Abstract
This study examined possible mechanisms by which Substance P (Sub P) assumes a pronociceptive role in the rostral ventromedial medulla (RVM) under conditions of peripheral inflammatory injury, in this case produced by intraplantar (ipl) injection of complete Freund’s adjuvant (CFA). In saline- and CFA-treated rats, neurokinin-1 receptor (NK1R) immunoreactivity was localized to neurons in the RVM. Four days after ipl injection of CFA, the number of NK1R immunoreactive neurons in the RVM was increased by 30%, and there was a concomitant increase in NK1R immunoreactive processes in CFA-treated rats. Although NK1R immunoreactivity was increased, tachykinin-1 receptor (Tacr1) mRNA was not increased in the RVM of CFA-treated rats. To assess changes in Sub P release, the number of RVM neurons that exhibited NK1R internalization was examined in saline- and CFA-treated rats following noxious heat stimulation of the hind paws. Only CFA-treated rats that experienced noxious heat stimulation exhibited a significant increase in the number of neurons showing NK1R internalization. These data suggest that tonic Sub P release is not increased as a simple consequence of peripheral inflammation, but that phasic or evoked release of Sub P in the RVM is increased in response to noxious peripheral stimulation in a persistent inflammatory state. These data support the proposal that an upregulation of the NK1R in the RVM, as well as enhanced release of Sub P following noxious stimulation underlie the pronociceptive role of Sub P under conditions of persistent inflammatory injury.
Keywords: complete Freund’s adjuvant, nociception, hyperalgesia, Tacr1, Substance P
INTRODUCTION
Substantial evidence supports a role of Substance P (Sub P) in the periphery and spinal cord in the induction and maintenance of heat hyperalgesia and mechanical hypersensitivity after peripheral injury (reviewed by (Baranauskas and Nistri, 1998; Sandkuhler et al., 2000; Mantyh, 2002; Keeble and Brain, 2004; Todd, 2010). However, the actions of Sub P at supraspinal sites remain comparatively unexplored. The rostral ventromedial medulla (RVM) plays a central role in the modulation of nociception by virtue of its direct projections to the spinal cord and its role as a relay nucleus for more rostral pain modulatory nuclei (Heinricher et al., 2009). The RVM contains high concentrations of Sub P, which originates from the nucleus cuneiformis and periaqueductal gray (Beitz, 1982; Chen et al., 2013), as well as moderate levels of the neurokinin-1 receptor (NK1R) (Saffroy et al., 1988; Nakaya et al., 1994). Microinjection of NK1R antagonists in the RVM (Pacharinsak et al., 2008; Hamity et al., 2010; Lagraize et al., 2010; Brink et al., 2012) can prevent or reverse mechanical hypersensitivity or heat hyperalgesia following peripheral inflammatory injury. These same antagonists are without effect in the absence of injury. Similarly, chemical ablation of RVM neurons that express the NK1R diminishes heat hyperalgesia and mechanical hypersensitivity evoked by intraplantar (ipl) injection of capsaicin, but does not alter responsiveness to heat or mechanical stimuli in the absence injury (Khasabov and Simone, 2013). Collectively, these findings strongly support the proposal that Sub P assumes a pronociceptive role in the RVM under conditions of inflammatory injury.
There are multiple mechanisms by which Sub P can act in the RVM to initiate and maintain heat hyperalgesia and mechanical hypersensitivity after injury. Iontophoretic application of Sub P excites on cells, a population of putative pain facilitatory neurons in the RVM (Budai et al., 2007). Moreover, the enhanced on cell activity observed in rats that received ipl injection of capsaicin can be blocked by iontophoresis of a NK1R antagonist in the RVM (Budai et al., 2007; Brink et al., 2012). However, several other mechanisms may contribute to the pronociceptive role of Sub P. One possibility is an upregulation in the number or affinity of NK1R in the RVM. Western blotting experiments support an upregulation of NK1R in the RVM after peripheral inflammatory injury (Lagraize et al., 2010). However, this approach cannot distinguish between neurons and glia. The latter is relevant because glia in the RVM play a role in nociceptive mechanisms (Ren and Dubner, 2008; Wei et al., 2008; Roberts et al., 2009). Another possibility is that persistent inflammatory injury results in an increased or sustained release of Sub P in the RVM.
Several approaches were used to investigate the possible mechanism(s) that underlie the pronociceptive role of Sub P in the RVM following peripheral inflammatory injury. The experiments were conducted four days after ipl injection of complete Freund’s adjuvant (CFA) in one hind paw, and modeled a subchronic inflammatory injury. This study first confirmed the neuronal expression of NK1R in the RVM, and determined whether astrocytes in the RVM express NK1R before or after inflammatory injury. Using a stereological approach, it then determined whether the numbers of NK1R immunoreactive neurons are increased in the RVM of CFA-treated rats. Copy numbers of Tacr1 were also determined. Finally, because internalization of NK1R is a well-accepted physiological measure of Sub P release in the spinal cord (Mantyh et al., 1995b; Allen et al., 1997; Marvizon et al., 1997; Marvizon et al., 2003), this study also examined the number of RVM neurons that exhibit internalization of NK1R with and without noxious heat stimulation of the hind paws.
MATERIALS AND METHODS
These experiments were approved by The University of Iowa Animal Care and Use Committee, and were conducted in accordance with the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health and the ethical guidelines of the International Association for the Study of Pain. Every effort was made to reduce the number and suffering of animals used in this study. Adult male Sprague-Dawley rats (Charles River, Raleigh, NC) weighing 275–325 g were used in these studies.
Model of Inflammatory Injury
Complete Freund’s adjuvant was used to model an immune-mediated inflammatory injury. The rats were lightly anesthetized with isoflurane and the thickness of the hind paw in the dorsoventral axis was measured with digital calipers. The left hind paw was then injected with 150 μl of CFA (150 μg of Mycobacterium butyricum, 85% Marcol 52, and 15% Aracel A mannide monoemulsifier; Calbiochem, San Diego, CA) or saline, pH 7.4. Rats were returned to their cages for four days.
Heat Stimulation
Rats were acclimated to the testing environment for 30 min and then placed in individual Plexiglas chambers situated on a 25°C glass surface for another 30-min period of acclimation. A high-intensity beam of light was focused on the plantar surface of each hind paw until the rat withdrew its hind paw from the heat stimulus. Two exposures were made on each hind paw. The heat stimulus elicited paw withdrawal latencies of 8 to 12 s in the hind paws of saline-treated rats and the contralateral hind paw of CFA-treated rats, and withdrawal latencies of 4 to 6 s on ipsilateral paws of CFA-treated rats.
Immunohistochemistry
Antibody characterization
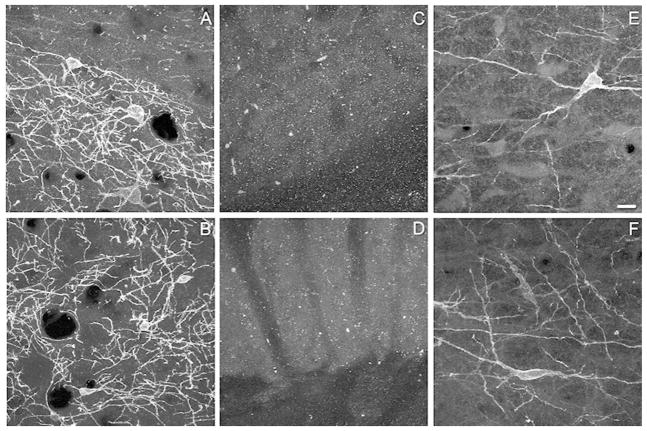
Table 1 identifies the antibodies used for immunohistochemistry. A rabbit polyclonal anti-NK1R antibody from Chemicon (Temecula, CA; Ab5060, lot LV1378369) and a rabbit polyclonal from Sigma (St. Louis, MO; S8305, lot 067K4885) were selected from the literature and evaluated for their suitability. Although preabsorption of the Chemicon antibody with an excess of a peptide corresponding to residues 393–407 of the NK1R abolishes immunostaining in the suprachiasmatic nuclei of the mouse and rat (Piggins et al., 2001), no information is available regarding its performance in Western blotting or NK1R−/− mice. The Sigma antibody recognizes a 46-kDa band in Western blots of rat brain, which is abolished by preabsorption of the antibody with the immunizing peptide (manufacturer’s data). This antibody also labels NK1R in sections of medulla and spinal cord from wild type mice, but not NK1R−/− mice (Ptak et al., 2002). The specificity of each antibody was further confirmed in our hands by an absence of labeling in the RVM of rats upon dilution or omission of the primary antibody, or by preabsorption of the primary antibody with a 10-fold excess of the immunizing peptide (data not shown). Furthermore, sections of striatum and RVM from C57BL6/J and NK1R−/− mice were processed in parallel for NK1R immunoreactivity. Each antibody labeled numerous neurons and processes in the striatum of the C57BL6/J mouse (Fig. 1A,B). This labeling was absent in the striatum of the NK1R−/− mouse (Fig. 1C,D); labeling was similarly absent in the RVM of the NK1R−/− mouse (data not shown). The Chemicon antibody labeled the cytoplasm as well, and appeared to have a somewhat higher level of nonspecific staining (Fig. 1E). The NK1R antibody from Sigma was used for the remainder of the experiments because non-specific labeling was less and the labeling was more restricted to the membrane of the soma and processes (Fig. 1F), which facilitated detection of immunoreactive endosomes.
