Abstract
Introduction
Little information is available on the outcomes of Hodgkin's lymphoma in Chinese patients. We analyzed the clinical and histopathological characteristics, treatment types, clinical course and treatment outcomes of Hong Kong Chinese patients.
Material and methods
Patients with Hodgkin's lymphoma diagnosed from January 1991 to December 2010 were recruited. A retrospective analysis of these patients was performed.
Results
Sixty-seven Chinese patients (38 males and 29 females) were identified and the median age was 36 (range 16–80). Nodular sclerosis was the most common histology (54%), followed by mixed cellularity (36%). Twenty-four patients had early favorable, 20 patients had early unfavorable and 23 patients had advanced-stage diseases. The most common presentation was palpable lymph node or mass (85%) followed by fever, weight loss, night sweating and mediastinal mass. Ninety percent of patients received chemotherapy and 40% received radiotherapy as consolidation. Seven patients with stage I lymphoma received radiotherapy alone. ABVD (doxorubicin, bleomycin, vinblastine and dacarbazine) was the most commonly used chemotherapeutic regimen. Following treatment, 87% of patients achieved complete remission. Six patients relapsed after first remission and 3 achieved second remission after re-induction therapy. The 5-year overall survival of the entire cohort was 89% and the freedom from treatment failure (FFTF) at 5 years was 82%. The 5-year overall survival rate for early favorable, early unfavorable and advanced stages was 95.7%, 95.0% and 74.7%, respectively.
Conclusions
Despite the relatively low incidence of Hodgkin's lymphoma in Hong Kong Chinese, the treatment outcomes are comparable to Caucasian patients.
Keywords: Hodgkin disease, treatment outcome
Introduction
Hodgkin's lymphoma (HL) constitutes about 30% of all cases of lymphoma in the Caucasian population [1]. The rate of HL in the United States is 2.8 per 100,000 person-years [2]. However, a low incidence of 8.6% (13% of HL cases) was found in Chinese patients [3–8]. The reason for the lower incidence in the Chinese population is not known. With the combined modality treatment and the effective chemotherapeutic regimens, treatment outcomes in Caucasian populations are satisfactory with high long-term cure rates [9, 10]. The report concerning its clinical features and treatment outcomes in Asians is limited. There is little information on the natural history and treatment outcomes of Hodgkin's lymphoma in Chinese patients or the prevalence of hepatitis B virus (HBV) infection in Hodgkin's lymphoma. Hepatitis B is endemic in Hong Kong. Since previous studies showed a higher association between HBV and B-cell non-Hodgkin's lymphoma, we would also like to test for any association between HBV and Hodgkin's lymphoma [11–13].
We analyzed the characteristics, clinical course and treatment outcomes of Hong Kong Chinese patients in a tertiary hospital in the past 20 years. Comparisons with Caucasian populations are made. We also studied the prevalence of hepatitis B in patients with Hodgkin's lymphoma.
Material and methods
Patients aged 16 or above with Hodgkin's lymphoma diagnosed from January 1991 to December 2010 in Tuen Mun Hospital, a tertiary centre in Hong Kong, were recruited. The pathological specimens were classified according to the WHO classification. Patients were staged according to the Ann Arbor Staging system [14]. Staging procedures used included history and physical examination, chest radiography, computed tomography (CT) or proton emission tomography (PET-CT) and bone marrow biopsy. Bulky disease was defined as mediastinal mass with a maximal width more than one third of the maximal diameter on a standing posteroanterior chest X-ray or any mass greater than 10 cm in diameter. The types of treatment and chemotherapy were recorded. The use of adjuvant radiotherapy was also documented. Patients were classified into early favorable stage, early unfavorable stage and advanced stage according to the German Hodgkin Study Group (GHSG) risk stratification [15, 16]. The response and survival rates were reported for the patients of different stages.
The prevalence of hepatitis B in patients with Hodgkin's lymphoma was assessed. The hepatitis markers including hepatitis B surface antigen (HBsAg) and antibody (anti-HBs) were checked in the patients.
