Abstract
The identification of the presence of active signaling between astrocytes and neurons in a process termed gliotransmission has caused a paradigm shift in our thinking about brain function. However, we are still in the early days of the conceptualization of how astrocytes influence synapses, neurons, networks and ultimately behavior. In this review, our goal is to identify emerging principles governing gliotransmission and consider the specific properties of this process that endow the astrocyte with unique functions in brain signal integration. We develop and present hypotheses aimed at reconciling confounding reports and define open questions to provide a conceptual framework for future studies. We propose that astrocytes mainly signals through high affinity slowly-desensitizing receptors to modulate neurons and perform integration in spatio-temporal domains complementary to those of neurons.
Introduction
Accumulating evidence supports the presence of a dynamic, bidirectional regulation of neuronal communication by astrocytes. Astrocytes detect synaptic activity through the activation of metabotropic or ionotropic receptors. For instance, synaptically released glutamate from Schaffer collaterals activates G protein-coupled receptors (GPCRs), such as the type 5 of the metabotropic glutamate receptors (mGluRs), localized on hippocampal astrocytes (Porter and McCarthy 1996; Pasti et al., 1997; Perea and Araque, 2005; Panatier et al., 2011). Activation of these receptors in turn causes variations of astrocytic intracellular Ca2+ that can trigger the release of various active substances, such as glutamate, ATP, and D-serine, the so-called gliotransmitters (Bezzi and Volterra, 2001). Such glia-derived transmitters have been shown to act on neurons in timescales ranging from seconds to minutes and to regulate synaptic transmission and plasticity through a wide variety of mechanisms (Araque et al., 1999b; Bezzi et al., 1998 ; Brockhaus and Deitmer, 2002; Henneberger et al., 2010; Jourdain et al., 2007; Panatier et al., 2006; Parpura et al., 1994 ; Pascual et al., 2005; Pasti et al., 1997; Perea and Araque, 2007; Serrano et al., 2006; Shigetomi et al., 2011; Zhang et al., 2003). These findings have established the concept of the “tripartite synapse”, which represents an integrative functional view of synaptic physiology that considers astrocytes as active protagonists regulating information transfer between neurons (Araque et al., 1999a). Indeed, the term “tripartite synapse” was coined to emphasize the modulation of the extracellular space around synapses by astrocytes, whether this modulation occurs via the clearance of synaptic transmitters or the delivery of signaling compounds to the synaptic, extrasynaptic or perisynaptic loci, and whether it produces a feedback mechanism, an homosynaptic modulation, or a feedforward, heterosynaptic action that might impact neuronal circuitry.
Although considerable progress has been made, a combination of conceptual and technical challenges needs to be overcome for a comprehensive understanding of how astrocytes impact and shape brain function. Our goal here is to critically evaluate the currently available findings and develop a conceptual framework to guide future work. In particular, we will emphasize that a detailed consideration of spatial and temporal properties and interactions is required to fully understand the reciprocal signaling between neurons and astrocytes and the physiological consequences of gliotransmission.
Ca2+ Signalling in Astrocytes: Decoding Neuronal Activity
Astrocytes possess Ca2+ excitability and display intracellular Ca2+ elevations in response to synaptic activity from physiological sensory and motor stimuli (Bekar et al., 2008; Nimmerjahn et al., 2009; Perea et al., 2009; Petzold et al., 2008; Schummers et al., 2008; Wang et al., 2006; Winship et al., 2007). The astrocyte Ca2+ signal that arises from synaptically-released neurotransmitters is not a stereotyped “on-off” response but rather has multiple and varied patterns and kinetics that depend on the synaptic system involved (Perea and Araque, 2005), the pattern and frequency of afferent input activity (Pasti et al., 1997; Todd et al., 2010), and include changes in amplitude, frequency, kinetics and spatial diffusion. Most importantly, since Ca2+ kinetics shape cell activity and responsiveness, the tight dependency of Ca2+ responses on the type and properties of neuronal signals indicate that Ca2+ responses in astrocytes encode neuronal information.
Most of our knowledge derives from monitoring Ca2+ signals in astrocyte somata as an indicator of astrocytic responsiveness. These slow Ca2+ events were observed in response to intense neuronal activity and led to the notion that while astrocytes can detect information conveyed by intense firing activity (although at a slower time scale with respect to fast responses at the synaptic sites), they lack sensitivity to low levels of synaptic activity. Recent studies revealed, however, that small, rapid and localised Ca2+ responses can be elicited in microdomains of astrocytic processes by minimal synaptic activity (Di Castro et al., 2011; Panatier et al., 2011). These data suggest that astrocytes may integrate the activity of several individual synapses to generate the larger Ca2+ responses observed upon sustained and intense stimulation. There are a number of observations that support such a possibility, although no direct evidence is yet available. For instance, Beierlein and Regehr (2006) showed that an increased number of stimuli generated Ca2+ responses that covered a larger area of a Bergmann glial cell process. However, it was not assessed whether the larger Ca2+ responses were directly the result of a summation of the smaller ones. Also, Di Castro et al. (2011) reported complex spatial-temporal properties of Ca2+ responses elicited by axonal firing in astrocytic processes, sometimes with multiple initiation points. Moreover, the rise phase of Ca2+ signals with slower and expanded kinetics appeared to be summative of smaller Ca2+ events. These observations argue against a simple propagation-dependent alteration of Ca2+response.
Therefore, it appears that astrocyte Ca2+ signaling is characterized by a complex spatial-temporal profile ranging from small, local fast responses to larger, global but slower responses that result from the integration of signals derived from restricted regions of processes close to synapses. This integration appears to be governed by a non-linear continuum of astrocyte excitability from which local changes can be incremented to larger and more global responses.
Synaptic Modulation and Plasticity
Release of gliotransmitters is a consequence of Ca2+ elevation in astrocytes. Different, but not mutually exclusive, Ca2+-dependent and Ca2+-independent mechanisms have been identified, including Ca2+-dependent release via exocytosis (Bezzi et al., 2004; Crippa et al., 2006; Montana et al., 2004; Zhang et al., 2004) and Ca2+ flux through plasma membrane ion channels (Woo et al., 2012) but the issue as to how astrocytes release transmitters remains a subject of debate (for reviews, see Hamilton and Attwell, 2010; Parpura and Zorec, 2010; Volterra and Meldolesi, 2005). These gliotransmittters activate neuronal receptors and account for astrocyte-mediated modulation of synaptic transmission and plasticity (Table 1). Our current understanding of astrocyte-mediated synaptic modulation, obtained from in situ and in vivo observations, reveals a high degree of richness in terms of the of signaling processes and physiological consequences of astrocyte neuromodulation. Here, we draw four general conclusions regarding gliotransmission.
Table 1.
