Abstract
Objective
Olfactory dysfunction is the most common pre-motor symptom in Parkinson’s disease, and smoking is known to be associated with lower risk of PD. This study tested the hypothesis that smoking is associated with better olfaction in PD.
Methods
Smoking history was obtained from 76 PD subjects [22 with a history of smoking (smokers), 54 who never smoked (non-smokers)], and 70 Controls (17 smokers, 53 non-smokers). Olfaction was assessed using the 40-item University of Pennsylvania Smell Identification Test (UPSIT). The olfactory scores between groups and subgroups were compared using analysis of covariance with adjustment for age, gender, and MAO-B inhibitor usage.
Results
Overall the olfactory score was lower in PD compared to Controls (olfactory scores: 21.54 vs. 33.45, p<0.0001). Among Controls, there was no significant difference in olfaction between smokers and non-smokers (olfactory scores: 33.2 vs. 34.2, p=0.95). Among PD subjects, however, smokers scored significantly better regarding olfaction compared to non-smokers (olfactory scores: 24.4 vs. 19.9, p=0.02).
Conclusions
These data suggest that history of smoking is associated with better olfaction among PD patients. The finding may be related to why smoking may be protective against PD. Further studies are needed to confirm this finding and investigate the underlying mechanism(s).
Keywords: Smoking, Parkinson’s disease, Olfactory, Cigarette
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder marked pathologically by loss of dopamine neurons in the substantia nigra of the basal ganglia. Although the cardinal clinical signs are defined by motor disabilities, many non-motor symptoms such as olfactory, gastrointestinal, and cognitive dysfunction are also recognized to be associated with PD. Some of this non-motor dysfunction, such as hyposmia and constipation, may develop during the prodromal stage of PD and precede PD diagnosis by years.1 As the nasopharynx and GI tract have direct contact with the environment, these areas can be the port-of-entry for neurotoxicants.2;3 Thus, there is an emerging concept that pre-motor symptoms represent intermediate phenotypes prior to overt PD, and may offer a vehicle to understand the role of genetics and the environment in the early stages of PD development.4 As such, a detailed understanding of these non-motor features and associated environmental factors may offer important clues.2–4
Olfactory dysfunction may affect 90% of early-stage PD cases,5 and according to the Braak hypothesis, α-synuclein-related pathology starts very early in the olfactory bulb.2;5 It has been hypothesized that one of the initial events in PD pathogenesis is pathogenic access to the brain through the nasopharynx, resulting in olfactory dysfunction.2 Although this is an intriguing hypothesis, little is known regarding causative factors associated with olfactory dysfunction in PD.
More than 60 epidemiological studies are consistent in reporting that smokers have a lower risk for developing PD,1;6 although the mechanism(s) by which cigarette smoking may confer a protective effect in PD is unknown. Past studies have focused on the potential influence of smoking or nicotine on the motor and dopamine system. For example, nicotine has been shown to stimulate dopamine release and up-regulate nicotinic receptors, inhibit free radical damage to nigral cells through carbon monoxide, and protect against neuronal damage by inhibiting monoamine oxidase B.7 Yet clinical trials have failed to demonstrate beneficial effects of nicotine alone.8;9
To our knowledge, the relationship between cigarette smoking and olfaction in subjects with PD has not been explored, although it may help in understanding the relationship between smoking and PD. For example, smoking may confer a neuroprotective effect non-selectively from dopaminergic to non-dopaminergic neurons, including neurons responsible for olfaction centrally. Another possibility is that smoke may act as a local irritant in the nasopharynx, thereby affecting the olfactory system10;11 and its ability to transfer environmental toxicants to the central nervous system. Thus, understanding if and how smoking interacts with olfaction may contribute to the understanding of PD etiology. Toward this goal, we conducted a case-control study to test the hypothesis that a past smoking history would result in better olfaction compared to PD patients who had never smoked.
Methods
Subjects
Seventy six PD subjects [22 with a history of smoking (referred to as smokers) and 54 with no history of smoking (referred to as non-smokers)] and 70 control subjects (17 smokers, 53 non-smokers) were recruited from a tertiary movement disorders clinic for an ongoing study approved by the Institutional Review Board/Human Subjects Protection Office (IRB/HSPO) of the Penn State Hershey Medical Center. Written informed consent in accordance with the Declaration of Helsinki was obtained for each subject.
