Abstract
Introduction
The epidemic of metabolic syndrome is increasing worldwide and correlates with elevation in serum uric acid and marked increase in total fructose intake. Fructose raises uric acid and the latter inhibits nitric oxide bioavailability. We hypothesized that fructose-induced hyperuricemia may have a pathogenic role in metabolic syndrome and treatment of hyperuricemia or increased nitric oxide may improve it.
Material and methods
Two experiments were performed. Male Sprague-Dawley rats were fed a control diet or a high-fructose diet to induce metabolic syndrome. The latter received either sodium nitrate or allopurinol for 10 weeks starting with the 1st day of fructose to evaluate the preventive role of the drugs or after 4 weeks to evaluate their therapeutic role.
Results
A high-fructose diet was associated with significant (p < 0.05) hyperuricemia (5.9 ±0.5 mg/dl), hypertension (125.2 ±7.8 mm Hg), dyslipidemia and significant decrease in tissue nitrite (27.4 ±2.01 mmol/l). Insulin resistance, as manifested by HOMAIR (20.6 ±2.2) and QUICKI (0.23 ±0.01) indices, as well as adiposity index (12.9 ±1.1) was also significantly increased (p < 0.1). Sodium nitrate or allopurinol was able to reverse these features significantly (p < 0.05) in the preventive study better than the therapeutic study.
Conclusions
Fructose may have a major role in the epidemic of metabolic syndrome and obesity due to its ability to raise uric acid. Either sodium nitrate or allopurinol can prevent this pathological condition by different mechanisms of action.
Keywords: fructose, hyperuricemia, insulin resistance, metabolic syndrome
Introduction
Metabolic syndrome is a worldwide problem, which refers to a constellation of coronary heart disease (CHD) risk factors including obesity and abdominal fat distribution, disorders of glucose and lipid metabolism, and hypertension [1, 2]. In addition, various other abnormalities of uric acid, inflammation, homeostasis, and fibrinolysis are often considered part of this syndrome [3].
The prevalence of metabolic syndrome is increasing and it is considered one of the main threats to human health worldwide [4]. The epidemic correlates with pronounced changes in the environment, behavior and lifestyle [5].
Several studies have suggested that fructose-induced hyperuricemia may play a pathogenic role in metabolic syndrome. This is consistent with the increased consumption of fructose-containing beverages and the epidemic of diabetes and obesity [6]. In contrast to the metabolic effects of other sugars, fructose induces hyperuricemia through the stimulation of nucleotide catabolism via the conversion of hepatic adenosine triphosphate to adenosine diphosphate by fructokinase and it may retard urinary excretion of uric acid [7].
Uric acid induces gene expression of chemokines and growth factors, such as monocyte chemoattractant protein-1 and platelet-derived growth factor, and stimulates proliferation of vascular smooth muscle cells [8].
Another potential mechanism activated by uric acid is oxidative stress. In the extracellular environment, urate can scavenge hydroxyl radical, singlet oxygen and peroxynitrite, especially when combined with ascorbic acid or thiols [9]. On the other hand, uric acid loses its antioxidant ability in the hydrophobic environment [10]. It was shown that soluble uric acid – alone or combined with peroxynitrite – stimulates an increase in nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity and increases production of reactive oxygen species in mature adipocytes, which result in increased protein nitrosylation and lipid oxidation [11, 12].
Uric acid also inhibits nitric oxide bioavailability and, because insulin requires nitric oxide to stimulate glucose uptake, there is a possibility that fructose-induced hyperuricemia and insulin resistance are partially prevented by lowering serum uric acid [13].
Recent studies have demonstrated that in the bioactivation of nitrate, nitrite anion is an intermediate and this more reactive compound is further metabolized to nitric oxide, nitrosothiols and other bioactive nitrogen oxide tissues [14].
Administration of nitrate or nitrite to humans and rodents is clearly associated with nitric oxide-like bioactivity, as demonstrated by increases in cyclic guanosine monophosphate formation [15], vasodilatation [16], reduction in blood pressure [17] and inhibition of platelet function [18].
Therefore, this study was designed to investigate and compare the role of the preventive and therapeutic effects of inorganic nitrate and allopurinol on metabolic syndrome features and renovascular complications to improve the role of NO and uric acid in pathogenesis of metabolic syndrome.
Material and methods
Chemicals and drugs
Allopurinol was purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). Normal saline solution (sodium chloride 0.9%) and fructose were purchased from AlGomhoria Pharmaceutical Co. (Cairo, Egypt). Sodium nitrate was obtained from MUP Pharmaceutical Co. (Ismailia, Egypt). All the commercial assay kits were purchased from Bio-diagnostic® (Cairo, Egypt).
Animals
Sixty-four Sprague-Dawley adult male rats (180-200 g) were purchased from the National Research Center, Cairo, Egypt. Animals were housed in wire-mesh cages with a normal light-dark cycle at a constant temperature of 22-24°C throughout the experiment. They were placed on a standard chow ad libitum for 1 week to adapt to the laboratory environment before experiments. The study was designed to avoid suffering and limit the number of animals used and all experimental protocols were approved by the institutional animal care and use committee at the Faculty of Medicine, Suez Canal University (Ismailia, Egypt).