| Gliotransmitter | Brain area | Neuromodulation | |
|---|---|---|---|
| Glutamate | Hippocampus | Depression of evoked EPSCs and IPSCs | Araque et al 1998a; Liu et al 2004a |
| Frequency increase of miniature PSCs | Araque et al 1998b, Santello et al. 2011 | ||
| Frequency increase of miniature IPSCs | Kang et al 1998 | ||
| Frequency increase of spontaneous EPSCs | Jourdain et al 2007, Fiacco and McCarthy 2004 | ||
| Frequency increase of spontaneous IPSCs | Liu et al. 2004b | ||
| Postsynaptic SIC | Araque et al., 1998a; Pasti et al. 2001; Sanzgiri et al. 1999; Angulo et al. 2004; Fellin et al. 2004; Cavelier and Attwell 2005; Kang et al. 2005; Perea and Araque 2005; Tian et al. 2005; Fellin et al. 2006; Nestor et al. 2007; Navarrete and Araque 2008; Shigetomi et al. 2008; Sasaki et al., 2011; Navarrete et al. 2013 | ||
| Increase of neuronal excitability | Bezzi et al. 1998 | ||
| Heterosynaptic depression | Andersson et al. 2007 | ||
| Modulation of LTD | Han et al. 2012 | ||
| Modulation of LTP | Navarrete et al. 2012 | ||
| Synaptic potentiation | Perea and Araque 2007; Navarrete and Araque 2010; Navarrete et al. 2012 | ||
| Modulation of Action-Potential | Sasaki et al. 2011 | ||
| Modulation of basal synaptic transmision | Bonansco et al. 2011 | ||
| Regulation of mEPSC kinetics | Han et al., 2013 | ||
| Cortex | Postsynaptic SIC | Ding et al. 2007; Gomez-Gonzalo et al., 2010; Navarrete et al. 2013; Chen et al. 2012 | |
| Modulation of LTD | Min and Nevian 2012 | ||
| Ventro basal thalamus | Postsynaptic SIC | Parri et al. 2001 | |
| Spinal cord dorsal horn | Postsynaptic SIC | Bardoni et al., 2010; Nie et al., 2010 | |
| Medial nucleus of the trapezoid body | Postsynaptic SIC | Reyes-haro et al., 2010 | |
| ATP/Adenosine | Hippocampus | Heterosynaptic depression of EPSCs | Serrano et al. 2006; Zhang et al. 2003; Chen et al. 2013; Pascual et al. 2005 |
| Modulation of LTP | Pascual et al. 2005; Schmitt et al. 2012; Lee HU et al. 2013 | ||
| Basal synaptic depresion | Pascual et al. 2005 | ||
| Regulation of basal neurotransmission | Di Castro et al. 2011; Panatier et al. 2011 | ||
| Depression of evoked EPSCs | Martin et al Glia 2007 | ||
| Cortex | Regulation of basal synaptic transmission | Halassa et al. 2009 | |
| Regulation of cortical slow oscilations | Fellin et al. 2009 | ||
| Cerebellum | Depression of spontaneous EPSCs | Brockhaus and Deitmer 2002 | |
| Retina | Light-evoked neuronal activity | Newman and Zahs, 1998 | |
| Depression of light-evoked EPSCs | Newman, 2003 | ||
| Nucleus accumbens | Postsynaptic SIC | D’Ascenzo et al. 2007 | |
| Hypothalamic paraventricular nucleus | Increase of EPSC amplitude | Gordon et al. 2005; Gordon et al. 2009 | |
| Medulla oblongata | Activation of chemoreceptor neurons | Gourine et al. 2010 | |
| D-Serine | Hippocampus | Modulation of LTP | Yang et al, 2003; Henneberger et al, 2010; Zhang et al, 2008 |
| Cortex | Modulation of LTP/LTD | Takata et al. 2011; Fossat et al. 2012 | |
| Retina | Potentiation of NMDA receptor transmission | Stevens et al. 2003 | |
| Hypothalamic supraoptic nucleus | Modulation of LTP/LTD | Panatier et al. 2006 | |
| Amygdala | Modulation of NMDA receptors | Li et al. 2013 | |
| TNFα | Hippocampus | Insertion of AMPA receptors | Beattie et al. 2002 |
| Increase of synaptic scaling | Stellwagen et al. 2006 | ||
| GABA | Hippocampus | Postsynaptic SOC | Le Meur et al. 2012 |
| Cerebellum | Tonic current | Lee et al. 2010 | |
| Olfactory bulb | Postsynaptic SOC | Kozlov et al. 2006 | |
| Undefined | Cortex | Regulation of cortical up states | Poskanzer and Yuste 2011 |
| Neuromuscular junction | Synaptic depression | Robitaille 1998; Perez- Gonzalez et al. 2008 | |
| Synaptic potentiation | Castonguay and Robitaille 2001 |
First, a single gliotransmitter acts on different targets. For instance, astrocytic glutamate transiently potentiates excitatory transmission in the hippocampal dentate gyrus by acting on presynaptic NMDARs (Jourdain et al., 2007), while at hippocampal CA3-CA1 synapses, it can activate presynaptic mGluRs (Navarrete and Araque, 2010; Navarrete et al., 2012; Perea and Araque, 2007). In the CA1 hippocampal region, astrocytic glutamate has been also reported to potentiate inhibitory transmission by acting on presynaptic kainate receptors (Kang et al., 1998; Liu et al., 2004) and to favor neuronal synchrony by acting on postsynaptic NMDARs (Fellin et al., 2004). Similarly, adenosine, produced via rapid ecto-nucleotidases-mediated ATP metabolism can act presynaptically to modulate presynaptic inhibition as well as postsynaptically to regulate NMDAR trafficking (Deng et al., 2011; Martin et al., 2007; Panatier et al., 2011; Pascual et al., 2005; Zhang et al., 2003). Hence, just like neurotransmitters, a single gliotransmitter can have multiple effects depending on the type of circuit and targeted neurons, the pre- or postsynaptic location of neuronal receptors, and the receptor subtype activated.
Second, astrocytes can release multiple gliotransmitters. For example, in addition to glutamate, astrocytes in CA1 can release the NMDA receptor co-agonist D-serine (Henneberger et al., 2010; Zhuang et al., 2010) and ATP (Zhang et al., 2003). After its conversion to adenosine, this latter gliotransmitter acts on either A1 or A2A receptors to depress or enhance excitatory synaptic transmission, respectively (Panatier et al., 2011; Pascual et al., 2005; Serrano et al., 2006). Thus, astrocytes immersed in the same circuit can release different types of gliotransmitters that exert diverse modulatory actions to influence synaptic transmission in multiple forms. A major challenge for future research will be to clarify the context-specificity of the different regulatory actions. For instance, are several transmitters released from the same astrocyte? If so, are they always co-released or do the specific features of the Ca2+ signals (their magnitude and spatial-temporal properties) govern the type of gliotransmitter that is released? Because of limitations in the approaches to studying signaling dynamics, our focus has necessarily been on Ca2+ as the proximate stimulus for gliotransmission. Are there additional second messengers that could selectively modulate gliotransmission? Are there Ca2+-independent gliotransmitter release pathways that operate under physiological conditions?
Third, gliotransmission can coordinate networks of neurons and synapses. Because the astrocytic Ca2+ signals evoked locally by active synapses can eventually expand intracellularly from their initial source towards different cell locations under different conditions, such as high frequency synaptic activity or concomitant activity of multiple synapses (see below), this implies that the coding signal travels throughout astrocytic processes and triggers gliotransmitter release at distant sites, affecting other synapses and circuits. Indeed, astrocytes activated by endocannabinoids released from neurons enhance synaptic efficacy at relatively distant synapses (several tens of micrometers away from the endocannabinoid source); stimulation of astrocytic CB1 receptors causes astrocytic glutamate release and neuronal mGluR activation, a different effect than the direct activation of presynaptic receptors by endocannabinoids that causes homosynaptic depression of neurotransmission (Navarrete and Araque, 2010). In hippocampal CA1, astrocytes stimulated by highly active synapses release ATP that after conversion to adenosine depresses other synapses through A1 receptor activation, leading to heterosynaptic depression (Pascual et al., 2005; Serrano et al., 2006). Hence, as a whole, these observations suggest that astrocytes operate as bridges for inter-synaptic communication.
Fourth, as a consequence of the diversity of gliotransmitters and their targets, there is also diversity in the forms of consequent modulation observed. In the CA1 region of the hippocampus in situ, a form of long-term potentiation (LTP) can be triggered by the coincidence of postsynaptic activity and astrocyte Ca2+ elevation that stimulates glutamate release. This form of LTP is independent of post-synaptic NMDAR-mediated signalling and requires presynaptic mGluR activation (Perea and Araque, 2007). In the same hippocampal CA1 region, astrocytes release the gliotransmitter D-serine that acts as the endogenous co-agonist of postsynaptic NMDARs necessary for the induction of NMDAR-mediated LTP at synapses located within the morphological territory of the D-serine releasing astrocyte (Henneberger et al., 2010). Basal levels of adenosine, derived from astrocytic ATP, regulate the dynamic range for LTP generation (Pascual et al., 2005). In contrast, glutamate released from stimulated astrocytes mediates the spike-timing dependent long-term depression (LTD) of excitatory transmission in the neocortex through activation of presynaptic NMDARs (Min and Nevian, 2012), again supporting the idea that the same gliotransmitter can have specific effects, depending on the circuit and the type and location of the targeted receptors. The involvement of astrocyte signalling in synaptic plasticity has been recently observed in vivo whereby cholinergic activity evoked during sensory stimulation induced LTP that required muscarinic receptor-dependent astrocyte Ca2+ elevations and gliotransmitter release (Chen et al., 2012; Navarrete et al., 2012; Takata et al., 2011). Astrocytes activated by nucleus basalis cholinergic afferents to the visual cortex have been also revealed to play a critical role in the selective potentiation of the neuronal response to specific visual stimuli (Chen et al., 2012).
