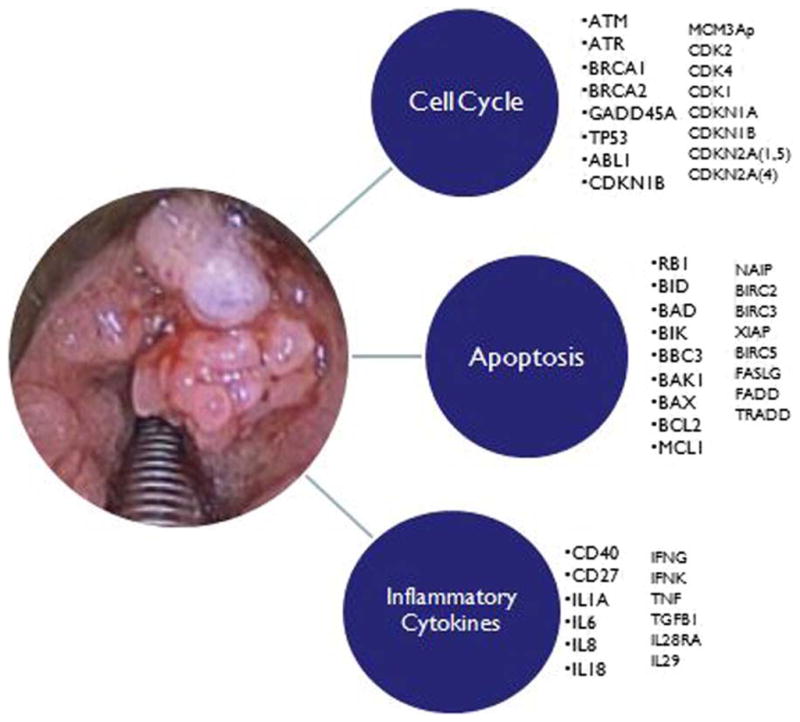
A visual representation of the genes tested for association with laryngeal papilloma. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
MATERIALS AND METHODS
Patients were enrolled from November 2010 through October 2012. Two research groups were identified; both groups were patients who consented to undergo surgery prior to enrollment in our study. The first group was patients with suspected laryngeal papilloma who were undergoing debridement. Inclusion criteria were papilloma lesions confirmed by pathology, and all ages, ethnicities, genders were included. After surgical removal, each specimen was split into two pieces; one was sent for pathology and one was stored in the preservative RNAlater (Life Technologies, Grand Island, NY) at −80°C for RNA extraction. Exclusion criteria were lesions not confirmed as papilloma on pathology. Each patient submitted only one tissue sample.
The second group of tissue samples (normal) was taken from patients undergoing uvulopalatopharyngoplasty (UPPP) for the surgical treatment of sleep apnea. Inclusion criteria were: consent for UPPP, and all ages, ethnicities, and genders included. Exclusion criteria were any patient who had laryngeal papilloma at anytime in their past medical history. After the uvula was excised, the mucosa was removed and stored in RNA-later at −80°C for processing.
RNA Extraction and Qualification
Tissues were processed and RNA extracted using the RNAEasy Mini kit (Qiagen, Valencia, CA). RNA was then quantitated using a NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE). Quality of the purified RNA was assessed by visualization of 18S and 28S RNA bands using an Agilent BioAnalyzer 2100 (Agilent Technologies, Chandler, AZ). Resulting electropherograms were used in the calculation of the 28S/18S ratio, and the RNA integrity number was confirmed for acceptability (>2).13
Assay Design
The microarrays were reverse transcriptase, real-time quantitative polymerase chain reaction (qPCR) assays, which allow for measuring the quantity of gene expression in papilloma tissue compared to the quantity of gene expression in control tissue. The real-time qPCR assays were designed from the coding sequence (CDS) of the gene of interest National Center for Biotechnology Information (NCBI) and exon-exon junctions mapped via the BLAT tool.14 Each array contains a panel of 96 primer sets for a set of 84 diseased focused genes, as well as five housekeeping genes and three RNA and polymerase chain reaction (PCR) quality controls. Primers were designed with Primer Express 2.0 (Applied Biosystems Inc., Foster City, CA) using default settings (primer Tm = 58°C–60°C, probe Tm = 10°C >primer Tm, GC content = 30%–80%, length = 9–40 nucleotides). Primers were synthesized and reconstituted to a final concentration of 100 μM prior to dilution to a working stock of 2 μM. Serial dilutions were performed as required by the plate design.