Study design
Two experiments were conducted in this study; rats were divided into four groups in each experiment.
Experiment I: treatment of fructose-induced hyperuricemia with inorganic nitrate or allopurinol (therapeutic regimen)
To assess the therapeutic effect of either inorganic nitrate or allopurinol in fructose-induced metabolic syndrome, two groups of rats were used. Rats (n = 24) were fed a high-fructose diet or a control diet (n = 8). The high-fructose diet contained 60% fructose, whereas the control diet contained 46% starch as the carbohydrate. The caloric content of these diets was 3.6 kcal/g and 3.1 kcal/g, respectively [19]. At 4 weeks, blood pressure was measured, blood samples were obtained at 11 a.m. after overnight fasting, and then the fructose-fed group was subdivided into 3 sub-groups of 8 animals each. The untreated fructose group served as a positive control group. The sodium nitrate and allopurinol groups received either sodium nitrate (150 mg/kg) [20] or allopurinol (10 mg/kg) [20] dissolved in normal saline by oral gavage once daily for an additional 6 weeks.
Experiment II: prevention of fructose-induced hyperuricemia with sodium nitrate or allopurinol (preventive regimen)
To assess their effect in preventing metabolic syndrome, sodium nitrate and allopurinol in the same mentioned doses were initiated on the day when the high-fructose diet was given from week 0 to week 10. Four groups were used in this preventive study: control, fructose, fructose sodium nitrate and fructose_allopurinol (n = 8 each).
Measurement of body weight
Rats were pair-fed to ensure equivalent caloric intake, thereby avoiding the influence of different food intake on the metabolic abnormalities. Body weight was measured weekly [21].
Measurement of mean blood pressure
All animals were preconditioned for mean blood pressure measurements one week before the experiment by the tail-cuff method (Data acquisition system mp140, USA). Blood pressure was measured at baseline, at 4 weeks and at 10 weeks. The mean of three successive measurements was taken [22].
Blood glucose determination and sample collection
At the end of the 4th week and at the end of the study, rats were fasted overnight and fasting blood glucose was determined with an automatic blood glucose meter (Super Glucocard, Japan) using blood samples from the tail vein. At the end of the study, rats were anesthetized with thiopental sodium (50 mg/kg) [23] and killed by decapitation. Blood samples were collected by cardiac puncture, centrifuged at 2000 rpm for 15 min within 30 min of collection and stored at –80°C until assayed.
Enzyme-linked immunosorbent assay for insulin
Serum insulin was determined at the 4th week and the end of the study using a rat insulin ultrasensitive ELISA kit (Crystal Chem Inc., Downers Grove, IL 60515, USA) according to the manufacturer's instructions. Reactions were quantified by optical density using an automated ELISA reader.
Calculation of insulin resistance
Insulin resistance was determined using the homeostasis model assessment index for insulin resistance (HOMA-IR) using the following formula: HOMA-IR index = [fasting glucose (mmol/l) × fasting insulin (µU/ml)]/22.5 [24]. To assess insulin sensitivity, the quantitative insulin sensitivity check index was used: (QUICKI) = 1/(log fasting insulin (µU/ml) + log fasting glucose (mg/dl)). QUICKI predicts insulin sensitivity, with lower values representing more insulin resistance [25].
Processing of adipose tissue
The retroperitoneal adipose tissue from each rat was totally removed and weighed at the end of the study to be used for calculation of the adiposity index: adipose tissue index = (retroperitoneal adipose tissue weight / body weight) × 100.
Biochemical assays
Serum uric acid, creatinine and urea
Serum uric acid was determined at 4 weeks and at the end of the study [26]. Serum creatinine [26] and urea [27] levels were measured at the end of the study.
Urinary uric acid
At the end of the study, rats in each group were individually housed in metabolic cages for 24 h urine collection, and continued to have free access to water and food. Urine samples were used for estimation of uric acid [26].
Lipid profile
Serum triglycerides (TGs) [28], total cholesterol [29] and high density lipoprotein cholesterol (HDL-C) [30] were measured at the end of the study. Low density lipoprotein (LDL) was calculated according to the following formula: serum LDL level = total cholesterol – ((triglycerides/5) + HDL) [31].
Assay of total nitrite in renal tissue homogenate (Griess reaction)
The total nitrite concentration level was measured in 100 mg renal tissue homogenate – as an indicator for nitric oxide production – at the end of the study according to the method described by Green et al. [32]. All the colorimetric assays were performed using a UV-visible spectrophotometer (UV-1601-PC, Shimadzu, Japan).
Statistical analysis
The data were coded and entered using the statistical package SPSS version 17 (Chicago, IL, USA). The results were expressed as mean ± S.E.M. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by post hoc multiple comparison Bonferroni test, to test the significance of differences among group means. P value of < 0.05 was considered statistically significant at confidence interval 95%.
