Abstract
Introduction
Standardization of care can reduce practice variation, optimize resource utilization and improve clinical outcomes. We have created a standardized clinical assessment and management plan (SCAMP) for patients having balloon aortic valvuloplasty (BAV) for congenital aortic stenosis (AS). This study compares acute outcomes of BAV at our institution before and after introduction of this SCAMP.
Methods
In this retrospective matched cohort study each SCAMP patient was matched to 4 historical controls. Outcomes were categorized based on the combination of residual AS and AR as: 1)Optimal: gradient ≤ 35 mmHg and trivial or no AR, 2)Adequate: gradient ≤ 35 mmHg and mild AR, 3)Inadequate: gradient > 35 mmHg and/or moderate or severe AR.
Results
All 23 SCAMP patients achieved a residual AS gradient ≤ 35 mmHg; the median residual AS gradient for the SCAMP group was lower (25 (10 – 35) mmHg) than in matched controls (30 (0 – 65) mmHg; p=0.005). The two groups did not differ with regard to degree of AR grade after BAV. Compared to controls, SCAMP patients were more likely to have an optimal result, and less likely to have an inadequate result (52% versus34% and 17% versus 45%, respectively; p=0.02)
Conclusions
A SCAMP for BAV resulted in optimal acute results in half of the initial 23 patients enrolled, and outcomes in this group were better than those of matched historical controls. Whether these improved acute outcomes translate into better long term outcomes for this patient population remains to be seen.
Keywords: aortic stenosis, balloon aortic valvuloplasty, congenital heart disease
Introduction
Practice variation can lead to higher resource utilization and can negatively affect the delivery of care.(1–3) This recognition has spawned concerted efforts to standardize care, with the ultimate goal of improving patient safety and clinical outcomes. Examples of standardization tools include clinical practice guidelines, algorithms of care, templated electronic medical records, and surgical checklists.(3–10)
In 2008, we created the Standardized Clinical Assessment and Management Plan (SCAMP), and introduced this quality improvement initiative into our academic center-based, pediatric cardiology practice. Each SCAMP targets a relatively heterogeneous patient population with a single underlying diagnosis or chief complaint, standardizing assessment and management through an iterative data collection and analysis process.(11–15)Early SCAMPs focused on outpatients,(12, 15) but as the tool matured and its utility became more evident, we tested the feasibility of inpatient and procedural SCAMPs. One of the early outpatient SCAMPs pertained to the management of patients with congenital aortic stenosis (AS), and one arm of the decision tree recommended referral for catheterization. Using this decision tree as a starting point, we then wrote a SCAMP to guide decision making during balloon aortic valvuloplasty (BAV) for AS.
The safety and efficacy of BAV for AS have been established over the last 25 years.(16–31) However, the implications of alternative management decisions with regard to timing and outcomes of interventions are not well understood. Long term follow up after BAV reveals an ongoing, steady hazard for repeat interventions, including aortic valve replacement (AVR).(32)In order to elucidate the interaction between residual AS, and post-dilation AR, as they affect referral for AVR, Brown, et al, modeled combinations of residual AS gradient and AR grade. Their analysis suggested that AS reduction during BAV might have greater impact than minimizing AR, with regard to delaying aortic valve surgery.(32)Based on this data, the BAV for AS SCAMP prioritized achieving a residual gradient ≤ 35 mmHg, while attempting to avoid moderate AR.
All SCAMPs undergo periodic data review after implementation. Thus, following initial implementation of the SCAMP, we can now report the results of the first analysis of data from the BAV for AS SCAMP, with attention to acute procedural outcomes. To assess the impact of the SCAMP on acute outcomes, we compared patients managed according to the SCAMP to patients with similar characteristics treated prior to the implementation of the SCAMP.
Methods
BAV for AS SCAMP
The BAV for AS SCAMP was written by an eight-member committee of experienced, academic pediatric cardiologists and pediatric cardiac surgeons through a consensus-based process, following extensive subject review and preparation of a background document(14). The decision tree focused on technical aspects of the procedure, specifying indications for repeated intervention (i.e. redilation with balloons of increasing diameter). As allowed by the design of the SCAMP, practitioners were permitted to deviate from the management recommendations, though they were required to document reasons for deviation to allow subsequent analysis and potential SCAMP modification.
Operators
All 5 catheterizers performing procedures in our catheterization laboratory during the SCAMP implementation period participated. All were familiarized with the SCAMP and had the document readily available through an online reference during the procedures. In addition, during early stages, a single operator who had played a key role in the writing of the SCAMP was on site during the procedures to serve as a resource and to ensure that data forms were completed.
Population
Patients referred for catheterization due to AS starting in December 2010 were prospectively identified during their pre-procedural evaluations, and were included in the SCAMP. As written, the SCAMP identified several exclusion factors, most of which marked patients with other left heart disease that might confound reoperation outcomes. These exclusions were: hypoplastic left ventricle (LV) (end diastolic dimension z-score < −2.5), hypoplastic or stenotic mitral valve (area z-score < −2.5 or mean mitral valve inflow Doppler gradient > 6 mmHg), sub or supravalvar AS, history of in utero aortic valvuloplasty, moderate or severe AR, associated congenital heart disease other than patent ductusarteriosus or coarctation of the aorta.
Controls were identified by a search of the Department of Cardiology database which yielded 453 patients prior to 2010 who would have met criteria for the SCAMP. From this group, and starting with the most recent patients, we performed a 4:1 match of variables that have been shown to affect acute procedural outcomes: age category at intervention (categories: <1 month, 1–11 months, 1–11 years, >11 years), echocardiographic aortic regurgitation grade before intervention (Grades: None/trivial, Mild, Moderate or severe), AS gradient: within 15 mmHg (peak-to-peak gradient measured at catheterization), history of prior BAV, history of surgical aortic valvuloplasty, presence of left ventricular dysfunction on pre-catheterization echocardiogram.
