Abstract
Background
Alcohol exposure during pregnancy results in an array of structural and functional abnormalities called Fetal Alcohol Spectrum Disorders (FASD). Alcohol dysregulates the exquisite coordination and regulation of gestational adaptations at the level of the uterine vasculature. We herein hypothesized that chronic binge-like alcohol impairs maternal uterine artery reactivity to vasoconstrictors and dilators and that alcohol-induced vascular dysfunction is dependent on the endothelium.
Methods
We utilized a once-daily binge alcohol (4.5 g/kg body weight) exposure paradigm (gestational day (GD) 7-17) in a pregnant rat model system and investigated primary uterine artery function in response to vasoconstrictors and vasodilators utilizing wire myography.
Results
Alcohol (peak blood alcohol concentration, 216 mg/dl) produced uterine vascular dysfunction in the absence of grossly observable growth deficits in maternal and fetal body weights, fetal crown-rump length and placental weight. Alcohol did not produce altered uterine vascular reactivity to α1 adrenergic agonist phenylephrine or the prostanoid thromboxane. However, alcohol specifically impaired endothelium-dependent acetylcholine (Ach)-mediated uterine artery vasodilation but exogenous endothelium-independent vasodilators like sodium nitroprusside exhibited no alcohol effect; Ach significantly decreased vessel relaxation (P=0.003; ↓pD2 (negative log molar Ach concentration producing the half maximum response), −7.004±0.215 vs. −6.310±0.208; EMax (maximal Ach response), 92% vs. 75%).
Conclusion
We conclude that moderate alcohol exposure impairs uterine vascular function in pregnant mothers. Alcohol specifically impairs endothelium-dependent agonist-induced uterine artery vasodilation. In summary, the maternal uterine compartment may play a significant role in the pathogenesis of FASD. Thus, the mechanistic targets of alcohol at the level of both the mother and the fetus need to be considered in order to develop effective therapeutic treatment strategies for FASD.
Keywords: FASD, alcohol, maternal, uterine, endothelium, vascular
INTRODUCTION
Alcohol exposure during pregnancy leads to a range of deficits in the developing offspring and is termed Fetal Alcohol Spectrum Disorders (FASD), a complex syndrome characterized by a range of structural (e.g. whole body growth restriction, craniofacial etc.) and functional (cardiovascular, endocrine, neurobehavioral etc.) abnormalities in the offspring (Riley and McGee, 2005, Ramadoss and Magness, 2012c, Sokol et al., 2003, Maier et al., 1999, Weinberg et al., 2008, Burd et al., 2007, Sawant et al., 2013). In spite of public health initiatives urging abstinence from alcohol during pregnancy, it is estimated that 2-5% of young children in the United States and Western Europe may suffer from FASD (May et al., 2009). Thus, it can be deduced that there is a need for deeper understanding of the mechanism of action of alcohol involving multiple organ systems in the mother, fetus, and the maternal-fetal interface (Cook et al., 2001, Ramadoss and Magness, 2011, Parnell et al., 2007).
Many FASD studies have primarily focused on alcohol-induced neuro-behavioral sequelae and few have examined the role of the mother particularly the maternal uterine vascular system in the pathogenesis of FASD (Cudd, 2005). In pregnant women, the maternal uterine artery regulates blood supply to the placenta where delivery of oxygen and nutrients take place. Following extraction of oxygen and nutrients into the fetal blood, the umbilical vein carries this blood into fetal systemic circulation. Uterine arteries are unique during pregnancy because over the course of a normal gestation, the uterine vascular resistance decreases by nearly 70 fold and the percent cardiac output to the uterus increases by 50 fold compared to the nonpregnant state (Rosenfeld, 1977, Magness, 1998) due to increased release of uterine artery endothelium-derived vasodilators (Ramadoss and Magness, 2012c, Magness, 1998). These maternal uterine vascular adaptations during pregnancy are critical for gas and nutrient delivery to the fetus and thus for normal development of the conceptus (Magness, 1998). Alcohol impairs these adaptations including uterine spiral artery remodeling (Gundogan et al., 2008), endothelial angiogenic gene expression (Ramadoss and Magness, 2012b), the endothelial proteome (Ramadoss and Magness, 2011, Ramadoss and Magness, 2012a) and blood flow (Falconer, 1990). However, no study has ever been conducted to test uterine vascular reactivity to vasoconstrictors or vasodilators following alcohol exposure. Even alcohol-related blood flow studies have been performed following a single acute dose of alcohol. Thus, there is a paucity of functional studies on effects of alcohol on the maternal uterine vascular system.
Therefore, we hypothesized that chronic binge-like alcohol exposure impairs maternal uterine artery reactivity to vasoconstrictors and dilators and that the vascular dysfunction is dependent on the endothelium. We herein utilized a moderate once-daily binge alcohol exposure in a rat model system and investigated uterine vascular function in response to pharmacologic agonists utilizing wire myography.