Real Time SYBR Green qPCR Gene Expression Analysis
Reverse transcription was performed on 1 μg of total RNA with random primers, utilizing TaqMan reverse transcription reagents (Applied Biosystems) as recommended by the manufacturer. The reverse transcription reaction was used as a template for the subsequent PCR reaction, consisting of SYBR Green PCR Master Mix, template cDNA, and assay primers in a total reaction volume of 25 μL. Thermal cycling was carried out with an ABI prism 7500 sequence detection system (Life Technologies) under factory defaults (50°C, 2 minutes; 95°C, 10 minutes; and 40 cycles at 95°C, 15 seconds; 60°C, 1 minute).
Gene Expression Data Analysis
Threshold cycle numbers (Ct) for both papilloma and control samples were defined as fluorescence values, generated by SYBR green binding to double stranded PCR products, exceeding baseline. Relative transcript levels for papillomas and controls were quantified as a comparison of measured Ct values for each reaction to a designated control via the ΔCt method.15 To normalize for template input, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcript levels were measured for each sample and used in these calculations. A Student t test was applied to GAPDH Ct values to rule out any change in expression of the endogenous controls due to treatment. The ΔCt for each papilloma was compared to the ΔCt of the paired control sample in each microarray, giving the ΔΔCt value. Fold change values were calculated as a log2 ratio of the ΔΔCt method averages of the biological replicates.
Data Analysis
Ct values that were either 0 or >37 were excluded, as such values are outside the detection limits of the machine. In addition, information for a gene was removed from a sample if it was shown to be derived from multiple peaks instead of just one. Q-Q plots and the Shapiro-Wilk test were performed on the data to check for normality. These tests failed, as did taking the logarithm transformation in an attempt to make the data more normally distributed. As a result, significant differences between normal versus papilloma samples were detected using the Mann-Whitney U test for non-normally distributed data. All analyses were performed using SPSS version 20 (IBM SPSS, Armonk, NY).
RESULTS
Fifteen papilloma samples were collected. Eleven confirmed papilloma samples had sufficient quality and quantity of RNA, and were randomly paired with 11 normals. The papilloma group was composed of nine adult patients, ranging in ages from 25 to 79 years (mean, 51.6 years) and two with childhood onset of disease (ages 7 and 19 years). In the adult group, the number of previous surgeries ranged from zero to nine, with a mean of 2.6. The two pediatric patients had more extensive disease, having had 13 and 57 surgeries for the 7 and 19 year old, respectively. One pediatric patient in our study had extralaryngeal spread and required a tracheostomy during the course of his disease. Six out of the 11 patients had been previously treated with cidofovir, and all had undergone at least five previous surgeries (Table I). Patients undergoing surgery for obstructive sleep apnea (OSA) were similar in demographics, with mean age of 48.5 years (Table II).
TABLE I.
Demographics of the Patients Included in the Study.
| Age, yr | Gender | Ethnicity | No. of Previous Surgeries | Most Recent Derkay Score | Extralaryngeal Spread | Required Tracheostomy | Cidofovir | Other Comorbidities |
|---|---|---|---|---|---|---|---|---|
| 47 | Female | White, not Hispanic | 0 | 4 | No | No | No | None |
| 44 | Male | White, not Hispanic | 5 | 6 | No | No | Yes | Severe dysplasia |
| 19 | Male | White, Hispanic | 57 | 31 | Yes | Yes | Yes | Recurrent pneumonia, pulmonary involvement |
| 60 | Male | White, not Hispanic | 5 | 17 | No | No | Yes | DM2, HTN, Afib, OSA |
| 79 | Female | Black, not Hispanic | 9 | 14 | No | No | Yes | HTN |
| 25 | Female | Black, not Hispanic | 0 | 3 | No | No | No | None |
| 40 | Male | White, not Hispanic | 5 | 8 | No | No | Yes | None |
| 7 | Male | White, Hispanic | 13 | 2 | No | No | Yes | None |
| 59 | Male | White, not Hispanic | 2 | 28 | No | No | No | None |
| 55 | Male | White, Hispanic | 0 | 5 | No | No | No | HepC, DM2, HTN |
Afib = atrial fibrillation; DM2 = diabetes mellitus type 2, HepC = hepatitis C; HTN = hypertension, OSA = obstructive sleep apnea.
TABLE II.