Does the above evidence imply that induction of synaptic plasticity requires astrocyte signalling? There is no simple yes or no answer to this question. Indeed, synaptic plasticity encompasses multiple phenomena. Whereas some forms of activity-dependent plasticity depend on NMDA receptors and are expressed postsynaptically through insertion or removal of AMPA receptors from synapses, others do not depend on NMDARs and/or are expressed presynaptically through changes in the probability of transmitter release. Likewise, whereas NMDAR-dependent plasticity is homosynaptic, other forms co-exist such as heterosynaptic plasticity that affects neighbouring inputs or homeostatic plasticity that impacts synapses on a given neuron in a global manner. Indeed, synaptic plasticity is diverse, and factors such as brain region, age, history of synaptic activity, afferent input stimulation, and circadian rhythm can influence the plasticity mechanisms observed. This has led to conflicting reports regarding the role of astrocytes in modulating synaptic plasticity and given rise to apparently paradoxical scenarios such as those concerning hippocampal LTP in IP3R2−/− mice that lack GPCR-mediated Ca2+ signalling in astrocytes (Petravicz et al., 2008). This deficiency does not prevent the induction of NMDAR-dependent LTP (Agulhon et al., 2010), but it abolishes cholinergic-induced presynaptic LTP in both hippocampus (Navarrete et al., 2012) and cortex (Takata et al., 2011) as well as nucleus basalis-induced stimulus-specific plasticity in visual cortical neurons (Chen et al., 2012). These observations suggest that IP3R2 expression in astrocytes is essential for some forms of LTP, but at the same time, that its genetic ablation does not eliminate all forms of LTP. Thus, some forms of synaptic plasticity may rely on purely neuronal mechanisms, while others may require or involve the contribution of signals from astrocytes. In addition, astrocytic signals may not always require IP3R2-dependent Ca2+ elevations. For instance, Ca2+ increases mediated through TRPA1 channels have been recently reported to occur in hippocampal astrocytes and to promote D-serine release thereby regulating NMDA receptor activation (Shigetomi et al., 2013). Given the complexity, it is not possible to draw broad conclusions based on the analysis of data from an individual phenomenon. It is clear that additional work is needed to clarify the relevance of astrocytic signalling to the diverse plasticity mechanisms operating in the brain
Emerging Hypotheses and Perspectives
Understanding glial regulation of neuronal function is both a conceptual and a technical challenge. Even though the body of evidence discussed above supports the existence of a dynamic, bidirectional regulation of neuronal communication by glial cells, the complexity and diversity of the mechanisms involved and the heterogeneity of astroglial cells make understanding and interpreting these phenomena a daunting task. Below we will present and discuss a number of hypotheses that need to be analysed, providing a framework for future studies aimed at more effectively integrating astrocytes into our current view of brain function.
Bidirectional Neuron-astrocyte Communication Granted by High Affinity, Slowly Desensitizing Receptors
Even though astrocytic processes are in close proximity to pre- and postsynaptic neuronal elements (Auld and Robitaille, 2003; Ventura and Harris, 1999), their relative distance to the synaptic cleft and the presence of very efficient neurotransmitter recapture systems might represent structural and functional limitations to effective astrocyte-to-neuron signaling. Neurotransmitters released at the synaptic cleft rapidly reach postsynaptic receptors, but they need to travel much longer distances to reach receptor targets on astrocytes (Fig. 1A, B). Consequently, neurotransmitter concentration drops rapidly away from the synaptic cleft, reaching very low levels in the vicinity of the astrocytic membrane. A detailed analysis of the mechanisms by which astrocytes detect neuronal activity reveals a common strategy in different synaptic contexts, finely tuned to allow astrocytes to overcome the problem of neurotransmitter concentration decay over distance (Rusakov and Kulmann, 1998). Indeed, many receptors involved in the astrocytic detection of synaptic activity, including metabotropic glutamate, muscarinic, CB1, P2Y and GABAB receptors, have been described as high-affinity, slowly-desensitizing receptors. This implies that low perisynaptic neurotransmitter concentrations may be sufficient to activate astrocytes. For instance, the receptor that senses the synaptic release of glutamate at astrocytic processes is the mGluR5 (Panatier et al., 2011), and not the rapidly desensitizing low-affinity AMPAR (Dingledine et al., 1999; Traynelis et al., 2010). Likewise, activation of astrocytes by synaptically released ACh is mediated by slowly desensitizing muscarinic receptors and not by rapidly inactivating nicotinic receptors (Araque et al., 2002; Giniatullin et al., 2005; Quick and Lester, 2002). A similar scenario seems to apply also to the GABAergic and purinergic signaling systems mediated by the activation of the slowly desensitizing P2Y and GABAB receptors (Bowser and Khakh, 2004; Guthrie et al., 1999; Venance et al., 1997; Waldo and Harden, 2004). All these receptors are characterized by high-affinity ligand binding (KD in the nanomolar/low micromolar range) that allows astrocytes to be activated by low concentrations of neurotransmitters. An alternative strategy is ectopic neurotransmitter release from sites located in axon terminals outside the synaptic cleft, as seen with Bergmann glia at climbing fiber-Purkinje cell synapses in the cerebellum, where the activation of lower affinity AMPA receptors appears to be mediated by glutamate released directly in face of the Bergmann glia processes (Matsui et al., 2005). The properties of the receptors mediating the astrocyte response to neurons are thus finely tuned to sense the low amounts of neurotransmitters and to avoid desensitization caused by a slow increase in neurotransmitter concentrations.
Figure 1. Bidirectional Neuron-astrocyte Communication Granted by High Affinity, Slowly Desensitizing Receptors.
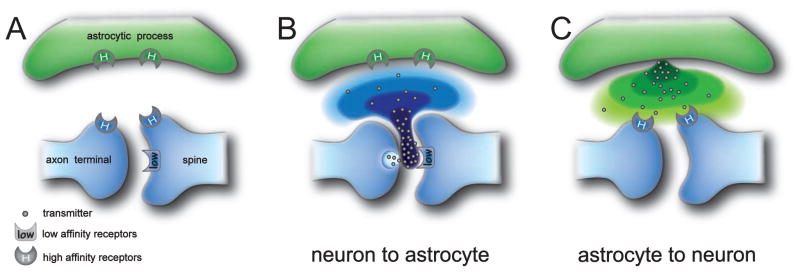
(A) Schematic drawing of the tripartite synapse illustrating the location of low and high affinity ligand receptors.
(B) Neurotransmitters rapidly activate low affinity receptors at the postsynaptic neuronal membrane and diffuse outside the synaptic cleft to activate high affinity receptors at the astrocytic membrane.
(C) Gliotransmitters activate high affinity receptors at perisynaptic locations in the neuronal membrane. Neurotransmitter (B) or gliotransmitter (C) decreasing concentrations over distance from release sites is illustrated by different color intensity.
The situation is similar when considering the possible actions of gliotransmitters on neurons (Fig. 1C). Indeed, owing to the same constraints, receptors within the synaptic cleft may hardly be sensitive to gliotransmitter released by astrocytes. In contrast, receptors located at perisynaptic axon terminals (e.g. presynaptic NMDA and mGluRs) and extrasynaptically at the postsynaptic membrane (e.g NR2B subunit-containing NMDARs) are likely to be more easily accessed by astrocytic glutamate. Most importantly, all these receptors have high binding affinities, slow deactivation and desensitization kinetics, and could thus be activated even by slowly increasing concentrations of gliotransmitters (Fig. 1C). A useful example of this is the unmasking of pure AMPAR-mediated responses triggered by astrocytic glutamate in CA1 pyramidal neurons when the desensitization of AMPARs is inhibited and NMDARs are blocked (Fellin et al., 2004).
