Abstract
Objectives
The aim of the study was to assess the significance of low-level viraemia (LLV) and the timing of treatment change in low/middle-income country (L/MIC) compared with high-income country (HIC) settings.
Methods
Patients with virological control following commencement of combination antiretroviral therapy (cART) were included in the study. LLV was defined as undetectable viral load (<50 HIV-1 RNA copies/mL) followed by confirmed detectable viral load < 1000 copies/mL. Virological failure was defined as viral load > 1000 copies/mL. Kaplan–Meier plots of time to virological failure by prior LLV and income category were generated. Regimen changes in the setting of LLV were compared between sites. Sensitivity analysis of rates of LLV and virological failure by person-years and number of tests was conducted for differing definitions of LLV and virological failure.
Results
A total of 1748 patients from HICs and 823 patients from L/MICs were included in the study. One hundred and ninety-six (11.2%) HIC participants and 36 (4.4%) L/MIC participants experienced at least one episode of LLV. Of the patients who underwent regimen switch in HIC settings, the majority changed from a nucleoside reverse transcriptase inhibitor (NRTI)/protease inhibitor (PI) regimen to an NRTI/nonnucleoside reverse transcriptase inhibitor (NNRTI) regimen (26.8%). Very few switches were made in L/MIC settings. Rates of LLV were significantly higher for HICs compared with L/MICs per 1000 person-years (28.6 and 9.9 per 1000 person-years, respectively), but not in terms of the number of tests (9.4 and 7.2 per 1000 tests, respectively). Rates of virological failure per test were significantly higher for L/MICs compared with HICs (30.7 vs. 19.6 per 1000 tests, respectively; P < 0.001). LLV was a significant predictor of virological failure at 2 years in L/MICs [0.25; 95% confidence interval (CI) 0.11–0.50; P = 0.043] but not in HICs (0.13; 95% CI 0.08–0.22; P = 0.523).
Conclusions
LLV is weakly predictive of virological failure at 2 years in L/MICs but not in HICs. This suggests that interventions targeted at subjects with LLV in L/MICs would help to improve treatment outcomes.
Keywords: HIV, low level viraemia, resource poor
Introduction
Globally, most HIV-infected patients in high-, middle- and low-income settings who receive antiretroviral therapy successfully achieve and maintain virological suppression [1–3]. Virological failure (VF) is often associated with a significant and increasing viral load (VL). However, there a number of patients who experience low-level viraemia (LLV) – a confirmed detectable HIV RNA level < 1000 HIV–1 RNA copies/mL [4]. The current Australasian Society for HIV Medicine (AHSM) guidelines recommend a change in antiretroviral therapy for those patients with persistent VL 200–1000 copies/mL [4]. It is unknown how this recommendation impacts clinical decision-making for patients presenting with LLV in settings with different economic constraints. In low- and middle-income settings, where changes in therapy have significant cost implications, understanding decision-making regarding VL cut-off and switching in the setting of LLV is important.
Several studies have reported limited association of LLV with increased VF [5–10] and increased mortality [11]; however, the studies were based on small numbers and were all performed in high-income settings. Accumulation of new resistance mutations has been associated with episodes of LLV in treatment-experienced patients with VL < 1000 copies/mL [12] and < 500 copies/mL [13–16]. It has also been postulated that LLV may affect non-AIDS-related outcomes [17] through the mechanism of chronic inflammation. The likelihood of LLV has been found to be higher in patients receiving combination antiretroviral therapy (cART) with ritonavir-boosted protease inhibitors compared with those receiving nonnucleoside reverse transcriptase inhibitors [8].
Evidence concerning the impact of low-level (< 200 copies/mL) but detectable viraemia on clinical and virological outcomes is scant. A large AIDS Clinical Trials Group (ACTG) analysis comparing VF definitions found that, of those with VF defined as > 50 copies/mL, 23–32% subsequently suppressed VL to < 50 copies/mL without a change in cART, compared with 11–12% of those with VF defined as > 200 copies/mL [18]. A recent study with comparable numbers of patients, utilizing an assay with a lower limit of detection of 40 copies/mL, found a significantly higher hazard ratio of virological rebound above 400 copies/mL [19] in those with persistently detectable viraemia < 50 copies/mL. Again, these studies were primarily carried out in high-income settings.
The impact of these findings on clinical management of LLV across different economic settings is unknown. The primary objective of this analysis was to assess the significance of LLV by high, middle and low income and timing of treatment change.
Study design and cohort description
Data for participants from the Treat Asia HIV Observational Database (TAHOD) and Australian HIV Observational Database (AHOD) were used in the analysis. TAHOD is an observational cohort of 17 low-, middle- and high-income clinical sites in the Asia and Pacific region [20]. AHOD is comprised of 27 high-income clinical sites throughout Australia [21]. Both databases have previously been described [22,23]. Sites were stratified into low, middle and high income based on gross national income per capita [24].
HIV-infected patients over 18 years old, with recorded viral suppression (≤50 copies/mL) within 1 year following commencement of cART, were included in the study. Patients were censored at > 7 days off treatment or > 7 days of mono/dual therapy; VF during these intervals was not included. Patients were also censored at VL > 1000 copies/mL, death or loss to follow-up.
LLV was defined as an interval of a measured VL of 51–1000 copies/mL in patients on cART, without cessation of treatment or reversion to mono/dual therapy during that interval, until the next measured VL < 51 or > 1000 copies/mL. VL results were carried forward the minimum of time to the next treatment change date, or 360 days from that test date.
We defined VF as a recorded VL > 1000 copies/mL. Treatment change was defined as the addition of at least two antiretrovirals and/or at least one new class of antiretroviral.
To account for intermittently used assays of different sensitivities at sites, test readings that were ‘undetectable’ for low-sensitivity assays (as indicated by VL = 199, 200, 399 or 400 according to site) were excluded from the analysis.
Patient selection and extraction of data occur at the data centres of the participating sites. Written informed consent is obtained from all patients at the time of enrolment. TAHOD and AHOD data are aggregated at The Kirby Institute, University of New South Wales. Ethical approval for the study was obtained from the University of New South Wales Ethics Committee. Each site also obtained approval from their local ethics committee.
