Abstract
Anti-Aβ immunotherapy provides potential benefits in Alzheimer’s disease patients. Nevertheless, strategies based on Aβ1-42 peptide induced encephalomyelitis and possible microhemorrhages. These outcomes were not expected from studies performed in rodents. It is critical to determine if other animal models better predict side effects of immunotherapies. Mouse lemur primates can develop amyloidosis with aging. Here we used old lemurs to study immunotherapy based on Aβ1-42 or Aβ-derivative (K6Aβ1-30). We followed anti-Aβ40 IgG and IgM responses as well as Aβ levels in plasma. In-vivo magnetic resonance imaging and histology were used to evaluate amyloidosis, neuroinflammation, vasogenic edema, microhemorrhages, and brain iron deposits. The animals responded mainly to the Aβ1-42 immunogen. This treatment induced immune response and increased Aβ levels in plasma but also microhemorrhages and iron deposits in the choroid plexus. A complementary study of untreated lemurs showed iron accumulation in the choroid plexus with normal aging. Worsening of iron accumulation is thus a potential side effect of Aβ-immunization at prodromal stages of Alzheimer’s disease, and should be monitored in clinical trials.
Keywords: Aβ-immunization, Aging, Alzheimer’s disease, ARIA (Amyloid Imaging Related Abnormalities), Choroid plexus, Iron, Lemur, Microcebus murinus, Microhemorrhages, MRI, Primate
1. Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease that is the most common cause of dementia. Anti-amyloid-β (Aβ) immunotherapies aim to reduce the Aβ lesions that are critical for the pathogenesis of this disease (Hardy and Selkoe, 2002). They can be dissociated into 1) active immunotherapies during which Aβ or Aβ derivative proteins are injected in order to activate the immune system and elicit anti-Aβ antibodies. 2) Passive immunotherapies that rely on the administration of anti-Aβ antibodies. The initial evaluation of these therapies in transgenic mouse models of β-amyloidosis, was based on active strategy with Aβ1-42 peptides in Freund’s adjuvant. The outcome was a reduction of Aβ plaques (Schenk et al., 1999) and a stabilization of cognitive performances in these models (Janus et al., 2000; Morgan et al., 2000). These successes led to a first clinical trial based on administration of synthetic Aβ1-42 peptide associated with the QS21 adjuvant (AN1792) in patients with clinical criteria for a diagnosis of AD. This trial decreased Aβ load (Ferrer et al., 2004; Masliah et al., 2005; Nicoll et al., 2003), reduced some but not all (Holmes et al., 2008) of the neuronal alterations that characterize AD (Boche et al., 2010; Serrano-Pozo et al., 2010), and provided some cognitive benefits in certain patients (Gilman et al., 2005; Hock et al., 2003). However, this first clinical trial induced meningoencephalomyelitis in some individuals (Orgogozo et al., 2003). This alteration was attributed to cytotoxic T cells and/or autoimmune reactions to AN1792. Other possible side effects of immunotherapies such as severe cerebral amyloid angiopathy (CAA) and microhemorrhages have also been reported during this trial (Ferrer et al., 2004; Uro-Coste et al., 2010). Also, in most patients without meningoencephalomyelitis from the AN1792 trial, cognitive outcomes were not modified by the therapy (Holmes et al., 2008). Since this first trial, several clinical trials have been initiated either by using active or passive immunotherapies (see Mangialasche et al., 2010; Aisen et al., 2013 for reviews). They provided interesting results such as a reduction of amyloid load (Rinne et al., 2010), but no significant improvement of cognitive outcomes (Aisen et al., 2013). They also reported side effects such as microhemorrhages and vasogenic edemas (Sperling et al., 2011), although the latter lesion seems to occur mainly during passive immunotherapy and not in active immunotherapy. The side effects that can be detected in vivo by MRI in humans have been called "Amyloid Imaging Related Abnormalities" (ARIA) (Sperling et al., 2011).
After these outcomes, several points became obvious for further trials. First, new trials should be administered in prodromal stages of the disease. Second, approaches based on active immunotherapy should selectively target B-cell epitopes leading to humoral (Th2) immunity and antibody production without stimulating T cells to avoid neuroinflammation and toxicity. This can be done by selecting appropriate adjuvants and vaccines. For example, the alum adjuvant may be better than Freund’s adjuvant as it promotes humoral immunity (Cribbs et al. 2003; Asuni et al., 2006). Regarding the vaccines, several developments tried to reduce or eliminate the mid-region and C-terminal part of Aβ because it contains T-cell epitopes while keeping the two major immunogenic sites of Aβ peptides i.e. the 1–11 and 22–28 residues (Cribbs et al., 2003; Jameson and Wolf, 1988). For example, some approaches were based on the use of the Aβ1-6 (Wiessner et al., 2011), Aβ1-15 (Ghochikyan et al., 2006; Muhs et al., 2007), Aβ1-15 derivatives (Maier et al., 2006), Aβ1-16 (Muhs et al., 2007) or Aβ1-28 (Petrushima et al., 2008) peptides. In a previous work, we designed the K6Aβ1-30, a non fibrillogenic, non toxic Aβ homologous peptide which has 6 lysines on the N-terminus to increase immunogenicity and enhance solubility. This modification in addition to removal of the C-terminal amino acids of Aβ also reduces its propensity to form β-sheets. This immunogen elicited a similar antibody response as Aβ1-42 in mice which resulted in a comparable therapeutic efficacy (Sigurdsson et al., 2001). Third, outcomes of the first trials also highlighted the need to test anti-Aβ vaccines in non transgenic animal models to better predict their efficiency and potential side effects. For example, Lemere et al. (Lemere et al., 2004) and Gandy et al. (Gandy et al., 2004) evaluated immunotherapy with Aβ1-42 in Freund’s adjuvant in old Caribbean Vervets and Rhesus Macaques, respectively. They showed that immunized primates generated anti-Aβ antibodies. Plasmatic Aβ levels were elevated in the immunized animals while, unlike control animals, they had no plaque deposition in the brain.
Here, we investigated immunotherapy based on Aβ1-42 or Aβ-derivative administered with alum adjuvant in old mouse lemurs. In this small primate (100g), 5 to 20% of aged animals develop Aβ amyloidosis (Mestre-Frances et al., 2000; Languille et al., 2012). A previous study in young animals, comparing Aβ1-42 and Aβ-derivatives, has shown that immunization promotes antibody response against Aβ1-40 and Aβ1-42 and increases plasmatic Aβ load (Trouche et al., 2009). Here, we studied animals without amyloid plaques or with a very small extracellular amyloid load, but presenting with intracellular and vascular amyloid deposits. We show that Aβ1-42 immunization increases plasmatic Aβ levels, but also microhemorrhages and iron deposition in the choroid plexus (CP) of aged animals including in Aβ-plaque free animals. The latter effect is a new potential side effect of anti-Aβ treatment administered at the prodromal stage of the disease.
2. Material and methods
2.1 Animals
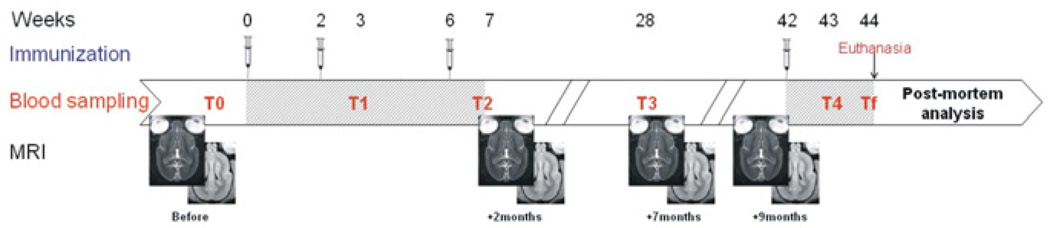
Our study evaluated the effects of immunotherapy and aging in mouse lemurs. First, the immunotherapy study was performed in 20 animals aged from 4.1 to 6.4 years: A first cohort of 8 animals (5.9±0.1 years) were treated with Aβ1-42 (n=4) or with K6Aβ1-30 (n=4) vaccines and were followed-up by MRI and biochemical parameters (antibody titers, Aβ levels in plasma) during 10 months (Fig. 1); A second cohort of 12 animals (4.7±0.2 years) were followed with the same protocol but treated with K6Aβ1-30 (n=6) or with the adjuvant alone (n=6). The brains of these 20 animals were then evaluated by histology. Second, the aging study was performed in 28 non-treated mouse lemurs aged from 1.6 to 6.4 years ((young adults (n=9; 1.9±0.2 years), middle-aged (n=11; 4.5±0.1 years), and old (n=8, 5.9±0.1 years)) that were studied by in vivo MRI. All the animals were born and raised in a laboratory breeding colony at Montpellier, France. Animal care was in accordance with institutional guidelines and animal protocol was approved by the local ethic committee (authorization #CEEA-LR-1002).