MATERIALS & METHODS
Animal protocols and alcohol dosing paradigm
All experimental procedures were in accordance with National Institutes of Health guidelines (NIH Publication No. 85–23, revised 1996) with approval by the Animal Care and Use Committee at the University of Texas Medical Branch at Galveston. Rats were housed in a temperature-controlled room (23°C) with a 12:12-h light-dark cycle with food and water available ad libitum. Timed pregnant Sprague-Dawley rats (gestation day (GD) 4) were purchased from Charles River (Wilmington, MA). Following acclimitization, on GD 7, rats were randomly divided into Control and Alcohol groups. A total of six animals in each treatment group were utilized for vascular function studies. Dams in the alcohol group received a moderate binge-like alcohol treatment of 4.5 g ethanol/kg body weight/day (22.5% w/v) via once-daily oral gavage from GD 7–17, (a total of 11 alcohol administrations) (Thomas et al., 2010, Thomas et al., 2008). The dose and pattern of exposure utilized in this study are highly relevant to pregnant women who consume alcohol during pregnancy (Church and Gerkin, 1988, Caetano et al., 2006, Gladstone et al., 1996, May et al., 2013). Daily feed consumption was monitored. Although based on the dose of alcohol utilized, the food intake, and growth parameters (example, maternal body weight, two way ANOVA, alcohol X GD effect, P=1.0; alcohol effect, P=0.97), a separate nutritional control group was not deemed necessary, we still measured growth measures to rule out effects of nutritional variables and so utilized six pair-fed rats that were each matched to an alcohol dam of similar weight with food intake correspondingly yoked. Further, the pair-fed dams received an isocaloric maltose dextrin solution through intragastric gavage to control for the calories derived from alcohol. Animals were sacrificed one day after the last alcohol exposure on GD 18 and maternal and fetal body weight, fetal crown-rump length, and placental weight were measured. We measured uterine vascular function at a time when substantial pregnancy-induced uterine artery adaptations and most pronounced vasodilation occur in the rat and before parturition starts (Ni et al., 1997, Scott et al., 2007, Alsip et al., 2000, Bruce, 1976, Dowell and Kauer, 1997). A separate cohort of pregnant rats (n=6) were utilized for blood alcohol concentration (BAC) measurement. For BAC studies, once daily alcohol was adminsitered beginning on GD 7. On GD 11 (fifth day of alcohol administration), blood from the orbital sinus was collected till 5h following alcohol administration. The plasma was separated (3,000 RPM, 4 °C, 15 min) and the alcohol concentration was measured using EnzyChrom™ Ethanol Assay Kit (ECET-100, BioAssay Systems).
Vascular function studies
Vascular reactivity studies were performed as previously described(Chinnathambi et al., 2013). In brief, one day after the last alcohol exposure on GD 18, rats were sacrificed by CO2 asphyxiation and the primary uterine artery was quickly removed and immersed in an ice-cold and oxygenated (95% O2-5% CO2) Krebs physiological saline solution (in mM; NaCl, 119; KCl, 4.7; CaCl2, 2.5; MgSO4, 1.17; NaHCO3, 25; KH2PO4, 1.18; EDTA, 0.026; and D-glucose, 5.5 (pH 7.4)). Arteries were cleaned of all surrounding adipose and connective tissues. Arterial segments of approximately 2 mm length were cut and mounted as ring preparations on two 25 μm tungsten wire on a wire myograph (DMT, Copenhagen, Denmark) for recording of isometric tension using Labchart software (AD Instruments, Colorado Springs, CO). As previously described, the wires were attached to the force transducer and the micrometer (Wang et al., 2000). Upon mounting, the uterine artery rings were warmed to 37 °C in Krebs buffer and allowed to equilibrate for 30 min. The ring internal circumference was set to give a wall tension of kPa0.9.
Morphologic measurement of vessel wall length was determined using a calibrated eyepiece with dissecting microscope. Arterial rings were stretched to their optimal tension using the normalization procedure; i.e. each vessel ring was placed under optimal stretch, equivalent to an in vivo arterial blood pressure using normalization software (Powerlab, ADInstruments, Colorado Springs, CO). As previously described, the internal circumference was set to 0.9 times the internal circumference of a relaxed vessel under in vivo condition under a transmural pressure of 100 mm Hg (Wang et al., 2000). Functional integrity of the vessels was assessed with 80 mM KCl solution. The uterine artery rings were exposed to KCl until depolarization-induced contractions could be reproduced. Arterial rings were equilibrated for at least 1 h before being challenged with pharmacological agents. After equilibration for a 60 min period, four different experimental protocols were followed to investigate effects of 1. phenylephrine, a potent α1 adrenergic receptor agonist and vasoconstrictor, 2) thromboxane, a prostanoid family vasoconstrictor, 3) sodium nitroprusside, an endothelium-independent exogenous vasodilator, and 4) acetylcholine, an endothelium-dependent vasodilator. The pharmacologic agonists were studied in the following order: phenylephrine, thromboxane, acetylcholine, and sodium nitroprusside with a wash period and equilibration time of 15 min between protocols.