The diagnosis of PD was confirmed by a movement disorder specialist according to published criteria.12 All subjects were negative for other major and acute neurological and psychological disorders including: hypothyroidism; vitamin B12 or folate deficiency; and kidney and liver disease. Duration of illness was defined as the number of years between the date of disease diagnosis and study visit date. Levodopa equivalent daily dose (LEDD) was calculated using published criteria.13 Unified Parkinson’s Disease Rating Scale section III motor scores (UPDRS-III) were obtained for each subject after withholding PD medications overnight (>12 hours).14 Because some monoamine oxidase (MAO)-B inhibitors are suggested to enhance olfaction,15 MAO-B inhibitor use was recorded for each subject and, coded “yes” for current use, or “no” for no use.
Smoking history
Cigarette smoking data were obtained as part of a comprehensive demographic survey. Subjects were considered to be smokers if they had smoked one cigarette per day for at least six months. Smokers completed questions regarding the number of cigarettes smoked and the duration of smoking. Pack years were calculated from the number of cigarette packs smoked per day and the number of years smoked.16 Among control smokers, the mean starting age was 16.3 years (S.D. ± 3.5). Three control subjects were current smokers. For the remaining control smokers, the mean time elapsed between smoking cessation and data collection was 26.2 years (S.D. ± 13.0) and mean age at time of cessation was 35.8 year (S.D. ± 11.5). Among PD smokers, the mean starting age was 18.7 years (S.D. ± 3.5). One PD subject was a current smoker. For the remaining PD smokers, the mean time elapsed between smoking cessation and data collection was 32.6 years (S.D. ± 13.2), mean age at time of cessation was 34.5 years (S.D. ± 12.9).
Neurobehavioral assessment
All subjects were screened for dementia using the Mini Mental Status Exam (MMSE). This examination is a 30-point test that assesses orientation, memory, and the ability to follow commands.17 Using a cutoff score of 23, the MMSE has been demonstrated to have 98% sensitivity and 77% specificity in detecting dementia among PD patients.18 To avoid a potential biasing effect of cognitive function upon olfactory examination performance, subjects were included in this study only if they had an MMSE score of ≥ 24.
Assessments of olfactory function
Olfactory function was evaluated with the University of Pennsylvania Smell Identification Test (UPSIT), a standardized forced-choice test comprised of four booklets containing ten odorants each, with one odorant per page. The stimuli are embedded in “scratch and sniff” microcapsules fixed and positioned on strips at the bottom of each page. A multiple-choice question with four response alternatives for each item is located above each odorant strip. Scores are calculated as the number of items correctly identified.19 Although normalized UPSIT scores (adjusted for age and gender) are utilized frequently, those scales were derived using broad population data. PD is known to affect olfactory function, and age-related olfactory decline among PD subjects may not progress at the same rate as that of typical individuals. The present study utilized raw UPSIT scores and adjusted statistically for age and gender using general linear modeling.
Assessing other non-motor symptoms
All subjects completed a standardized neuropsychological battery. The Montreal Cognitive Assessment (MoCA) was used to detect mild cognitive impairment (MCI),20 and has been shown to have higher sensitivity for detecting PD-related cognitive deficits than the MMSE.21 The Dementia Rating Scale-2 (DRS2) examines a broader range of cognitive functions (attention, initiation and perseveration, conceptualization, construction, and memory), and is used to detect other subtle cognitive impairments in PD.22 Symptoms of constipation were extracted from UPDRS-I (“1.11 Over the past week have you had constipation troubles that cause you difficulty moving your bowels? Patients could answer: Normal: No constipation. Slight: I have been constipated. I use extra effort to move my bowels. However, this problem does not disturb my activities or my being comfortable. Mild: Constipation causes me to have some troubles doing things or being comfortable. Moderate: Constipation causes me to have a lot of trouble doing things or being comfortable. However, it does not stop me from doing anything. Severe: I usually need physical help from someone else to empty my bowels.).”
Statistical analyses
Demographic and clinical parameters were compared between groups and subgroups using two-sample t-tests with pooled variance (age, age at diagnosis), Fisher’s exact test (gender, MAO-B inhibitor use), and one-way analysis of covariance (ANCOVA) with adjustment for age and gender (LEDD, UPDRS-III scores, duration of illness, MMSE scores). Relative risk for PD according to history of previous smoking was determined using logistic regression with adjustment for age and gender.