Treatment
The treatment plan for patients with early stage lymphoma was four cycles of chemotherapy followed by involved field radiotherapy. Stage IIB lymphoma and advanced stages (stage III or IV) were treated with 6 to 8 cycles of chemotherapy and radiotherapy for bulky tumors. Patients with refractory disease were given salvage chemotherapy. High-dose therapy followed by autologous stem cell transplant was done whenever possible.
Response
The response rate, overall survival and freedom from treatment failure (FFTF) were assessed. Complete remission was defined as disappearance of all detectable disease. Partial remission was defined as regression of tumor of more than 50%. Overall survival was calculated from the date of lymphoma diagnosis till death or censored at last date of follow-up. Freedom from treatment failure was defined as time from start of treatment to relapse or progressive disease, no complete remission (CR) at the end of first-line treatment, or death due to any cause.
Statistical analysis
Survival was estimated by the Kaplan-Meier method and the log-rank test was used to identify prognostic factors. Multivariate Cox regression analysis was done to identify independent predictors. The χ2 test was used to compare complete remission rate. Univariate analysis was performed to assess the prognostic factors including age, sex, stage, bulky disease, B symptoms, bone marrow involvement and elevation of lactate dehydrogenase (LDH) level for the overall survival. Then a Cox proportional hazards model that included factors with p < 0.05 in the univariate analysis was done. All p values were 2-sided and p values < 0.05 were considered statistically significant. Data were analyzed using SPSS version 11.
Results
Patient demographic and clinical features
A total of 67 Hong Kong Chinese patients were identified within the study period. There were 38 males and 29 females. The male to female ratio was 1.3 : 1. The median age was 36 (range 16–80). There was also an early peak in young adults aged 16–30, but there was not another peak at advanced age. Most of the patients had the histological subtypes of nodular sclerosis (54%), followed by mixed cellularity (36%), nodular lymphocyte predominant Hodgkin's lymphoma (9%) and lymphocyte rich (1%). None of the patients had the histology of lymphocyte depleted. The most common presentation was palpable lymph node or mass (85%) followed by fever, weight loss and night sweating (Table I). Five patients (7%) developed a mediastinal mass with superior vena cava obstruction at presentation. The median follow-up time was 92 months (range: 11–241 months). At the time of diagnosis, 24 patients (36%) had early favorable disease, 20 patients (30%) had early unfavorable disease and 23 patients (34%) had advanced-stage disease. Ten patients (15%) underwent PET-CT scan for staging.
Table I.
Baseline patient characteristics
| Characteristics | Results, n (%) |
|---|---|
| Age [years]: | |
| 16–30 | 26 (39) |
| 31–45 | 15 (22) |
| 46–60 | 11 (17) |
| > 60 | 15 (22) |
| Gender: | |
| Male | 38 (57) |
| Female | 29 (43) |
| Symptoms: | |
| Palpable lymph node or mass | 57 (85) |
| Fever | 7 (10) |
| Weight loss | 6 (9) |
| Night sweating | 4 (6) |
| Mediastinal mass with superior vena cava obstruction | 5 (7) |
| Histology type: | |
| Nodular lymphocyte predominant | 6 (9) |
| Nodular sclerosis | 36 (54) |
| Mixed cellularity | 24 (36) |
| Lymphocyte rich | 1 (1) |
| Lymphocyte depleted | 0 (0) |
| Stage: | |
| I | 17 (25) |
| II | 29 (43) |
| III | 15 (23) |
| IV | 6 (9) |
| B symptoms: | |
| Yes | 17 (25) |
| No | 50 (75) |
| Bulky disease: | |
| Yes | 5 (7) |
| No | 62 (93) |
| Bone marrow involvement: | |
| Yes | 3 (4) |
| No | 64 (96) |
| LDH elevation: | |
| Yes | 26 (39) |
| No | 41 (61) |
LDH – lactate dehydrogenase
Treatment
Chemotherapy
Sixty patients received chemotherapy. They received various chemotherapeutic regimens including ABVD (doxorubicin, bleomycin, vinblastine and dacarbazine) (n = 40), ChlVPP (chlorambucil, vinblastine, procarbazine and prednisolone) (n = 13), Stanford V (mechlorethamine, doxorubicin, vinblastine, vincristine, bleomycin, etoposide and prednisolone) (n = 2), MOPP/ABV (mechlorethamine, vincristine, procarbazine, prednisolone/doxorubicin, bleomycin, vinblastine) (n = 1), ChlVPP/EVA (ChlVPP/etoposide, vincristine, doxorubicin) (n = 1), and escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisolone) (n = 3). The ABVD was the most commonly used regimen (67% of all chemotherapeutic regimens).
