Abstract
Background
The visual temporal discrimination threshold (TDT) is the shortest time interval at which one can determine two stimuli to be asynchronous and meets criteria for a valid endophenotype in adult-onset idiopathic focal dystonia, a poorly penetrant disorder. Temporal discrimination is assessed in the hospital laboratory; in unaffected relatives of multiplex adult-onset dystonia patients distance from the hospital is a barrier to data acquisition. We devised a portable headset method for visual temporal discrimination determination and our aim was to validate this portable tool against the traditional laboratory-based method in a group of patients and in a large cohort of healthy controls.
Methods
Visual TDTs were examined in two groups 1) in 96 healthy control participants divided by age and gender, and 2) in 33 cervical dystonia patients, using two methods of data acquisition, the traditional table-top laboratory-based system, and the novel portable headset method. The order of assessment was randomized in the control group. The results obtained by each technique were compared.
Results
Visual temporal discrimination in healthy control participants demonstrated similar age and gender effects by the headset method as found by the table-top examination. There were no significant differences between visual TDTs obtained using the two methods, both for the control participants and for the cervical dystonia patients. Bland–Altman testing showed good concordance between the two methods in both patients and in controls.
Discussion
The portable headset device is a reliable and accurate method for visual temporal discrimination testing for use outside the laboratory, and will facilitate increased TDT data collection outside of the hospital setting. This is of particular importance in multiplex families where data collection in all available members of the pedigree is important for exome sequencing studies.
Keywords: Cervical dystonia, temporal discrimination threshold, headset
Introduction
Dystonia is a movement disorder, characterized by “sustained muscle contractions, frequently causing twisting and repetitive movements, or abnormal postures.”1 Adult-onset idiopathic isolated focal dystonia (AOIFD) is inherited in an autosomal dominant manner with a reduced penetrance of 12–15%;2,3 this lack of penetrance poses difficulties for genetic studies as identification of gene carriers is challenging. Recent advances in next-generation genetic sequencing have facilitated the discovery of a number of adult-onset idiopathic focal dystonia (AOIFD) genes,4–7 but these affect relatively few families and overall gene discovery in AOIFD has been slow.
Tools to identify non-manifesting gene carriage have been extensively studied in the form of endophenotypes,8–10 traits that are subclinical markers of gene carriage.11,12 We have suggested that an abnormal temporal discrimination threshold (TDT) fulfills the criteria for an endophenotype in AOIFD,13,14 and could significantly increase the yield from genetic studies. The neural circuitry involved in the TDT is thought to involve a sub-cortical–basal ganglia circuit,12 and it is postulated that the main input to this circuit is the superior colliculus.15 TDT abnormalities are not specific to AOIFD, however, and are present in other disorders that are characterized by basal ganglia pathology.16,17
Distance from the hospital laboratory has meant that families living in remote areas have been unable to participate in TDT testing, despite wishing to do so. In order to facilitate data acquisition in those who were unable to attend the hospital, we constructed a portable headset device for measurement of the visual TDT. The aim of this study was to assess the reliability and validity of measurement of the visual TDT using the novel headset device compared to the standard table-top method in healthy control participants and patients with cervical dystonia.
Methods
The study received ethical approval from the St Vincent's University Hospital Research Ethics committee and was carried out between February 2009 and November 2013.
Participants tested
Healthy control participants
Ninety-six healthy control participants (48 females) were recruited from hospital staff and visitors; informed written consent was obtained from each individual. The controls were divided into four subgroups in accordance with our most recently published control values that are age and gender dependent.18 The subgroups were (1) males aged 18–35 years, (2) males aged 36–65 years, (3) females aged 18–35 years, (4) females aged 36–65 years. Exclusion criteria were: history of any condition resulting in loss of visual acuity that might affect ability to perceive the visual stimulus (excluding visual refractory disorders such as myopia or hyperopia that are correctable with lenses); any history of a neurological disorder known to affect the basal ganglia including dystonia or a family history of dystonia, or parkinsonism of any cause; any history of cognitive impairment that may affect ability to understand and participate in the analysis.
Cervical dystonia patients
Thirty-three cervical dystonia patients (18 females) attending the botulinum toxin clinic were examined by both techniques at separate times, prior to their therapeutic injection. All of these patients had their TDT determined by the standard table-top method at variable intervals up to three years previously.