Results
Effect of the high-fructose diet in induction of features of metabolic syndrome
Oral administration of a high-fructose diet for 4 weeks in rats resulted in development of features of metabolic syndrome manifested by significant increase (p < 0.05) in mean blood pressure (BP), serum uric acid and fasting insulin levels with mean values of 125.2 ±7.8 mm Hg, 5.9 ±0.5 mg/dl and 53 ±1.7 µU/l respectively as compared with rats fed a control diet with mean values of 62.7 ±4.7 mm Hg, 1.8 ±0.2 mg/dl and 42.2 ±1.8 µU/l respectively. On the other hand, the body weight of fructose-fed rats did not differ significantly compared with rats fed a normal diet: 225 ±10.2 g vs. 231 ±10.1 g respectively (data not shown).
Mean blood pressure
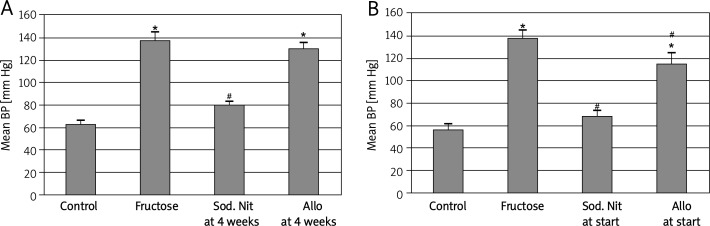
To examine the effect of sodium nitrate and allopurinol after development of metabolic syndrome, one-third of the fructose-fed rats were treated with sodium nitrate and one-third were treated with allopurinol for 6 additional weeks. Sodium nitrate significantly (p < 0.05) reduced mean blood pressure while allopurinol induced a non-significant decrease in blood pressure compared to fructose-fed rats (Figure 1A). The present study also examined the effectiveness of sodium nitrate and allopurinol in preventing the development of metabolic syndrome. The drugs were given simultaneously with the high-fructose diet from the starting point to avoid fructose-induced metabolic syndrome. As shown in Figure 1B, the elevation of mean blood pressure induced by the high-fructose diet was significantly (p < 0.05) decreased over the 10-week period by using any of the drugs.
Figure 1.
Mean blood pressure (BP) of experimental groups after 6 weeks (A) or 10 weeks (B) oral therapy of sodium nitrate (sod. Nit) 150 mg/kg/day or allopurinol (Allo) 10 mg/kg/day in rats with metabolic syndrome induced by 60% fructose in diet. Results are mean ± SEM and analyzed by one-way ANOVA and Bonferroni post-hoc test
*Significant compared to control group, #significant compared to fructose group
Fasting blood glucose, insulin and insulin resistance
Although fructose-fed rats did not develop significant fasting hyperglycemia, they developed significant (p < 0.05) fasting hyperinsulinemia and a significant (p < 0.05) increase in insulin resistance compared to controls. Insulin resistance, as measured by HOMA-IR and QUICKI, was reversed significantly by either sodium nitrate or allopurinol compared to the fructose group in the therapeutic or preventive regimen. In addition, both drugs also induced a significant decrease in insulin level in the preventive regimen (Table I).
Table I.
Fasting blood glucose, insulin, HOMA-IR and QUICKI in fructose-induced metabolic syndrome in rats after therapeutic and preventive interventions with either sodium nitrate or allopurinol
| Parameter | Therapeutic regimen | Preventive regimen | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Fructose | Sodium nitrate | Allo | Value of F | Control | Fructose | Sodium nitrate | Allo | Value of F | |
| Fasting glucose [mmol/l] | 5.9 ±0.4 | 7.5 ±1.2 | 6.2 ±0.9 | 6.0 ±1.3 | 5.54 | 5.9 ±0.4 | 7.2 ±0.4 | 6.2 ±0.3 | 6.3 ±0.4 | 6.54 |
| Fasting insulin [µU/ml] | 49.4 ±3.5 | 61.8 ±1.4* | 59.3 ±3.1* | 61.2 ±3.1* | 14.56 | 49.4 ±3.5 | 61.8 ±4.4* | 55.8 ±2.1* # | 52.9 ±2.7# | 18.35 |
| HOMA-IR | 13 ±0.9 | 20.6 ±2.2* | 16.3 ±1.9*,# | 16.3 ±1.4*,# | 16.32 | 13 ±0.9 | 19.8 ±0.9* | 15.3 ±0.4# | 14.8 ±0.8# | 16.52 |
| QUICKI | 0.27 ±0.02 | 0.23 ±0.01* | 0.25 ±0.01*,# | 0.25 ±0.00*,# | 17.11 | 0.27 ±0.02 | 0.24 ±0.09* | 0.26 ±0.01# | 0.26 ±0.02# | 16.39 |
| Body weight [g] | 287.5 ±8 | 255.0 ±12.4 | 270.3 ±8 | 284.5 ±7.4 | 2.64 | 287.5 ±8 | 280.0 ±3.8 | 285.3 ±4.5 | 270.0 ±3.9 | 1.07 |
| Adipose tissue [g] | 16.0 ±1.2 | 32.9 ±1.5* | 29.5 ±0.8* | 21.1 ±1.9*,#,@ | 30.837 | 16.0 ±1.2 | 32.7 ±1.5* | 24.1 ±0.8*,# | 21.1 ±1.9*,# | 34.0 |
| Adiposity index | 5.6 ±0.4 | 12.9 ±1.1* | 10.9 ±0.3* | 7.4 ±0.6#,@ | 27.06 | 5.6 ±0.4 | 11.7 ±0.9* | 8.4 ±0.6*,# | 7.8 ±0.1*,# | 27.82 |
HOMA-IR – homeostatic model assessment for insulin resistance. QUICKI – quantitative insulin sensitivity check index. Rats fed 60% fructose diet or a control diet for 10 weeks. Oral sodium nitrate 150 mg/kg/d or allopurinol (Allo) 10 mg/kg/day was used for 6 weeks in the therapeutic regimen or for 10 weeks in the preventive regimen. Results are mean ± SEM and analyzed by one-way ANOVA and Bonferroni post-hoc test. N = 8.