Radiotherapy
A total of 31 patients received radiation therapy. Radiotherapy alone was given to 7 patients with early stage (stage IA) disease. Among those treated with chemotherapy, 24 patients (40%) also received radiotherapy. Seventy-one percent received involved field radiotherapy (IFRT) and 29% received mantle field RT. The radiotherapy doses ranged from 20 to 40 Gy.
Outcomes
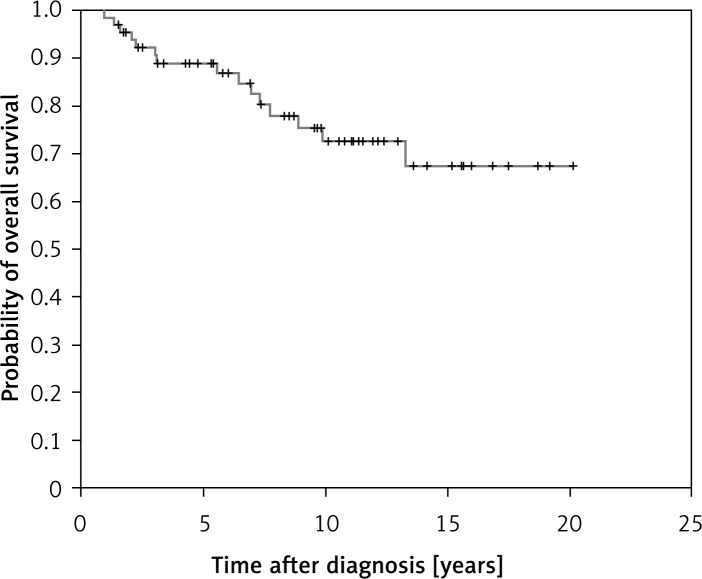
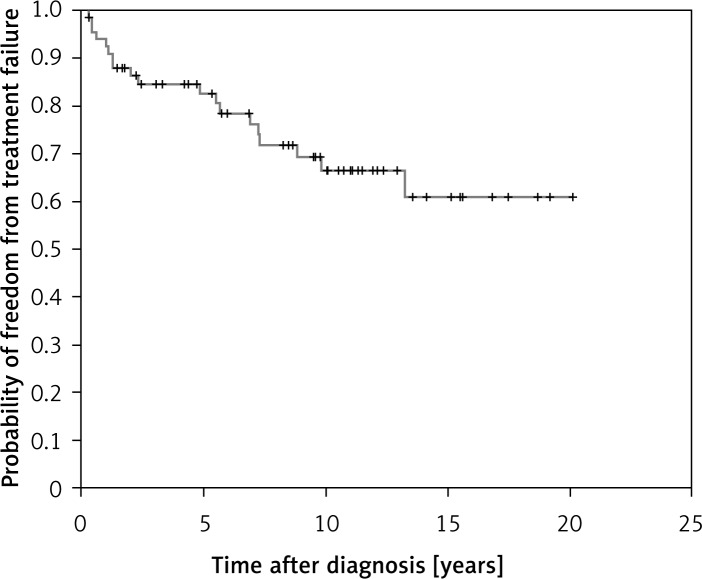
Eighty-seven percent of patients achieved CR after treatment. The CR rate for early favorable, early unfavorable and advanced stages was 96%, 95% and 71%, respectively. The 5-year overall survival was 89% for all the patients (Figure 1). The FFTF at 5 years was 82% (Figure 2). The 5-year overall survival rate for early favorable, early unfavorable and advanced stages was 95.7%, 95.0% and 74.7%, respectively. The rate of FFTF at 5 years for early favorable, early unfavorable and advanced stages was 94.7%, 91.5% and 68.6%, respectively. Three patients had disease progression and were still refractory to salvage chemotherapy and died of lymphoma. Six patients relapsed after first remission and three achieved second remission after re-induction therapy and they were still alive. One patient underwent autologous stem cell transplant and complete remission was subsequently achieved. Another 3 patients were still refractory to chemotherapy and died of disease progression.