Statistical methods
Patient demographics were summarized for each variable by income category by LLV status and at-risk population. Variables included were total number of person-years of follow-up, gender, age, exposure [men who have sex with men (MSM), injecting drug use (IDU), heterosexual, other and missing], CD4 cell count (≤ 200, 201–350, 351–500 and > 500 cells/μL), prior AIDS-defining illness, tuberculosis (TB) ever, year of cART (< 2000, 2000–2005 and ≥ 2006), hepatitis C virus (HCV) infection ever and hepatitis B virus (HBV) infection ever. Mosaic plots of detectable viral load by calendar year for at-risk populations were developed by income.
Treatment changes were summarized by prior switches/regimen line number. Rates of LLV were described in at-risk patients per 1000 person-years and per number of tests conducted by income. Kaplan–Meier plots of time to first LLV by income category and time to first VF by prior LLV (time-updated) and income were generated. Receiver operating characteristic (ROC) analyses of optimum LLV predictive of VF were conducted based on the mean tested VL during the first LLV episode. Sensitivity analysis for rates of LLV and VF by person-years and by number of tests was conducted for differing definitions of LLV and VF: LLV = 50–200 copies/mL, VF ≥ 200 copies/mL; LLV = 200–400 copies/mL, VF ≥ 400 copies/mL; LLV ≤ 5000 copies/mL, VF ≥ copies/mL, and persistent LLV defined as at least four consecutive measurements of LLV (50–1000 copies/mL).
Results
A total of 2571 adult patients were included in the study, 68% from high-income countries (HICs) and 32% from low- and middle-income countries (L/MICs). Patient demographics are outlined in Table 1.
Table 1.
Patient demographic characteristics
| Low/middle income
|
High income
|
|||||
|---|---|---|---|---|---|---|
| LLV ever n (%) |
No LLV ever n (%) |
All at risk n (%) |
LLV ever n (%) |
No LLV ever n (%) |
All at risk n (%) |
|
| Total | 36 (100) | 787 (100) | 823 (100) | 196 (100) | 1552 (100) | 1748 (100) |
| Gender | ||||||
| Male | 27 (75) | 469 (59.6) | 496 (60.3) | 183 (93.4) | 1435 (92.5) | 1618 (92.6) |
| Female | 9 (25) | 318 (40.4) | 327 (39.7) | 13 (6.6) | 117 (7.5) | 130 (7.4) |
| Age | ||||||
| <20 years | 0 (0) | 2 (0.3) | 2 (0.2) | 0 (0) | 8 (0.5) | 8 (0.5) |
| 20–29 years | 3 (8.3) | 152 (19.3) | 155 (18.8) | 28 (14.3) | 195 (12.6) | 223 (12.8) |
| 30–39 years | 23 (63.9) | 376 (47.8) | 399 (48.5) | 72 (36.7) | 552 (35.6) | 624 (35.7) |
| 40–49 years | 8 (22.2) | 171 (21.7) | 179 (21.8) | 54 (27.6) | 482 (31.1) | 536 (30.7) |
| 50–59 years | 1 (2.8) | 64 (8.1) | 65 (7.9) | 35 (17.9) | 227 (14.6) | 262 (15) |
| 60–69 years | 1 (2.8) | 15 (1.9) | 16 (1.9) | 7 (3.6) | 70 (4.5) | 77 (4.4) |
| ≥ 70 years | 0 (0) | 7 (0.9) | 7 (0.9) | 0 (0) | 18 (1.2) | 18 (1) |
| Exposure | ||||||
| MSM | 5 (13.9) | 70 (8.9) | 75 (9.1) | 127 (64.8) | 991 (63.9) | 1118 (64) |
| IDU | 1 (2.8) | 39 (5) | 40 (4.9) | 7 (3.6) | 39 (2.5) | 46 (2.6) |
| Heterosexual | 26 (72.2) | 600 (76.2) | 626 (76.1) | 47 (24) | 297 (19.1) | 344 (19.7) |
| Other | 2 (5.6) | 71 (9) | 73 (8.9) | 13 (6.6) | 202 (13) | 215 (12.3) |
| Missing | 2 (5.6) | 7 (0.9) | 9 (1.1) | 2 (1) | 23 (1.5) | 25 (1.4) |
| CD4 count | ||||||
| ≤200 cells/μL | 5 (13.9) | 109 (13.9) | 114 (13.9) | 31 (15.8) | 218 (14) | 249 (14.2) |
| 201–350 cells/μL | 8 (22.2) | 190 (24.1) | 198 (24.1) | 44 (22.5) | 288 (18.6) | 332 (19) |
| 351–500 cells/μL | 7 (19.4) | 183 (23.3) | 190 (23.1) | 54 (27.6) | 341 (22) | 395 (22.6) |
| >500 cells/μL | 12 (33.3) | 243 (30.9) | 255 (31) | 51 (26) | 586 (37.8) | 637 (36.4) |
| Missing | 4 (11.1) | 62 (7.9) | 66 (8) | 16 (8.2) | 119 (7.7) | 135 (7.7) |
| Prior ADI | ||||||
| No | 12 (33.3) | 416 (52.9) | 428 (52) | 125 (63.8) | 1095 (70.6) | 1220 (69.8) |
| Yes | 24 (66.7) | 371 (47.1) | 395 (48) | 71 (36.2) | 457 (29.4) | 528 (30.2) |
| TB ever | ||||||
| No | 32 (88.9) | 693 (88.1) | 725 (88.1) | 189 (96.4) | 1515 (97.6) | 1704 (97.5) |
| Yes | 4 (11.1) | 94 (11.9) | 98 (11.9) | 7 (3.6) | 37 (2.4) | 44 (2.5) |
| Year of first cART | ||||||
| <2000 | 8 (22.2) | 32 (4.1) | 40 (4.9) | 69 (35.2) | 265 (17.1) | 334 (19.1) |
| 2000–2005 | 20 (55.6) | 469 (59.6) | 489 (59.4) | 82 (41.8) | 601 (38.7) | 683 (39.1) |
| ≥ 2006 | 8 (22.2) | 286 (36.3) | 294 (35.7) | 45 (23) | 686 (44.2) | 731 (41.8) |
| HCV ever | ||||||
| No | 19 (52.8) | 413 (52.5) | 432 (52.5) | 160 (81.6) | 1277 (82.3) | 1437 (82.2) |
| Yes | 1 (2.8) | 71 (9) | 72 (8.8) | 15 (7.7) | 100 (6.4) | 115 (6.6) |
| Missing | 16 (44.4) | 303 (38.5) | 319 (38.8) | 21 (10.7) | 175 (11.3) | 196 (11.2) |
| HBV ever | ||||||
| No | 17 (47.2) | 468 (59.5) | 485 (58.9) | 170 (86.7) | 1201 (77.4) | 1371 (78.4) |
| Yes | 1 (2.8) | 56 (7.1) | 57 (6.9) | 7 (3.6) | 82 (5.3) | 89 (5.1) |
| Missing | 18 (50) | 263 (33.4) | 281 (34.1) | 19 (9.7) | 269 (17.3) | 288 (16.5) |
| First regimen | ||||||
| NRTI/PI | 14 (38.9) | 167 (21.2) | 181 (22) | 90 (45.9) | 582 (37.5) | 672 (38.4) |
| NRTI/NNRTI | 22 (61.1) | 617 (78.4) | 639 (77.6) | 84 (42.9) | 816 (52.6) | 900 (51.5) |
| NRTI | 0 (0) | 0 (0) | 0 (0) | 9 (4.6) | 44 (2.8) | 53 (3) |
| NRTI/NNRTI/PI | 0 (0) | 2 (0.3) | 2 (0.2) | 7 (3.6) | 53 (3.4) | 60 (3.4) |
| II | 0 (0) | 0 (0) | 0 (0) | 5 (2.6) | 55 (3.5) | 60 (3.4) |
| Other | 0 (0) | 1 (0.1) | 1 (0.1) | 1 (0.5) | 2 (0.1) | 3 (0.2) |
The analysis included all patients who had achieved undetectable viral load (≤ 50 copies/mL) within 1 year of starting combination antiretroviral therapy (cART) and prior to subsequent virological failure and excluded periods off treatment or on mono/duo for > 7 days.
