Abstract
We present in this paper a novel neuroinformatic platform, the BAMS2 Workspace (http://brancusi1.usc.edu), designed for storing and processing information about gray matter region axonal connections. This de novo constructed module allows registered users to directly collate their data by using a simple and versatile visual interface. It also allows construction and analysis of sets of connections associated with gray matter region nomenclatures from any designated species. The Workspace includes a set of tools allowing the display of data in matrix and networks formats, and the uploading of processed information in visual, PDF, CSV, and Excel formats. Finally, the Workspace can be accessed anonymously by third party systems to create individualized connectivity networks. All features of the BAMS2 Workspace are described in detail, and are demonstrated with connectivity reports collated in BAMS and associated with the rat sensory-motor cortex, medial frontal cortex, and amygdalar regions.
Keywords: neuroinformatics, rat, amygdala, cerebral cortex
Introduction
The advent of new technologies for identification and validation of structural neuroanatomical connections (Huang and Zheng, 2013; Osten and Margrie 2013), and the use of combinations of classic pathway tracing methods (Thompson and Swanson, 2010; Hintiryan et al., 2012) led to the emergence of several large scale projects with the common goal of producing the complete, systematic, and detailed set of neuroanatomical connections of the rodent central nervous system (CNS), that is, its connectome (Sporns et al., 2005). This is done both at the level of gray matter regions (the macroconnectome; Bota and Swanson 2012), and at the level of neuron types and classes (the mesoconnectome; Bota and Swanson 2012). At least three such large scale projects have already made publicly available the results of hundreds, if not thousands, of pathway tracing experiments: the Mouse Connectivity Project of the Allen Brain Institute (AIBS; http://connectivity.brain-map.org), the Mouse Connectome Project at USC (iConnectome; http://www.mouseconnectome.org/iConnectome, RRID: nlx_143548; Hintiryan et al., 2012), and the Cold Spring Harbor (CSHL) Mouse Brain Architecture Project (http://brainarchitecture.org/mouse, RRID:nlx_146201).
At the same time, several neuroinformatics projects actively collate neuroanatomical connectional information from the published literature, in rats (TemporalLobe of Sugar et al., 2011; BAMS of Bota et al., 2005), monkeys (CoCoMac of Stephan et al., 2000; Bakker et al., 2012), and other species. The common denominator of these two major research directions is the annotation and collation of connectional data and metadata, either from unpublished online high resolution images, or from the published research literature. This process requires expert knowledge, is time consuming and manual, and usually requires at least two iterations. It constitutes one of the most serious bottlenecks for large sets of publicly accessible connectivity data and there are no publicly available tools to accelerate the manual process of data annotation and collation. In addition, tools for visualizing nervous system networks are available only in generic public software platforms like Cytoscape (http://www.cytoscape.org, RRID:nif-0000-30404), or are embedded in downloadable programs (neuroVIISAS; Schmitt and Eipert, 2012) that cannot be used directly on the Web by the neuroscience community.
The Brain Architecture Knowledge Management System Version 2 (BAMS2; htttp://brancusi1.usc.edu) is the second iteration of the neuroinformatics platform, BAMS (http://brancusi.usc.edu, RRID: nif-0000-00018), that we have been developing and populating since 2003 (Bota et al., 2003), and which will named here for convenience Classic BAMS. BAMS2 is not a simple repetition of the Classic BAMS with an improved interface, but rather a system constructed de novo from the computer hardware to its Web accessible tools. BAMS2 and its functionality were briefly described in Swanson and Bota (2010), in the context of the Foundational Model of Connectivity (FMC; http://brancusi1.usc.edu/thesaurus/principles/).
We describe here the infrastructure and interface of a BAMS2 module, Workspace, which was designed and implemented for handling connectional data and metadata, either collated from the literature, or inserted directly by neuroanatomists from raw data. The infrastructure and interface of BAMS2 Workspace allow handling of neuroanatomical connectional information in any designated species, and at different resolution levels: from reports annotated on standard Atlas or Plate Levels, to pre-processed connectivity data associated with chosen sets of gray matter regions. Furthermore, the Workspace includes a rich set of tools for visualizing connectivity matrices and networks of neural nodes, and for exporting processed information in the most commonly used formats. Finally, the Workspace can be used to construct connectivity matrices and neural networks by third party systems.
Methods
1.BAMS2 general structure and design
Here, we briefly describe the most important aspects of the publicly accessible BAMS2, and compare them with the Classic BAMS.
Briefly, with respect to Classic BAMS, BAMS2 introduces RDF storage, full-text search, interactive connectome (connections) grids with a public API for third party data sources, connectome creation and management tools, connectivity graphs and multiple live data exports.
While the original Classic BAMS was implemented in a relational MySQL database, the structure of BAMS2 public interface is based on a single standard RDF file, which is queried using SPARQL 1.1 (http://www.w3.org/TR/rdf-sparql-query/) to construct the displayed content. The structure of this file follows the general logic of Classic BAMS infrastructure (Bota et al., 2005), and it is compliant with the requirements of the Semantic Web syntax. This RDF file and its structural specifications are publicly available for download (http://brancusi1.usc.edu/ontology/). BAMS2 publicly available structure and data can be therefore used by members of the neuroscience community in a standard format to build on its other applications, replicate the system, or integrate it with other data sources. A unique module of BAMS2 is the Foundational Model of Connectivity (FMC; http://brancusi1.usc.edu/thesaurus), which is also based onto an RDF schema. FMC has two main components: its construction Principles and Thesaurus. The structure and content of the Thesaurus, as well as its search tools, are described in detail in Swanson and Bota (2010). Briefly, the FMC Thesaurus is made of a growing set of completely defined neuroanatomical concepts, related terms and their explicitly stated relationships, which are valid across mammalian species. Each concept and term included in the Thesaurus is historically referenced. Registered users in BAMS2 are allowed to add new terms, edit the existing terms, establish new relationships between terms and concepts, as well as add textual comments. As with other public components of BAMS2, Thesaurus can be downloaded in RDF format: http://brancusi1.usc.edu/thesaurus/.
Another difference with Classic BAMS is the mode of search for information in BAMS2, which is performed in several fields simultaneously, thus minimizing the number of pages and allowing a faster retrieval of the relevant information. Another new feature of the second iteration of our platform is a module dedicated to the rat macroconnectome constructed annually with the data inserted in Classic BAMS, and based on the Swanson 2004 rat Atlas (Swanson-04; Swanson, 2004). Thus far, BAMS2 includes five versions of the rat macroconnectome, arranged historically from 2009 to 2013. Each version is created in a matrix (grid) format using technology described in the sections below. This historic versioning allows anonymous users to inspect the evolution of the Classic BAMS rat macroconnectome from the content point of view. The most recent version of the Classic BAMS rat macroconnectome is constructed on the Swanson-2004 nomenclature, and includes 496 brain regions. Thus, the rat CNS macroconnectome can be represented as a 496 × 496 matrix, each cell of it representing a connection between two gray matter regions. The newest version of it includes more than 36k connections shown in the literature to be either present or absent and inserted in BAMS, hence is more than 15% complete. One percent of the entire matrix (2455 cells in a column, or a row, respectively), can be translated as the complete inputs or outputs of five regions. The rat macroconnectome CNS matrix is therefore sparsely populated with several exceptions, which include the rat cerebral cortex, the bed nuclei of stria terminalis, and the amygdala.
Finally, BAMS2 includes a fully developed Workspace that is accessible to registered users and described in detail below. The Workspace and Thesaurus modules are inter-related in such way that the user-defined neuroanatomical nomenclatures used in Workspace (see below) can be added automatically to the Thesaurus.
2. The BAMS2 Workspace Module: creation of a new group
The logical structure of the Workspace module follows the workflow of a generic neuroanatomy laboratory. The Workspace backend architecture is centered around the concepts of group and case. The additional criteria we considered in the design of this module are the ease of use and agnosticism, that is, the Workspace should accommodate any designated species and neuroanatomical nomenclature. The first criterion is necessary for rapid insertion, retrieval and manipulation of connectivity data into BAMS2, while the latter is necessary for universality.