Endothelium-intact uterine arterial rings were stimulated with increasing concentration of phenylephrine (10−9 to 10−4 M) followed by thromboxane (10−10 to 10−5 M). The bath was rinsed with Krebs buffer three times and allowed to equilibrate for 15min between each protocols. A cumulative concentration-response curve (CRC) was constructed to determine the negative logarithm of the molar concentration of the agonist that produced 50% (pD2) of the maximum response (Emax). To investigate endothelium-dependent relaxation, vessels were pre-contracted by incubating them with 10−7 M thromboxane, and vascular relaxation was measured by adding cumulatively increasing concentration of acetylcholine (10−10 to 10−5 M). Similarly, sodium nitroprusside (10−10 to 10−4 M) was then used to assess the endothelium-independent relaxation response in uterine arterial rings.
Statistical Analysis
Data are presented as Mean ± SEM. Maternal body weight was analyzed by one way ANOVA and fetal growth measures were analyzed by two way ANOVA with numbers in litter and treatment groups as two independent factors. For uterine vascular reactivity studies, a twoway ANOVA was performed with alcohol as the between factor and the dose of the agonist as the within factor. Level of significance was established a priori at P<0.05.
RESULTS
Effects of alcohol exposure on growth parameters
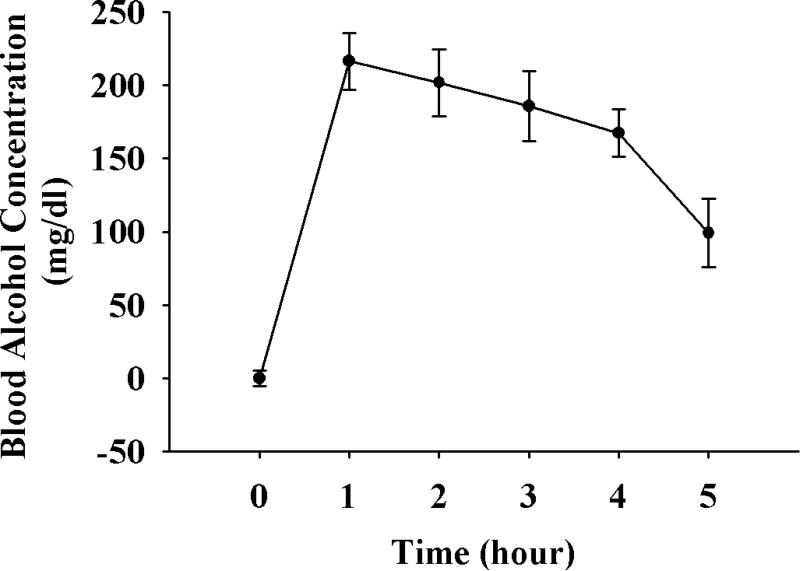
Peak BAC was measured in a separate cohort of rats upto 5 h following alcohol administration. The peak BAC was 216 mg/dl on GD 11 (Figure 1). Animals were sacrificed on GD 18, one day after the last chronic once-daily binge alcohol administration paradigm between GD 7-17. Maternal body weight, fetal body weight, placental weight, and fetal crown-rump length were not different among Control, Pairfed and Alcohol treatment groups. A litter effect was also not observed.
Figure 1.
Blood Alcohol Concentration (BAC) measurement. Once daily alcohol (4.5 g/kg body weight) was adminsitered beginning on GD 7. On GD 11 (fifth day of alcohol administration), blood from the orbital sinus was collected till 5h following alcohol administration for BAC measurement.
Vasoconstrictor responses
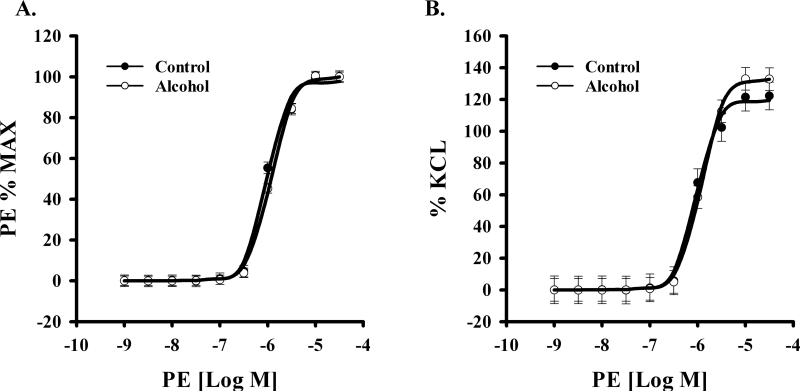
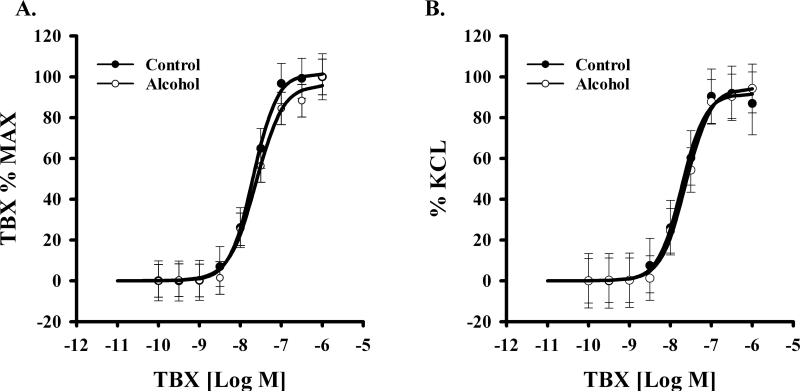
We examined the effect of alcohol on uterine vascular dysfunction in pregnant rats. Alcohol treatment of pregnant rats during gestation, did not alter phenylephrine-induced contractile response in uterine arteries between groups (Figure 2). Data were expressed as the percentage of maximum phenylephrine contraction (% max) or as percentage of KCl (to produce initial contraction) response (%KCl). Neither an interaction of phenylephrine dose and alcohol effect (% max, p=0.652; % KCl, p=0.960) nor a main effect of alcohol (% max, p=0.4; % KCl, p=0.54) was detected. A significant phenylephrine dose effect was noted (% max & % KCl, P<0.001) in both Control and Alcohol groups. Likewise the effect of thromboxane on uterine artery contractile response was not altered between groups (Figure 3). Neither an interaction of thromboxane dose and alcohol effect (% max, p=0.995; % KCl, p=1.000) nor a main effect of alcohol (% Max, p=0.331; % KCl, p=0.85) was detected. A significant thromboxane dose effect was noted (% Max & % KCl, P<0.001) in both Control and Alcohol groups.