Fig. 1.
Diagram depicting the timeline of the immunizations, bleeds for measurements of antibody response and Aβ levels, and MRI sessions. Hatched areas correspond to immunization and second phase of immunization.
2.2 Peptides
The peptides used for the immunization were synthesized by the solid-phase technique at the Keck Foundation at Yale University, as previously described (Asuni et al., 2006; Sigurdsson et al., 2004).
2.3 Injections and bleeds
Animals vaccinated with Aβ1-42 received Aβ1-42 in alum adjuvant (100 µl subcutaneous injections; Alhydrogel®; Brenntag Biosector, Frederiksund, Denmark). Animals vaccinated with K6Aβ1-30 received K6Aβ1-30 in alum adjuvant (100 µl subcutaneous injections; Adju-Phos®; Brenntag Biosector, Frederiksund, Denmark). Alum adjuvant was chosen because it is the most common adjuvant in human vaccines (Gupta, 1998) and because it promotes humoral (Th2) immunity (Asuni et al., 2006). In the context of the vaccine, aluminium is used at low dose that should not be toxic for the organism, and that is why it is approved for clinical use in humans. Animals treated with the alum adjuvant alone received Adju-Phos® (100 µl subcutaneous injections; Brenntag Biosector, Frederiksund, Denmark). Aβ1-42 and K6Aβ1-30 peptides were mixed with the alum adjuvant at a concentration of 1 mg/ml and the solution was rotated overnight at 4°C prior to administration to allow the peptide to adsorb onto the aluminum particles, which have an opposite charge to the peptide.
The animals received four injections. The second, third and fourth injection were administered 2, 6, and 42 weeks after the first injection (Fig. 1). The primates were bled prior to the first immunization (T0), 1 week following the second (T1, 3 weeks) and third injection (T2, 7 weeks). T3 was at 28 weeks (22 weeks following the third injection). T4 and Tf were performed at 43 and 44 weeks, respectively (1 week and 2 weeks following the fourth injection, respectively). The Tf was performed at the euthanasia of the animal. The mouse lemurs went through several MRI sessions, before and 2, 7, and 9 months after the injections (Fig. 1).
2.4 Antibody levels
Anti Aβ1-40 as well as anti K6Aβ1-30 IgM and IgG antibodies were evaluated from the plasma of mouse lemurs. IgM antibodies are usually produced immediately after an exposure to antigens, while IgG antibodies are associated to a later response. Anti Aβ1-40 and anti K6Aβ1-30 antibody levels were determined at 1:200 dilution of plasma using an ELISA assay as described previously (Asuni et al., 2006; Sigurdsson et al., 2001), where the full-length Aβ1-40 or K6Aβ1-30 peptides were coated onto microtiter wells (Immulon 2 HB, ThermoScientific, Waltham, MA). Antibody levels were detected by an anti-primate IgG and IgM linked to a horseradish peroxidase (Alpha Diagnostics, San Antonio, TX) (Trouche et al., 2009).
2.5 Aβ1-40 levels in plasma
For the measurement of free Aβ1-40 in plasma, 10% dilution of untreated plasma was used, and the detection was performed by an ELISA kit (Biosource, Camarillo, CA) as previously described (Trouche et al., 2009). Aβ1-42 levels in plasma were below the limit of detection.
2.6 Magnetic resonance image (MRI) acquisition and image processing
T2-weighted (T2w) and T2*-weighted (T2*w) images were recorded on a 7 Tesla spectrometer (Bruker Pharmascan) with an isotropic resolution of 234 µm. The T2w sequence (TR/TE=2500/69.2 ms, TI=60 ms, RARE-factor=12) was used to evaluate cerebral inflammation (hyperintense signal). The T2*w sequence (TR/TE=40/8 ms, flip angle=12°) was used to evaluate iron deposits and microhemorrhages (hypointense signal).
Four MRI sessions (before, 2, 7, and 9 months after the beginning of immunization, Fig. 1) were performed for the longitudinal follow-up of the vaccinated primates. Animals were pre-anesthetized with a subcutaneous injection of atropine (0.025 mg/kg). Twenty minutes later, they were anesthetized by isoflurane (5% for induction and 1% during the MRI scans as described in Dhenain et al., 2003). Respiration rate and body temperature were monitored to insure stability of the animal. Body temperature was maintained stable with water-heating bed and air-heating ventilation.
On T2w images, cerebral inflammation can be detected as hyperintense regions. Such signal alterations were evaluated by visual inspection. On T2*w images, iron deposits and microhemorrhages lead to hypointense signal while CSF accumulation within the ventricles leads to hyperintense signal. Voxels with hypointense signal were quantified by using the following method (Anatomist-freeware, http://brainvisa.info/): first, a threshold (T = M × 0.5) was calculated for each image by using the mean intensity (M) of a cortical region of the image and a constant coefficient of 0.5. The cortical region was selected with constant landmarks in the parietal cortex and was exempt of hypointense voxels. Voxels with signal intensity below the calculated threshold were considered as hypointense. These voxels were painted by using a graphic tablet. They were classified as belonging to the CP or brain parenchyma on the basis of their anatomical location (Bons et al., 1998). The volumes of hypointense voxels belonging to the CP or brain parenchyma (index of cerebral microhemorrhages) were then automatically calculated by the image analysis freeware.
2.7 Histology
The animals were euthanized with an overdose of ketamine (~0.03 ml/100g). Their brains were post-fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) at 4°C. The mouse lemur hemi-brains were embedded in paraplast (MM France, Francheville, France) and cut into 6-µm serial sagittal sections and used for iron staining and immunohistochemistry.
2.7.1 Iron staining
Microhemorrhages and other iron deposits were analyzed with Perls’ staining which reveals ferric ions. Sagittal brain sections were incubated in a solution composed of 5% potassium ferrocyanide and 10% hydrochloric acid (v/v) for 30 minutes and rinsed in distilled water. Subsequently, nuclear fast red (0.1%) counterstain was performed, and the sections were rinsed again in distilled water. After dehydration, sections were coverslipped with Mountex (Histolab, Gothenburg, Sweden).
2.7.2 Immunohistochemistry
Sagittal brain sections were stained for amyloid. Amyloid detection was based on Aβ1-42 rabbit polyclonal (FCA3542, Calbiochem, Merck, Darmstadt, Germany) that binds to C-terminus of the Aβ1-42 peptide and on 4G8 monoclonal antibody (Covance, Emeryville, CA) that recognizes the middle region (amino acid residues 17–24) of Aβ. FCA3542 is specific for Aβ ending at residue 42 and does not stain APP (Barelli et al, 1997). A pre-treatment with formic acid for 15 minutes was used for Aβ1-42 and 4G8 staining. Endogenous peroxidase was quenched by treating the sections with distilled water containing 1% H2O2 for 30 minutes at room temperature. Sections were blocked in 3% goat serum. Products were diluted in Tris Buffered Saline (TBS), pH 7.6. Primary antibodies were diluted at 1:1000. Slices were incubated with secondary biotinylated antibodies (anti-rabbit and anti-mouse antibodies for Aβ1-42 and 4G8, respectively) for 30 minutes at room temperature. Next the signal was amplified by using avidin-peroxidase complex standard (ABC-kit, Vectastain, Vector Laboratories, Burlingame, CA). Final reaction used 3,3’-diaminobenzidine tetrahydrochloride (DAB, Vector laboratories, Burlingame, CA) as chromogen for peroxidase activity. All washing steps (3 times for 3 minutes each) and antibody dilution were done using TBS, pH 7.6. Incubation with the ABC complex and detection with DAB were done according to the manufacturer’s manual. Hematoxylin counterstaining was performed according to a standard procedure. After dehydration, sections were coverslipped with Mountex.