All 92 historical controls underwent BAV between December of 1993 and May of 2009, and have been reported previously.(32) To assess for an era effect within the control group, we compared the outcomes of patients undergoing BAV before 2005 to those undergoing BAV between 2005 and the introduction of the SCAMP.
As with other SCAMPs at our institution, the data were collected as part of a quality improvement project. Retrospective analysis and reporting of the data was approved by the Committee on Clinical Investigation.
Data Collection
Data for the control group were obtained by retrospective review of the medical records and cine angiograms. For SCAMP patients, data related to the procedure were obtained prospectively through the use of the data collection forms.
Measurements of balloon sizes used during the procedure are reported as the manufacturer-specified nominal balloon size. Because one of the SCAMP recommendations was to increase balloon-to-annulus ratio (BAR) by approximately 10% when further dilations were indicated, and sometimes that required inflating the same nominal size balloon at a higher pressure, we also report the measured balloon size. This size was obtained from the recorded angiograms by a single investigator andwas used to calculate the measured BAR applied during each inflation. The decision to use rapid ventricular pacing for balloon stabilization was left to the provider’s discretion.
The reported gradients before and after BAV were peak-to-peak gradients (AS gradient) obtained by simultaneous measurements in the LV and aorta or by direct catheter pullback across the aortic valve. Patients with AS gradients ≤ 35 mmHg at the end of the procedure were considered to have acutely successful gradient reduction.
For purposes of this study, the degree of AR for both groups before and after BAV was assessed by a single angiographic reviewer in blinded fashion, and classified according to the scale defined in Table 1. The degree of AR before and after BAV as reported in the catheterization report and pre- and post-BAV echocardiograms are also reported for comparison purposes.
Table 1.
Angiographic Assessment of Aortic Regurgitation
| Descriptor | Angiographic appearance on aortic root angiogram |
|---|---|
| None/trivial | No contrast, or a tiny jet of contrast is seen entering the LVOT |
| Mild | A small amount of contrast enters the left ventricle during diastole and clears with each systole. |
| Moderate | More contrast enters with each diastole and faint opacification of the entire left ventricular chamber occurs |
| Moderately severe | Left ventricular chamber is well opacified and equal in density when compared with the ascending aorta |
| Severe | Complete, dense opacification of the ventricular chamber on the first beat, and the left ventricle is more densely opacified than the ascending aorta |
The composite outcomes of BAV were classified into the following categories based on the reported post-BAV AS gradient and the blinded assessment of post-BAV angiographic AR:
-
-
Optimal: gradient ≤ 35 mmHg and trivial or no AR
-
-
Adequate: gradient ≤ 35 mmHg and mild AR
-
-
Inadequate: gradient > 35 mmHg and/or moderate or severe AR
This hierarchy of outcomes is based on the freedom from AVR 10 years post dilation associated with each category.(32) Freedom from AVR after BAV for the cohort reported by Brown et.al. with the patients categorized into these three combined outcome categories can be found in supplemental figure 1.
Balloon Aortic Valvuloplasty
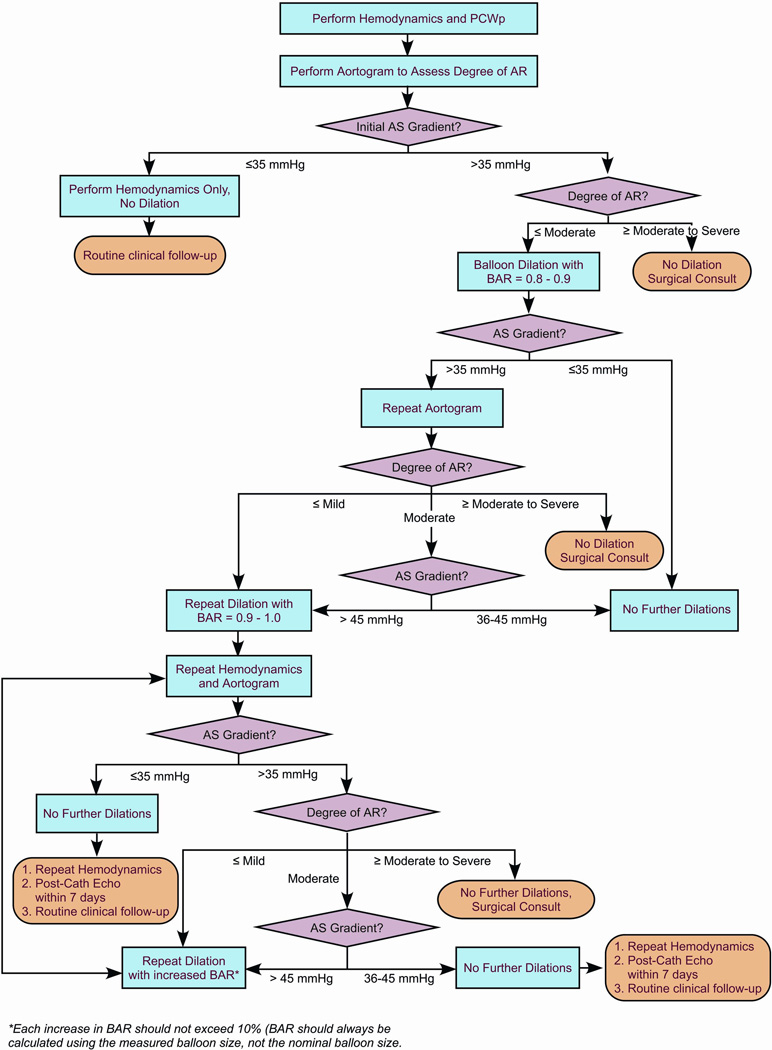
The technical details of aortic valve dilation have been described previously.(19, 26, 30) The decision support algorithm for the SCAMP (Figure 1) provides recommendations for some of the major technical aspects of the procedure, including initial BAR, criteria for repeat dilation, incremental increase in BAR with subsequent dilations, and performance of aortic root angiograms to evaluate AR. Our institution’s overall approach to BAV has remained largely unchanged over the last two decades. The main changes in practice incorporated into the SCAMP were the following: intervening for gradients less than 50 mmHg, repeated dilation to achieve residual AS gradient ≤35 mmHg, and more gradual increase in the balloon to annulus ratio, increasing the measured balloon size to aortic valve annulus ratio by ~10% with each dilation. In small children and infants, this may require taking advantage of compliance characteristics of the balloons beyond their nominal pressures to deliver specific dilating diameters.