Significant compared to control group at p < 0.05
Significant compared to fructose group at p < 0.05
Significant compared to sodium nitrate group at p < 0.05
Adiposity index
Although body weight did not change significantly between fructose-fed rats and controls, the adipose tissue index was significantly (p < 0.05) increased in fructose-fed rats compared to controls. In the therapeutic regimen, allopurinol significantly (p < 0.05) prevented the increase in adiposity index compared to fructose-fed rats and sodium nitrate-treated rats. On the other hand, both drugs resulted in a decrease in the adiposity index in the preventive regimen (Table I).
Serum and urinary uric acid and serum creatinine and urea
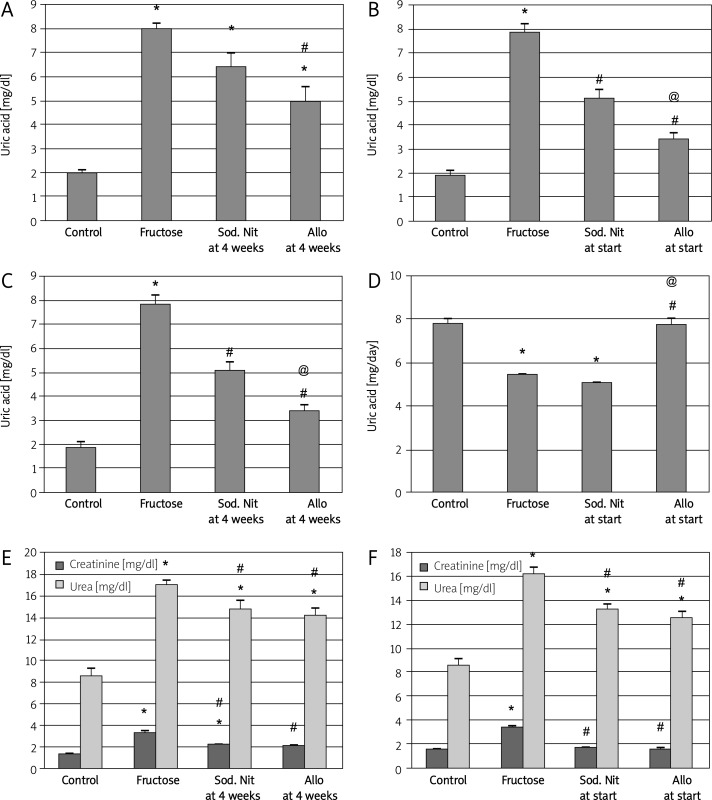
In accordance with the mechanism of action of allopurinol, in the therapeutic regimen, it showed a significant (p < 0.05) decrease in uric acid serum level, whereas the fructose-fed rats that did not receive treatment or received sodium nitrate continued to be hyperuricemic (Figure 2A).
Figure 2.
Serum uric acid (mg/dl), urinary uric acid (mg/day) and serum creatinine and urea (mg/dl) of experimental groups. Serum uric acid (A, B), urinary uric acid (mg/day) (C, D) and serum creatinine and urea (E, F) after 6 weeks (A, C, E) or 10 weeks (B, D, F) oral therapy of sodium nitrate (sod. Nit) 150 mg/kg/day or allopurinol (Allo) 10 mg/kg/day in rats with metabolic syndrome induced by 60% fructose in diet
Results are mean ± SEM and analyzed by one-way ANOVA and Bonferroni post-hoc test. *Significant compared to control group, #significant compared to fructose group, @significant compared to sodium nitrate group
On the other hand, in the preventive regimen, both sodium nitrate and allopurinol resulted in a significant decrease in uric acid serum level compared to fructose-fed non-treated rats and the beneficial effect of allopurinol was significant compared to sodium nitrate (p < 0.05, Figure 2B). In addition, we examined the urinary excretion of uric acid in these animals to clarify the mechanisms of hyperuricemia in fructose-fed rats. Fructose-fed rats had lower urinary excretion of uric acid. Interestingly, allopurinol – but not sodium nitrate – significantly prevented and reversed the reduced excretion of uric acid in fructose-fed rats (p < 0.05, Figures 2C, 2D). The urinary excretion of uric acid was normalized in the preventive regimen.
Again, the high-fructose diet induced a significant (p < 0.05) increase in serum creatinine and urea compared to controls. Administration of either sodium nitrate or allopurinol in both regimens resulted in improvement of renal function as manifested by a significant decrease in these serum levels compared to fructose-fed rats (p < 0.05, Figures 2E, 2F).