Table 1.
Primary antibodies used for immunohistochemistry
| Antigen | Immunogen | species | Source |
|---|---|---|---|
| Neurokinin-1 receptor (NK1R) | Synthetic peptide corresponding to amino acids 385–407 from the C-terminus of the rat NK1R | Rabbit polyclonal | Chemicon, Temecula, CA; Ab5060, lot LV1378369 |
| Neurokinin-1 receptor (NK1R) | Synthetic peptide corresponding to amino acids 393–407 from the C-terminus of the rat NK1R; conjugated to keyhole limpet hemocyanin | Rabbit polyclonal | Sigma-Aldrich, St. Louis, MO; S8305, lot 067K4885 |
| NeuN | Purified cell nuclei | Mouse monoclonal | Millipore, Temecula, CA; MAB377, lot LV1634819 |
| GFAP | Full length native protein isolated from a Triton X-100 extract of myelin associated material and then purified by centrifugation and ion exchange chromatography | Chicken | Abcam, Cambridge, MA; ab4674, lot 923263 |
Figure 1.
Representative images demonstrating the specificity of the two neurokinin-1 receptor (NK1R) antibodies. Panels A, C and E were obtained with antisera from Chemicon (Ab5060 lot number LV1378369). Panels B, D and F were obtained using antisera from Sigma-Aldrich (S8305, lot number 067K4885). In the striatum of wild type mice both antibodies produced strong labeling of soma and processes (A,B). Specific labeling was absent in the striatum of a NK1R −/− mouse (C,D). Using the Chemicon antisera, NK1R immunoreactivity was present on the membrane and processes, as well as within the cytoplasm (E) of neurons in the rostral ventromedial medulla (RVM). In contrast, in sections processed with the Sigma antisera (F), NK1R immunoreactivity was largely restricted to the membrane of the soma and processes of neurons in the RVM. Images in panels A–D are projections of four confocal sections taken at an interval of 0.51 or 0.63 microns. Panel E is a projection of three confocal sections taken at an interval of 0.84 microns, while panel F is a projection of four confocal sections taken at an interval of 0.63 microns. Scale bar is 20 microns and applies to each panel.
Antibodies to NeuN and to glial fibrillary acid protein (GFAP) were used to identify neurons and astrocytes, respectively. The mouse monoclonal antibody NeuN (Millipore, Temecula, CA; MAB377, lot LV1634819) reacts with a protein specific to neurons (Mullen et al., 1992). The antibody to GFAP (Abcam, Cambridge, MA; ab4674, lot 923263) labels a 50 kDa band in Western blots of Apteronotus leptorhynchus brain tissue lysate (manufacturer’s data), as well as rat brainstem in our hands (data not shown).
Secondary antibodies were purchased from Jackson ImmunoResearch (West Grove, PA) and were highly cross absorbed for minimal species cross-reactivity. The secondary antibodies were donkey anti-rabbit DyLight 549 (711-505-152; lot 94382), donkey anti-mouse DyLight 488 (715-485-150, lot 92290), and donkey anti-chicken DyLight 649 (703-495-155, lot 92438).
Tissue Processing
Between 5 and 15 minutes after behavioral testing, rats were deeply anesthetized with sodium pentobarbital (75 mg/kg i.p.). Each rat was perfused through the proximal ascending aorta with 100 ml of 0.9 % saline pH 7.4 at 37°C followed by 300 ml of ice-cold 4% paraformaldehyde in phosphate buffer pH 7.4. The brain was removed and placed in 30% sucrose phosphate buffer at 4°C for 48 hours for cryoprotection. Coronal sections of 50-μm thickness were cut through the rostral-caudal extent of the RVM using a cryostat microtome. Sections were collected into 0.1 M phosphate-buffered saline (PBS) pH 7.4 and processed free-floating in individual wells (Netwell™, Electron Microscopy Sciences, Fort Washington, PA) to minimize handling and to preserve the order in which they were obtained. Sections were rinsed twice in 0.1 M PBS and then incubated for 2 hr in 2% normal donkey serum (Lampire, Pipersville, PA) with 0.3% Triton X-100 prepared in 0.1 M PBS pH 7.4, which was also used as the diluent for all antibody solutions. The sections were then incubated in primary antibody solutions for 40 hr at 4°C on an orbital shaker. For experiments that determined the number of NK1R positive neurons, sections were labeled with rabbit anti-NK1R (4.85 μg/ml) and mouse anti-NeuN (1 μg/ml). For experiments in which endosomes were analyzed, a ten-fold lower concentration of NK1R antibody (0.48 μg/ml) was used. For sections that evaluated colocalization of NK1R with GFAP, the anti-GFAP antibody was used at a concentration of 6.6 μg/ml. After four washes in 0.1 M PBS, the sections were incubated in secondary antibody solutions for 1 hr at room temperature at a concentration of 1.9 μg/ml. Following incubation in secondary antibody, the sections were washed thrice with 0.1 M PBS, mounted from distilled water onto slides, and allowed to dry overnight at room temperature. Sections were cleared in xylenes for 1 min and coverslipped with DPX.
Quantification
The RVM extends from the rostral pole of the inferior olive and the beginning of the VII motor nucleus to the caudal pole of the trapezoid body (Leong et al., 2011). The number of coronal 50-μm sections that could be obtained through the length of the RVM was determined. The total number of possible slices was then divided by the number of desired disectors (5–6) to establish the sampling interval (k), which was determined as every fifth section. A table of random integers between the number 1 and 5 (k) was consulted to determine the section number that would serve as the first disector. Hence, sections were obtained through the RVM in accordance with unbiased stereological principles (Coggeshall, 1992; Coggeshall and Lekan, 1996; West, 1999)
Confocal scanning of the sections confirmed that all antibodies uniformly penetrated the full thickness of each section. The optical fractionator probe of Stereoinvestigator software (Microbrightfield, Colchester, VT) was therefore used for analysis of neuron number. A Nikon E800 epifluorescence microscope equipped with a Nikon 40X oil Planapoachromat lens (N.A. 1.3) was used. This objective afforded sufficient resolution to identify tops of individual somata as they came into focus as the focal plane descended through the tissue. The tissue thickness was measured using the 40X objective for every section from every rat. The average thickness was 28.6 ± 0.4 μm in the z-axis after processing. For quantification, a rectangle was centered over the RVM with the top edge aligning with the dorsal edge of the facial nucleus, the base at the dorsal edge of the pyramidal tracts and the lateral edges aligned with the lateral edge of the pyramids. For some experiments, the rectangle was divided into ipsilateral and contralateral halves. The size of the counting frame was 140 μm by 140 μm, and approximately 40 counting frames per section were analyzed. The disector depth was 20 μm with a minimum of 3-μm guard zones at the top and the bottom of the section. The average volume of reference used to calculate the number of neurons expressing NK1R was 2.56 mm3 and 2.36 mm3 for number of neurons with internalization of the receptor. All NK1R immunoreactive profiles were confirmed to be neurons by the presence of NeuN immunoreactivity. In this and all subsequent analyses, the investigator was blinded to the treatment conditions. The rats were assigned ID numbers unrelated to their treatment condition. After the immunohistochemical staining was completed, the slides were blinded by a member of the laboratory not involved in the study and the stereology was then performed by the first author. The senior author monitored for statistical power. When an increased N was required, the N in each treatment condition was increased as a cohort. The first author was not informed of findings until the data were unblinded as a group at the completion of all the stereology. Each treatment group was comprised of five rats.
Internalization
Rats were euthanized immediately after behavioral testing as prior work in spinal cord slices indicated that maximal numbers of NK1R immunoreactive neurons exhibited internalization within 2 min of Sub P application persisting through 15 min (Wang and Marvizon, 2002). To count the numbers of NK1R immunoreactive neurons that exhibited receptor internalization we proceeded as described above, but used a 60X oil Planapoachromat objective (N.A. 1.4). The percentage of NK1R immunoreactive neurons that exhibited internalization of the receptor was quantified using a standard method (Mantyh et al., 1995b; Abbadie et al., 1997; Marvizon et al., 1997; Honor et al., 1999; Marvizon et al., 2003) with some modifications that reflect the characteristics of the RVM. Preliminary analysis indicated that 81% of NK1R immunoreactive neurons in the RVM of saline-treated rats had fewer than five endosomes. A neuron was considered to have internalized receptors if it contained six or more labeled endosomes within the soma and contiguous proximal dendrites. The largest number of labeled endosomes observed in neurons of any treatment group after noxious heat stimulation was 26.