Regimen at time of first undetectable viral load within 1 year after cART commencement.
ADI, AIDS-defining illness; IDU, injecting drug use; HBV, hepatitis B virus; HCV, hepatitis C virus; LLV, low-level viraemia; MSM, men who have sex with men; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; II, integrase inhibitor; TB, tuberculosis.
A total of 5024 VL tests were performed in L/MICs compared with 20 829 tests in HICs. The median time between tests was 103 days [interquartile range (IQR) 84–154 days] for HICs and 182 days (IQR 149–283 days) for L/MICs. The rate of LLV was significantly higher for HIC settings compared with L/MIC settings per 1000 person-years [28.6 (95% confidence interval (CI) 24.8–32.8) and 9.9 (95% CI 7.2–13.8) per 1000 person-years, respectively] but not in terms of the number of tests [9.4 (95% CI 8.1–10.7) and 7.2 (95% CI 4.8–9.5) per 1000 tests, respectively]. The median duration of episodes of LLV was 268 days (IQR 167–421 days) in HICs compared with 363 days (IQR 252–537 days) in L/MICs, with a median magnitude of 149 copies/mL (IQR 87–290 copies/mL) in HICs and 193 copies/mL (IQR 78–331 copies/mL) in L/MICs.
Mosaic plots (Fig. 1, Appendix 3) demonstrate an increasing distribution of LLV 50–199 copies/mL in recent calendar years for HICs. This is less apparent for L/MICs. The number of VL tests per calendar year per patient was higher in HICs (2.40 tests per patient; 95% CI 2.37–2.43) compared with LICs (1.41 tests per patient; 95% CI 1.38–1.43).
Fig. 1.
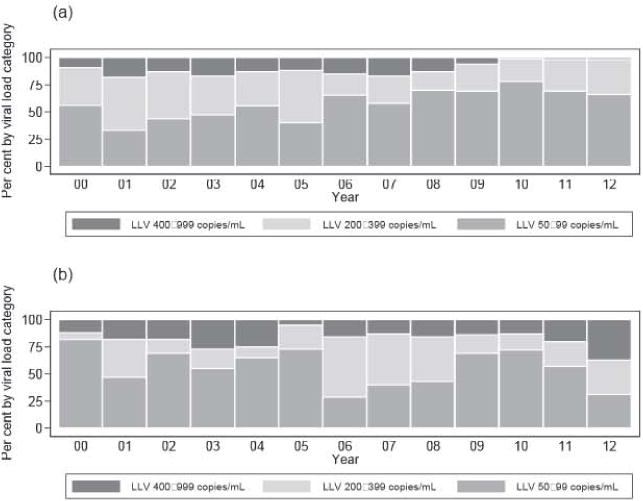
Distribution of low-level viraemia (LLV) by duration for given calendar year and income, (a) High-income countries (b) Low/middle-income countries
Higher rates of regimen change were observed in HICs (Table 2) compared with L/MICs in the setting of LLV. Time to regimen change following first virological control post cART differed between HICs and L/MICs. The median lime to regimen change for those with no prior episodes of LLV was 732 days (IQR 284–1522 days) in HICs compared with 614 days (IQR 365–1518 days) in L/MICs. In those with one prior episode of LLV, the median time to regimen change was 728 days (IQR 359–1604 days) in HICs compared with 896 days (IQR 252–1410 days) in L/MICs. Sixty-four patients from L/MICs and 294 patients from HICs underwent a regimen switch with no prior LLV. Similar proportions of patients in L/MICs underwent changes irrespective of LLV; 7.8% with no prior LLV compared with 8.3% with one episode of LLV. The majority of changes were from a nucleoside reverse transcriptase inhibitor (NRTI)/protease inhibitor (PI) regimen to an NRTI/nonnucleoside reverse transcriptase inhibitor (NNRTI) regimen In L/MICs (55.8%) and HICs (62.4%).
Table 2.