For implementation we used the Django framework (https://www.djangoproject.com/). The backend of the Workspace was implemented in PostgreSQL (http://www.postgresql.org), and we used Python (http://www.python.org/), JavaScript, jQuery, and CSS to construct its interfaces. This software combination is one of the most powerful available, and is primarily used in science and information technology communities.
The insertion of neuroanatomical connectional information in BAMS2 Workspace can be performed in two different ways: the first is generic and allows data entry either via a simple visual interface, or via uploading of Excel or CSV files. The second way allows insertion of connectivity data by Plate or Level of a standard neuroanatomical Atlas. Both methods use the concepts of group and case.
A BAMS2 Workspace group is made of any number of registered users (collators), with a single leader, who use a single designated neuroanatomical nomenclature. Any member of the group can directly insert or upload connectivity annotation files for any number of neuroanatomical experiments (or cases) as well as view, process, and edit the files. The group leader is by default the individual who creates the group, and chooses the species and the neuroanatomical nomenclature that will be used across all experiments collated or inserted by each member. However, the leadership can be transferred at any later time to any group member. The group leader has full access to all experiments inserted by the group members.
The nomenclature to be used by the entire group can already reside in Classic BAMS, or if not present can be uploaded by the group leader in CSV or Excel formats. To date, BAMS includes 5 species: mouse, rat, cat, macaque, and human, and 32 associated nomenclatures. Whenever the group is created using a custom nomenclature, the default species is rat. This choice was made based on the species used most often for neuroanatomical experiments in the Swanson laboratory. However, this property can be extended to include a different species when the nomenclature file is uploaded. The general form of an uploadable nomenclature file is as simple as possible: it includes a numerical and unique ID for each included gray matter region, the name of the region, and its abbreviation. The structure of the Workspace is constructed such that gray matter region names and abbreviations are case insensitive. From our experience, typing errors are common in the initial loading of a new nomenclature. The nomenclatures that are uploaded in the Workspace can be made of any set of gray matter regions, thus allowing registered users to insert data and construct customized connectivity matrices at different granularity levels.
The group leader also establishes the number of ranked qualitative strengths of the neuroanatomical connections that will be used to collate the experiments The results of a neuroanatomical experiment can be measured in qualitative, semi-quantitative, or quantitative variables, as well as any combinations of them. However, the ranked qualitative description of neuroanatomical connections is the common denominator for the vast majority of experiments to date. Hence, for simplicity and ease of use, we implemented thus far only the ranked qualitative strengths as measures of the neuroanatomical connections. The BAMS2 Workspace interface includes the option of using the standard set of qualitative strengths of BAMS (However, the color code can also be changed by the group leader. Finally, the group leader must specify whether the right or left side of the nervous system was injected, and whether the group will be public
Once a group is created, the user is returned to the list of groups where the user is leader (Fig. 1).
Figure 1.
The BAMS2 Workspace interface as it appears to a registered user. Note that the “actions” column contains the functionality necessary to view, create, edit, and delete experiments (cases). If a group contains at least two experiments, then a new link becomes accessible: “subset matrix”. This functionality allows group members to view and process only those experiments of interest (see Text for details). The “case” column allows direct access to those experiments associated with a group. The “strengths” column allows editing the associated color codes of connectional strengths. Those groups constructed on customized nomenclatures (from file) have an additional feature that allows the user to download the nomenclature file in Excel format, which can be further edited by their leaders.
3. The BAMS2 Workspace interface: connections data insertion
Once a new user is registered and inserts at least one neuroanatomical nomenclature, the interface of the Workspace, which includes all its functionalities, becomes visible (Fig. 1). The interface shown in Fig. 1 allows registered users to add, edit, or delete individual experiments (cases), as well as view individual experiments in grid (matrix) format. Additionally, the link “subset matrix” allows them to organize their experiments of interest (see below). The interface for inserting a new experiment in a group is shown in Figure 2.
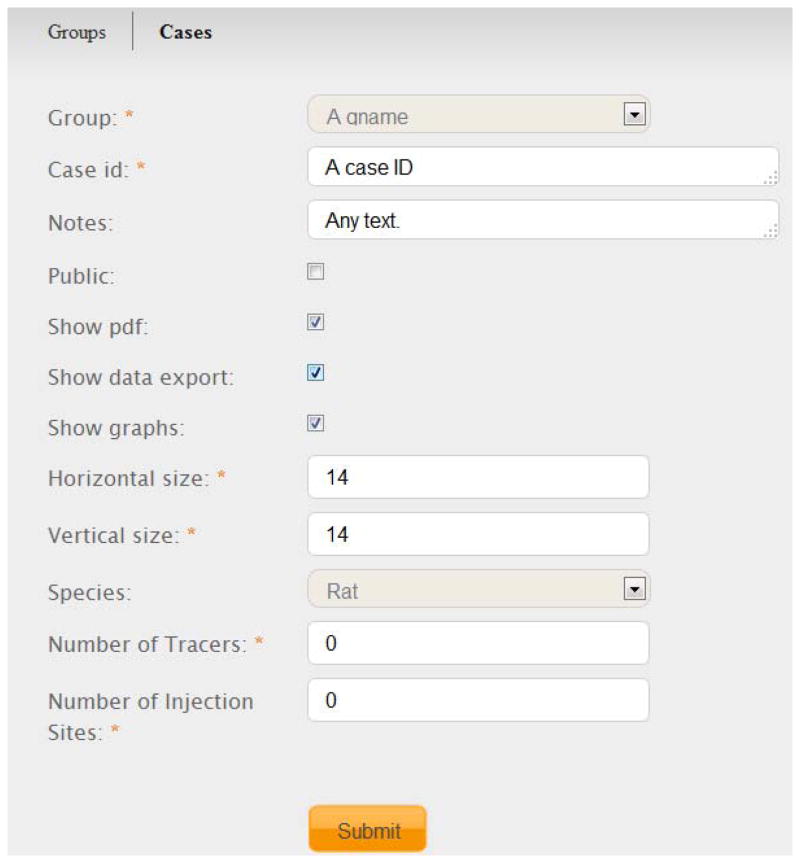
Figure 2.
The interface for adding a new case (a set of connections annotated and collated from a neuroanatomical experiment) in BAMS2 Workspace. The mandatory fields are starred. Registered users who create cases can choose the degree of functionality associated with each inserted one, as well as its status: public or private. See Text for details.
Besides registration of the most important metadata that are associated with an experiment, registered users can manipulate (reduce) the size of the gray matter region matrix, and specify the number of injection sites and tracers used in the experiment to be inserted. Additionally, they can customize the export functionalities of the newly inserted case (see below), and whether the results will be public or private. Thus, each group has the option of making public only a subset of experiments.
The BAMS2 Workspace allows insertion of connectivity data collated from the published literature. The default values of variables “numbers of tracers” and of “number of injection sites” (0; see Fig. 3), respectively, allow registered users to insert already pre-processed connectional data, such as that collated from the published literature. This data can be inserted in the Workspace in the simplest possible format, as Excel lists of efferent (origin) and afferent (termination) regions, and the ranked qualitative weights of connections. Thus, the data inserted by a registered user may include either the results of unpublished neuroanatomical experiments, or sets of connections collated from sets of published references. However, each type of data must be associated with different groups. This rule eliminates the risk of combining data of different provenances.