Testing conditions
Although the headset followed the table-top data acquisition in all patients, the order of testing in the healthy control participants by table-top and headset methods was counterbalanced within the subgroups in order to minimize any bias. The traditional table-top method required testing in a sound-proofed, air-conditioned, darkened room in the hospital. The novel headset method was tested, either in the same single session or at a different time point, in a bright, quiet office simulating the home environment as the device is enclosed; therefore, the ambient lighting conditions do not affect its use. Testing was carried out by research registrars (A.M., O.K., L.W.) according to a standard protocol. One demonstration run was done prior to each test in order to ensure that the participant knew what to expect, and understood the test. Inter-rater reliability determined by repeat examination of the TDT in 30 control patients and relatives showed no evidence of any significant inter-rater variability among the three raters (intraclass correlation coefficient = 0.8).18
Device design
Table-top method
The standard table-top method was created by the Trinity Centre for Bioengineering, Trinity College Dublin, employing commercial software presentation (Neurobehavioural Systems, www.neurobs.com) installed on a desktop computer and programmed to control the illumination of two light-emitting diodes (LED) via the parallel port of the computer. The two LED lights were positioned 7 degrees into the visual field of the participant and horizontally orientated on the table in front of the subject. The participants were asked to focus on a focal point in the midline and to try not to look directly at the flashing lights. Pairs of lights were illuminated synchronously for 5 ms initially, and thereafter were progressively separated in time by 5-ms steps every 5 s. When the subject reported that pairs of lights were flashing asynchronously on three consecutive occasions, the first of these was taken as the visual TDT. The median of four trials on each side was used for each subject in order to allow for practice effect, and these two results (one from each side) were averaged to obtain a summary visual TDT score (ms).
Headset device
The headset device (see Figure 1), also created by the Trinity Centre for Bioengineering, Trinity College Dublin, was made from laser-sintered nylon plastic and weighed 0.70 kg; the device is strong, flexible and has a low transparency index. Mirrors reflect the LEDs, yellow lights with a 5 mm diameter, with a red focal point of 3 mm diameter, from the back of the unit to 7 degrees into the subject's visual field. A rubber sealing system surrounded the unit-to-head interface to ensure that little light entered the device while the test was running. A focal distance of 350 mm was found to be sufficient to ensure focus, regardless of age of the participant. A strapping system ensured that the device was fixed securely to the participant's head in a comfortable manner. A compact control unit centered on a microcontroller (Arduino ATmega328) connected to the device was developed so that no external computer connection was needed to execute the experiment. Pairs of lights were presented in the same way as with the table-top method.
Figure 1. The Headset Device from the Rear (from the Patient's Perspective).
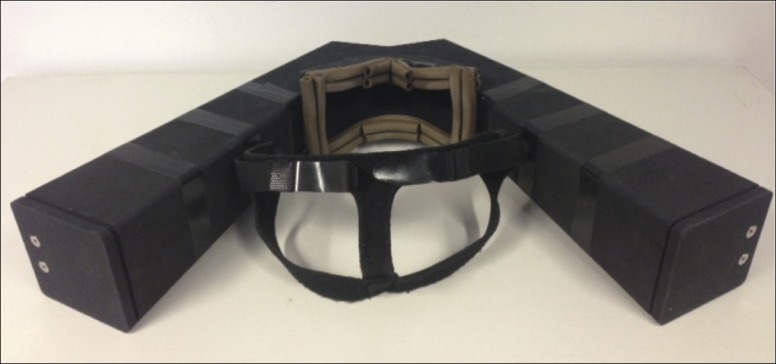
The device is made of nylon plastic and is lightweight and flexible. Two mirrors reflect the LEDs from the back of the unit on each side to 7 degrees into the subject's visual field. A comfortable rubber sealing system that surrounds the unit-to-head interface ensures that little light enters the device and that consistent background luminance is maintained. A flexible, elasticated strapping system ensures that the device is fixed securely to the participant's head comfortably during the test.
The luminance of the LEDs with both devices was 90 cd/m2, with an additional small amount of background luminance with the table-top method, to enable the operator to see just enough in the dark environment to run the experiment.