Lipid profile
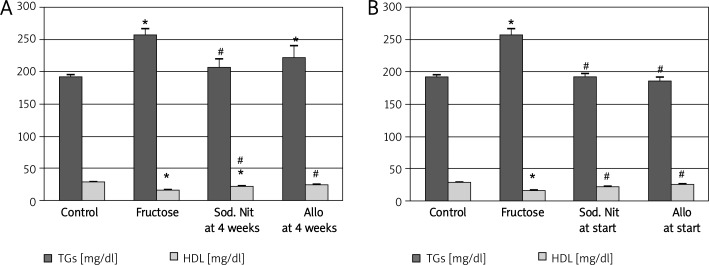
Regarding lipid profile, the results of the current study showed that fructose-fed rats developed a significant (p < 0.05) increase in serum TGs, cholesterol and LDL and a significant (p < 0.05) decrease in HDL serum levels compared to controls. Administration of sodium nitrate resulted in a significant decrease in TGs in both therapeutic and preventive regimens and both sodium nitrate and allopurinol significantly normalized HDL serum level in both regimens compared to fructose-fed rats (p < 0.05, Figures 3A, 3B). On the other hand, the tested drugs resulted in a non-significant improvement in total cholesterol and LDL serum levels compared to fructose alone-fed rats (p > 0.05, data not shown).
Figure 3.
Serum triglycerides (TGs) and serum high-density lipoproteins (HDL) after 6 weeks (A) or 10 weeks (B) oral therapy of sodium nitrate (sod. Nit) 150 mg/kg/day or allopurinol (Allo) 10 mg/kg/day in metabolic syndrome induced by 60% fructose in diet
Results are mean ± SEM and analyzed by one-way ANOVA and Bonferroni post-hoc test. *Significant compared to control group, #significant compared to fructose group
Nitrite level in renal tissue homogenate
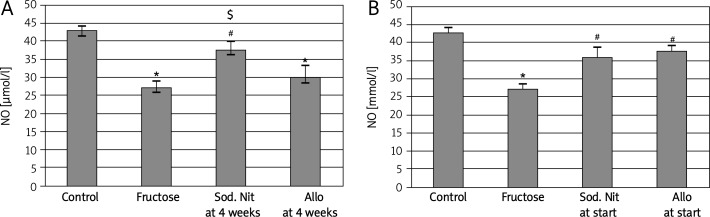
A shown in Figure 4, nitric oxide level was measured in renal tissue homogenate. Induction of metabolic syndrome by 60% fructose in diet induced a significant (p < 0.05) decrease in this level. In the therapeutic regimen, administration of sodium nitrate significantly (p < 0.05) increased its level again compared to the fructose-fed group and compared to the allopurinol-treated group (Figure 4A), while in the preventive regimen, both drugs induced a significant increase in its level compared to the fructose-fed group (p < 0.05, Figure 4B).
Figure 4.
Nitrite level (NO) in 100 mg renal tissue homogenate of experimental groups after 6 weeks (A) or 10 weeks (B) oral therapy of sodium nitrate (sod. Nit) 150 mg/kg/day or allopurinol (Allo) 10 mg/kg/day in rats with metabolic syndrome induced by 60% fructose in diet
Results are mean ± SEM and analyzed by one-way ANOVA and Bonferroni post-hoc test. *Significant compared to control group, #significant compared to fructose group, $significant compared to allopurinol group
Discussion
We have succeeded in inducing metabolic syndrome in experimental rats by administering 60% fructose for 4 weeks in the diet. Features of the syndrome were in the form of hypertension, hyperuricemia and hyperinsulinemia in accordance with Roncal et al. [22].
In an attempt to compare the effect of sodium nitrate and allopurinol on attenuating these mentioned features, sodium nitrate showed a significantly higher effect in reducing the mean arterial blood pressure compared to allopurinol. This could be attributed to the effect of sodium nitrate in inducing nitric oxide-like bioactivity in humans including a robust reduction in blood pressure, inhibition of platelet aggregation and improvement of endothelial function [15, 20, 33]. On the other hand, and in accordance with our study, it was found that allopurinol had a partial effect in preventing the rise in arterial blood pressure when compared to captopril, a potent antihypertensive drug [22].
However, allopurinol was a potent drug in reducing both the hyperuricemia and the increase in the adipose tissue index induced by the metabolic syndrome compared to the effect of sodium nitrate. There is supporting evidence that uric acid may have a pathogenic role in metabolic syndrome. Hyperuricemia has been found to predict the development of both obesity and type II diabetes [34], as well as in secondary insulin resistance syndromes such as that associated with gout [35] or diuretic usage [36]. These data introduce the novel concept that uric acid may have a causal role in metabolic syndrome.
It is known that although fructose likely increases the production of uric acid, decreased urinary uric acid excretion could be due to an increase in uric acid reabsorption in proximal tubules [25]. The beneficial hypouricemic effect of allopurinol may be explained either by decreased uric acid synthesis [5] or according to Johnson et al. [37], who stated that hyperuricemic rats are known to develop renal vasoconstriction with a reduction of renal blood flow, and this is reversed by allopurinol. In turn, an increase in renal blood flow will result in increased uric acid excretion. According to that, by improving endothelial dysfunction and renal blood flow, the lowering of uric acid could paradoxically enhance excretion.