2.7.3 Quantification of histological sections
Histological sections were analyzed with Leitz Laborlux S (Leica microsystems, Nanterre, France), using the Mercator software (ExploraNova, La Rochelle, France). This software permits quantification of histological sections and can generate maps of counted objects such as extracellular Aβ1-42 deposits (12 sagittal brain sections per animal), intracellular 4G8 positive objects (30 sagittal brain sections per animal) or microhemorrhages (7 sagittal brain sections separated by 300 µm per animal). Counting was performed on each section per cortical area (frontal, parietal, and occipital) for the intracellular 4G8 positive deposits (fields of 500 × 500 µm2) or on whole brain sections for microhemorrhages. A global semi-quantitative evaluation of vessels stained by Aβ1-42 was also performed. The following scoring scale was used based on the number of Aβ stained vessels per slide: (0) zero-, (+) one to three-, (++) four to six- and (+++) more than six Aβ-stained vessels detected per slide (Table 1).
Table 1.
Semi-quantitative evaluation of vascular Aβ in the different animals. Score scale: few (+), moderate (++), high (+++).
| Animal | Vaccine group | Vascular Aβ (score) |
|---|---|---|
| 184 | Aβ1-42 | + |
| 189 | Aβ1-42 | + |
| 192 | Aβ1-42 | + |
| 194 | Aβ1-42 | + |
| 190 | K6Aβ1-30 | ++ |
| 191 | K6Aβ1-30 | + |
| 193 | K6Aβ1-30 | +++ |
| 195 | K6Aβ1-30 | +++ |
2.8 Statistical analysis
Data were analyzed with Statistica (Statsoft France, Maisons-Alfort, France). Microhemorrhages and intracellular Aβ were analyzed with Student’s t-test. Antibody level, plasmatic Aβ and volume of hypointense regions were analyzed by repeated measures ANOVA and LSD test for post-hoc analysis. The conditions required to use parametric statistical tests (normality, homoscedasticity, sphericity of the data) were respected. Correlative studies were based on non parametric Spearman's rank correlation.
3. Results
3.1 Aβ1-42 immunization modulates immune responses and plasmatic amyloid levels
Before immunization, the animals from the cohorts (treated with Aβ1-42, K6Aβ1-30 or adjuvant alone) had similar low levels of anti-Aβ1-40 IgG and IgM antibodies (Fig. 2A and 2C) or anti-K6Aβ1-30 IgG and IgM antibodies (data not shown). They also had similar plasma levels of Aβ1-40 (Fig. 2E and 2F).
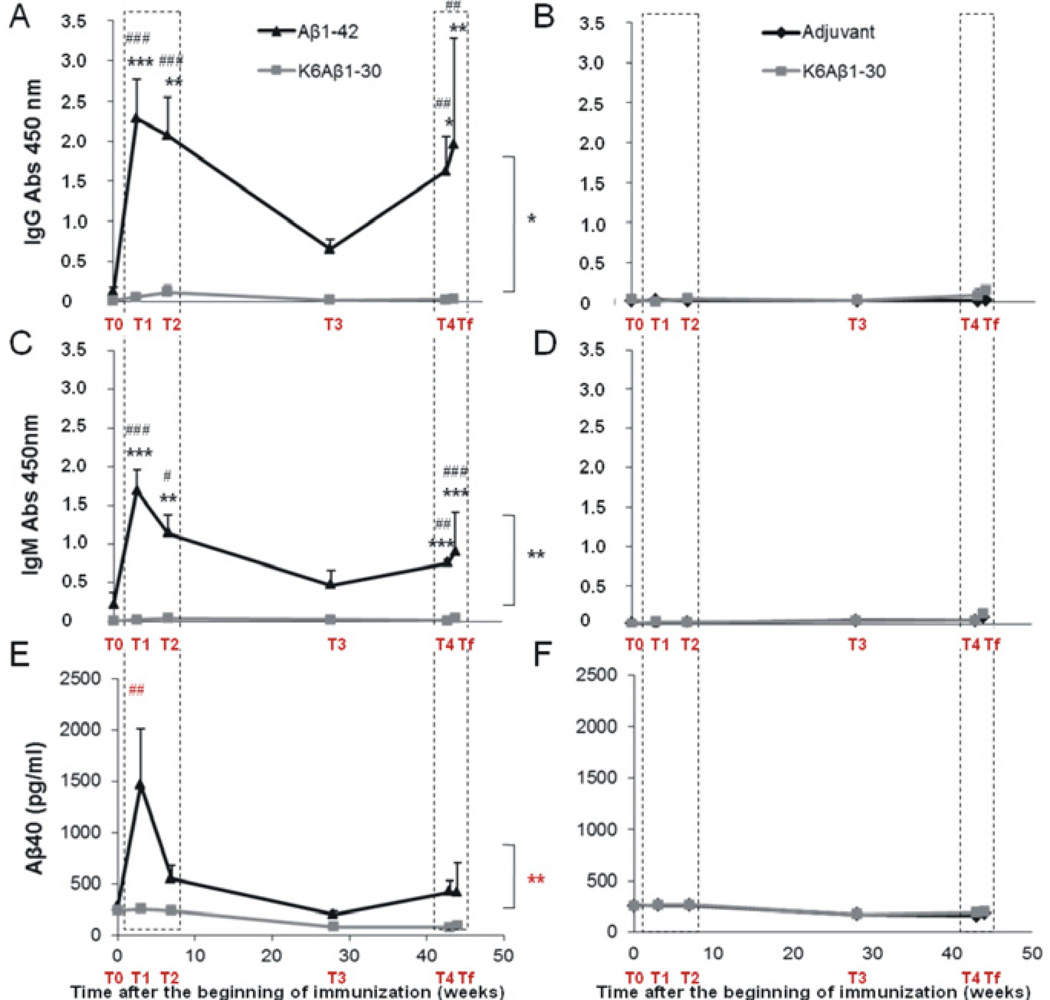
Fig. 2.
Anti-Aβ1-40 IgG and IgM antibody responses and plasma Aβ1-40 in the mouse lemurs treated with Aβ1-42, K6Aβ1-30, and adjuvant.
A–D – The Aβ1-42 vaccinated lemurs (black triangle) developed more anti-Aβ1-40 IgG (A) and IgM (B) compared to the K6Aβ1-30 group (grey square) (FIgG vaccine effect(1,3)=23; *p<0.05 and FIgM vaccine effect(1,3)=182; **p<0.001) during immunization phases (dotted line frames). The K6Aβ1-30 vaccinated lemurs (grey square) did not develop more anti-Aβ1-40 IgG (B) and IgM (D) compared to the adjuvant group (black rhomb) (ns). (A) IgG responses were higher in the Aβ1-42 group (post-hoc analyses at T1K6Aβ1-30 vs Aβ1-42, ***p<0.0001; T2K6Aβ1-30 vs Aβ1-42, **p<0.005; T4K6Aβ1-30 vs Aβ1-42, *p<0.01; TfK6Aβ1-30 vs Aβ1-42, **p<0.005). Their values were significantly increased compared to their basal levels (post-hoc analyses IgGT0 vs T1, ###p<0.0005; IgGT0 vs T2, ###p<0.0005; IgGT0 vs T4, ##p<0.005; IgGT0 vs Tf, ##p<0.005). (B) IgM responses were higher in the Aβ1-42 group (post-hoc analyses at T1K6Aβ1-30 vs Aβ1-42, ***p<0.000001; T2K6Aβ1-30 vs Aβ1-42, **p<0.005; T4K6Aβ1-30 vs Aβ1-42, ***p<0.0005; TfK6Aβ1-30 vs Aβ1-42, ***p<0.0005). Their values were significantly increased compared to their basal levels (post-hoc analyses IgMT0 vs T1, ###p<0.0005; IgMT0 vs T2, #p<0.01; IgMT0 vs T4, ##p<0.005; IgMT0 vs Tf, ###p<0.0005).