Statistical analysis
Demographic information was expressed as means and standard deviations (SD). Mean visual TDT scores including range and standard deviation were obtained for 1) the healthy control participant population as a whole and for each of the four subgroups, and 2) the cervical dystonia patients. Differences in means between subgroups were analyzed using one-way analysis of variance (ANOVA). Paired t-testing was used to assess differences in table-top versus headset results within each subgroup and for the population as a whole. Bland–Altman testing was done in each of the four control subgroups in order to assess for a systematic bias between methods. The effect of age and gender on the visual TDT result was determined using linear regression modeling.
Results
Control participants
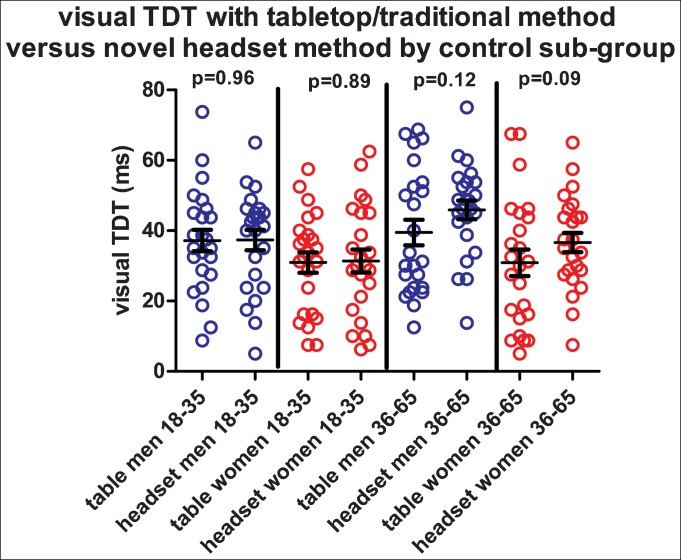
The 96 (48 females) healthy control participants had a mean age of 38.6 years (SD: 11.9, range 21.6–64.9 years). Each of the four subgroups (divided by gender and age, 18–35 and 36–65 years) included 24 individuals: 12 who carried out the table-top method first, and 12 who carried out the headset method first. The mean time difference between the two tests was 1.6 months (SD: 3.0, range 0–15.8 months) and on linear regression testing there was no significant effect of time difference on TDT result (p = 0.2). Mean visual TDT results, by gender, age, and method, including SD and upper limit of normal (mean+2.5 SD) are given in Table 1. Overall, using the average TDT of both testing methods, there was a significant difference in mean visual TDT between the four subgroups (one-way ANOVA [F(3,92) = 5.11, p = 0.002]): males 18–35 years: 37.3 ms (SD: 14.4), females 18–35 years: 31.1 ms (SD: 14.9); males 36–65 years: 42.7 ms (SD: 15.75), females 36–65 years: 33.75 (SD: 16.2). Paired t-testing (see Figure 2) showed no significant differences in the visual TDT between table-top versus headset methods within the subgroups: males 18–35 years (p = 0.96), females 18–35 years (p = 0.89); males 36–65 years (p = 0.12), females 36–65 years (p = 0.09). Using Bland–Altman testing, there was good concordance between the two methods with a homogeneous scatter around the mean in each group: in younger males the bias was 0.16 ms, in younger females 0.41 ms; in older males it was 6.44 ms and in older females it was 5.73 ms. In each group, the bias was in favor of the headset (the headset result was marginally longer). A significant practice effect was found regardless of method used first (first test mean visual TDT: 39.1 ms; second test mean visual TDT: 33.4 ms) (paired t-test, p = 0.012). Linear regression modeling showed a significant association of gender (p = 0.001), but not age (p = 0.43) with the visual TDT result.
Table 1. The Visual TDT in Healthy Control Participants, by Gender and Age subgroup, using Headset and Table-top Methods.
| Males18–35 years (n = 24) | Females 18–35 years (n = 24) | Males 36–65 years (n = 24) | Females 36–65 years (n = 24) | |
|---|---|---|---|---|
| Headset method | ||||
| Mean TDT (ms) | 37.3 | 31.4 | 45.9 | 36.6 |
| SD TDT (ms) | 14.2 | 15.9 | 13.1 | 13.4 |
| ULN (mean+2.5 SD) | 72.8 | 71.2 | 78.7 | 70.1 |
| Table-top method | ||||
| Mean TDT (ms) | 37.2 | 30.9 | 39.5 | 30.9 |
| SD table-top TDT | 14.9 | 14.2 | 17.8 | 18.5 |
| ULN (mean+2.5 SD) | 74.5 | 66.4 | 84.0 | 77.2 |
SD, Standard Deviation; TDT, Temporal Discrimination Threshold; ULN, Upper Limit of Normal.