Quantification of Immunoreactive Processes
To quantify NK1R immunoreactive processes in the RVM, the Autoneuron module from Neurolucida software (Microbrightfield) was used. The same tissue sections (disectors) that were used for stereological counting of immunoreactive neurons were also used for quantification of immunoreactive processes. Using the Stereoinvestigator module, counting frames were randomly placed within the RVM of each disector and images were obtained of each counting frame. The Autoneuron module was then used to trace and reconstruct the immunoreactive processes in a single plane; the length of the processes in the z-axis was not determined. The length of processes in each counting frame was then summed for that disector, and the average of the disectors was calculated for the rat. The reference area for analysis of the processes was 2.75 mm2.
RT-qPCR for Tacr1
Rats were euthanized by CO2 inhalation followed by decapitation, a 2-mm wide transverse section containing the RVM was isolated, and the RVM was removed with a 1.5-mm diameter punch centered on the midline immediately above the pyramids. The tissue punches were stored in RNAlater (Ambion, Austin; TX). Preliminary experiments using the NK1R primers described by Heng et al. (2011) indicated no difference in the number of transcripts for β-actin or Tacr1 as a function of heat stimulation in either saline or CFA-treated rats (data not shown). However, concerns about the size of the product (~570 bp) and the need to pool tissue to obtain sufficient product dictated the design and use of an alternate primer and protocol.
For these experiments, the primers used to amplify Tacr1 were 5′-CAGAGTCGTGTGCATGATCG (forward) and 5′-CACCAGTAGAGGCAGGAAGT (reverse), giving a PCR product of 105 bp. The primers used to amplify Actb were 5′-CCGCGAGTACAACCTTCTTG (forward) and 5′-GCAGCGATATCGTCATCCAT (reverse), giving a product of 81 bp. Both sets of primers were designed to span introns to exclude genomic DNA contamination. Sufficient amounts of total RNA could be isolated from individual tissue punches using the RNeasy lipid tissue mini kit (Qiagen; Valencia, CA). Briefly, the RVM punches were disrupted and homogenized in 1 ml of QIAzol Lysis Reagent. The lysate was extracted with chloroform, then centrifuged and the supernatant saved in a clean tube. The RNA was precipitated using an equal volume of 70% ethanol and loaded on the column. After two washes, 80 μl of buffer containing 1 unit DNase I was applied to the column and allowed to incubate for 15 min at room temperature. The DNase was then removed by extensive washing. The RNA was eluted using RNase free water, and RNA concentration was determined by spectrometry. The integrity of the RNA (Fleige and Pfaffl, 2006) of six randomly selected samples was further assessed by resolving the 28S and 18S ribosomal RNA bands using an Agilent 2100 bioanalyzer (Agilent Technologies; Santa Clara, CA). The mean RNA integrity number of these six samples was 8.3 ± 0.2. Reverse transcription was performed using 50 ng of purified RNA and the SuperScript VILO cDNA synthesis kit (Invitrogen; Grand Island, NY) in a 20 μl reaction at 42° for 1 hr. The PCR was performed using 2 μl of the RT product in a 20 μl reaction with primers complementary to rat Tacr1 or Actb and IQ™ SYB Green Supermix (BioRad, Hercules, CA). Reactions were performed in triplicate and analyzed using a Bio-Rad MY-IQ thermocycler. Cycle conditions were 50°C for 2 min, 95° for 10 minutes, 40 cycles of 95° for 15 sec, 60° for 1 min, and 72° for 1 min. No reverse transcriptase and no template controls were also run in triplicate. At the end of amplification, a thermal melting curve was generated. Samples that did not yield a homogenous melt curve were not included. Both primers were cloned using Strata Clone™ PCR cloning kit (Stratagene, La Jolla, CA) and verified by DNA sequencing. Cycle thresholds (CT) for Tacr1 and Actb were converted to absolute copy numbers using standard curves generated with serial dilutions of pSCa Actb plasmids and of pSCa Tacr1, respectively, in 10 ng/mL yeast tRNA. The amplification efficiency of the Tacr1 assay was 100%, r2= 0.998, slope =-3.270; and for the Actb assay, efficiency = 100%, r2 = 0.997, slope = −3.282. Copy numbers of Tacr1 are expressed as a ratio of Tacr1 to Actb. For this set of experiments, CFA- and saline-treated rats did not undergo heat stimulation.
Western Blotting for GFAP
Rats were euthanized by CO2 inhalation followed by decapitation. A 2-mm thick coronal block of brainstem was dissected on an ice-cold platform and a triangular region containing the RVM obtained and immediately frozen on dry ice. The triangle was centered with its apex on the midline, bounded ventrally by the pyramids, dorsally by the dorsal edge of the facial motor nucleus and laterally by the lateral edge of the pyramids. The tissue was homogenized in an excess of ice-cold lysis buffer (5 mM Tris pH 7.4, 150 mM NaCl, 1% Triton X-100) with protease inhibitors (Roche Complete Mini protease cocktail; Indianapolis, IN, USA) using an Argos Pestle motor mixer and plastic pestles. The solution was centrifuged at 16,200 g for 30 min at 4° C. The resulting supernatant was decanted and the protein concentration in the supernatant was determined using the micro bicinchoninic acid protein assay reagent (Pierce, Rockford, IL). Twenty-five μg of protein was then added to sample buffer (1M DTT; 4X LDS and lysis buffer) and the samples were heated for 10 min at 70°C. Samples were separated on a 10% NuPAGE gel (Novex, Carlsbad, CA) in 1X MOPS buffer and transferred to a PVDF membrane. The membranes were blocked with TBS and 0.1% Tween 20 (TBST) containing 5% nonfat milk for 1 hr at room temperature. Using the visible ladder on the gel, the membranes were then cut in two. The upper part, which contained the 50 kD band (GFAP), was incubated overnight at 4°C with mouse monoclonal anti-GFAP (2.08 μg/ml; anti-GFAP clone N206A/8, Lot 4472JH75; UC Davis/NIH NeuroMab Facility) diluted in blocking solution. This antibody was raised against the synthetic amino acid 411–422 of human GFAP and recognizes a band at 50 kDa that is absent in tissue from GFAP null mice (manufacturer’s data). After three rinses with TBST, the membranes were then incubated with the appropriate horseradish peroxidase-linked secondary antibodies (goat anti-mouse 0.2 μg/ml, UC Davis/NIH NeuroMab Facility) diluted in TBST containing 5% nonfat milk for 2 hr at room temperature. To verify equal loading of protein, the lower half of the membrane was incubated overnight at 4°C with mouse monoclonal anti-β-actin (0.15 μg/ml; Sigma, St. Louis, MO) diluted in blocking solution and followed by the corresponding horseradish peroxidase-linked secondary antibody (0.5 μg/ml; Millipore, Billerica, MA), diluted in TBST containing 5% nonfat milk for 30 min at room temperature. After thorough washing, the membranes were developed with the Amersham ECL Plus reagent according to manufacturer’s instructions (GE Healthcare, Piscataway, NJ). Images were acquired and analyzed using an EC3 darkroom image station (UVP, Upland, CA). Multiple exposure times were acquired to ensure signals were not saturated. Gels were scanned and images analyzed in Image J (National Institutes of Health, Bethesda, MD) for densitometric quantification of the immunoreactive bands.
Statistical Analyses
Values are presented as mean ± SEM. Two-way analyses of variance were used to compare the number of NK1R immunoreactive neurons, number of neurons with internalized NK1R or length of immunoreactive processes among the four different treatment groups. Two-way analyses of variance were also used to compare ipsilateral and contralateral sides of saline- and CFA-treated rats within heat tested and untested groups. A Bonferroni test was used for post-hoc comparisons. Levels of transcript were compared by Student’s t-test. P < 0.05 was considered significant.