E–F – The plasmatic Aβ1-40 was modulated following the profile of immune responses in the animals vaccinated with Aβ1-42 (E) but no modulation in the animal vaccinated with K6Aβ1-30 compared to adjuvant (F). The lemurs vaccinated with Aβ1-42 (black triangle) had an increased plasmatic Aβ1-40 compared to the K6Aβ1-30 group (grey square) (FplasmAβ1-40 vaccine effect(1,3)=100; **p<0.005). In this group, the increase in plasma Aβ1-40 levels was particularly high at T1 during the first immunization phase (post-hoc analysis, ##p<0.005) and had subsided at T2 (ns). Re-immunization did not lead to as robust anti-Aβ1-40 antibody response at T4 and Tf (ns). In the other group, K6Aβ1-30 was not immunogenic and the level of Aβ1-40 in the plasma did not change (ns). Statistics are indicated on the right side of the graph for global vaccine effect from ANOVA and above the curves for post-hoc analyses. Statistical annotations: asterisks represent the significant differences between the groups (*, **, or ***); sharps represent the significant differences between T0 and other time points after immunization (#, ##, or ###). *, #: p<0.05; **, ##: p<0.005; ***, ###: p<0.0005.
In the Aβ1-42 vaccine group, the anti-Aβ1-40 antibody titers were highly increased at T1, T2, T4, and Tf after immunization, compared to their levels before immunization (respectively 17, 15, 12, and 14-fold for IgG levels and 8, 5, 3, and 4-fold for the IgM levels (all ps<0.01)). Anti-K6Aβ1-30 antibody titers of the Aβ1-42 vaccine group were also increased, compared to their levels before immunization (at T1 and Tf for IgG and T1, T4, and Tf for IgM (all ps<0.05)). In this group, plasma levels of Aβ1-40 were also increased, in particular during the first immunization phase (T1 about 465% increase (p<0.005), T2 about 108% increase (non-significant (ns)), T4 about 48% increase (ns) and Tf about 32% increase (ns)). The increased levels of Aβ1-40 in plasma paralleled within the same time frame the increased levels of anti-Aβ1-40 IgG and IgM. These data suggest that the Aβ1-42 group responded to immunization.
In the K6Aβ1-30 groups, the anti-Aβ1-40 IgG and IgM antibody titers were low and not significantly modified at any time point after immunization, compared to their levels before immunization (all ps>0.05, see Fig. 2A and 2C for the first cohort and Fig. 2B and 2D for the second cohort). The animals vaccinated with Aβ1-42 had thus higher anti-Aβ1-40 IgG and IgM levels compared to the animals treated with K6Aβ1-30 (respectively F(1,3)=23; p<0.05 and F(1,3)=182; p<0.001). Also, the anti-K6Aβ1-30 IgG or IgM antibody titers from animals treated with K6Aβ1-30 vaccine were not modified at any time after the immunization, compared to their levels before immunization (all ps>0.05, blood analysis performed on the first cohort). Plasma levels of Aβ1-40 were also not significantly altered in the K6Aβ1-30 groups, compared to their levels before immunization (Fig. 2E and 2F). Thus, Aβ1-42 group presented higher Aβ1-40 levels in plasma, compared to the K6Aβ1-30 group (F(1,3)=100; p<0.005; Fig. 2E).
In the adjuvant group, the anti-Aβ1-40 IgG and IgM levels as well as the plasma levels of Aβ1-40 were not modified, compared to their levels before immunization (all ps>0.05; Fig. 2B, 2D, and 2F).
Because of the lack of immune response and Aβ modulation in plasma in the K6Aβ1-30 groups and the similar lack of response in the K6Aβ1-30 and adjuvant groups, the animals treated with K6Aβ1-30 vaccine in the first cohort were considered as non-responders during our study. Note that for ethic reasons and because we had strong arguments showing that K6Aβ1-30 vaccine did not induce a significant immune response, an additional group of adjuvant animals, age-matched to the groups of first cohort (Aβ1-42 versus K6Aβ1-30), was not used in the current study.
3.2 Aβ1-42 and its derivative do not induce meningoencephalitis or vasogenic edema
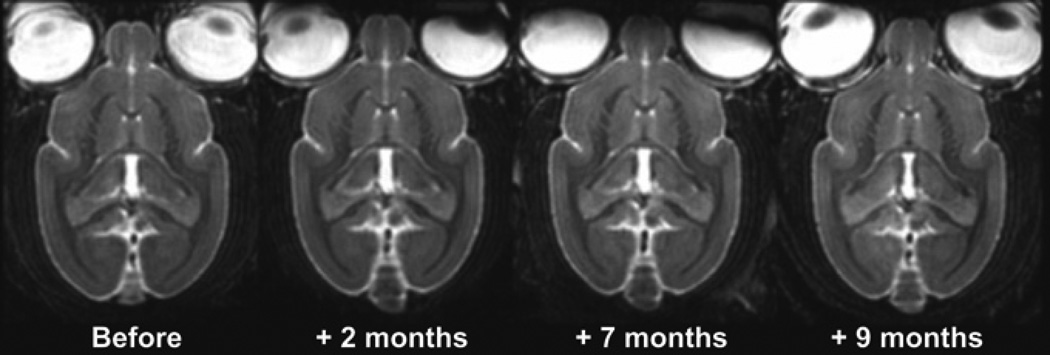
All the animals involved in the current study were followed longitudinally by in vivo MRI. None of the animals displayed hyperintense T2w MR signals that might suggest vasogenic edema or neuroinflammatory processes (Fig. 3).
Fig. 3.
Assessment of vasogenic edema or neuroinflammation by MRI before and during Aβ immunization.
MRI does not highlight hyperintense signal characteristic of vasogenic oedema or neuroinflammation on T2w images, irrespective of the MRI session or the vaccine group. The hyperintense signal visible on these images corresponds to cerebrospinal fluid (CSF).
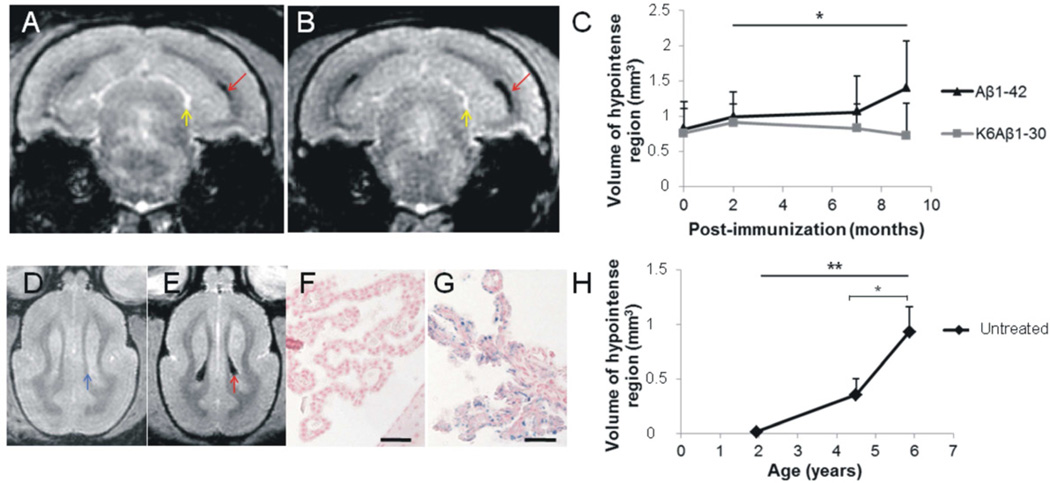
3.3 Aβ1-42 vaccine worsens age-associated iron accumulation in the choroid plexus
T2*w images revealed hypointense signals in the ventricles of old animals, before and during immunization (Fig. 4A, 4B, and 4E). Quantification revealed an increased size of these hypointense signals in animals treated with the Aβ1-42 vaccine compared to the K6Aβ1-30 group (Fvaccine*session (2,10)=5; p<0.05; Fig. 4C). Such signal changes on MR images are characteristic of iron deposition. Histological analysis confirmed that the hypointense regions detected on MRI corresponded to iron accumulation in epithelial cells of the CP (Fig. 4G). Hypointense signal changes were not detected in the second cohort (K6Aβ1-30 versus adjuvant) (Fvaccine*session (2,20)=0.03; n.s.).