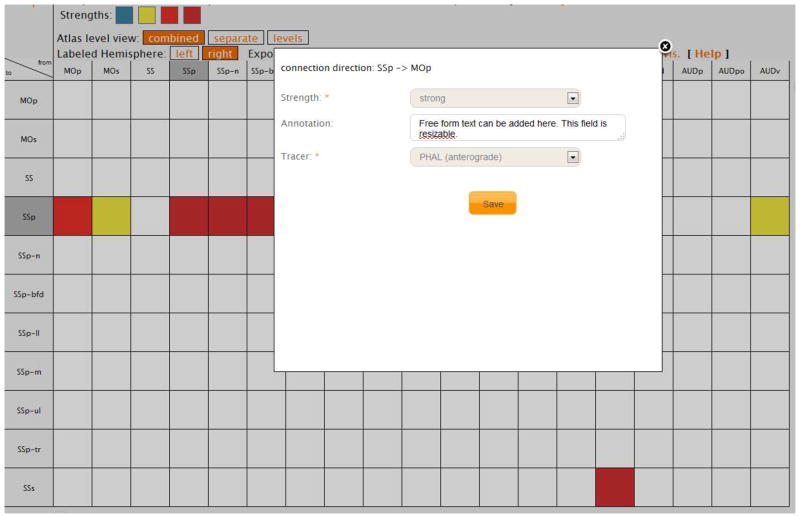
Figure 3.
The generic matrix representation of a new case (experiment), filled directly in the Workspace. The inset shows the form to be filled for each connection (cell) of the matrix. This matrix is updated after the user hits the “Save” button, showing the newly inserted report.
Once these preliminary steps are completed and the connections data were not uploaded in Excel format, the user is presented with an empty matrix made of the gray matter regions forming the associated nomenclature, which is a visual data entry form. Directions of neuroanatomical connections are represented by the columns and the rows of the matrix, respectively. The columns represent the efferent regions (origins) while the rows represent the afferent regions (terminations), and each cell represents the connection between the respective column and row. An example of the connectivity data entry form is shown in Figure 3. Clicking on a white (empty) cell will return a very simple entry form for insertion of that particular connection.
Because our aim was to create a simple and versatile connectivity data entry tool, the mandatory fields for inserting a connection between a pair of regions in BAMS2 Workspace include qualitative strength and tracer used. Importantly, however, an additional optional field was added: a free text form for textual description of the collated connection. The two mandatory fields are designed as simple drop-down boxes. The “Tracer” field includes the acronyms of the tracers used in neuroanatomical experiments, and their directionality (anterograde vs. retrograde vs. both). Directionality was added to eliminate possible mistakes by collators. Once this form is filled and submitted, the matrix updates itself and the inserted connection will appear as a colored square. The color represents the visual code of the qualitative connectional strengths chosen for the associated group (Fig. 3). Connectivity data of any pathway tracing experiment can thus be rapidly inserted in the BAMS2 Workspace using this very simple visual interface. This interface may become important for rapid collation and recording in the context of the publicly available resources with large data sets like the Allen Brain Mouse Connectivity Atlas (http://connectivity.brain-map.org/), the Mouse Brain Architecture Project (http://brainarchitecture.org/mouse), or the LONI iConnectome Project (http://www.mouseconnectome.org/iConnectome).
A second, and more detailed, method of inserting connectional data allows recording by Levels or Plates of standard neuroanatomical atlases. Because the manual insertion of data per Atlas Level using the Workspace visual interface can become time consuming, we implemented an additional engine that automatically detects in each uploaded Excel (XSLX) or CSV file the data and metadata of an experiment (case). The format of files that can be uploaded is generic, and follows the general arrangement of registered data and metadata of an experiment (case). Thus, the engine extracts the unique alphanumeric experiment ID from the first row of any uploaded file, and the tracers and the injection sites from the second row, respectively. For double injections, co-injections, or double coinjection experiments, the second injection site and the associated tracers are extracted from the third row of the file. The terminal fields and the associated Atlas Levels where label was detected are listed as the first columns in the uploaded files.
Each data set revealed by a tracer has three columns: the right and the left side strengths for each terminal field and associated Atlas Level, respectively, and an additional field for free text annotations. These columns are ordered by the registered tracers. Thus, the first three columns are associated with the first tracer recorded in the uploaded file, and so on. The allowed codes of the ranked qualitative connection strengths are either text (for example, “weak”, “strong”) or strings of identical repetitive characters, for example, ‘+’ for weak connections, ‘++’ for moderate connections. Text values are encoded in the engine, whereas the strings are processed once the experiment files are uploaded. A string made by a single character (for example, ‘+’) is associated automatically with the lowest connection strength assigned in the group (for example, weak). The next strength value will be associated with those strings made by two identical characters, and so on. The strings that are made of different characters are interpreted by the engine as mistakes, and are therefore ignored. The same rule is applied to those strings with numbers of identical characters higher than the number of strengths allowed in the group. The strings used by each group to encode for the qualitative strengths are recorded in BAMS2, and will thus be used as one output modality of the Workspace. Finally, each pathway tracer experiment constructed from an uploaded file can be further modified in the grid (matrix) representation, as described above. When this is done, files uploaded earlier are also updated.
4. The Workspace Interface: viewing connections data
Figure 1 shows the main menu of the Workspace as seen by registered users. The main menu has two view modes: groups and cases (experiments). Group leaders are able to access both their groups and cases, while group members can access only the cases they insert.
The group view includes links to forms that allow editing the group information and its associated neuroanatomical nomenclature, adding new experiments (cases), and viewing and editing existing experiments. All inserted experiments can be viewed by default in a single matrix representation by clicking on the number of the experiments, displayed in the “cases” column of Fig. 1. A refinement of this option is described below.
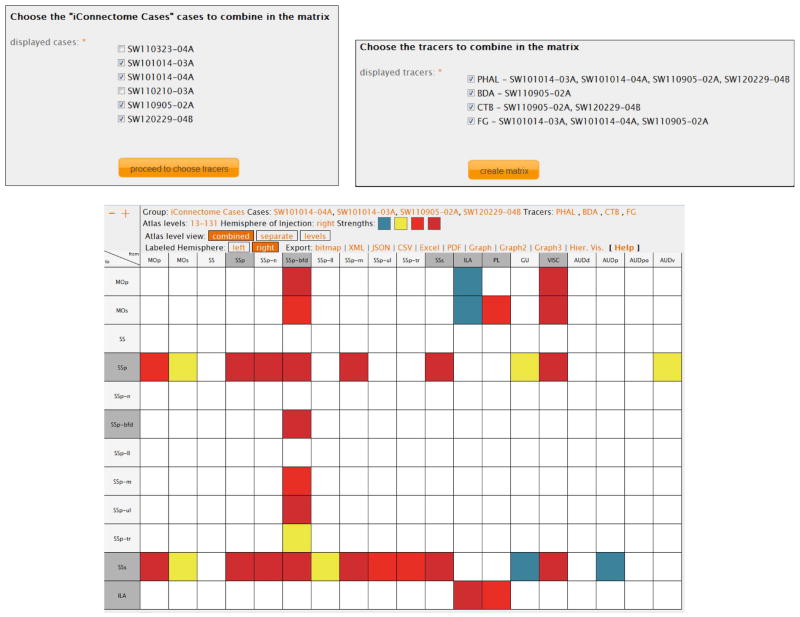
If a group includes more than one case, then an additional link, called “subset matrix”, which allows users to view only a subset of their inserted experiments, becomes available (Fig. 4 left inset). The group leaders can choose any number of experiments associated to a group, the default option being all experiments checked. After choosing the cases of interest, they can further refine their searches by choosing only the tracers of interest used in the previously selected experiments, as shown in the right inset of Fig. 4. The same functionality is available to the group members, but only restricted to their inserted cases.
Figure 4.
An example of Workspace functionality. Registered users can choose subsets of their inserted cases (left inset) and can further refine their search by choosing specific tracers (right inset). Only the connections associated with the selected cases and tracers will be displayed. The default display shows all this data in matrix format. See Text for details regarding the functionality of the icons displayed in the header of the matrix. The view mode of the data in this Figure is “combined”, which is encoded in the Atlas Level View header (white font and orange background).