RESULTS
Distribution of NK1R immunoreactivity in the RVM
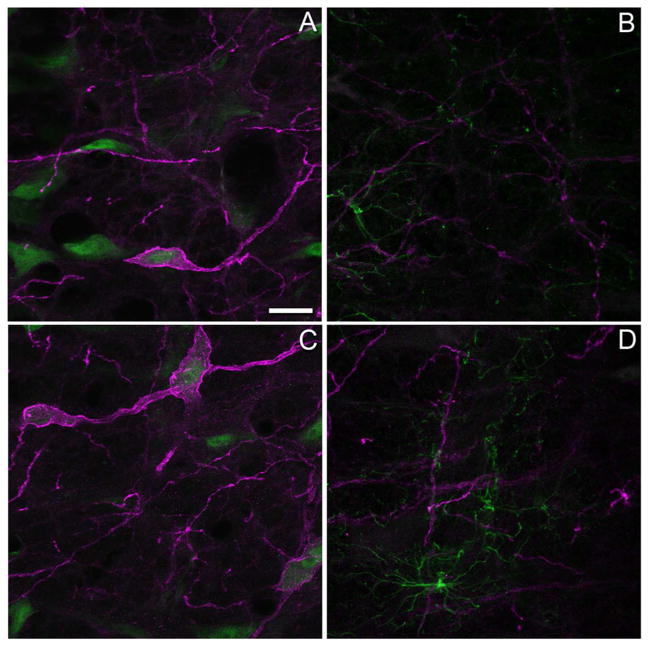
In saline- and CFA-treated rats, NK1R immunoreactivity was predominantly localized to the membrane of the soma and processes of neurons in the RVM when visualized with the Sigma antibody (Fig. 2A, C). An ancillary analysis was conducted to confirm that the immunoreactivity was largely neuronal given that nerve injury can induce an early activation of microglia that is later followed by activation of astrocytes in the RVM (Wei et al., 2008). Little to no colocalization of NK1R immunoreactivity with GFAP labeling was observed four days after injection of saline or CFA (Fig. 2B, D). Of note, levels of GFAP determined by Western blotting did not differ in saline and CFA-treated rats (median and interquartile range: 0.66 (0.35 – 1.22) vs 0.83 (0.71 – 3.66), respectively (P >0.1, Mann-Whitney test).
Figure 2.
Neurokinin-1 receptor (NK1R) immunoreactivity is largely neuronal in nature. Distribution of NK1R immunoreactivity (magenta) in the rostral ventromedial medulla of rats four days after an intraplantar injection of (A) saline or (C) complete Freund’s adjuvant (CFA) in the left hind paw. These tissue sections were also processed with an antibody to NeuN (green) to identify neurons. NK1R immunoreactivity rarely co-localized with immunoreactivity for glial fibrillary acidic protein (green) in saline-treated (B) or CFA-treated (D) rats. Images in panels A and C are the projections of a stack of 3–4 confocal sections taken at an interval of 0.76 microns while panels B and D are the projections of 3–4 stacks taken at 0.72 and 0.74 microns respectively. Scale bar for all panels is 20 microns. The gamma was adjusted by 10% for images in panels B and D.
Number of NK1R immunoreactive neurons in the RVM increases in CFA-treated rats
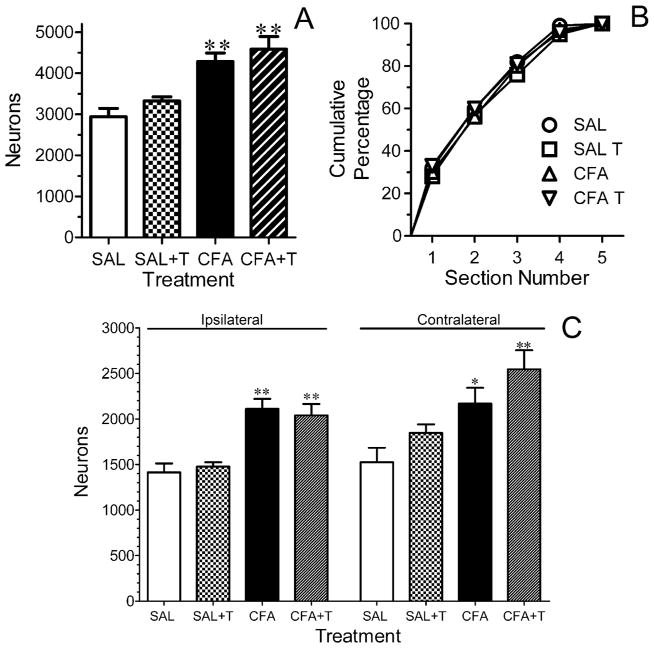
Stereological analysis indicated that the RVM normally contains ~ 3,000 NK1R immunoreactive neurons (Fig. 3A), with larger numbers of immunoreactive neurons present in the more caudal aspects of the region (Fig. 3B). The number of NK1R immunoreactive neurons in saline-treated rats was similar whether or not the hind paw was exposed to a noxious heat stimulus. Four days after ipl injection of CFA, the number of NK1R immunoreactive neurons in the RVM increased by 31.9% in rats that did not experience noxious heat stimulation and by 39.6% in those that did experience noxious heat stimulation (Fig. 3A). The increase occurred throughout the RVM as indicated by the similar rostral-caudal distribution of immunoreactive neurons in all treatment groups (Fig. 3B). To determine whether the unilateral injury affected one side of the RVM, the region was subdivided into ipsilateral and contralateral halves for further analysis. Figure 3C illustrates that CFA treatment increased the number of NK1R immunoreactive neurons both ipsilateral and contralateral to the injured hind paw irrespective of heat stimulation. Numbers of neurons were similar on both sides of the RVM for each treatment group, with one exception. In CFA-treated rats that experienced noxious heat stimulation, the number of neurons was greater on the contralateral than ipsilateral side (P < 0.05).
Figure 3.
The total number of neurokinin-1 receptor (NK1R) immunoreactive neurons is increased in the rostral ventromedial medulla (RVM) of rats that received an intraplantar injection of complete Freund’s adjuvant (CFA) in the left hind paw four days earlier. (A) The rats were subdivided into those that underwent heat stimulation of both hind paws (SAL-T and CFA-T) and those that did not (SAL and CFA). The number of NK1R immunoreactive neurons was greater in both subsets of CFA-treated rats. (B) The rostro-caudal distribution of NK1R immunoreactive neurons in the RVM does not differ among any of the treatment groups. The ordinate is the cumulative number of NK1R immunoreactive neurons. The number of neurons was counted in five sections through the RVM beginning at the very caudal pole (section 1) and ending at the very rostral tip (section 5) of the VIIth motor nucleus. (C) Further subdivision of the data revealed that the number of NK1R immunoreactive neurons was increased in ipsilateral RVM of both subsets of CFA-treated rats. In the contralateral RVM the number of NK1R immunoreactive neurons was significantly greater in the group of CFA-treated rats that underwent heat stimulation when compared with the corresponding saline-treated group. Data are expressed as the mean ± S.E.M. of determinations in four rats in each treatment group. For all panels, * P < 0.05; ** P < 0.01 compared to the corresponding saline-treated group.
The morphology and density of NK1R immunoreactive processes in the RVM changes in CFA-treated rats
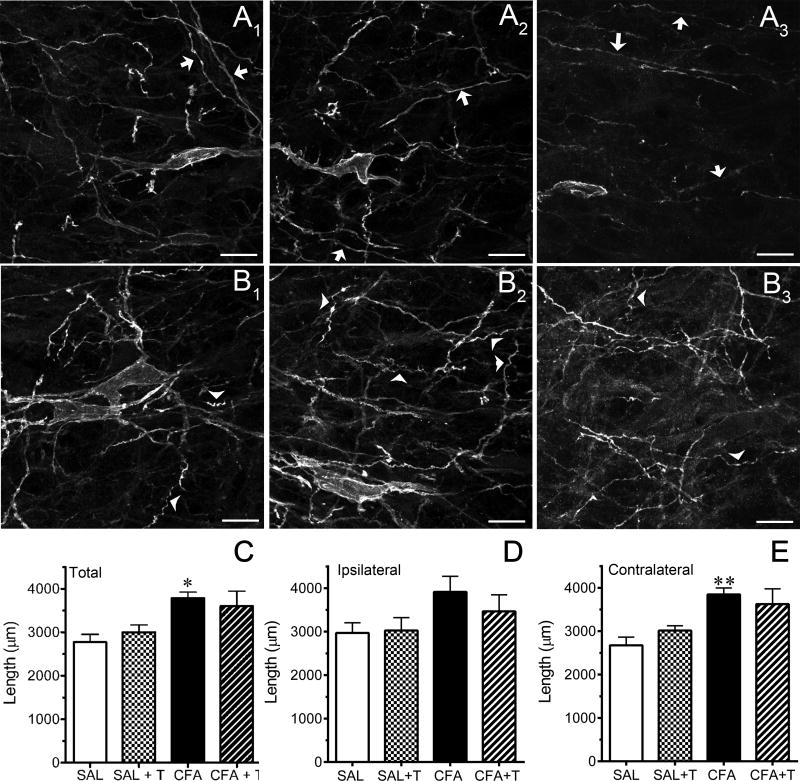
In saline-treated rats, the majority of NK1R immunoreactive processes in the neuropil of the RVM were thin and smooth in appearance (Fig. 4A1–2, arrows) whether or not they had experienced heat stimulation of the hind paws. In contrast, in CFA-treated rats, NK1R immunoreactive processes often appeared thicker with a more tortuous trajectory or corkscrew appearance (Fig. 4, B1–2, arrowheads). This morphology was particularly evident when the hindpaw of the rat had been stimulated with noxious heat (compare Fig. 4B3 with Fig. 4B1–2). As there was little colocalization of NK1R immunoreactivity with GFAP labeling after CFA treatment (Fig. 2D), these thicker processes are unlikely to be astrocytes.