Figure 1.
Decision Support Algorithm for the Balloon Aortic Valvuloplasty SCAMP. AR: aortic regurgitation; AS: aortic stenosis; BAR: balloon-to-annulus ratio; Echo: echocardiogram; PCWp: pulmonary capillary wedge pressure.
Statistical Analysis
The primary outcome measure was the composite outcome after BAV, using the blinded assessment of AR. Secondary outcome measures include the residual AS gradient and degree of AR after BAV as assessed by post-procedure echocardiogram and as reported in the catheterization report. Data are presented as mean ±SD or median (range), as appropriate. To account for the matching of SCAMP patients and controls, comparisons were performed using conditional logistic regression analysis, with the exception of the comparison of post-BAV AS gradient category, which was performed using Fisher’s exact test (since there were no patients with a residual gradient >35 mmHg in the SCAMP group). The variability around minimal and maximal BAR between the two groups was compared using the F test of equality of variances. A p-value of less than 0.05 was considered statistically significant.For comparison of AR grading between different modalities (blinded angiographic assessment, angiographic assessment as reported by the operator, and echocardiographic assessment) Spearman rank correlation coefficients and percent agreement were calculated.
Results
Pre Intervention Characteristics
The demographic and clinical characteristics of the 23 patients enrolled in the SCAMP from December 2010 until April 2012 and their 92 matched historical controls are summarized in Table 2. Forty eight percent of the cohort was ≤ 1 year of age. Peak gradients were greater than 50 mm Hg in 61% with no or trivial AR present in the majority (83%) and mild AR in the remaining. Left ventricular function was normal in 82%. Although the median gradient before BAV was lower in the SCAMP group, categorical groups were similar due to the matching of gradients within 15 mm Hg.
Table 2.
Demographic and clinical characteristics of SCAMP patients compared to historical controls
| Variable | SCAMP | Control | p value |
|---|---|---|---|
| N | 23 | 92 | |
| Age | 1.3 (0–22.5) | 0.9 (–0.9) | 0.59 |
| 1= <1m | 5 (22%) | 21 (23%) | |
| 2= 1–11m | 6 (26%) | 26 (28%) | >0.99 |
| 3= 1–11y | 6 (26%) | 19 (21%) | |
| 4= >11y | 6 (26%) | 26 (28%) | |
| Weight (Kg) | 9.9 (2.5–118) | 10.4 (1.7–138.5) | 0.54 |
| BSA (m2) | 0.65 (.18–2.21) | 0.46 (.15–2.57) | 0.20 |
| History of prior BAV | 6 (26%) | 22 (24%) | 0.79 |
| History of prior surgical aortic valvuloplasty | 3 (13%) | 6 (7%) | 0.38 |
| Ejection fraction (%) Echo Pre Cath | 63 (24–74) | 67 (10–80) | 0.05 |
| LV dysfunction By Pre Cath echo | 4 (18%) | 16 (18%) | >0.99 |
| LVEDV z score | −.44 (−1.78–5.77) | −.41 (−4.01–6.08) | 0.64 |
| LVEDp before BAVP | 17±5.6 | 15±4.7 | 0.06 |
| Aortic Valve Diameter (mm) | 9.7 (5–26) | 10.6 (3.8–26) | 0.54 |
| AS gradient pre BD (mmHg) | 55 (35–95) | 60 (35–100) | 0.02 |
| ≤50 | 9 (39%) | 28 (30%) | |
| 51–79 | 11 (48%) | 48 (53%) | 0.10 |
| ≥80 | 3 (13%) | 16 (17%) | |
| AR pre BD by Echo | |||
| None/trace | 19 (83%) | 76 (83%) | |
| Mild | 4 (17%) | 16 (13%) | >0.99 |
| Moderate or severe | 0 (0%) | 0 (0%) | |
Adherence to SCAMP recommendations
Deviations from the SCAMP recommendations were identified and reviewed. Overall adherence to the SCAMP decision support algorithm was 78%, and the point of deviation had to do with either balloon size or performance of aortography. There were no deviations on the initial decision to proceed with BAV. Among deviations related to balloon size, the initial balloon used was larger than recommended in 2 patients (larger size was chosen due to knowledge of balloon size during BAV only 6 months prior, n=1; “per operator preference”, n=1), and was smaller than recommended in one (operator preference). Omission of the recommended angiogram between the first and second dilation occurred in 4 cases, because no hemodynamic change was seen and the patient was deemed to be too unstable by the operator (n=3) or because the aortic valve had been challenging to cross and operator did not want to give up LV wire position (n=1).