The result of this refined search is a matrix containing the connection information for only the selected experiments and tracers, along with their associated injection sites. This search can also be used for user-driven analysis of cases of interest (see below). For faster identification, the injection sites are marked by a darker shade of gray (Fig 4).
The collated connectivity data can be viewed by registered users in three different ways: combined, separate, and Atlas Level (Plate), regardless of the number of selected experiments. The active view modality is encoded by the white font and the orange background color of the variable “Atlas level view” (Figs. 4–6), displayed above the connections matrix, while the other two modalities are written in black onto a gray background. The default mode view of experiments or combinations of them is referred to as “combined”. Registered users can switch the display views by clicking on the categories listed in the “Atlas level view” tab (Figs. 4–6).
Figure 6.
The display mode of data by Atlas Level. The connections matrix of five mouse experiments inserted in the Workspace, Atlas Level 43, Dong nomenclature. As in Figs. 4 and 5, this mode is encoded in the Atlas Level view header: white font and orange background. The inset shows the connections reports details across all chosen experiments (Fig. 5). See Text for details.
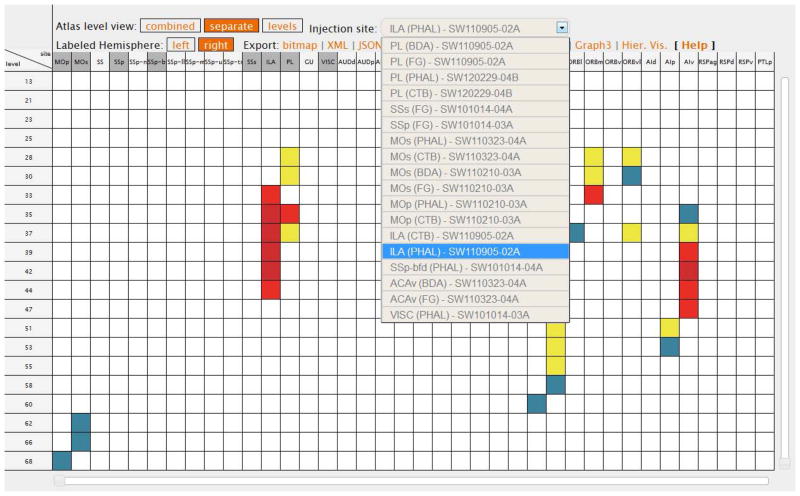
The combined view displays all data in a single matrix (Fig. 4). The separate view becomes available when the connectivity data have been collated on Atlas Levels or Plates for individual injections. This option displays all gray matter regions on the columns of the matrix, and all the Atlas Levels associated with at least one connection report, on rows, for a single injection site that can be chosen from a drop-down menu (Fig. 5). A colored cell in this view means that a terminal field was collated and annotated in the gray matter region shown on the column and in the Atlas Level on the respective row, for the experiment (injection) chosen from the drop-down menu.
Figure 5.
The separate display modality shows only the data associated with a certain injection site in a specific experiment, by Atlas Levels. All injection sites shown in the drop-down menu are marked by a darker shade of gray. Note the encoding for this view mode, in the Atlas Level view tab: white font and orange background. See Text for details.
This modality therefore allows users to view all terminal fields of a certain injection site across all Atlas Levels or Plates where label was found. This modality becomes particularly useful in double co-injection experiments where four tracers, two anterograde and two retrograde, are injected in a pair of gray matter regions (Thompson and Swanson 2010; Hintiryan et al., 2012). In this way, the results from each injection are shown separately, making them easier to view by users. It also can be used as a first level and user-driven identification of connectional patterns (analysis), because it enables identification of particular groupings of terminal fields over the specific sets of regions identified in a nomenclature (atlas), and across the entire plane used to section the nervous system. A set of Atlas Levels or Plates is defined in relation to a specific plane of sectioning used in neuroanatomy (Paxinos and Watson, 1998, Swanson, 2004, Dong, 2007). Because the majority of current neuroanatomical atlases are sectioned in the transverse plane, the separate view shows the terminal fields of an injection over the entire set of regions, rostrocaudally (Fig. 5). This view modality is unique to BAMS2, to our knowledge.
The third view mode for results of tract tracing experiments is by Atlas Level (Plate) (Fig. 6). This interface becomes functional only for those connections data sets that have been registered with standard neuroanatomical atlases Levels or Plates. The Workspace automatically reconstructs in a grid (matrix) format those regions displayed in each standard Level, displays the associated connections of any set of experiments included in a certain group. This modality becomes very useful for data aggregation across experiments in specific spatial locations, and for detailed analysis and comparison of connections patterns in different parts (e.g. rostral vs. caudal) of gray matter regions of interest.
For each displayed Atlas Level, the returned grid (matrix) is made of the associated output/origin and input/termination regions on the vertical and horizontal axes, respectively. For easier identification, the abbreviations of injection sites of all chosen experiments are displayed in a darker shade of gray. The connections recorded with the pair of regions on vertical and horizontal axes, for the specific Atlas Level, are shown as a colored cell. As with the other display modalities, the highest value of the displayed connection strength is shown. However, registered users have the option of viewing all their recorded connections reports for that specific Atlas Level, by clicking on the cells of the matrix (Fig. 6 inset).
The connectional data displayed in any combination of experiments or any Atlas Level can also be viewed as networks. For construction of networks of gray matter regions we used the light version of Cytoscape (http://www.cytoscape.org; Saito et al., 2012). Cytoscape is an open platform for visualizing complex networks, and it includes a desktop distributable, as well as a set of Javascript libraries. We implemented these libraries for the Force directed, graph r. (http://www.cytoscape.org; Cline et al., 2007; Saito et al., 2012), for both ipsilateral and contralateral connectivity data. Figure 7 shows an example of a network representation of connections associated with the rat sensory-motor cortex, as collated from BAMS. This graphic network representation adds another viewing dimension for recorded connectivity data, regardless of granularity (gray matter region, or Atlas Level). The reconstructed rat sensory-motor network shown in Fig. 7 will be discussed in detail in the Results section.
Figure 7.
A. The set of connectivity matrices (connectomes) available in the Classic BAMS (http://brancusi.usc.edu) that access the BAMS2 Workspace, as a third party source. All connectivity data reside in Classic BAMS. B. The connections of the rat sensory-motor cortex as defined in Swanson-04 nomenclature, in matrix representation, automatically created in Workspace. Code of colors: red – very strong connection; intense pink – strong connection; pink – strong/moderate connection, yellow – moderate connection; faded yellow – moderate/weak connection, intense blue – weak connection; faded blue – very weak connection, green – connection exists, but its strength is not assessed, black – connection shown to be absent, white – no data. C. The same set of connections represented as a network. The ILA, VISC and GU are displayed over a gray area. Code of colors: red – very strong, strong and moderate-strong connections, yellow – moderate and moderate/weak connections, blue – weak and very weak connections; green – connection exists but its strength is not assessed. See Text for details.
To date, BAMS2 Workspace has 20 groups and 47 cases, which include our own analyses of rat connections inserted in BAMS, the results of 6 experiments (1 group) of iConnectome, 6 experiments (1 group) of NeSys (http://www.nesys.uio.no), and 3 experiments (1 group) of the CSHL Mouse Brain Architecture Project.
5. Exporting connections data from Workspace and third party linking
The BAMS2 Workspace interface includes a comprehensive set of modalities for exporting back processed data to registered users and third parties. This functionality of the Workspace was designed so that export modalities to them are complementary to each other.
Any connectivity matrix constructed in the Workspace can be exported as a high resolution bitmap image, which can be customized by choosing the width of columns and rows. Any combination of experiments can be exported in PDF format, which has as columns the abbreviations of injection sites, the tracers, and the experiment/case ID’s. The rows are made of the entire set of terminal fields for the chosen set of experiments, and the qualitative connectional weights are written in literal format. Finally, the Excel and CSV formats export connectivity data in literal and numerical formats, respectively. Connectivity data exported in Excel format can be used for directly adding tables to manuscripts, while the CSV format is better suited for further processing and analysis.