UPSIT scores were first compared between PD and control subjects using one-way ANCOVA. The general linear model was used to obtain least-squares means of UPSIT scores in each group, and included age, gender, history of smoking, and the interaction term between PD diagnosis and previous smoking. Subgroup comparisons among controls were adjusted for age and gender. Among PD patients, the appropriate model for subgroup comparisons was selected by examining the dependency of UPSIT scores on disease-specific covariates and interactions (i.e., duration of illness, age at diagnosis, LEDD, and MAO-B inhibitor use and dose). The final ANCOVA model for PD subgroup comparisons included age, gender, and MAO-B inhibitor use as covariates. Subgroup comparisons of UPSIT scores according to smoking status were adjusted for multiple comparisons using Bonferroni correction.
For correlation analysis, Spearman’s partial correlation coefficients were obtained after correction for age, gender, and monoamine oxidase inhibitor usage, as appropriate. All analyses were performed using SAS 9.3.
Results
As shown in Table 1, the mean age of PD subjects was slightly higher than that of Controls (p=0.08). Among PD cases, the average age of smokers was 4.8 years older than non-smokers (p=0.02). Similarly, the age of PD onset in smokers was also significantly greater than non-smokers (p=0.02). Among PD subjects, there were no significant differences by smoking status regarding LEDD (p=0.36), UPDRS-III scores (p=0.84), duration of illness (p=0.11), MMSE scores (p=0.74), or the proportion of subjects using MAO inhibitors (p=1.00) [rasagiline (p=0.44) or selegiline (p=0.60)] (Table 2).
Table 1.
Demographic and smoking history of Control and Parkinson’s disease subjects
| Previous Smoker | Gender | N | Age (y)* | Smoking* (pack-years) | |
|---|---|---|---|---|---|
| Control | No | F | 32 | 58.9±7.0 | NA |
| M | 21 | 61.1±8.6 | NA | ||
| Yes | F | 3 | 61.7±13.2 | 18.5±10.9 | |
| M | 14 | 60.6±7.1 | 8.5±8.9 | ||
| PD | No | F | 20 | 59.4±7.8 | NA |
| M | 34 | 62.0±8.8 | NA | ||
| Yes | F | 8 | 61.0±9.4 | 1.6±1.8 | |
| M | 14 | 68.6±4.6 | 9.9±11.2 |
Values are mean±SD.
Table 2.
Clinical information on PD subgroups according to history of smoking
| History of Smoking | Yes | No | P value |
|---|---|---|---|
| Number (F/M) | 22 (8/14) | 54 (20/34) | 1.00 |
| Age (y; mean±SD) | 65.8±7.5 | 61.0±8.5 | 0.02 |
| Duration of Illness (y; mean±SD) | 4.4±5.2 | 5.2±5.7 | 0.11 |
| Age at Diagnosis (y; mean±SD) | 61.4±7.8 | 55.9± 7.6 | 0.02 |
| LEDD (mg; mean±SD) | 520±433 | 559± 435 | 0.36 |
| UPDRS-III (mean±SD) | 24.5±13.8 | 22.3± 14.8 | 0.84 |
| MMSE (mean±SD) | 29.1±1.1 | 29.2±1.1 | 0.74 |
| MAOI (%, rasagiline/selegiline) | 31.8% (9:7) | 31.5% (17:22) | 1.00 |
Abbreviations: LEDD: Levodopa equivalent daily dose; MAOI: Monoamine oxidase B inhibitors; MMSE: Mini Mental Status Examination; UPDRS-III: Unified Parkinson’s Disease Rating Scale section III motor scores; UPSIT: University of Pennsylvania Smell Identification Test
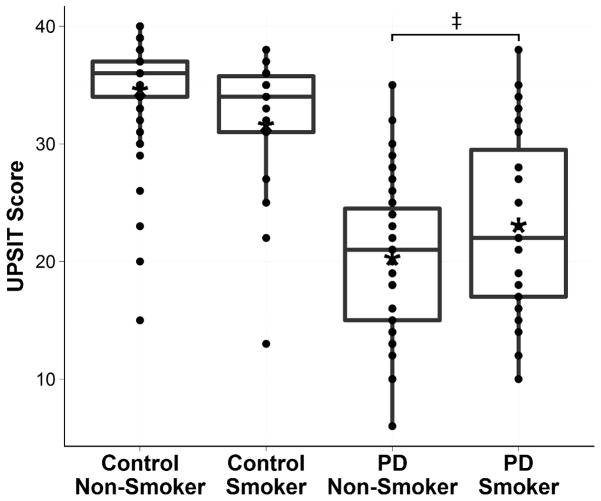
UPSIT scores were significantly higher among Controls compared to PD subjects (33.5 [95% CI 32.1 – 34.8] vs. 21.5 [20.2 – 22.9], p<0.0001) (Figure 1). Among Controls, there was no significant difference in UPSIT scores between smokers and non-smokers (33.2 [95% CI 30.7 – 35.7] vs. 34.2 [95% CI 32.9 – 35.6], p=0.95). Female controls scored significantly higher than male controls (35.6 vs. 32.3, p=0.011). Among PD subjects, UPSIT scores were negatively correlated with age (ρ=−0.45, p=0.0003), but showed only a negative trend among control subjects (ρ=−0.20, p=0.098). There were no associations between UPSIT scores and duration of illness (ρ=−0.083, p=0.48) or age at diagnosis (ρ=0.14, p=0.25) among PD subjects. UPSIT scores were not correlated with pack years of smoking among control (ρ=−0.26, p=0.36) or PD (ρ=−0.27, p=0.25) smokers. Time elapsed since smoking cessation did not correlate with UPSIT scores among control smokers (ρ=0.0039, p=0.89) or PD smokers (ρ=−0.23, p=0.33). The UPSIT score of rasagiline users did not differ significantly from that of non-users in the overall PD group (p=0.29). Within the PD non-smokers subgroup, however, UPSIT scores were 4.17 points lower (95% CI 0.71–7.62) for rasagiline users than non-users (p=0.019), and the daily rasagiline dose correlated negatively with UPSIT scores (ρ=−0.30, p=0.029). In the PD smokers subgroup, no associations between UPSIT scores and rasagiline use were found (p=0.43). Selegiline usage and dosage were not associated with olfactory function in either PD subgroup.