Balloon Aortic Valvuloplasty
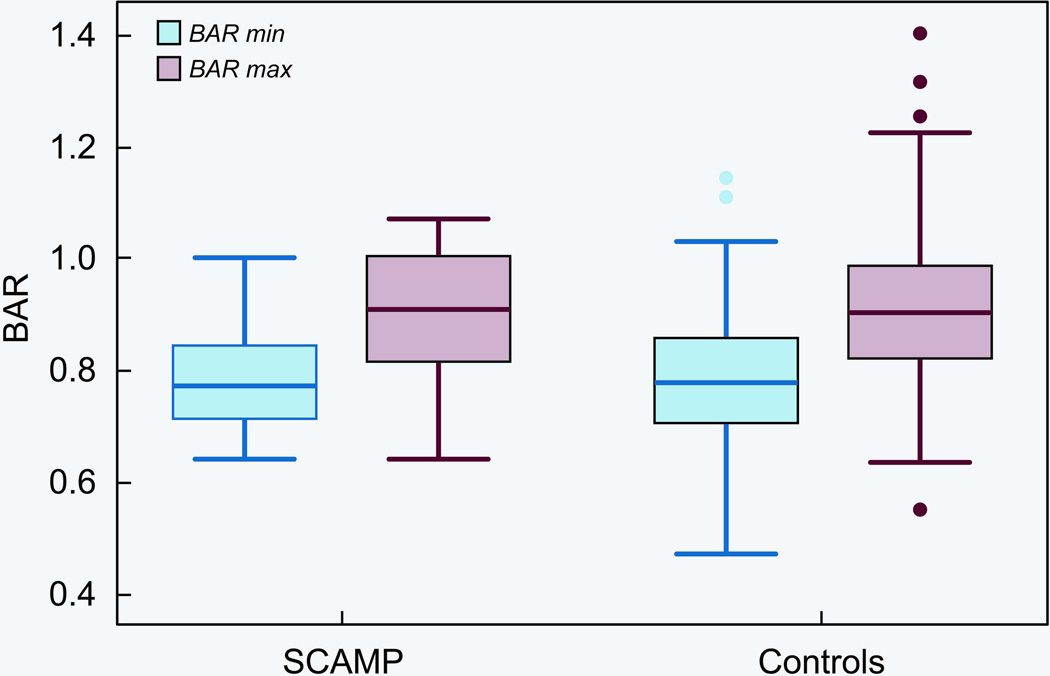
Technical variables related to BAV are summarized in Table 3. Procedures were similar with regard to number of balloons used and number of inflations. Although there was no difference in the mean of the BAR in the SCAMP vs. control groups, there was a trend towards less variability in the BAR in the SCAMP group (p=0.07, Figure 2). Rapid cardiac pacing for balloon stabilization was used more frequently in the SCAMP patients (39 versus 10%; p=0.002). No procedure related adverse events were reported in the 23 SCAMP patients.
Table 3.
Technical Variables in the SCAMP patients compared to historical controls
| Variable | SCAMP | Control | p value |
|---|---|---|---|
| N | 23 | 92 | |
| Nominal Minimal Balloon (mm) | 9 (4–24) | 9.5 (3–22) | 0.08 |
| Nominal Maximal Balloon (mm) | 12 (5–25) | 11 (4.5–28) | 0.11 |
| Nominal Minimal BAR | 0.83±0.10 | 0.83±0.10 | 0.17 |
| Nominal Maximal BAR | 0.98±0.10 | 0.95±0.12 | 0.27 |
| Measured Minimal Balloon (mm) | 9.3 (4.1 – 19.3) | 8.6 (2.6 – 24.8) | 0.36 |
| Measured Maximal Balloon (mm) | 11.5 (5.4 – 21.1) | 12.2 (2.8 – 24.8) | 0.78 |
| Measured Minimal BAR | 0.79 ± 0.10 | 0.77 ± 0.13 | 0.68 |
| Measured Maximal BAR | 0.91 ± 0.11 | 0.92 ± 0.16 | 0.83 |
| # of balloons used | 2 (1–3) | 2 (1–6) | 0.15 |
| # of inflations | 2 (1–4) | 3 (1–11) | 0.07 |
| Balloon stabilization | 9 (39%) | 9 (10%) | 0.002 |
Figure 2.
Box Plot Showing balloon to annulus ratios (BAR) for the smallest (min) and largest (Max) balloons used during BAV for patients in the SCAMP and control groups.
Acute Outcomes
The acute outcomes of BAV for both groups are summarized in Table 4. The median post-BAV gradient was lower in SCAMP patients, and all SCAMP patients had a residual gradient ≤35 mmHg. Although SCAMP patients more often had none/trace AR post BAV (52% versus 41%) and less often had moderate or severe AR (18% versus 31%), this difference is not significant. (p=0.33)However, the composite outcome was better in the SCAMP group, with a higher proportion of patients having an optimal result, and a lower proportion of patients having an inadequate result (52% versus 34% and 17% versus 45%, respectively; p=0.02).
Table 4.
Acute Outcomes after Balloon Aortic Valvuloplasty for Congenital Aortic Stenosis in SCAMP patients and historical controls
| Variable | SCAMP | Control | p value |
|---|---|---|---|
| N | 23 | 92 | |
| AS gradient post BD (mmHg) | |||
| Median (range) | 25 (10–35) | 30 (0–65) | 0.005 |
| ≤35 | 23 (100%) | 74 (80%) | 0.02* |
| >35 | 0 (0%) | 18 (20%) | |
| AR Post BD | |||
| Unable to evaluate | 0 (0%) | 1 (1%) | |
| None/trivial | 12 (52%) | 38 (41%) | 0.33 |
| Mild | 7 (30%) | 25 (27%) | |
| Moderate or severe | 4 (18%) | 28 (31%) | |
| Final result Category (AS gradient, AR grade) | 0.02 | ||
| Unable to evaluate | 0 (0%) | 1 (1%) | |
| Optimal (≤35 mmHg, none/trivial) | 12 (52%) | 31 (34%) | |
| Adequate (≤35 mmHg, Mild) | 7 (30%) | 19 (21%) | |
| Inadequate (>35 mmHg ± moderate or severe) | 4 (17%) | 41 (45%) | |
Obtained using Fisher’s exact test.
Most (83%) of the patients in the control group underwent BAV within 10 years of the introduction of the SCAMP. Within the control group, there was no difference in the proportion of patients in each combined outcome category between the group of patients undergoing BAV before 2005 and those undergoing BAV between 2005 and the introduction of the SCAMP (p=0.31).