Exceptionally, the gliotransmitter D-serine has a high affinity for the co-agonist binding sitesof synaptic NMDARs, which are almost fully occupied under basal conditions (Henneberger et al., 2010). This suggests the presence of ambient D-serine within the cleft possibly due to tonic release and/or inefficient clearance, as no known uptake system controls the spatial diffusion of this gliotransmitter.
Hence, the first unifying hypothesis is that the presence of slowly desensitizing high affinity receptors determines both the selectivity and the sensitivity of astrocyte activation and dictates the regulation of synaptic transmission and plasticity by astrocytes.
Besides receptor characteristics, the precise spatiotemporal properties of gliotransmitter release are currently poorly defined. For example, it is not clear whether there is a co-localization of hot spots of intracellular Ca2+ elevation with release sites that trigger gliotransmission. In addition, gliotransmitter release may be influenced by the different Ca2+ signalling properties and/or location of different astrocytic receptors. For example, stimulation of both PAR-1 and P2Y1 receptors evokes Ca2+-dependent glutamate release, but only PAR-1 receptor-evoked release enhances post-synaptic excitability (Shigetomi et al., 2008), while P2Y1 receptor stimulation has mainly presynaptic effects (Jourdain et al., 2007; Pascual et al., 2012; Santello et al., 2011). It is also known that changes in the spatiotemporal properties of glutamate release can alter astrocyte-induced synaptic regulation, particularly because of the dynamic competition with the uptake mechanism that shapes extracellular glutamate levels (Santello et al., 2011).
In summary, spatiotemporal properties of astrocyte Ca2+ signals and gliotransmitter release combined with actions on high-affinity, slowly desensitizing neuronal receptors located at perisynaptic sites allow gliotransmission to influence synaptic transmission.
Astrocytes as Spatial and Temporal Integrators
Astrocytes have been proposed to be involved in a large array of synaptic events, from the regulation of basal synaptic transmission to various types of synaptic plasticity. At first glance, the extent of all these astrocytic actions coupled with a large array of modulatory mechanisms may appear counterintuitive and confusing. For instance, how could astrocytes regulate local synaptic events and heterosynaptic, network-based phenomena? How could astrocytes contribute to antagonistic plasticity events such as heterosynaptic depression (Pascual et al., 2005; Serrano et al., 2006) and long-term potentiation (Henneberger et al., 2010; Navarrete et al., 2012 ; Perea and Araque, 2007; Takata et al., 2011)? How can one reconcile the variety of gliotransmitters and mechanisms involved in the regulation of the different synaptic events?
To reconcile the available information, we propose as a second unifying hypothesis that astrocytes act as time and space integrators, decoding neuronal information occurring in a large array of neuronal activity. This time and space integration encompasses faster and more local changes based on the rapid activation of small compartments along the astrocytic processes (Di Castro et al., 2011; Grosche et al., 1999; Panatier et al., 2011; Pasti et al., 1997) up to complex multi-astrocytic and neuronal interactions that are induced by sustained, intense and extended activity resulting in long-term changes in the synaptic network properties. There are a number of common properties that emerge from the multiplexing capabilities of astrocytes.
Spatial threshold mechanisms
The spatial and temporal properties of the Ca2+ dynamics triggered by the neuronal activation of the astrocyte may lead to different modulatory effects on neuronal and synaptic activity. For instance, astrocytic regulation may be confined to a small functional compartment if synaptic transmission remains below a certain level (Fig. 2A)(Di Castro et al., 2011; Panatier et al., 2011; Pasti et al., 1997). Upon an increase in the frequency of synaptic activity (or the recruitment of multiple synapses), the intracellular astrocytic Ca2+ activation, initially restricted to a microdomain, expands beyond the local subcompartment into another process (Fig. 2B) and eventually the whole cell (Fig. 2C)(Castonguay et al, 2001; Di Castro et al., 2011; Panatier et al., 2011; Pasti et al., 1997; Zonta et al., 2003). The spatial extension of the astrocyte Ca2+ signal may also be regulated by the spatial and temporal integration of the synaptic inputs from different neurotransmitter signaling pathways, which may control the spatial extension of the regulatory consequences on specific synapses (Fellin and Carmignoto, 2004; Perea and Araque, 2005), revealing synaptic information processing by astrocytes (De Pittà et al., 2012; Perea and Araque, 2005).
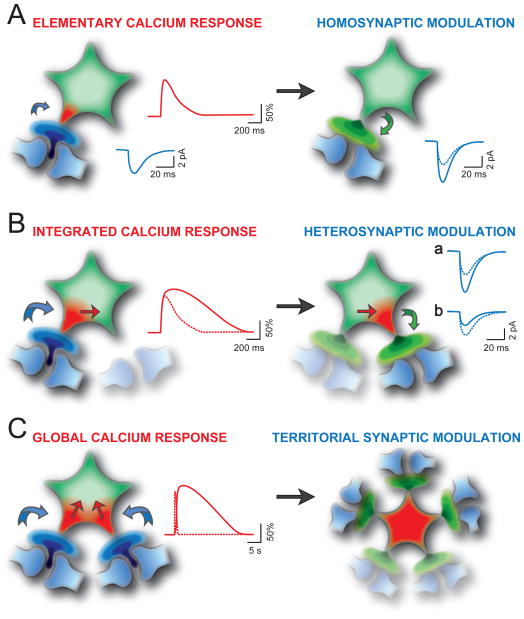
Figure 2. Synaptic Modulatory Actions of Gliotransmitters Depend on Integration by Astrocytes of the Ca2+ Changes Evoked by Different Levels of Neuronal Activity.
(A) Low levels of synaptic activity (blue arrow, left) evoke rapid, spatially restricted Ca2+ elevations at an astrocytic process (red trace) resulting in a gliotransmitter release that locally modulates synaptic transmission (green arrow, right) (Jourdain et al., 2007; Perea and Araque, 2007; Pascual et al., 2012; Santello et al., 2011; Panatier et al., 2011). The change in synaptic efficacy due to gliotransmitter-mediated regulation of the probability of release is illustrated as an increase in the mean amplitude of excitatory postsynaptic events(dashed and solid blue line).
(B) Ca2+ elevations evoked at an astrocytic process by an intense activity of an individual synapse diffuse to a nearby process (red arrow) to trigger gliotransmitter release that affects nearby synapses (right). The red superimposed traces are the integrated Ca2+ response (solid line) and the elementary Ca2+ response (dashed line, same as solid line in A). As a result of this astrocyte modulatory action, synaptic transmission (solid blue line) can be either potentiated (Navarrete and Araque, 2010) or depressed (Zhang et al., 2003; Pascual et al., 2005; Andersson et al., 2007; Serrano et al., 2006)(dashed blue line in a and b, respectively). As in (C), the focus of the phenomenon being described is indicated in colour, while the elements that are not the focus are greyed out (but are not necessarily inactive).
(C) Multiple Ca2+ events at different processes evoked by simultaneously active synapses are spatially and temporally integrated (left) resulting in a global, long lasting Ca2+ elevation that can affect synaptic transmission in the territory of individual astrocytes (right) (Henneberger et al., 2010). The global Ca2+ response (solid line) and the integrated Ca2+ response (dashed line, same as solid line in B) are reported. Note the different time scale of Ca2+ traces in (A), (B) and (C).
Astrocyte domains and spatial extent of neuromodulation
It is well established that each astrocyte occupies a determined volume that defines an exclusive astrocytic territory (Bushong et al., 2002; Halassa et al., 2007). As a result, a given astrocyte will be the only one to interact with a determined set of several thousands of synapses and dendrites (Fig. 2C). The large diversity of astrocytic receptors, their spatial location, and the spatiotemporal properties of the synaptic-dependent Ca2+ signals provide the necessary properties that allow a single astrocyte to detect, process and decode the activity of a variety of synapses upon which it can provide distinct feedback and feedforward modulations.