Figure 1.
Overall survival of the entire cohort
Figure 2.
Freedom from treatment failure of the entire cohort
The ABVD was the most commonly used chemotherapeutic regimen. The CR rate for patients given ABVD was 85%. The CR rate for non-ABVD regimens was 80%.
Prognostic factors
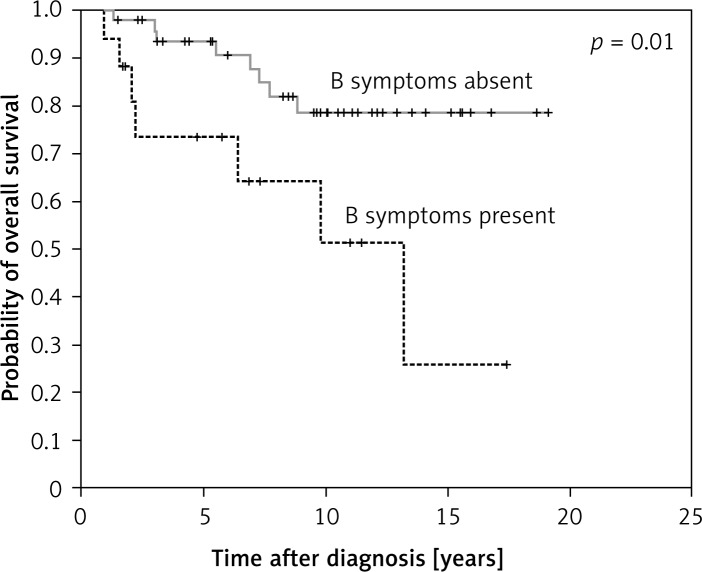
Of the potential prognostic factors analyzed, presence of B symptoms was found to adversely affect overall survival (p = 0.01, hazard ratio 3.65 (CI 1.32–10.11)) (Figure 3).
Figure 3.
Presence of B symptoms adversely affect the overall survival of the patients
Toxicities and secondary malignancies
Two patients (3%) developed secondary malignancies after chemotherapy. One patient developed carcinoma of the colon 5 years after therapy and she received chemotherapy (ChlVPP for 6 courses) and involved field radiotherapy. Another patient developed carcinoma of the lungs 13 years after treatment for lymphoma and she received chemotherapy (ChlVPP for 6 courses) only. The patient with carcinoma of the colon was cured and the other one died of carcinoma of the lung.
Six patients (9%) developed neutropenic fever that was controlled by antibiotics. Two patients (3%) developed cardiotoxicity after ABVD chemotherapy. There was no pulmonary complication documented in the patients.
Hepatitis B in Hodgkin's lymphoma
The rate of hepatitis B infection was 1.5% (1/67) in our cohort. Anti-HBs were detected in 32% (22/67) of our patients. There was a flare-up of hepatitis B in that patient with stage II lymphoma and positive HBsAg after receiving 3 courses of Stanford V chemotherapy. The alanine aminotransferase rose to 1000 U/l. His clotting profile was normal and ultrasound of the abdominal system did not show any hepatic lesion. Chemotherapy was stopped and the patient was given lamivudine 100 mg daily and liver enzymes gradually normalized. He was also in complete remission for the lymphoma.
Discussion
The relatively low incidence of Hodgkin's lymphoma in Hong Kong has been a consistent finding. The cause of the low incidence is not known and has been postulated to be due to genetic and viral factors. Orientals may have a relatively high incidence of T-cell lymphoma, especially the NK-T cell lymphoma.
The distribution of histologic subtypes was quite similar to the pattern found in Caucasians with nodular sclerosis being the most common histology followed by mixed cellularity. The incidence was higher in male patients. There was also an early peak in young adults but we did not observe a bimodal age distribution in our cohort. In the Caucasian population, there was an early peak in young adulthood and another peak at advanced age.