Fig. 4.
Iron deposition in the choroid plexus of mouse lemurs. Effects of Aβ immunization and age.
A–B – The T2*w images show hypointense signal (red arrows), characteristic of iron deposition, distributed inside the ventricles of the old animals. On these images, CSF appeared with a hyperintense signal (yellow arrows). The hypointense signal was increased in the same animal after 9 months of vaccination with Aβ1-42 vaccine (B), compared to before immunization (A). C – The volume of hypointense signal increased in old lemurs vaccinated with Aβ1-42 (black triangle) compared to animals vaccinated with K6Aβ1-30 (grey square) (ANOVA, Fvaccine*time effect(2,10)=5; *p<0.05). The volume of hypointense signal did not change in the animals from the second cohort (K6Aβ1-30 versus adjuvant, data not shown).
D–G – Comparison of young and old naive animals. Hypointense signal corresponding to iron in the choroid plexus was observed in old (E (red arrow) and G (blue stain on Perls’s stained sections)) but not in young animals (D and F). In these latter, iron-free choroid plexus had the same intensity as surrounding tissue (blue arrow). H – The analysis of young (1.9±0.2), middle-aged (4.5±0.1), and old (5.9±0.1) lemurs showed an age-associated increased of the volume of hypointense regions characteristic of iron deposits in the choroid plexus (ANOVA, F(2,25)=8; **p<0.005 and post-hoc analyses comparing middle-aged versus old, t(25)=2; *p<0.05). Scale bars = 50µm.
To further evaluate iron accumulation in the CP, T2*w MRI were recorded in a cohort of young adults (n=9; 1.9±0.2 years) and middle-aged animals (n=11; 4.5±0.1 years). MRI from these young and middle-aged animals were compared to MRI from the old animals (n=8; 5.9±0.1 years) of the first cohort before immunization. In vivo T2*w MRI of the young animals did not show any hypointense signal at the level of the CP (Fig. 4D and 4F). The size of the hypointense signal at the level of the CP increased in the middle-aged compared to young animals and further increased in old mouse lemurs (F(2,25)=8; p<0,005; post hoc analyses, ps<0.05; Fig. 4H).
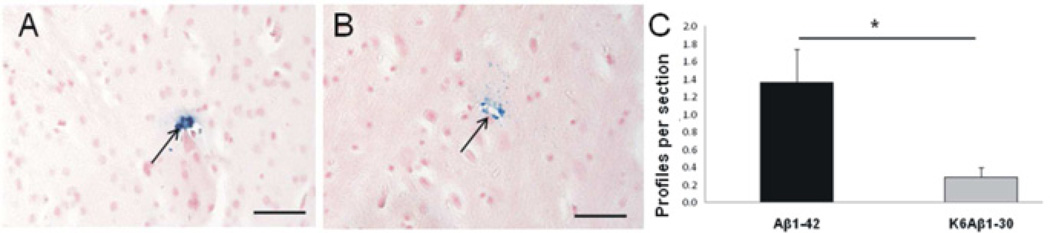
3.4 Aβ1-42 vaccine increases microhemorrhages
Histological studies revealed microhemorrhages (<10 µm) in the cortex (Fig. 5A) and other brain regions (globus pallidus, thalamus (Fig. 5B), subthalamic regions, ventral hippocampus, amygdala). The subcortical microhemorrhages were more numerous than the cortical microhemorrhages. The Aβ1-42 group had more parenchymal microhemorrhages, compared to the K6Aβ1-30 group (t(6)=2.72; p<0.05; Fig. 5C). The number of microhemorrhages was correlated with the anti-Aβ1-40 IgG response and plasma Aβ1-40 levels at the late stages of immunization (mean values for T0, T4 and Tf, ps<0.005 and T4 and Tf, ps<0.005, respectively). No significant difference of number of microhemorrhages could be detected in the second cohort (K6Aβ1-30 versus adjuvant, data not shown).
Fig. 5.
Microhemorrhages in the brain parenchyma of the lemurs after ten months of vaccination.
A–B – Example of Perls-positive microhemorrhages located in the occipital cortex (A, arrow) and thalamus (B, arrow). C – More microhemorrhages were detected in the old lemurs vaccinated with Aβ1-42 (black) compared to the K6Aβ1-30 group (grey) (t(6)=2.72; *p<0.05). Scale bars = 50µm.
The microhemorrhages detected on histological sections could, however, not be detected (as hypointense spots) on in vivo MRI, which can be explained by their small size.
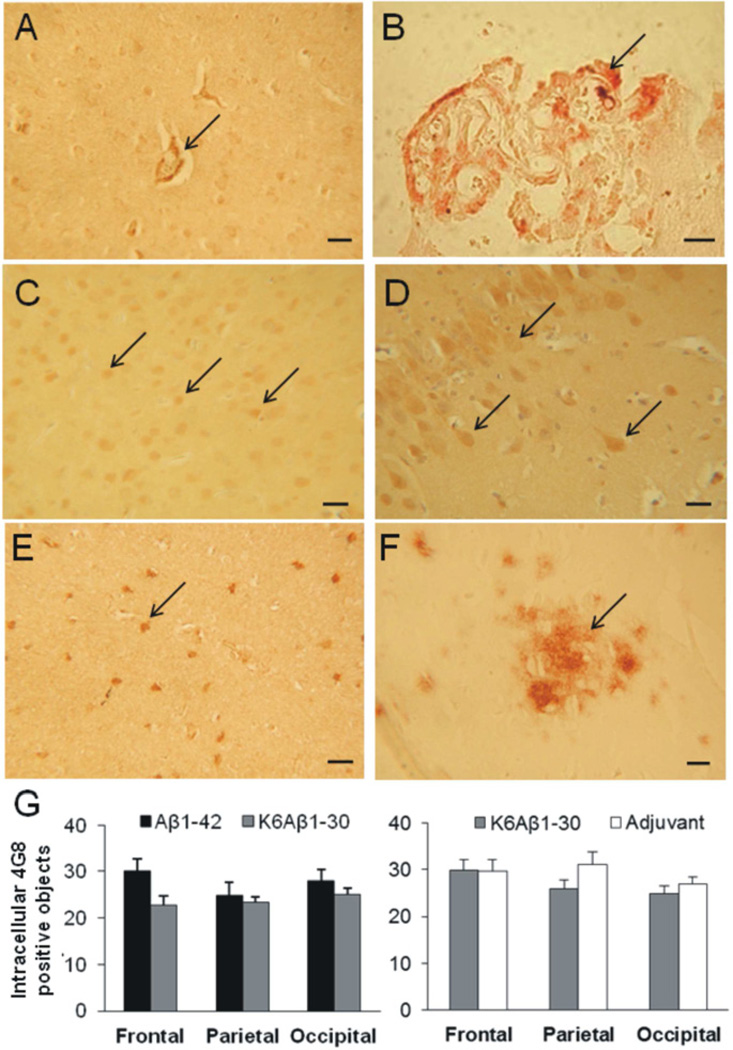
3.5 Neuropathological evaluation of vascular, intracellular and extracellular amyloidosis
Aβ deposits were detected in the vasculature of several animals (Fig. 6A). The Aβ1-42 responders had fewer vascular Aβ deposits compared to the K6Aβ1-30 animals (Table 1). These Aβ deposits were never detected in the vicinity of microhemorrhages. Aβ immunoreactivity was also detected in the epithelial cells of the CP (Fig. 6B) but was not colocalized with the iron deposits. However, no obvious difference between animals from the Aβ1-42, K6Aβ1-30 or adjuvant groups could be detected by visual inspection.
Fig. 6.
Amyloidosis in vaccinated mouse lemurs.