The export formats to third parties are written in two of the most commonly used formats, XML and JSON. Either format allows seamless integration of BAMS Workspace experiments or combinations of experiments with other platforms. For example, JSON is used by iConnectome (see Fig. 4 left inset for the list of the cases collated into the Workspace) to display the collated mouse connectivity experiments in their viewer.
5.1 Connections matrices constructed by third parties
A more complex method of using the BAMS2 Workspace functionalities is described below. This method allows displaying connectional data stored on third party sites, and not in BAMS2’s database. First, the cross-platform communication is established using standard JSON (JavaScript Object Notation; http://www.json.org/). Documentation for the JSON syntax accepted by BAMS2, as well as script examples, are at the URL: http://brancusi1.usc.edu/grid_API. JSON files including the data and metadata necessary to create connectivity grids (matrices) can be instantiated using any programming language to access the third party backend databases. In other words, anonymous systems can use BAMS2′ functionalities by writing the outputs of queries of connections in JSON and using their query language of choice. Because the majority of public neuroinformatics platforms that include connectional reports (NIF, http://www.neuinfo.org; CoCoMac, http://cocomac.g-node.org; Temporal-Lobe, http://www.temporal-lobe.com) have interfaces and functionalities written in PHP (http://www.php.net), we have created a cross-platform set of scripts written in PHP that can be downloaded from the URL provided above. This object-oriented library is generic and includes no details of the third party systems, thus allowing it to be recoded in any programming language (for example, Python; http://www.python.org).
To demonstrate this feature, we implemented cross-platform communication using Classic BAMS (http://brancusi.usc.edu/) to generate a set of networks involving rat CNS connections (connectomes), extracted from the backend database of the Classic BAMS (Fig. 7A). All networks are freely accessible.
The result of calling of any of the links listed in Fig. 7A will be a grid (case) constructed de novo by the interface of the Workspace. Figure 7 shows the connections of the rat sensory-motor cortex from the data collated in BAMS in matrix (Fig. 7B), and network (Fig. 7C) formats, respectively. As with other matrices and networks constructed in Classic BAMS (Bota et al., 2005, Bota and Swanson, 2012), the rat CNS connectomes display the highest strengths of connections collated in BAMS. However, the reports associated with each of the connections graphically reconstructed can be accessed by clicking on the cells of the matrices. There are 9 such rat connections matrices (connectomes) that have been created in the Classic BAMS to date, and which can be directly accessed at the URL: http://brancusi.usc.edu/connectomes/standard_rat.php.
Perhaps the most important feature of this method is that the connectivity data displayed in matrix format in Fig. 7 is not recorded in BAMS2, but is kept on the client’s side. This function of BAMS2 Workspace is agnostic of the nomenclature used by the client, and of species. Thus, it can be used to display and further process connectivity data in any species and it can be instantiated to handle other datatypes, such as gene expression ranked qualitative assertions.
Finally, BAMS2 Workspace includes a simple, autocomplete search form by names of groups and by injections sites. The result of this search will return those cases that are associated with the searched group and injection site. The search is made over all cases inserted by the user, therefore it will return both the results of fiber tracing experiments, and the cases constructed from preprocessed connections data. This simple functionality become particularly useful to those registered users that inserted many cases in the Workspace.
Results
To demonstrate the two methods of construction of connections matrices implemented in the Workspace and described in the previous section, we created two neuronal networks from the Classic BAMS connections data. The first was constructed as an individual group and case in the Workspace, while the second network was created directly in the Classic BAMS, using the third party library described above.
1. The rat medial frontal cortex and amygdala network
This network includes the connections between the rat amygdala and a set of cortical areas named here for convenience the “medial frontal cortex”. All gray matter regions included in this analysis are defined in the Swanson-04 nomenclature (Swanson, 2004). The rat medial frontal cortex includes the prelimbic area (PL); infralimbic area (ILA); lateral, medial, ventral, and ventrolateral parts of the orbital area (ORBl, ORBm, ORBm, ORBvl, respectively); dorsal and ventral and parts of the anterior cingulate area (ACAd, ACAv, respectively); and secondary somatomotor areas (MOs). The amygdalar gray matter regions included in the analysis are those defined in the Swanson-04 nomenclature (Petrovich and Swanson, 1998; Swanson, 2004). The entire set of nodes used in this example is formed by 31 gray matter regions.
Construction of the gray matter region level macroconnectivity network used more than 2,000 reports expertly and manually collated and then inserted in BAMS, from more than 100 published original research references. To our knowledge, this is the most complete set of rat connections involving the medial frontal cortex and amygdalar region (MFC-AMY) that has been compiled in a public neuroinformatics platform from the published literature.
Because the pathway tracing literature is rife with contradictory reports, and the results of a neuroanatomical pathway tracing experiment depend both on the size and relative position of the injection site with respect to the region of interest (and, of course, on the method used), we manually and expertly evaluated each connection of the rat MFC-AMY recorded in BAMS and included in this analysis. For reliability assessments of neuroanatomical connections as a function of methods used see Bota et al. (2003, 2005). The method employed in collating data and metadata in BAMS from the published literature is described in detail in Bota et al. (2005, 2012). We describe now the method of manually pre-processing MFC-AMY connectivity data extracted from the system.
In the triage phase, we eliminated all connectivity reports recorded in BAMS that are associated with the Nauta (Nauta, 1952) and Fink-Heimer degeneration techniques (Fink and Heimer, 1967), and any of their variants (Nauta and Gygax, 1951). We also eliminated all connectivity reports based on electrophysiological experiments. Then, we took into account only those experiments with large tracer injections centered in the region of interest with only minimal spread beyond its borders. We also included the results of small injection sites restricted entirely within particular MFC-AMY regions. After this initial triage, all reports were ordered by their strengths and techniques. The most accurate connections included in the present analysis were those revealed by anterograde Phaseolus vulgaris leucoagglutinin (PHAL) experiments and confirmed by retrograde tracer experiments.
After this initial triage, all reports were ordered by their strengths and techniques. The connections included in the present analysis were first those revealed by Phaseolus vulgaris leucoagglutinin (PHAL) experiments that were confirmed by retrograde tracer experiments. If a connection between a pair of regions was not reported by PHAL experiments, we added a second subset, which was made of those connections reported in experiments that used as anterograde tracers biocytin and cholera toxin subunit b (CTB), and True Blue, Fast Blue and different amines (e.g., Rhodamine, Tetramethylrhodamine dextran amine, Biotinylated dextran amine, etc.) as retrograde traces. The second subset also included connections revealed by tracers that label both anterogradely and retrogradely, such as Fluororuby, or selenite salts. Finally, the third and least accurate subset included connections based on the anterograde autoradiographic method or the anterograde/retrograde horseradish peroxidase (HRP) method, used alone, or in combination with wheat germ agglutinin (WGA).
We followed the same procedure for evaluating connections shown to be absent in the recorded literature. Finally, we considered only those connections with highest strengths for each pair of regions, ordered and evaluated as described above. This manually evaluated set of connections was inserted in BAMS2 Workspace as a separate group and case that had the nomenclature made of the regions of MFC-AMY, ordered as in the rat nomenclature Swanson-04 (http://brancusi1.usc.edu/connections/grid/122/). The matrix representation of the rat MFC-AMY is shown in Figure 8A. The size of this matrix is 31×31 regions, having thus 961 cells, each one representing a connection from the region on the associated column to the region on the associated row. The degree of coverage of this network is 77%.
Figure 8.