There was a higher proportion of early stage diseases (68% of patients at stage I and II) in our patients. This may be due to the lower proportion of elderly patients (22% with age more than 60) in our study. The elderly HL patients commonly present with advanced stage disease and have a worse prognosis [16, 17]. The lower number of elderly HL patients may be due to the under-diagnosis of lymphoma in this age group. They were also diagnosed at the advanced stage.
Ten patients underwent PET-CT scan for staging and it was used more commonly in our patients in the last few years. Positron emission tomography plays an important role in evaluation of Hodgkin lymphoma, for staging and therapeutic assessment [18, 19]. The PET scan has a prognostic value for evaluation of therapy [20–22]. It also has a high negative predictive value. The German Hodgkin Study Group (GHSG) HD 15 study showed that the patients who were in PET-negative partial remission after chemotherapy had a prognosis similar to that of patients in complete remission [23].
The CR rate, the 5-year overall survival and FFTF in our cohort were comparable to Caucasian patients, especially for patients of the early favorable and early unfavorable stages [24–27]. It was found that the overall survival and FFTF at 5 years were 94.5% and 85% respectively for patients of early unfavorable stage in the GHSH HD 11 study [26]. The 5-year overall survival was 96–97% in patients of early favorable stage as shown in the GHSG HD 10 study. Two cycles of ABVD followed by 20 Gy IFRT is regarded the standard of treatment in early favorable HL patients based on the results of the GHSH HD 10 study [24]. The outcomes of the patients of advanced stage in our cohort were inferior to those of the Caucasian patients [28, 29].
The ABVD chemotherapy was the most commonly used chemotherapeutic regimen in our patients. The complete remission rate of patients given ABVD in this study was satisfactory, comparable to the results in Caucasian patients [30, 31]. The ABVD is the most commonly used regimen for the treatment of Hodgkin's lymphoma in most countries. It is the treatment of choice due to its efficacy and more favorable toxicity profile [10, 32]. It has been compared with a number of regimens such as Stanford V, alternating and hybrid multidrug regimens, and they did not demonstrate a clear superiority over ABVD [30–35]. Escalated BEACOPP was shown to have a better survival rate than ABVD in advanced stage patients but also associated with increased toxicities and infections [28, 36].
Presence of B symptoms was identified as a poor prognostic factor for overall survival. We could not prove other prognostic factors such as advanced age and male sex [37] and it is likely due to the small sample size of our patient group.
The rate of secondary malignancy was 3% and was comparable to Caucasian patients [31]. The 2 affected patients both received ChlVPP chemotherapy and developed solid tumors after chemotherapy.
The rate of HBV infection was low (1.5%) in our cohort. The prevalence of HBV carrier has been estimated to be 10% in Hong Kong Chinese. Previous studies showed a higher association between HBV and B-cell non-Hodgkin's lymphoma [11–13]. The outcome of B-cell non-Hodgkin's lymphoma has been improved by rituximab-based chemotherapy [38]. It was proposed that HBV can integrate in the host genome, leading to overexpression of cellular oncogenes or downregulation of the expression of tumor suppression genes [39]. Another possible mechanism is chronic antigenic stimulation and proliferation of B-lymphocytes. This may predispose to genetic aberrations or induction of double-strand DNA breaks and events that would induce neoplastic transformation and proliferation.
The low prevalence of HBV in Hodgkin's lymphoma in our patients did not suggest an association between these two diseases. This low prevalence could possibly be a protective factor for the development of lymphoma, resulting in lower incidence of Hodgkin's lymphoma in the Chinese population.
The reactivation of hepatitis B after giving chemotherapy in our patient and the subsequent liver impairment showed the importance of preemptive treatment with an anti-viral agent. The patient received three courses of Stanford V therapy. Previous studies showed that lamivudine prophylaxis could reduce the incidence and severity of HBV infection for patients receiving chemotherapy for lymphoma [40–42]. The disruption of chemotherapy could also be reduced.
The study is limited by its retrospective nature and the relatively small sample size, reflecting the low incidence of Hodgkin's lymphoma in Hong Kong Chinese. Although the sample size is small, it can give more information on Hodgkin's lymphoma in the Asian population.