Distribution of regimen changes by number of prior low-level viraemia (LLV) episodes
| Low/middle income
|
High income
|
|||||
|---|---|---|---|---|---|---|
| No prior LLV | First episode | Second episode | No prior LLV | First episode | Second episode | |
| n | 823 | 36 | 3 | 1748 | 194 | 36 |
| No change [n (%)] | 759 (92.2) | 33 (91.7) | 3 (100) | 1454 (83.2) | 153 (78.9) | 26 (72.2) |
| Change [n (%)] | 64 (7.8) | 3 (8.3) | 0 (0) | 294 (16.8) | 41 (21.1) | 10 (27.8) |
| Change from [n (%)] | ||||||
| NRTI/PI | 36 (56.3) | 2 (66.7) | – | 149 (50.7) | 14 (34.1) | 5 (50) |
| NRTI/NNRTI | 28 (43.8) | 1 (33.3) | – | 106 (36.1) | 21 (51.2) | 3 (30) |
| NRTI | – | – | – | 31 (10.5) | 3 (7.3) | 2 (20) |
| NRTI/NNRTI/PI | – | – | – | 4 (1.4) | 1 (2.4) | 0 (0) |
| II | – | – | – | 3 (1) | 1 (2.4) | 0 (0) |
| Other | – | – | – | 1 (0.3) | 1 (2.4) | 0 (0) |
| Change to [n (%)] | ||||||
| NRTI/PI | 8 (12.5) | 0 (0) | – | 75 (25.5) | 12 (29.3) | 1 (10) |
| NRTI/NNRTI | 52 (81.3) | 2 (66.7) | – | 149 (50.7) | 17 (41.5) | 2 (20) |
| NRTI | – | – | – | – | – | – |
| NRTI/NNRTI/PI | 4 (6.3) | 1 (33.3) | – | 25 (8.5) | 7 (17.1) | 4 (40) |
| II | – | – | – | 42 (14.3) | 3 (7.3) | 3 (30) |
| Other | – | – | – | 1 (0.3) | 0 (0) | 0 (0) |
The analysis included all patients who had achieved undetectable viral load (≤ 50 copies/mL) within 1 year of starting combination antiretroviral therapy (cART) and prior to subsequent virological failure, and excluded periods off treatment or on mono/duo for > 7 days. Analysis time commenced at date of viral load test.
Regimen change was defined as a change in class or the addition of two new antiretrovirals, and did not include changes from on to off treatment or mono/duo therapy.
Six patients from high-income countries had three or more episodes.
NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; II, integrase inhibitor.
Kaplan–Meier curves of time to LLV demonstrate a higher risk of LLV prior to VF in HICs than in L/MICs (Fig. 2 and Table 3). There was a marginally significant difference in rates of VF by income by lime at risk. VF occurred al a rate of 41.2 and 53.5 per 1000 person-years in L/MICs and HICs, respectively. The rate of VF per test was significantly higher for L/MICs compared with HICs (30.7 vs. 19.6 per 1000 tests, respectively; P < 0.001).
Fig. 2.
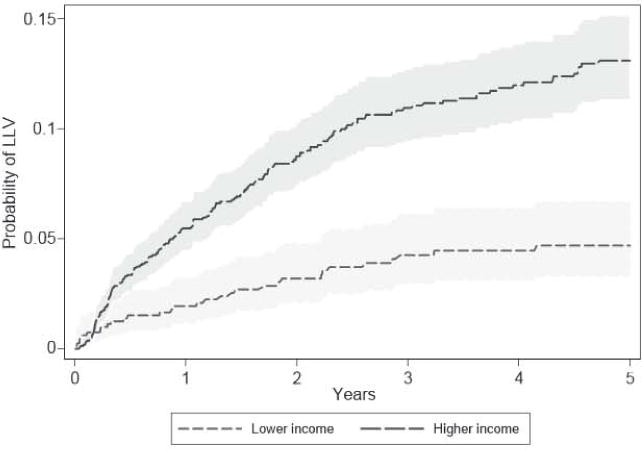
Kaplan–Meier low-level viraemia (LLV) by income status for a duration of up to 5 years from viral control to censor or first virological failure with 95% confidence intervals (shaded).
Table 3.
Kaplan–Meier low-level viraemia (LLV) probabilities by income status for selected years of viral control
| Income | Time (years) | Begin | Failure (95% CI) |
|---|---|---|---|
| Low/middle | 1 | 664 | 0.019 (0.012, 0.032) |
| Low/middle | 2 | 574 | 0.032 (0.021, 0.048) |
| Low/middle | 3 | 506 | 0.043 (0.03, 0.061) |
| Low/middle | 4 | 410 | 0.045 (0.031, 0.063) |
| Low/middle | 5 | 355 | 0.047 (0.033, 0.067) |
| High | 1 | 1370 | 0.055 (0.045, 0.067) |
| High | 2 | 1090 | 0.087 (0.074, 0.103) |
| High | 3 | 867 | 0.109 (0.094, 0.127) |
| High | 4 | 703 | 0.12 (0.103, 0.139) |
| High | 5 | 550 | 0.131 (0.113, 0.151) |
The analysis included all patients who had achieved undetectable viral load (≤ 50 copies/mL) within 1 year of starting combination antiretroviral therapy (cART) and prior to subsequent virological failure, and excluded periods off treatment or on mono/duo for > 7 days.
CI, confidence interval.
Kaplan–Meier curves of time to VF (Fig. 3 and Table 4) demonstrate similar probability of VF by LLV status although probability of VF was moderately higher in LLV patients in L/MICs after 2 years duration. Log rank tests indicated that LLV was moderately predictive of VF at 2 years duration in L/MICs (p=0.041) but not in HICs (p=0.523). Findings are limited by the low number of patients in L/MICs with LLV.
Fig. 3.
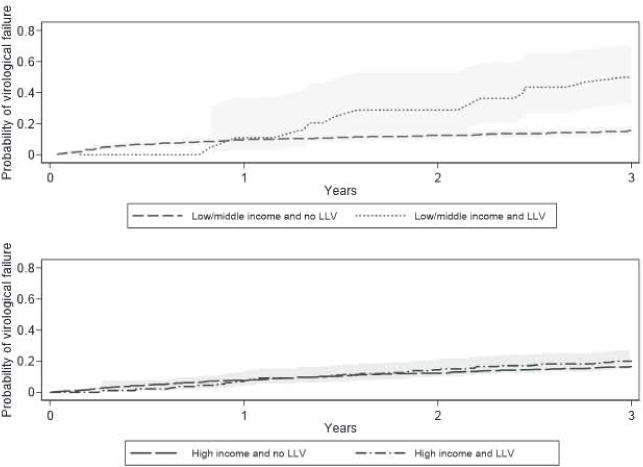
Kaplan–Meier virological failure by income status and low-level viraemia (LLV) status with 95% confidence intervals (shaded).
Table 4.