Figure 4.
Neurokinin-1 R (NK1R) immunoreactive processes in the rostral ventromedial medulla (RVM) of rats that received an intraplantar injection of complete Freund’s adjuvant (CFA) are increased and have a more tortuous appearance compared to saline-treated rats. Panels A1–2 and B1–2 are representative examples of NK1R immunoreactive processes in the RVM of saline-treated rats and CFA-treated rats, respectively, which did not undergo heat stimulation of the hind paws. Representative examples of NK1R immunoreactive processes in the RVM of saline- and CFA-treated rats that underwent heat stimulation of the hind paws are shown in panels A3 and B3, respectively. Arrows identify the thinner, smoother processes frequently observed in tissue from saline-treated rats, while arrowheads identify examples of more tortuous and convoluted processes observed in CFA-treated rats. Panels C–E summarize the quantitative analysis. Data are expressed as the mean ± S.E.M. of determinations in four rats in each treatment group. * P < 0.05; ** P < 0.01 compared to the corresponding saline-treated group. Scale bar is 20 microns and applies to each panel. The gamma of the image in panel B3 was adjusted by 20%.
The density of NK1R immunoreactive processes appeared greater in CFA-treated rats. The length of NK1R immunoreactive processes was therefore traced, summed for each disector taken through the RVM and the average of the disectors was calculated for each rat. Figures 4C–E illustrate the results for the total length, as well as for the ipsilateral and contralateral sides of the RVM. In rats that did not experience heat stimulation, the total length of NK1R immunoreactive processes in CFA-treated rats was greater than saline-treated (Figure 4C, P < 0.01). In rats that experienced heat stimulation, the length of NK1R immunoreactive processes in CFA-treated rats was not significantly different from saline-treated rats (Figure 4C, P = 0.075). The variance in the CFA-treated group that experienced heat stimulation was nearly twice that of other groups, which likely reflects the increased difficulty in tracing processes in which the receptor had internalized and as a consequence did not “outline” the processes as clearly. Because the inflammatory injury was unilateral, further analysis of ipsilateral and contralateral sides of the RVM was conducted. In the ipsilateral RVM (Fig. 4D), the length of NK1R immunoreactive processes in CFA-treated rats that did not experience heat stimulation was not significantly greater than their corresponding saline-control treatment group (P = 0.055). Process length did not differ between CFA- and saline-treated rats that experienced noxious heat stimulation (P > 0.3). In the contralateral RVM (Fig 4E), the length of NK1R immunoreactive processes in CFA-treated rats that did not experience heat stimulation was significantly greater than corresponding saline-treated rats (P < 0.01). However, in rats that experienced heat stimulation, the length of NK1R immunoreactive processes in CFA-treated rats was not statistically different from saline-treated rats (P = 0.068). Process length was comparable on the ipsilateral and contralateral sides of the RVM in each treatment group (P > 0.4 all groups).
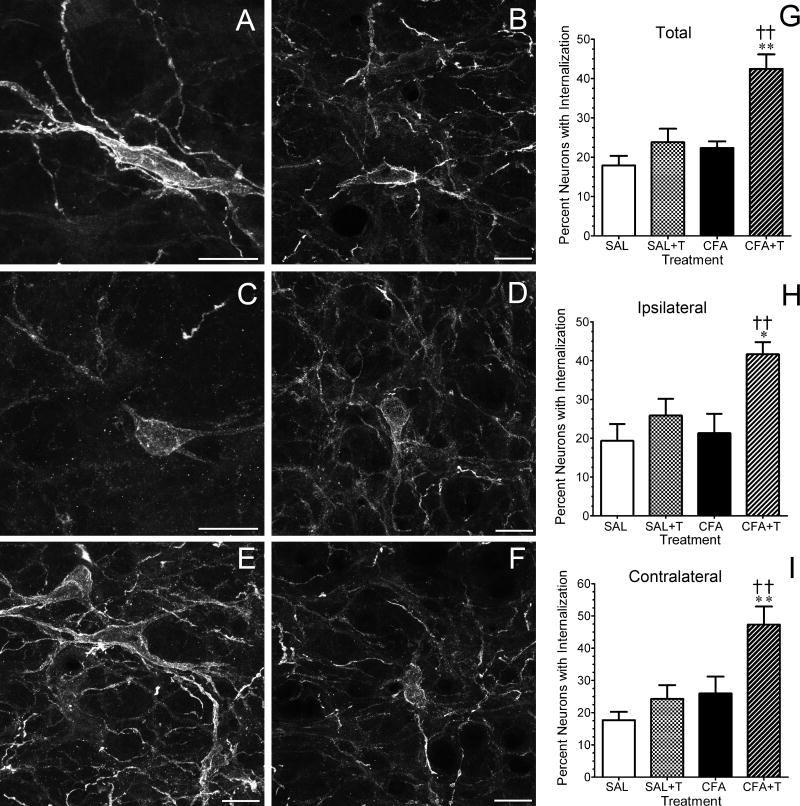
Noxious heat stimulation increases the percentage of NK1R immunoreactive neurons in CFA-treated rats that exhibit internalization
Figure 5 illustrates two representative examples of NK1R internalization in saline-treated rats (Fig. 5A,B) that experienced noxious heat stimulation of the hind paws and four examples of NK1R internalization in CFA-treated rats that experienced noxious heat stimulation of the hind paws. In the absence of injury, approximately 20% of NK1R immunoreactive neurons in the RVM exhibited NK1R internalization, defined as six or greater labeled endosomes (Fig. 5G–I). Brief heat stimulation of the hind paws did not increase the percentage of NK1R immunoreactive neurons that exhibited internalization in saline-treated rats (Fig. 5G–I). In CFA-treated rats without heat stimulation, the percentage of NK1R immunoreactive neurons with receptor internalization was the same as saline-treated rats that did or did not experience noxious heat stimulation. In contrast, the percentage of NK1R immunoreactive neurons that exhibited receptor internalization increased from 20% to 40% in CFA-treated rats that underwent brief noxious heat stimulation of both hind paws (Fig. 5G–I). The number of neurons with NK1R internalization was comparable on the ipsilateral and contralateral sides of the RVM in each treatment group (P > 0.3 all groups).
Figure 5.
The number of neurokinin-1 receptor (NK1R) immunoreactive neurons in the rostral ventromedial medulla (RVM) that exhibit internalization of the receptor is increased in rats that received an intraplantar injection of complete Freund’s adjuvant (CFA) and additionally underwent heat stimulation of the hind paws. Images in panels A and B are representative examples of NK1R immunoreactive neurons in the RVM of saline-treated rats that underwent heat stimulation. The neurons exhibit little to no internalization of receptor. Images in panels C–F are representative examples of NK1R immunoreactive neurons in the RVM of CFA-treated rats that underwent heat stimulation. Neurons with > 5 labeled endosomes were considered to have internalized the receptor. Images in panels A and C are projections of 3–4 stacks taken at intervals of 0.48 microns, while panels B, D, E and F are projections of 3–4 stacks taken at intervals of 0.41 microns. All scale bars are 20 microns. Panels G–I summarize the quantitative analysis. Data are expressed as the mean ± S.E.M. of determinations in four rats in each treatment group. * P < 0.05; ** P < 0.01 compared to the corresponding saline-treated group. † P < 0.05; †† P < 0.01 compared to the CFA-treated rats that did not undergo heat stimulation.
Tacr1 and Actb transcripts in the RVM are unchanged in CFA-treated rats
The numbers of Tacr1 and Actb transcripts were determined in the RVM of rats four days after ipl injection of CFA or saline. In both the CFA- and the saline-treatment groups, the levels of Actb transcript in rats that received noxious heat stimulation of the hind paw were similar to those that did not undergo hind paw stimulation (P > 0.3; data not shown). The data from the two subsets were therefore pooled for each treatment group. The number of Actb transcripts in CFA-treated rats (395,428 ± 53,034 N=6) and saline-treated rats (294,793 ± 35,686 N=7) were comparable (P > 0.1). Therefore, for analysis, the number of Tacr1 transcripts was normalized to the number of Actb transcripts in the respective sample. Levels of Tacr1 transcript, normalized to Actb, in CFA- and saline-treated rats were 0.0054 ± 0.0019 (N=6) and 0.0084 ± 0.0021 (N = 7), respectively. These values did not differ (P > 0.3) indicating that persistent inflammatory injury did not cause transcriptional upregulation of Tacr1. A similar conclusion was reached using the Tacr1 primer described by Heng et al. (2011).