Figure 1.
UPSIT scores of control and PD smoking subgroups. PD smokers had significantly higher UPSIT scores compared to PD non-smokers (p=0.02). Asterisks (*) denote raw means. Double cross (‡) denotes statistical significance.
According to above results, the final model for comparing UPSIT scores between PD smokers and PD non-smokers included age, gender, and rasagiline usage as covariates. PD smokers scored 4.50 (95% CI 1.17–7.83) points (22.6%) higher on the UPSIT compared to PD non-smokers (24.4 vs. 19.9, p=0.018) (Figure 1). Time elapsed since smoking cessation did not correlate with UPSIT scores among control smokers (ρ=0.0039, p=0.89) or PD smokers (ρ=−0.23, p=0.36). Among PD smokers, neither pack-years nor duration of smoking was correlated with UPSIT score.
Lastly, we explored the relationship between smoking and other non-motor symptoms. PD smokers and non-smokers did not differ with regards to history of constipation (p=0.65) or cognitive measurements (MoCA, DRS2, MMSE) (p=0.095, p=0.33, p=0.91, respectively). In addition, no correlations between olfactory function and cognitive or motor measurements were found.
Discussion
Few previous studies have examined the associations that might exist between smoking and non-motor symptoms in PD. Alves et al.23 followed the progression of non-motor features, including cognition and mood, for eight years in a group of Parkinson’s patients that included smokers and non-smokers, but they did not assess olfaction. To our knowledge, the current study is the first to investigate the effects of smoking on olfaction in PD, and supports the hypothesis that smoking may protect against PD-related olfactory dysfunction.
Previous studies on smoking and olfaction in the general elderly population have generated inconsistent results. Overall, those data have not shown an association, although several studies have found a poorer sense of smell among smokers.10;11 In the current study, we did not find a statistical difference in UPSIT scores between smokers and non-smokers among controls. Conversely, among PD patients, UPSIT scores in smokers were significantly better than in the non-smokers.
An inverse association with smoking is one of the most robust epidemiological findings in PD.6 It is unknown why this association occurs, but the potential neuroprotective effects of smoking or nicotine on the nigrostriatal pathway are obvious candidates. In toxicant-induced parkinsonian animal models, nicotine administration protects against nigrostriatal damage,24 yet in PD patients neither nicotine patches nor gum have shown benefit, and even worsened motor function.8;9 Furthermore, there were no significant differences in motor progression 23 or mortality 25;26 between smokers and non-smokers in a longitudinal study. These human experiments suggest that cigarette smoking may not have a major neuroprotective effect in patients after PD diagnosis.23 Interestingly, a recent large prospective analysis of smoking history found that the duration of smoking, not the smoking intensity, was responsible for the lower PD risk among smokers.1 In the current study, we noted that PD smokers had a later age of onset compared to PD non-smokers. Together, these data suggest that if the association of smoking and PD is biological, the mechanism may involve gradual changes taking place over many years prior to disease diagnosis, and is unlikely to be reflected in many of the human studies given the short length of pharmacological treatment after PD diagnosis.