A–F – Aβ immunoreactivity was detected in the vessels (A, 4G8, arrow) and choroid plexus (B, 4G8, arrow) of vaccinated animals. Intracellular Aβ was observed in various brain regions such as the frontal cortex (C, Aβ1-42 staining (FCA35-42), arrows), the hippocampus (D, Aβ1-42 staining (FCA35-42), arrows) or the parietal cortex (E, 4G8, arrow). Two of the immunized lemurs had extracellular diffuse Aβ plaques (F, 4G8, arrow). G – Quantification of the intracellular staining (4G8 staining) in the frontal, parietal, and occipital cortices revealed no significant differences between the two groups whatever the cohort (Aβ1-42 (black) versus K6Aβ1-30 (grey) or adjuvant (dark grey) versus K6Aβ1-30 (grey)). Scale bars = 25µm.
Intracellular Aβ immunoreactivity was also detected in neurons of the frontal, parietal, and occipital cortical regions as well as in the hippocampus from Aβ1-42, K6Aβ1-30 or adjuvant treated primates (Fig. 6C and 6E). In the two cohorts, the degree of 4G8 positive objects was similar, irrespective of the region and experimental group (Fig. 6G).
It is noteworthy that extracellular Aβ deposits were only detected in two animals of the study which were treated with K6Aβ1-30. The deposits were diffuse and restricted to the subiculum in one animal and were rare and localised in several cortical regions (frontal, parietal) and in the subiculum in the second animal (Fig. 6F).
4. Discussion
The current study evaluated Aβ immunization in aged mouse lemur primates, an animal which has the same Aβ sequence as humans and can spontaneously develop intracellular, extracellular, and vascular Aβ deposits with age (Bons et al., 1994; Kraska et al., 2011; Languille et al., 2012). In mouse lemur primates, the Aβ load is much lower than in transgenic mouse models of amyloidosis that over express mutated forms of human APP. The latter models have a very high Aβ load and are largely used to evaluate various immunotherapy protocols. In mouse lemurs, only a small proportion of aged animals (5 to 20%) naturally develop extracellular Aβ plaques (Mestre-Frances et al., 2000; Languille et al., 2012). In our two cohorts of eight and twelve aged lemurs we could thus expect that one or two animals had extracellular Aβ plaques before immunization or were in the process of developing such plaques. Our immunohistological study on twenty animals revealed amyloid plaques in only two animals (10% of animals). This value is consistent with the prevalence of amyloid plaques in lemurs even without treatment. We can thus consider that the number of animals presenting with plaques in the treated population was not strongly reduced compared to the number of animals presenting with plaques in the general population. All the aged animals, however, had intracellular Aβ immunoreactivity and Aβ in plasma. It seems that the Aβ plaque load in lemurs mimics normal aging in humans, i.e. a situation with low Aβ plaque load. Results from our study might thus be predictive of the consequence of Aβ immunotherapy in normal aged, non-AD, humans.
We showed that active immunization with the full-length Aβ1-42, but not with a derivative, K6Aβ1-30, elicits an immune response in our cohort of old mouse lemurs. This immune response was associated with increased Aβ1-40 levels in the plasma. As the antibodies generated by the immunization process bound at least some Aβ in the blood, we can speculate that our measured plasma levels of Aβ were underestimated compared to the real Aβ load in the plasma. In addition, in the animals treated with Aβ1-42, we observed increased levels of microhemorrhages and an increased accumulation of iron in the CP. No sign of meningoencephalomyelitis or vasogenic edema could be detected in the vaccinated animals. In humans, during passive immunotherapies based on monoclonal antibodies, the presence of microhemorrhages is a risk factor for developing vasogenic edema, but other risk factors such as the APOE E4 status are also associated with the risk of vasogenic edema (Sperling et al., 2011). Vasogenic edemas are however not reported during active immunotherapies such as ours (Sperling et al., 2011). The lack of vasogenic edema in our study is thus consistent with studies in humans. Meningoencephalomyelitis is the major side effect reported in active immunotherapies (Orgogozo et al., 2003). The mechanisms associated with the occurrence of microhemorrhages and meningoencephalomyelitis are however different. Microhemorrhages are expected to be associated with redistribution of Aβ into cerebral blood vessels (Wilcock et al., 2004) or with binding of antibodies to existing CAA (Racke et al., 2005) while meningoencephalomyelitis is related to the hyperactivation of T cells, a process that was minimised in the design of our immunotherapy trial by using alum adjuvant that promotes humoral (Th2) immunity (Asuni et al., 2006). In our study, the lack of meningoencephalomyelitis despite the presence of microhemorrhages is thus consistent with the expected lack of T cell hyperactivation with our vaccine.
In the present study, the response to the K6Aβ1-30 vaccine was much lower than that induced by the Aβ1-42 vaccine and was similar to that induced by the adjuvant. In our previous study in young animals both Aβ1-42 and K6Aβ1-30 immunotherapies administered with alum adjuvants were associated with a robust antibody response and increased level of Aβ1-40 in plasma (Trouche et al., 2009). Of these two, Aβ1-42 elicited a stronger immune response against Aβ as expected. In another previous study we also showed high antibody response towards K6Aβ1-30 in old lemurs (Mestre-Frances et al., 2010), an outcome that is converse to the result from the current study. These apparently opposite results are explained by the lower immunogenicity of the K6Aβ1-30, compared to Aβ1-42, which leads to more variable responses in different cohorts of old animals as antibody response generally subsides with aging. This lower immunogenicity of K6Aβ1-30 is however of interest as it makes K6Aβ1-30 potentially safer than Aβ1-42. K6Aβ1-30 might thus be a good treatment to initiate in middle-aged subjects. It is also interesting to outline that the variable response to K6Aβ1-30 has also been reported in transgenic mice, where a cohort of 11–24 months old mice were shown to respond to treatment by K6Aβ1-30 while 19–24 months old animals did not respond (Asuni et al., 2006).
The Aβ1-42 responder animals presented a transient IgM response and a long-lasting higher IgG response. The transient plasma Aβ increase paralleled within the same time-frame the IgG and IgM responses. A possible explanation is that immunization led to a clearance of Aβ from the brain via a peripheral sink process (DeMattos et al., 2001; Lemere et al., 2003). However, the extracellular aggregated Aβ load is small in lemurs and we did not find any difference of extracellular Aβ or intracellular 4G8 positive objects load in the two groups of treated animals. Another possible explanation for the high Aβ level in plasma is that immunoglobulins sequestered Aβ and increased its half-life (Levites et al., 2006).
Our study highlighted that vaccination with Aβ1-42 vaccine increases iron accumulation in the epithelial cells of the CP. To our knowledge, this is the first study showing a link between iron deposits in CP and Aβ immunotherapy. It has been shown that epithelial cells of the CP are involved in iron exchanges between the blood and the brain (Deane et al., 2004). Moreover, it has been proposed that the CP plays a role in the modulation of iron metabolism in aging and AD (Mesquita et al., 2012). Also, peripheral inflammation is known to modulate expression of choroidal genes implicated in immune response cascade, in barrier integrity (Marques et al., 2009b) and iron homeostasis (Marques et al., 2009a). The presumed inflammation induced by immunotherapy might thus modulate the function of the CP and lead to increased iron accumulation in the CP.
To date, in humans, iron accumulation in the CP has never been reported after Aβ immunotherapy. However, during pathological conditions, iron accumulates in the CP of humans (Kira et al., 2000), which is reminiscent to the increase in iron that we detected in the CP of lemurs. Because of these similarities, we suggest that iron accumulation in the CP may also occur during Aβ immunotherapy in humans. This potential effect should be further evaluated in MR images of anti-Aβ immunized human subjects, as MRI does detect iron accumulation in human CP (Kira et al., 2000).