DISCUSSION
This study identified several mechanisms by which Sub P in the RVM may assume a pronociceptive role after peripheral inflammatory injury. First, it determined that the number of NK1R immunoreactive neurons, as well as the density of NK1R immunoreactive processes in the RVM increased four days after CFA treatment. An increase in the number or accessibility of NK1R would amplify the actions of Sub P in the RVM. Second, it determined that brief noxious heat stimulation of the hind paws caused internalization of NK1R in a subpopulation of RVM neurons in CFA-, but not in saline-treated rats. These data are consistent with an increased release or an enhanced action of Sub P in the RVM following peripheral inflammatory injury.
The number of NK1R immunoreactive neurons and length of process increases after CFA
Western blotting has documented an increase in NK1R in the RVM of CFA-treated rats (Lagraize et al., 2010), but was unable to ascribe the increase to neurons or glia. This study demonstrated that the number of NK1R immunoreactive neurons in the RVM increased by ~30% four days after CFA treatment. Whether the increased number reflects an increased expression of NK1R by the neurons, which could facilitate their detection by the antibody, or the expression of NK1R by a new population of neurons is unclear. Interestingly, the number of NK1R immunoreactive spinoparabrachial neurons increases in the superficial laminae of the dorsal horn two weeks after intraplantar injection of CFA (Almarestani et al., 2009) or 10 days after constriction injury of the sciatic nerve (Saeed and Ribeiro-da-Silva, 2013). In both studies, NK1R immunoreactivity was detected in pyramidal-shaped neurons that do not normally express the NK1R. The authors posited that the increase represented de novo synthesis and a possible change in the function of the neuron from encoding innocuous cooling stimuli to nociceptive stimuli. It is tempting to speculate that the increase in NK1R expressing neurons in the RVM represents a new population of pain facilitatory neurons. Although the number of NK1R transcripts was unchanged in CFA-treated rats, it is now well accepted that protein levels can increase in the absence of a corresponding increase in mRNA (Khositseth et al., 2011; Vogel and Marcotte, 2012). However, this proposal has to be tempered by reports that the relative percentages of on, off and neutral cells in the RVM are unchanged after CFA treatment (Khasabov et al., 2012; Cleary and Heinricher, 2013; but see Miki et al., 2002).
There was also a substantial increase in NK1R immunoreactive processes in the RVM of CFA-treated rats. This increase could be secondary to an increase in the number of NK1R immunoreactive neurons, or due to increased trafficking of receptor to the dendrites. There was an impression that processes in CFA-treated rats were thicker and more tortuous in appearance, a morphological change that was particularly noticeable in CFA-treated rats that underwent heat stimulation. Others have also noted morphological changes in NK1R immunoreactive dendrites in the dorsal horn (Mantyh et al., 1995b; Allen et al., 1997), striatum (Mantyh et al., 1995a; 1995b) and RVM (Hahm et al., 2011) following Sub P application or release.
Sub P release in the RVM is activity dependent under conditions of persistent inflammation
Microinjection of NK1 receptor antagonists reverses CFA-induced heat hyperalgesia or mechanical hypersensitivity, but does not alter nociceptive threshold in the absence of injury. This observation suggests that there is increased release of Sub P, possibly tonic in nature, in the RVM after inflammatory injury. We expected that a greater proportion of NK1R immunoreactive neurons would exhibit internalization in CFA-treated rats, irrespective of heat stimulation, than in saline-treated rats. This proved not be the case. NK1R internalization was observed only in CFA-treated rats that had undergone heat stimulation of the hind paw. Although the percentage is small, it is likely an underestimate because the area of heat stimulation was small (40 mm2), and the total duration of stimulation was quite brief, less than 30 sec as experienced during nociceptive testing in the behavioral studies. The percentage of neurons with NK1R internalization was similar in saline-treated rats that did or did not experience heat stimulation. Of note, responsiveness to noxious stimuli was not altered by microinjection NK1R receptor antagonists in saline-treated rats (Hamity et al., 2010) or ablation of NK1R immunoreactive neurons (Khasabov and Simone, 2013). Taken together, these observations suggest that in the absence of injury Sub P is neither tonically nor phasically released to a significant extent in the RVM. Sub P is also unlikely to be tonically released in the inflammatory state because the percentages of neurons exhibiting NK1R internalization in CFA-treated rats that did not experience heat stimulation and saline-treated rats were equivalent. Rather, the finding that NK1R internalization is activity dependent indicates that Sub P is phasically released in the RVM in response to noxious peripheral stimulation after injury. Concordant with this proposal, microinjection of a NK1R antagonist in the RVM of CFA-treated rats did not alter the spontaneous activity of either on or off cells, but did attenuate the enhanced responsiveness of on cells to heat and mechanical stimuli (Khasabov et al., 2012).
The increased internalization of NK1R in the RVM is strongly suggestive of an increased release of Sub P in the RVM. Peripheral inflammatory injury increases the number of c-Fos-immunoreactive neurons in the nucleus cuneiformis and the periaqueductal gray (Lanteri-Minet et al., 1994; Loyd and Murphy, 2006). As Sub P neurons in these nuclei project to the RVM (Beitz, 1982; Chen et al., 2013), they could be the source of increased release of Sub P in the RVM. Another mechanism could involve an action of glutamate at N-methyl-D-aspartate receptors (NMDAR) situated on the axon terminals of Sub P afferents in the RVM. Activation of presynaptic NMDAR facilitates Sub P release from primary afferents (Marvizon et al., 1997; Chen et al., 2010; but see Nazarian et al., 2008) and there is electrophysiologic evidence for a similar action in the RVM (Zhang and Hammond, 2009). Indeed, mRNA for NMDA receptor subunits GluN1, GluN2A and GluN2B increased in the RVM, as did GluN2A protein and phosphorylated GluN2A 3–7 days after ipl injection of CFA (Miki et al., 2002; Guo et al., 2006). The increase in NK1R internalization could also be secondary to an increase in the affinity or number of the receptor. Unfortunately, attempts to quantitate changes in affinity in the adult by analyzing the effects of different concentrations of Sub P on internalization of NK1R in RVM neurons were unsuccessful due to poor viability of the slices. In slices of brainstem from neonatal rats, Sub P produced smaller, rather than larger, inward currents in spinally-projecting RVM neurons of CFA-treated rats (Zhang and Hammond, 2009), which could reflect receptor downregulation or increased internalization of receptor as a result of sustained release in the slice.
NK1R immunoreactivity is neuronal in nature
Western blotting documented a two-fold increase in NK1R (Lagraize et al., 2010) up to three days after CFA injection, which contrasts with the 30% increase observed with an immunohistochemical approach four days after CFA. Although the disparity may stem from the different experimental methods and time points assessed, it also suggests that there could be an upregulation of NK1R by non-neuronal structures. Astrocytes are one possibility. The number of activated microglia and astrocytes in the RVM increases within three hrs of the ipl injection of a high concentration of carrageenan (Roberts et al., 2009). Chronic constriction of the infraorbital nerve is associated with a near immediate activation of microglia followed sometime later (after three but before 14 days) by activation of astrocytes (Wei et al., 2008). Sub P binds to astrocytes in the glial scar that forms after optic nerve transection (Mantyh et al., 1989). Substance P also binds to astrocytes in primary cultures from embryonic or neonatal rats, although the level of binding varied greatly by the brain region harvested (Beaujouan et al., 1990; Marriott and Wilkin, 1993). Although no significant increase in GFAP was detected by Western blotting, modest numbers of activated astrocytes were observed in the RVM of CFA-treated rats. Nonetheless, no significant colocalization of GFAP and NK1R immunoreactivity was observed in the RVM of CFA-treated rats. Similarly, no colocalization of NK1R with GFAP-positive structures was evident in the trigeminal nucleus of rats with CFA-induced inflammation of the masseter muscle (Guo et al., 2007).