Mean visual TDT with standard deviations by table-top and headset methods in each of four control groups (24 participants in each group) (males 18–35; females 18–35; males 36–65; females 36–65 years). The ULN for each group is the mean TDT plus 2.5 SD.
Figure 2. Illustrating the Visual TDT (in ms) Determined by the Traditional Table-top and Novel Headset Methods in Each of the Four Control Groups.
Paired t-tests of differences in means with headset and table-top values within each group are shown, all p-values are non-significant, consistent with no significant difference in means between each device in each group. Males 18–35, blue circles; females 18–35, red circles; males 36–65, blue circles; females 36–65, red circles. ms: Milliseconds; TDT, Temporal Discrimination Threshold.
Cervical dystonia patients
The 33 patients (18 females) with cervical dystonia had a mean age of 55.3 years (SD: 9.7, range 36.9–71.3 years). All had performed the table-top method previously (mean time between table-top and headset method 21.6 months, range 0–60 months). There was no significant difference in mean visual TDT between table-top (mean 70.2 ms, SD: 26.2) and headset methods (mean 69.0 ms, SD: 28.0) (p = 0.72). Bland–Altman analysis showed a bias of 1.2 ms in favor of the headset device in this group with a homogeneous scatter around the mean. The device was deemed to be light-weight, and was tolerated by all participants, and each individual completed the procedure.
Discussion
The aim of this study was to examine the utility of a portable device to measure visual temporal discrimination in unaffected relatives of AOIFD patients who lived some distance from the hospital laboratory. Such a device would particularly help in collecting data from unaffected relatives of multiplex AOIFD families in their homes. We have shown that this novel, portable device produces visual TDT results comparable to the traditional laboratory-based method.
Visual temporal discrimination in control participants
Control participants were divided into groups according to age and gender; we had previously found in 192 healthy control participants, using the table-top method, that TDTs showed significant independent age- and gender-related effects (temporal discrimination increased with age and was longer in men than in women).18 Thus, in the current headset study, we similarly subdivided our control participants; the visual TDT by the headset method behaved similarly in relation to age and gender and, within the four control sub-groups, showed good concordance and consistency.
Practice effect
We found a significant practice effect between the first and second test in this study, as we noted previously.18 This is relatively small (approximately 6 ms; <0.5 SD). However, this finding implies that in any individual, if a mildly abnormal visual TDT (TDT Z-score: 2.5–3.0) is found, it would be prudent and important to repeat the test. It is possible that the ascending nature of the inter-stimulus interval between light flashes might lend itself to pattern recognition among subjects. We plan to explore various alternative algorithms for presentation of stimuli in future studies.
TDT methods in this study compared to published studies
In this study we have tried to replicate the stimulus set-up we used in the table-top system. The luminance of both our table–top and headset LEDs is 90 cd/m2, which is lower than that reported in other studies (140 cd/m2).19,20 This may be important in measurement of the visual TDT, as the perception of an interval between sequential flashes of a bright stimuli could possibly differ with changes in luminance. Some variability exists between studies in relation to calculation of the TDT19,20 and, although not relevant in our analysis here, in studies that have employed the tactile TDT the location of electrodes for tactile stimuli has varied.21 Many studies used tactile stimuli alone, but in those that have employed visual stimuli for a combined TDT, the location of the LEDs has remained relatively consistent.13,19,20
Limitations of the current study
Both men and women in the 36–65 years age subgroup had slightly longer mean visual TDTs using the headset (males: 45.9 ms, females: 36.6 ms) compared to the table-top (males: 39.5 ms, females: 30.9 ms); smaller LEDs in the headset than in the table-top apparatus might be a possible reason. As noted above, any individual with a borderline abnormal visual TDT result using the headset should be re-tested with the table-top method. The headset was generally well tolerated by the control participants; however, a few cervical dystonia patients reported increased head tremor due to anxiety and the requirement for concentration; this occurs with the table-top examination also. An advantage of the headset system is that it moves with head tremor, presenting a static image to the subject compared to the table-top system.