In parallel, in mouse lemurs we found, in a small cohort of animals, that Aβ1-42 treatment increases microhemorrhages, despite the lack of extracellular Aβ plaques and the low level of vascular Aβ in the brain. In a previous study based on post-mortem MRI, we already showed that aged lemurs can develop spontaneously microhemorrhages during normal aging (Bertrand et al., 2013). Here we confirmed this study and showed however that the number of microhemorrhages is relatively low in control animals (0.29±0.10 profile per section). The microhemorrhage load found in our study was comparable to data reported in transgenic mouse models of AD (see for example 0.5 profile per section in Wilcock et al., 2004). However, the number of microhemorrhages in our treated animals (1.36±0.38 profiles per section) was lower than those reported in transgenic mice (3.4 profiles per section in Wilcock et al., 2004). This can be related to a different treatment, but also probably to the lower global amyloid load in lemurs compared to transgenic mice. In most studies in transgenic mice, microhemorrhages have been associated with increased level of vascular amyloidosis (Thakker et al., 2009; Wang et al., 2011; Wilcock et al., 2007; Wilcock et al., 2004). From these studies it has been suggested that vascular amyloidosis is a prerequisite for Aβ-immunization-associated microhemorrhages. However, data from a preventative study in mice showed that Aβ immunization at early stage increases microbleeds without increasing CAA (Schroeter et al., 2008). Our data in primates are consistent with this latter study and suggest that vascular Aβ deposits are not mandatory to induce microhemorrhages after Aβ-immunization. One possible explanation is that T cell response induced by the epitopes such as 16–24 and 34–42 of Aβ occurred in mouse lemurs even if the adjuvant was designed to minimize this response. This T cell response could induce microbleeds in the absence of vascular Aβ deposits.
To conclude, we have evaluated Aβ-immunization in the mouse lemur, a primate model of normal aging or prodromal stage of AD with minimal extracellular Aβ deposition. We have shown that even in the absence of severe β-amyloidosis, Aβ-immunization can lead to iron deposition in the CP and microhemorrhages. This study suggests that Aβ-immunization of normal aged subjects or of aged patients at very early stage of AD can induce side effects that may have clinical relevance. This should be taken into account in the design of future clinical evaluations in patients at a very early AD stage.
Acknowledgements
This work was supported by the France-Alzheimer association; the longevity program from the CNRS; the Regional Council of Martinique; the National Foundation for Alzheimer’s Disease and Related Disorders; and the National Institute on Aging [R01-AG020197]. O.D. was financed by Hoffmann-La Roche.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statements
There is no actual or potential conflict of interest for this study.
References
- Aisen PS, Vellas B. Editorial: passive immunotherapy for Alzheimer's disease: what have we learned, and where are we headed? The journal of nutrition, health & aging. 2013;17:49–50. doi: 10.1007/s12603-013-0001-3. [DOI] [PubMed] [Google Scholar]
- Asuni AA, Boutajangout A, Scholtzova H, Knudsen E, Li YS, Quartermain D, et al. Vaccination of Alzheimer's model mice with Abeta derivative in alum adjuvant reduces Abeta burden without microhemorrhages. Eur J Neurosci. 2006;24:2530–2542. doi: 10.1111/j.1460-9568.2006.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barelli H, Lebeau A, Vizzavona J, Delaere P, Chevallier N, Drouot C, Marambaud P, Ancolio K, Buxbaum JD, Khorkova O, Heroux J, Sahasrabudhe S, Martinez J, Warter J-M, Mohr M, Checler F. Characterisation of new polyclonal specific for 40 and 42 amino acid-long amyloid β peptides: their use to examine the cell biology of presenilins and the immunochemistry of sporadic Alzheimer's disease and cerebral amyloid angiopathy cases. Mol Med. 1997;3:695–707. [PMC free article] [PubMed] [Google Scholar]
- Bertrand A, Pasquier A, Petiet A, Wiggins C, Kraska A, Joseph-Mathurin N, Aujard F, Mestre-Francés N, Dhenain M. Micro-MRI study of cerebral aging: Detection of hippocampal subfield reorganization, microhemorrhages, and amyloid plaques in mouse lemur primates. PlosOne. 2013;8:e56593. doi: 10.1371/journal.pone.0056593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boche D, Donald J, Love S, Harris S, Neal JW, Holmes C, et al. Reduction of aggregated Tau in neuronal processes but not in the cell bodies after Abeta42 immunisation in Alzheimer's disease. Acta Neuropathol. 2010;120:13–20. doi: 10.1007/s00401-010-0705-y. [DOI] [PubMed] [Google Scholar]
- Bons N, Mestre N, Ritchie K, Petter A, Podlisny M, Selkoe D. Identification of amyloid beta protein in the brain of the small, short-lived lemurian primate Microcebus murinus. Neurobiol Aging. 1994;15:215–220. doi: 10.1016/0197-4580(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Bons N, Sihol S, Barbier V, Mestre-Frances N, Albe-Fessard D. A stereotaxic atlas of the grey lesser mouse lemur brain (Microcebus murinus) Brain Res Bull. 1998;46:1–173. doi: 10.1016/s0361-9230(97)00458-9. [DOI] [PubMed] [Google Scholar]
- Cribbs DH, Ghochikyan A, Vasilevko V, Tran M, Petrushina I, Sadzikava N, Babikyan D, Kesslak P, Kieber-Emmons T, Cotman CW, Agadjanyan MG. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. Intern immunol. 2003;15:505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Zheng W, Zlokovic BV. Brain capillary endothelium and choroid plexus epithelium regulate transport of transferrin-bound and free iron into the rat brain. J Neurochem. 2004;88:813–820. doi: 10.1046/j.1471-4159.2003.02221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhenain M, Chenu E, Hisley CK, Aujard F, Volk A. Regional atrophy in the brain of lissencephalic mouse lemur primates: measurement by automatic histogram-based segmentation of MR images. Magn Res Med. 2003;50:984–992. doi: 10.1002/mrm.10612. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Boada Rovira M, Sanchez Guerra ML, Rey MJ, Costa-Jussa F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer's disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandy S, DeMattos RB, Lemere CA, Heppner FL, Leverone J, Aguzzi A, et al. Alzheimer A beta vaccination of rhesus monkeys (Macaca mulatta) Alzheimer Dis Assoc Disord. 2004;18:44–46. doi: 10.1097/00002093-200401000-00009. [DOI] [PubMed] [Google Scholar]
- Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- Ghochikyan A, Mkrtichyan M, Petrushina I, Movsesyan N, Karapetyan A, Cribbs DH, Agadjanyan MG. Prototype Alzheimer's disease epitope vaccine induced strong Th2-type anti-Abeta antibody response with Alum to Quil A adjuvant switch. Vaccine. 2006;24:2275–2282. doi: 10.1016/j.vaccine.2005.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, et al. Antibodies against beta-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, et al. Long-term effects of Abeta42 immunisation in Alzheimer's disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- Jameson BA, Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput Appl Biosci. 1988;4:181–186. doi: 10.1093/bioinformatics/4.1.181. [DOI] [PubMed] [Google Scholar]
- Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, et al. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- Kira R, Ohga S, Takada H, Gondo K, Mihara F, Hara T. MR choroid plexus sign of iron overload. Neurology. 2000;55:1340. doi: 10.1212/wnl.55.9.1340. [DOI] [PubMed] [Google Scholar]
- Kraska A, Dorieux O, Picq J-L, Petit F, Bourrin E, Chenu E, et al. Age associated cerebral atrophy in mouse lemur Primates. Neurobiol Aging. 2011;32:894–906. doi: 10.1016/j.neurobiolaging.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Languille S, Blanc S, Blin O, Canale CI, Dal-Pan A, Devau G, et al. The grey mouse lemur: A non-human primate model for ageing studies. Ageing Res Rev. 2012;11:150–162. doi: 10.1016/j.arr.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Lemere CA, Beierschmitt A, Iglesias M, Spooner ET, Bloom JK, Leverone JF, et al. Alzheimer's disease abeta vaccine reduces central nervous system abeta levels in a non-human primate, the Caribbean vervet. Am J Pathol. 2004;165:283–297. doi: 10.1016/s0002-9440(10)63296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemere CA, Spooner ET, LaFrancois J, Malester B, Mori C, Leverone JF, et al. Evidence for peripheral clearance of cerebral Abeta protein following chronic, active Abeta immunization in PSAPP mice. Neurobiol Dis. 2003;14:10–18. doi: 10.1016/s0969-9961(03)00044-5. [DOI] [PubMed] [Google Scholar]