Comparison of the Demographics of the Papilloma Population and Control Group.
| Papilloma Group | Sleep Apnea (Control) Group | |
|---|---|---|
| Mean age, yr | 51.6 +2 pediatric patients | 48.5 |
| Gender | 4 female, 7 male | 4 female, 7 male |
| Ethnicity | 4 white non-Hispanic, 3 white Hispanic, 2 black non-Hispanic | 5 white non-Hispanic, 3 white Hispanic, 3 black non-Hispanic |
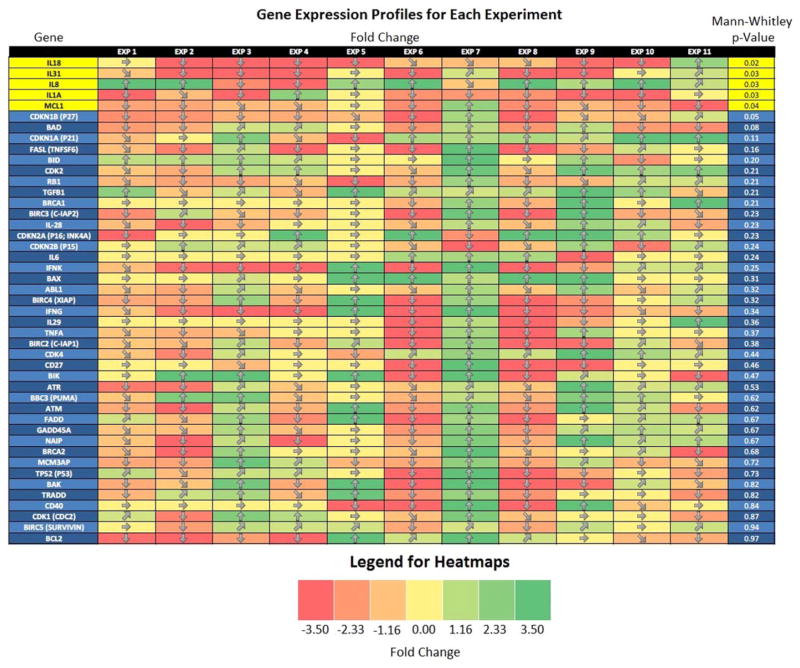
The log2 ratio of the ΔΔCt for each of 11 microarrays, each with one papilloma sample compared to one control, was calculated for all genes analyzed. Several genes (MCL1, IL1A, IL8, IL18, and IL31) were found to be significantly different between the normal and papilloma groups (P <.05). Figure 3 shows the genes analyzed and whether the gene is upregulated or downregulated in papilloma versus its paired control. The column on the left shows the P value from the Mann-Whitney U test comparing the gene expression profiles within each group.
Fig. 3.
Heat map giving a visual representation of gene expression in papilloma tissue compared to control. The y-axis shows each of the genes that were analyzed in this study. The x-axis represents each of the paired 11 microarrays. A comparison of papilloma to control is made for each gene, for each of 11 paired microarrays. The colors represent the “fold change,” which is the log2 ratio of the ΔΔCt. Upregulation is represented with green, and downregulation is represented with red. Significant difference in gene expression is demonstrated by the P value in the far right column. Genes with significant difference were MCL-1, IL-1A, IL-8, IL-18, and IL-31.
DISCUSSION
Our results demonstrate significant differences between diseased papilloma tissues and controls in MCL-1, IL1-A, IL-8, IL-18, and IL-31 gene expression profiles. MCL-1, an antiapoptosis gene, was downregulated in our papilloma samples. The proinflammatory cytokine genes IL1A, IL18, and IL-31 were also downregulated. Cytokine IL-8, which is involved in inflammation but also in angiogenesis, was upregulated.
The MCL-1 gene encodes an antiapoptotic protein, which is a member of the Bcl-2 family and is important for the regulation of apoptosis.16 Interestingly, alternative splicing results in multiple transcript variants. Isoform 1 enhances cell survival by inhibiting apoptosis, whereas isoforms 2 and 3 promote programmed cell death.17 Previously examined in HPV 16-associated lung cancer,18,19 an increase in IL-6 and IL-17 was associated with a downstream increase in MCL-1 expression. Interestingly, in our study, this gene was significantly downregulated in papillomas. Because the presence of the viral oncoprotein, E6, from multiple HPV types often blunts the apoptotic response,20 it is possible that isoforms 2 and 3 were the significant forms detected in our microarrays.