Figure 2.
Effect of in vivo chronic once-daily binge alcohol exposure (GD 7-17) to pregnant rats on α1 adrenergic phenylephrine (PE)-induced maternal uterine artery contraction. A main effect of alcohol (% max, p=0.4; % KCl, p=0.54) was not detected. A significant PE dose effect was noted (P<0.001) in both Control and Alcohol groups. Data were expressed as the percentage of maximum PE contraction (% max) or as percentage of KCl. Values are expressed as Mean ± SEM.
Figure 3.
Effect of in vivo chronic once-daily binge alcohol exposure (GD 7-17) to pregnant rats on thromboxane (TBX)-induced maternal uterine artery contraction. A main effect of alcohol (% Max, p=0.331; % KCl, p=0.85) was not detected. A significant TBX dose effect was noted (P<0.001) in both Control and Alcohol groups. Data were expressed as the percentage of maximum TBX contraction (% max) or as percentage of KCl. Values are expressed as Mean ± SEM.
Endothelium-independent vasodilatory response
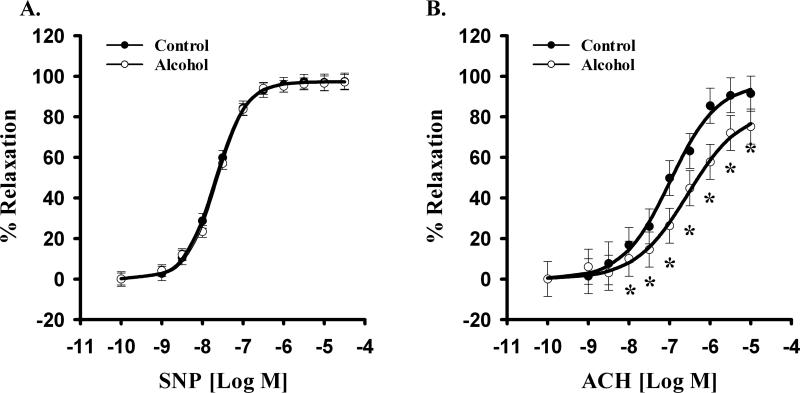
Further, alcohol did not alter endothelium-independent sodium nitroprusside-induced relaxation (Figure 4A). Neither an interaction of sodium nitroprusside dose and alcohol effect (p=0.998) nor a main effect of alcohol (p=0.648) was detected. A significant sodium nitroprusside dose effect was noted (p<0.001) in both Control and Alcohol groups.
Figure 4.
Effect of in vivo chronic once-daily binge alcohol exposure (GD 7-17) to pregnant rats on maternal uterine artery relaxation. (A) Alcohol did not alter endothelium-independent sodium nitroprusside (SNP)-induced relaxation. A significant SNP dose effect was noted (p<0.001) in both Control and Alcohol groups. (B) Alcohol selectively impaired uterine endothelium-dependent acetylcholine (ACh)-induced relaxation. A significant main effect of alcohol (P=0.003) and ACh dose effect were noted (p<0.001). Values are expressed as Mean ± SEM.