Assessment of aortic regurgitation
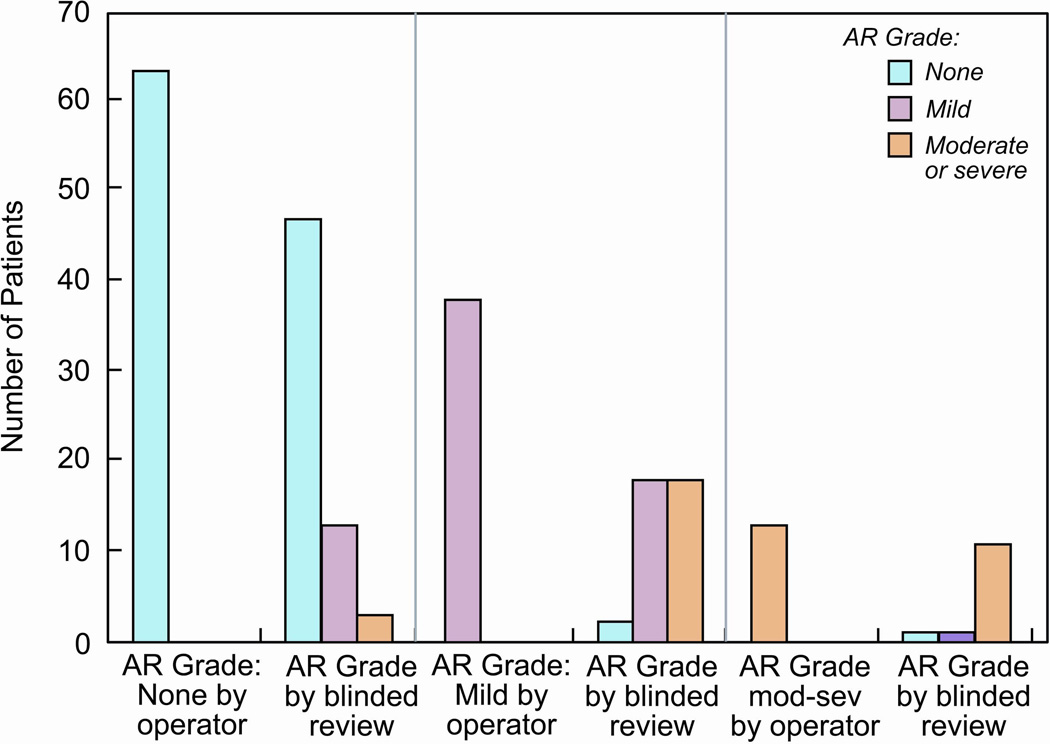
Outcomes based on AR grade obtained from the catheterization report and the post-BAV echocardiograms are shown in Table 5. Independent of the source of AR grading, the degree of AR between SCAMP and control patients was not different, and the combined outcomes were better for SCAMP patients. However, the degree of AR as assessed by the blinded angiographic reviewer was greater than that reported by operator in 36% of the cases (35% for the SCAMP group and 36% for the control group), whereas the reviewer found less AR in only 3% of cases (Figure 3).
Table 5.
Acute Outcomes after Balloon Aortic Valvuloplasty for Congenital Aortic Stenosis in SCAMP patients and historical controls based on reported (non-blinded) angiographic and echocardiographic assessment of AR.
| Variable | SCAMP | Control | p value |
|---|---|---|---|
| N | 23 | 92 | |
| AR Post BD (per cathreport) | |||
| None/trivial | 16 (70%) | 47 (51%) | 0.15 |
| Mild | 5 (22%) | 33 (36%) | |
| Moderate or severe | 2 (8%) | 12 (13%) | |
| AR Post BD (per post BAV echo report) | |||
| No echo within 3 months | 4 (17%) | 21 (23%) | |
| None/trivial | 8/19 (42%) | 24/71 (34%) | 0.78 |
| Mild | 9/19 (47%) | 40/71 (56%) | |
| Moderate or severe | 2/19 (11%) | 7/71 (10%) | |
| Final result Category (AS gradient, AR grade)(Using cath report AR) | 0.01 | ||
| Optimal (≤35 mmHg, none/trivial) | 16 (69%) | 37 (40%) | |
| Adequate (≤35 mmHg, Mild) | 5 (22%) | 27 (29%) | |
| Inadequate (>35 mmHg ± moderate or severe) | 2 (9%) | 28 (30%) | |
| Final result Category (AS gradient, AR grade)(Using post BAV echo report AR) | |||
| Unable to evaluate | 4 (17%) | 18 (20%) | 0.07 |
| Optimal (≤35 mmHg, none/trivial) | 8/19 (42%) | 18/74 (24%) | |
| Adequate (≤35 mmHg, Mild) | 9/19 (47%) | 33/74 (45%) | |
| Inadequate (>35 mmHg ± moderate or severe) | 2/19 (11%) | 23/74 (31%) | |
Figure 3.
Histogram showing correlation between the angiographic aortic regurgitation (AR) grade at the end of the procedure as assessed by a blinded reviewer and the operator (non-blinded). As compared to the blinded reviewer, the operator underestimated the degree of AR in 34 cases (30%), and overestimated it in 4 cases (3%). Mod-sev: moderate or severe.
The Spearman rank correlation coefficient between the blinded and non-blinded assessments of angiographic AR was 0.73, and the percent agreement (how often do patients end up in the same category) between the two methods was 67%. The Spearman rank correlation coefficient between the blinded angiographic assessment of AR and the reported echocardiographic assessment of AR was 0.55, and the percent agreement was 48%.
Discussion
In this study we report our initial experience with a standardized approach to BAV for congenital AS. The ultimate goal of the SCAMP is to improve long term outcomes. Therefore, we based the design on available data correlating acute results after BAV with long term outcomes.(32)The result is a SCAMP which prioritizes achieving a residual gradient ≤ 35 mmHg, while attempting to avoid moderate AR. All patients in the SCAMP group achieved a final gradient ≤ 35 mmHg. Importantly, there was no statistically significant difference in the degree of AR at end of the procedure in the SCAMP group, when compared to matched historical controls with higher residual gradients. This finding suggests that attempting to achieve a lower gradient using this SCAMP methodology does not necessarily result in greater AR.