Since astrocytic Ca2+ can stimulate gliotransmission, the spatially dynamic nature of the Ca2+ signal necessarily provides a spatially diverse potential for gliotransmission. For example, low frequency synaptic activity that leads to local astrocytic Ca2+ signals is likely to lead to localized gliotransmission (Fig. 2A) that will be restricted to exerting feedback modulation of the active synapse. However, with an increased frequency of synaptic activity (Fig. 2B, C), the capability of the astrocytic Ca2+ signal to spread through the processes and to even fill the entire astrocyte, now imparts the potential for the resulting gliotransmission to exert feedforward actions on other synapses at distant locations. According to this notion, the spatial extent of the astrocytic activation is conditional on the activity of associated synapses: under some conditions gliotransmitters act in a highly localized manner, while under others they act on larger neuronal domains. Therefore, they can exert qualitatively different effects, such as the modulation of neighboring synapses (Fig 2B) (Navarrete and Araque, 2010; Pascual et al., 2005; Serrano et al., 2006) and synaptic domains defined by the morphological territory of individual astrocytes (Fig. 2C) (Henneberger et al., 2010). This set of observations would argue that neuronal activity-dependent Ca2+ changes in astrocytes could convey specific informative signals to neurons.
A Need to Understand Receptor Coupling to Ca2+
Based on this analysis and on the evidence of multiple receptors and gliotransmitters, there are a number of fundamental questions to be answered. First, we need to determine the distribution of receptors along the astrocytic processes and the mechanisms that govern this distribution. Second, there is an urgent need to understand better the molecular mechanisms underlying the different modes of Ca2+-dependent (and possibly also Ca2+-independent) activation of astrocytes by different types of receptors. Third, it is equally important to determine the association between a set of astrocytic receptors and the selective mechanisms regulating the release of a gliotransmitter. Each of these questions represents a major technological and conceptual challenge that must be tackled in order to provide a solid basis for our understanding of the astrocytic regulation of neuronal communication.
A clearer understanding of these aspects would also allow us to more critically examine the conclusions of studies that have argued against a role for astrocytes in modulating neuronal activity. For example, over-expression and pharmacological activation of the foreign receptor Mas-related gene A1 (MrgA1) receptor in astrocytes produced long-lasting (minutes) and cell ubiquitous Ca2+ elevations that had no impact on synaptic functions (Agulhon et al., 2010; Fiacco et al., 2007). Intriguingly, prolonged stimulations of endogenous GPCRs producing long-lasting and widespread Ca2+ elevations similar to those evoked via MrgA1 stimulation, were also synaptically ineffective (Agulhon et al., 2010; Fiacco et al., 2007). These long-lasting and widespread Ca2+ elevations are not observed in astrocytes during physiological activity in the brain and may represent an abnormal and possibly pathological response in these cells that does not necessarily reflect the effects that would be observed after activation of astrocytes by physiological stimuli. Consistent with this hypothesis, a long lasting Ca2+ increase evoked in cultured astrocytes by GPCR overstimulation was observed to trigger a solitary episode of glutamate release at the onset of the Ca2+ change only (Pasti et al., 2001). In contrast, short-lasting Ca2+ transients, that mimic the typical oscillatory behaviour of astrocyte Ca2+ signals both at rest (Nett et al., 2002) and in response to neuronal activity (Di Castro et al., 2011; Pasti et al., 1997), resulted in multiple glutamate release episodes and, in turn, repetitive activation of neuronal receptors.
If we consider that the type of receptor, its location and its mode of activation influence the properties of the downstream signalling, we can reasonably expect that all these other parameters, in addition to the duration of the astrocytes response, also profoundly affect gliotransmitter release and its functional consequences. Accordingly, the lack of effects reported in the above studies must be consideredin their specific context and carefully weighed.
Glial and Neuronal Modulation: Convergence of Two Different Time and Functional Domains
Since gliotransmitters are similar to known neurotransmitters and target receptors similar to those targeted by neurotransmitters, one is left wondering whether it is possible for gliotransmission to provide unique encoding in the brain. As a third unifying hypothesis, we propose that astrocytes represent an additional neuromodulatory system that acts in complement to the neuronal ones, but with its own time and space domains based upon the particular intrinsic properties of Ca2+ signaling that encode and integrate incoming inputs from neurons and other environmental sources.
The glial regulation provides an intermediate regulation between the direct neuronal modulation and the very slow and chronic “hormonal-like” regulation carried out by general brain homeostasis. Indeed, owing to their proximity to neurons and synapses, as well as the kinetics of the Ca2+-dependent decoding of neuronal activity and glial elaboration, astrocytes can provide a balanced and easily tunable feedback or feedforward response that regulates neuronal communication in a different time domain. Moreover, astrocytes are in contact with thousands of synaptic inputs targeting many dendrites of several neurons. It has been estimated that an individual astrocyte contacts 300–600 neuronal dendrites in the cortex (Halassa et al., 2007) and oversees ~140,000 hippocampal synapses in the hippocampus (Bushong et al., 2002). This allows the astrocyte to integrate and filter a unique volume of synaptic activity. Hence, astrocytic integration and modulation encompasses neuron and synapse types to provide an analysis and output reflecting a unique complex neuronal and glial network.
Finally, in addition to synaptic inputs, astrocytes receive multiple signals and homeostatic information from different cellular sources, including neurons, vascular cells, other astrocytes and even different types of glial cells. They process this diverse information to produce output signals that convey integrated information reflecting the complex microenvironment. Indeed, astrocytes play fundamental roles linking neuronal metabolic requirements and supply, sensing neuronal activity and providing energy support to neurons through the glucose/glycogen pathways (Magistretti et al., 1999; Pellerin and Magistretti, 1994) and the regulation of blood flow for oxygen consumption and nutrients (Attwell et al., 2010; Gordon et al., 2008; Haydon and Carmignoto, 2006; Mulligan and MacVicar, 2004; Takano et al., 2006; Zonta et al., 2003). Similarly, astrocytes can exchange information concerning immune state with microglia and detect local pH and osmolality changes to control breathing (Gourine et al., 2010) and water homeostasis, respectively (Haj-Yasein et al., 2011). As a whole, astrocytes act as multiplexer integrators of metabolic, neuronal and other cell signals.
In fact, the different time and space domains of neuronal encoding coupled with the diversity of their interactions, would allow astrocytes to perform complex and diversified modulation of neuronal functions that would contribute to the enrichment of information processing in the brain. For instance, this integration could feed back in a non-specific, more homeostatic manner tuned with metabolic regulation. This would be quite powerful in setting a balanced tone of neuronal activity across large areas (Rouach et al., 2008). However, on the other hand of this spectrum, the multiplexing integrations in space and time by astrocytes would allow them also to perform very fine and selective regulation of neuronal activity generating various gradients of plasticity depending on location and properties of the glial and neuronal elements. Hence, astrocytes act as multiplexer integrators of multiple complex cell signals that would influence information processing in a wide array of time and space domains domains possibly complementing the neuronal processing.
Conclusions
The field of neuron-glia interactions has grown enormously over the past two decades. In addition, the breath of techniques and approaches has also exploded, revealing the involvement of astrocytes from local synaptic circuitries (Di Castro et al., 2011; Fellin and Carmignoto, 2004; Henneberger et al., 2010 ; Navarrete et al., 2012; Panatier et al., 2011; Perea and Araque, 2007; Serrano et al., 2006; Takata et al., 2011) up to behavior (Halassa et al., 2009; Han et al., 2012; Saab et al., 2012; Tanaka et al., 2013). It is quite clear that astrocytes play a very large array of roles in multiple brain regions, utilizing a multitude of functional membrane receptors and signaling molecules. Yet, given the complexity and diversity at play, it is no surprise that the literature also reports some discrepant results regarding the roles played by astrocytes in the regulation of synaptic functions. These data highlight our limited understanding of the true nature of astrocytes and their interactions with neurons and point to future directions for research on neuron-glia interactions.