Summary and conclusions
To date, only candidate gene-association studies in dystonia have been reported22 and the highest yield in gene discovery so far has been in multiplex dystonia pedigrees. In our experience, recruitment of all members of large, geographically dispersed, kindreds for research studies is challenging; using the headset, any consenting adult family member will be able to participate in testing the visual TDT in the community. The researcher can now travel to the family member, making participation more convenient and less time-consuming for these individuals. We have shown that a portable headset device is a valid alternative tool for visual TDT measurement. We recommend that dystonia research groups using the visual TDT might consider such a device in their daily practice as it has the potential to increase data collection and study participation.
Footnotes
Funding: This study was supported by grants from Dystonia Ireland, a non-profit patient information and support organization; the Irish Institute for Clinical Neuroscience; the Health Research Board, Ireland, Clinical Scientist Award (CSA-2012/5) the Foundation for Dystonia Research; Science Foundation Ireland (09/RFP/NE2382) and the Trinity Centre for Bioengineering.
Financial Disclosures: R.B.R is in receipt of funding from the European Commission (FP7-288914-VERVE), Health Research Board (J120,987), Science Foundation Ireland ( 09/RFP/NE2382) and Enterprise Ireland ( CS/2012/1007 and CF/2013/0058Y) and Cochlear Research and Development Ltd. S.O’R. reports receiving an honorarium from Abbott (advisory board) and speaker’s honorarium from Lundbeck. M. Hutchinson serves on a medical advisory board (BG00012) for Biogen-Idec; serves as associate editor of the Multiple Sclerosis Journal, has received speaker’s honoraria from Biogen-Idec, Bayer- Schering and Novartis and receives research grants from Dystonia Ireland, the Health Research Board of Ireland (CSA-2012-5) and the Irish Institute of Clinical Neuroscience.
Conflict of Interests: R.B.R is in receipt of research grants from Health Research Board Ireland: FP7-288914-VERVE, “VERVE: “Vanquishing Fear and apathy through E-inclusion: Personalised and populated Realistic Virtual Environments for clinical, home and mobile platforms” co-PI with Prof C. O’Sullivan and Prof F. Newell. Total project €4.6M October 2012 to October 2014; HRB: “INCA: Inhaler device for objective analysis of medication adherence” with Professor Richard Costello, Beaumont Hospital. €120,987, October 2011 to October 2013. S.O’R. reports receiving an honorarium from Abbott (advisory board) and speaker's honorarium from Lundbeck. M. Hutchinson serves on a medical advisory board (BG00012) for Biogen-Idec; serves as associate editor of the Multiple Sclerosis Journal, has received speaker's honoraria from Biogen-Idec, Bayer- Schering and Novartis and receives research grants from Dystonia Ireland, the Health Research Board of Ireland (CSA-2012-5) and the Irish Institute of Clinical Neuroscience.
References
- 1.Fahn S. Concept and classification of dystonia. In: Fahn S, Marsden CD, Calne DB, editors. Dystonia 2. Advances in neurology. New York Raven Press; 1988. pp. p 1–8. [PubMed] [Google Scholar]
- 2.Waddy HM, Fletcher NA, Harding AE, Marsden CD. A genetic study of idiopathic focal dystonias. Ann Neurol. 1991;29:320–324. doi: 10.1002/ana.410290315. [DOI] [PubMed] [Google Scholar]
- 3.Leube B, Kessler KR, Goecke T, Auburger G, Benecke R. Frequency of familial inheritance among 488 index patients with idiopathic focal dystonia and clinical variability in a large family. Mov Disord. 1997b;12:1000–1006. doi: 10.1002/mds.870120625. [DOI] [PubMed] [Google Scholar]