Procedural success for BAV has been defined in different ways, mostly involving a combination of residual AS gradient and AR at the end of the procedure(19, 23, 26, 29, 30). Here, we also use residual AS gradient and AR to classify acute outcomes into three categories. However, the hierarchy of these categories is based on the associated risk of AVR on long term follow up. Using this novel combined outcome, we find that patients who underwent BAV at our institution after introduction of the SCAMP had better acute outcomes than those that underwent BAV before the SCAMP was introduced.Whether these improved acute outcomes translate into better long term outcomes for this patient population will be the focus of future research.
Standardization of processes in medicine can lead to reductions in errors and improvement in outcomes. An excellent example of this is the implementation of surgical checklists, which has been shown to decrease morbidity and mortality in surgical patients.(5) Standardized clinical care pathways for surgical procedures providing a goal-directed approach for initial assessment, procedure selection, intraoperative management, and post-operative care have been demonstrated to reduce length of stay and costs and improve peri-operative outcomes.(6–10)Clinical practice guidelines have also been shown to standardize care, diminish local variation of practice and improve health care outcomes.(3) Unfortunately, for rare conditions, evidence to support “best clinical practice” is often non-existent, and clinical decision making can become idiosyncratic.(33)
The three goals of a SCAMP are to reduce practice variation, to optimize resource utilization, and to improve patient care.(13)Importantly, the implementation of SCAMPs includes a process of iterative analysis and modification.(12–15) Use of a SCAMP for evaluation of chest pain in pediatric patients has been shown to decrease practice variation and resource utilization.(12, 15) In the present study, we show an improvement in acute procedural outcomes after implementation of the BAV for AS SCAMP, along with a trend toward standardization of the technical aspects of the procedure.
Another unique feature of a SCAMP is that there is active collection of data on deviations from the recommended algorithm, which takes advantage of the idiosyncratic nature of clinical practice in a heterogeneous population and provides opportunities for improving patient care.(11) The overall adherence to the SCAMP recommendations in the present series was 78%. This is similar to that reported for other SCAMPs, which has varied between 70 and 92%.(11, 13, 15) The deviations recorded in our initial experience with the BAV for AS SCAMP have not been of major clinical significance, and have been too few to be able to draw any conclusions that would lead to major changes in the SCAMP recommendations. Only two minor changes to this SCAMP have occurred in this first cycle. First, procedurally, the recommendation for a baseline aortogram has been eliminated for patients who have trivial or no AR echocardiographically prior to the procedure, thus reducing total contrast burden of the intervention. Second, elements of pre- and post-procedural echocardiographic data assessing left ventricular function have been added to the data collection forms.
Assessment of AR for this study was performed by a blinded observer in order to avoid bias introduced by inter-rater variability. It is interesting to note that agreement between the blinded angiographic AR grade, non-blinded angiographic AR grade (as reported by the operator) and the echocardiographic AR grade was only between 48 and 67%. This study was not designed to assess the accuracy of AR grading by any one modality, and therefore these data should be interpreted with caution. However, it does illustrate the problem with subjective methods of AR assessment.
Limitations
Some of the limitations of this study relate to the difference in criteria for intervention between the two groups. There were no pre-defined standards for intervention in the control group, whereas the SCAMP patients had a well-defined set of criteria for intervention. Overall, patients in the SCAMP group had a lower median pre-BAV AS gradient. This is an expected finding, since one of the main changes in practice incorporated into the SCAMP was intervening for lower gradients. We tried to account for this by matching historical controls with AS gradients within 15 mmHg of the corresponding SCAMP patient. In fact, there was no difference in AS gradient category between the two groups. Despite these efforts, it is possible that the patients in the SCAMP group represent a less severe spectrum of disease. Given this limitation, the data that suggests that this SCAMP improved care is not conclusive. However, we believe it is persuasive, and should guide further care in these patients.
Another limitation is that the control group spans a period of over 15 years prior to the introduction of the SCAMP to our practice. There is a possibility that there were trends towards improvement in the procedure over time that were unrelated to the SCAMP. We tried to account for this by matching for the most recent cases (83% of the cases in the control group were performed within 10 years of introduction of the SCAMP). We also did not observe an era effect in acute procedural outcomes within the control group. However, it is possible that the improved outcomes observed in the SCAMP cohort are a reflection of secular trends and not the introduction of the SCAMP itself.
Adverse events were not compared between the two groups in this study because a comprehensive, prospective, adverse event reporting system was not established in our program until 2005. Therefore, capture of adverse events before that may be incomplete. Also, because there are only 23 patients in the SCAMP group and adverse events related to BAV are rare, it would be difficult to draw valid conclusions from this kind of analysis. To date, there have been no reported adverse events in the 23 SCAMP patients.
Conclusions
In conclusion, we report the use of a standardized methodology for BAV which aims to increase the proportion of patients with acute procedural results which have been shown to be associated with improved long-term outcomes. The initial 23 patients that have undergone BAV at our institution since implementation of this SCAMP have had improved compositeacute outcomes, as compared to matched historical controls. Whether these improved acute outcomes translate into better long term outlook for this patient population, as suggested by previous reports, remains to be seen.
Supplementary Material
Acknowledgements
we thank Emily Harris for artistic support.
Funding Sources: NIH grant number T32HLO7572-27
Footnotes
Disclosures: The authors do not have any relevant relationships with industry to disclose.
Author Contributions
Diego Porras: conceptualized and designed the study, collected the data and drafted the initial manuscript.
David W Brown: collected the data on the control subjects, reviewed and critically revised the manuscript.
Rahul Rathod: collected the echocardiographic data and reviewed and critically revised the manuscript.
Kevin Friedman: contributed to conceptualization and design of the study, reviewed and critically revised the manuscript.
Kimberly Gauvreau: contributed to conceptualization and design of the study, performed the statistical analysis of the data and reviewed and critically revised the manuscript.