The matrix and network representations of rat MFC-AMY connections. A. Matrix representation. The code of colors is identical to that in Figure 8B. B. Network representation of MFC-AMY color and line width encoded. The color code is identical to that in Figure 8C. Weak connections have 1pt width, moderate connections 2, and strong connections 3pt. Connections just assessed as “exists” have 1 pt width. C. The same network, only width encoded. D. MFC-AMY network reconstructed in BAMS, showing only those connections that are at least moderate in strength (Bota and Swanson, 2007). See Text for details.
The MFC-AMY system is displayed as a network representation in two formats using the web version of Cytoscape’s Force directed algorithm: the qualitative strengths of connections are either color and line width encoded (Fig. 8B) or only line width encoded (Fig. 8C). Finally, Figure 8D displays the same network automatically constructed in Classic BAMS (Bota and Swanson, 2007).
In all three directed network representations, the most connected medial frontal gray matter region is the ILA, followed by the PL. The force directed algorithm (Fig. 8B, C) associates a gravitational field to each node and edge (reference). The initial configuration of nodes (gray matter regions) is random, but the nodes of the network with the most number of common edges (connections) will tend to group together, while the nodes with the least number of edges will tend to occupy the periphery of the configuration. The latter is true for the ORBm, ORBv, and ACAv. However, the connections of these three regions, and especially the ORBm and ORBv, have not been thoroughly analyzed (Vertes, 2011). Therefore, the configuration of this network may change with future published pathway tracing experiments inserted in BAMS.
For the amygdalar region, the most connected node is the anterior part of the basolateral nucleus (BLAa) followed by its posterior part (BLAp). The least connected amygdalar regions are the bed nucleus of the accessory olfactory tract (BA), the anterior amygdalar area (AAA), and intercalated amygdalar nuclei (IA; Fig. 8). These three amygdalar regions do not send established connections to the medial frontal region. The single medial frontal region that sends outputs (projects) to all these amygdalar regions is the ILA. However, these results are also likely to change when the entire published literature of pathway tracing experiments in rats is recorded in BAMS.
Because the MFC-AMY matrix includes many connections with the ranked qualitative strength “exists” (Bota et al., 2005), and not all relevant original research literature has been inserted to date, we reduced the matrix to give a more restricted and complete description. For this, we constructed the network of interactions between rat medial frontal cortex as defined above and those amygdalar gray matter regions included in the cerebral cortex, as defined in the Swanson-04 nomenclature. A subset of rat amygdalar gray matter regions is included in two subdivisions of the cerebral cortex: the sensory-motor cortex of the cortical plate, and the cortical subplate. The matrix and network representations of this reduced circuit are shown in Figure 9.
Figure 9.
The reduced rat MFC-AMY network in matrix (A) and network (B) representation, respectively. The code of colors is identical to those in Figs. 8 and 9. See Text for details.
This macrocircuit consists of 21 gray matter regions (nodes) and 441 connections (edges) and its coverage is 92%, making it one of the most complete networks constructed from the data recorded in BAMS. Because the aim of this paper is to describe the BAMS2 Workspace as neuroinformatics resource, and the reduced MFC-AMY network is not 100% complete (Fig. 10A), we will describe below only the connections of the primary motor areas (Mop), ILA, PL, and ACAd to and from the amygdalar regions of this circuit.
As in the entire MFC-AMY circuit, the most connected medial frontal cortical regions are the ILA and PL. The ILA has connections to and from all cortical amygdalar gray matter regions or areas. In contrast, the PL sends moderate-to-weak outputs (projections) to any amygdalar regions generally included in the main and accessory olfactory systems (Haberly and Price, 1978; Luskin and Price, 1983; Swanson and Petrovich, 1998): the piriform-amygdalar area (PAA), the anterior part of the cortical amygdalar area (COAa), the lateral and medial zones of the posterior part of the cortical amygdalar area (COApl, COApm, respectively), the postpiriform transitional area (TR), and the posterior amygdalar nucleus (PA). The only exception is a weak projection to the nucleus of the lateral olfactory tract (NLOT).
The third medial frontal cortical region that receives substantial but restricted amygdalar output is the MOs, which in turn sends outputs only to three amygdalar gray matter regions: the lateral amygdalar nucleus (LA), BLAa, and BLAp. Moderate to strong amygdalar outputs to the MOs arise from the BLAa, BLAp, and BMAp, but not from the LA, and weaker outputs to the MOs arise from the PAA and PA. PAA is the single node included in the rat main olfactory-vomeronasal (accessory olfactory) system (Canteras et al., 1992; Swanson 2004) that projects weakly to the MOs. Thus, amygdalar components of the main olfactory-vomeronasal system do not generally communicate directly with the MOs. This implies that MOs premotor body representations (Reep et al, 1984) do not integrate main olfactory and pheromonal information directly via the amygdalar region, and that premotor input is not processed in main olfactory-vomeronasal components of the amygdalar region.
More generally, connections between MOs and amygdalar region can be characterized as asymmetrical with respect to sets of amygdalar regions receiving and sending connections, and to the relative strengths associated with these connections. As mentioned above, the BLAa and BLAp are the only two amygdalar gray matter regions that share bidirectional connections with the MOs. The projections of the BLAa and BLAp to the MOs are at least moderate-strong, and they seem to arise from a population of neurons distributed throughout the entire BLAa, and the rostromedial half of the BLAp (Sripanidkulcai et al., 1984; Conde et al., 1995; Hoover and Vertes, 2007; our annotation). However, outputs of the MOs innervate only the rostral half of the BLAa, and a continuous rostromedial zone of the BLAp extending from the lateral border of the BLAa (Reep et al., 1984; McDonald et al., 1999; our annotation). Thus, the bidirectional connections between the MOs and the BLAa and BLAp can be characterized as topographically asymmetrical: neurons that both receive axons from, and send axons to, the MOs appear to lie within the BLAa and rostromedial BLAp, whereas neurons that innervate the MOs but do not receive inputs from the MOs are located in the rostrolateral and caudal BLAp, as well as in the BLAa. One should mention here that the BLAa may also receive an additional weak input from the primary motor cortex while BLAp does not receive any input from it (Ottersen, 1982, Reep et al., 1987).
Finally, the ACAd does not send, nor receive axons to and from the amygdalar regions of the olfactory-vomeronasal system. Instead, it moderately projects to the BLAa and BLAp, and receives strong projections from BLAa, BLAp and from the BMAa (Hoover and Vertes, 2007).
To summarize, the connections of the reduced MFC-amygdala network follow the general topographical-hodological dissociation between the “limbic” and the more sensory-motor parts of the cortex (Hoover and Vertes, 2007). The ILA sends and receives connections to and from all amygdalar nuclei included in this analysis. The connections patterns of the PL and the ORBm can be considered as complementary: the PL has a restricted set of targets of the cortical part of the amygdala, but receives inputs from both the olfactory-vomeronasal and cortical amygdalar regions. Conversely, the ORBm sends connections to several nuclei of the olfactory amygdala and to the cortical amygdala, but receives major connections only from the COApl and BLAa (Fig. 10A). More dorsally and dorsolaterally, the ACAd and MOs have reciprocal connections only with the anterior and posterior divisions of the BLA. Thus, we note here a general trend of more restricted sets of connections of the cortical areas with the olfactory-vomeronasal and cortical amygdalar nuclei, ventromedially to dorsolaterally.
2. The rat sensory-motor cortex network
The second example described here is the connectivity of the rat sensory-motor cortex as defined in the Swanson-04 nomenclature, directly created from the BAMS data. The connections of the rat sensory-motor cortex network have the maximal values recorded in BAMS. As shown in Fig. 8B-C, both the matrix and circuit representations of the sensory-motor regions connections indicate they are grouped in two basic modules. The first module is made exclusively of the regions processing the olfactory-vomeronasal information (Fig. 8C). The second module includes all the other sensory modalities and the primary (MOp) and secondary motor cortices (MOs), the ILA, the gustatory areas (GU), and the visceral cortex (VISC). Within the second group one may observe the dense and relatively strong connectivity patterns between somatosensory, visual, and auditory areas. There are at least two functional implications of this grouping: 1. the cortical processing of the sensory input is multimodal from its early stages at least for the rat; 2. the ILA, GU and VISC integrate multimodal somatosensory information. Hence, a possible set of experiments testing neurons for multimodal properties in the sensory and in the insular (GU, VISC) regions of the second module, is warranted. In fact, such experiments have been performed (Brett-Green et al., 2004, Rodgers et al., 2008, Kimura et al., 2010), but we do not know of any large scale, comprehensive, and detailed approach except the AIBS project for the mouse visual system (Koch and Reid, 2012).