Regarding insulin resistance, our results were in accordance with previous studies which suggested that insulin resistance or hyperinsulinemia is associated with metabolic syndrome [38], enhanced urate reabsorption and reduced urate excretion in the kidney, leading to elevated serum uric acid concentrations [39]. Consistent with this observation is the finding that drugs which improve insulin sensitivity and lower insulin levels also reduce the level of serum uric acid in diabetic patients [40].
Uric acid potently reduces endothelial NO bioavailability in both cell culture and in experimental animal models [41]. In turn, reducing endothelial NO levels is a known mechanism for inducing insulin resistance [42]; thus endothelial NOS-deficient mice exhibit the features of metabolic syndrome [20]. This can be explained as insulin stimulates glucose uptake in skeletal muscle by increasing blood flow to these tissues through a nitric oxide-dependent pathway [42]. According to this, our study showed that allopurinol or sodium nitrate may have a beneficial role in metabolic syndrome by blocking hyperuricemia-induced endothelial dysfunction or providing nitric oxide to tissues respectively, a finding which was previously supported by many studies [5, 19].
Recent epidemiological evidence indicated that hyperuricemia might be a risk factor for renal dysfunction [43, 44]. In accordance with the previous studies, the current study showed that fructose resulted in hyperuricemia and attenuated renal function manifested by increase in serum creatinine and urea. On the other hand, allopurinol and sodium nitrate resulted in improvement of kidney function, probably due to hypouricemic properties and increased nitric oxide production respectively.
A recent study demonstrated that fructose up-regulated the expression levels of renal specific transporters (rRST) and down-regulated the expression levels of organic anion transporters (rOAT) and organic cation transporters (rOCT1 and rOCT2), with elevation of the regulator prostaglandin E2 (PGE2) and nitric oxide reduction, which might lead to excessive accumulation of endogenous and exogenous toxins, resulting in renal damage in fructose-fed rats. The dysregulation of these transporters, in addition to PGE2 elevation and nitric oxide reduction, was reversed by allopurinol, rutin and quercetin [21].
Reviewing the effect on the lipid profile, we noticed that both drugs significantly increased HDL serum level but no significant effect was reported in the case of cholesterol or low-density lipoprotein serum levels. For the triglycerides, only sodium nitrate showed a significant decrease in its level in both therapeutic and prophylactic intervention. This is very consistent with the results of Carlström et al. [20], who reported that nitrate-treated endothelial nitric oxide synthase-deficient mice displayed lower levels of circulating triglycerides compared to untreated animals.
Although the role of uric acid in the metabolism of triglycerides remains unknown, uric acid might be involved in either the overproduction or the reduction of clearance of triglycerides. A decrease in the clearance of triglycerides in fructose-fed rats has been attributed to a reduction in lipoprotein lipase activity in endothelial cells [44]. An alternative explanation is the possibility that the de novo increase in purine synthesis observed in fructose-fed rats may be pathogenetically linked to hepatic fatty acid synthesis, resulting in overproduction of triglycerides [46].
In previous studies, it was reported that lowering uric acid improves dyslipidemia, which is consistent with our work [5, 47].
Regarding the potent effect of nitrates in increasing the tissue nitric oxide compared to allopurinol, we can explain that by the recently discovered ability of inorganic nitrates to be reduced to nitrite and then nitric oxide and other bioactive nitrogen oxides. In that way the deficiency in nitric oxide accompanying the metabolic syndrome could be replenished. It was assumed by certain studies that this deficiency is the result of polymorphism in the endothelial nitric oxide synthase gene in humans [20, 48, 49].
Allopurinol also blocks oxidants generated by the xanthine oxidase pathway. Oxidants are involved in the pathogenesis of diabetes and its complications. It is therefore possible that the beneficial effects of allopurinol in metabolic syndrome may be attributed, in part, to the lowering of oxidants in addition to its effect on uric acid [10].
To test their prophylactic effects, we introduced both drugs from the beginning of the experiment with the same high-fructose diet in two other groups. All the features of metabolic syndrome were prevented: elevation of uric acid and mean arterial blood pressure, insulin resistance, hypertriglyceridemia and decreased HDL, disturbance in adipose tissue index, elevated serum creatinine and urea, decrease in renal nitric oxide. These results turn our attention to the importance of enriching the daily diet with extensive intake of vegetables, the dominant dietary source of nitrate compounds [20, 50].
In conclusion, from the foregoing we can conclude that in metabolic syndrome we cannot count on allopurinol alone in dealing with the elevated blood pressure, but sodium nitrate should be considered first. At the same time, when considering hyperuricemia and dyslipidemia as fundamental disturbances in that syndrome, we proved that allopurinol is the treatment of choice. Our study proved the efficacy of each tested drug to partially attenuate the components of the metabolic syndrome, but in different ways.
Our fundamental recommendation is to test the efficacy of combining both of them. We hypothesize that adding their effects together could have great promise in reversing most of the features of metabolic syndrome.