Kaplan–Meier virological failure probabilities by income status
| Income | LLV ever | Time (years) | Begin | Failure (95% CI) |
|---|---|---|---|---|
| Low/middle | No | 1 | 661 | 0.097 (0.078, 0.12) |
| Low/middle | No | 2 | 573 | 0.126 (0.104, 0.152) |
| Low/middle | No | 3 | 505 | 0.154 (0.129, 0.182) |
| Low/middle | Yes | 1 | 16 | 0.059 (0.009, 0.35) |
| Low/middle | Yes | 2 | 16 | 0.249 (0.112, 0.497) |
| Low/middle | Yes | 3 | 16 | 0.469 (0.296, 0.681) |
| High | No | 1 | 1356 | 0.075 (0.063, 0.089) |
| High | No | 2 | 1082 | 0.126 (0.11, 0.144) |
| High | No | 3 | 864 | 0.169 (0.15, 0.19) |
| High | Yes | 1 | 95 | 0.067 (0.03, 0.142) |
| High | Yes | 2 | 114 | 0.134 (0.082, 0.217) |
| High | Yes | 3 | 106 | 0.207 (0.144, 0.294) |
The analysis included all patients who had achieved undetectable viral load (≤ 50 copies/mL) within 1 year of starting combination antiretroviral therapy (cART), and excluded periods off treatment or on mono/duo for > 7 days.
CI. confidence interval; LLV, low-level viraemia.
ROC analysis to determine which LLV RNA level was predictive of VF found no significant differences for each of the thresholds examined (Appendix 1). Fifty percent sensitivity was attained for RNA thresholds at 180 copies/mL for HICs and 375 copies/mL for LICs.
Sensitivity analyses assessing the significance of LLV with differing definitions of VF and LLV found no significant differences. Rates of VF per 1000 tests were lower for HICs compared with L/MICs for all analyses (Appendix 2). Sensitivity analyses assessing the significance of at least four episodes of LLV found no qualitative differences in HICs compared with L/MICs either by rate per 1000 person-years or rate per number of tests.
Discussion
This analysis found that the rate of LLV was significantly higher for HICs compared with L/MICs per 1000 person-years (28.6 and 9.9 per 1000 person-years, respectively). No significant difference in the rate of LLV was found per number of VL tests in the differing settings. After 2 years from the initial viraemia event, LLV was a weak predictor of failure in L/MICs, but not in HICs. LLV episodes had a higher median magnitude and longer median duration in L/MICs compared with HICs. The median time between tests was vastly longer in L/MTCs compared with HICs.
Much higher rates of regimen change occurred in HICs, with a greater range of changes to patient regimens in these settings compared with L/MICs. Most patients who experienced LLV in HICs were switched from an NRTI/PI regimen to an NRTI/NNRTI regimen (26.8%), in keeping with previous studies [8]. Very few regimen changes occurred in L/MICs in the setting of LLV. In L/MICs, NRT1/NNRTI regimens were used as the initial regimen 77.6% of the lime vs. 51.5% of the time in HICs (Table 1). The median time to regimen change after virological control had been established (within 1 year following cART initiation) was much longer in L/MICs compared with HICs, for patients with and without prior episodes of LLV. The proportions of patients in L/MICs who underwent a change in treatment were very similar for those with and without LLV, suggesting that LLV may not be the major factor driving regimen switch in these settings.
This is the first analysis to assess the significance of LLV in resource-limited settings with less frequent access to VL testing. It reflects ‘real-world decision-making’ in an area of clinical uncertainty across resource-limited and resource-replete settings regarding the timing and choice of regimen changes. It is the first to assess the significance of different definitions of LLV, VF and persistent LLV in L/MICs. A limitation of the analysis is the low number of LLV events in L/MIC settings, which limits the development of appropriately adjusted prognostic models. A further limitation is the omission of ‘undetectable’ VL measures with less sensitive assays (with lower limits of detection of 200 or 400 copies/mL). The omission of these measures, rather than the omission of these patients, potentially biased the results to higher levels of detectable VL. A further limitation is the inability to examine adherence as a factor in the development of LLV, although it is likely to play a role in some individuals.
This analysis supports findings from other studies in resource-replete settings regarding the low probability of developing VF in the setting of LLV [18]. Very few patients in this analysis experienced persistent LLV. Of note, rates of VF were slightly higher for patients with prior LLV in L/MICs, but not in HICs. However, it should be noted that a recent Canadian study [25] of 2416 patients found an association with VF regardless of how low the persistent viraemia had been, although the trend was lower at lower VL levels.
LLV appears to occur more frequently in HICs; however, in these settings VL is tested more frequently, and thus rebound viraemia is detected earlier and at lower levels. More patients are switched, and in a shorter time, in HICs compared with L/MICs in the setting of LLV. Fewer patients in HICs developed VF. It is possible that this may be attributable to an intervention (switching or improved adherence) prompted by detection of LLV. It does seem likely that the threshold for changing therapy because of VL elevation has been higher in poorer countries, in keeping with World Health Organization (WHO) guidelines which have only recently suggested using a level of 1000 copies/mL for treatment failure, with a previous level of 5000 copies/mL [26]. Over time, as with CD4 thresholds for therapy, in L/MICs this may also change to reflect practice in HICs. Viral resistance data, which were largely unavailable for this study, would be useful in helping to understand these phenomena and their impact on practice, in particular the question of resistance vs. adherence.
Several questions remain for further study. It remains unclear whether the lower VF rates in HICs are caused by early regimen switch or improved adherence. The cost-effectiveness of increased VL testing to support such interventions, in HICs and L/MICs, also warrants further investigation. Further studies are likely to be needed to determine best practice in all settings in this difficult and relatively common problem.
Table A4.
Rate of low-level viraemia (LLV) per 1000 tests by income
| Income | Number of tests | LLV (n) | Crude rate (95% CI) (per 1000 tests) |
|---|---|---|---|
| Low/middle | 4485 | 15 | 3.345 (1.655, 5.034) |
| High | 17956 | 90 | 5.012 (3.979, 6.045) |
Virological failure = 200 copies/mL; LLV = 50–200 copies/mL.
The analysis included all tests in patients who had achieved undetectable viral load (≤ 50 copies/mL) within 1 year of starting combination antiretroviral therapy (cART) and up to and including first virological failure, and excluded counts of virological failure when off treatment or on mono/duo for > 7 days.