James E Lock: contributed to conceptualization and design of the study, reviewed and critically revised the manuscript.
Jesse J Esch: contributed to conceptualization and design of the study, reviewed and critically revised the manuscript.
Lisa Bergersen: contributed to conceptualization and design of the study, reviewed and critically revised the manuscript.
Audrey C Marshall: contributed to conceptualization and design of the study, provided the blinded review of all angiographic images, and reviewed and critically revised the manuscript.
References
- 1.Wennberg J, Gittelsohn A. Small area variations in health care delivery. Science. 1973;182(4117):1102–1108. doi: 10.1126/science.182.4117.1102. Epub 1973/12/14. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg EP. Improving the quality of care--can we practice what we preach? The New England journal of medicine. 2003;348(26):2681–2683. doi: 10.1056/NEJMe030085. Epub 2003/06/27. [DOI] [PubMed] [Google Scholar]
- 3.Steinbrook R. Guidance for guidelines. The New England journal of medicine. 2007;356(4):331–333. doi: 10.1056/NEJMp068282. Epub 2007/01/26. [DOI] [PubMed] [Google Scholar]
- 4.Shelley D, Tseng TY, Matthews AG, Wu D, Ferrari P, Cohen A, et al. Technology-driven intervention to improve hypertension outcomes in community health centers. The American journal of managed care. 2011;17(12 Spec No.):SP103–SP110. Epub 2012/06/27. [PubMed] [Google Scholar]
- 5.Haynes AB, Weiser TG, Berry WR, Lipsitz SR, Breizat AH, Dellinger EP, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. The New England journal of medicine. 2009;360(5):491–499. doi: 10.1056/NEJMsa0810119. Epub 2009/01/16. [DOI] [PubMed] [Google Scholar]
- 6.Low DE, Kunz S, Schembre D, Otero H, Malpass T, Hsi A, et al. Esophagectomy--it's not just about mortality anymore: standardized perioperative clinical pathways improve outcomes in patients with esophageal cancer. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2007;11(11):1395–1402. doi: 10.1007/s11605-007-0265-1. discussion 402. Epub 2007/09/04. [DOI] [PubMed] [Google Scholar]
- 7.Muehling B, Schelzig H, Steffen P, Meierhenrich R, Sunder-Plassmann L, Orend KH. A prospective randomized trial comparing traditional and fast-track patient care in elective open infrarenal aneurysm repair. World journal of surgery. 2009;33(3):577–585. doi: 10.1007/s00268-008-9892-2. Epub 2009/01/13. [DOI] [PubMed] [Google Scholar]
- 8.Munitiz V, Martinez-de-Haro LF, Ortiz A, Ruiz-de-Angulo D, Pastor P, Parrilla P. Effectiveness of a written clinical pathway for enhanced recovery after transthoracic (Ivor Lewis) oesophagectomy. The British journal of surgery. 2010;97(5):714–718. doi: 10.1002/bjs.6942. Epub 2010/02/27. [DOI] [PubMed] [Google Scholar]
- 9.Preston SR, Markar SR, Baker CR, Soon Y, Singh S, Low DE. Impact of a multidisciplinary standardized clinical pathway on perioperative outcomes in patients with oesophageal cancer. The British journal of surgery. 2012 doi: 10.1002/bjs.8974. Epub 2012/11/20. [DOI] [PubMed] [Google Scholar]
- 10.Wang G, Jiang ZW, Xu J, Gong JF, Bao Y, Xie LF, et al. Fast-track rehabilitation program vs conventional care after colorectal resection: a randomized clinical trial. World journal of gastroenterology : WJG. 2011;17(5):671–676. doi: 10.3748/wjg.v17.i5.671. Epub 2011/02/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farias M, Friedman KG, Powell AJ, de Ferranti SD, Marshall AC, Brown DW, et al. Dynamic evolution of practice guidelines: analysis of deviations from assessment and management plans. Pediatrics. 2012;130(1):93–98. doi: 10.1542/peds.2011-3811. Epub 2012/06/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman KG, Kane DA, Rathod RH, Renaud A, Farias M, Geggel R, et al. Management of pediatric chest pain using a standardized assessment and management plan. Pediatrics. 2011;128(2):239–245. doi: 10.1542/peds.2011-0141. Epub 2011/07/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman KG, Rathod RH, Farias M, Graham D, Powell AJ, Fulton DR, et al. Resource utilization after introduction of a standardized clinical assessment and management plan. Congenital heart disease. 2010;5(4):374–381. doi: 10.1111/j.1747-0803.2010.00434.x. Epub 2010/07/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rathod RH, Farias M, Friedman KG, Graham D, Fulton DR, Newburger JW, et al. A novel approach to gathering and acting on relevant clinical information: SCAMPs. Congenital heart disease. 2010;5(4):343–353. doi: 10.1111/j.1747-0803.2010.00438.x. Epub 2010/07/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verghese GR, Friedman KG, Rathod RH, Meiri A, Saleeb SF, Graham DA, et al. Resource Utilization Reduction for Evaluation of Chest Pain in Pediatrics Using a Novel Standardized Clinical Assessment and Management Plan (SCAMP) Journal of the American Heart Association. 2012;1(2) doi: 10.1161/JAHA.111.000349. Epub 2012/11/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Justo RN, McCrindle BW, Benson LN, Williams WG, Freedom RM, Smallhorn JF. Aortic valve regurgitation after surgical versus percutaneous balloon valvotomy for congenital aortic valve stenosis. The American journal of cardiology. 1996;77(15):1332–1338. doi: 10.1016/s0002-9149(96)00201-9. Epub 1996/06/15. [DOI] [PubMed] [Google Scholar]