The complexity of such interactions is increasingly appreciated, with functional specificities possibly determined by the type of transmitter, synaptic circuit or brain region involved, as well as by diversities ascribable to the physiological context of the studies and the age of the animals. For example, consider a recent study (Sun et al., 2013) reporting that expression of the astrocyte mGluR5 receptor decreases with age and that its pharmacological stimulation fails to produce somatic Ca2+ responses in mature brain astrocytes. From these observations the authors concluded that glutamatergic tripartite synapses operate only during development. However, other data have demonstrated that Ca2+ elevations evoked in mature astrocytes by whisker stimulation are predominantly mediated by the synaptic release of glutamate and activation of astrocytic mGluR5 (Wang et al., 2006) and that the mGluR5 is expressed in the perisynaptic processes of mature brain astrocytes (Di Castro et al., 2011; Lavialle et al., 2011). These latter data are not inconsistent with those of Sun et al, who did not study Ca2+ responses in astrocytic processes, and they suggest a different interpretation forthe decrease in astrocyte mGluR5 expression in the mature brain, i.e. that the refinement of the synaptic circuitry leads to a restriction in the expression of the receptor to perisynaptic processes where it is needed for tripartite modulation (Arizono et al., 2012). This example highlights the current difficulties in correctly understanding the synaptic roles of astrocytes, and we must take a holistic approach to interpreting the literature, aiming to better understand the technical, physiological, and even interpretational reasons behind such discrepant results.
Following this reasoning, in this review we have attempted a conceptual synthesis by proposing that astrocytes contribute to information processing by linking neuronal activities (as well as activities in other cell types) that occur on different spatial and temporal dimensions to achieve a higher level of integration of brain function. We offer three unifying concepts that we hope will provide a useful framework for the studies to come. First, astrocytes participate in synaptic integration differently from neurons. Their activation and output modulatory responses occur on temporal and spatial scales distinct from those of synaptic transmission and rely on the perisynaptic expression of high-affinity slow-desensitizing receptors in both astrocytes and neurons. Second, as integrative and regulatory entities, astrocytes offer a flexible system that can process information on multiple scales and cover spatial territories and temporal frames different from those offered by purely neuronal circuits. Third, during this function, astrocytes can enrich the integration by incorporating information coming from outside the synaptic world (e.g., from vascular, immune and other cells), to fine tune the synaptic circuitry according to the environmental state.
With the present review we have tried to outline the complex choreography that exists between neurons and astrocytes, focusing on specific characteristics of the latter that render them central actors in brain function. Indeed, in our view, astrocyte signalling and gliotransmission represent the highly evolved integrative interface in brain communication that couples slow modulatory signalling from multiple sources with fast synaptic transmission.
Acknowledgments
For the kind hospitality, the authors would like to thank Francesco Pasti and La Frassina, in the quiet countryside near Venice, where we met to discuss and write the main body of this manuscript. We also thank Micaela Zonta for her support in the preparation of figures, and Paulo Magalhães for critical reading of the manuscript. The original work by the authors was supported by the following grants: MINECO (BFU2010-15832; CSD2010-00045) and Cajal Blue Brain (AA); European Union HEALTH-F2-2007-202167 (AA and GC); Telethon Italy GGP10138B/GGP12265, Cariparo Foundation and FIRB RBAP11X42L (GC); National Institutes of Health R01NS037585, R01AA020183 and R01MH095385 (PGH); INSERM, Conseil Régional d’Aquitaine, Équipe FRM and the Labex BRAIN (SHRO); Canadian Institute of Health Research (MOP-14137 and MOP-111070), a Discovery group grant from the National Science and Engineering Research Council (RGPGP 203729) and a group infrastructure grant from Fonds de recherche du Québec-Santé (RR); ERC Advanced, grant 340368 “Astromnesis”, Swiss National Science Foundation, grant 31003A-140999 and National Centres of Competence in Research (NCCR) “Synapsy” and “Transcure” (AV).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agulhon C, Fiacco TA, McCarthy KD. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science. 2010;327:1250–1254. doi: 10.1126/science.1184821. [DOI] [PubMed] [Google Scholar]
- Andersson M, Blomstrand F, Hanse E. Astrocytes play a critical role in transient heterosynaptic depression in the rat hippocampal CA1 region. J Physiol. 2007;585:843–852. doi: 10.1113/jphysiol.2007.142737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo MC, Kozlov AS, Charpak S, Audinat E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J Neurosci. 2004;24:6920–6927. doi: 10.1523/JNEUROSCI.0473-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Martín ED, Perea G, Arellano JI, Buño W. Synaptically released acetylcholine evokes Ca2+ elevations in astrocytes in hippocampal slices. J Neurosci. 2002;22:2443–2450. doi: 10.1523/JNEUROSCI.22-07-02443.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998a;10:2129–2142. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999a;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Araque A, Sanzgiri RP, Parpura V, Haydon PG. Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. J Neurosci. 1998b;18:6822–6829. doi: 10.1523/JNEUROSCI.18-17-06822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Sanzgiri RP, Parpura V, Haydon PG. Astrocyte-induced modulation of synaptic transmission. Can J Physiol Pharmacol. 1999b;77:699–706. [PubMed] [Google Scholar]
- Arizono M, Bannai H, Nakamura K, Niwa F, Enomoto M, Matsu-Ura T, Miyamoto A, Sherwood MW, Nakamura T, Mikoshiba K. Receptor-selective diffusion barrier enhances sensitivity of astrocytic processes to metabotropic glutamate receptor stimulation. Sci Signal. 2012;5:ra27. doi: 10.1126/scisignal.2002498. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld DS, Robitaille R. Glial cells and neurotransmission: an inclusive view of synaptic function. Neuron. 2003;40:389–400. doi: 10.1016/s0896-6273(03)00607-x. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Ghirri A, Zonta M, Betelli C, Vitale G, Ruggieri V, Sandrini M, Carmignoto G. Glutamate-mediated astrocyte-to-neuron signalling in the rat dorsal horn. J Physiol. 2010;588:831–846. doi: 10.1113/jphysiol.2009.180570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFα. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Regehr WG. Brief bursts of parallel fiber activity trigger calcium signals in bergmann glia. J Neurosci. 2006;26:6958–6967. doi: 10.1523/JNEUROSCI.0613-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekar LK, He W, Nedergaard M. Locus coeruleus α-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb Cortex. 2008;18:2789–2795. doi: 10.1093/cercor/bhn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhäuser C, Pilati E, Volterra A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–620. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Volterra A. A neuron-glia signalling network in the active brain. Curr Opin Neurobiol. 2001;11:387–394. doi: 10.1016/s0959-4388(00)00223-3. [DOI] [PubMed] [Google Scholar]
- Bonansco C, Couve A, Perea G, Ferradas CA, Roncagliolo M, Fuenzalida M. Glutamate released spontaneously from astrocytes sets the threshold for synaptic plasticity. Eur J Neurosci. 2011;33:1483–1492. doi: 10.1111/j.1460-9568.2011.07631.x. [DOI] [PubMed] [Google Scholar]
- Bowser DN, Khakh BS. ATP excites interneurons and astrocytes to increase synaptic inhibition in neuronal networks. J Neurosci. 2004;24:8606–8620. doi: 10.1523/JNEUROSCI.2660-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus J, Deitmer JW. Long-lasting modulation of synaptic input to Purkinje neurons by Bergmann glia stimulation in rat brain slices. J Physiol. 2002;545:581–593. doi: 10.1113/jphysiol.2002.028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castonguay A, Lévesque S, Robitaille R. Glial cells as active partners in synaptic functions. Prog Brain Res. 2001;132:227–240. doi: 10.1016/S0079-6123(01)32079-4. [DOI] [PubMed] [Google Scholar]