Table A5.
Rate of first virological failure per 1000 person-years by income
| Income | Virological failure (n) | Person-years (1000’s) | Crude rate (95% CI) (per 1000 person-years) |
|---|---|---|---|
| Low/middle | 233 | 3.360 | 69.345 (60.989, 78.846) |
| High | 593 | 6.601 | 89.834 (82.887, 97.363) |
Virological failure = 200 copies/mL; low-level viraemia (LLV) = 50–200 copies/mL.
The analysis included all patients who had achieved undetectable viral load (≤ 50 copies/mL) within 1 year of starting combination antiretroviral therapy (cART) and prior to subsequent virological failure, and excluded periods off treatment or on mono/duo for > 7 days.
Table A6.
Rate of first virological failure per 1000 tests by income
| Income | Number of tests | LLV (n) | Crude rate (95% CI) (per 1000 tests) |
|---|---|---|---|
| Low/middle | 4485 | 233 | 51.951 (45.456, 58.446) |
| High | 17956 | 593 | 33.025 (30.411, 35.639) |
Virological failure = 200 copies/mL; low-level viraemia (LLV) = 50–200 copies/mL.
The analysis included all tests in patients who had achieved undetectable viral load (≤ 50 copies/mL) within 1 year of starting combination antiretroviral therapy (cARTI and up to and including first virological failure, and excluded counts of virological failure when off treatment or on mono/duo for > 7 days.
Table A7.
Rate of first low-level viraemia (LLV) per 1000 person-years by income
| Income | LLV (n) | Person-years (1000’s) | Crude rate (95% CI) (per 1000 person years) |
|---|---|---|---|
| Low/middle | 4 | 3.583 | 1.116 (0.419, 2.974) |
| High | 24 | 6.918 | 3.469 (2.325, 5.176) |
Virological failure = 400 copies/mL; LLV = 200–400 copies/mL.
The analysis included all patients who had achieved undetectable viral load (≤ 50 copies/mL) within 1 year of starting combination antiretroviral therapy (cART) and prior to subsequent virological failure, and excluded periods off treatment or on mono/duo for > 7 days.
Table A8.
Rate of low-level viraemia (LLV) per 1000 tests by income
| Income | Number of tests | LLV (n) | Crude rate (95% CI) (per 1000 tests) |
|---|---|---|---|
| Low/middle | 4784 | 4 | 0.836 (0.017, 1.655) |
| High | 19084 | 24 | 1.258 (0.755, 1.76) |
Virological failure = 400 copies/mL; LLV = 200–400 copies/mL.
The analysis included all tests in patients who had achieved undetectable viral load (≤50 copies/mL) within 1 year of starting combination antiretroviral therapy (cART) and up to and including first virological failure, and excluded counts of virological failure when off treatment or on mono/duo for > 7 days.
Table A9.
Rate of first virological failure per 1000 person-years by income
| Income | Virological failure (n) | Person-years (1000’s) | Crude rate (95% CI) (per 1000 person years) |
|---|---|---|---|
| Low/middle | 187 | 3.586 | 52.145 (45.182, 60.18) |
| High | 509 | 7.002 | 72.69 (66.642, 79.288) |
Virological failure = 400 copies/mL; low-level viraemia (LLV) = 200–400 copies/mL.
The analysis included all patients who had achieved undetectable viral load (≤ 50 copies/mL) within 1 year of starting combination antiretroviral therapy (cART) and prior to subsequent viral failure, and excluded periods off treatment or on mono/duo for > 7 days.
Table A11.
Rate of first low-level viraemia (LLV) per 1000 person-years by income
| Income | LLV (n) | Person-years (1000’s) | Crude rate (95% CI) (per 1000 person years) |
|---|---|---|---|
| Low/middle | 56 | 3.660 | 15.302 (11.776, 19.883) |
| High | 294 | 7.025 | 41.849 (37.329, 46.917) |
Virological failure = 5000 copies/mL; LLV ≤ 5000 copies/mL.
The analysis included all patients who had achieved undetectable viral load (≤ 50 copies/mL) within 1 year of starting combination antiretroviral therapy (cART) and prior to subsequent viral failure, and excluded periods off treatment or on mono/duo for > 7 days.
Table A12.
Rate of low-level viraemia (LLV) per 1000 tests by income
| Income | Number of tests | LLV (n) | Crude rate (95% CI) (per 1000 tests) |
|---|---|---|---|
| Low/middle | 5285 | 56 | 10.596 (7.836, 13.357) |
| High | 22764 | 294 | 12.915 (11.448, 14.382) |
Virological failure = 500 copies/mL; LLV≤ 5000 copies/mL.
The analysis included all tests in patients who had achieved undetectable viral load (≤ 50 copies/mL) within 1 year of starting combination antiretroviral therapy (cART) and up to and including first viral failure, and excluded counts of virological failure when off treatment or on mono/duo for > 7 days.
Table A13.
Rate of first virological failure per 1000 person-years by income
| Income | Virological failure (n) | Person-years (1000’s) | Crude rate (95% CI) (per 1000 person years) |
|---|---|---|---|
| Low/middle | 128 | 3.884 | 32.954 (27.712, 39.188) |
| High | 302 | 8.265 | 36.54 (32.642, 40.902) |
Virological failure = 5000 copies/mL; LLV ≤ 5000 copies/mL.
The analysis included all patients who had achieved undetectable viral load (≤ 50 copies/mL) within 1 year of starting combination antiretroviral therapy (cART) and prior to subsequent viral failure, and excluded periods off treatment or on mono/duo for > 7 days.
Table A14.
Rate of first virological failure per 1000 tests by income
| Income | Number of tests | Virological failure (n) | Crude rate (95% CI) (per 1000 tests) |
|---|---|---|---|
| Low/middle | 4485 | 128 | 24.22 (20.075, 28.364) |
| High | 17956 | 302 | 13.267 (11.78, 14.753) |
Virological failure = 5000 copies/mL; LLV ≤ 5000 copies/mL.
The analysis included all tests in patients who had achieved undetectable viral load (≤ 50 copies/mL) within 1 year of starting combination antiretroviral therapy (cART) and up to and including first viral failure, and excluded counts of virological failure when off treatment or on mono/duo for > 7 days.
Table A15.