Endothelium-dependent vasodilatory response
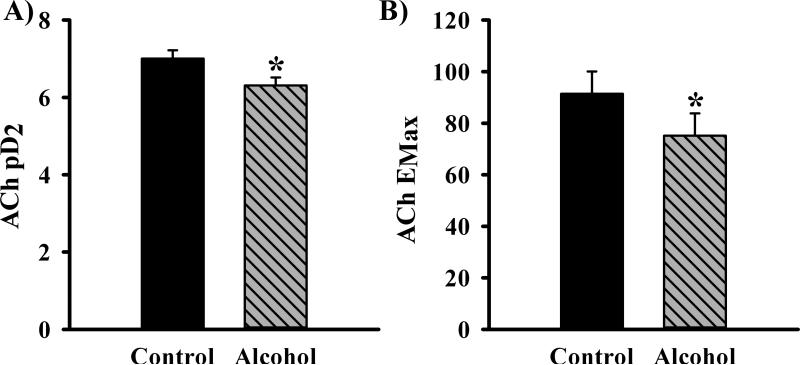
Alcohol selectively impaired uterine endothelium-dependent relaxation. A significantly decreased acetylcholine-induced vasodilation was noted in the Alcohol group compared to the Controls (Figure 4B). A significant main effect of alcohol and acetylcholine dose were noted (alcohol main effect, P=0.003; acetylcholine dose effect, p<0.001). Alcohol produced a rightward shift in the acetylcholine-induced uterine artery vasodilatory response. The sensitivity to acetylcholine was decreased in alcohol treated-pregnant rats (↓pD2, −7.004±0.215 vs. −6.310±0.208; Figure 5A). Further, alcohol decreased the maximal vascular response to acetylcholine (EMax, 92% vs. 75%; Figure 5B). These data show for the first time that alcohol exposure during pregnancy leads to uterine artery endothelium-dependent vasodilatory impairment.
Figure 5.
(A) In vivo chronic once-daily binge alcohol exposure (GD 7-17) to pregnant rats resulted in decreased uterine artery sensitivity (pD2) to endothelium-dependent acetylcholine (ACh)-induced relaxation (↓pD2, −7.004±0.215 vs. −6.310±0.208) (B) The maximal uterine vascular responses to Ach (EMax) was also decreased in alcohol treated-pregnant rats (Emax, 92% vs. 75%) Values are expressed as Mean ± SEM.
DISCUSSION
The main goal of the study was to determine the effect of alcohol exposure on maternal uterine artery function. The major findings of the present study are: 1) A moderate dose of alcohol produces maternal uterine vascular dysfunction in the absence of gross growth deficits such as maternal body weight, fetal body weight, fetal crown rump length, and placental weight; 2) There were no alcohol effects on uterine artery vasoconstrictive actions of α1 adrenergic agonist phenylephrine or the potent prostanoid thromboxane; 3) Alcohol specifically impaired endothelium-dependent acetylcholine-mediated uterine artery vasodilation but exogenous endothelium-independent vasodilators like sodium nitroprusside exhibited no alcohol effect.
As expected, the moderate doses of alcohol utilized in this study did not lead to any deficit in fetal whole body growth parameters (weight and crown-rump length) and placental mass. Although the focus of this study is on effects of alcohol maternal uterine vasodilation, a dose of 4.5 g/kg of alcohol administered to maternal rats till GD 20 is reported to produce significant loss of cerebellar and olfactory granule neurons in the offspring (Maier and West, 2001). Although FAS, an extreme form of the span of deficits produced by FASD is characterized by prenatal growth restriction (Sokol et al., 2003), low and moderate alcohol exposure levels may not lead to grossly observable growth deficits in humans (Henderson et al., 2007), rats (Rosenberg et al., 2010), and sheep (Ramadoss et al., 2007). However, it should be noted that it is possible that if the duration of alcohol exposure is longer than that utilized in this study (GD 7-17), or if a higher dose (6g/kg) that is utilized in many other FAS studies (Thomas et al., 2009, Thomas et al., 2010), gowth deficits are likely to be observed. Nonetheless it is interesting that maternal uterine vascular dysfunction occurs even in the absence of feto-placental growth deficiency.
The current study demonstrates that alcohol exposure during pregnnacy leads to decreased uterine vasodilation. Uterine arteries exhibit substantial decreases in their resistance over the course of gestation leading to about 50 fold increase in blood flow to the uterus (Rosenfeld, 1977, Magness, 1998). Studies have reported that alcohol impairs uterine spiral artery remodeling (Gundogan et al., 2008), angiogenic gene expression (Ramadoss and Magness, 2012b), endothelial proteomic profile (Ramadoss and Magness, 2011, Ramadoss and Magness, 2012a) and blood flow (Falconer, 1990). In summary, alcohol has negative impact on the maternal uterine artery adaptations during pregnnacy.