- Cavelier P, Attwell D. Tonic release of glutamate by a DIDS-sensitive mechanism in rat hippocampal slices. J Physiol. 2005;564:397–410. doi: 10.1113/jphysiol.2004.082131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Sugihara H, Sharma J, Perea G, Petravicz J, Le C, Sur M. Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes. Proc Natl Acad Sci USA. 2012;109:E2832–2841. doi: 10.1073/pnas.1206557109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippa D, Schenk U, Francolini M, Rosa P, Verderio C, Zonta M, Pozzan T, Matteoli M, Carmignoto G. Synaptobrevin2-expressing vesicles in rat astrocytes: insights into molecular characterization, dynamics and exocytosis. J Physiol. 2006;570:567–582. doi: 10.1113/jphysiol.2005.094052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, Moss SJ, Haydon PG. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci USA. 2007;104:1995–2000. doi: 10.1073/pnas.0609408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pittà M, Volman V, Berry H, Parpura V, Volterra A, Ben-Jacob E. Computational quest for understanding the role of astrocyte signaling in synaptic transmission and plasticity. Front Comput Neurosci. 2012;6:98. doi: 10.3389/fncom.2012.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Terunuma M, Fellin T, Moss SJ, Haydon PG. Astrocytic activation of A1 receptors regulates the surface expression of NMDA receptors through a src kinase dependent pathway. Glia. 2011;59:1084–1093. doi: 10.1002/glia.21181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Castro MA, Chuquet J, Liaudet N, Bhaukaurally K, Santello M, Bouvier D, Tiret P, Volterra A. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat Neurosci. 2011;14:1276–1284. doi: 10.1038/nn.2929. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Fellin T, Carmignoto G. Neurone-to-astrocyte signalling in the brain represents a distinct multifunctional unit. J Physiol. 2004;559:3–15. doi: 10.1113/jphysiol.2004.063214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Halassa MM, Terunuma M, Succol F, Takano H, Frank M, Moss SJ, Haydon PG. Endogenous nonneuronal modulators of synaptic transmission control cortical slow oscillations in vivo. Proc Natl Acad Sci USA. 2009;106:15037–15042. doi: 10.1073/pnas.0906419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Fellin T, Pozzan T, Carmignoto G. Purinergic receptors mediate two distinct glutamate release pathways in hippocampal astrocytes. J Biol Chem. 2006;281:4274–4284. doi: 10.1074/jbc.M510679200. [DOI] [PubMed] [Google Scholar]
- Fiacco TA, Agulhon C, Taves SR, Petravicz J, Casper KB, Dong X, Chen J, McCarthy KD. Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron. 2007;54:611–626. doi: 10.1016/j.neuron.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Fossat P, Turpin FR, Sacchi S, Dulong J, Shi T, Rivet JM, Sweedler JV, Pollegioni L, Millan MJ, Oliet SH, Mothet JP. Glial D-serine gates NMDA receptors at excitatory synapses in prefrontal cortex. Cereb Cortex. 2012;22:595–606. doi: 10.1093/cercor/bhr130. [DOI] [PubMed] [Google Scholar]
- Giniatullin R, Nistri A, Yakel JL. Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci. 2005;28:371–378. doi: 10.1016/j.tins.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Gomez-Gonzalo M, Losi G, Chiavegato A, Zonta M, Cammarota M, Brondi M, Vetri F, Uva L, Pozzan T, de Curtis M, et al. An excitatory loop with astrocytes contributes to drive neurons to seizure threshold. PLoS Biol. 2010;8:e1000352. doi: 10.1371/journal.pbio.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci. 2005;8:1078–1086. doi: 10.1038/nn1498. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Iremonger KJ, Kantevari S, Ellis-Davies GC, MacVicar BA, Bains JS. Astrocyte-mediated distributed plasticity at hypothalamic glutamate synapses. Neuron. 2009;64:391–403. doi: 10.1016/j.neuron.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H. Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat Neurosci. 1999;2:139–143. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Yasein NN, Vindedal GF, Eilert-Olsen M, Gundersen GA, Skare O, Laake P, Klungland A, Thoren AE, Burkhardt JM, Ottersen OP, Nagelhus EA. Glial-conditional deletion of aquaporin-4 (Aqp4) reduces blood-brain water uptake and confers barrier function on perivascular astrocyte endfeet. Proc Natl Acad Sci USA. 2011;108:17815–17820. doi: 10.1073/pnas.1110655108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. J Neurosci. 2007;27:6473–6477. doi: 10.1523/JNEUROSCI.1419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci. 2010;11:227–238. doi: 10.1038/nrn2803. [DOI] [PubMed] [Google Scholar]
- Han J, Kesner P, Metna-Laurent M, Duan T, Xu L, Georges F, Koehl M, Abrous DN, Mendizabal-Zubiaga J, Grandes P, et al. Acute Cannabinoids Impair Working Memory through Astroglial CB1 Receptor Modulation of Hippocampal LTD. Cell. 2012;148:1039–1050. doi: 10.1016/j.cell.2012.01.037. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Kang N, Xu J, Xu Q, Nedergaard M, Kang J. Astrocytic glutamate release-induced transient depolarization and epileptiform discharges in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2005;94:4121–4130. doi: 10.1152/jn.00448.2005. [DOI] [PubMed] [Google Scholar]
- Kozlov AS, Angulo MC, Audinat E, Charpak S. Target cell-specific modulation of neuronal activity by astrocytes. Proc Natl Acad Sci USA. 2006;103:10058–10063. doi: 10.1073/pnas.0603741103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavialle M, Aumann G, Anlauf E, Prols F, Arpin M, Derouiche A. Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc Natl Acad Sci USA. 2011;108:12915–12919. doi: 10.1073/pnas.1100957108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Meur K, Mendizabal-Zubiaga J, Grandes P, Audinat E. GABA release by hippocampal astrocytes. Front Comput Neurosci. 2012;6:59. doi: 10.3389/fncom.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS, Augustine GJ, Lee CJ. Channel-mediated tonic GABA release from glia. Science. 2010;330:790–796. doi: 10.1126/science.1184334. [DOI] [PubMed] [Google Scholar]
- Li Y, Sacchi S, Pollegioni L, Basu AC, Coyle JT, Bolshakov VY. Identity of endogenous NMDAR glycine site agonist in amygdala is determined by synaptic activity level. Nat Commun. 2013;4:1760. doi: 10.1038/ncomms2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QS, Xu Q, Arcuino G, Kang J, Nedergaard M. Astrocyte-mediated activation of neuronal kainate receptors. Proc Natl Acad Sci USA. 2004;101:3172–3177. doi: 10.1073/pnas.0306731101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–497. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- Martin ED, Fernandez M, Perea G, Pascual O, Haydon PG, Araque A, Cena V. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia. 2007;55:36–45. doi: 10.1002/glia.20431. [DOI] [PubMed] [Google Scholar]
- Matsui K, Jahr CE, Rubio ME. High-concentration rapid transients of glutamate mediate neural-glial communication via ectopic release. J Neurosci. 2005;25:7538–7547. doi: 10.1523/JNEUROSCI.1927-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min R, Nevian T. Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat Neurosci. 2012;15:746–753. doi: 10.1038/nn.3075. [DOI] [PubMed] [Google Scholar]
- Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci. 2004;24:2633–2642. doi: 10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Navarrete M, Araque A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron. 2010;68:113–126. doi: 10.1016/j.neuron.2010.08.043. [DOI] [PubMed] [Google Scholar]
- Navarrete M, Perea G, de Sevilla DF, Gomez-Gonzalo M, Nunez A, Martin ED, Araque A. Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS Biol. 2012;10:e1001259. doi: 10.1371/journal.pbio.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor MW, Mok LP, Tulapurkar ME, Thompson SM. Plasticity of neuron-glial interactions mediated by astrocytic EphARs. J Neurosci. 2007;27:12817–12828. doi: 10.1523/JNEUROSCI.2442-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nett WJ, Oloff SH, McCarthy KD. Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. J Neurophysiol. 2002;87:528–537. doi: 10.1152/jn.00268.2001. [DOI] [PubMed] [Google Scholar]