- Levites Y, Smithson LA, Price RW, Dakin RS, Yuan B, Sierks MR, et al. Insights into the mechanisms of action of anti-Abeta antibodies in Alzheimer's disease mouse models. Faseb J. 2006;20:2576–2578. doi: 10.1096/fj.06-6463fje. [DOI] [PubMed] [Google Scholar]
- Maier M, Seabrook TJ, Lazo ND, Jiang L, Das P, Janus C, Lemere CA. Short amyloid-beta (Abeta) immunogens reduce cerebral Abeta load and learning deficits in an Alzheimer's disease mouse model in the absence of an Abeta-specific cellular immune response. J Neurosci. 2006;26:4717–4728. doi: 10.1523/JNEUROSCI.0381-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer's disease: clinical trials and drug development. Lancet Neurol. 2010;9:702–716. doi: 10.1016/S1474-4422(10)70119-8. [DOI] [PubMed] [Google Scholar]
- Marques F, Falcao AM, Sousa JC, Coppola G, Geschwind D, Sousa N, et al. Altered iron metabolism is part of the choroid plexus response to peripheral inflammation. Endocrinology. 2009a;150:2822–2828. doi: 10.1210/en.2008-1610. [DOI] [PubMed] [Google Scholar]
- Marques F, Sousa JC, Coppola G, Geschwind DH, Sousa N, Palha JA, et al. The choroid plexus response to a repeated peripheral inflammatory stimulus. BMC Neurosci. 2009b;10:135. doi: 10.1186/1471-2202-10-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, et al. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005;64:129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- Mesquita SD, Ferreira AC, Sousa JC, Santos NC, Correia-Neves M, Sousa N, et al. Modulation of iron metabolism in aging and in Alzheimer's disease: relevance of the choroid plexus. Front Cell Neurosci. 2012 doi: 10.3389/fncel.2012.00025. 6 - article 25: 10 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre-Frances N, Keller E, Calenda A, Barelli H, Checler F, Bons N. Immunohistochemical analysis of cerebral cortical and vascular lesions in the primate Microcebus murinus reveal distinct amyloid beta 1–42 and beta 1–40 immunoreactivity profiles. Neurobiol Dis. 2000;7:1–8. doi: 10.1006/nbdi.1999.0270. [DOI] [PubMed] [Google Scholar]
- Mestre-Frances N, Trouche S, Boutajangout A, Asuni AA, Arribat Y, Rouland S, Wisniewski T, Frangione B, Maurice T, Sigurdsson EM, Verdier JM. Abeta derivative vaccination in old mouse lemur primates. Alzheimer's Dement. 2010;6:S223. [Google Scholar]
- Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- Muhs A, Hickman DT, Pihlgren M, Chuard N, Giriens V, Meerschman C, van der Auwera I, van Leuven F, Sugawara M, Weingertner MC, Bechinger B, Greferath R, Kolonko N, Nagel-Steger L, Riesner D, Brady RO, Pfeifer A, Nicolau C. Liposomal vaccines with conformation-specific amyloid peptide antigens define immune response and efficacy in APP transgenic mice. Proc Natl Acad Sci USA. 2007;104:9810–9815. doi: 10.1073/pnas.0703137104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- Petrushina I, Ghochikyan A, Mkrtichyan M, Mamikonyan G, Movsesyan N, Ajdari R, Vasilevko V, Karapetyan A, Lees A, Agadjanyan MG, Cribbs DH. Mannan-Abeta28 conjugate prevents Abeta-plaque deposition, but increases microhemorrhages in the brains of vaccinated Tg2576 (APPsw) mice. J Neuroinflam. 2008;5:42. doi: 10.1186/1742-2094-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racke MM, Boone LI, Hepburn DL, Parsadainian M, Bryan MT, Ness DK, Piroozi KS, Jordan WH, Brown DD, Hoffman WP, Holtzman DM, Bales KR, Gitter BD, May PC, Paul SM, DeMattos RB. Exacerbation of cerebral amyloid angiopathy-associated microhemorrhage in amyloid precursor protein transgenic mice by immunotherapy is dependent on antibody recognition of deposited forms of amyloid beta. J Neurosci. 2005;25:629–636. doi: 10.1523/JNEUROSCI.4337-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, Mathis CA, Blennow K, Barakos J, Okello AA, Rodriguez Martinez de Liano S, Liu E, Koller M, Gregg KM, Schenk D, Black R, Grundman M. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9:363–372. doi: 10.1016/S1474-4422(10)70043-0. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Schroeter S, Khan K, Barbour R, Doan M, Chen M, Guido T, et al. Immunotherapy reduces vascular amyloid-beta in PDAPP mice. J Neurosci. 2008;28:6787–6793. doi: 10.1523/JNEUROSCI.2377-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Pozo A, William CM, Ferrer I, Uro-Coste E, Delisle MB, Maurage CA, et al. Beneficial effect of human anti-amyloid-beta active immunization on neurite morphology and tau pathology. Brain. 2010;133:1312–1327. doi: 10.1093/brain/awq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson EM, Knudsen E, Asuni A, Fitzer-Attas C, Sage D, Quartermain D, et al. An attenuated immune response is sufficient to enhance cognition in an Alzheimer's disease mouse model immunized with amyloid-beta derivatives. J Neurosci. 2004;24:6277–6282. doi: 10.1523/JNEUROSCI.1344-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson EM, Scholtzova H, Mehta PD, Frangione B, Wisniewski T. Immunization with a nontoxic/nonfibrillar amyloid-beta homologous peptide reduces Alzheimer's disease-associated pathology in transgenic mice. Am J Pathol. 2001;159:439–447. doi: 10.1016/s0002-9440(10)61715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Jack CR, Jr, Black SE, Frosch MP, Greenberg SM, Hyman BT, Scheltens P, Carrillo MC, Thies W, Bednar MM, Black RS, Brashear HR, Grundman M, Siemers ER, Feldman HH, Schindler RJ. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer's Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367–385. doi: 10.1016/j.jalz.2011.05.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakker DR, Weatherspoon MR, Harrison J, Keene TE, Lane DS, Kaemmerer WF, et al. Intracerebroventricular amyloid-beta antibodies reduce cerebral amyloid angiopathy and associated microhemorrhages in aged Tg2576 mice. Proc Natl Acad Sci U S A. 2009;106:4501–4506. doi: 10.1073/pnas.0813404106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouche SG, Asuni A, Rouland S, Wisniewski T, Frangione B, Verdier JM, et al. Antibody response and plasma Abeta1–40 levels in young Microcebus murinus primates immunized with Abeta1–42 and its derivatives. Vaccine. 2009;27:957–964. doi: 10.1016/j.vaccine.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uro-Coste E, Russano de Paiva G, Guilbeau-Frugier C, Sastre N, Ousset PJ, da Silva NA, et al. Cerebral amyloid angiopathy and microhemorrhages after amyloid beta vaccination: case report and brief review. Clin Neuropathol. 2010;29:209–216. doi: 10.5414/npp29209. [DOI] [PubMed] [Google Scholar]
- Wang A, Das P, Switzer RC, 3rd, Golde TE, Jankowsky JL. Robust amyloid clearance in a mouse model of Alzheimer's disease provides novel insights into the mechanism of amyloid-beta immunotherapy. J Neurosci. 2011;31:4124–4136. doi: 10.1523/JNEUROSCI.5077-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Jantzen PT, Li Q, Morgan D, Gordon MN. Amyloid-beta vaccination, but not nitro-nonsteroidal anti-inflammatory drug treatment, increases vascular amyloid and microhemorrhage while both reduce parenchymal amyloid. Neuroscience. 2007;144:950–960. doi: 10.1016/j.neuroscience.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Rojiani A, Rosenthal A, Subbarao S, Freeman MJ, Gordon MN, et al. Passive immunotherapy against Abeta in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J Neuroinflammation. 2004;1:24. doi: 10.1186/1742-2094-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiessner C, Wiederhold KH, Tissot AC, Frey P, Danner S, Jacobson LH, Jennings GT, Luond R, Ortmann R, Reichwald J, Zurini M, Mir A, Bachmann MF, Staufenbiel M. The second-generation active Abeta immunotherapy CAD106 reduces amyloid accumulation in APP transgenic mice while minimizing potential side effects. J Neurosci. 2011;31:9323–9331. doi: 10.1523/JNEUROSCI.0293-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]