We herein report for the first time that alcohol does not impair uterine artery vasoconstrictive actions of α1 adrenergic agonist phenylephrine or the prostanoid thromboxane. Phenylephrine is a powerful vasoconstrictor that produces arterial constriction by its action on the α1 adrenergic receptors located on the vascular smooth muscle cell. Thromboxane is a critical endogenous modulator of vascular tone (Feletou et al., 2011). Endothelium-derived thromboxane is produced by the catalytic activity of prostaglandin H synthase and thromboxane synthase which subsequently acts on its receptors on the vascular smooth muscle cell and produces vasoconstriction through coupling to Gq/11 or G12/13 and phospholipase C pathway (Ellinsworth et al., 2014). We herein report an absence of alcohol effect on phenylephrine and thromboxane-induced maternal uterine artery vasoconstriction. Similarly, in adult male rats, daily alcohol (7.2% v/v) administration for four weeks resulted in no difference in phenylephrine-induced vasoconstrictor response (EMax or pD2) in the aorta (Utkan et al., 2001). Pregnant mice fed alcohol (25% ethanol-derived calories) between GD 6-18 did not exhibit any difference in phenylephrine-induced EMax but blunted the sensitivity (pD2) response in the systemic mesenteric vasculature (Cooke and Davidge, 2003). In contrast, the offspring (25 weeks of age) of mothers administered alcohol (ethanol-containing diet had 36% of total calories) during pregnancy exhibited significant attenuation of norepinephrine vasoconstrictive response in the aortic rings (Turcotte et al., 2002). Although no one has reported alcohol effects on thromboxane vasoconstrictive response during pregnancy to our knowledge, alcohol perfusion (240 min or 60 min; 200 or 300 mg%) of human cotyledons increased the ratio of thromboxane (vasoconstrictor) to prostacyclin (vasodilator) at a few time points (Siler-Khodr et al., 2000). Randall and colleagues (Randall and Saulnier, 1995) have demonstrated that alcohol perfusion (100 mM) in human umbilical veins showed an increased thromboxane-to-prostacyclin ratio at all time points. In summary, alcohol mediated uterine vascular dysfunction does not arise from impairing α1 adrenergic agonist phenylephrine or the prostanoid thromboxane response.
This is the first study to report that moderate alcohol exposure impairs endothelium-dependent acetylcholine-mediated uterine artery relaxation. Acetylcholine acts on Muscarinic receptors located on the surface of the endothelial cells and leads to release of major endothelium-derived vasodilators including nitric oxide, prostacyclin, and endothelium-derived hyperpolarizing factor which subequently act on the smooth muscle and produce potent vasodilation (Chinnathambi et al., 2013). No differences were noted in the vasodilatory response of sodium nitroprusside (exogenous vasodilator that does not require endothelial release of vasodilator) between Control and Alcohol groups. Sodium nitroprusside, an exogenous vasodilator is a nitric oxide donor and acts directly on the vascular smooth muscle cell independent of the endothelium and produces vasodilation. No study to date has investigated uterine artery reactivity to vasodilatory agonists like acetylcholine. However, it has been reported that a single acute dose of alcohol (1g alcohol/min) decreased ovine uterine blood flow from 1477 ± 169 ml/min to 1180 ± 195 ml/min and the decreases were maintained for 2 hours post-infusion (Falconer, 1990). In C57BL/6J pregnant mice, chronic alcohol exposure reduced maximal relaxation response to methacholine in maternal systemic mesenteric artery (Cook et al., 2001). Our data show that alcohol specifically impairs endothelial function in primary uterine arteries. This is supported by our in vitro binge-like alcohol exposure findings; alcohol impaired uterine artery endothelial angiogenic gene expression (Ramadoss and Magness, 2012b), vasodilatory nitric oxide-related protein expression levels (Ramadoss and Magness, 2012a), and redox balance-related protein levels (Ramadoss and Magness, 2011). In the current study, we administered an exogenous NO donor sodium nitroprusside and showed that alcohol did not impair sodium nitroprusside-induced uterine artery vasodilation and demonstrated that uterine vascular dysfunction is related to endothelial tissue dysfunction. Furthermore, we will utilize pharmacologic inhibitors in the future to inhibit endothelial production of NO, prostacyclin and endothelium-derived hyperpolarizing factor to dilineate underlying endothelial pathways impaired by alcohol. Alcohol-induced impairment of uterine vascular function has a direct effect on the delivery of oxygen and nutrients to the developing fetus. Our ongoing studies will investigate alcohol-induced maternal-fetal nutrient homeostasis and brain development in relation to uterine vascular adaptations utilizing mechanistic studies that block vasodilatory pathways in the uterine artery endothelium.
The current study demonstrates that moderate alcohol exposure impairs uterine vascular function in pregnant mothers. We herein show that it is important to consider effects of alcohol on the maternal system in addition to the local effects on the fetal brain to understand the pathogenesis of FASD. The mother, the maternal uterine vasculature, the placenta, and the fetus need to be collectively considered in order to develop effective therapeutic strategies for FASD.
Acknowledgements
We thank Ms. Liz Powell (Department of Obstetrics & Gynecology, University of Texas Medical Branch-Galveston) for her support in this study.
Source of Funding: NIH AA19446
Footnotes
Disclosure: None of the authors have a conflict of interest.