The unilateral nature of the inflammatory injury prompted further examination of the potential laterality of changes in NK1R expression and Sub P release in the RVM. With the sole exception of a greater number of NK1R immunoreactive neurons in the contralateral RVM of CFA-treated rats that experienced noxious heat stimulation, ipsilateral and contralateral sides of the RVM exhibited similar increases in neuron number, receptor internalization and immunoreactive processes in each treatment group. Similarly, Imbe and colleagues (2005; 2008) did not report any differences in the numbers of neurons immunoreactive for phosphorylated extracellular-signal regulated kinase or p38-mitogen activated protein kinase in the RVM after CFA treatment. In contrast, unilateral spinal nerve ligation caused a preferential loss of neurons in the ipsilateral RVM (Leong et al., 2011).
The phenotype of NK1R immunoreactive neurons in the RVM, and particularly those neurons in which NK1R expression is increased is not yet known. It is likely that many project to the spinal cord (Pinto et al., 2008; Zhang and Hammond, 2009), and some neurons may project to other supraspinal nuclei. Although work by LaGraize et al. (Lagraize et al., 2010) indicates that the hyperalgesic effects of Sub P are mediated by spinal 5HT3 receptors, electrophysiological and immunohistochemical studies in neonatal rats determined that serotonergic neurons in the RVM did not respond Sub P or express NK1R (Zhang and Hammond, 2009). Serotonergic neurons in the adult rat, naive or CFA-treated, also were not immunoreactive for the NK1R (Hamity, unpublished observations), similar to reports for the raphe dorsalis (Commons and Valentino, 2002; Leger et al., 2002; but see Lacoste et al., 2006). It will be of interest to determine the neurotransmitter phenotype and projections of NK1R expressing neurons in the RVM, as potential markers for an activity dependent pain facilitatory pathway.
Conclusions
Persistent inflammatory injury increases the number of NK1R immunoreactive neurons in the RVM, increases the density of NK1R immunoreactive process and produces morphological changes in these processes consistent with those produced by application of Sub P. The evidence suggests little if any tonic or stimulus-evoked release of Sub P in the RVM in the absence of injury and that the simple presence of inflammatory injury is not sufficient to cause an increased basal release of Sub P. However, following injury, the evoked release of Sub P is enhanced. This increased release coupled with an increased number of NK1R immunoreactive neurons in the RVM may be the basis by which Sub P exerts its pronociceptive actions in the RVM following inflammatory injury.
Acknowledgments
We thank Stephanie White for expert technical assistance. We thank Dr. Norma P. Gerard for providing tissue from the NK1R −/− mouse. We thank Dr. Blanca Marquez de Prado for early guidance regarding Western blotting and RT-qPCR experiments. We thank Frank Jareczek and Dr. Anne-Sophie Wattiez for critical comments.
Footnotes
Supported by 1R01DA023576 to DLH
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ROLE OF AUTHORS
All authors had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: MVH, DLH. Aquisition of data: MVH, RYW. Analysis and interpretation of data: MVH, RYW, DLH. Writing the manuscript and critical revision for important intellectual content: MVH, RYW, DLH. Statistical analysis: MVY, DLH. Obtained funding: DLH.
References
- Abbadie C, Trafton J, Liu H, Mantyh PW, Basbaum AI. Inflammation increases the distribution of dorsal horn neurons that internalize the neurokinin-1 receptor in response to noxious and non-noxious stimulation. J Neurosci. 1997;17:8049–8060. doi: 10.1523/JNEUROSCI.17-20-08049.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen BJ, Rogers SD, Ghilardi JR, Menning PM, Kuskowski MA, Basbaum AI, Simone DA, Mantyh PW. Noxious cutaneous thermal stimuli induce a graded release of endogenous substance P in the spinal cord: imaging peptide action in vivo. J Neurosci. 1997;17:5921–5927. doi: 10.1523/JNEUROSCI.17-15-05921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almarestani L, Waters SM, Krause JE, Bennett GJ, Ribeiro-da-Silva A. De novo expression of the neurokinin 1 receptor in spinal lamina I pyramidal neurons in polyarthritis. J Comp Neurol. 2009;514:284–295. doi: 10.1002/cne.22024. [DOI] [PubMed] [Google Scholar]
- Baranauskas G, Nistri A. Sensitization of pain pathways in the spinal cord: cellular mechanisms. Prog Neurobiol. 1998;54:349–365. doi: 10.1016/s0301-0082(97)00067-1. [DOI] [PubMed] [Google Scholar]
- Beaujouan JC, Daguet de Montety MC, Torrens Y, Saffroy M, Dietl M, Glowinski J. Marked regional heterogeneity of 125I-Bolton Hunter substance P binding and substance P-induced activation of phospholipase C in astrocyte cultures from the embryonic or newborn rat. J Neurochem. 1990;54:669–675. doi: 10.1111/j.1471-4159.1990.tb01923.x. [DOI] [PubMed] [Google Scholar]
- Beitz AJ. The nuclei of origin of brain stem enkephalin and substance P projections to the rodent nucleus raphe magnus. Neuroscience. 1982;7:2753–2768. doi: 10.1016/0306-4522(82)90098-7. [DOI] [PubMed] [Google Scholar]
- Brink TS, Pacharinsak C, Khasabov SG, Beitz AJ, Simone DA. Differential modulation of neurons in the rostral ventromedial medulla by neurokinin-1 receptors. J Neurophysiol. 2012;107:1210–1221. doi: 10.1152/jn.00678.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budai D, Khasabov SG, Mantyh PW, Simone DA. NK-1 receptors modulate the excitability of ON cells in the rostral ventromedial medulla. J Neurophysiol. 2007;97:1388–1395. doi: 10.1152/jn.00450.2006. [DOI] [PubMed] [Google Scholar]
- Chen T, Wang XL, Qu J, Wang W, Zhang T, Yanagawa Y, Wu SX, Li YQ. Neurokinin-1 receptor-expressing neurons that contain serotonin and gamma-aminobutyric Acid in the rat rostroventromedial medulla are involved in pain processing. J Pain. 2013;14:778–792. doi: 10.1016/j.jpain.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Chen W, Zhang G, Marvizon JC. NMDA receptors in primary afferents require phosphorylation by Src family kinases to induce substance P release in the rat spinal cord. Neuroscience. 2010;166:924–934. doi: 10.1016/j.neuroscience.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary DR, Heinricher MM. Adaptations in responsiveness of brainstem pain-modulating neurons in acute compared with chronic inflammation. Pain. 2013;154:845–855. doi: 10.1016/j.pain.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall RE. A consideration of neural counting methods. Trends Neurosci. 1992;15:9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Commons KG, Valentino RJ. Cellular basis for the effects of substance P in the periaqueductal gray and dorsal raphe nucleus. J Comp Neurol. 2002;447:82–97. doi: 10.1002/cne.10228. [DOI] [PubMed] [Google Scholar]
- Fleige S, Pfaffl MW. RNA integrity and the effect on the real-time qRT-PCR performance. Molecular aspects of medicine. 2006;27:126–139. doi: 10.1016/j.mam.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Guo W, Robbins MT, Wei F, Zou S, Dubner R, Ren K. Supraspinal brain-derived neurotrophic factor signaling: a novel mechanism for descending pain facilitation. J Neurosci. 2006;26:126–137. doi: 10.1523/JNEUROSCI.3686-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm ET, Hammond DL, Proudfit HK. Substance P induces the reversible formation of varicosities in the dendrites of rat brainstem neurons. Brain Res. 2011;1369:36–45. doi: 10.1016/j.brainres.2010.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamity MV, White SR, Hammond DL. Effects of neurokinin-1 receptor agonism and antagonism in the rostral ventromedial medulla of rats with acute or persistent inflammatory nociception. Neuroscience. 2010;165:902–913. doi: 10.1016/j.neuroscience.2009.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng YJ, Saunders CI, Kunde DA, Geraghty DP. TRPV1, NK1 receptor and substance P immunoreactivity and gene expression in the rat lumbosacral spinal cord and urinary bladder after systemic, low dose vanilloid administration. Reg Peptides. 2011;167:250–258. doi: 10.1016/j.regpep.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Honor P, Menning PM, Rogers SD, Nichols ML, Basbaum AI, Besson JM, Mantyh PW. Spinal substance P receptor expression and internalization in acute, short-term, and long-term inflammatory pain states. J Neurosci. 1999;19:7670–7678. doi: 10.1523/JNEUROSCI.19-17-07670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbe H, Kimura A, Okamoto K, Donishi T, Aikawa F, Senba E, Tamai Y. Activation of ERK in the rostral ventromedial medulla is involved in hyperalgesia during peripheral inflammation. Brain Res. 2008;1187:103–110. doi: 10.1016/j.brainres.2007.10.075. [DOI] [PubMed] [Google Scholar]