IL1-A, significantly downregulated compared to controls, is a pleiotropic cytokine involved in various immune responses, inflammatory processes, and hematopoiesis. IL1-A plays a key role in the regulation of our immune response, binding to the interleukin-1 receptor21,22 and activating tumor necrosis factor-α. When studied in HPV-positive cervical cancer patients, IL1-A was found to be downregulated.23 Similarly, its downregulation in laryngeal papilloma disease may explain the lack of an inflammatory response during persistent HPV infection.
IL-8 is one of the major mediators of the inflammatory response, and is a chemotactic factor that attracts neutrophils, basophils, and T cells. It is also involved in neutrophil activation and is a potent angiogenic factor. It is released from several cell types in response to an inflammatory stimulus.24 IL-8 has been found to be upregulated in HPV-associated lung cancers, presumably due to its angiogenic properties.25 It has also been studied in patients with RRP. One study found that IL-8 was expressed at low levels in normal mucosa in healthy individuals, but was modestly upregulated in papilloma patients.26 Our study confirms this finding, as IL-8 was also upregulated in our papilloma samples. We hypothesize that IL-8 predominantly plays a critical role in angiogenesis for the development of papillomatosis.
We also found IL-18 to be downregulated in papilloma patients. The IL-18 gene encodes a proinflammatory cytokine that augments NK cell activity and stimulates interferon gamma production in T-helper type I cells.27 Multiple studies have examined IL-18 in HPV-associated cervical cancers finding it to be downregulated.28,29 It has also been shown that extracellular E6 and E7 proteins inhibit IL-18-induced interferon-γ production locally in HPV16 lesions through inhibition of IL-18 binding to its receptor.30 The downregulation of IL-18, along with a lack of cell-mediated immunity, may be a key to why a Th2 type response occurs when a Th1 response is expected.
Finally, IL-31 was significantly downregulated in the papilloma group. IL-31, which is made principally by activated Th2-type T cells, may be involved in the promotion of allergic skin disorders and in regulating other allergic diseases such as asthma.31 The lack of IL-31, which is specific to epithelial cell inflammation, also may indicate the overall lack of an adequate inflammatory response in RRP.
Recent research on cytokine levels in patients with sleep apnea brings into question whether the OSA population is truly normal. Studies have shown that patients with OSA have elevated levels of IL-6 and TNFα.32,33 Interestingly, it has been shown that IL-31 can significantly induce the release of proinflammatory IL-6.34 In our study, IL-31 was downregulated in comparison to our control group. It is feasible that the downregulation of IL-31 in our papilloma group might conversely be an upregulation in the OSA patients. The other inflammatory cytokines we examined have not been shown to be dysregulated in OSA.
There are, however, some limitations of this type of study. Because the study was designed to deidentify patient information from the collected samples immediately, comparisons between pediatric versus adult onset of disease are impossible. It is certainly plausible that the more aggressive clinical behavior in pediatric patients may correlate with a variant gene expression profile, and is a topic that should be addressed in future studies. Our study is also limited by a small sample size and the paucity of tissue obtained from the biopsies. As such, the division of patients into smaller groups according to HPV type, age of onset, or clinical course would lose any significance. Because of the small size of the study and multiple genes tested, statistical significance is weakened after multiple comparison analysis.
CONCLUSION
It is known that RRP patients do not seem to mount an expected cell-mediated immune response. These patients also seem to develop a tolerance to the virus and are unable to clear the HPV infection. Our study contributes to the growing body of research suggesting that a lack of proinflammatory cytokines is largely responsible for this ineffective immune response. In particular, our study reveals that the transcription of inflammatory cytokine genes IL1-A, IL-18, and IL-31 is significantly downregulated in papilloma diseased tissue. We also show that MCL-1, a gene regulating apoptosis, is significantly downregulated. We further show that IL-8, important for angiogenesis, is upregulated. Future studies with larger sample sizes in both adult and juvenile RRP patients will likely reveal other cellular pathways that are affected by HPV infection, or perhaps additional gene dysregulation within the cell cycle, apoptosis, or inflammatory cytokine pathways. Most importantly, additional studies are needed to better understand the cellular mechanisms of this devastating disease, which will likely lead to novel therapeutic targets for more effective treatments for RRP.
Acknowledgments
This research was conducted at the University Texas Medical Branch, Galveston, Texas.
All financial and material support for this research was provided by the Department of Otolaryngology, The University of Texas Medical Branch.
Footnotes
Presented as an oral presentation at the Laryngology/Bronchoeso-phagology portion of the Triological Society Meeting at COSM, Orlando, Florida, U.S.A., April 10–14 2013.