REFERENCES
- Alsip NL, Hornung JW, Henzel MK, Asher EF. Pregnancy-induced alterations of uterine arteriolar reactivity in the rat: observations with a new in vivo microcirculatory preparation. Am. J. Obstet. Gynecol. 2000;183:621–626. doi: 10.1067/mob.2000.106074. [DOI] [PubMed] [Google Scholar]
- Bruce NW. The distribution of blood flow to the reproductive organs of rats near term. J. Reprod. Fertil. 1976;46:359–362. doi: 10.1530/jrf.0.0460359. [DOI] [PubMed] [Google Scholar]
- Burd L, Deal E, Rios R, Adickes E, Wynne J, Klug MG. Congenital heart defects and fetal alcohol spectrum disorders. Congenit Heart Dis. 2007;2:250–255. doi: 10.1111/j.1747-0803.2007.00105.x. [DOI] [PubMed] [Google Scholar]
- Caetano R, Ramisetty-Mikler S, Floyd LR, McGrath C. The epidemiology of drinking among women of child-bearing age. Alcohol. Clin. Exp. Res. 2006;30:1023–1030. doi: 10.1111/j.1530-0277.2006.00116.x. [DOI] [PubMed] [Google Scholar]
- Chinnathambi V, Balakrishnan M, Ramadoss J, Yallampalli C, Sathishkumar K. Testosterone alters maternal vascular adaptations: role of the endothelial NO system. Hypertension. 2013;61:647–654. doi: 10.1161/HYPERTENSIONAHA.111.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church MW, Gerkin KP. Hearing disorders in children with fetal alcohol syndrome: findings from case reports. Pediatrics. 1988;82:147–154. [PubMed] [Google Scholar]
- Cook JL, Zhang Y, Davidge ST. Vascular function in alcohol-treated pregnant and nonpregnant mice. American journal of physiology. Regulatory, integrative and comparative physiology. 2001;281:R1449–1455. doi: 10.1152/ajpregu.2001.281.5.R1449. [DOI] [PubMed] [Google Scholar]
- Cooke CL, Davidge ST. Pregnancy-induced alterations of vascular function in mouse mesenteric and uterine arteries. Biol. Reprod. 2003;68:1072–1077. doi: 10.1095/biolreprod.102.009886. [DOI] [PubMed] [Google Scholar]
- Cudd TA. Animal model systems for the study of alcohol teratology. Exp Biol Med (Maywood) 2005;230:389–393. doi: 10.1177/15353702-0323006-06. [DOI] [PubMed] [Google Scholar]
- Dowell RT, Kauer CD. Maternal hemodynamics and uteroplacental blood flow throughout gestation in conscious rats. Methods Find. Exp. Clin. Pharmacol. 1997;19:613–625. [PubMed] [Google Scholar]
- Ellinsworth DC, Shukla N, Fleming I, Jeremy JY. Interactions between thromboxane A2, thromboxane/prostaglandin (TP) receptors and endothelium-derived hyperpolarization. Cardiovasc. Res. 2014 doi: 10.1093/cvr/cvu015. [DOI] [PubMed] [Google Scholar]
- Falconer J. The effect of maternal ethanol infusion on placental blood flow and fetal glucose metabolism in sheep. Alcohol Alcohol. 1990;25:413–416. [PubMed] [Google Scholar]
- Feletou M, Huang Y, Vanhoutte PM. Endothelium-mediated control of vascular tone: COX-1 and COX-2 products. Br. J. Pharmacol. 2011;164:894–912. doi: 10.1111/j.1476-5381.2011.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone J, Nulman I, Koren G. Reproductive risks of binge drinking during pregnancy. Reprod. Toxicol. 1996;10:3–13. doi: 10.1016/0890-6238(95)02024-1. [DOI] [PubMed] [Google Scholar]
- Gundogan F, Elwood G, Longato L, Tong M, Feijoo A, Carlson RI, Wands JR, de la Monte SM. Impaired placentation in fetal alcohol syndrome. Placenta. 2008;29:148–157. doi: 10.1016/j.placenta.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J, Gray R, Brocklehurst P. Systematic review of effects of low-moderate prenatal alcohol exposure on pregnancy outcome. BJOG : an international journal of obstetrics and gynaecology. 2007;114:243–252. doi: 10.1111/j.1471-0528.2006.01163.x. [DOI] [PubMed] [Google Scholar]
- Magness RR. The Endocrinology of Pregnancy. Humana Press; Bazer: 1998. Maternal cardiovascular and other physiologic responses to the endocrinology of pregnancy. pp. 507–539. [Google Scholar]
- Maier SE, Miller JA, West JR. Prenatal binge-like alcohol exposure in the rat results in region-specific deficits in brain growth. Neurotoxicol. Teratol. 1999;21:285–291. doi: 10.1016/s0892-0362(98)00056-7. [DOI] [PubMed] [Google Scholar]
- Maier SE, West JR. Regional differences in cell loss associated with binge-like alcohol exposure during the first two trimesters equivalent in the rat. Alcohol. 2001;23:49–57. doi: 10.1016/s0741-8329(00)00133-6. [DOI] [PubMed] [Google Scholar]
- May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Barnard R, De Vries M, Robinson LK, Adnams CM, Buckley D, Manning M, Jones KL, Parry C, Hoyme HE, Seedat S. Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol. Clin. Exp. Res. 2013;37:818–830. doi: 10.1111/acer.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Developmental disabilities research reviews. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- Ni Y, Meyer M, Osol G. Gestation increases nitric oxide-mediated vasodilation in rat uterine arteries. Am. J. Obstet. Gynecol. 1997;176:856–864. doi: 10.1016/s0002-9378(97)70611-2. [DOI] [PubMed] [Google Scholar]