- Imbe H, Okamoto K, Okamura T, Kumabe S, Nakatsuka M, Aikawa F, Iwai-Liao Y, Senba E. Effects of peripheral inflammation on activation of ERK in the rostral ventromedial medulla. Brain Res. 2005;1063:151–158. doi: 10.1016/j.brainres.2005.09.057. [DOI] [PubMed] [Google Scholar]
- Keeble JE, Brain SD. A role for substance P in arthritis? Neurosci Lett. 2004;361:176–179. doi: 10.1016/j.neulet.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Khasabov SG, Brink TS, Schupp M, Noack J, Simone DA. Changes in response properties of rostral ventromedial medulla neurons during prolonged inflammation: modulation by neurokinin-1 receptors. Neuroscience. 2012;224:235–248. doi: 10.1016/j.neuroscience.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasabov SG, Simone DA. Loss of neurons in rostral ventromedial medulla that express neurokinin-1 receptors decreases the development of hyperalgesia. Neuroscience. 2013;250:151–165. doi: 10.1016/j.neuroscience.2013.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khositseth S, Pisitkun T, Slentz DH, Wang G, Hoffert JD, Knepper MA, Yu MJ. Quantitative protein and mRNA profiling shows selective post-transcriptional control of protein expression by vasopressin in kidney cells. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.004036. M110 004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste B, Riad M, Descarries L. Immunocytochemical evidence for the existence of substance P receptor (NK1) in serotonin neurons of rat and mouse dorsal raphe nucleus. Eur J Neurosci. 2006;23:2947–2958. doi: 10.1111/j.1460-9568.2006.04833.x. [DOI] [PubMed] [Google Scholar]
- Lagraize SC, Guo W, Yang K, Wei F, Ren K, Dubner R. Spinal cord mechanisms mediating behavioral hyperalgesia induced by neurokinin-1 tachykinin receptor activation in the rostral ventromedial medulla. Neuroscience. 2010;171:1341–1356. doi: 10.1016/j.neuroscience.2010.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanteri-Minet M, Weil-Fugazza J, de Pommery J, Menetrey D. Hindbrain structures involved in pain processing as revealed by the expression of c-Fos and other immediate early gene proteins. Neuroscience. 1994;58:287–298. doi: 10.1016/0306-4522(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Leger L, Gay N, Cespuglio R. Neurokinin NK1- and NK3-immunoreactive neurons in serotonergic cell groups in the rat brain. Neurosci Lett. 2002;323:146–150. doi: 10.1016/s0304-3940(01)02543-5. [DOI] [PubMed] [Google Scholar]
- Leong ML, Gu M, Speltz-Paiz R, Stahura EI, Mottey N, Steer CJ, Wessendorf M. Neuronal loss in the rostral ventromedial medulla in a rat model of neuropathic pain. J Neurosci. 2011;31:17028–17039. doi: 10.1523/JNEUROSCI.1268-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd DR, Murphy AZ. Sex differences in the anatomical and functional organization of the periaqueductal gray-rostral ventromedial medullary pathway in the rat: a potential circuit mediating the sexually dimorphic actions of morphine. J Comp Neurol. 2006;496:723–738. doi: 10.1002/cne.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW. Neurobiology of substance P and the NK1 receptor. J Clin Psychiatry. 2002;63(Suppl 11):6–10. [PubMed] [Google Scholar]
- Mantyh PW, Allen CJ, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE. Rapid endocytosis of a G protein-coupled receptor: substance P evoked internalization of its receptor in the rat striatum in vivo. Proc Natl Acad Sci USA. 1995a;92:2622–2626. doi: 10.1073/pnas.92.7.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW, DeMaster E, Malhotra A, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE, et al. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science. 1995b;268:1629–1632. doi: 10.1126/science.7539937. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Johnson DJ, Boehmer CG, Catton MD, Vinters HV, Maggio JE, Too HP, Vigna SR. Substance P receptor binding sites are expressed by glia in vivo after neuronal injury. Proc Natl Acad Sci USA. 1989;86:5193–5197. doi: 10.1073/pnas.86.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott DR, Wilkin GP. Substance P receptors on O-2A progenitor cells and type-2 astrocytes in vitro. J Neurochem. 1993;61:826–834. doi: 10.1111/j.1471-4159.1993.tb03593.x. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, Martinez V, Grady EF, Bunnett NW, Mayer EA. Neurokinin 1 receptor internalization in spinal cord slices induced by dorsal root stimulation is mediated by NMDA receptors. J Neurosci. 1997;17:8129–8136. doi: 10.1523/JNEUROSCI.17-21-08129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizon JC, Wang X, Matsuka Y, Neubert JK, Spigelman I. Relationship between capsaicin-evoked substance P release and neurokinin 1 receptor internalization in the rat spinal cord. Neuroscience. 2003;118:535–545. doi: 10.1016/s0306-4522(02)00977-6. [DOI] [PubMed] [Google Scholar]
- Miki K, Zhou QQ, Guo W, Guan Y, Terayama R, Dubner R, Ren K. Changes in gene expression and neuronal phenotype in brain stem pain modulatory circuitry after inflammation. J Neurophysiol. 2002;87:750–760. doi: 10.1152/jn.00534.2001. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nakaya Y, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat. J Comp Neurol. 1994;347:249–274. doi: 10.1002/cne.903470208. [DOI] [PubMed] [Google Scholar]
- Nazarian A, Gu G, Gracias NG, Wilkinson K, Hua XY, Vasko MR, Yaksh TL. Spinal N-methyl-D-aspartate receptors and nociception-evoked release of primary afferent substance P. Neuroscience. 2008;152:119–127. doi: 10.1016/j.neuroscience.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacharinsak C, Khasabov SG, Beitz AJ, Simone DA. NK-1 receptors in the rostral ventromedial medulla contribute to hyperalgesia produced by intraplantar injection of capsaicin. Pain. 2008;139:34–46. doi: 10.1016/j.pain.2008.02.032. [DOI] [PubMed] [Google Scholar]
- Piggins HD, Samuels RE, Coogan AN, Cutler DJ. Distribution of substance P and neurokinin-1 receptor immunoreactivity in the suprachiasmatic nuclei and intergeniculate leaflet of hamster, mouse, and rat. J Comp Neurol. 2001;438:50–65. doi: 10.1002/cne.1301. [DOI] [PubMed] [Google Scholar]
- Pinto M, Sousa M, Lima D, Tavares I. Participation of mu-opioid, GABA(B), and NK1 receptors of major pain control medullary areas in pathways targeting the rat spinal cord: implications for descending modulation of nociceptive transmission. J Comp Neurol. 2008;510:175–187. doi: 10.1002/cne.21793. [DOI] [PubMed] [Google Scholar]
- Ptak K, Burnet H, Blanchi B, Sieweke M, De Felipe C, Hunt SP, Monteau R, Hilaire G. The murine neurokinin NK1 receptor gene contributes to the adult hypoxic facilitation of ventilation. Eur J Neurosci. 2002;16:2245–2252. doi: 10.1046/j.1460-9568.2002.02305.x. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21:570–579. doi: 10.1097/ACO.0b013e32830edbdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J, Ossipov MH, Porreca F. Glial activation in the rostroventromedial medulla promotes descending facilitation to mediate inflammatory hypersensitivity. Eur J Neurosci. 2009;30:229–241. doi: 10.1111/j.1460-9568.2009.06813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed AW, Ribeiro-da-Silva A. De novo expression of neurokinin-1 receptors by spinoparabrachial lamina I pyramidal neurons following a peripheral nerve lesion. J Comp Neurol. 2013;521:1915–1928. doi: 10.1002/cne.23267. [DOI] [PubMed] [Google Scholar]
- Saffroy M, Beaujouan J-C, Torrens Y, Besseyre J, Bergstrom L, Glowinski J. Localization of tachykinin binding sites (NK1, NK2, NK3 ligands) in the rat brain. Peptides. 1988;9:227–241. doi: 10.1016/0196-9781(88)90255-0. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J, Benrath J, Brechtel C, Ruscheweyh R, Heinke B. Synaptic mechanisms of hyperalgesia. Prog Brain Res. 2000;129:81–100. doi: 10.1016/S0079-6123(00)29007-9. [DOI] [PubMed] [Google Scholar]
- Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012;13:227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Marvizon JC. Time-course of the internalization and recycling of neurokinin 1 receptors in rat dorsal horn neurons. Brain Res. 2002;944:239–247. doi: 10.1016/s0006-8993(02)02918-9. [DOI] [PubMed] [Google Scholar]
- Wei F, Guo W, Zou S, Ren K, Dubner R. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J Neurosci. 2008;28:10482–10495. doi: 10.1523/JNEUROSCI.3593-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- Zhang L, Hammond DL. Substance P enhances excitatory synaptic transmission on spinally projecting neurons in the rostral ventromedial medulla after inflammatory injury. J Neurophysiol. 2009;102:1139–1151. doi: 10.1152/jn.91337.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]