Level of Evidence: NA
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Marsico M, Mehta V, Wentworth C, Chastek B, Derkay CS. Estimating the disease burden of juvenile onset RRP in the US using large administrative databases—preliminary pilot results. Paper presented at: International Papillomavirus Conference; May 2009; Malmo, Sweden. [Accessed on March 23, 2013]. Abstract P-03.58. Available at: http://www.hpv2009.org. [Google Scholar]
- 2.Gissmann L, Wolnik L, Ikenberg H, et al. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983;80:560–563. doi: 10.1073/pnas.80.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derkay CS, Darrow DH. Recurrent Respiratory Papillomatosis. Ann Otol Rhinol Laryngol. 2006;115:1–11. doi: 10.1177/000348940611500101. [DOI] [PubMed] [Google Scholar]
- 4.Venkatesan N, Pine H, Underbrink M. Recurrent respiratory papillomatosis. Otolaryngol Clin North Am. 2012;45:671–694. doi: 10.1016/j.otc.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helt AM, Galloway DA. Mechanisms by which tumor virus oncoproteins target the Rb family of pocket proteins. Carcinogenesis. 2003;24:159–169. doi: 10.1093/carcin/24.2.159. [DOI] [PubMed] [Google Scholar]
- 6.Miller CS, Johnstone BM. Human papilloma virus as a risk factor for oral squamous cell carcinoma: a meta analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:622–635. doi: 10.1067/moe.2001.115392. [DOI] [PubMed] [Google Scholar]
- 7.Bonnez W, Kashima HK, Leventhal B, et al. Antibody response to human papillomavirus (HPV) type 11 in children with juvenile onset recurrent respiratory papillomatosis (RRP) Virology. 1992;188:384–387. doi: 10.1016/0042-6822(92)90770-p. [DOI] [PubMed] [Google Scholar]
- 8.Sameshima A, Fujiyoshi T, Pholampaisathit S, et al. Demonstration of antibodies against human papilloma virus type 11 E6 and L2 proteins in patients with recurrent respiratory papillomatosis. Auris Nasus Larynx. 1997;24:185–191. doi: 10.1016/s0385-8146(96)00000-4. [DOI] [PubMed] [Google Scholar]
- 9.Tachezy R, Hamsikova E, Valvoda J, et al. Antibody response to synthetic peptide derived from the human papilloma virus type 6/11 L2 protein in recurrent respiratory papillomatosis: correlation between Southern blot hybridization, polymerase chain reaction, and serology. J Med Virol. 1994;42:52–59. doi: 10.1002/jmv.1890420111. [DOI] [PubMed] [Google Scholar]
- 10.Bonagura VR, Hatam LJ, Rosenthal DW, et al. Recurrent respiratory papillomatosis: a complex defect in immune responsiveness to human papillomavirus 6 and 11. APMIS. 2010;118:455–470. doi: 10.1111/j.1600-0463.2010.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonagura VR, Hatam LJ, DeVoti J, Zeng F, Steinberg BM. Recurrent respiratory papillomatosis: altered CD8(+) T cell subsets and T(H)1/T(H)2 cytokine imbalance. Clin Immunol. 1993;93:302–311. doi: 10.1006/clim.1999.4784. [DOI] [PubMed] [Google Scholar]
- 12.DeVoti JA, Rosenthal DW, Wu R, Abramson AL, Steinberg BM, Bonagura VR. Immune dysregulation and tumor associated gene changes in respiratory papillomatosis: a paired microarray analysis. Mol Med. 2008;14:608–617. doi: 10.2119/2008-00060.DeVoti. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroeder A, Mueller O, Stocker S, et al. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol. 2006;7:3. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kent WJ. BLAT—the BLAST-like alignment tool. Genome Res. 2002;12:656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.MCL-1 Human 907820. [Accessed on March 23, 2013];UniProtKB/Swiss-Pot. Available at: www.uniprot.org. Published December 6, 2005. Updated March 6, 2013.
- 17.RefSeq. [Accessed on March 23, 2013];Entrez Gene. 2010 Oct; Available at: www.ncbi.nlm.nih.gov/gene. Updated March 19, 2013.