- Parnell SE, Ramadoss J, Delp MD, Ramsey MW, Chen WJ, West JR, Cudd TA. Chronic ethanol increases fetal cerebral blood flow specific to the ethanol-sensitive cerebellum under normoxaemic, hypercapnic and acidaemic conditions: ovine model. Exp. Physiol. 2007;92:933–943. doi: 10.1113/expphysiol.2007.038091. [DOI] [PubMed] [Google Scholar]
- Ramadoss J, Lunde ER, Pina KB, Chen WJ, Cudd TA. All three trimester binge alcohol exposure causes fetal cerebellar purkinje cell loss in the presence of maternal hypercapnea, acidemia, and normoxemia: ovine model. Alcohol. Clin. Exp. Res. 2007;31:1252–1258. doi: 10.1111/j.1530-0277.2007.00422.x. [DOI] [PubMed] [Google Scholar]
- Ramadoss J, Magness RR. 2-D DIGE uterine endothelial proteomic profile for maternal chronic binge-like alcohol exposure. J Proteomics. 2011;74:2986–2994. doi: 10.1016/j.jprot.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Magness RR. Alcohol-Induced Alterations in Maternal Uterine Endothelial Proteome: A Quantitative iTRAQ Mass Spectrometric Approach. Reprodictive Toxicology. 2012a;34:538–544. doi: 10.1016/j.reprotox.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Magness RR. Multiplexed digital quantification of binge-like alcohol-mediated alterations in maternal uterine angiogenic mRNA transcriptome. Physiol Genomics. 2012b;44:622–628. doi: 10.1152/physiolgenomics.00009.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Magness RR. Vascular effects of maternal alcohol consumption. American journal of physiology. Heart and circulatory physiology. 2012c;303:H414–421. doi: 10.1152/ajpheart.00127.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall CL, Saulnier JL. Effect of ethanol on prostacyclin, thromboxane, and prostaglandin E production in human umbilical veins. Alcohol. Clin. Exp. Res. 1995;19:741–746. doi: 10.1111/j.1530-0277.1995.tb01576.x. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Rosenberg MJ, Wolff CR, El-Emawy A, Staples MC, Perrone-Bizzozero NI, Savage DD. Effects of moderate drinking during pregnancy on placental gene expression. Alcohol. 2010;44:673–690. doi: 10.1016/j.alcohol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CR. Distribution of cardiac output in ovine pregnancy. Am. J. Physiol. 1977;232:H231–235. doi: 10.1152/ajpheart.1977.232.3.H231. [DOI] [PubMed] [Google Scholar]
- Sawant OB, Lunde ER, Washburn SE, Chen WJ, Goodlett CR, Cudd TA. Different patterns of regional Purkinje cell loss in the cerebellar vermis as a function of the timing of prenatal ethanol exposure in an ovine model. Neurotoxicol. Teratol. 2013;35:7–13. doi: 10.1016/j.ntt.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott PA, Tremblay A, Brochu M, St-Louis J. Vasorelaxant action of 17 -estradiol in rat uterine arteries: role of nitric oxide synthases and estrogen receptors. American journal of physiology. Heart and circulatory physiology. 2007;293:H3713–3719. doi: 10.1152/ajpheart.00736.2007. [DOI] [PubMed] [Google Scholar]
- Siler-Khodr TM, Yang Y, Grayson MH, Henderson GI, Lee M, Schenker S. Effect of ethanol on thromboxane and prostacyclin production in the human placenta. Alcohol. 2000;21:169–180. doi: 10.1016/s0741-8329(00)00084-7. [DOI] [PubMed] [Google Scholar]
- Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. JAMA. 2003;290:2996–2999. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Abou EJ, Dominguez HD. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol. Teratol. 2009;31:303–311. doi: 10.1016/j.ntt.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Idrus NM, Monk BR, Dominguez HD. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth defects research. Part A, Clinical and molecular teratology. 2010;88:827–837. doi: 10.1002/bdra.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Sather TM, Whinery LA. Voluntary exercise influences behavioral development in rats exposed to alcohol during the neonatal brain growth spurt. Behav. Neurosci. 2008;122:1264–1273. doi: 10.1037/a0013271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte LA, Aberle NS, Norby FL, Wang GJ, Ren J. Influence of prenatal ethanol exposure on vascular contractile response in rat thoracic aorta. Alcohol. 2002;26:75–81. doi: 10.1016/s0741-8329(01)00198-7. [DOI] [PubMed] [Google Scholar]
- Utkan T, Yildiz F, Ilbay G, Ozdemirci S, Erden BF, Gacar N, Ulak G. Blood pressure and vascular reactivity to endothelin-1, phenylephrine, serotonin, KCl and acetylcholine following chronic alcohol consumption in vitro. Fundam. Clin. Pharmacol. 2001;15:157–165. doi: 10.1046/j.1472-8206.2001.00024.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Iversen J, Strandgaard S. Endothelium-dependent relaxation of small resistance vessels is impaired in patients with autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 2000;11:1371–1376. doi: 10.1681/ASN.V1181371. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J. Neuroendocrinol. 2008;20:470–488. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]