Although research on the interaction between smoking and non-motor symptoms among PD subjects may help to understand the relationship between smoking and premorbid PD, few studies have done this. Weisskopf et al. 27 reported worse cognition among smokers, whereas Alves et al. 23 found no association between smoking and measurements of cognition and mood. To our knowledge, none of the previous studies included olfaction. In the current study, we did not find any association between history of smoking and any of the cognitive measurements among PD subjects. We, however, found significantly better UPSIT scores in PD subjects with a history of smoking, despite those subjects being older in age, compared to those of non-smokers.
Because olfactory and GI dysfunctions are among the earliest manifestations of PD, a “dual-hit hypothesis” for PD etiology has been proposed.28;29 It is hypothesized that an environmental neurotropic pathogen may enter the brain via a nasal and/or GI route.2 Our finding that PD smokers had a better sense of smell than PD non-smokers is intriguing in this context. One possibility is that smoke may act as a local irritant in the nasopharynx, thus attenuating the ability of olfactory terminals to transport environmental toxicants to the olfactory bulb. Therefore, smoking may prevent one of the “hits” via the respiratory tract as proposed by Hawkes et al.2 The GI measurements in our study, however, were limited, and PD subjects with a history of smoking did not report significantly more GI symptoms. Future studies with more detailed assessments of GI function are needed.
Although the interaction between smoke and the olfactory system at a peripheral level is a very intriguing hypothesis, it is also possible that cigarette smoke may protect olfactory structures within the brain. It has been proposed that smoking may protect against PD by inhibiting MAO-B.30 Rasagiline, one of the MAO-B inhibitors that is approved to treat PD motor symptoms, has been reported to protect PD-like olfactory loss in a mouse model,15 but conflicting results have been found in PD patients.31;32 In our study, selegiline-users were similar in UPSIT scores to selegiline non-users in both PD smoker and non-smoker groups. Interestingly, rasagiline-users showed a worse sense of smell in non-smokers. The current study was not designed to study effects of MAO-B inhibition on olfaction, and the negative association between rasagiline usage and olfaction may reflect prescriber bias (i.e., rasagiline is more expensive, and more likely to be used in subjects who are vulnerable to selegiline-related side effects such as hallucinations and confusion). In our analyses, we adjusted for use of MAO-B inhibitors. Future studies investigating MAO-B inhibitor effects in olfaction should account for history of smoking as a confounding factor.
There are a number of limitations in our study. First, the sample size is relatively small and larger studies with detailed assessment of smoking habits will allow for more solid answers and exploration of possible mechanisms. A larger study will also help balance out the differences of smoking history among groups. Second, we did not ask our subjects about a history of head trauma, and it is possible that this could have been a confounding factor in some subjects. Third, though the UPSIT is a widely used test for olfaction identification, future studies might benefit from a more comprehensive measurement of olfaction modalities, such as olfactory threshold testing. Lastly, it has been proposed that there is a neurobiological link between low sensation-seeking traits and the “parkinsonian personality”. 33 Thus, it is possible that PD smokers are inherently different, biologically or otherwise, from PD non-smokers. Future studies may benefit from the assessment of personality traits to examine this issue further.
Despite these limitations, we found that smoking was associated with a better sense of smell identification among PD patients. Further studies are needed to confirm this finding since this may help elucidate the mechanisms of the protective effect that smoking confers against PD.
Acknowledgments
This work was supported in part by NIH NS060722 and NS082151, Penn State Clinical & Translational Science Institute (NIH UL1 TR000127, TL1 TR000125) and GCRC Construction (C06 RR016499) research grants. We would like to thank the study participants and research coordinators (Ms. Eleanore Hernandez and Mrs. Brittany Jones) for their contributions to this research. Dr. Chen is supported by the intramural research program of the NIH, the National Institute of Environmental Health Sciences (Z01-ES-101986). We also would like to thank Dr. Richard Mailman for his critique and commentary.
Footnotes
Disclosure: The authors report no conflicts of interest.