In conclusions, despite the lower incidence of Hodgkin's lymphoma in Hong Kong Chinese, the treatment outcomes are comparable to Caucasian patients. The low prevalence of hepatitis B infection in the Hodgkin's lymphoma patients of an endemic area did not suggest any association between Hodgkin's lymphoma and HBV infection.
Acknowledgments
Man Fai Law and Ting Ying Ng contributed equally to this work.
References
- 1.Jaffe ES, Harris LN, Stein H, Vatdiman JW. IARC Press; 2001. Tumours of haematopoietic and lymphoid tissues. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; pp. 240–53. [Google Scholar]
- 2.Altekruse S, Kosary C, Krapcho M, et al. SEER cancer statistics review, 1975-2007; Bethesda: National Cancer Institute; [Google Scholar]
- 3.Liang R, Choi P, Todd D, et al. Hodgkin's disease in Hong Kong Chinese. Hematol Oncol. 1989;7:395–403. doi: 10.1002/hon.2900070602. [DOI] [PubMed] [Google Scholar]
- 4.Yang QP, Zhang WY, Yu JB, et al. Subtype distribution of lymphomas in Southwest China: analysis of 6,382 cases using WHO classification in a single institution. Diagn Pathol. 2011;6:77. doi: 10.1186/1746-1596-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Au WY, Gascoyne RD, Gallagher RE, et al. Hodgkin's lymphoma in Chinese migrants to British Columbia: a 25-year survey. Ann Oncol. 2004;15:626–30. doi: 10.1093/annonc/mdh132. [DOI] [PubMed] [Google Scholar]
- 6.Sun J, Yang Q, Lu Z, et al. Distribution of lymphoid neoplasms in China: analysis of 4,638 cases according to the World Health Organization classification. Am J Clin Pathol. 2012;138:429–34. doi: 10.1309/AJCP7YLTQPUSDQ5C. [DOI] [PubMed] [Google Scholar]
- 7.Huang X, Nolte I, Gao Z, et al. Epidemiology of classical Hodgkin lymphoma and its association with Epstein Barr virus in Northern China. PLoS One. 2011;6:e21152. doi: 10.1371/journal.pone.0021152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu YJ, Sun YL, Xia Y, et al. Clinical characteristics and prognostic factors in Chinese patients with Hodgkin's lymphoma. Med Oncol. 2012;29:1127–33. doi: 10.1007/s12032-011-9902-3. [DOI] [PubMed] [Google Scholar]
- 9.Bonadonna G, Zucali R, Monfardini S, et al. Combination chemotherapy of Hodgkin's disease with adriamycin, bleomycin, vinblastine and imidazole carboxamide versus MOPP. Cancer. 1975;36:252–9. doi: 10.1002/1097-0142(197507)36:1<252::aid-cncr2820360128>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Canellos GP, Niedzwiecki D, Johnson JL. Long-term follow-up of survival in Hodgkin's lymphoma. N Engl J Med. 2009;361:2390–1. doi: 10.1056/NEJMc0906731. [DOI] [PubMed] [Google Scholar]
- 11.Wang F, Xu RH, Han B, et al. High incidence of hepatitis B virus infection in B-cell subtype non-Hodgkin lymphoma compared with other cancers. Cancer. 2007;109:1360–4. doi: 10.1002/cncr.22549. [DOI] [PubMed] [Google Scholar]
- 12.Park SC, Jeong SH, Kim J, et al. High prevalence of hepatitis B or C virus infection in patients with B-cell non-Hodgkin's lymphoma in Korea. J Med Virol. 2008;80:960–6. doi: 10.1002/jmv.21168. [DOI] [PubMed] [Google Scholar]
- 13.Engels EA, Cho ER, Jee SH. Hepatitis B virus infection and risk of non-Hodgkin lymphoma in South Korea: a cohort study. Lancet Oncol. 2010;11:827–34. doi: 10.1016/S1470-2045(10)70167-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carbone PP, Kaplan HS, Musshoff K, et al. Report of the committee on Hodgkin's disease staging. Cancer Res. 1971;31:1860–1. [PubMed] [Google Scholar]