Mosaic plot source data percentage for figure 1
| Year | Income | % of patients
|
||
|---|---|---|---|---|
| LLV
| ||||
| 50–199 copies/mL |
200–399 copies/mL |
400–999 copies/mL |
||
| 2000 | High | 56 | 35 | 9 |
| 2001 | High | 33 | 49 | 18 |
| 2002 | High | 44 | 44 | 13 |
| 2003 | High | 48 | 36 | 17 |
| 2004 | High | 55 | 31 | 13 |
| 2005 | High | 40 | 48 | 12 |
| 2006 | High | 66 | 20 | 15 |
| 2007 | High | 58 | 25 | 17 |
| 2008 | High | 70 | 17 | 13 |
| 2009 | High | 69 | 25 | 6 |
| 2010 | High | 78 | 21 | 1 |
| 2011 | High | 69 | 29 | 2 |
| 2012 | High | 66 | 32 | 2 |
| 2000 | Low/middle | 82 | 6 | 12 |
| 2001 | Low/middle | 47 | 35 | 18 |
| 2002 | Low/middle | 69 | 13 | 18 |
| 2003 | Low/middle | 55 | 18 | 27 |
| 2004 | Low/middle | 65 | 10 | 25 |
| 2005 | Low/middle | 73 | 22 | 5 |
| 2006 | Low/middle | 29 | 55 | 16 |
| 2007 | Low/middle | 40 | 47 | 13 |
| 2008 | Low/middle | 43 | 41 | 16 |
| 2009 | Low/middle | 69 | 17 | 14 |
| 2010 | Low/middle | 72 | 15 | 13 |
| 2011 | Low/middle | 57 | 23 | 20 |
| 2012 | Low/middle | 31 | 32 | 37 |
LLV, low-level viraemia.
Appendix 1
Tables A1 and A2 show the results from ROC analyses for HIC and M/LIC countries respectively for a range of RNA thresholds defining the lower limit of positive LLV status predictive of subsequent VF.
Table A1.
Analysis of low-level viraemia (LLV) episode mean RNA threshold to determine patients from high-income counties with subsequent virological failure
| RNA threshold (copies/mL) | Sensitivity (%) | Specificity (%) | Area under the receiver operating characteristic curve (95% CI) |
|---|---|---|---|
| 150 | 58.49 | 52.11 | 0.553 (0.481, 0.625) |
| 200 | 49.06 | 55.63 | 0.524 (0.451, 0.595) |
| 250 | 37.74 | 67.61 | 0.527 (0.456, 0.6) |
| 300 | 33.96 | 74.65 | 0.543 (0.471, 0.615) |
| 350 | 32.08 | 83.10 | 0.576 (0.502, 0.645) |
| 400 | 22.64 | 89.44 | 0.560 (0.486, 0.63) |
| 450 | 18.87 | 93.66 | 0.563 (0.491, 0.635) |
| 500 | 13.21 | 97.89 | 0.556 (0.481, 0.625) |
Sensitivity = 50% for RNA test result > 180 copies/mL
Table A2.
Analysis of low-level viraemia (LLV) mean RNA threshold to determine patients from low/middle-income countries with subsequent virological failure
| RNA threshold (copies/mL) | Sensitivity (%) | Specificity (%) | Area under the receiver operating characteristic curve (95% CI) |
|---|---|---|---|
| 150 | 76.92 | 47.83 | 0.624 (0.435, 0.769) |
| 200 | 76.92 | 65.22 | 0.711 (0.548, 0.858) |
| 250 | 69.23 | 69.57 | 0.694 (0.519, 0.837) |
| 300 | 61.54 | 82.61 | 0.721 (0.548, 0.858) |
| 350 | 61.54 | 86.96 | 0.743 (0.578, 0.879) |
| 400 | 30.77 | 86.96 | 0.589 (0.408, 0.745) |
| 450 | 23.08 | 95.65 | 0.594 (0.408, 0.745) |
| 500 | 23.08 | 100.00 | 0.615 (0.435, 0.769) |
Sensitivity = 50% for RNA > 375 copies/mL
Appendix 2
Tables A3–A10 show the rates of VF (based on person years and on number of tests) by income group (L/MIC and HIC) and specified thresholds for LLV and VF.
Table A3.
Rate of first low-level viraemia (LLV) per 1000 person-years by income
| Income | Virological failure (n) | Person-years (1000’s) | Crude rate (95% CI) (per 1000 person-years) |
|---|---|---|---|
| Low/middle | 15 | 3.310 | 4.532 (2.732, 7.517) |
| High | 90 | 6.350 | 14.174 (11.528, 17.426) |
Virological failure = 200 copies/ml; LLV = 50–200 copies/ml.
The analysis included all patients who had achieved undetectable viral load (≤ 50 copies/ml) within 1 year of starting combination antiretroviral therapy (cART) and prior to subsequent virological failure, and excluded periods off treatment or on mono/duo for > 7 days.
Table A10.
Rate of first virological failure per 1000 tests by income
| Income | Number of tests | Virological failure (n) | Crude rate (95% CI) (per 1000 tests) |
|---|---|---|---|
| Low/middle | 4784 | 187 | 39.089 (33.597, 44.581) |
| High | 19084 | 509 | 26.672 (24.386, 28.958) |
Virological failure = 400 copies/mL; LLV = 200–400 copies/mL.
The analysis included all tests in patients who had achieved undetectable viral load (≤ 50 copies/mL) within 1 year of starting combination antiretroviral therapy (cART) and up to and including first virological failure, and excluded counts of virological failure when off treatment or on mono/duo for > 7 days.
Sensitivity analysis: use of different thresholds of low-level viraemia (LLV) and virological failure (VF).
Appendix 3
This table shows source data for Figure 1.