- 18.Chang YH, Yu CW, Lai LC, et al. Up regulation of interleukin-17 expression by human papillomavirus type 16 E^ in nonsmall cell lung cancer. Cancer. 2010;116:4800–4809. doi: 10.1002/cncr.25224. [DOI] [PubMed] [Google Scholar]
- 19.Cheng TW, Lee H, Shiau MY, et al. Human papillomavirus type 16/18 up regulates the expression of interleukin-6 and antiapoptotic Mcl-1 in non-small cell lung cancer. Clin Cancer Res. 2008;14:4705–4712. doi: 10.1158/1078-0432.CCR-07-4675. [DOI] [PubMed] [Google Scholar]
- 20.Underbrink MP, Howie HL, Bedard KM, Koop JI, Galloway DA. E6 proteins from multiple human betapapillomavirus types degrade Bak and protect keratinocytes from apoptosis after UVB irradiation. J Virol. 2008;82:10408–10417. doi: 10.1128/JVI.00902-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bankers-Fulbright JL, Kalli KR, McKean DJ. Interleukin-1 signal transduction. Life Sci. 1996;59:61–83. doi: 10.1016/0024-3205(96)00135-x. [DOI] [PubMed] [Google Scholar]
- 22.Dinarello CA. Induction of interleukin-1 and interleukin-1 receptor antagonist. Semin Oncol. 1997;24(3 suppl 9):S9-81–S9–93. [PubMed] [Google Scholar]
- 23.Hudelist G, Czerwenka K, Singer C, Pischinger K, Kubista E, Manavi M. cDNA array analysis of cytobrush-collected normal and malignant cervical epithelial cells: a feasibility study. Cancer Genet Cytogenet. 2005;158:35–42. doi: 10.1016/j.cancergencyto.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 24.IL-8. [Accessed on March 24, 2013];UniProtKB/Swiss-Pot. Available at: www.uniprot.org. Published December 6, 2005. Updated March 6, 2013.
- 25.Shia MY, Fan LC, Yang AC, Tsao CH, Lee H, Cheng YW. Human papillomavirus up-regulates MMP-2 and MMP-9 expression and activity by inducing interleukin-8 in lung adenocarcinomas. PLoS One. 2013;8:1–8. doi: 10.1371/journal.pone.0054423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chong K, Xiang L, Wang X, Jun E, Xi L, Schweinfurth J. High level expression of human epithelial B-Defensins (hBD-1,2 and 3) in papillomavirus induced lesions. Virology. 2006;3:75–83. doi: 10.1186/1743-422X-3-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.RefSeq. IL-18. [Accessed on March 24, 2013];Entrez Gene. 2010 Oct; Available at: www.ncbi.nlm.nih.gov/gene.
- 28.Lee Ka, Cho KJ, Shim JH, et al. IL-18 E42A mutant is resistant to the inhibitory effects of HPV-16 E^ and E7 oncogenes on the IL-18 mediated immune response. Cancer Lett. 2005;18:229, 261–270. doi: 10.1016/j.canlet.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 29.Cho YS, Kang JW, Cho M, et al. Down modulation of IL-18 expression by human papilloma virus type 16 E6 oncogene via binding to IL-18. FEBS Lett. 2001;501:139–145. doi: 10.1016/s0014-5793(01)02652-7. [DOI] [PubMed] [Google Scholar]
- 30.Lee SJ, Cho YS, Cho MC, et al. Both E6 and E7 oncoproteins of human papillomavirus 16 inhibit IL-18-induced IFN-gamma production in human peripheral blood mononuclear and NK cells. J Immunol. 2001;167:497–504. doi: 10.4049/jimmunol.167.1.497. [DOI] [PubMed] [Google Scholar]
- 31.RefSeq. IL-31. [Accessed on March 24, 2013];Entrez Gene. 2010 Oct; Available at: www.ncbi.nlm.nih.gov/gene. Updated March 19, 2013.
- 32.Kapsimalis F, Basta M, Varouchakis G, Gourgoulianis K, Vgontzas A, Kryger M. Cytokines and pathologic sleep; a review article. Sleep Med. 2008;9:603–614. doi: 10.1016/j.sleep.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 33.Ciftci TU, Kokturk O, Bukan N, Bilgihan A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine. 2004;28:87–91. doi: 10.1016/j.cyto.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Cheung P, Wong CK, Ho A, Hu S, Chen DP, Lam C. Activation of human eosinophils and epidermal keratinocytes by Th2 cytokine IL-31: implication for the immunopathogenesis of atopic dermatitis. Int Immunol. 2010;22:453–467. doi: 10.1093/intimm/dxq027. [DOI] [PubMed] [Google Scholar]