- 15.Eichenauer DA, Engert A, Dreyling M. Hodgkin's lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(Suppl 6):vi55–8. doi: 10.1093/annonc/mdr378. [DOI] [PubMed] [Google Scholar]
- 16.Böll B, Bredenfeld H, Görgen H, et al. Phase 2 study of PVAG (prednisone, vinblastine, doxorubicin, gemcitabine) in elderly patients with early unfavorable or advanced stage Hodgkin lymphoma. Blood. 2011;118:6292–8. doi: 10.1182/blood-2011-07-368167. [DOI] [PubMed] [Google Scholar]
- 17.Evens AM, Sweetenham JW, Horning SJ. Hodgkin lymphoma in older patients: an uncommon disease in need of study. Oncology (Williston Park) 2008;15:1369–79. [PubMed] [Google Scholar]
- 18.Ansquer C, Hervouët T, Devillers A, et al. 18-F FDG-PET in the staging of lymphocyte-predominant Hodgkin's disease. Haematologica. 2008;93:128–31. doi: 10.3324/haematol.11661. [DOI] [PubMed] [Google Scholar]
- 19.Weihrauch MR, Re D, Bischoff S, et al. Whole-body positron emission tomography using 18F-fluorodeoxyglucose for initial staging of patients with Hodgkin's disease. Ann Hematol. 2002;81:20–5. doi: 10.1007/s00277-001-0390-y. [DOI] [PubMed] [Google Scholar]
- 20.Zinzani PL, Tani M, Fanti S, et al. Early positron emission tomography (PET) restaging: a predictive final response in Hodgkin's disease patients. Ann Oncol. 2006;17:1296–300. doi: 10.1093/annonc/mdl122. [DOI] [PubMed] [Google Scholar]
- 21.Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin's lymphoma: a report from a joint Italian-Danish study. J Clin Oncol. 2007;25:3746–52. doi: 10.1200/JCO.2007.11.6525. [DOI] [PubMed] [Google Scholar]
- 22.Mocikova H, Obrtlikova P, Vackova B, Trneny M. Positron emission tomography at the end of first-line therapy and during follow-up in patients with Hodgkin lymphoma: a retrospective study. Ann Oncol. 2010;21:1222–7. doi: 10.1093/annonc/mdp522. [DOI] [PubMed] [Google Scholar]
- 23.Engert A, Haverkamp H, Kobe C, et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin's lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379:1791–9. doi: 10.1016/S0140-6736(11)61940-5. [DOI] [PubMed] [Google Scholar]
- 24.Engert A, Plütschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin's lymphoma. N Engl J Med. 2010;363:640–52. doi: 10.1056/NEJMoa1000067. [DOI] [PubMed] [Google Scholar]
- 25.Meyer RM, Gospodarowicz MK, Connors JM, et al. ABVD alone versus radiation based therapy in limited-stage Hodgkin's lymphoma. N Engl J Med. 2012;366:399–408. doi: 10.1056/NEJMoa1111961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eich HT, Diehl V, Gorgen H, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin's lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol. 2010;28:4199–206. doi: 10.1200/JCO.2010.29.8018. [DOI] [PubMed] [Google Scholar]
- 27.von Tresckow B, Plütschow A, Fuchs M, et al. Dose-intensification in early unfavorable Hodgkin's lymphoma: final analysis of the German hodgkin study group HD14 trial. J Clin Oncol. 2012;30:907–13. doi: 10.1200/JCO.2011.38.5807. [DOI] [PubMed] [Google Scholar]
- 28.Engert A, Diehl V, Franklin J, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin's lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27:4548–54. doi: 10.1200/JCO.2008.19.8820. [DOI] [PubMed] [Google Scholar]
- 29.Borchmann P, Haverkamp H, Diehl V, et al. Eight cycles of escalated-dose BEACOPP compared with four cycles of escalated-dose BEACOPP followed by four cycles of baseline-dose BEACOPP with or without radiotherapy in patients with advanced-stage hodgkin's lymphoma: final analysis of the HD12 trial of the German Hodgkin Study Group. J Clin Oncol. 2011;29:4234–42. doi: 10.1200/JCO.2010.33.9549. [DOI] [PubMed] [Google Scholar]