- 4.Charlesworth G, Plagnol V, Holmstrom KM, et al. Mutations in ANO3 cause autosomal dominant craniocervical dystonia: Ion channel implicated in pathogenesis. Am J Hum Genet. 2012;91:1041–1050. doi: 10.1016/j.ajhg.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs T, Saunders-Pullman R, Masuho I, et al. Mutations in GNAL cause primary torsion dystonia. Nat Genet. 2012;45:88–92. doi: 10.1038/ng.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hersheon J, Menacci NE, Davis M, et al. Mutations in the autoregulatory domain of beta-tubulin 4a cause hereditary dystonia. Ann Neurol. 2013;73:546–553. doi: 10.1002/ana.23832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao J, Uitti RJ, Zhao Y, et al. Mutations in CIZ1 cause adult-onset primary cervical dystonia. Ann Neurol. 2012;71:458–469. doi: 10.1002/ana.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamura Y, Matsuhashi M, Lin P, et al. Impaired intracortical inhibition in the primary somatosensory cortex in focal hand dystonia. Mov Disord. 2008;23:558–565. doi: 10.1002/mds.21870. [DOI] [PubMed] [Google Scholar]
- 9.Molloy FM, Carr TD, Zeuner KD, Dambrosia JM, Hallett M. Abnormalities of spatial discrimination in focal and generalized dystonia. Brain. 2003;126:2175–2182. doi: 10.1093/brain/awg219. [DOI] [PubMed] [Google Scholar]
- 10.Meunier S, Garnero L, Ducorps A, et al. Human brain mapping in dystonia reveals both endophenotypic traits and adaptive reorganization. Ann Neurol. 2001;50:521–527. doi: 10.1002/ana.1234. [DOI] [PubMed] [Google Scholar]
- 11.Kendler KS, Neale MC. Endophenotype: A conceptual analysis. Mol Psychiatry. 2010;15:789–797. doi: 10.1038/mp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutchinson M, Kimmich O, Molloy A, et al. The endophenotype and the phenotype: Temporal discrimination and adult onset dystonia. Mov Disord. 2013;28:1766–1774. doi: 10.1002/mds.25676. [DOI] [PubMed] [Google Scholar]
- 13.Bradley D, Whelan R, Walsh R, et al. Temporal discrimination threshold: VBM evidence for an endophenotype in adult onset primary torsion dystonia. Brain. 2009;132:2327–2335. doi: 10.1093/brain/awp156. [DOI] [PubMed] [Google Scholar]
- 14.Kimmich O, Bradley D, Whelan R, et al. Sporadic adult onset primary torsion dystonia is a genetic disorder by the temporal discrimination test. Brain. 2011;134:2656–2663. doi: 10.1093/brain/awr194. [DOI] [PubMed] [Google Scholar]
- 15.Hutchinson M, Isa T, Molloy A, et al. Cervical dystonia: A disorder of the midbrain network for covert attentional orienting. Front Neurol. 2014;5:54. doi: 10.3389/fneur.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Artieda J, Pastor MA, Lacruz F, Obeso J. Temporal discrimination is abnormal in Parkinson's disease. Brain. 1992;115((Pt 1)):119–210. doi: 10.1093/brain/115.1.199. [DOI] [PubMed] [Google Scholar]
- 17.Lyoo C, Lee S, Song T, Lee M. Abnormal temporal discrimination thresholds in patients with multiple systems atrophy. Mov Disord. 2007;22:556–559. doi: 10.1002/mds.21111. [DOI] [PubMed] [Google Scholar]
- 18.Kimmich O, Molloy A, Whelan R, et al. Temporal discrimination, a cervical dystonia endophenotype: Penetrance and functional correlates. Mov Disord. 2014;29:804–811. doi: 10.1002/mds.25822. [DOI] [PubMed] [Google Scholar]
- 19.Tinazzi M, Fiorio M, Bertolasi L, Aglioti S. Timing of tactile and visuo-tactile events is impaired in patients with cervical dystonia. J Neurol. 2004;251:85–90. doi: 10.1007/s00415-004-0282-x. [DOI] [PubMed] [Google Scholar]
- 20.Fiorio M, Gambarin M, Valente E, et al. Defective temporal processing of sensory stimuli in DYT1 mutation carriers: A new endophenotype of dystonia? Brain. 2007;130:134–142. doi: 10.1093/brain/awl283. [DOI] [PubMed] [Google Scholar]
- 21.Tinazzi M, Fasano A, Di Matteo A, et al. Temporal discrimination in patients with dystonia and tremor and patients with essential tremor. Neurology. 2013;80:1–9. doi: 10.1212/WNL.0b013e31827b1a54. [DOI] [PubMed] [Google Scholar]
- 22.Charlesworth G, Bhatia K, Wood N. The genetics of dystonia: New twists in an old tale. Brain. 2013;136:2017–2037. doi: 10.1093/brain/awt138. [DOI] [PMC free article] [PubMed] [Google Scholar]