References
- 1.Bao Y, Shang X, Zhou L, Hu R, Li Y, Ding W. Relationship between N-terminal pro-B-type natriuretic peptide levels and metabolic syndrome. Arch Med Sci. 2011;7:247–56. doi: 10.5114/aoms.2011.22075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gluba A, Mikhailidis DP, Lip GY, Hannam S, Rysz J, Banach M. Metabolic syndrome and renal disease; Int J Cardiol; 2012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.De Moura RF, Ribeiro C, De Oliveira JA, De Mello MA. Metabolic syndrome signs in Wister rats submitted to different high-fructose ingestion protocols. Br J Nutr. 2009;101:1178–84. doi: 10.1017/S0007114508066774. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Li C, Cook S, Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115:2526–32. doi: 10.1161/CIRCULATIONAHA.106.657627. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290:625–31. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- 6.Stanhope KL, Havel PJ. Endocrine and metabolic effects of consuming beverages sweetened with fructose, glucose, sucrose, or high-fructose corn syrup. Am J Clin Nutr. 2008;88:1733–7. doi: 10.3945/ajcn.2008.25825D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao X, Qi L, Qiao N, et al. Intake of added sugar and sugar-sweetened drink and serum uric acid concentration in US men and women. Hypertension. 2007;50:306–12. doi: 10.1161/HYPERTENSIONAHA.107.091041. [DOI] [PubMed] [Google Scholar]
- 8.Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J Hypertens. 2008;26:269–75. doi: 10.1097/HJH.0b013e3282f240bf. [DOI] [PubMed] [Google Scholar]
- 9.Lin SD, Tsai DH, Hsu SR. Association between serum uric acid level and components of the metabolic syndrome. J Chin Med Assoc. 2006;69:512–6. doi: 10.1016/S1726-4901(09)70320-X. [DOI] [PubMed] [Google Scholar]
- 10.Sui X, Church TS, Meriwether RA, Lobelo F, Blair SN. Uric acid and the development of metabolic syndrome in women and men. Metabolism. 2008;57:845–52. doi: 10.1016/j.metabol.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao HH, Liu JC, Lin J, Chen CH, Wu CH, Cheng TH. Uric acid stimulates endothelin-1 gene expression associated with NADPH oxidase in human aortic smooth muscle cells. Acta Pharmacol Scin. 2008;29:1301–12. doi: 10.1111/j.1745-7254.2008.00877.x. [DOI] [PubMed] [Google Scholar]
- 12.Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acidin adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293:584–96. doi: 10.1152/ajpcell.00600.2006. [DOI] [PubMed] [Google Scholar]
- 13.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300:924–32. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–1. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 15.Webb AJ, Patel N, Loukogeorgakis S, et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–90. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansson EA, Huang L, Malkey R, et al. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat Chem Biol. 2008;4:411–7. doi: 10.1038/nchembio.92. [DOI] [PubMed] [Google Scholar]
- 17.Kapil V, Milsom AB, Okorie M, et al. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension. 2010;56:274–81. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 18.Zharikov SI, Krotova K, Hu H, et al. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol. 2008;295:1183–90. doi: 10.1152/ajpcell.00075.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sánchez-Lozada LG, Le M, Segal M, Johnson RJ. How safe is fructose for persons with or without diabetes? Am J Clin Nutr. 2008;88:1189–90. doi: 10.3945/ajcn.2008.26812. [DOI] [PubMed] [Google Scholar]
- 20.Carlström M, Larsen FJ, Nyström T, et al. Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitricoxide synthase-deficient mice. Proc Natl Acad Sci U SA. 2010;107:17716–20. doi: 10.1073/pnas.1008872107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Q, Wang C, Li J, Zhang D, Kong L. Allopurinol, rutin, and quercetin attenuate hyperuricemia and renal dysfunction in rats induced by fructose intake: renal organic ion transporter involvement. Am J Physiol Renal Physiol. 2009;297:1080–91. doi: 10.1152/ajprenal.90767.2008. [DOI] [PubMed] [Google Scholar]
- 22.Roncal CA, Reungjui S, Sánchez-Lozada LG, et al. Combination of captopril and allopurinol retards fructose-induced metabolic syndrome. Am J Nephrol. 2009;30:399–404. doi: 10.1159/000235731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogler GA. Anesthesia and analgesia. In: Suckow MA, Weisbroth S, Franklin CL, editors. The laboratory rat. New York: Elsevier Academic Press; 2006. pp. 627–95. [Google Scholar]
- 24.Rudenski AS, Matthews DR, Levy JC, Turner R. Understanding insulin resistance: both glucose resistance and insulin resistance are required to model human diabetes. Metabolism. 1991;40:908–17. doi: 10.1016/0026-0495(91)90065-5. [DOI] [PubMed] [Google Scholar]
- 25.Reungjui S, Roncal CA, Mu W, et al. Thiazide diuretics exacerbate fructose-induced metabolic syndrome. J Am Soc Nephrol. 2007;18:2724–31. doi: 10.1681/ASN.2007040416. [DOI] [PubMed] [Google Scholar]