- 30.Hoskin PJ, Lowry L, Horwich A, et al. Randomized comparison of the stanford V regimen and ABVD in the treatment of advanced Hodgkin's Lymphoma: United Kingdom National Cancer Research Institute Lymphoma Group Study ISRCTN 64141244. J Clin Oncol. 2009;27:5390–6. doi: 10.1200/JCO.2009.23.3239. [DOI] [PubMed] [Google Scholar]
- 31.Johnson PW, Radford JA, Cullen MH, et al. Comparison of ABVD and alternating or hybrid multidrug regimens for the treatment of advanced Hodgkin's lymphoma: results of the United Kingdom Lymphoma Group LY09 Trial (ISRCTN97144519) J Clin Oncol. 2005;23:9208–18. doi: 10.1200/JCO.2005.03.2151. [DOI] [PubMed] [Google Scholar]
- 32.Duggan DB, Petroni GR, Johnson JL, et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin's disease: report of an intergroup trial. J Clin Oncol. 2003;21:607–14. doi: 10.1200/JCO.2003.12.086. [DOI] [PubMed] [Google Scholar]
- 33.Canellos GP, Gollub J, Neuberg D, et al. Primary systemic treatment of advanced Hodgkin's disease with EVA (etoposide, vinblastine, doxorubicin): 10-year follow-up. Ann Oncol. 2003;14:268–72. doi: 10.1093/annonc/mdg076. [DOI] [PubMed] [Google Scholar]
- 34.Martinelli G, Cocorocchio E, Peccatori F, et al. ChlVPP/ABVVP, a first line ‘hybrid'combination chemotherapy for advanced Hodgkin's lymphoma: a retrospective analysis. Br J Haematol. 2004;125:584–9. doi: 10.1111/j.1365-2141.2004.04962.x. [DOI] [PubMed] [Google Scholar]
- 35.Horning SJ, Hoppe RT, Breslin S, et al. Stanford V and radiotherapy for locally extensive and advanced Hodgkin's disease: mature results of a prospective clinical trial. J Clin Oncol. 2002;20:630–7. doi: 10.1200/JCO.2002.20.3.630. [DOI] [PubMed] [Google Scholar]
- 36.Federico M, Luminari S, Iannitto E, et al. ABVD compared with BEACOPP compared with CEC for the initial treatment of patients with advanced Hodgkin's lymphoma: results from the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. J Clin Oncol. 2009;27:805–11. doi: 10.1200/JCO.2008.17.0910. [DOI] [PubMed] [Google Scholar]
- 37.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease. N Engl J Med. 1998;19:1506–14. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 38.Doumas S, Sakkas L, Panayiotidis P, Wozniak G, Vlychou M, Vassilopoulos G. Favorable outcome in non-Hodgkin lymphoma of the maxillary sinus treated with R-CHOP. Arch Med Sci. 2014;10:406–9. doi: 10.5114/aoms.2013.34986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang XW, Gibson MK, Vermeulen W, et al. Abrogation of p53-induced apoptosis by the hepatitis B virus X gene. Cancer Res. 1995;55:6012–6. [PubMed] [Google Scholar]
- 40.He YF, Li YH, Wang FH, et al. The effectiveness of lamivudine in preventing hepatitis B viral reactivation in rituximab-containing regimen for lymphoma. Ann Hematol. 2008;87:481–5. doi: 10.1007/s00277-008-0454-3. [DOI] [PubMed] [Google Scholar]
- 41.Ziakas PD, Karsaliakos P, Mylonakis E, et al. Effect of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in lymphoma: a meta-analysis of published clinical trials and a decision tree addressing prolonged prophylaxis and maintenance. Haematologica. 2009;94:998–1005. doi: 10.3324/haematol.2009.005819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen XQ, Peng JW, Lin GN, et al. The effect of prophylactic lamivudine on hepatitis B virus reactivation in HBsAg-positive patients with diffuse large B-cell lymphoma undergoing prolonged rituximab therapy. Med Oncol. 2012;29:1237–41. doi: 10.1007/s12032-011-9974-0. [DOI] [PubMed] [Google Scholar]