- Newman EA. Glial cell inhibition of neurons by release of ATP. J Neurosci. 2003;23:1659–1666. doi: 10.1523/JNEUROSCI.23-05-01659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA, Zahs KR. Modulation of neuronal activity by glial cells in the retina. J Neurosci. 1998;18:4022–4028. doi: 10.1523/JNEUROSCI.18-11-04022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Mukamel EA, Schnitzer MJ. Motor behavior activates Bergmann glial networks. Neuron. 2009;62:400–412. doi: 10.1016/j.neuron.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Toquet B, Pollegioni L, Poulain DA, Oliet SHR. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Panatier A, Vallee J, Haber M, Murai KK, Lacaille JC, Robitaille R. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell. 2011;146:785–798. doi: 10.1016/j.cell.2011.07.022. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Parpura V, Zorec R. Gliotransmission: Exocytotic release from astrocytes. Brain Res Rev. 2010;63:83–92. doi: 10.1016/j.brainresrev.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci. 2001;4:803–812. doi: 10.1038/90507. [DOI] [PubMed] [Google Scholar]
- Pascual O, Ben Achour S, Rostaing P, Triller A, Bessis A. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci USA. 2012;109:E197–205. doi: 10.1073/pnas.1111098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci. 1997;17:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasti L, Zonta M, Pozzan T, Vicini S, Carmignoto G. Cytosolic calcium oscillations in astrocytes may regulate exocytotic release of glutamate. J Neurosci. 2001;21:477–484. doi: 10.1523/JNEUROSCI.21-02-00477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Araque A. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J Neurosci. 2005;25:2192–2203. doi: 10.1523/JNEUROSCI.3965-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317:1083–1086. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32:421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Perez-Gonzalez AP, Albrecht D, Blasi J, Llobet A. Schwann cells modulate short-term plasticity of cholinergic autaptic synapses. J Physiol. 2008;586:4675–4691. doi: 10.1113/jphysiol.2008.160044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petravicz J, Fiacco TA, McCarthy KD. Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci. 2008;28:4967–4973. doi: 10.1523/JNEUROSCI.5572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold GC, Albeanu DF, Sato TF, Murthy VN. Coupling of neural activity to blood flow in olfactory glomeruli is mediated by astrocytic pathways. Neuron. 2008;58:897–910. doi: 10.1016/j.neuron.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskanzer KE, Yuste R. Astrocytic regulation of cortical UP states. Proc Natl Acad Sci USA. 2011;108:18453–18458. doi: 10.1073/pnas.1112378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick MW, Lester RA. Desensitization of neuronal nicotinic receptors. J Neurobiol. 2002;53:457–478. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- Reyes-Haro D, Muller J, Boresch M, Pivneva T, Benedetti B, Scheller A, Nolte C, Kettenmann H. Neuron-astrocyte interactions in the medial nucleus of the trapezoid body. J Gen Physiol. 2010;135:583–594. doi: 10.1085/jgp.200910354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R. Modulation of synaptic efficacy and synaptic depression by glial cells at the frog neuromuscular junction. Neuron. 1998;21:847–855. doi: 10.1016/s0896-6273(00)80600-5. [DOI] [PubMed] [Google Scholar]
- Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- Rusakov DA, Kullmann DM. Extrasynaptic glutamate diffusion in the hippocampus: Ultrastructural constraints, uptake and receptor activation. J Neurosci. 1998;18:3158–3170. doi: 10.1523/JNEUROSCI.18-09-03158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab AS, Neumeyer A, Jahn HM, Cupido A, Simek AA, Boele HJ, Scheller A, Le Meur K, Gotz M, Monyer H, et al. Bergmann glial AMPA receptors are required for fine motor coordination. Science. 2012;337:749–753. doi: 10.1126/science.1221140. [DOI] [PubMed] [Google Scholar]
- Santello M, Bezzi P, Volterra A. TNFα controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron. 2011;69:988–1001. doi: 10.1016/j.neuron.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Sanzgiri RP, Araque A, Haydon PG. Prostaglandin E(2) stimulates glutamate receptor-dependent astrocyte neuromodulation in cultured hippocampal cells. J Neurobiol. 1999;41:221–229. [PubMed] [Google Scholar]
- Sasaki T, Kuga N, Namiki S, Matsuki N, Ikegaya Y. Locally Synchronized Astrocytes. Cereb Cortex. 2011;21:1889–1900. doi: 10.1093/cercor/bhq256. [DOI] [PubMed] [Google Scholar]
- Schmitt LI, Sims RE, Dale N, Haydon PG. Wakefulness affects synaptic and network activity by increasing extracellular astrocyte-derived adenosine. J Neurosci. 2012;32:4417–4425. doi: 10.1523/JNEUROSCI.5689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320:1638–1643. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- Serrano A, Haddjeri N, Lacaille JC, Robitaille R. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J Neurosci. 2006;26:5370–5382. doi: 10.1523/JNEUROSCI.5255-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Bowser DN, Sofroniew MV, Khakh BS. Two forms of astrocyte calcium excitabilty have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J Neurosci. 2008;28:6659–6663. doi: 10.1523/JNEUROSCI.1717-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Jackson-Weaver O, Huckstepp RT, O’Dell TJ, Khakh BS. TRPA1 Channels Are Regulators of Astrocyte Basal Calcium Levels and Long-Term Potentiation via Constitutive D-Serine Release. J Neurosci. 2013;33:10143–10153. doi: 10.1523/JNEUROSCI.5779-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci. 2012;15:70–80. doi: 10.1038/nn.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-α. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Stevens ER, Esguerra M, Kim PM, Newman EA, Snyder SH, Zahs KR, Miller RF. D-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc Natl Acad Sci USA. 2003;100:6789–6794. doi: 10.1073/pnas.1237052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W, Lovatt D, Han X, Smith Y, Nedergaard M. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science. 2013;339:197–200. doi: 10.1126/science.1226740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Takata N, Mishima T, Hisatsune C, Nagai T, Ebisui E, Mikoshiba K, Hirase H. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J Neurosci. 2011;31:18155–18165. doi: 10.1523/JNEUROSCI.5289-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Shih PY, Gomi H, Yoshida T, Nakai J, Ando R, Furuichi T, Mikoshiba K, Semyanov A, Itohara S. Astrocytic Ca2+ signals are required for the functional integrity of tripartite synapses. Mol Brain. 2013;6:6. doi: 10.1186/1756-6606-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian GF, Azmi H, Takano T, Xu Q, Peng W, Lin J, Oberheim N, Lou N, Wang X, Zielke HR, et al. An astrocytic basis of epilepsy. Nat Med. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd KJ, Darabid H, Robitaille R. Perisynaptic glia discriminate patterns of motor nerve activity and influence plasticity at the neuromuscular junction. J Neurosci. 2010;30:11870–11882. doi: 10.1523/JNEUROSCI.3165-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venance L, Stella N, Glowinski J, Giaume C. Mechanism involved in initiation and propagation of receptor-induced intercellular calcium signaling in cultured rat astrocytes. J Neurosci. 1997;17:1981–1992. doi: 10.1523/JNEUROSCI.17-06-01981.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci. 1999;19:6897–6906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Waldo GL, Harden TK. Agonist binding and Gq-stimulating activities of the purified human P2Y1 receptor. Mol Pharmacol. 2004;65:426–436. doi: 10.1124/mol.65.2.426. [DOI] [PubMed] [Google Scholar]
- Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- Winship IR, Plaa N, Murphy TH. Rapid astrocyte calcium signals correlate with neuronal activity and onset of the hemodynamic response in vivo. J Neurosci. 2007;27:6268–6272. doi: 10.1523/JNEUROSCI.4801-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo DH, Han KS, Shim JW, Yoon BE, Kim E, Bae JY, Oh SJ, Hwang EM, Marmorstein AD, Bae YC, et al. TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell. 2012;151:25–40. doi: 10.1016/j.cell.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, Poo M, Duan S. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc Natl Acad Sci USA. 2003;100:15194–15199. doi: 10.1073/pnas.2431073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J-m, Wang H-k, Ye C-q, Ge W, Chen Y, Jiang Z-l, Wu C-p, Poo M-m, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40:971–982. doi: 10.1016/s0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Fukuda M, Van Bockstaele E, Pascual O, Haydon PG. Synaptotagmin IV regulates glial glutamate release. Proc Natl Acad Sci USA. 2004;101:9441–9446. doi: 10.1073/pnas.0401960101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Z, Yang B, Theus MH, Sick JT, Bethea JR, Sick TJ, Liebl DJ. EphrinBs regulate D-serine synthesis and release in astrocytes. J Neurosci. 2010;30:16015–16024. doi: 10.1523/JNEUROSCI.0481-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]