Author Roles:
Dr. Elisabeth Lucassen: research project execution, manuscript writing of the first draft, review, and critique.
Nicholas Sterling: research project conception and execution, statistical analysis execution, manuscript writing of the first draft, review, and critique.
Dr. Eun-Young Lee: manuscript review and critique.
Dr. Honglei Chen: statistical analysis review and critique, manuscript review and critique.
Dr. Mechelle Lewis: research project organization and execution, manuscript organization, review, and critique.
Dr. Lan Kong: statistical analysis design, review, and critique.
Dr. Xuemei Huang: research project conception, organization, and execution; manuscript organization, review, and critique.
Financial Disclosures:
The authors have no financial disclosures to report.
References
- 1.Chen H, Huang X, Guo X, Mailman RB, Park Y, Kamel F, et al. Smoking duration, intensity, and risk of Parkinson disease. Neurology. 2010;74(11):878–884. doi: 10.1212/WNL.0b013e3181d55f38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawkes CH, Del TK, Braak H. Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33(6):599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawkes CH, Del TK, Braak H. Parkinson’s disease: the dual hit theory revisited. Ann N Y Acad Sci. 2009;1170:615–622. doi: 10.1111/j.1749-6632.2009.04365.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Burton EA, Ross GW, Huang X, Savica R, Abbott RD, et al. Research on the Pre-Motor Symptoms of Parkinson’s Disease: Clinical and Etiological Implications. Environ Health Perspect. 2013 doi: 10.1289/ehp.1306967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doty RL. Olfactory dysfunction in Parkinson disease. Nat Rev Neurol. 2012;8(6):329–339. doi: 10.1038/nrneurol.2012.80. [DOI] [PubMed] [Google Scholar]
- 6.Allam MF, Campbell MJ, Hofman A, Del Castillo AS, Fernandez-Crehuet NR. Smoking and Parkinson’s disease: systematic review of prospective studies. Mov Disord. 2004;19(6):614–621. doi: 10.1002/mds.20029. [DOI] [PubMed] [Google Scholar]
- 7.Hernan MA, Takkouche B, Caamano-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Ann Neurol. 2002;52(3):276–284. doi: 10.1002/ana.10277. [DOI] [PubMed] [Google Scholar]
- 8.Ebersbach G, Stock M, Muller J, Wenning G, Wissel J, Poewe W. Worsening of motor performance in patients with Parkinson’s disease following transdermal nicotine administration. Mov Disord. 1999;14(6):1011–1013. doi: 10.1002/1531-8257(199911)14:6<1011::aid-mds1016>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 9.Vieregge A, Sieberer M, Jacobs H, Hagenah JM, Vieregge P. Transdermal nicotine in PD: a randomized, double-blind, placebo-controlled study. Neurology. 2001;57(6):1032–1035. doi: 10.1212/wnl.57.6.1032. [DOI] [PubMed] [Google Scholar]
- 10.Schriever VA, Reither N, Gerber J, Iannilli E, Hummel T. Olfactory bulb volume in smokers. Exp Brain Res. 2013;225(2):153–157. doi: 10.1007/s00221-012-3356-5. [DOI] [PubMed] [Google Scholar]
- 11.Vent J, Robinson AM, Gentry-Nielsen MJ, Conley DB, Hallworth R, Leopold DA, et al. Pathology of the olfactory epithelium: smoking and ethanol exposure. Laryngoscope. 2004;114(8):1383–1388. doi: 10.1097/00005537-200408000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatr. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25(15):2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 14.Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, Freeman T, et al. Core assessment program for intracerebral transplantations (CAPIT) Mov Disord. 1992;7(1):2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- 15.Petit GH, Berkovich E, Hickery M, Kallunki P, Fog K, Fitzer-Attas C, et al. Rasagiline ameliorates olfactory deficits in an alpha-synuclein mouse model of Parkinson’s disease. PLoS One. 2013;8(4):e60691. doi: 10.1371/journal.pone.0060691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brigham J, Lessov-Schlaggar CN, Javitz HS, Krasnow RE, McElroy M, Swan GE. Test-retest reliability of web-based retrospective self-report of tobacco exposure and risk. J Med Internet Res. 2009;11(3):e35. doi: 10.2196/jmir.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Hobson P, Meara J. The detection of dementia and cognitive impairment in a community population of elderly people with Parkinson’s disease by use of the CAMCOG neuropsychological test. Age Ageing. 1999;28(1):39–43. doi: 10.1093/ageing/28.1.39. [DOI] [PubMed] [Google Scholar]