References
- 1.Bartlett JA, Fath MJ, Demasi R, et al. An updated systematic overview of triple combination therapy in antiretroviral-naive HIV-infected adults. AIDS. 2006;20:2051–2064. doi: 10.1097/01.aids.0000247578.08449.ff. [DOI] [PubMed] [Google Scholar]
- 2.Barth RE, van der Loeff MF, Schuurman R, Hoepelman AI, Wensing AM. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. Lancet Infect Dis. 2010;10:155–166. doi: 10.1016/S1473-3099(09)70328-7. [DOI] [PubMed] [Google Scholar]
- 3.Grennan JT, Loutfy MR, Su D, et al. Magnitude of virologic blips is associated with a higher risk for virologic rebound in HIV-infected individuals: a recurrent events analysis. J Infect Dis. 2012;205:1230–1238. doi: 10.1093/infdis/jis104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panel on antiretroviral guidelines for adults and adolescents Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Available at http://arv.ashm.org.au (accessed 21 January 2014) [Google Scholar]
- 5.Karlsson AC, Younger SR, Martin JN, et al. Immunologic and virologic evolution during periods of intermittent and persistent low-level viremia. AIDS. 2004;18:981–989. doi: 10.1097/00002030-200404300-00005. [DOI] [PubMed] [Google Scholar]
- 6.Greub G, Cozzi-Lepri A, Ledergerber B, et al. Intermittent and sustained low-level HIV viral rebound in patients receiving potent antiretroviral therapy. AIDS. 2002;16:1967–1969. doi: 10.1097/00002030-200209270-00017. [DOI] [PubMed] [Google Scholar]
- 7.Sungkanuparph S, Overton ET, Seyfried W, Groger RK, Fraser VJ, Powderly WG. Intermittent episodes of detectable HIV viremia in patients receiving nonnucleoside reverse-transcriptase inhibitor-based or protease inhibitor-based highly active antiretroviral therapy regimens are equivalent in incidence and prognosis. Clin Infect Dis. 2005;41:1326–1332. doi: 10.1086/496985. [DOI] [PubMed] [Google Scholar]
- 8.Geretti AM, Smith C, Haberl A, et al. Determinants of virological failure after successful viral load suppression in first-line highly active antiretroviral therapy. Antivir Ther. 2008;13:927–936. [PubMed] [Google Scholar]
- 9.Maggiolo F, Callegaro A, Cologni G, et al. Ultrasensitive assessment of residual low-level HIV viremia in HAART-treated patients and risk of virological failure. J Acquir Immune Dcfic Syndr. 2012;60:473–482. doi: 10.1097/QAI.0b013e3182567a57. [DOI] [PubMed] [Google Scholar]
- 10.Pilcher CD, Miller WC, Beatty ZA, Eron JJ. Detectable HIV–1 RNA at levels below quantifiable limits by amplicor HIV-1 monitor is associated with virologic relapse on antiretroviral therapy. AIDS. 1999;13:1337–1342. doi: 10.1097/00002030-199907300-00010. [DOI] [PubMed] [Google Scholar]
- 11.Chao C, Tang B, Towner W, Silverberg MJ, Hurley L, Horberg M. Short-term clinical outcomes among treatment-experienced HIV-positive patients with early low level viremia. AIDS Patient Care STDS. 2012;26:253–255. doi: 10.1089/apc.2012.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li JZ, Gallien S, Do TD, et al. Prevalence and significance of HIV-1 drug resistance mutations among patients on antiretroviral therapy with detectable low-level viremia. Antimicrob Agents Chemother. 2012;56:5998–6000. doi: 10.1128/AAC.01217-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delaugerre C, Gallien S, Flandre P, et al. Impact of low-level-viremia on HIV-1 drug-resistance evolution among antiretroviral treated-patients. PLoS ONE. 2012;7:e36673. doi: 10.1371/journal.pone.0036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallien S, Delaugerre C, Charreau I, et al. Emerging integrase inhibitor resistance mutations in raltegravir-treated HIV-1-infected patients with low-level viremia. AIDS. 2011;25:665–669. doi: 10.1097/QAD.0b013e3283445834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nettles RE, Kieffer TL, Simmons RP, et al. Genotypic resistance in HIV-1-infected patients with persistently detectable low-level viremia while receiving highly active antiretroviral therapy. Clin Infect Dis. 2004;39:1030–1037. doi: 10.1086/423388. [DOI] [PubMed] [Google Scholar]
- 16.Taiwo B, Gallien S, Aga E, et al. Antiretroviral drug resistance in HIV-1-infected patients experiencing persistent low-level viremia during first-line therapy. J Infect Dis. 2011;204:515–520. doi: 10.1093/infdis/jir353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang S, van Sighem A, Kesselring A, et al. Episodes of HIV viremia and the risk of non-AIDS diseases in patients on suppressive antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;60:265–272. doi: 10.1097/QAI.0b013e318258c651. [DOI] [PubMed] [Google Scholar]
- 18.Ribaudo H, Lennox J, Currier J, et al. Virologic failure endpoint definition in clinical trials: is using HIV-1 RNA threshold <200 copies/mL better than <50 copies/mL? An analysis of ACTG studies. 16th Conference on Retroviruses and Opportunistic Infections; Montreal. 2009. [Google Scholar]
- 19.Doyle T, Smith C, Vitiello P, et al. Plasma HIV-1 RNA detection below 50 copies/ml and risk of virologic rebound in patients receiving highly active antiretroviral therapy. Clin Infect Dis. 2012;54:724–732. doi: 10.1093/cid/cir936. [DOI] [PubMed] [Google Scholar]
- 20.Anonymous. Available at http://www.amfar.org/Around_the_World/TREAT_Asia/Research_and_Treatment/TREAT_Asia_HIV_AIDS_observational_Database/ (accessed 13 January 2014)
- 21.Anonymous. Available at http://www.med.unsw.edu.au/nchecrweb.nsf/page/AustHIVObservationalDb (accessed 13 January 2014)
- 22.Austin D, Baker D, Block M, et al. Rates of combination antiretroviral treatment change in Australia, 1997–2000. HIV Med. 2002;3:28–36. doi: 10.1046/j.1464-2662.2001.00094.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhou J, Kumarasamy N, Ditangco R, et al. The TREAT Asia HIV Observational Database: baseline and retrospective data. J Acquir Immune Defic Syndr. 2005;38:174–179. doi: 10.1097/01.qai.0000145351.96815.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anonymous. Available at http://data.worldbank.org/about/country-classifications (accessed 13 January 2014)
- 25.Laprise C, de Pokomandy A, Baril JG, Dufresnc S, Trottier H. Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients: results from 12 years of observation. Clin Infect Dis. 2013;57:1489–1496. doi: 10.1093/cid/cit529. [DOI] [PubMed] [Google Scholar]
- 26.Anonymous. Available at http://www.who.int/hiv/pub/guidelines/arv2013/art/artmonitoring/en/index3.html (accessed 13 January 2014)