- 26.Spincer K. Analytical reviews in clinical biochemistry. Ann Clin Biochem. 1986;23:1–25. doi: 10.1177/000456328602300101. [DOI] [PubMed] [Google Scholar]
- 27.Fawcett JK, Scott JE. A rapid and precise method for the determination of urea. J Clin Pathol. 1960;13:156–9. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buccolo G, David H. Quantitative determination of serum triglycerides by use of enzymes. Clin Chem. 1973;19:476–82. [PubMed] [Google Scholar]
- 29.Fasce CF, Vanderlinde RE. Factors affecting the results of serum cholesterol determinations: an interlaboratory evaluation. Clin Chem. 1972;118:901–8. [PubMed] [Google Scholar]
- 30.Warnick GR, Nauck M, Rifai N. Evolution of methods for measurement of HDL-cholesterol: from ultracentrifugation to homogeneous assays. Clin Chem. 2001;47:1579–96. [PubMed] [Google Scholar]
- 31.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 32.Green LC, Wagner DA, Gloowski J, Skipper DL, Wishnok JS, Tannenbaum S. Analysis of nitrite, nitrate and [15N] nitrite in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 33.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med. 2006;355:2792–3. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 34.Nakanishi N, Okamoto M, Yoshida H, Matsuo Y, Suzuki K, Tatara K. Serum uric acid and risk for development of hypertension and impaired fasting glucose or type II diabetes in Japanese male office workers. Eur J Epidemiol. 2003;18:523–30. doi: 10.1023/a:1024600905574. [DOI] [PubMed] [Google Scholar]
- 35.Miao Z, Yan S, Wang J, et al. Insulin resistance acts as an independent risk factor exacerbating high-purine diet induced renal injury and knee joint gouty lesions. Inflam Res. 2009;58:659–68. doi: 10.1007/s00011-009-0031-9. [DOI] [PubMed] [Google Scholar]
- 36.Lindholm LH, Persson M, Alaupovic P, Carlberg B, Svensson A, Samuelsson O. Metabolic outcome during 1 year in newly detected hypertensives: results of the Antihypertensive Treatment and Lipid Profile in a North of Sweden Efficacy Evaluation (ALPINE study) J Hypertens. 2003;21:1563–74. doi: 10.1097/01.hjh.0000084723.53355.76. [DOI] [PubMed] [Google Scholar]
- 37.Johnson RJ, Kang DH, Feig D, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41:1183–90. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- 38.Athyros VG, Giouleme O, Ganotakis ES, et al. Safety and impact on cardiovascular events of long-term multifactorial treatment in patients with metabolic syndrome and abnormal liver function tests: a post hoc analysis of the randomised ATTEMPT study. Arch Med Sci. 2011;7:796–805. doi: 10.5114/aoms.2011.25554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am J Physiol Regul Integr Comp Physiol. 2008;295:1370–5. doi: 10.1152/ajpregu.00195.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwatani M, Wasada T, Katsumori K, Watanabe-Takahashi C, Kamatani N, Iwamoto Y. Troglitazone decreases serum uric acid concentrations in type II diabetic patients and non-diabetics. Diabetologia. 2000;43:814–5. doi: 10.1007/s001250051380. [DOI] [PubMed] [Google Scholar]
- 41.Khosla UM, Zharikov S, Finch JL, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–42. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 42.Lundberg JO, Gladwin MT, Ahluwalia A, et al. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol. 2009;5:865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ritz E. Metabolic syndrome and kidney disease. Blood Purif. 2008;26:59–62. doi: 10.1159/000110566. [DOI] [PubMed] [Google Scholar]
- 44.Ruilope LM, Garcia-Puig J. Hyperuricemia and renal function. Curr Hypertens Rep. 2007;3:197–202. doi: 10.1007/s11906-001-0038-2. [DOI] [PubMed] [Google Scholar]
- 45.Parks EJ, Hellerstein MK. Carbohydrate-induced hypertriacylglycerolemia: historical perspective and review of biological mechanisms. Am J Clin Nutr. 2000;71:412–33. doi: 10.1093/ajcn/71.2.412. [DOI] [PubMed] [Google Scholar]
- 46.Roncal-Jimenez CA, Lanaspa MA, Rivard CJ, et al. Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake. Metabolism. 2011;60:1259–70. doi: 10.1016/j.metabol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cirillo P, Gersch MS, Mu W, et al. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J Am Soc Nephrol. 2009;20:457–9. doi: 10.1681/ASN.2008060576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monti LD, Barlassina C, Citterio L, et al. Endothelial nitric oxide synthase polymorphisms are associated with type 2 diabetes and the insulin resistance syndrome. Diabetes. 2003;52:1270–5. doi: 10.2337/diabetes.52.5.1270. [DOI] [PubMed] [Google Scholar]
- 49.Fernandez ML, Ruiz R, Gonzalez MA, et al. Association of NOS3 gene with metabolic syndrome in hypertensive patients. Thromb Haemost. 2004;92:413–8. doi: 10.1160/TH04-02-0103. [DOI] [PubMed] [Google Scholar]
- 50.Hord NG, Tang Y, Ryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr. 2009;90:1–10. doi: 10.3945/ajcn.2008.27131. [DOI] [PubMed] [Google Scholar]