- 19.Segura B, Baggio HC, Solana E, Palacios EM, Vendrell P, Bargallo N, et al. Neuroanatomical correlates of olfactory loss in normal aged subjects. Behav Brain Res. 2013;246:148–153. doi: 10.1016/j.bbr.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 20.Hoops S, Nazem S, Siderowf AD, Duda JE, Xie SX, Stern MB, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73(21):1738–1745. doi: 10.1212/WNL.0b013e3181c34b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marras C, Armstrong MJ, Meaney CA, Fox S, Rothberg B, Reginold W, et al. Measuring mild cognitive impairment in patients with Parkinson’s disease. Mov Disord. 2013 doi: 10.1002/mds.25426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown GG, Rahill AA, Gorell JM, McDonald C, Brown SJ, Sillanpaa M, et al. Validity of the Dementia Rating Scale in assessing cognitive function in Parkinson’s disease. J Geriatr Psychiatry Neurol. 1999;12(4):180–188. doi: 10.1177/089198879901200403. [DOI] [PubMed] [Google Scholar]
- 23.Alves G, Kurz M, Lie SA, Larsen JP. Cigarette smoking in Parkinson’s disease: influence on disease progression. Mov Disord. 2004;19(9):1087–1092. doi: 10.1002/mds.20117. [DOI] [PubMed] [Google Scholar]
- 24.Quik M, Perez XA, Bordia T. Nicotine as a potential neuroprotective agent for Parkinson’s disease. Mov Disord. 2012;27(8):947–957. doi: 10.1002/mds.25028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Ascherio A. Survival of Parkinson’s disease patients in a large prospective cohort of male health professionals. Mov Disord. 2006;21(7):1002–1007. doi: 10.1002/mds.20881. [DOI] [PubMed] [Google Scholar]
- 26.Elbaz A, Bower JH, Peterson BJ, Maraganore DM, McDonnell SK, Ahlskog JE, et al. Survival study of Parkinson disease in Olmsted County, Minnesota. Arch Neurol. 2003;60(1):91–96. doi: 10.1001/archneur.60.1.91. [DOI] [PubMed] [Google Scholar]
- 27.Weisskopf MG, Grodstein F, Ascherio A. Smoking and cognitive function in Parkinson’s disease. Mov Disord. 2007;22(5):660–665. doi: 10.1002/mds.21373. [DOI] [PubMed] [Google Scholar]
- 28.Ross GW, Petrovitch H, Abbott RD, Tanner CM, Popper J, Masaki K, et al. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann Neurol. 2008;63(2):167–173. doi: 10.1002/ana.21291. [DOI] [PubMed] [Google Scholar]
- 29.Ross GW, Abbott RD, Petrovitch H, Tanner CM, White LR. Pre-motor features of Parkinson’s disease: the Honolulu-Asia Aging Study experience. Parkinsonism Relat Disord. 2012;18 (Suppl 1):S199–S202. doi: 10.1016/S1353-8020(11)70062-1. [DOI] [PubMed] [Google Scholar]
- 30.Khalil AA, Davies B, Castagnoli N., Jr Isolation and characterization of a monoamine oxidase B selective inhibitor from tobacco smoke. Bioorg Med Chem. 2006;14(10):3392–3398. doi: 10.1016/j.bmc.2005.12.057. [DOI] [PubMed] [Google Scholar]
- 31.Alvarez MV, Grogan PM. Hyposmia in Parkinson’s disease. Psychiatry Clin Neurosci. 2012;66(4):370. doi: 10.1111/j.1440-1819.2012.02339.x. [DOI] [PubMed] [Google Scholar]
- 32.Haehner A, Hummel T, Wolz M, Klingelhofer L, Fauser M, Storch A, et al. Effects of rasagiline on olfactory function in patients with parkinson’s disease. Mov Disord. 2013 Nov 4; doi: 10.1002/mds.25661. [DOI] [PubMed] [Google Scholar]
- 33.Evans AH, Lawrence AD, Potts J, MacGregor L, Katzenschlager R, Shaw K, et al. Relationship between impulsive sensation seeking traits, smoking, alcohol and caffeine intake, and Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77(3):317–321. doi: 10.1136/jnnp.2005.065417. [DOI] [PMC free article] [PubMed] [Google Scholar]