A third observation with respect to the cortical sensory-motor cortex network is that there are only three cortical regions that share connections with both sensory-motor modules: ILA, GU, and VISC. Network-wise, these three regions may be interpreted as a cortical interface between the two sensory-motor modules. Here one should also note the presence of the cardiovascular pressor and depressor sites in the dysgranular insular cortex, which corresponds to the VISC (Yasui et al., 1991, Swanson, 2004; our annotation).
Finally, and perhaps most importantly, two of the networks discussed in detail here, the reduced MFC-AMY circuit and the sensory-motor cortex circuit, support each other in the assertion of two parallel systems of sensory information processing in the rat cerebral cortex. The only direct connections between MOp and MOs and olfactory related regions are a projection from the MOp to the ventral part of the taenia tecta (TTv), a weak projection from PAA to MOs, and a projection from the dorsal part of the taenia tecta (TTd) to the MOs.
Discussion
Any mature publicly available neuroinformatic platform should include more than the basic functionalities for recording and displaying data and metadata. Such platform has to allow registered or anonymous users to handle data in different ways, to both analyze and synthesize them. Moreover, a mature neuroinformatic platform has to assist them to detect or infer new patterns in the inserted data.
The BAMS2 Workspace is unique in several aspects. It is the first neuroinformatic platform that includes both the infrastructure and the visual interface necessary to enable registered users to simultaneously collate data and construct connectivity matrices, to our knowledge. These generic visual interfaces can be used to handle connectivity data in any species and can be associated with any neuroanatomical nomenclature. Registered users can use the Workspace to combine experiments of interest, to display data by Atlas Levels (Plates), and to export the processed connections data as complex reports in CSV, Excel and PDF formats. Moreover, the inserted connections data can be visualized in networks (circuits) formats, automatically created by freely available tools. These tools use publicly accessible algorithms that tend to group the regions of interest according to their common sets of connections. Thus, the circuits produced inside the Workspace are a first step toward automatic analysis of connections.
The connectivity data collated in BAMS can be used with the Workspace interface to visualize and describe the input and output patterns of regions of interest. As shown above, users can construct within the Workspace customized sets of gray matter regions names as nomenclatures and use them to create their own groups and cases as well as share data among group members. We presented and described above the examples of the rat MFC-AMY network manually extracted and processed from the Classic BAMS, and of the rat sensory-motor cortex that was automatically created from the data stored in the backend database of the Classic BAMS. These results may be construed not so much as novel findings, but more as confirmation and synthesis of the rat connectivity data reported in the published rat primary research literature that was comprehensively and systematically manually inserted in a neuroinformatic platform (BAMS).
Since the sensory-motor cortex network was automatically constructed from the publicly accessible database of BAMS used as a third party system, it can be replicated by any user, and therefore this knowledge is accessible to the entire community. Moreover, as described above, the scripts for third party access to the BAMS Workspace matrices and circuits interfaces can be implemented in any system that stores connectivity data and similar descriptions can be made for functionally meaningful connectivity sets, regardless of the species. However, the interpretation of the data is dependent on the set of brain regions of interest. For example, the networks described here may change if the connections of the polymodal association cortical regions (Swanson, 2004) are added.
Most importantly, the two rat neuronal networks discussed here are examples of user-driven analysis of connections data aided by a neuroinformatic platform. Conversely, the tools of BAMS Workspace have been designed to assist both its registered users and the neuroscience community to synthesize, and analyze brain-region level connections data sets. The new display modalities of connections data by Levels, and the possibility of combining the results of different experiments and injections in the same grid (matrix) format were specifically designed to help detection of novel patterns, and thus identify specific spatial locations for new experiments. Finally, to address the problem of replication of our analysis by the neuroscience community, we implemented a method of connections data and metadata export in the Classic BAMS. The results of search for connections by references in Classic BAMS can be downloaded by anonymous users in Excel tabular formats, which include reference details, and the following details of the retrieved reports: the output and input regions, the qualitative connections strengths, the tracer (technique), and the connection description as collated from the associated reference. Hence the connections data recorded in Classic BAMS can be anonymously downloaded, and subsequently processed either as described above, or in any other way. Finally, the processed sets of connections can be inserted in the BAMS2 Workspace as described in this article.
We continuously add, update, and refine the BAMS2 Workspace functionalities. One direction of development is related to the statistical processing of connectivity data. In the near future, the Workspace will include algorithms for automatic clustering and network analysis (Sporns et al., 2007, Langferder et al., 2008, Kaiser, 2011). For implementation, we will use the programming language R (Erkstrom, 2012), which is one of the most developed freely available tools for statistical analysis. A second direction of development will be the implementation of interfaces for automatic and user-guided evaluation of neuroanatomical experiments. These evaluations will take into account the relationships of injection sites with the regions of interests, the reliabilities of the employed techniques (Bota et al., 2003), as well as statements related to similar experiments. Finally, the Workspace interface described here will be extended to allow handling gene expression patterns and refined in such way that will allow matrix and networks representations of zones of brain regions (e.g., the medial and lateral parts of the BLAa and BLAp, cortical layers).
Acknowledgments
M.B. and L.W.S. are supported by the National Institutes of Health Grant NS050792. H.H and H-W.D are supported by the National Institutes of Health Grant 5R01MH094360-02.
Footnotes
M.B, H.H. and H-W.D. annotated and collated connectional data in BAMS2 Workspace, S.T. and M.B designed and implemented it, and all authors had equal participation to writing the paper.
The authors declare no conflicts of interests.
Literature cited
- Allen Brain Institute Mouse Connectivity Project. http://connectivity.brain-map.org.
- Bakker R, Wachtler T, Diesmann M. CoCoMac 2.0 and the future of tract-tracing databases. Front Neuroinform. 2012;6 doi: 10.3389/fninf.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bota M, Dong H-W, Swanson LW. From gene networks to brain networks. Nat Neurosci. 2003;6 :795–799. doi: 10.1038/nn1096. [DOI] [PubMed] [Google Scholar]
- Bota M, Dong H-W, Swanson LW. Brain architecture management system. Neuroinformatics. 2005;3:15–48. doi: 10.1385/NI:3:1:015. [DOI] [PubMed] [Google Scholar]
- Bota M, Dong HW, Swanson LW. Combining collation and annotation efforts toward completion of the rat and mouse connectomes in BAMS. Front Neuroinform. 2012;6 doi: 10.3389/fninf.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett-Green B, Paulsen M, Staba RJ, Fifková E, Barth DS. Two distinct regions of secondary somatosensory cortex in the rat: topographical organization and multisensory responses. J Neurophysiol. 2004;91:1327–1336. doi: 10.1152/jn.00905.2003. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Connections of the posterior nucleus of the amygdala. J Comp Neurol. 1992;324:143–179. doi: 10.1002/cne.903240203. [DOI] [PubMed] [Google Scholar]
- Cline MS, Smoot M, Cerami E, Kuchinsky A, Landys N, Workman C, Christmas R, Avila-Campilo I, Creech M, Gross B, Hanspers K, Isserlin R, Kelley R, Killcoyne S, Lotia S, Maere S, Morris J, Ono K, Pavlovic V, Pico AR, Vailaya A, Wang PL, Adler A, Conklin BR, Hood L, Kuiper M, Sander C, Schmulevich I, Schwikowski B, Warner GJ, Ideker T, Bader GD. Integration of biological networks and gene expression data using Cytoscape. Nat Protoc. 2007;2:2366–2382. doi: 10.1038/nprot.2007.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CoCoMac. http://cocomac.g-node.org.