- 17.Alva C, Sanchez A, David F, Jimenez S, Jimenez D, Ortegen J, et al. Percutaneous aortic valvoplasty in congenital aortic valvar stenosis. Cardiology in the young. 2002;12(4):328–332. doi: 10.1017/s1047951100012919. Epub 2002/09/11. [DOI] [PubMed] [Google Scholar]
- 18.Balmer C, Beghetti M, Fasnacht M, Friedli B, Arbenz U. Balloon aortic valvoplasty in paediatric patients: progressive aortic regurgitation is common. Heart. 2004;90(1):77–81. doi: 10.1136/heart.90.1.77. Epub 2003/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egito ES, Moore P, O'Sullivan J, Colan S, Perry SB, Lock JE, et al. Transvascular balloon dilation for neonatal critical aortic stenosis: early and midterm results. Journal of the American College of Cardiology. 1997;29(2):442–447. doi: 10.1016/s0735-1097(96)00497-4. Epub 1997/02/01. [DOI] [PubMed] [Google Scholar]
- 20.Fratz S, Gildein HP, Balling G, Sebening W, Genz T, Eicken A, et al. Aortic valvuloplasty in pediatric patients substantially postpones the need for aortic valve surgery: a single-center experience of 188 patients after up to 17.5 years of follow-up. Circulation. 2008;117(9):1201–1206. doi: 10.1161/CIRCULATIONAHA.107.687764. Epub 2008/02/21. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins JA, Minich LL, Shaddy RE, Tani LY, Orsmond GS, Sturtevant JE, et al. Aortic valve repair and replacement after balloon aortic valvuloplasty in children. The Annals of thoracic surgery. 1996;61(5):1355–1358. doi: 10.1016/0003-4975(96)00018-5. Epub 1996/05/01. [DOI] [PubMed] [Google Scholar]
- 22.Keane JF, Bernhard WF, Nadas AS. Aortic stenosis surgery in infancy. Circulation. 1975;52(6):1138–1143. doi: 10.1161/01.cir.52.6.1138. Epub 1975/12/01. [DOI] [PubMed] [Google Scholar]
- 23.McCrindle BW. Independent predictors of immediate results of percutaneous balloon aortic valvotomy in children. Valvuloplasty and Angioplasty of Congenital Anomalies (VACA) Registry Investigators. The American journal of cardiology. 1996;77(4):286–293. doi: 10.1016/s0002-9149(97)89395-2. Epub 1996/02/01. [DOI] [PubMed] [Google Scholar]
- 24.McCrindle BW, Blackstone EH, Williams WG, Sittiwangkul R, Spray TL, Azakie A, et al. Are outcomes of surgical versus transcatheter balloon valvotomy equivalent in neonatal critical aortic stenosis? Circulation. 2001;104(12 Suppl 1):I152–I158. doi: 10.1161/hc37t1.094837. Epub 2001/09/25. [DOI] [PubMed] [Google Scholar]
- 25.McElhinney DB, Lock JE, Keane JF, Moran AM, Colan SD. Left heart growth, function, and reintervention after balloon aortic valvuloplasty for neonatal aortic stenosis. Circulation. 2005;111(4):451–458. doi: 10.1161/01.CIR.0000153809.88286.2E. Epub 2005/02/03. [DOI] [PubMed] [Google Scholar]
- 26.Moore P, Egito E, Mowrey H, Perry SB, Lock JE, Keane JF. Midterm results of balloon dilation of congenital aortic stenosis: predictors of success. Journal of the American College of Cardiology. 1996;27(5):1257–1263. doi: 10.1016/0735-1097(95)00608-7. Epub 1996/04/01. [DOI] [PubMed] [Google Scholar]
- 27.Mosca RS, Iannettoni MD, Schwartz SM, Ludomirsky A, Beekman RH, 3rd, Lloyd T, et al. Critical aortic stenosis in the neonate. A comparison of balloon valvuloplasty and transventricular dilation. The Journal of thoracic and cardiovascular surgery. 1995;109(1):147–154. doi: 10.1016/S0022-5223(95)70430-2. Epub 1995/01/01. [DOI] [PubMed] [Google Scholar]
- 28.Pedra CA, Sidhu R, McCrindle BW, Nykanen DG, Justo RN, Freedom RM, et al. Outcomes after balloon dilation of congenital aortic stenosis in children and adolescents. Cardiology in the young. 2004;14(3):315–321. doi: 10.1017/S1047951104003105. Epub 2005/02/01. [DOI] [PubMed] [Google Scholar]
- 29.Reich O, Tax P, Marek J, Razek V, Gilik J, Tomek V, et al. Long term results of percutaneous balloon valvoplasty of congenital aortic stenosis: independent predictors of outcome. Heart. 2004;90(1):70–76. doi: 10.1136/heart.90.1.70. Epub 2003/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sholler GF, Keane JF, Perry SB, Sanders SP, Lock JE. Balloon dilation of congenital aortic valve stenosis. Results and influence of technical and morphological features on outcome. Circulation. 1988;78(2):351–360. doi: 10.1161/01.cir.78.2.351. Epub 1988/08/01. [DOI] [PubMed] [Google Scholar]
- 31.Zeevi B, Keane JF, Castaneda AR, Perry SB, Lock JE. Neonatal critical valvar aortic stenosis. A comparison of surgical and balloon dilation therapy. Circulation. 1989;80(4):831–839. doi: 10.1161/01.cir.80.4.831. Epub 1989/10/01. [DOI] [PubMed] [Google Scholar]
- 32.Brown DW, Dipilato AE, Chong EC, Lock JE, McElhinney DB. Aortic valve reinterventions after balloon aortic valvuloplasty for congenital aortic stenosis intermediate and late follow-up. Journal of the American College of Cardiology. 2010;56(21):1740–1749. doi: 10.1016/j.jacc.2010.06.040. Epub 2010/11/13. [DOI] [PubMed] [Google Scholar]
- 33.Darst JR, Newburger JW, Resch S, Rathod RH, Lock JE. Deciding without data. Congenital heart disease. 2010;5(4):339–342. doi: 10.1111/j.1747-0803.2010.00433.x. Epub 2010/07/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.