- Condé F, Maire-Lepoivre E, Audinat E, Crépel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol. 1995;352:567–593. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- CSHL Mouse Brain Architecture Project. http://brainarchitecture.org/mouse.
- Dong HW. Allen Reference Atlas : A Digital Color Brain Atlas of the C57Black/67 Male Mouse. Wiley; Hoboken: 2007. [Google Scholar]
- Erkstrom CT. The R Primer. CRC Press; Boca Tato: 2012. [Google Scholar]
- Fink RP, Heimer L. Two methods for selective silver impregnation of degenerating axons and their synaptic endings in the central nervous system. Brain Res. 1967;4:369–374. doi: 10.1016/0006-8993(67)90166-7. [DOI] [PubMed] [Google Scholar]
- Fisk GD, Wyss JM. Descending projections of infralimbic cortex that mediate stimulation-evoked changes in arterial pressure. Brain Res. 2000;859:83–95. doi: 10.1016/s0006-8993(00)01935-1. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, van Weeghel R, Hersh LB, Luiten PG. Prefrontal cortical projections to the cholinergic neurons in the basal forebrain. J Comp Neurol. 1991;303:563–583. doi: 10.1002/cne.903030405. [DOI] [PubMed] [Google Scholar]
- Haberly LB, Price JL. Association and commissural fiber systems of the olfactory cortex of the rat. II. Systems originating in the olfactory peduncle. J Comp Neurol. 1978;181:781–807. doi: 10.1002/cne.901810407. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hintiryan H, Gou L, Zingg B, Yamashita S, Lyden HM, Song MY, Grewal AK, Zhang X, Toga AW, Dong HW. Comprehensive connectivity of the mouse main olfactory bulb: analysis and online digital atlas. Front Neuroanat. 2012;6 doi: 10.3389/fnana.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Projections of the medial orbital and ventral orbital cortex in the rat. J Comp Neurol. 2011;519:3766–3801. doi: 10.1002/cne.22733. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Zeng H. Genetic approaches to the neural circuits in the mouse. Ann Rev Neurosci. 2013;36:283–315. doi: 10.1146/annurev-neuro-062012-170307. [DOI] [PubMed] [Google Scholar]
- Kaiser M. A tutorial in connectome analysis: topological and spatial features of brain networks. Neuroimage. 2011;57:892–907. doi: 10.1016/j.neuroimage.2011.05.025. [DOI] [PubMed] [Google Scholar]
- Kimura A, Imbe H, Donishi T. Efferent connections of an auditory area in the caudal insular cortex of the rat: anatomical nodes for cortical streams of auditory processing and cross-modal sensory interactions. Neuroscience. 2010;166:1140–1157. doi: 10.1016/j.neuroscience.2010.01.032. [DOI] [PubMed] [Google Scholar]
- Koch C, Reid C. Observatories of the mind. Nature. 2012;483:397–398. doi: 10.1038/483397a. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Price JL. The topographic organization of associational fibers of the olfactory system in the rat, including centrifugal fibers to the olfactory bulb. J Comp Neurol. 1983;216:264–291. doi: 10.1002/cne.902160305. [DOI] [PubMed] [Google Scholar]
- Langfelder P, Zhang B, Horvath S. Defining clusters from a hierarchical cluster tree: the Dynamic Tree Cut package for R. Bioinformatics. 2008;24:719–720. doi: 10.1093/bioinformatics/btm563. [DOI] [PubMed] [Google Scholar]
- iConnectome Project. http://www.mouseconnectome.org/iConnectome.
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann N Y Acad Sci. 1999;877:309–338. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- Nauta W. Selective silver impregnation of degenerating axons in the central nervous system. Stain Technology. 1952;27:175–179. doi: 10.3109/10520295209105080. [DOI] [PubMed] [Google Scholar]
- Nauta W, Gygax PA. Silver impregnation of degenerating axon terminals in the central nervous system. Stain Technol. 1951;26:5–11. doi: 10.3109/10520295109113170. [DOI] [PubMed] [Google Scholar]
- NIF. http://neuinfo.org.
- Ottersen OP. Connections of the amygdala of the rat. IV: Corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horseradish peroxidase. J Comp Neurol. 1982;205:30–48. doi: 10.1002/cne.902050104. [DOI] [PubMed] [Google Scholar]
- Osten P, Margrie TW. Mapping brain circuitry with a light microscope. Nat Methods. 2013;10:515–523. doi: 10.1038/nmeth.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Reep RL, Corwin JV, Hashimoto A, Watson RT. Afferent connections of medial precentral cortex in the rat. Neurosci Lett. 1984;44:247–52. doi: 10.1016/0304-3940(84)90030-2. [DOI] [PubMed] [Google Scholar]
- Reep RL, Corwin JV, Hashimoto A, Watson RT. Efferent connections of the rostral portion of medial agranular cortex in rats. Brain Res Bull. 1987;19:203–221. doi: 10.1016/0361-9230(87)90086-4. [DOI] [PubMed] [Google Scholar]
- Rodgers KM, Benison AM, Klein A, Barth DS. Auditory, somatosensory, and multisensory insular cortex in the rat. Cereb Cortex. 2008;18:2941–2951. doi: 10.1093/cercor/bhn054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito R, Smoot ME, Ono K, Ruscheinski J, Wang PL, Lotia S, Pico AR, Bader GD, Ideker T. A travel guide to Cytoscape plugins. Nat Methods. 2012;11:1069–1076. doi: 10.1038/nmeth.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt O, Eipert P. neuroVIISAS: approaching multiscale simulation of the rat connectome. Neuroinformatics. 2012;10:243–267. doi: 10.1007/s12021-012-9141-6. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Sporns O, Tononi G, Kötter R. The human connectome: A structural description of the human brain. PLoS Comput Biol. 2005;1:e42. doi: 10.1371/journal.pcbi.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Honey CJ, Kötter R. Identification and classification of hubs in brain networks. PLoS One. 2007;2:e1049. doi: 10.1371/journal.pone.0001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripanidkulchai K, Sripanidkulchai B, Wyss JM. The cortical projection of the basolateral amygdaloid nucleus in the rat: a retrograde fluorescent dye study. J Comp Neurol. 1984;229:419–431. doi: 10.1002/cne.902290310. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Kamper L, Bozkurt A, Burns GA, Young MP, Kötter R. Advanced database methodology for the Collation of Connectivity data on the Macaque brain (CoCoMac) Phil Trans R Soc Lond B Biol Sci. 2000;356:1159–1186. doi: 10.1098/rstb.2001.0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugar J, Witter MP, van Strien NM, Cappaert NL. The retrosplenial cortex: intrinsic connectivity and connections with the (para)hippocampal region in the rat. An interactive connectome. Front Neuroinform. 2011;5 doi: 10.3389/fninf.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. A Laboratory Guide with Printed and Electronic Templates for Data, Models and Schematics. 3. Elsevier; Amsterdam: 2004. [Google Scholar]
- Swanson LW, Bota M. Foundational model of structural connectivity in the nervous system with a schema for wiring diagrams, connectome, and basic plan architecture. Proc Natl Acad Sci. 2010;107 :20610–20617. doi: 10.1073/pnas.1015128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–31. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Temporal-Lobe. http://www.temporal-lobe.com.
- Thompson RH, Swanson LW. Hypothesis-driven structural connectivity analysis supports network over hierarchical model of brain architecture. Proc Natl Acad Sci. 2010;107:15235–15239. doi: 10.1073/pnas.1009112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y, Breder CD, Saper CB, Cechetto DF. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol. 1991;303:355–374. doi: 10.1002/cne.903030303. [DOI] [PubMed] [Google Scholar]